Abstract
Extracorporeal life support (ECLS) has been proven to be very useful in the neonatal period. For reversible respiratory and cardiac disorders, when maximal conventional measures have failed to provide life support, extracorporeal membrane oxygenation (ECMO) becomes the treatment of choice. The indications, contra-indications for ECMO, optimization of the care prior to embracing ECMO, cannulation techniques, daily management of ECMO from the practical standpoint, weaning and decannulation, complications, and special circumstances in neonatal period have been described. The follow-up of neonatal ECMO and various system manifestations necessitating careful review will be highlighted.
Keywords: Neonatal ECMO, ECLS, Cannulation, Monitoring, Follow-up
Introduction
When maximal conventional support fails in rescuing sick neonates, extracorporeal membrane oxygenation (ECMO) will be the option in treating reversible respiratory and cardiac pathologies. ECMO in the neonatal period was done for the first time by Dr. Robert Bartlett in the year 1975. Since 1989, 32,385 neonates required ECMO for respiratory causes, 8830 neonates were treated for cardiac causes, and 2035 neonates were resuscitated with extracorporeal cardiopulmonary resuscitation (ECPR) as reported to the Extracorporeal Life Support Organization (ELSO) registry (International ELSO Registry, Jan 2020) [1].
Extracorporeal life support has been proved to be very useful in the neonatal period . Interestingly, placental circulation is similar to the ECMO design. Neonatal age group is phenomenal for its plasticity, rapid replication of the tissues, and the influence of hypoxia on developing organs, more so on the brain. The issues range from human anatomy to economics and emotional bonding. Neonatal lung growth continues until 2 years of age and beyond. It will be interesting to note the effect of fibrosis developing within the neonatal lung as a result of baro-trauma and volu-trauma, as a reflection of ventilator-induced lung injury. As we all know, humans breath and support their respiratory function as negative pressure ventilation. It makes sense to consider ECMO therapy before causing irreplaceable lung injury for avoiding ventilator-related lung injury, by giving time for the recovery of the injured lung.
Neonatal ECMO in premature babies deals with a newborn with increasing fragility, urging us to keep away from them as the tissues could be very porous and immature. They tend not to tolerate high pressures as the cerebral blood vessels could rupture easily. Added to that is the small size of the vasculature making the cannulation process difficult as the available cannulae only permit venoarterial (VA) cannulation. Hence, 34 weeks period of gestation is taken as a criterion for defining the lower age group for consideration of ECMO.
In resource-limited countries where ECMO is possible on self-funding and the health insurance is not geared up to cover the substantially huge bills of ECMO, probability of minimal bonding to an unborn baby might play a role in parents not choosing ECMO.
In our practice, ECMO in the neonatal age group has been very satisfying as most of the conditions in this group are reversible. Historically, we have been seeing increasing survival figures in this age group, as expected.
Neonatal ECMO trend in the world and the randomized controlled trials (RCTs)
The ECMO community will be thankful to Dr. Robert Bartlett for treating the baby of the immigrant mother, Esperanza, on ECMO for meconium aspiration syndrome, while she was unresponsive to maximal conventional measures. Esperanza in Spanish means hope. She remained as “Hope” to the ECMO community despite the ups and downs faced by the RCTs in the Western world. Though Dr. Donald Hill did the first case of adult ECMO in 1972, Esperanza remains as the first case of neonatal ECMO. Richard Firmin, former chairman of European ELSO chapter and Founding ECMO consultant at Glenfield Hospital, Leicester, United Kingdom (UK), initiated the first neonatal ECMO trial in the UK.
Neonatal ECMO indications
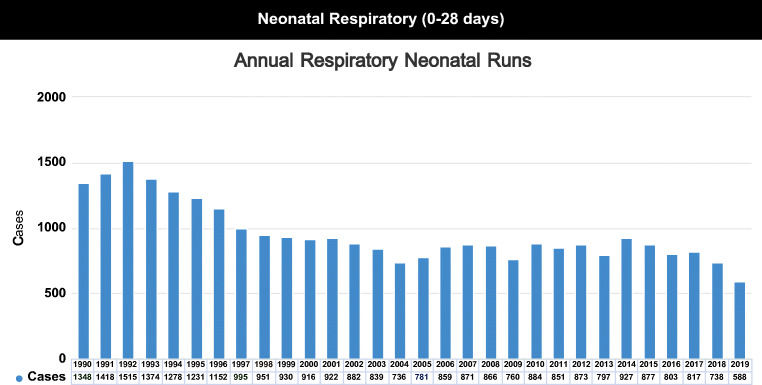
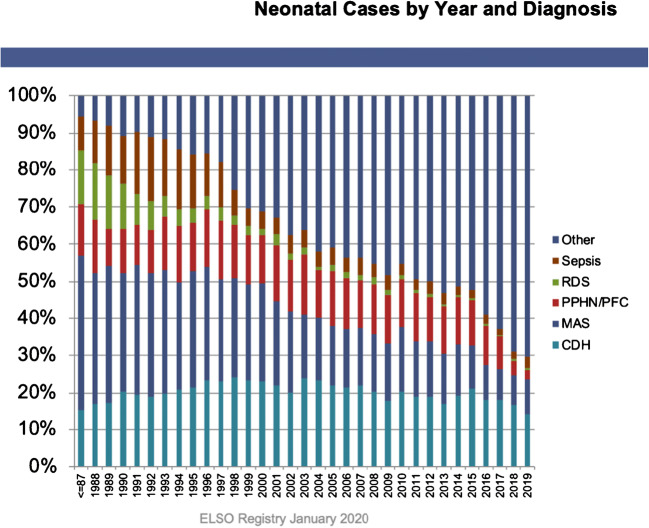
ECMO in the neonatal period will be required for respiratory reasons and sparingly for cardiac stabilization. ELSO registry data on annual neonatal respiratory ECMOs done over the years and the percentage of cases as per indication have been shown in Appendix 1 and 2 Figs. 1 and 2.
| Respiratory causes of ECMO: | |
| Meconium aspiration syndrome | |
| Severe respiratory distress syndrome not responding to surfactant | |
| Persisting pulmonary hypertension of newborn | |
| Septicemia with shock | |
| Pneumonias after failure of conventional management | |
| Neonatal pertussis | |
| Congenital diaphragmatic hernia (CDH) | |
| Multiple pneumothoraces ± bronchopleural fistula | |
| Cardiac ECMO: | |
| Pre-stabilization: | |
| Cyanotic congenital heart disease in decompensated shock | |
| Resistant arrythmias in cardiogenic shock | |
| Neonatal myocardial infarction | |
| Myocarditis | |
| Post-operative: | |
| Inability to come off cardio-pulmonary bypass | |
| Failing heart after cardiac surgery |
Fig. 1.
Annual Respiratory Neonatal Runs. Courtesy of Peter Rycus: ELSO (Extracorporeal Life Support Organization, Ann Arbor Michigan) International Summary. Jan 2020
Fig. 2.
Neonatal Cases by Year and Diagnosis. Courtesy of Peter Rycus: ELSO (Extracorporeal Life Support Organization, Ann Arbor Michigan) International Summary. Jan 2020
Contra-indications for ECMO:2
| Major intraventricular/cerebral bleed (grade 3 or 4 intraventricular hemorrhage) | |
| Not able to cope with anti-coagulation | |
| Prematurity: less than 32 weeks gestation | |
| Established chronic lung disease | |
| Lethal malformations or congenital anomalies |
Optimizing conservative measures of management
To date, we have been following the concept that maximal conventional management should have been tried prior to putting a child on ECMO. Some of the measures worth considering in the case of meconium aspiration syndrome are to stretch the alveolus reasonably, for example with an inspiratory time of 0.6 s on a respiratory rate of 40/min, without resorting to inverse ratio ventilation. Inspiratory time of 0.35 s and respiratory rates of more than 50/min are less successful in improving gas exchange in this condition. As ventilation, perfusion is crucial for achieving adequate gas exchange; it is very important to provide optimal hydration to the child, and at the same time avoiding overhydration as this could be the time Systemic Inflammatory Response Syndrome (SIRS) and capillary leak will set in. The above settings have to be individualized as the compliance varies. As we are likely to be working on 100% FiO2 at this time on high ventilator pressures, it is very important to avoid any asynchrony from the child’s point of view and use the right amount of sedation, analgesia, and paralysis to get the best results of gas exchange. Once these measures show hypoxia and hypercapnia for more than 4 hours, immediately, support should be sought from the ECMO center. High-frequency oscillatory ventilation and inhaled nitric oxide can be tried too, while the baby is being evaluated.
How long to wait before putting the patient on ECMO?
There have been debates about the ideal time before putting a child on ECMO. Once it is clear that the child is deteriorating and maximal conventional management was tried, it is the right time to put the child on ECMO. As far as possible, the physician must be satisfied that the underlying condition making the child unstable is reversible. Certain indices such as the oxygenation index (OI) will help as an objective measure in deciding the suitability of a patient to consider initiating ECMO (Appendix 3). If the oxygenation index is more than 40, it is an indication for putting the child on ECMO. If the patient is on additional nitric oxide, conventionally, another 10 points will be added to the oxygenation index total score. In practice, once the patient starts to deteriorate, the fall in gas exchange parametres will be very fast. Traditionally, patients should be placed within a week to 10 days after resorting to high pressure ventilation. Recent information is indicating that the results of ECMO outcome would be much better if ECMO is started early without undue delay.
Cannulation
Peripheral
In neonates with a birth weight of less than 4 kg, resorting to VA ECMO for respiratory as well as cardiac reasons is ideal. This is in view of the non-availability of appropriate sized cannulae to fit the smaller blood vessels. Usually, in infants weighing around 4 kg, 13 F Avalon Elite™ Bi-Caval Dual Lumen Catheter (Maquet Cardiovascular) for insertion is available. If double lumen cannulae are not available, that is another reason to choose VA cannulation as femoral vessels are too small below 2 years of age. Internal carotid artery for arterial access and internal jugular vein for venous access are the preferred vessels. Usually, the semi-Seldinger technique is used.
Central
If peripheral cannulation is technically difficult/not possible, then central cannulation with the help of the pediatric cardiac surgical team would be the procedure of choice.
Sedatives and analgesics
We use ketamine 1 to 2 mg/kg and rocuronium 1 mg/kg for cannulation. Additional doses may be required as per individual patient’s tolerance. Fentanyl can be considered as an addition. Propofol is useful but can cause hypotension.
We continue with infusions of morphine and midazolam to achieve adequate sedation and analgesia. We use chloral hydrate sparingly as it is an oral medication. Other medications to be considered are fentanyl, ketamine, dexmedetomidine, and clonidine. We focus on avoiding cumulative toxicity.
Anti-coagulation
Heparin is the anti-coagulant of choice in many centers. A stat dose of 75 mg/kg heparin is given at the time of cannulation. Activated clotting time (ACT) is measured as a bedside test. Once ACT starts coming down to 300, continuous heparin infusion can be started in the dosage of 13 units/kg/h (to be modified as per the requirements).
Antithrombin 3 (AT) in ECMO
Heparin covalently bonds to antithrombin and induces a conformational change in the protein structure that profoundly increases the anti-coagulant activity [2]. Theoretically, antithrombin III administration after checking the levels of AT 3 may reduce heparin utilization and bring down the dosage requirement of heparin in ECMO patients. But, studies have been showing that it has no effect on clinical outcomes [3]. Heparin resistance is defined as the inability to reach target monitoring levels with heparin doses > 35–40 units/kg/h. Blood exposure to heparin and circuit causes consumption of endogenous AT.
Children are born with an immature coagulation system, including blood antithrombin activity [3]. Neonates and Infants < 6 months of age have decreased antithrombin III levels, and further decreases in AT3 levels are noted in children with congenital heart disease [4, 5]. Neonates have approximately 30% of adult levels [6]. Activity of coagulation factors normalizes asynchronously over the first few years of life.
There are multiple variables affecting ACT measurement. In patients with inherited (e.g., hemophilia, von Willebrand disease type 3) or acquired (e.g., vitamin K antagonist anti-coagulation, liver failure, dilutional coagulopathy) coagulation disorders, low heparin concentrations/dosages will be required to prolong the clotting time within the targeted ranges. However, other situations such as hyperfibrinogenemia, antithrombin (AT) deficiency, and thrombocytosis may call for increased, sometimes excessive, dosages of unfractionated heparin (UFH). Lupus anti-coagulant (LA) also prolongs initial ACT values, and other strategies could be considered for heparin monitoring in patients to monitor anti-coagulation [7]. Severe deficiency of contact activation factors (factor XII, prekallikrein, high molecular weight kininogen) does not cause increased bleeding risk during heparin therapy but does prolong the ACT. AT III deficiency prolongs ACT. Fresh frozen plasma administration reduces ACT by increasing pro-coagulant factors.
As an approximate guidance, it is customary for ACTs to be in the range of 160–180 in veno-venous (VV) ECMO and 180–200 in VA ECMO. Devices from different manufacturers use different activators and different methodologies of assessing the formation of clot; thus, ACT values from different machines cannot be used interchangeably. Each facility should follow a standard protocol and ideally define its own reference interval, in guidance with the above recommended values. In case of any adverse reactions to heparin such as heparin-induced thrombocytopenia, other anti-coagulants like bivalirudin, lepirudin, and argatroban can be considered.
Infection management
Strict asepsis should be carried out throughout the ECMO run. The skin should be thoroughly cleaned with chlorhexidine and prepped prior to cannulation. A single dose of antibiotic to cover skin flora (such as cefotaxime/flucloxacillin) is given at the time of cannulation. Depending upon the infection risk, etiology of respiratory failure, and possibility of sepsis, suitable antibiotics can be used. It is very important to evaluate the blood and source cultures for the presence of infection. Septic markers such as white cell count, C-reactive protein, and pro-calcitonin are watched frequently (12 hourly). There could be an overlap between infection and inflammation while on ECMO circuit. If there is no evidence of sepsis, antibiotics can be stopped with meticulous monitoring for new onset of sepsis. Clinical interpretation and best practice guidelines can help in deciding the choice and duration of antibiotic therapy.
Respiratory management
The most important reason for initiating ECMO is correcting gas exchange and giving rest to the lungs. On optimal ECMO flow, ventilatory settings should be brought down to rest ventilatory settings. Ideal rest ventilatory settings are still not clear. On pressure support/synchronized intermittent mandatory ventilation (SIMV), peak inspiratory pressure of 20 cm H2O pressure, PEEP of 10 cm H2O pressure, inspiratory time of 1 second, and rate of 10 breaths/min in FiO2 30% is one such recommendation. It may or may not be possible to reduce ventilatory settings immediately going on to ECMO but every effort should be made to reduce the settings as soon as possible. Chest X-ray confirmation of cannula position is mandatory.
Cardiac issues
Evaluation of cardiac anatomy and physiology are mandatory while a neonate is on ECMO. Electrocardiogram (ECG) and echocardiography are mandatory. At times, cardiac catheterization may be needed in difficult cases. Understanding cardiopulmonary interactions is important.
Cardiac evaluation is important because of the following reasons
Indications for cardiac evaluation in neonates on ECLS:
| Exclude any significant congenital cardiac anomalies | |
| Patent ductus arteriosus (PDA) and the direction of flow | |
| Atrial septal defect (ASD)/patent foramen ovale (PFO) status and the quantification of shunt | |
| Left atrial distention | |
| Cardiac function | |
| Exclusion of pericardial effusion |
Gastrointestinal management:
We give a lot of importance in starting feeds as soon as possible and escalate to reach the required volume. The only contra-indication would be having any indicators for necrotizing enterocolitis following severe hypoxic ischemic encephalopathy or a surgical reason. Nasogastric aspirate should be watched for any reasons suggestive of non-tolerance of feeds.
Anatomical and surgical reasons causing delayed gastric emptying should be considered. Hypokalemia has to be excluded. In the absence of the above, therapeutic trial of erythromycin, metaclopramide, or domperidone can be attempted. Naso-jejunal continuous feeds are another effective way to tide over gastric atony. Feeding is essential for maintaining good calorific intake and keeping the gastrointestinal tract healthy, avoiding intestinal atrophy.
Central nervous system
Neonates tend to bleed if there is a significant ischemic focus prior to going on to ECLS. Prematurity could be another contributing factor to cerebral bleeds; hence, as stated earlier, neonates younger than 32 weeks period of gestation are usually excluded for ECLS support [8]. It is good practice to watch neuro-behavior regularly. Some of the important aspects to watch are the following: activity of the baby (sensorium), pupillary sizes and reaction to light, mobility of extremities. Our therapeutic goal is to maintain a pain-free state but not deep sedation or paralysis. Muscle paralysis is used rarely, if the child is hyperventilating despite adequate sedation and analgesia, resulting in significant hypocarbia or obstruction to the ECMO flows. A thorough check for co-existing ECMO circuit-related factors should be looked for carefully. It is a good practice to keep a record of daily neurosonograms and near-infrared spectroscopy (NIRS) monitoring. Whenever there is a concern about intracranial bleed, the baby should be transported to computerized tomography (CT) scanner on ECMO for having a detailed examination as cerebral bleeds tend to get worse on continuous anti-coagulation. In-house, intra-hospital transport on ECMO support is an expected task for any unit doing ECMO. Careful planning, simulations, dry runs, and practice will help in making this process safe as it involves multi-disciplinary team management. Meticulous attention should be paid to cannula position to avoid any accidental disconnection. Electro-encephalography (EEGs) can be done as per clinical need. Cerebral function analyzing monitor (CFAM) is a bedside tool for monitoring global cerebral electrical activity and detection of seizures [9]. Magnetic resonance imaging (MRI) scans are contra-indicated while on ECMO due to metallic and magnetic compatibility issues.
Renal issues
Fluid balance (including cumulative) is very important for effective outcome and the duration of ECMO run. An initial period of capillary leak in systemic inflammatory response conditions is expected. Individuals will need fluid and inotropes during this phase. Once the capillary leak phase is resolving, effective diuresis to get rid of excess fluid is an important step in moving toward recovery of lungs.
Daily management
ECMO specialists are the expert critical care nurses looking after the care of the circuit and the neonate round the clock in shifts. Whenever the nursing shifts are changing and in between, it is customary to focus attention in minute detail to the following aspects:
Inspection of circuit: The ECMO circuit and membrane should be inspected for any clots, air bubbles with attention to propagation of clots if there are any.
Cannula position: Position of the cannula tip is very important to stay as it is and not move. Factors such as movement, proning, and physiotherapy tend to cause migration of the cannula. Proper securement with ties and sutures is mandatory. Any such movement should be brought to the notice of senior staff immediately.
Hemoglobin
At the initiation of ECMO, it is ideal to keep Hb levels between 12 and 14 g/dl. There are some studies suggesting ECMO can be maintained on Hb levels of more than 7 g/dl [10–12]. As discussed with Prof. Robert Bartlett, keeping the Hb high, above 12 g/dl, makes the physiological sense to maintain adequate oxygen carrying capacity.
Platelets
Platelet numbers are expected to go down as a result of circuit/membrane trapping and infection. Heparin-induced thrombocytopenia can be suspected if the clinical condition and investigations denote the possibility. In the absence of active bleeding tendency, a platelet count of above 40,000 can be watched without further transfusions. If there is a need to supplement platelets as per clinical indication, it is justified to give platelets.
Blood gases
In veno-venous ECMO, we monitor patient’s arterial blood gases for oxygenation. In venoarterial ECMO, we monitor mixed venous blood gases. Blood lactate values and serial trends are very useful markers of tissue oxygenation and prognosis on ECMO.
Circulation
Minimal inotropic requirement to promote myocardiac contractility is usually encouraged in VA ECMO. If the ventricles are not contracting, clots tend to develop due to sluggish circulation. Serial echocardiograms are necessary during the ECMO run. The cardiologist should understand ECMO physiology to interpret the views as the pump is actively pumping blood off the right atrium to the membrane oxygenator.
Physiotherapy
It is very important for lung recovery. Mobilization of secretions and keeping the lungs healthy by avoiding stasis are some of the goals. Physiotherapy could be gentle so as not to cause cannula dislodgement or unblocking a healing bronchopleural fistula (for example).
Proning
It helps by recruitment of posterior, dependent parts of the lung. Pressure effects and cannula dislodgement are to be avoided.
Permissive settings
Permissive hypoxia, hypercarbia, and hypotension are relevant in the practice of ECMO, to help with the optimal outcomes.
Weaning
As the lungs and heart function improve, ECMO flows can be brought down serially along with reduction in FiO2. Simultaneous improvement in chest X-ray picture and cardiac function can be seen.
Decannulation
Once the ECMO support is brought down to minimal settings, under aseptic precautions, double lumen veno-venous ECMO can be removed and hemostasis secured. If it is veno-arterial ECMO, surgical help will be needed in removing the arterial cannula and either repairing or ligating the vessel as the suitability of vessel wall at the time of decannulation.
Counseling parents
Parents and family need extensive counseling and lot of support during and after the ECMO run. The anticipated complications and expected outcomes have to be carefully shared with them. ECMO centers do provide written information for parents to understand ECMO.
Second run of ECMO
In rare circumstances, a neonate who got better earlier can deteriorate again needing re-cannulation. In such circumstances, we did ECMO for a second time.
Special circumstances
Surfactant protein B deficiency
It is an autosomal recessive disorder. The incidence is approximately 1 per million live births. It usually presents at full-term gestational age with severe respiratory failure needing ventilation. With administration of surfactant, there will be transient improvement. The degree of respiratory compromise often demands ECMO, presenting as pulmonary hypertension. The ECMO run tends to be long with protracted course. In cases where lung biopsy is done, microscopy demonstrates features suggestive of alveolar type II cell hyperplasia, interstitial thickening and/or fibrosis, and areas of alveolar proteinosis. Electron microscopy demonstrates disorganized or unrecognizable lamellar bodies and an accumulation of abnormal-appearing multivesicular bodies. Genetic test is confirmatory. 121ins2, I73T, and E292V mutations in SFTPB, SFTPC, and ABCA3 respectively provide the initial screens which must be confirmed with direct sequencing if present [13]. There is no treatment apart from lung transplantation.
Alveolar capillary dysplasia (ACD)
It is an interesting condition presenting with neonatal respiratory failure. The neonate manifests with multiple congenital nonlethal anomalies such as micropthalmia or anopththalmos, duodenal atresia, anorectal anomalies, intestinal malrotation and total colonic Hirschsprung’s disease, cardiovascular, urogenital, and musculoskeletal abnormalities. It presents as persistent pulmonary hypertension of the newborn (PPHN) unresponsive to treatment.
The pathognomonic feature of this disorder is alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV; OMIM number 265380) [14]. There are areas of alveoli, not surrounded by capillaries, and areas of crowding of capillary network unassociated with alveoli. It can be diagnosed by genetic confirmation/lung biopsy. Treatment is lung transplantation in centers of expertise. St. Louis Children’s Hospital in St. Louis, MO, USA, has the most active pediatric lung transplant program in the world. According to a 2013 case series publication by St. Louis, “If they survive to bilateral lung transplantation (BLT), patients with Alveolar Capillary Dysplasia/Misalignment of Pulmonary Veins (ACDMPV) can have successful outcomes” and the ACDMPV patients “are alive at last follow-up at 1, 8, 9 and 12 years of age” (as of May 2013) [15]. In “Infants with Atypical Presentations of Alveolar Capillary Dysplasia with Misalignment of the Pulmonary Veins Who Underwent Bilateral Lung Transplantation,” a 2017 update case study found similar results: “The 1- and 5-year survival rates for infants with atypical ACDMPV are similar to infants transplanted for other indications.” [16]
Congenital diaphragmatic hernia (CDH)
Following failure of maximal conventional ventilation, ECMO support is an accepted modality of life support in CDH cases. ECMO facilitates gas exchange, minimizes baro-trauma, and facilitates resolution of pulmonary hypertension and corrective surgery. There are two schools of thought in repair of congenital diaphragmatic hernia: while (1) on ECMO, (2) off ECMO. Surgeons who prefer to do surgery off ECMO will have the advantage of operating off heparin and not having the need to go back on to heparin. The overall survival in children with diaphragmatic hernia depends on the degree of pulmonary hypoplasia. Cannulation in cases of CDH is also one of the difficult tasks due to underdevelopment of blood vessels and distortion of anatomy following herniation of abdominal contents into the chest. Systematic reviews into the benefits of ECMO in CDH concluded that any benefit is unclear. Few randomized control trials exist to demonstrate clear benefit and guide management. However, ECMO may have its uses in those that have reversibility of their respiratory disease [17]. McHoney et al. state that the optimal timing of surgery for patients on ECMO is difficult to establish, but it seems that repair at an early stage (with careful perioperative management) is becoming less of a taboo, and may improve outcome and help with either coming off ECMO or decisions on withdrawal later. Rafat et al. mentioned in their paper [18] that survival might be influenced by the timing of ECMO initiation and the timing of surgical repair. In this regard, a trend toward early initiation of ECMO and early surgery on ECMO exists. Once the thoracic contents are reduced, there will be more space for the native lungs to expand and an opportunity for pulmonary hypertension to get better (to the extent possible). It is essential to plan heparin management meticulously (avoiding bleeding/clotting), provide gentle ventilation of the lungs as they are very likely to be hypoplastic, and be watchful for possible complications.
Neonatal pertussis
When pertussis affects the neonate in the early postnatal period, the outcome may not be very favorable [19]. The poor outcomes are due to lack of passive immunity passed over to the neonate in either unimmunized mothers or due to lack of herd immunity. Severe Bordetella pertussis infection in infants is characterized by severe respiratory failure, pulmonary hypertension, leukocytosis, and death. We reported 60% mortality in this series of cases with neonatal/infantile pertussis. From the ELSO registry database, Domico et al. analyzed the clinical course and demographic details of neonates that went through ECMO following severe pertussis between 2002 and 2015. Of the 200 infants who received extracorporeal membrane oxygenation for pertussis, only 56 survived (28%) [20]. They concluded by mentioning the survival rate for infants with pertussis who received extracorporeal membrane oxygenation support remains poor. Among their observations, younger age, lower PaO2/FiO2 ratio, vasoactive use, pulmonary hypertension, and a rapidly progressive course were associated with increased mortality.
E-CPR
ECMO as extended support of cardiopulmonary resuscitation has been gaining momentum in centers which can afford to provide ECPR. Most of the times, ECPR is advocated in cases of in-house cardiac arrest. If the patient cannot be resuscitated following cardiac arrest with 15 min, he/she will be cannulated onto ECMO. The teams should be prepared to be alerted as soon as the cardiopulmonary resuscitation (CPR) starts. The outcomes in neonatal ECPR are 42% compared with neonatal cardiac ECMO outcomes of 43% as per the published ELSO registry information (Appendix 4 Table 1). Lasa et al. concluded that for children with in-hospital CPR of ≥ 10 min duration, E-CPR was associated with improved survival to hospital discharge and survival with favorable neurological outcome compared with conventional cardiopulmonary resuscitation (C-CPR) [21].
Table 1
| Neonatal cases | Total runs | Survived ECLS | Survived to Discharge or transfer | ||
| Pulmonary | 32,385 | 28,417 | 87% | 23,675 | 73% |
| Cardiac | 8,830 | 6,097 | 69% | 3,818 | 43% |
| E-CPR | 2,035 | 1427 | 70% | 861 | 42% |
| Total Neonatal ECLS cases reported to ELSO Registry 1989-2020 | |||||
Courtesy of Peter Rycus: ELSO (Extracorporeal Life Support Organization, Ann Arbor Michigan) International Summary. Jan 2020
Follow-up after neonatal ECMO
Neonates should be carefully and periodically followed up after ECMO therapy. Critical care tends to be a traumatic period for patients and their families.
The important aspects to plan during the follow-up starts with preparation for discharge. Few units do MRI scan of the brain routinely at the time of discharge.
Neuro-developmental assessment
Post discharge, children should be followed up with assessment of neuro-behavior at clinics along with ultrasound scans and CT/MRI scans as per the need. Neurological problems might include bleeding, ischemia to parts of the brain, and seizure activity, which happens to 8% percent of patients (collectively), as per published ELSO registry data (2015 to 2020 figures). Mild learning difficulties to severe neurological impairment have been noticed. Severe impairment is more likely if a neuroimaging abnormality was already detected at the time of ECMO discharge. These figures do not differ from those babies treated conventionally without ECMO, as shown by the UK trial follow-up study in neonates [22]. Studies indicate that developmental problems which are not apparent at 1 year of age may show by 2 years, and more subtle changes, such as learning difficulties, may not become apparent until school age.
Particular attention should be paid to the reconstruction site of carotid artery (if done) to exclude stenosis or aneurysm formation.
Sensory neural deafness was reported among the ECMO survivors, up to 15% of patients. Formal hearing test should be part of the follow-up.
Respiratory
Higher incidence of respiratory infections was reported in ECMO as well as in non-ECMO patients. Lung function tests can be done as part of the follow-up.
Cardiac
Neonates undergoing cardiac ECMO or those following CDH repair need close follow-up by the cardiac team.
Feeding difficulties
Feeding history and weight gain/loss pattern should be recorded as neonates often have difficulty establishing feeds after ECMO.
Vaccinations
Post ECMO, children should have vaccination as per the usual schedule.
Conclusion
For reversible respiratory conditions, ECMO is a viable option resulting in best outcomes among different age groups. Neonatal cardiac ECMO is very helpful in post-cardiac surgical conditions when the babies cannot come off cardiopulmonary bypass following major complex cardiac surgery or in those neonates needing cardiac support for major inotropic requirement. We showed that the neonates needing minor inotropic requirement (as measured by inotropic score) can be supported by VV ECMO as well. Once the hypoxia gets resolved, the myocardium usually recovers. [23]
Complications on ECMO could be ascribed to mechanical issues or patient related. The ELSO registry data on reported incidence and survival statistics as per various ECMO complications have been described in Appendix 5 Table 2. Meticulous training of the ECMO teams including the critical care nurses who will be caring for the neonates bedside round the clock, will avert many of the complications by prevention, early detection, and resolution.Simulation should be an integral part of any neonatal ECMO program. Simulation helps in improving the promptness of ECMO teams, procedures such as cannulation, decannulation and resolving the emergencies. Quick response and effectiveteamwork are essential for patient care.[24] Further advances with regard to availability of the equipment within the country and improving the affordability of the life-saving technology will help in saving lives in critical circumstances.
Table 2
| Neonatal Respiratory Complications from 2015 to Present | Total number reported | % reported | survived | %survived |
| Mechanical: Oxygenator failure | 157 | 4.1% | 85 | 54% |
| Mechanical: Pump Failure | 29 | 0.8% | 14 | 48% |
| Mechanical: Heat exchanger malfunction | 14 | 0.4% | 5 | 36% |
| Mechanical: Clots: Circuit Component Clots | 1,146 | 30% | 617 | 54% |
| Mechanical: Air in circuit | 126 | 3.3% | 60 | 48% |
| Mechanical: Cannula problems | 495 | 12.9% | 293 | 59% |
| Mechanical: Circuit change | 320 | 8.4% | 145 | 45% |
| Mechanical: Clots and Air Emboli | 6 | 0.2% | 4 | 67% |
| Mechanical: Thrombosis/Clots: circuit component | 112 | 2.9% | 51 | 46% |
| Hemorrhagic: GI hemorrhage | 56 | 1.5% | 18 | 32% |
| Hemorrhagic: Surgical site bleeding | 228 | 6% | 86 | 38% |
| Hemorrhagic: Peripheral cannulation site bleeding | 68 | 1.8% | 46 | 68% |
| Neurologic: Brain death | 7 | 0.2% | 0 0% | |
| Neurologic: Seizures: clinically determined | 105 | 2.7% | 47 | 45% |
| Neurologic: Seizures Confirmed by EEG | 170 | 4.4% | 77 | 45% |
| Neurologic: CNS Infarction (US or CT or MRI) | 119 | 3.1% | 39 | 33% |
| Neurologic: Intraventricular CNS hemorrhage (US or CT or MRI) | 22 | 0.6% | 6 | 27% |
| Neurologic: Intra/extra parenchymal CNS Hemorrhage (US or CT or MRI) | 22 | 0.6% | 8 | 36% |
| Neurologic: CNS diffuse ischemia (CT/MRI) | 6 | 0.2% | 1 | 17% |
| Renal: Creatinine 1.5 - 3.0 | 135 | 3.5% | 48 | 36% |
| Renal: Creatinine > 3.0 | 12 | 0.3% | 2 | 17% |
| Renal: Renal Replacement Therapy Required | 1,070 | 28% | 509 | 48% |
| Cardiovascular: CPR required | 88 | 2.3% | 26 | 30% |
| Cardiovascular: Cardiac arrhythmia | 164 | 4.3% | 79 | 48% |
| Cardiovascular: Tamponade (blood) | 63 | 1.6% | 25 | 40% |
| Cardiovascular: Tamponade (not blood) | 18 | 0.5% | 5 | 28% |
| Pulmonary: Pneumothorax requiring treatment | 166 | 4.3% | 84 | 51% |
| Pulmonary: Pulmonary hemorrhage 170 4.4% 68 40% | 170 | 4.4% | 68 | 40% |
| Infectious: WBC < 1,500 | 25 | 0.7% | 14 | 56% |
| Metabolic: Hyperbilirubinemia | 346 | 9.1% | 163 | 47% |
| Metabolic: Severe hemolysis | 40 | 1% | 16 | 40% |
| Limb: Ischemia | 15 | 0.4% | 2 | 13% |
Courtesy of Peter Rycus: ELSO (Extracorporeal Life Support Organization, Ann Arbor Michigan) International Summary. Jan 2020
Appendix 1
Appendix 2
Appendix 3
Oxygenation index= Mean airway pressure x FiO2/ PaO2 in mm Hg. If the child is on inhaled Nitric Oxide, add another 10 points to the oxygenation index.
Appendix 4
Appendix 5
Complications while on ECMO
Funding
No funding was received.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our paper doesn’t need ethics approval.
Research involving human participants and/or animals
We are not reporting new research involving human participants and/ or animals.
Informed consent
Obtained.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ELSO Registry data. International summary https://www.elso.org/Registry/Statistics/InternationalSummary.aspx
- 2.Pol-Fachin L, Franco Becker C, Almeida Guimaraes J, Verli H. Effects of glycosylation on heparin binding and antithrombin activation by heparin. Proteins. 2011;79:2735–2745. doi: 10.1002/prot.23102. [DOI] [PubMed] [Google Scholar]
- 3.Ignjatovic V, Lai C, Summerhayes R, et al. Age-related differences in plasma proteins: how plasma proteins change from neonates to adults. PLoS One. 2011;6:e17213. [DOI] [PMC free article] [PubMed]
- 4.Odegard KC, Zurakowski D, DiNardo JA, Castro RA, McGowan FX, Jr, Neufeld EJ, Laussen PC. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage I through Fontan completion. J Thorac Cardiovasc Surg. 2009;137:934–941. doi: 10.1016/j.jtcvs.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Kaltman JR, Andropoulos DB, Checchia PA, Gaynor JW, Hoffman TM, Laussen PC, Ohye RG, Pearson GD, Pigula F, Tweddell J, Wernovsky G, del Nido P. Report of the Pediatric Heart Network and National Heart, Lung, and Blood Institute Working Group on the Perioperative Management of Congenital Heart Disease and for the Perioperative Working Group. Circulation. 2010;121:2766–2772. doi: 10.1161/CIRCULATIONAHA.109.913129. [DOI] [PubMed] [Google Scholar]
- 6.Smedley L. Antithrombin III in pediatric ECMO: our perfect teenage heart .sites. utexas.edu › _FinalHandout_12.7.17-without-pic.pdf
- 7.Vadim Kostousov, 2019. Activated clotting time (online) E medicine. medscape.com. Available at: < https://emedicine.medscape.com/article/2084818-overview#a4
- 8.Van Ommen CH, Neunert CE, Chitlur MB. Neonatal ECMO. Front Med (Lausanne) 2018;5:289. doi: 10.3389/fmed.2018.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorusso R , Taccone FS, Belliato M, et al. Brain monitoring in adult and pediatric ECMO patients: the importance of early and late assessments. Minerva Anestesiol. 2017;83:1061–1074. [DOI] [PubMed]
- 10.Kim HS, Park S. Blood transfusion strategies in patients undergoing extracorporeal membrane oxygenation; Korean J Crit Care Med. 2017;32:22–8. [DOI] [PMC free article] [PubMed]
- 11.ELSO anticoagulation guideline. Patient care practice guidelines 2014. [cited 2016 06/29/2016] http://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf
- 12.Muszynski J, Reeder R, Hall M, et al. RBC Transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med. 2018; 46:e552-e559. [DOI] [PMC free article] [PubMed]
- 13.Nogee L.M. (2010) Surfactant deficiency disorders: SP-B and ABCA3. In: McCormack F., Panos R., Trapnell B. (eds) Molecular basis of pulmonary disease. Respiratory Medicine. Humana Press. 10.1007/978-1-59745-384-4_11
- 14.Miranda J, Rocha G, Soares H, Vilan A, Brandão O, Guimarães H. Alveolar capillary dysplasia with Misalignment of pulmonary veins (ACD/MPV): A case series. Case Rep Crit Care. 2013;2013:327250. [DOI] [PMC free article] [PubMed]
- 15.Pediatric lung transplantation: 10 years of experience: Camargo,P., Pato,E,Campos,S., Afonso, J et al. Clinics (2014),69(Suppl 1):51–54 10.6061/clinics/2014(Sup01)10 [DOI] [PMC free article] [PubMed]
- 16.Towe CT, White FV, Grady RM, Sweet SC, Eghtesady P, Wegner DJ, Sen P, Szafranski P, Stankiewicz P, Hamvas A, Cole FS, Wambach JA (2017): J Pediatr. 2017 Nov 30. 10.1016/j.jpeds.2017.10.026. [DOI] [PMC free article] [PubMed]
- 17.McHoney M, Hammond P. Role of ECMO in congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2018;103:F178–F181. [DOI] [PubMed]
- 18.Rafat N, Schaible T. Extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Front Pediatr. 2019;7:336. doi: 10.3389/fped.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pooboni S, Roberts N, Westrope C, et al. Extracorporeal life support in pertussis: Pediatr Pulmonol. 2003;36:310–5. [DOI] [PubMed]
- 20.Domico M, Ridout D, MacLaren G, et al. Extracorporeal membrane oxygenation for pertussis: predictors of outcome including pulmonary hypertension and leukodepletion. Pediatr Crit Care Med. 2018;19:254–261. [DOI] [PubMed]
- 21.Lasa JJ, Rogers RS, Localio R, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge: a report from the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation. 2016;133:165–76. doi: 10.1161/CIRCULATIONAHA.115.016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally H, Bennett CC, Elbourne D, Field DJ. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics. 2006;117:e845–54. [DOI] [PubMed]
- 23.Roberts N, Westrope C, Pooboni SK, et al. Venovenous extracorporeal membrane oxygenation for respiratory failure in inotrope dependent neonates. ASAIO J. 2003;49:568–571. [DOI] [PubMed]
- 24.ELSO guidelines for neonatal respiratory failure https://www.elso.org/Portals/0/ELSOGuidelinesNeonatalRespiratoryFailurev1_4.pdf [DOI] [PubMed]