Abstract
The recognition of the rare but serious and potentially lethal complication of vaccine induced thrombotic thrombocytopenia (VITT) raised concerns regarding the safety of COVID-19 vaccines and led to the reconsideration of vaccination strategies in many countries. Following the description of VITT among recipients of adenoviral vector ChAdOx1 vaccine, a review of similar cases after Ad26.COV2·S vaccination gave rise to the question whether this entity may constitute a potential class effect of all adenoviral vector vaccines. Most cases are females, typically younger than 60 years who present shortly (range: 5–30 days) following vaccination with thrombocytopenia and thrombotic manifestations, occasionally in multiple sites. Following initial incertitude, concrete recommendations to guide the diagnosis (clinical suspicion, initial laboratory screening, PF4-polyanion-antibody ELISA) and management of VITT (non-heparin anticoagulants, corticosteroids, intravenous immunoglobulin) have been issued. The mechanisms behind this rare syndrome are currently a subject of active research and include the following: 1) production of PF4-polyanion autoantibodies; 2) adenoviral vector entry in megacaryocytes and subsequent expression of spike protein on platelet surface; 3) direct platelet and endothelial cell binding and activation by the adenoviral vector; 4) activation of endothelial and inflammatory cells by the PF4-polyanion autoantibodies; 5) the presence of an inflammatory co-signal; and 6) the abundance of circulating soluble spike protein variants following vaccination. Apart from the analysis of potential underlying mechanisms, this review aims to synopsize the clinical and epidemiologic features of VITT, to present the current evidence-based recommendations on diagnostic and therapeutic work-up of VITT and to discuss new dilemmas and perspectives that emerged after the description of this entity.
Keywords: Adenoviral vector, Adenovirus, COVID-19, SARS-CoV-2, Vaccine induced thrombotic thrombocytopenia, Vaccine
Abbreviations: aPTT, activated partial thromboplastin time; CAR, Coxsackie-adenovirus receptor; CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; CVST, cerebellar sinus thrombosis; ΕΜΑ, European Medicines Agency; FDA, Food and Drug Administration; HIT, Heparin-induced thrombocytopenia; ICU, Intensive Care Unit; IVIG, Intravenous immunoglobulin; LMWH, low molecular weight heparin; PF4, Platelet factor 4; PLT, Platelet; PRAC, Pharmacovigilance Risk Assessment Committee; PT, prothrombin time; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis; TTS, thrombosis-thrombocytopenia-syndrome; VCAM-1, vascular cell adhesion molecule 1; VIPIT, vaccine-induced prothrombotic immune thrombocytopenia; VITT, vaccine induced thrombotic thrombocytopenia
1. Introduction
The successful genome sequencing of SARS-CoV-2 already in the early stages of the pandemic, initiated a frantic race towards the development of vaccines against COVID-19. Following the results of phase 3 clinical trials on vaccines demonstrating extremely high efficacy rates for symptomatic and serious COVID-19, international drug regulation agencies issued emergency use authorizations for several novel vaccines; between December 2020 and March 2021, four vaccine preparations were granted marketing authorization by the European Medicines Agency (EMA). Within this timeframe, national vaccination programs of unprecedented speed and thoroughness were worldwide implemented, resulting in vaccinations of several millions of individuals from all age adult groups. The recognition of the rare but serious and potentially lethal complication of vaccine induced thrombotic thrombocytopenia (VITT) brought concern regarding the safety of COVID-19 vaccines and led to the reconsideration of vaccination strategies in many countries. Following the description of VITT among recipients of adenoviral vector ChAdOx1 vaccine, a review of similar cases after Ad26.COV2·S vaccination gave rise to the question whether this entity may constitute a potential class effect of all adenoviral vector vaccines. The aim of the present brief review is to analyze the proposed underlying mechanisms of VITT, summarize the clinical and epidemiologic features of VITT and to present the current evidence-based recommendations on the diagnostic and therapeutic approach to VITT. Furthermore, the dilemmas and further perspectives that emerged after the description of this entity are also discussed.
2. An account of early VITT-related events
In March 2021, EMA's Pharmacovigilance Risk Assessment Committee (PRAC) began an assessment οn signals of increased incidence of thrombotic events, including splanchnic vein and cerebellar sinus thrombosis (SVT and CVST, respectively) accompanied by thrombocytopenia, especially among females aged less than 60 years, within the 2 weeks following the 1st dose of adenoviral vector ChAdOx1 vaccine Vaxzevria® (Oxford/AstraZeneca).
The clinical and laboratory features of 16 of 25 cases of this complication which were reported in Germany and Austria were described in two case series [1,2]. The presented cases of both reports consisted of typically young patients (range: 22–49 years old/y. o.), most commonly females (13 of 16, 81.25%) who presented shortly after ChAdOx1-vaccination (median 8 days, range 5–16 days). In total 14 patients had CVST, 4 SVT, 3 pulmonary embolism, 4 other thrombotic complications, with 9 of the presented 16 cases (56.3%) having a fatal outcome. At presentation, elevated D-dimer levels, and variable degrees of typically severe thrombocytopenia (median PLT count 19 × 103/μl) were most commonly noted. Patients showed detectable antibodies against Platelet factor 4 (PF4)-Heparin complexes although none had received heparin in the preceding days, while functional assays revealed a stark platelet activation from patient's serum, in the presence of buffer in most, and in the presence of PF4 in virtually all cases [1,2]. Due to the striking clinical and laboratory similarities of the syndrome to heparin-induced thrombocytopenia, the term vaccine-induced immune thrombotic thrombocytopenia – VITT was coined [2], later also referred to as the more euphonic “vaccine-induced prothrombotic immune thrombocytopenia”-VIPIT. These terms have been used interchangeably with thrombosis-thrombocytopenia-syndrome (TTS). Furthermore, individuals with VITT often presented features of disseminated intravascular coagulation apart from thrombopenia, namely greatly elevated D-dimer levels (>10.0 mg/L), low fibrinogen concentration and variably prolonged prothrombin and activated partial thromboplastin time (PT and aPTT) [2].
On April 7, 2021, the committee concluded that a causal link between this rare side-effect and the vaccination could not be ruled out. Up to that date, 169 CVST and 53 SVT cases had been reported among around 34 million vaccinated individuals in the European Economic Area and the United Kingdom [3]. Because of concerns regarding ChAdOx1 safety, temporary halts of ChAdOx1 pending PRAC assessment were followed by permanent stops of its administration by national drug agencies in some countries (Norway, Denmark) or by restriction of its usage to older age groups in others (e.g. Iceland, France, Germany).
In a similar course of events, on April 13, 2021 the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) in the USA recommended a pause of the administration of another adenovirus vector vaccine, Ad26.COV2·S (Johnson & Johnson/Janssen) [4] pending further assessment of a case report of CVST/SVT and thrombocytopenia in a 48-year female recipient [5], while a similar case occurred in a 25-year participant during the clinical trial testing [4]. After review of 8 cases of thrombosis with thrombocytopenia, both FDA/CDC and the EMA concluded that in total, Ad26.COV2·S outweigh its risks, and accordingly advised in favor of resumption of vaccination programs although noting that high-risk recipients (i.e. women under the age of 50) should be aware of this rare complication, as well as of the availability of other vaccines which do not seem to carry that risk [6,7]. A series of 12 Ad26.COV2·S – related VITT cases published shortly after by See et al. [8], revealed features remarkably similar to that related with ChAdOx1; all 12 cases were Caucasian females, 11 of 12 (91.7%) were younger than 50 y. o. and all were younger than 60 years, the median vaccination-symptom onset interval was 8 (range 6–15) days, while all had CSVT, 8 had additional non-CSVT thrombotic manifestations while death had occur in 3 cases by the time of publication [8]. The median (nadir) platelet count was 19 × 103/μl and the D-dimer elevated in all cases. Likewise, antibodies against PF4-heparin were positive in all 11 tested patients. However, in contrast to the Vaxzevria®-related cases, functional platelet assays were mostly negative; although, as the authors have stressed, standardization issues between different laboratories may at least partly account for this phenomenon [8].
3. Potential mechanisms inducing VITT
Since this otherwise particularly infrequent complication has to date not been observed among other vaccine types (although, cases of clinically significant thrombocytopenia, likely of autoimmune mechanism, have been described following mRNA-based vaccination [9]), the question arose whether VITT/TTS observed across two independent adenoviral vector vaccines may represent a side effect unique to this vaccine category, and to what extent this may represent a class effect affecting all agents of this family. Vaccines of this type use recombinant, non-replicative adenoviruses which act as shells for the carriage of the DNA strand that codes for the SARS-CoV-2 spike protein, which is necessary for its pathogenicity. Upon intramuscular administration, recombinant viruses enter local cells expressing, among others, the Coxsackie-adenovirus receptor (CAR) via clathrin-mediated endocytosis [10], the carried episomal DNA enters the nucleus, is transcribed into mRNA coding for viral spike protein and is the translated in the endoplasmic reticulum [11]. A number of mechanisms implicating adenoviral vectors and/or other vaccine ingredients as triggers of prothrombotic states have been proposed:
-
1.
The striking clinical and laboratory similarities between VITT/TTS and HIT may imply that a similar autoimmune mechanism may underly both conditions. In HIT, the pathogenic autoantibodies target PF4 complexes with heparin, which is chemically a polyanion itself. A syndrome similar to HIT without prior heparin therapy (termed autoimmune HIT - aHIT) can follow the exposure to a variety of other large polyanions such as hypersulfated chondroitin sulfate, DNA or RNA and bacterial wall components [12]. In any case, the PF4-polyanion-autoantibody complexes activates platelets through their Fcγ-receptors resulting in an increased risk of thrombosis. In the case of VITT/TTS, the adenoviral DNA content or other currently unaccounted for polyanionic vaccine contents could bind to PF4, and hence induce autoantibody production and subsequent platelet activation, which would essentially render VITT/TTS a subtype of aHIT. This mechanism was supported by Greinacher et al. in one of the original case series of ChAdOx1 recipients [2]. In favor of this mechanism is the universal demonstration of circulating PF4/Polyanion autoantibodies as well as the predominance of young females among cases, a population which is most susceptible to autoimmunity.
-
2.
Platelets also express CAR [13], so it can be hypothesized that megakaryocytes, their nucleated precursors may be susceptible to recombinant adenovirus infection. A subsequent SARS-CoV-2 spike protein expression could render platelets primary antibody targets or enhance thromboxane A2 production [14].
-
3.
Following intravenous administration, adenoviruses may bind to circulating platelets in a von Willebrand Factor- and P-selectin mediated fashion, causing their activation and sequestration in the reticuloendothelial system [15,16]. Furthermore, injection through this route induces endothelium activation as indexed by increased VCAM-1 (vascular cell adhesion molecule 1) expression, which may further contribute to the establishment of a prothrobotic milieu [15]. Although such phenomena are not expected after intramuscular administration, an intravenous injection of a smaller or larger volume of the vaccine solution cannot be definitely excluded in some cases, especially when administering the vaccines under immense time pressure conditions as is often the case in vaccination centers.
-
4.
Apart from platelets, anti-PF4 antibodies may also bind to and activate various other cell types, such as neutrophils [17], monocytes (hence inducing the expression of tissue factor) [18,19], as well as endothelial cells [20], therefore further promoting thrombotic manifestations.
5. Greinacher et al. have proposed that the Ethylenediaminetetraacetic acid (EDTA) contained in the vaccine preparation may increase local vascular permeability in the injection site and cause the systematic dissemination of vaccine components which interact with preformed natural antibody, inducing a serum sickness-like illness. This inflammatory state may act as a co-signal to augment the antibody production of anti PF4-antibody-producing B-cells [21].
6. Finally, currently unpublished data from an in vitro study by Kowarz E. et al., point towards the presence of alternative splicing events following adenoviral vector vaccination, owing to the entry and transcription of the delivered episomal DNA into the cell nucleus, rather than the direct mRNA transcription in the endoplasmic reticulum as is the case with mRNA vaccines. This may in turn lead to the production of shorter spike protein variants deprived of their c-terminal membrane anchor, which circulate in the bloodstream and bind to the ACE2 receptors on endothelial cells, thus triggering thrombotic events [11].
4. Is VITT observed in all marketed adenovirus-vector vaccines?
There are currently four marketed adenovirus-vector vaccines against SARS-Cov-2 which utilize a different adenovirus vector (non-replicating chimpanzee adenovirus in ChAdOx1, Ad5 in CanSino Biologicals Ad5-nCoV and first Sputnik V dose, Ad26 in Johnson & Johnson/Janssen and in the booster Sputnik V dose). No corresponding signals have emerged for Sputnik 5 or CanSino or even the since 2020 licensed adenoviral Vector vaccine Ad26. ZEBOV against Ebola virus disease for that matter. However, there are no sufficient safety data regarding other adenovirus-vector vaccines used in COVID-19 prevention in light also of concerns of Ad-5 replication [22]. Furthermore, there is lack of detailed information on the adverse events of Sputnik V reported during the clinical trial [23].
Despite a considerable and growing cumulative number of vaccinations, it would seem at first unlikely that the entity constitutes a class effect for all adenoviral vector vaccines. Differences among vaccines regarding the spike protein inserts and utilized adenoviral vector shells may theoretically affect the risk of VITT/TTS manifestation [4]. Furthermore, different adenovirus strains may bind to variable cellular receptors, and hence may infect a different spectrum of host cells [4].
5. Impact of VITT on COVID-19 vaccination strategies
However infrequent, the severity of this potentially fatal complication which essentially evaded detection during clinical testing worsened preexisting public skepticism not only towards these vaccines but also against COVID-19 vaccination in general. Primary care physicians and immunology experts were overwhelmed by questions from patients and medical colleagues, regarding the safety of vaccination among those with known or suspected prothrombotic medical conditions and/or platelet abnormalities. Vaccination appointments were frequently cancelled which, together with the vaccination pauses pending assessment results from the responsible committees, newly implemented age restrictions and the logistic difficulties of replenishing retracted batches with vaccines from other manufacturers landed decisive blows on national vaccination programs, delaying the progress towards the establishment of herd immunity, possibly with a heavy cost of lives in a particularly active stage of the pandemic course worldwide [24].
Recent reports have indicated that the incidence of VITT/TTS may be in reality considerably higher than previously presumed [25], and this may possibly further rise as awareness and understanding of the syndrome becomes more widespread among physicians. The estimated incidence of VITT varies between reports from 1 for every roughly 25,000 individuals vaccinated with ChAdOx1 [1] to 1 for every more than 500,000 Ad26.COV2·S vaccinations [26]. These estimates are by all accounts lower than the mortality rates of SARS-Cov-2 infection, essentially in all age groups [27], and undoubtedly less likely than the rates of thromboses complicating actual SARS-CoV-2 infection, especially those with a severe disease course [28]. Based on one meta-analysis, the rate of thrombosis (including fatal thrombosis) was estimated at 8% in subjects hospitalized for COVID-19 and 23% in subjects at the intensive care unit (ICU) [28,29]. The overall risk-benefit-ratio of continued use of the vaccines implicated in VITT/TTS or conversely, their restriction to specific age groups or complete withdrawal is a close function of the status of viral shedding in the community, and thus constitutes a dynamic, constantly varying balance. Furthermore, a strategy of adenovirus vector vaccine withdrawal and their replacement with mRNA-based ones, as was implemented in certain European countries may be not easily feasible or even completely unrealistic in other areas. It should be taken into account that the costs of a full vaccination course per recipient for both ChAdOx1 and Ad26.COV2·S are substantially lower and their storage and transport conditions considerably simpler than those of their mRNA-counterparts [29]. For these reasons -among others-adenoviral vector vaccines constitute the bulk of the vaccination program in many countries globally. Furthermore, the single-dose Ad26.COV2·S has provided a viable solution for the rapid achievement of adequate coverage rates of geographically remote areas, as is the case in certain islands of Greece.
Self-evidently, adequate information about the (remote, albeit existing) possibility of VITT/TTS should be provided to adenoviral vector vaccine recipients. The choice of an alternative agent (e.g. mRNA-based) should be weighted for the high-risk groups, predominantly for females of younger age, until further predisposing factors are recognized. Individuals should be advised to immediately seek medical attention in case that alarming clinical symptoms appear, especially within the time frame that VITT/TTS would be expected to manifest following vaccination. This should ideally occur in health care providing services with access to expert hematology consultation and specialized diagnostic laboratories.
6. Diagnostic and therapeutic considerations
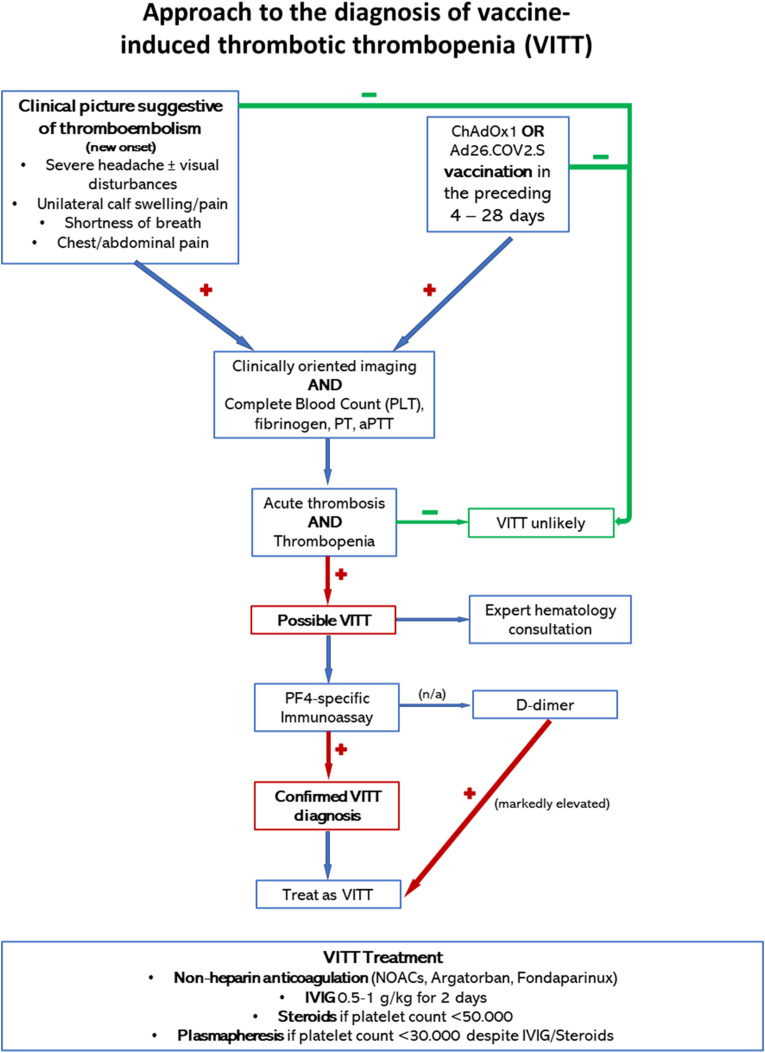
In order for suspected cases to be systemically identified, interim case definition criteria network for definite, probable or possible VITT/TTS have been developed the Brighton Collaboration vaccine safety research. The algorithms are updated on the basis of new knowledge and as of May 19, 2021 the version 10.16.3 is available [30]. Furthermore, numerous national and international societies have issued clinical practice algorithms for the diagnostic approach and treatment of VITT/TTS. A modified algorithm based on the recommendations of the International Society on Thrombosis and Hemostasis is presented in Fig. 1.
Fig. 1.
A proposed diagnostic approach to suspected VITT/TTS. Modified from Ref. [35] Abbreviations: IVIG: intravenous immunoglobulin; n/a: not available; NOACs: novel oral anticoagulants.
The presentation window after vaccination may span a broader time range that reported in the original case series, especially when underlying thrombotic events lead to milder, less acute clinical manifestations hence leading to delayed diagnosis. Furthermore, a non-specific, flu-like illness may precede or accompany the syndrome at the time of presentation [31]. A presumptive diagnosis of VITT is confirmed with the demonstration of circulating PF4/polyanion antibodies (usually with high optical density readings [1,2]) via enzyme-linked immunosorbent assay (ELISA), in the absence of prior heparin exposure. Unfortunately, the rapid immunoassays used for HIT screening (including latex-enhanced, lateral flow and particle gel immunoassays) especially in non-tertiary centers, do not appear to be sensitive for the detection of PF4-polyanion-autoantibodies in VITT/TTS, and are therefore not useful in the diagnostic workup outside specialized departments [32]. In that case, readily available standard coagulation studies should be ordered awaiting further testing. A reasonable clinical suspicion of VIΤΤ even pending definite laboratory confirmation mandates the initiation of anticoagulation therapy. While there is no evidence for the notion that unfractionated or low molecular weight heparin (LMWH) may negatively impact the course of the syndrome, due to its similarities with HIT, most societies advise in favor of the use of non-heparin anticoagulants. A short course of intravenous immunoglobulin (IVIG) in the acute phase of the syndrome may hinder platelet activation and lower thrombosis risk through the blockade of platelet surface Fcγ-receptors [33].
7. Conclusion
Deciphering the pathogenetic process behind VITT/TTS would not only help optimize therapeutic strategies but would also aid the identification of high-risk groups as well as the optimization and cost-effectiveness of nation-wide vaccination strategies, especially during pandemic peaks and/or vaccine shortage. Further issues to be addressed include the safety of adenoviral vector vaccines among those with known thrombophilia or risk factors for thrombosis or chronic thrombocyte disorders and whether diagnostic and/or therapeutic preventive measures would be beneficial among such recipients. The vaccination programs are likely to continue dominating the strategies against the ongoing COVID-19 pandemic, especially since the efforts towards the development of safe and effective therapies against SARS-Cov-2 infection have yet to yield concrete and clinically applicable results [34]. Besides, with the issue of the potential necessity of additional boosters or even of repeat vaccinations on an annual basis still remaining open, further reasons for debate on the subject of VITT/TTS management are bound to emerge.
Financial support
None.
Declaration of competing interest
No conflict of interest to disclose
References
- 1.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J., Carpena-Ruiz M., Montero-Errasquin B., Sanchez-Castellano C., Sanchez-Garcia E. Exclusion of older adults from ongoing clinical trials about type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61:734–738. doi: 10.1111/jgs.12215. [DOI] [PubMed] [Google Scholar]
- 4.Sadoff J., Davis K., Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination - response from the manufacturer. N Engl J Med. 2021;384:1965–1966. doi: 10.1056/NEJMc2106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir K.L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EMA COVID-19 vaccine janssen. https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen2021
- 7.Rooney M.R., Rawlings A.M., Pankow J.S., Echouffo Tcheugui J.B., Coresh J., Sharrett A.R. Risk of progression to diabetes among older adults with prediabetes. JAMA internal medicine. 2021 doi: 10.1001/jamainternmed.2020.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. Jama. 2021 doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier O., Greber U.F. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 11.Kowarz E.K.L., Reis J., Bracharz S., Kochanek S., Marschalek R. “Vaccine-Induced Covid-19 Mimicry” Syndrome:Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Research Square. 2021 doi: 10.21203/rs.3.rs-558954/v1. [DOI] [Google Scholar]
- 12.Greinacher A., Selleng K., Warkentin T.E. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemostasis : JTH. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 13.Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kate Chander Chiang A.G. Mechanism of thrombosis with AstraZeneca and J & J vaccines. Expert Opinion. 2021 MD. [Google Scholar]
- 15.Othman M., Labelle A., Mazzetti I., Elbatarny H.S., Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 16.Stone D., Liu Y., Shayakhmetov D., Li Z.Y., Ni S., Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81:4866–4871. doi: 10.1128/JVI.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Z., Visentin G.P., Dayananda K.M., Neelamegham S. Immune complexes formed following the binding of anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood. 2008;112:1091–1100. doi: 10.1182/blood-2008-04-153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouplard C., Iochmann S., Renard B., Herault O., Colombat P., Amiral J. Induction of monocyte tissue factor expression by antibodies to heparin-platelet factor 4 complexes developed in heparin-induced thrombocytopenia. Blood. 2001;97:3300–3302. doi: 10.1182/blood.v97.10.3300. [DOI] [PubMed] [Google Scholar]
- 19.Kasthuri R.S., Glover S.L., Jonas W., McEachron T., Pawlinski R., Arepally G.M. PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcgammaRI. Blood. 2012;119:5285–5293. doi: 10.1182/blood-2011-06-359430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visentin G.P., Ford S.E., Scott J.P., Aster R.H. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93:81–88. doi: 10.1172/JCI116987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greinacher A.S.K., Wesche J., Handtke S., Palankar R., Aurich K., Lalk M., Methling K., Völker U., Hentschker C., Michalik S., Steil L., Schönborn L., Beer M., Franzke K., Rangaswamy C., Mailer R.K., Thiele T., Kochanek S., Krutzke L., Siegerist F., Endlich N., Warkentin T.E., Renné T. Towards understanding ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) Research Square. 2021 doi: 10.21203/rs.3.rs-440461/v1. [DOI] [Google Scholar]
- 22.Moutinho S., Wadman M. Brazil and Russia face off over vaccine contamination charge. Science. 2021;372:554. doi: 10.1126/science.372.6542.554-a. [DOI] [PubMed] [Google Scholar]
- 23.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver S. 2021. Risk/benefit assessment of thrombotic thrombocytopenic events after janssen COVID-19 vaccines: applying evidence to recommendation framework. April 21. [Google Scholar]
- 25.Pottegard A., Lund L.C., Karlstad O., Dahl J., Andersen M., Hallas J. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tom Shimabukuro M., MPH, MBA CDC COVID-19 Vaccine Task Force Vaccine Safety Team . 2021. Thrombosis with thrombocytopenia syndrome (TTS) following Janssen COVID-19 vaccine. [Google Scholar]
- 27.Meyerowitz-Katz G., Merone L. International journal of infectious diseases : IJID. Vol. 101. official publication of the International Society for Infectious Diseases; 2020. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates; pp. 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenner W.J., Kanji R., Mirsadraee S., Gue Y.X., Price S., Prasad S. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;51:595–607. doi: 10.1007/s11239-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyer O. Covid-19: countries are learning what others paid for vaccines. BMJ. 2021;372:n281. doi: 10.1136/bmj.n281. [DOI] [PubMed] [Google Scholar]
- 30.Collaboration B . 17 May 2021. TTS interim case definition v10.16.3. [Google Scholar]
- 31.Bayas A., Menacher M., Christ M., Behrens L., Rank A., Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397:e11. doi: 10.1016/S0140-6736(21)00872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vayne C., Rollin J., Gruel Y., Pouplard C., Galinat H., Huet O. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021 doi: 10.1056/NEJMc2106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padmanabhan A., Jones C.G., Pechauer S.M., Curtis B.R., Bougie D.W., Irani M.S. IVIg for treatment of severe refractory heparin-induced thrombocytopenia. Chest. 2017;152:478–485. doi: 10.1016/j.chest.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallianou N.G., Tsilingiris D., Christodoulatos G.S., Karampela I., Dalamaga M. Anti-viral treatment for SARS-CoV-2 infection: a race against time amidst the ongoing pandemic. Metabolism open. 2021;10:100096. doi: 10.1016/j.metop.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haemostasis ISoTa. Vaccine-induced immune thrombotic thrombocytopenia (VITT) diagnostic flow chart (Updated 20 April, 2021).