Abstract
Background: C-x-C motif chemokine ligands (CXCLs) are critical regulators of cancer immunity and angiogenesis, which affect disease progression and treatment responses. The character of each CXCL in the prognosis and immune infiltration of hepatocellular carcinoma (HCC) patients is unclear yet. Methods: Differentially expressed CXCLs between HCC and normal control were screened by Oncomine and GEPIA2. Genetic alternations of CXCLs in HCC were analyzed by cBioPortal. Clinicopathological relevance of CXCLs in HCC patients was analyzed using UALCAN. The prognostic value of CXCLs was evaluated using univariate and multivariate analyses. Correlations of CXCLs’ expression with immune infiltration, chemokines and their receptors were assessed integrating TIMER, TISIDB, and GEPIA2. The co-expressed genes of CXCLs were discovered, and functional enrichment analysis was performed for them. Results: CXCL9/10 was significantly higher expressed while CXCL2/12/14 was lower expressed in HCC than normal tissues, but they didn’t show significant clinicopathological relevance in HCC patients. High-expression of CXCL2/10/12/14 indicated favorable outcomes of HCC patients. The expression of CXCL9/10/12/14 was significantly positively correlated with not only the infiltration and biomarkers’ expression of various tumor-infiltrating immune cells but also the abundance of chemokines and their receptors. The co-expressed genes of the five CXCLs were extracellular components and regulated immune or inflammatory responses and signaling pathways of chemokine, Toll-like receptor and tumor necrosis factor might be involved. Conclusion: The present study proposed CXCL2/10/12/14 might predict outcomes of HCC patients and were extensively related with the immune microenvironment in HCC. It would be a prospective therapeutic strategy for HCC to enhance effective immunity surveillance through intervening in these CXCLs.
Keywords: chemokines, CXCL, hepatocellular carcinoma, immune infiltration, prognostic biomarker
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequently diagnosed and the fourth lethal cancer globally [1]. Due to occult symptoms, a majority of HCC patients are diagnosed at advanced stages when curative surgery is unavailable. Although dramatic achievements have been made in comprehensive treatments, frequent therapy resistance, recurrence, and metastasis lead to the poor prognosis of patients with a 5-year survival rate of about 12% [2]. Over the last 10 years, immunotherapies, especially immune checkpoint inhibitors, have revolutionized the field of cancer. However, the general clinical response of immunotherapy is unsatisfying due to cancer immune escape [3].
Chemokines are small secreted proteins that can induce migration of various cells, including immune cells, epithelial cells, and cancer cells; thus, they are important modulators of cancer immunity, angiogenesis, progression, and therapy [4]. The chemokine superfamily is divided into CC, CXC, CX3C, and XC subfamilies, based on the position of conserved cysteine residues [4]. C-x-C motif chemokine ligand (CXCL), in which the ‘C’s stand for two N-terminal cysteines separated by a random amino acid (‘x’). Sixteen CXCL family members have been identified in human and are named by identifying numbers from CXCL1 to CXCL17, except for CXCL15, which is only reported in mice [5]. CXCLs are further divided into two subgroups, depending on the presence of a Glu-Leu-Arg (ELR) motif at the first conserved cysteine residue. ELR+ CXCLs, including CXCL1-3, CXCL5-8, and CXCL17, bind to CXC chemokine receptor 2 (CXCR2) to promote endothelial cell survival and tumor angiogenesis, whereas most ELR− CXCLs, including CXCL4, CXCL9-11, and CXCL16, bind to CXCR3 to inhibit endothelial cell proliferation and angiogenesis [6].
Immune cells recruited into the tumor microenvironment (TME) by chemokines are called tumor-infiltrating immune cells (TIICs), which play critical roles in the initiation, progression, metastasis, and therapeutic response of cancers [7]. Distinct subsets of TIICs can act at opposite poles. For example, CD8+ T cells and natural killer (NK) cells exert effective anticancer immunity surveillance, whereas regulatory T cells (Tregs) and M2 tumor-associated macrophages (TAMs) foster immunosuppression in HCC [4,8]. Some CXCLs had been reported to promote cancer by stimulating immune-suppressive TIICs. For instance, CXCL16 might induce tumoral phenotypes in solid tumors by stimulating macrophage polarization [9]. By contrast, elevated expression of CXCL10 in tumor cells could increase NK cell infiltration in tumors and prolong NK cell-dependent survival of mice [10]. Therefore, it is a promising strategy to enhance the immunotherapy efficacy by increasing the infiltration of effective TIICs in HCC trough regulating CXCLs [11]. However, the roles of diverse CXCL family members and their interactions with TIICs in HCC are not fully elucidated.
In the present study, we comprehensively analyzed the expression and prognostic values of CXCLs along with their correlations with immune infiltration in HCC patients. Moreover, a co-expression network of CXCLs was constructed, whose biological functions were explored. The findings of our study might provide an overview of CXCLs’ roles in the development and immune microenvironment of HCC.
Materials and methods
Analysis of CXCLs’ differential expression between HCC and normal liver samples
Gene Expression Profiling Interactive Analysis 2 (GEPIA2) (http://gepia2.cancer-pku.cn/) is a web portal for analyzing the transcriptome data of 9736 tumors and 8587 normal samples from the Cancer Genome Atlas (TCGA) and the GTEx projects [12]. The expression of CXCL family genes in HCC compared with normal liver samples was analyzed by GEPIA2 using HCC data (n=369) from TCGA and normal liver data (n=160) combined TCGA and GTEx datasets.
The differential mRNA expression of CXCLs between HCC and adjacent normal tissues was further confirmed using Oncomine (https://www.oncomine.org/) server, which analyzes gene expression integrating data from published literature, the Stanford Microarray Database, and the NCBI Gene Expression Omnibus (GEO) [13]. CXCLs meeting |log2(fold change)| > 1 and P value < 0.05 were considered significantly differentially expressed between HCC and normal liver tissues. The overlapping CXCLs between the above two databases were included in our following investigations.
Analysis of genomic alternations of CXCLs in HCC
cBioPortal (http://www.cbioportal.org/) is a comprehensive web resource providing visual and multidimensional cancer genomics data [14,15]. Genomic alternation profiles including mutations, putative copy-number alterations, and mRNA expression were analyzed by cBioPortal using the data of 360 complete HCC samples from ‘TCGA, Firehose Legacy’ dataset.
Analysis of CXCLs’ expression in HCC patients with distinct clinicopathological features
UALCAN (http://ualcan.path.uab.edu) is an interactive platform for in-depth analysis of cancer omics data from TCGA [16]. Associations between CXCLs’ expression with distinct clinicopathological features of HCC patients, including genders, ages, pathological stages, and tumor grades were analyzed using UALCAN.
Analysis of the prognostic significance of CXCLs in HCC patients
Kaplan–Meier (KM) Plotter (http://www.kmplot.com/) is an online tool providing gene expression profiles with patients’ survival information of various cancers [17]. KM Plotter was applied to evaluate associations of CXCLs’ expression and overall survival (OS), relapse-free survival (RFS), progression-free survival (PFS), and disease-free survival (DSS) of HCC patients. All cases were split into two groups by the median of a gene’s expression level to conduct univariate analysis.
The prognostic value of CXCLs was also validated using SurvExpress (http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp) [18], a web tool providing multivariate survival analysis and risk assessment for a list of genes in human cancer datasets. Here, we used the data from ‘LIHC-TCGA-Liver hepatocellular carcinoma June 2016’ (n=361) to perform a multivariate Cox proportional hazards regression. A prognostic risk score was calculated for each HCC patient, and the patients were divided into high- and low-risk groups by the best cutoff of the scores.
Analysis of correlations between CXCLs’ expression and immune infiltration in HCC
Correlations between CXCLs’ expression and infiltration levels of diverse TIICs, including CD8+ T cells, CD4+ T cells, B cells, neutrophils, macrophages, dendritic cells (DCs), NK cells, and myeloid-derived suppressor cells (MDSCs) in HCC were assessed using Tumor IMmune Estimation Resource (TIMER) (http://timer.cistrome.org) [19]. Correlations between CXCLs’ expression and abundance of subsets of TIICs, chemokines, and chemokine receptors were further explored using TISIDB (http://cis.hku.hk/TISIDB) [20]. The two web portals both facilitate the investigation of tumor-immune interactions covering multiple cancer types. Moreover, correlations between the expression of CXCLs and biomarkers of TIICs were analyzed using GEPIA2.
Co-expression network of CXCLs and functional enrichment analysis
A co-expression network of CXCLs was constructed using the GeneMANIA plugin of Cytoscape software (Version 3.7.0) [21]. Then, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed for all genes in the co-expression network, using Database for Annotation, Visualization, and Integrated Discovery (DAVID) server (https://david.ncifcrf.gov/home.jsp) [22]. GO enrichment analysis annotated the biological functions of genes in three aspects: biological process (BP), cellular component (CC), and molecular function (MF).
Statistical analysis
The comparison of gene expression levels in HCC and normal tissues in Oncomine and UALCAN was performed using Student’s t-test, which by GEPIA2 was conducted using one-way ANOVA test. For KM Plotter database, log-rank test was performed to compare the prognostic difference between two groups and generate hazard ratio (HR), 95% confidence interval (CI), and P values. For SurvExpress platform, a Cox proportional hazard regression model was used to evaluate the prognostic value of CXCLs’ signature. Survival curves were generated applying Kaplan–Meier method. Spearman’s method was performed to analyze correlations between a gene’s expression and immune infiltration or chemokines or TIIC biomarkers’ expression. Correlation strength was measured by correlation coefficient (r): 0.00–0.19 was ‘very weak’, 0.20–0.39 was ‘weak’, 0.40–0.59 was ‘moderate’, 0.60–0.79 was ‘strong’, and 0.80–1.0 is ‘very strong’ [23,24]. For all two-tailed analyses, P values < 0.05 were considered statistically significant, and false discovery rates (FDRs) < 0.05 were additional criteria for functional enrichment analyses.
Results
Differentially expressed CXCLs between HCC and normal liver samples
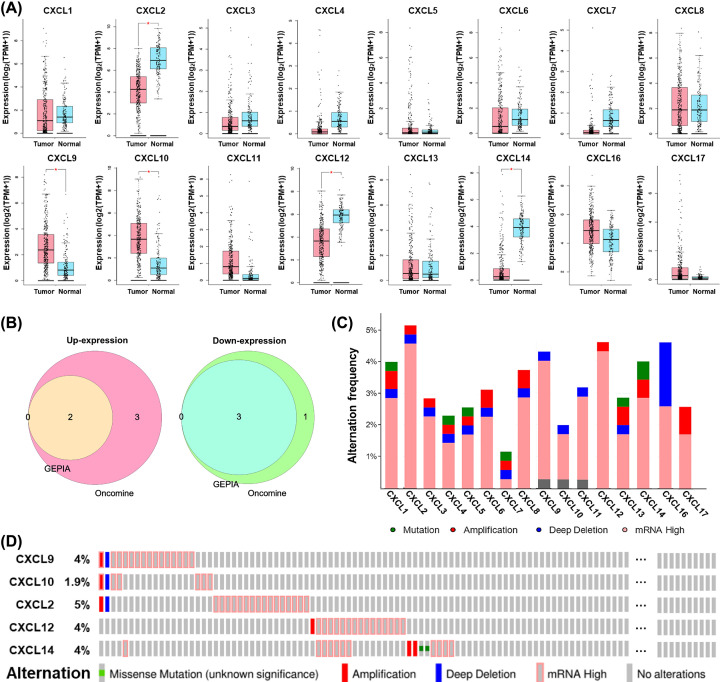
First, the differential expression of CXCL family genes between HCC and normal liver tissues was analyzed integrating GEPIA2 and Oncomine databases. The results from GEPIA2 database showed CXCL9/10 expression was significantly higher, whereas CXCL2/12/14 was lower in HCC, compared with normal liver tissues (Figure 1A). In Oncomine, CXCL6/8/9/10/11 was significantly up-expressed in HCC in one, one, one, two, and one datasets, while CXCL1/2/12/14 was down-expressed in HCC in one, five, four, and four datasets, respectively, versus adjacent normal tissues (Table 1). Therefore, CXCL9/10 was consistently significantly up-expressed, whereas CXCL2/12/14 was down-expressed in HCC versus normal controls in the two databases (Figure 1B), the five CXCLs were included in our further study.
Figure 1. Expression and alternations of CXCL family genes in HCC.
(A) The significant differential expression of CXCL family genes between HCC (n = 369) and normal liver tissues (n = 160) (GEPIA). *log2(fold change)| > 1 and P value < 0.05; TPM, transcript per million. (B) Venn diagrams show the overlapping up-expressed and down-expressed CXCLs between Oncomine and GEPIA databases. (C) The alternation frequency of CXCL family members in HCC (n = 360). (D) An overview of the alternations occurring in CXCL9/10/2/12/14 in HCC samples (cBioPortal).
Table 1. The significantly differentially expressed CXCLs between HCC and normal liver tissues (Oncomine).
| Gene name | Fold change | P value | Sample size | Reference (PMID) | |
|---|---|---|---|---|---|
| HCC | Normal | ||||
| CXCL1 | -4.16 | 1.76E-20 | 102 | 72 | 12058060 |
| -6.54 | 8.33E-60 | 225 | 220 | 21159642 | |
| -3.60 | 4.65E-17 | 102 | 72 | 12058060 | |
| CXCL2 | -5.74 | 1.45E-07 | 35 | 10 | 17393520 |
| -7.40 | 2.59E-07 | 225 | 220 | 21159642 | |
| -2.27 | 9.36E-07 | 19 | 38 | 19098997 | |
| CXCL6 | 3.75 | 2.15E-07 | 19 | 38 | 19098997 |
| CXCL8 | 3.32 | 7.84E-06 | 19 | 38 | 19098997 |
| CXCL9 | 5.33 | 6.20E-17 | 19 | 38 | 19098997 |
| CXCL10 | 9.27 | 6.33E-17 | 19 | 38 | 19098997 |
| 5.93 | 1.78E-04 | 35 | 10 | 17393520 | |
| CXCL11 | 2.19 | 2.26E-06 | 19 | 38 | 19098997 |
| CXCL12 | -5.34 | 2.32E-93 | 225 | 220 | 21159642 |
| -2.39 | 4.32E-31 | 102 | 72 | 12058060 | |
| -4.04 | 4.43E-11 | 225 | 220 | 21159642 | |
| -4.23 | 3.82E-09 | 35 | 10 | 17393520 | |
| CXCL14 | -10.94 | 6.98E-154 | 225 | 220 | 21159642 |
| -9.67 | 1.24E-21 | 225 | 220 | 21159642 | |
| -12.90 | 1.96E-43 | 102 | 72 | 12058060 | |
| -13.98 | 6.81E-10 | 35 | 10 | 17393520 | |
Note: The screening threshold was set as: data type of mRNA, |fold change| of 2.0, P value of 0.05, and the rank of top 10% gene; PMID, PubMed Unique Identifier.
Genetic alternations of CXCLs in HCC
Following, genetic alternations of CXCL family in HCC patients were analyzed using cBioPortal. Overall, missense mutation, putative copy-number alterations including amplification and deep deletion, together with mRNA overexpression were detected in a total of 114 out of 360 (32%) HCC samples, and mRNA overexpression was the most frequent alteration (Figure 1C). To be specific, 16 (4%), 7 (1.9%), 18 (5%), 16 (4%), and 15 (4%) patients were observed with alternations of CXCL9, CXCL10, CXCL2, CXCL12, and CXCL14, respectively (Figure 1D).
Clinicopathological relevance of CXCLs
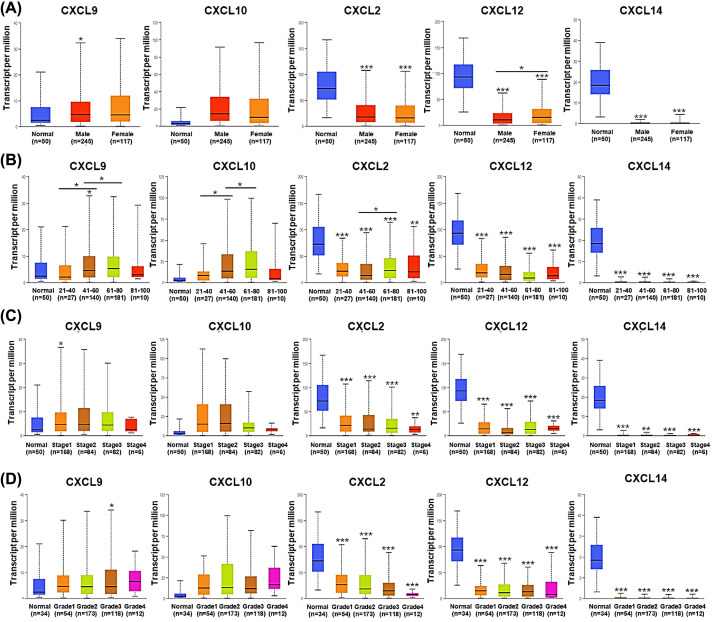
Associations of the expression of the five CXCLs with clinicopathological characteristics of HCC patients were investigated using UALCAN. In the aspect of genders, CXCL12 was expressed higher in females (P<0.05) than males (Figure 2A). Speaking of ages, middle-aged and senile HCC patients (41–80 years old) tended to have higher expression levels of CXCL9/10, compared with those in youth (21–40 years old) (P<0.05) (Figure 2B). However, the expression of the five CXCLs showed no significant difference among diverse pathological stages and tumor grades (Figure 2C,D).
Figure 2. Expression of the five CXCLs in HCC patients with distinct clinicopathological parameters (UALCAN).
Expression of CXCLs in HCC patients classified by (A) genders, (B) ages, (C) pathological stages, and (D) tumor grades. (*P<0.05, **P<0.01, ***P<0.001).
Prognostic significance of CXCLs in HCC patients
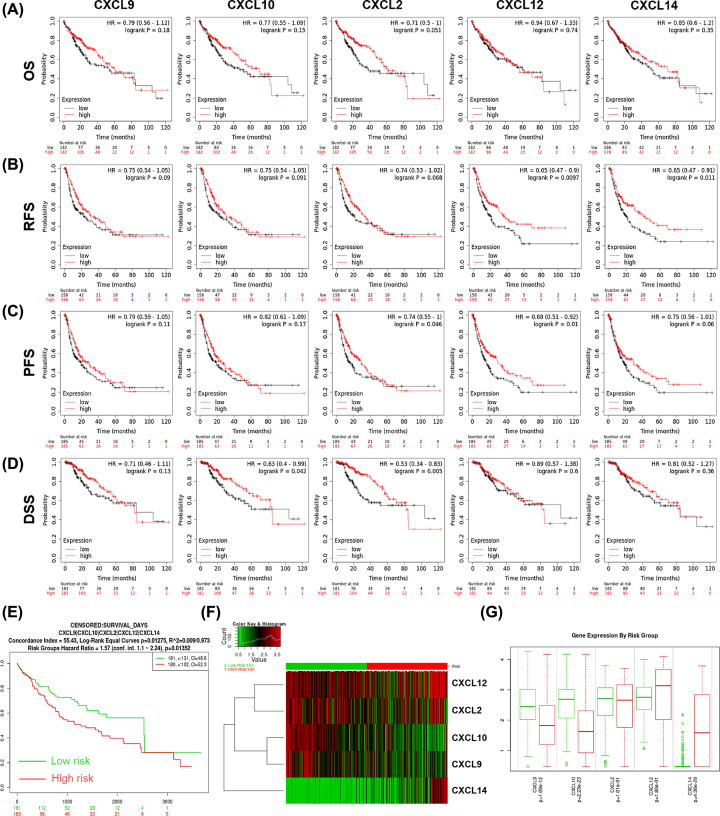
Wondering the prognostic significance of the five CXCLs in HCC patients, survival analysis was performed using KM Plotter. As shown in Figure 3A–D, higher expression of CXCL10 was associated with better DSS (HR = 0.63, P=0.042); overexpression of CXCL2 was associated with longer PFS and DSS (PFS: HR = 0.74, P=0.046; DDS: HR = 0.67, P=0.016); up-expression of CXCL12 was related to favorable RFS and PFS (RFS: HR = 0.65, P=0.0097; PFS: HR = 0.68, P=0.01); and up-regulation of CXCL14 was linked to better RFS (HR = 0.65, P=0.011) of HCC patients.
Figure 3. Prognostic significance of the five CXCLs in HCC patients.
The survival curves showed the associations between the expression of CXCLs with (A) OS, (B) RFS, (C) PFS, and (D) DDS of HCC patients (KM Plotter). (E-G) The prognostic value of CXCLs’ signature in HCC (SurvExpress). (E) Survival curves of the low- (green, n = 181) and high- (red, n = 180) risk groups. (F) A heat map showing the clustered expression of CXCLs between the low- and high-risk groups. (G) Comparison of the expression of each CXCL member between low- and high-risk groups. OS, overall survival; RFS, relapse-free survival; PFS, progression-free survival; DDS, disease-free survival; HR, hazard ratio; CI, confidence interval.
In addition, the results from SurExpress showed the low-risk group displayed significant favorable OS compared with the high-risk group (HR = 1.57, 95% CI = 1.1-2.24, P=0.0135) (Figure 3E). In the high-risk group, the expression of CXCL9 (P=1.09E-12) and CXCL10 (P=2.23E-23) was significantly lower, while CXCL14 (P=4.36E-29) was higher than that in the low-risk group (Figure 3 F,G).
Correlations between CXCLs’ expression and immune infiltration in HCC
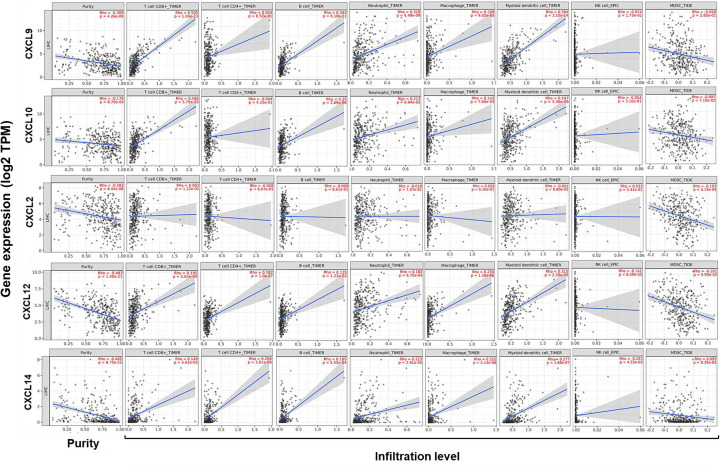
Subsequently, correlations between the five CXCLs’ expression and immune infiltration in HCC were investigated combined TIMER and TISIDB databases. Tumor purity is defined as the proportion of cancer cells in tumor admixture, which influence the evaluation of immune infiltration. All analyses about immune infiltration were adjusted with the corresponding tumor purity in the present study [25]. As presented in Figure 4, the five CXCLs’ expression was consistently negatively correlated to the tumor purity.
Figure 4. Correlations between CXCLs’ expression with the immune infiltration in HCC (TIMER).
Correlations between CXCLs’ expression with tumor purity, and infiltration levels of CD8+ T cells, CD4+ T cells, B cells, neutrophils, macrophages, DCs, NK cells, and MDSCs in HCC. DCs, dendritic cells; NK cells, natural killer cells; MDSCs, myeloid-derived suppressor cells, Th, helper T cell.
According to the results from TIMER, CXCL9/10/12/14 expression was positively correlated with the infiltration of CD8+ T cells, B cells, neutrophils, macrophages, and DCs (P ≤ 8.70E-04). CXCL12/14 expression was positively correlated with the infiltration of CD4+ T cells but negatively correlated with that of NK cells (P ≤ 8.59E-03). CXCL2 expression presented a negative correlation with the infiltration of MDSCs (P = 3.15E-04). Noteworthy, correlation strength of the infiltration of CD8+ T cells with the expression of CXCL9 (r=0.505, P=1.09E-23) and CXCL10 (r=0.466, P=5.79E-20) was moderate (Figure 4).
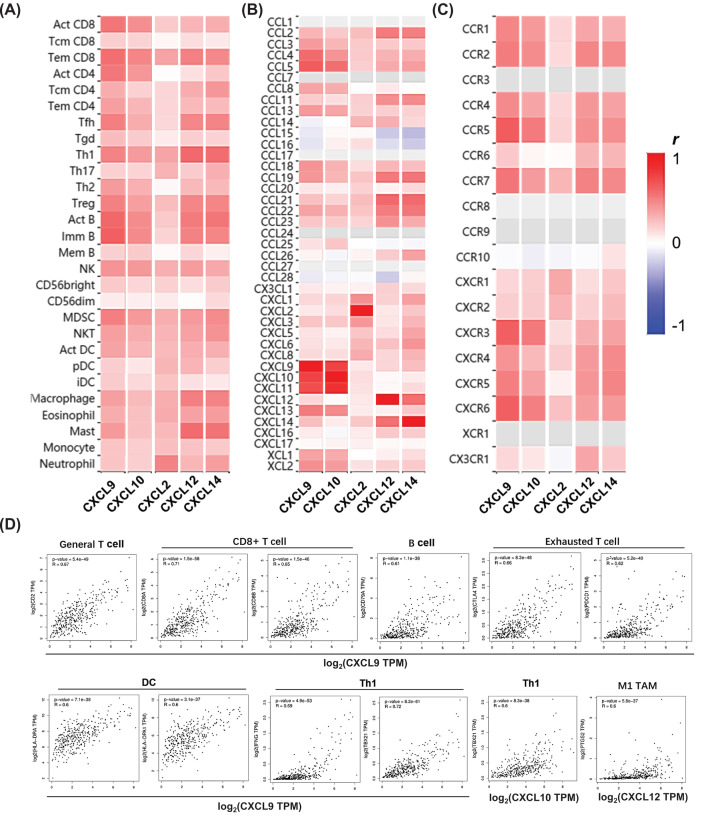
As exhibited in Figure 5A, the expression of these CXCLs was generally positively correlated with the abundance of 28 kinds of TIICs in TISIDB. Consistent with the findings from TIMER, CXCL9/10/12/14 expression showed moderate to strong positive correlations with the abundance of effector memory CD8+ T cells, along with activated and immature B cells. Moderate positive correlations were also found between CXCL9/10 expression and the abundance of activated CD8+ T cells; CXCL9/12/14 expression and the abundance of follicular helper T cell (Tfh), type-1 helper T cell (Th1), and Tregs; CXCL12/14 expression and the abundance of macrophages and mast cells; CXCL2 expression and the abundance of neutrophils.
Figure 5. Correlations between CXCLs’ expression with the abundance of TIICs, chemokines, chemokine receptors, and biomarkers’ expression of some TIICs in HCC.
Correlations between CXCLs’ expression with (A) the abundance of TIIC subsets, (B) chemokines, and (C) chemokine receptors (TISIDB). (D) Strong correlations between the expression of CXCL9/10/12 and biomarkers of T cells, CD8+ T cells, B cells, exhausted T cells, DCs, Th1 cells, and M1 TAMs in HCC (GEPIA). “Act”, “Tcm”, and “Tem” CD8/CD4 represent activated, central memory, and effector memory CD8+/CD4+ T cells respectively. Tfh, follicular helper T cell; Tgd, gamma delta T cells. “Act”, “Imm”, and “Mem” B represent activated, immune, and memory B cells respectively. “CD56bright” and “CD56dim” mean CD56bright and CD56dim natural killer cells respectively. NKT, natural killer T cell. “Act DC”, “pDC”, and “iDC” mean activated, plasmacytoid, and immune dendritic cells respectively.
As for relations among CXCLs and other chemokines and their receptors, CXCL9-11 highly interacted with each other, CXCL12/14 connected with a moderate degree. Positive connections with moderate to strong strength were observed between CXCL9/10 and CCL4/5, also CXCL12/14 and CCL2/11/19/21/22/23 (Figure 5B). Moderate to strong correlations were found in CXCL9/10/12/14 with CCR1/2/4/5/7 also CXCR3/4/5/6 (Figure 5C).
Correlations between the expression of CXCLs and biomarkers of TIICs in HCC
To confirm the participation of TIICs, correlations between the expression of CXCLs and biomarkers of TIICs in HCC were further analyzed using GEPIA2. The biomarkers of all TIICs mentioned above were investigated, together with monocytes, M1/M2 TAMs, and subsets of T cells, including Th1, Th2, Th17, Tfh, Tregs, and exhausted T cells. A little bit different from the results from TIMER, it showed that CXCL9/10/12/14 expression was not only positively correlated with the expression of almost all biomarkers of B cells, CD8+ T cells, neutrophils, TAMs, and DCs, but also subsets of CD4+T cells, monocytes, and NK cells, implying the potential involvement of these TIICs (P<0.05) (Table 2). Notably, strongly positive correlations were observed between the expression of CXCL9 and signatures of B cells (CD79A), general T cells (CD2), Th1 cells (TBX21 and IFNG), CD8+ T cells (CD8A/B), exhausted T cells (PDCD1 and CTLA4), and DCs (HLA-DRA and HLA-DPA1) (P<3.1E-37); CXCL10 and Th1 (TBX21), as well as CXCL12 and M1 TAMs (PTGS2) (P<5.8E-37) (Figure 5D). Till now, the above findings indicated CXCLs' regulations on immune infiltrate of various TIICs in HCC.
Table 2. Correlations of the expression of CXCLs and biomarkers of TIICs in HCC (GEPIA).
| Types of TIICs | Gene markers | CXCL9 | CXCL10 | CXCL2 | CXCL12 | CXCL14 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | R | P | ||
| B cell | CD19 | 0.4 | 1.6E−15 | 0.3 | 7.3E−09 | 0.033 | 0.53 | 0.32 | 2.2E−10 | 0.41 | 1.2E−16 |
| CD79A | 0.61 | 1.1E−38 | 0.42 | 5.0E−17 | 0.11 | 0.03 | 0.5 | 3E−24 | 0.52 | 6.4E−27 | |
| T cell (general) | CD3D | 0.56 | 1.8E−32 | 0.44 | 1.7E−18 | 0.098 | 0.059 | 0.25 | 8.7E−07 | 0.4 | 2.6E−15 |
| CD2 | 0.67 | 5.4E−49 | 0.53 | 1.9E−28 | 0.19 | 0.00035 | 0.44 | 3E−19 | 0.47 | 8E−22 | |
| Th1 | TBX21 | 0.72 | 8.2E−61 | 0.6 | 8.3E−38 | 0.18 | 0.00073 | 0.42 | 5.4E−17 | 0.35 | 6.3E−12 |
| STAT4 | 0.37 | 1.4E−13 | 0.4 | 2.7E−15 | 0.27 | 2.3E−07 | 0.32 | 2.5E−10 | 0.32 | 3.5E−10 | |
| STAT1 | 0.57 | 5.9E−33 | 0.56 | 1E−31 | 0.059 | 0.26 | 0.32 | 6E−10 | 0.25 | 1.3E−06 | |
| TNF | 0.51 | 7.4E−26 | 0.37 | 1.9E−13 | 0.15 | 0.0046 | 0.38 | 8.5E−14 | 0.38 | 2.3E−14 | |
| IFNG | 0.69 | 4.9E−53 | 0.55 | 2.5E−30 | 0.016 | 0.76 | 0.15 | 0.0029 | 0.26 | 4.7E−07 | |
| Th2 | GATA3 | 0.53 | 5.9E−28 | 0.37 | 1.4E−13 | 0.19 | 0.00017 | 0.49 | 1.7E−23 | 0.53 | 3.3E−28 |
| STAT6 | 0.15 | 0.0029 | 0.14 | 0.0065 | 0.11 | 0.03 | 0.12 | 0.024 | 0.098 | 0.059 | |
| IL13 | 0.19 | 0.00019 | 0.16 | 0.0022 | 0.025 | 0.63 | 0.029 | 0.58 | 0.13 | 0.013 | |
| STAT5A | 0.39 | 4.5E−15 | 0.36 | 1.8E−12 | 0.088 | 0.091 | 0.3 | 4.2E−09 | 0.29 | 1.1E−08 | |
| Tfh | BCL6 | 0.23 | 6.4E−06 | 0.18 | 0.00038 | 0.076 | 0.14 | 0.084 | 0.11 | 0.045 | 0.39 |
| IL21 | 0.36 | 1.4E−12 | 0.29 | 9.4E−09 | −0.066 | 0.2 | 0.11 | 0.03 | 0.14 | 0.0087 | |
| Th17 | STAT3 | 0.22 | 1.3E−05 | 0.18 | 0.00059 | 0.33 | 8.9E−11 | 0.26 | 3.1E−07 | 0.23 | 9.8E−06 |
| IL17A | 0.066 | 0.21 | 0.11 | 0.035 | 0.076 | 0.15 | 0.033 | 0.52 | 0.035 | 0.51 | |
| Treg | FOXP3 | 0.48 | 3.3E−22 | 0.46 | 6.4E−21 | 0.13 | 0.014 | 0.15 | 0.0049 | 0.19 | 0.00017 |
| CCR8 | 0.55 | 2.2E−30 | 0.47 | 1.1E−21 | 0.18 | 0.00036 | 0.32 | 3.6E−10 | 0.31 | 2.1E−09 | |
| TGFB1 | 0.32 | 2.3E−10 | 0.13 | 0.015 | 0.075 | 0.15 | 0.48 | 3.9E−23 | 0.51 | 3.6E−26 | |
| CD8+ T | CD8A | 0.71 | 1.5E−58 | 0.54 | 1.2E−29 | 0.16 | 0.0024 | 0.44 | 1.6E−18 | 0.42 | 1.9E−17 |
| CD8B | 0.65 | 1.5E−46 | 0.49 | 1.4E−23 | 0.11 | 0.043 | 0.35 | 2.4E−12 | 0.39 | 1E−14 | |
| Exhausted T cell | PDCD1 | 0.62 | 5.2E−40 | 0.42 | 1.4E−17 | 0.058 | 0.27 | 0.37 | 3.9E−13 | 0.4 | 1.3E−15 |
| CTLA4 | 0.66 | 8.3E−48 | 0.47 | 6.6E−22 | 0.15 | 0.0035 | 0.24 | 3.4E−06 | 0.35 | 2.3E−12 | |
| LAG3 | 0.48 | 3.8E−23 | 0.38 | 6.8E−14 | −0.017 | 0.75 | 0.15 | 0.0035 | 0.24 | 3.3E−06 | |
| TIM3 | 0.54 | 5.5E−29 | 0.4 | 9.5E−16 | 0.18 | 0.00052 | 0.42 | 2.6E−17 | 0.47 | 2.7E−21 | |
| GZMB | 0.58 | 1.1E−34 | 0.42 | 2.2E−17 | 0.094 | 0.072 | 0.23 | 9.5E−06 | 0.28 | 3.5E−08 | |
| NK cell | KIR2DL1 | 0.26 | 2.8E−07 | 0.19 | 0.00033 | 0.15 | 0.0036 | 0.12 | 0.023 | 0.12 | 0.022 |
| KIR2DL3 | 0.4 | 1.6E−15 | 0.31 | 8.7E−10 | 0.045 | 0.82 | 0.16 | 0.002 | 0.19 | 0.00033 | |
| KIR3DL1 | 0.27 | 1.9E−07 | 0.25 | 1.3E−06 | 0.13 | 0.015 | 0.13 | 0.01 | 0.052 | 0.32 | |
| KIR3DL2 | 0.43 | 2.3E−18 | 0.3 | 4E−09 | 0.024 | 0.65 | 0.29 | 2.1E−08 | 0.27 | 1E−07 | |
| KIR3DL3 | 0.16 | 0.0019 | 0.13 | 0.012 | −0.012 | 0.82 | −0.01 | 0.84 | 0.073 | 0.16 | |
| KIR2DS4 | 0.26 | 3E−07 | 0.22 | 1.4E−05 | 0.062 | 0.24 | 0.16 | 0.0025 | 0.083 | 0.11 | |
| Neutrophil | CD11b | 0.32 | 4.8E−10 | 0.35 | 4.9E−12 | 0.22 | 1.6E−05 | 0.24 | 1.9E−06 | 0.28 | 7.5E−08 |
| CCR7 | 0.57 | 7.4E−33 | 0.42 | 1.4E−17 | 0.29 | 1.2E−08 | 0.55 | 3.7E−30 | 0.5 | 1.6E−24 | |
| CD66b | 0.081 | 0.12 | 0.036 | 0.49 | 0.046 | 0.38 | 0.033 | 0.53 | 0.055 | 0.29 | |
| M1 TAM | NOS2 | 0.13 | 0.016 | 0.13 | 0.015 | 0.11 | 0.039 | 0.25 | 1.2E−06 | 0.14 | 0.0053 |
| PTGS2 | 0.3 | 2.6E−09 | 0.19 | 0.00018 | 0.34 | 2.2E−11 | 0.6 | 5.8E−37 | 0.53 | 3.7E−28 | |
| IRF5 | 0.18 | 0.00061 | 0.19 | 0.00024 | 0.035 | 0.5 | 0.078 | 0.13 | 0.12 | 0.017 | |
| M2 TAM | CD163 | 0.43 | 1.2E−17 | 0.39 | 3.8E−15 | 0.24 | 2.2E−06 | 0.42 | 5.3E−17 | 0.4 | 7.3E−16 |
| VSIG4 | 0.38 | 5.9E−14 | 0.36 | 8.4E−13 | 0.28 | 5.2E−08 | 0.42 | 2.3E−17 | 0.4 | 2.6E−15 | |
| MS4A4A | 0.48 | 6.8E−23 | 0.42 | 3.4E−17 | 0.28 | 7.8E−08 | 0.45 | 1.4E−19 | 0.34 | 2.6E−11 | |
| TAM | CCL2 | 0.37 | 4.2E−13 | 0.3 | 6.9E−09 | 0.27 | 1.7E−07 | 0.58 | 6.8E−35 | 0.54 | 2.3E−29 |
| CD68 | 0.37 | 3.0E−13 | 0.28 | 4.5E−08 | 0.11 | 0.037 | 0.37 | 1.4E−13 | 0.26 | 2.5E−07 | |
| IL10 | 0.46 | 3.5E−21 | 0.35 | 4.4E−12 | 0.13 | 0.016 | 0.37 | 1E−13 | 0.29 | 1.9E−08 | |
| Monocyte | CD86 | 0.58 | 9.5E−35 | 0.46 | 1.2E−20 | 0.18 | 0.00038 | 0.48 | 3.5E−22 | 0.45 | 5E−20 |
| CD115 | 0.49 | 3.7E−24 | 0.4 | 1.1E−15 | 0.23 | 5.6E−06 | 0.53 | 8.7E−28 | 0.46 | 1.3E−20 | |
| DC | HLA-DPB1 | 0.57 | 1.6E−33 | 0.47 | 3E−21 | 0.23 | 7.1E−06 | 0.53 | 1.3E−27 | 0.47 | 9.3E−22 |
| HLA-DRA | 0.6 | 7.1E−38 | 0.5 | 6.1E−25 | 0.28 | 7.5E−08 | 0.49 | 8.7E−24 | 0.43 | 2.8E−18 | |
| HLA-DQB1 | 0.43 | 6.8E−18 | 0.3 | 5E−09 | 0.13 | 0.013 | 0.28 | 3.3E−08 | 0.32 | 4.6E−10 | |
| HLA-DPA1 | 0.6 | 3.1E−37 | 0.5 | 3.3E−24 | 0.27 | 9.4E−08 | 0.5 | 6.7E−25 | 0.43 | 6.9E−18 | |
| CD1C | 0.38 | 2.1E−14 | 0.31 | 1.1E−09 | 0.2 | 8.8E−05 | 0.57 | 1.8E−33 | 0.45 | 1.3E−19 | |
| NRP1 | 0.24 | 2E−06 | 0.14 | 0.0058 | 0.071 | 0.18 | 0.33 | 4.9E−11 | 0.28 | 3.3E−08 | |
| CD11c | 0.51 | 1.3E−25 | 0.41 | 1.1E−16 | 0.22 | 1.3E−05 | 0.4 | 2.4E−15 | 0.41 | 3.8E−16 | |
Note: The correlation analysis was adjusted for the tumor purity. P values with statistical significance are shown in bold. TAM, tumor-associated macrophage; Tfh, follicular helper T cell; Th, helper T cell; Treg, regulatory T cell; NK cell, natural killer cell; r, the correlation coefficient of Spearman’s analysis.
Functions of the co-expression network of CXCLs
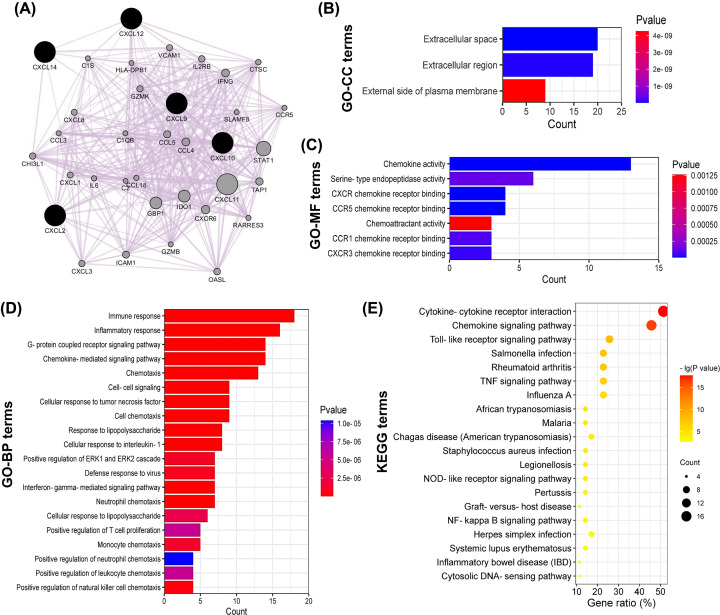
To understand the functional mechanisms of the five CXCLs, functional enrichment analysis was performed for the co-expression network of CXCLs. Thirty co-expressed genes of the five CXCLs were identified, so the co-expression network was composed of totally 35 genes (Figure 6A). And 15 genes out of them were CXCLs or C-C motif chemokine ligands (CCLs) or their receptors.
Figure 6. Functions of the co-expression network of CXCLs.
(A) The co-expression network of the five CXCLs. (B) All GO-CC terms, (C) all GO-MF terms, (D) the top 20 GO-BP terms, and (E) the top 20 KEGG pathway terms enriched for the co-expressed genes.
Subsequently, functional enrichment analysis was conducted for all genes in the co-expression network using DAVID platform. The significantly enriched GO-CC (Figure 6B), GO-MF (Figure 6C) and GO-BP (Figure 6D) terms elucidated the co-expressed genes were components of the extracellular region and participated in chemokine-mediated immune or inflammatory responses, as well as G-protein coupled receptor activity through binding with the corresponding chemokine receptors. Moreover, the enriched KEGG pathway terms elucidated these genes mainly involved in cytokine–cytokine receptor interaction and signaling pathways of chemokine, Toll-like receptor, tumor necrosis factor (TNF), and nucleotide-binding oligomerization domain (NOD)-like receptor (NLR), etc. (Figure 6E).
Discussion
To begin with, five significantly differentially expressed CXCLs overlapping between GEPIA2 and Oncomine databases were screened out, among which CXCL 9/10 was elevated, while CXCL2/12/14 was decreased in HCC versus normal control. Interestingly, although mRNA overexpression was the most frequent alternation of CXCL2/12/14, they were still low-expressed in HCC. Different from two recent bioinformatics studies, in which CXCL1/3/5/8 overexpression was reported as unfavorable prognostic indicators of HCC [26,27]. We found high expression of CXCL2/10/12/14 implied favorable survivals of HCC patients using univariate analysis, even though their expression was not significantly associated with pathological stages and histological grades of HCC patients. In addition, CXCL9/10 up-regulation indicated lower risk in the multivariate analysis. However, the results on CXCL14 were contradictory, since low-risk HCC patients presented extremely low CXCL14 expression.
After that, we observed the five CXCLs’ expression was uniformly negatively correlated to the tumor purity, suggesting their expression might be mainly from immune cells or stromal cells in the TME rather than cancer cells. Generally speaking, CXCL9/10/12/14 was conformably positively correlated with the infiltration and (or) biomarkers’ expression of diverse TIICs, including CD8+ T cells, B cells, neutrophils, TAMs, DCs, and NK cells. In particular, CXCL9/10 expression showed moderate correlations with the infiltration of activated CD8+ T cells. CXCL9/12/14 expression presented quite close relationships with the abundance of subsets of CD4+ T cells, especially Th1 cells. Besides, the expression of CXCL9/10/12/14 was correlated with CCR1/2/4/5/7 and CXCR3/4/5/6 with moderate to strong extents.
It is acknowledged that CD8+ cytotoxic T cells and NK cells are the main undertakers of anticancer immunity in the TME, both can be stimulated by pro-inflammatory cytokines secreted by Th1 cells. A higher density of CD8+ T cells and NK cells in tumor tissues could predict improved treatment responses and the prognosis of HCC patients [28,29]. Consistent with our results, CXCL9/10 can recruit CD8+ T cells, Th1 cells, and NK cells by binding to the common receptor CXCR3 [10,30]. A retrospective study showed HCC patients with higher serum CXCL9 levels had better survivals under sorafenib therapy [31]. And the down-regulation of CXCL9/CXCR3 axis might contribute to HCC recurrence after partial hepatectomy, since it remarkably reduced the proportion of intrahepatic NK cells [32]. DCs are the most potent antigen-presenting cells to motivate effective immune responses of T cells and NK cells once activated by antigens [33,34]. The role of tumor-infiltrating B cells in HCC remains controversial, since it seems to work dually depending to the secretion of inflammatory factors [35]. It was ever reported that the proximity between T cells and B cells indicated a functional interaction that might lead to a better prognosis [36]. Nevertheless, it was also demonstrated that CXCL9-11 would induce the M2-type polarization of TAMs by binding to CXCR3+ B cells, which were correlated with early recurrence of HCC [37]. Overall, the up-regulation of CXCL9/10 could enhance immune response and the subsequent apoptosis to augment immunotherapy effects [38,39].
MDSCs exert tumor-promoting and immunosuppressive roles in cancers through inducing differentiation and expansion of Tregs, inhibiting DCs, NK cells, and T cells [40–42]. In accordance with our findings, the earlier studies expounded CXCL2/14 were both stably down-regulated in HCC specimens compared with adjacent normal tissues, and whose overexpression might profoundly inhibit angiogenesis and aggressiveness of HCC cells, partly through apoptosis pathways [43,44]. Our results showed CXCL2 expression was negative correlated with the infiltration of MDSCs, suggesting CXCL2 might ameliorate host immunosurveillance by reducing MDSC generation [45,46]. It had been ever proved that CXCL14 overexpression could attract effective DCs, NK cells, and T cells [47]. CXCL12 is a common ligand of CXCR4 and CXCR7, which was expressed at significantly lower levels in HCC compared with adjacent normal liver tissues. Controversial to our observations, CXCL12/CXCR4 axis was reported to promote angiogenesis and aggressiveness of HCC cells and facilitate immune escape via inducing MDSCs and plasmacytoid DCs [48–51]. However, the activation of CXCL12/CXCR7 did not affect the prognosis of HCC patients [52]. Hereto, we could deduce the regulations of CXCLs to the tumor immune microenvironment might partly explain their influence on HCC patients’ prognosis. The up-regulation of CXCL9/10/12/14 might improve outcomes of HCC patients through reinforcing immune surveillance of CD8+ T cells, NK cells, DCs, and Th1 cells, etc., while CXCL2 might mainly be through reducing MDSCs. It appeared that CXCL12 functioned dually in HCC according to the receptors to which it bound, thus, more explorations are still required.
Finally, a co-expression network of the five CXCLs was constructed and functional enrichment analysis was performed for these co-expressed genes to elucidate their biological functions. The enrichment analysis uncovered these co-expressed genes were components of the external cellular region, and were responsible for chemokine-mediated immune or inflammatory responses, along with G-protein coupled receptor activity. And the signaling pathways of chemokine, Toll-like receptor, TNF, and NLR were involved. All these pathways play vital roles in the inflammation engaged in cancer malignant progress [35,42].
Conclusion
The present study proposed that elevated CXCL2/10/12/14 expression might serve as favorable prognostic indicators of HCC patients. The critical regulatory mechanisms might lie in their beneficial modulations of multiple TIICs in the TME, particularly CD8+ T cells, NK cells, DCs, Th1 cells, and MDSCs. The inflammation-related signaling pathways of chemokine, Toll-like receptor, TNF, and NLR were potentially involved. Therefore, it’s enlightened the five CXCLs might exert as possible therapeutic targets to regulate anticancer immunity in the TME of HCC to improve the efficacy of immunotherapy. However, further validated experiments and clinical studies are still required.
Abbreviations
- CI
confidence interval
- CXCL
C-x-C motif chemokine ligand
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- NLR
NOD-like receptor
- NOD
nucleotide-binding oligomerization domain
- TIICs
tumor-infiltrating immune cells
- TNF
tumor necrosis factor
- TME
tumor microenvironment
Data Availability
All the data that support the findings of this study are publicly available in: https://www.oncomine.org/, http://gepia2.cancer-pku.cn/, http://www.cbioportal.org/, http://ualcan.path.uab.edu, http://www.kmplot.com/, http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp, http://timer.cistrome.org, http://cis.hku.hk/TISIDB, and https://david.ncifcrf.gov/home.jsp.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
CRediT Author Contribution
Tong Lin: Formal analysis, Methodology, Writing—original draft, Writing—review and editing. E. Zhang: Investigation, Writing—review and editing. Pei-pei Mai: Investigation, Writing—review and editing. Ying-zhao Zhang: Visualization, Writing—review and editing. Xiang Chen: Visualization, Writing—review and editing. Li-sheng Peng: Conceptualization, Supervision, Writing—review & editing.
Funding
The authors declare that there are no sources of funding to be acknowledged.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A. and Jemal A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Duran S.R. and Jaquiss R.D.B. (2019) Hepatocellular Carcinoma. New Engl. J. Med. 381, e2 10.1056/NEJMc1906565 [DOI] [PubMed] [Google Scholar]
- 3.Joyce J.A. and Fearon D.T. (2015) T cell exclusion, immune privilege, and the tumor microenvironment. Science (New York, N.Y.) 348, 74–80 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 4.Nagarsheth N., Wicha M.S. and Zou W. (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 10.1038/nri.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone M., Hayward J., Huang C., Huma Z.E. and Sanchez J. (2017) Mechanisms of regulation of the chemokine-receptor network. Int. J. Mol. Sci. 18, 342 10.3390/ijms18020342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandercappellen J., Van Damme J. and Struyf S. (2008) The role of CXC chemokines and their receptors in cancer. Cancer Lett. 267, 226–244 10.1016/j.canlet.2008.04.050 [DOI] [PubMed] [Google Scholar]
- 7.Liang C. (2015) Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J. Hepatol. 7, 1390 10.4254/wjh.v7.i10.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y., Ye Y.C., Chen Y., Zhao J.L., Gao C.C., Han H.et al. (2018) Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 9, 793 10.1038/s41419-018-0818-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S.W., Kim Y.A., Sun H.J., Kim Y.A., Oh B.C., Yi K.H.et al. (2016) CXCL16 signaling mediated macrophage effects on tumor invasion of papillary thyroid carcinoma. Endocr. Relat. Cancer 23, 113–124 10.1530/ERC-15-0196 [DOI] [PubMed] [Google Scholar]
- 10.Wendel M., Galani I.E., Suri-Payer E. and Cerwenka A. (2008) Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 68, 8437–8445 10.1158/0008-5472.CAN-08-1440 [DOI] [PubMed] [Google Scholar]
- 11.Fu Y., Liu S., Zeng S. and Shen H. (2019) From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 38, 396 10.1186/s13046-019-1396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z., Kang B., Li C., Chen T. and Zhang Z. (2019) GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic. Acids. Res. 47, W556–W560 10.1093/nar/gkz430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes D.R., Kalyana-Sundaram S., Mahavisno V., Varambally R., Yu J., Briggs B.B.et al. (2007) Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9, 166–180 10.1593/neo.07112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A.et al. (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O.et al. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, l1 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrashekar D.S., Bashel B., Balasubramanya S., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B.et al. (2017) UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19, 649–658 10.1016/j.neo.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Györffy B., Lanczky A., Eklund A.C., Denkert C., Budczies J., Li Q.et al. (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 123, 725–731 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- 18.Aguirre-Gamboa R., Gomez-Rueda H., Martínez-Ledesma E., Martínez-Torteya A., Chacolla-Huaringa R., Rodriguez-Barrientos A.et al. (2013) SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS ONE 8, e74250 10.1371/journal.pone.0074250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T., Fan J., Wang B., Traugh N., Chen Q., Liu J.S.et al. (2017) TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77, e108–e110 10.1158/0008-5472.CAN-17-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ru B., Wong C.N., Tong Y., Zhong J.Y., Zhong S., Wu W.C.et al. (2019) TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics 35, 4200–4202 10.1093/bioinformatics/btz210 [DOI] [PubMed] [Google Scholar]
- 21.Franz M., Rodriguez H., Lopes C., Zuberi K., Montojo J., Bader G.D.et al. (2018) GeneMANIA update 2018. Nucleic Acids Res. 46, W60–W64 10.1093/nar/gky311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang D.W., Sherman B.T. and Lempicki R.A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 23.Pan J., Zhou H., Cooper L., Huang J., Zhu S., Zhao X.et al. (2019) LAYN Is a prognostic biomarker and correlated with immune infiltrates in gastric and colon cancers. Front. Immunol. 10, 10.3389/fimmu.2019.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Z., Hu L., Yang L., Wang S., Gao Y., Zhu Q.et al. (2020) TGFβ2 is a prognostic‐related biomarker and correlated with immune infiltrates in gastric cancer. J. Cell. Mol. Med. 24, 7151–7162 10.1111/jcmm.15164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aran D., Sirota M. and Butte A.J. (2015) Systematic pan-cancer analysis of tumour purity. Nat. Commun. 6, 8971 10.1038/ncomms9971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Q., Hu G., Wu J. and Lv L. (2020) A new clinical prognostic nomogram for liver cancer based on immune score. PLoS ONE 15, e236622 10.1371/journal.pone.0236622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Huang J., Tian Z., Zhou Y. and Yang J. (2020) The role of CXC cytokines as biomarkers and potential targets in hepatocellular carcinoma. Math. Biosci. Eng. 17, 1381–1395 10.3934/mbe.2020070 [DOI] [PubMed] [Google Scholar]
- 28.Lee J.H., Lee J.H., Lim Y.S., Yeon J.E., Song T.J., Yu S.J.et al. (2015) Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148, 1383–1391 10.1053/j.gastro.2015.02.055 [DOI] [PubMed] [Google Scholar]
- 29.Endig J., Buitrago-Molina L.E., Marhenke S., Reisinger F., Saborowski A., Schütt J.et al. (2016) Dual role of the adaptive immune system in liver injury and hepatocellular carcinoma development. Cancer Cell 30, 308–323 10.1016/j.ccell.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 30.Namkoong H., Song M.Y., Seo Y.B., Choi D.H., Kim S.W., Im S.J.et al. (2014) Enhancement of antigen-specific CD8 T cell responses by co-delivery of Fc-fused CXCL11. Vaccine 32, 1205–1212 10.1016/j.vaccine.2013.07.066 [DOI] [PubMed] [Google Scholar]
- 31.Hayashi T., Yamashita T., Terashima T., Suda T., Okada H., Asahina Y.et al. (2017) Serum cytokine profiles predict survival benefits in patients with advanced hepatocellular carcinoma treated with sorafenib: a retrospective cohort study. BMC Cancer 17, 870 10.1186/s12885-017-3889-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano T., Ohira M., Nakano R., Tanaka Y. and Ohdan H. (2017) Hepatectomy leads to loss of TRAIL-expressing liver NK cells via downregulation of the CXCL9-CXCR3 axis in mice. PLoS ONE 12, e186997 10.1371/journal.pone.0186997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F. and Sancho D. (2020) Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 34.Osada T., Clay T., Hobeika A., Lyerly H.K. and Morse M.A. (2006) NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity. Cancer Immunol. Immunother. 55, 1122–1131 10.1007/s00262-005-0089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringelhan M., Pfister D., O'Connor T., Pikarsky E. and Heikenwalder M. (2018) The immunology of hepatocellular carcinoma. Nat. Immunol. 19, 222–232 10.1038/s41590-018-0044-z [DOI] [PubMed] [Google Scholar]
- 36.Garnelo M., Tan A., Her Z., Yeong J., Lim C.J., Chen J.et al. (2017) Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 66, 342–351 10.1136/gutjnl-2015-310814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R.X., Wei Y., Zeng Q.H., Chan K.W., Xiao X., Zhao X.Y.et al. (2015) Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 62, 1779–1790 10.1002/hep.28020 [DOI] [PubMed] [Google Scholar]
- 38.Hong Y.K., Li Y., Pandit H., Li S., Pulliam Z., Zheng Q.et al. (2019) Epigenetic modulation enhances immunotherapy for hepatocellular carcinoma. Cell. Immunol. 336, 66–74 10.1016/j.cellimm.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 39.Hirano S., Iwashita Y., Sasaki A., Kai S., Ohta M. and Kitano S. (2007) Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes. J. Gastroenterol. Hepatol. 22, 690–696 [DOI] [PubMed] [Google Scholar]
- 40.Lu C., Rong D., Zhang B., Zheng W., Wang X., Chen Z.et al. (2019) Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol. Cancer 18, 130 10.1186/s12943-019-1047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar V., Patel S., Tcyganov E. and Gabrilovich D.I. (2016) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 10.1016/j.it.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y., Kim S. and Seki E. (2019) Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin. Liver Dis. 39, 26–42 10.1055/s-0038-1676806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y., Chang Q., Wu X., Yu Y. and Zhang H. (2020) Effect of chemokine CXCL14 on in vitro angiogenesis of human hepatocellular carcinoma cells. Arch. Physiol. Biochem. 17, 1–7 10.1080/13813455.2020.1769677 [DOI] [PubMed] [Google Scholar]
- 44.Wang W., Huang P., Zhang L., Wei J., Xie Q., Sun Q.et al. (2013) Antitumor efficacy of C-X-C motif chemokine ligand 14 in hepatocellular carcinoma in vitro and in vivo. Cancer Sci. 104, 1523–1531 10.1111/cas.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi H., Han X., Sun Y., Shang C., Wei M., Ba X.et al. (2018) Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells. Cancer Sci. 109, 3826–3839 10.1111/cas.13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J., Pei S., Wang Y., Liu J., Qian Y., Huang M.et al. (2019) Tpl2 protects against fulminant hepatitis through mobilization of myeloid-derived suppressor cells. Front. Immunol. 10, 1980 10.3389/fimmu.2019.01980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westrich J.A., Vermeer D.W., Colbert P.L., Spanos W.C. and Pyeon D. (2020) The multifarious roles of the chemokine CXCL14 in cancer progression and immune responses. Mol. Carcinog. 59, 794–806 10.1002/mc.23188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng Y., Cheng J., Fu B., Liu W., Chen G., Zhang Q.et al. (2017) Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene 36, 1090–1101 10.1038/onc.2016.273 [DOI] [PubMed] [Google Scholar]
- 49.Ghanem I., Riveiro M.E., Paradis V., Faivre S., de Parga P.M. and Raymond E. (2014) Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am. J. Transl. Res. 6, 340–352 [PMC free article] [PubMed] [Google Scholar]
- 50.Shah A.D., Bouchard M.J. and Shieh A.C. (2015) Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK signaling. PLoS ONE 10, e142337 10.1371/journal.pone.0142337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn H.J., Hwang S.Y., Nguyen N.H., Lee I.J., Lee E.J., Seong J.et al. (2019) Radiation-induced CXCL12 upregulation via histone modification at the promoter in the tumor microenvironment of hepatocellular carcinoma. Mol. Cells 42, 530–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neve P.M., Ierano C., D'Alterio C., Simona L.N., Napolitano M., Portella L.et al. (2015) CXCR4 expression affects overall survival of HCC patients whereas CXCR7 expression does not. Cell. Mol. Immunol. 12, 474–482 10.1038/cmi.2014.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data that support the findings of this study are publicly available in: https://www.oncomine.org/, http://gepia2.cancer-pku.cn/, http://www.cbioportal.org/, http://ualcan.path.uab.edu, http://www.kmplot.com/, http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp, http://timer.cistrome.org, http://cis.hku.hk/TISIDB, and https://david.ncifcrf.gov/home.jsp.