Abstract
Scurvy is a disease caused by chronic vitamin C deficiency. The greater prevalence was found in the paediatric population with neurodevelopmental disorders such as autism spectrum disorders due to their restricted dietary intake. Our case reported a child with autism who presented with arthralgia and anaemia. Systemic lupus erythematosus was the first diagnostic impression, resulting in over investigation and delayed diagnosis of vitamin C deficiency. After the child was treated with ascorbic acid, the child’s symptoms resolved. This case highlighted the importance of developmental and nutritional history taking in the paediatric population. Furthermore, parents and physicians should be concerned about nutritional status, especially in children with restrictive dietary intake.
Keywords: paediatrics, developmental paediatrocs
Background
Scurvy is a disease resulting from chronic vitamin C deficiency. The manifestation of vitamin C deficiency has a wide spectrum of symptoms from early minor illnesses, such as loss of appetite and bleeding per gums, to the advanced stage of internal organs bleeding, and ultimately death if left untreated.1 2 Vitamin C plays essential roles involved in collagen biosynthesis.3 It also acts as an antioxidant, enzymatic cofactor and immune system booster.4 5 Owing to the inability to convert or synthesise vitamin C in the human body, this vitamin is exclusively obtained from foods.
Scurvy is rare in healthy typically developmental children.6 7 The greater prevalence was found in the particular paediatric population with special needs such as cerebral palsy and autism spectrum disorders (ASD) because of their restricted dietary intake.8 Thus, this leads to subsequently unfavourable outcomes of certain types of diet restriction. However, scurvy is often overlooked and misdiagnosed due to its less fatal and low incidence. Here, we present a case of children with autism who presented with arthralgia and anaemia. The autoimmune disease such as systemic lupus erythematosus (SLE) was suspected at the first admission. Thus, laboratory tests for autoimmune diseases were conducted before the diagnosis of vitamin C deficiency was made.
Case presentation
An 11-year-old girl had a problem of joint pain and limping gait for 1 week. She had pain at her right ankle, which added to develop the pain at the other side of the ankle 2 days later. Her mother noticed that her daughter was pale and looked fatigued. There were bruises on her legs. The mother said the symptoms of joint pain, pallor and fatigue were progressive during 1 week, ultimately causing her daughter to refuse to walk. One day before the admission, she started to throw up, limited food intake due to loss of appetite, and presented with myalgia. At this time, she had pain of the fingers and hands. Her mother gave her analgesia and blood tonic. She denied a history of fever, diarrhoea, rash or oral ulcer.
Her mother denied a history of recent illnesses in her daughter and current medicine intake. She also rejected a history of trauma and injury. The girl was generally healthy except for her previous admission to another provincial hospital last year. At that time, she had similar symptoms of joint pain, pallor and fatigue. Her mother did not recall the definite diagnosis but reported that her daughter received a blood transfusion and some medications. The child was born full-term and delivered by elective caesarean section with normal Apgar scores. Her birth weight was 3800 g. There were no complications at birth. She was the only child in the family. Her parents divorced several years ago. She and her mother relocated for work last year. There was no family history of autoimmune diseases, haematologic diseases or malignancies.
On physical examination, the child was crying, screaming and refusing to cooperate with the examination and peripheral intravenous cannulation. She was orientated only to time and person. She had pale conjunctivae and anicteric sclerae. Lymph nodes could not be palpated. Heart and lung sounds were within normal limits. There was mild tenderness at the periumbilical abdomen without guarding or palpable liver and spleen. All joints were thoroughly examined with mild swelling, tenderness and warmth around both knees and ankles, and left wrist. No swelling, tenderness and warmth of the fingers were noted. There were no oral ulcers and skin rash, except for a bruise on her left leg. A complete neurological examination was conducted and found within normal limits. Growth parameters were within the normal range for her age and sex.
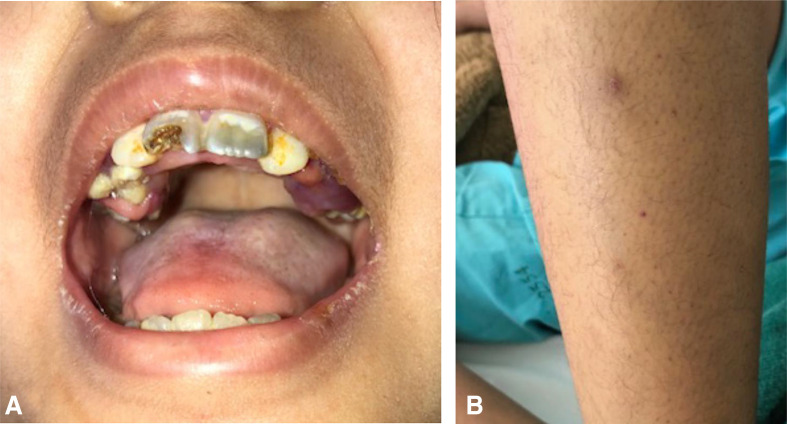
The first diagnostic impression was SLE with alteration of consciousness, her symptoms were possible a lupus psychosis. The laboratory investigations involving such disease and paediatric neurologist consultation promptly proceeded. During admission, the doctor noticed that the patient had abnormal behaviours and tone of voice and speech. This time she was orientated to time, place and person. She could answer questions correctly but sometimes repeated sentences right after the doctor asked. Since then, the developmental history was reviewed and found that she had below-average performance than her peers. Although she could study and pass the grades, she rarely got along well with her friends. Her aggressive behaviours were often precipitated by fear and anxiety. She began walking at 1 year of age and began talking 2 years later. When she was a toddler, she had repetitive and restrictive behaviours such as playing with the same toys and listening to the same songs repeatedly. She was hypersensitive to every hard and rough-textured food. Thus, she would eat only ground food or rice porridge and ultra-high temperature processing (UHT) milk, six boxes per day. She had limited preferences for some fruits and vegetables. ASD with avoidant/restrictive food intake disorder (ARFID) and vitamin C deficiency were new possible diagnoses. The complete physical examination was repeated and evidence supporting scurvy was found, for example, swollen and bleeding per gums and perifollicular haemorrhage (figure 1).
Figure 1.
Characteristic signs of vitamin C deficiency. (A) Swollen inflamed gums. (B) Perifollicular haemorrhage.
Investigations
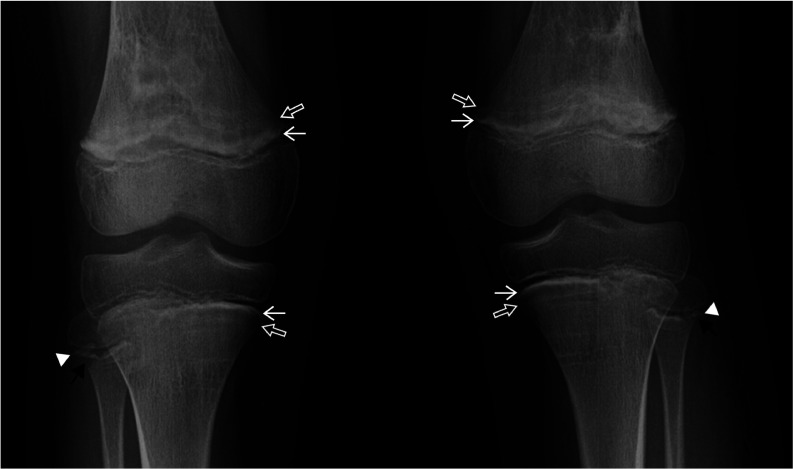
The laboratory results for autoimmune diseases were unremarkable. The antinuclear antibody was negative. C3 and C4 complements were not low levels. The anti-double stranded DNA antibody was <10 IU/mL. Serum lactate dehydrogenase was 368 U/L and total creatine kinase was 104 U/L. To confirm scurvy diagnosis, the vitamin C level was revealed a level of <0.1 mg/dL (reference range 0.2–4.0 mg/dL). In addition, the radiographic evaluation of long bones showed the signs that were compatible with scurvy (figure 2).
Figure 2.
Radiographic features of scurvy. Radiograph of the knees shows thickened zone of provisional calcification (white line of Fränkel; white arrows) and subjacent prominent lucent zone (Trümmerfeld zone) in the metaphyses of femurs and tibiae (open arrows). Note: metaphyseal spurs (Pelkan spurs; arrowheads) and peripheral lucent cleft near the provisional zone of calcification of fibulae (corner sign; black arrows).
The anaemia laboratory investigations, prior to blood transfusion, reported low haemoglobin (Hb) and haematocrit (Hct) levels (Hb 58 g/L (reference range: 120–160 g/L) and Hct 18.1% (reference range: 36.0%–48.0%)). Direct and indirect antiglobulin tests were negative. Red blood cell indices, peripheral blood smear and iron studies indicated iron deficiency anaemia as low mean corpuscular volume (69.1 fL); high red cell distribution width (17.2%); low serum iron (18 µg/dL) and low transferrin saturation (8.45%).
For ASD, the doctor who was in charge of the ward consulted the developmental-behavioural paediatrician. The diagnosis of ASD and ARFID were made by meeting the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria.
Differential diagnosis
Apart from the joint pain, complaining of pain in extremities raised concern of myositis at the first presentation because the children may have difficulties localising pain precisely. In this case, we were concerned about viral myositis. However, the combination of lack of prodrome symptoms, disease progression and no elevation of muscle enzymes could exclude the viral myositis.
Juvenile dermatomyositis is another suspicious autoimmune disease, but it was ruled out by the absence of typical rash, muscle weakness and increase of muscle enzymes.
Despite the absence of hepatosplenomegaly and blast cells on peripheral blood smear, progressive anaemia and bone pain still lead to consideration of acute leukaemia with leukaemic arthritis. This patient did not have highly suggestive evidence of leukaemia, such as bicytopenia or pancytopenia and significant increase of lactate dehydrogenase. Nevertheless, we could not explicitly rule out acute leukaemia until we received confirmation of vitamin C deficiency.
Finally, we excluded SLE because it did not meet the criteria for SLE. The vitamin C deficiency was a definite diagnosis from the characteristic clinical pictures, history of insufficient vitamin C intake, radiographic findings and confirmed low serum vitamin C level.
Treatment
The patient was treated with vitamin C supplementation as a recommendation of 500 mg/day. Vitamin C 250 mg two times per day was prescribed for at least 2 weeks or until the symptoms resolved.
For iron deficiency anaemia, the patient was given a red blood cell transfusion because of her significant anaemic symptoms. An iron supplement was also provided to the patient at discharge.
Furthermore, the doctor advised the patient’s mother to limit milk consumption not to exceed >3 cups per day and set regularly scheduled meals and snack times. The patient was also scheduled to consult an occupational therapist to help desensitise some specific types of food that the patient was hypersensitive to.
Outcome and follow-up
The symptoms of swollen inflamed gums and perifollicular haemorrhage were resolved after vitamin C supplementation. At 3-month follow-up, she could eat a variety of foods and textures. Moreover, she was sent to school for children with special needs.
Discussion
We report the case of a child with autism who presented with arthralgia and anaemia. The autoimmune disease such as SLE was the first suspected disease, resulting in over investigation and delayed diagnosis of vitamin C deficiency.
Vitamin C deficiency was missed in this case for several reasons. First, the delayed developmental history was concealed. Certainly, the known underlying neurodevelopmental disorder such as autism could help the physician’s vigilance regarding the potential nutritional deficiency at an early visit due to the hint of their food restriction. In children with autism, it is common to have feeding problems. They are usually highly restricted to some particular foods, and also have hypersensitivity to some textured foods, as seen in some case reports and series.9–13 Most cases were children approximately aged 6–14 years, which was consistent with this case. The possible explanation was the children with autism would have surveillance for nutrition in the regular visits at the early period of ASD diagnosis. As they get older, they might rarely adhere to the scheduled visits and follow the doctor’s suggestions. In contrast to children with neurodevelopmental disorders, the typically developmental children with vitamin C deficiency often present symptoms at younger ages.14–16 Those typically developmental children who had scurvy potentially related to either having organic diseases such as allergies to specific foods or living in poor environments such as low socioeconomic status and extremely permissive parenting.17 18
Second, the physician was diverged to autoimmune disease due to the female sex and age at the onset of presentation usually found in childhood-onset SLE.19 The symptoms of symmetrical polyarthralgia affecting large and small joints, fatigue and anaemia in this patient are the most common presenting complaints of children with SLE. Bizarre behaviours accompanied by alteration of consciousness on the first day of admission misled the physician to think of the neuropsychiatric complications of SLE that may occur in the early years of disease.20
To the best of our knowledge, this case was the first reported vitamin C deficiency mimicking SLE in children. One similar adult case of vitamin C deficiency presented with joint pain and purpuric vasculitic rash misdiagnosed as active SLE was previously reported.21 However, there are evidences of clinical symptom mimicry between vitamin C deficiency and other diseases. A 9-year-old boy refused to walk. The examination revealed swelling and warmth of the knee joint and purulent discharge per gums. Due to the initial diagnosis of osteomyelitis, he was given antibiotics and aspirated fluid collection. Until restrictive diet history was revealed, the doctor stopped antibiotics and treated him with vitamin C supplement.7 The similar scenarios were reported in children and young adult with vitamin c deficiency, but confusing with juvenile idiopathic arthritis and reactive arthritis.22 23
The lessons learnt in this case were we conducted costly unnecessary investigations, unrelated treatments and delayed diagnosis. Scurvy should be a differential diagnosis of musculoskeletal complaints and petechial/purpuric skin manifestations. This case report emphasised the importance of developmental and nutritional history taking in the paediatric population. In addition, parents and physicians taking care of such children should be aware of nutritional status, especially in children with restrictive dietary intake.
Patient’s perspective.
At first, I was afraid that my daughter might have some severe illnesses because of her progressive symptoms. When the doctor said that my daughter had to be consulted with a paediatric neurologist made me feel nervous. After my daughter was consoled, she was pretty normal. My anxious feeling was relieved when we have known the exact cause of the illness. The doctor explained to me about the diseases and treatments extensively. I appreciated the medical team for helping me through all this.
Learning points.
Scurvy should be considered in a case presenting with musculoskeletal manifestations such as joint/leg pain, limping gait and refusal to walk.
Developmental and dietary history should be reviewed in all paediatric patients, although they have normal growth parameters.
Feeding difficulties are common problems among children with neurodevelopmental disorders, especially highly selective intake that usually presents in children with autism. Hence, the physician should assess the dietary intake and nutritional status of such patients.
Footnotes
Contributors: NL: collected data, reviewed the literature and wrote the manuscript. WM: provided and described the X-ray images. NB and OL: supervised and edited the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Vannier S, Thomas R, Jean-christophe F. Vitamin C depletion is spontaneous intracerebral hemorrhage risk factor. Neurology 2014:82:S25.005. [Google Scholar]
- 2.Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group - A disease often forgotten? J Clin Orthop Trauma 2015;6:101–7. 10.1016/j.jcot.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients 2017;9:866. 10.3390/nu9080866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 2009;46:719–30. 10.1016/j.freeradbiomed.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr AC, Maggini S. Vitamin C and immune function. Nutrients 2017;9:1211. 10.3390/nu9111211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solanki M, Baweja DK, Patil SS, et al. Ascorbic acid deficiency: a case report. J Dent Child 2011;78:115–9. [PubMed] [Google Scholar]
- 7.Harknett KMW, Hussain SK, Rogers MK, et al. Scurvy mimicking osteomyelitis: case report and review of the literature. Clin Pediatr 2014;53:995–9. 10.1177/0009922813506609 [DOI] [PubMed] [Google Scholar]
- 8.Field D, Garland M, Williams K. Correlates of specific childhood feeding problems. J Paediatr Child Health 2003;39:299–304. 10.1046/j.1440-1754.2003.00151.x [DOI] [PubMed] [Google Scholar]
- 9.Lim PP, Juanna B, Bahadun J. Scurvy: a case report on a child with autism. Int J Clin Pediatr 2018;7:59–62. 10.14740/ijcp321 [DOI] [Google Scholar]
- 10.Rafee Y, Burrell K, Cederna-Meko C. Lessons in early identification and treatment from a case of disabling vitamin C deficiency in a child with autism spectrum disorder. Int J Psychiatry Med 2019;54:64–73. 10.1177/0091217418791443 [DOI] [PubMed] [Google Scholar]
- 11.Saavedra MJ, Aziz J, Cacchiarelli San Román N. Scurvy due to restrictive diet in a child with autism spectrum disorder: case report. Arch Argent Pediatr 2018;116:e684–7. 10.5546/aap.2018.eng.e684 [DOI] [PubMed] [Google Scholar]
- 12.Fortenberry M, Rucker H, Gaines K. Pediatric scurvy: how an old disease is becoming a new problem. J Pediatr Pharmacol Ther 2020;25:735–41. 10.5863/1551-6776-25.8.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp WG, Berry RC, Burrell L, et al. Scurvy as a sequela of avoidant-restrictive food intake disorder in autism: a systematic review. J Dev Behav Pediatr 2020;41:397–405. 10.1097/DBP.0000000000000782 [DOI] [PubMed] [Google Scholar]
- 14.Hahn T, Adams W, Williams K. Is vitamin C enough? A case report of scurvy in a five-year-old girl and review of the literature. BMC Pediatr 2019;19:74. 10.1186/s12887-019-1437-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambilla A, Pizza C, Lasagni D, et al. Pediatric scurvy: when contemporary eating habits bring back the past. Front Pediatr 2018;6:126. 10.3389/fped.2018.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alten ED, Chaturvedi A, Cullimore M, et al. No longer a historical ailment: two cases of childhood scurvy with recommendations for bone health providers. Osteoporos Int 2020;31:1001–5. 10.1007/s00198-019-05264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popovich D, McAlhany A, Adewumi AO, et al. Scurvy: forgotten but definitely not gone. J Pediatr Health Care 2009;23:405–15. 10.1016/j.pedhc.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 18.Bacci C, Sivolella S, Pellegrini J, et al. A rare case of scurvy in an otherwise healthy child: diagnosis through oral signs. Pediatr Dent 2010;32:536–8. [PubMed] [Google Scholar]
- 19.Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am 2012;59:345–64. 10.1016/j.pcl.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro-Checa C, Zirkzee EJ, Huizinga TW, et al. Management of neuropsychiatric systemic lupus erythematosus: current approaches and future perspectives. Drugs 2016;76:459–83. 10.1007/s40265-015-0534-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadia T, Lopez MC, Joks R, et al. P297 Topsy-turvy over scurvy: vitamin C deficiency masquerading as systemic lupus erythematosus in a 20-year-old female. Ann Allergy, Asthma Immunol 2017;119:S76. 10.1016/j.anai.2017.08.213 [DOI] [Google Scholar]
- 22.Perkins A, Sontheimer C, Otjen JP, et al. Scurvy masquerading as juvenile idiopathic arthritis or vasculitis with elevated inflammatory markers: a case series. J Pediatr 2020;218:234–7. 10.1016/j.jpeds.2019.10.059 [DOI] [PubMed] [Google Scholar]
- 23.Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis 2019;103:E21–3. [PubMed] [Google Scholar]