Abstract
Background:
Comparisons of patient-reported outcomes (PROs) based on surgical approach for total hip arthroplasty (THA) in the United States are limited to series from single surgeons or institutions. Using prospective data from a large, multicenter study, we compare preoperative to postoperative changes in PROs between posterior, transgluteal, and anterior surgical approaches to THA.
Methods:
Patient-reported function, global health, and pain were systematically collected preoperatively and at 1, 3, and 6 months postoperatively from patients undergoing primary THA at 26 sites participating in the Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement (ClinicalTrials.gov: NCT02810704). Outcomes consisted of the brief Hip disability and Osteoarthritis Outcome Score, the Patient-Reported Outcomes Measurement Information System Physical Health score, and the Numeric Pain Rating Scale. Operative approaches were grouped by surgical plane relative to the abductor musculature as being either anterior, transgluteal, or posterior.
Results:
Between 12/12/2016 and 08/31/2019, outcomes from 3018 eligible participants were examined. At 1 month, the transgluteal cohort had a 2.2-point lower improvement in Hip disability and Osteoarthritis Outcomes Score (95% confidence interval, 0.40–4.06; P = .017) and a 1.3-point lower improvement in Patient-Reported Outcomes Measurement Information System Physical Health score (95% confidence interval, 0.48–2.04; P = .002) compared to posterior approaches. There was no significant difference in improvement between anterior and posterior approaches. At 3 and 6 months, no clinically significant differences in PRO improvement were observed between groups.
Conclusion:
PROs 6 months following THA dramatically improved regardless of the plane of surgical approach, suggesting that choice of surgical approach can be left to the discretion of surgeons and patients without fear of differential early outcomes.
Keywords: hip arthroplasty, patient-reported outcomes, transgluteal, posterior, anterior, HOOS
Total hip arthroplasty (THA) offers substantial pain relief and improved quality of life for patients with hip osteoarthritis [1]. Innovations in implant technology, perioperative management, and operative technique have reduced complications, shortened length of stay, decreased costs, and improved patient outcomes. None-theless, the aging US population is expected to drive a 174% increase in procedure volume between 2005 and 2030 [2], indicating the need to innovate toward increased efficiency and improved outcomes.
Surgical approach toward hip arthroplasty may be an important factor in determining clinical outcomes and efficiency. Surgical approach is conventionally classified by the location of the plane of dissection relative to the abductor musculature, an essential muscle to preserve for optimal functional recovery. In the United States, the most common techniques are posterior approaches, variations of which are known as the Southern, Moore, or posterolateral approaches [3,4]. Transgluteal approaches, also known as the direct lateral or Hardinge approaches, are alternatives that provide improved joint access at the expense of violating the abductor musculature [5,6]. More recently, increased interest in anterior approaches, including the Smith-Peterson, Hueter, or Watson-Jones approaches, has been driven by the belief that they may reduce soft tissue trauma and provide more reliable component positioning [7]. These approaches follow internervous and/or intranervous planes with the patient in a supine position with the goal of improved early functional recovery and reduced pain [8].
Previous studies have attempted to determine the superiority of a single surgical approach, but no consensus has emerged [7]. One framework for comparison relies on patient-reported outcomes (PROs), a valuable tool for assessing patient recovery and function after orthopedic procedures [9]. While registries in Norway [10] and the Netherlands [11] have compared nationwide PROs based on surgical approach, similar studies within the United States have been limited to single surgeons or institutions. To improve the understanding of patient recovery after THA based on surgical approach in the United States, we assess longitudinally collected PROs from a multi-institutional consortium.
Methods
Study Design
As part of the Comparative Effectiveness of Pulmonary Embolism Prevention after Hip and Knee Replacement (PEPPER) trial [12], longitudinally collected PROs, baseline demographics, and surgical approach were collected from patients undergoing THA at 27 North American hospitals. All approaches were categorized by the operating surgeon as traversing anterior, through, or posterior to the gluteal abductor musculature, and the approaches were grouped and classified as “anterior,” “transgluteal,” and “posterior,” respectively. We compared preoperative to postoperative changes in PROs among patients undergoing primary or revision THA based on this categorization of surgical approach.
Settings
PROs were collected through the PEPPER trial, a large, pragmatic, multicenter, randomized, clinical trial evaluating safety and effectiveness of prophylactic antithrombotic medication after total hip and knee arthroplasty [12]. While randomization and safety outcomes with respect to antithrombotic regimen were unrelated to our analysis, PEPPER’s longitudinal collection of PROs from study participants enrolled through multiple institutions provided an opportunity to evaluate the influence of surgical approach on PROs. The PEPPER trial is an ongoing study registered with ClinicalTrials.gov (NCT02810704), enrolling subjects from 26 hospitals in North America.
Participants
We included study participants enrolled in PEPPER between 12/12/2016 and 08/31/2019. Appendix A outlines the full inclusion and exclusion criteria for study participants. Briefly, adults undergoing elective primary THA who were able to provide informed consent were included. Patients with comorbidities that confounded the assessment of antithrombotic medication were excluded. All included participants in the study cohort underwent THA using a surgical approach categorized as anterior, transgluteal, or posterior to the abductor musculature. Although PEPPER enrollment included subjects with hip revision or resurfacing procedures, these subjects were excluded from our analysis.
Outcomes
Outcomes included validated measures of hip function, general health, and pain. Function was assessed using the Hip disability and Osteoarthritis Outcome Score (HOOS Jr), which asks 6 questions prompting assessment of pain, function, and quality of daily living within the past week [13]. The HOOS Jr is scored on a 0–100 scale, with a higher score indicating a higher level of function, and a 7-point difference is smallest reported estimate of a minimum clinically important difference (MCID) using an anchor-based approach [14,15]. The Patient-Reported Outcomes Measurement Information System Physical Health Summary (PROMIS-PH) was used to assess global pain and general health. The PROMIS-PH measure is evaluated using a T-score set at a mean of 50, with greater scores representing improved health [16]; the MCID for PROMIS-PH scores is estimated to be 7.9 points [14]. The Numeric Pain Rating Scale (NPRS) was scored on a scale of 0–10 with lower values indicating less pain; a difference of 2 points is considered clinically meaningful [17]. The HOOS Jr, PROMIS-PH, and NPRS outcomes were collected due to their common use in joint arthroplasty studies and registries. Outcome surveys with individual items that were missing were not scored. Given our large sample and difference between surgical approaches, we had greater than 80% power to detect a MCID for each outcome. In addition to PROs and baseline characteristics, participant discharge disposition was collected and categorized as either routine (“home or home health agency”) or transfer (“skilled nursing facility, inpatient rehab, or other facility”).
A web-enabled database maintained by an independent contractor housed eligibility and screening detail, baseline information, and operative data [18]. Postoperative outcomes were centrally collected using telephone interviews, web-based surveys, and postage-paid reply mail surveys. PROs were collected over a 1-month (37 days postoperative −7/+10 days), a 3-month (90 days postoperative −10/+14 days), and a 6-month window (180 days postoperative −28/+28 days). Patients were contacted for follow-up irrespective of complications. Data systems, procedures, and policies were compliant with the Health Insurance Portability and Accountability Act, the Code of Federal Regulations Title 21 Part 11, the Federal Information Security Modernization Act, and computing principles of minimum necessity, separation of duties, and least privilege.
Covariates
Patient demographics and comorbidities were collected at baseline (preoperatively) to adjust for varying patient risk associated with adverse outcomes and poor initial functional status [19–27]. Patient characteristics included age (continuous), sex (female, male), race (white, black, other/multiple), ethnicity (Hispanic, not Hispanic), smoking (never, current, former), alcohol use (never, monthly or less, 2–4 times a month, 2–3 times a week, 4 or more times a week), work status (working, unemployed looking, sick on leave, disabled due to hip or knee, disabled for other reasons, student/homemaker/retired), and the Charlson Comorbidity Index [28]. Participant height and weight were collected to calculate body mass index (BMI) [29].
Statistical Analysis Methods
Differences in patient demographics and comorbidity were summarized based on surgical approach with differences tested using χ-square statistics for categorical data and analysis of variance for continuous variables. A linear mixed-effects regression was used to compare preoperative to postoperative changes in PROs based on surgical approach using the posterior group as the reference and adjusting for baseline measures of participant health, age, sex, race, ethnicity, work status, alcohol use, smoking status, and comorbidity. The standard errors of model coefficients were adjusted for correlation of serially repeated outcomes collected over time within patients, and for patients nested within hospitals. Age, BMI, and baseline health were included as continuous variables; all other covariates, including surgical approach and time (month), were included as categorical variables. Adjusted outcomes were estimated by setting covariates to their mean distributions, and inferences about the effect of surgical approach were based on evaluating the statistical significance of the coefficient of the interaction term between surgical approach and time (month).
Associations between predictor variables and outcomes were examined for influential data points with high leverage and nonlinearity. No other polynomial, transformation, or interaction terms were required. Participants with missing baseline data were dropped from the regression models. Mixed effects regression models implicitly impute outcomes for participants when missing for a given time point, due to survey nonresponse, due to individual item missingness that prevented scoring, or because they had not passed through the survey window at the time of analysis (eg, had not yet reached the 6-month follow-up window). All analyses were performed using STATA-MP-15 software (College Station, TX) with hypothesis testing based on an alpha level of 0.05. Ethical approval was obtained from the Medical University of South Carolina, which served as a central institutional review board for all but 3 participant sites.
Results
Participants
Of the 3018 participants enrolled in PEPPER who met criteria for this analysis (Fig. 1), 1452 participants (48.1%) underwent THA via posterior approaches, 633 participants (21.0%) via transgluteal approaches, and 933 (30.9%) via anterior approaches. Participants who withdrew at any time during the study were excluded. Those who withdrew were similar in all characteristics and comorbidity except for work status; withdrawn patients were less likely to be working (41% working compared to 44% working in study cohort, P = .004). Overall, 2640 of 2847 participants (92.7%) who passed through the 6-month follow-up window at the time of the analysis completed preoperative PRO collection and at least 1 postoperative PRO collection survey (Fig. 1). The median number of days between baseline survey completion and THA was 8 days. Missing responses in baseline characteristics did not exceed 3% for any single survey item. Within the 27 PEPPER participant sites, 26 contributed participants to our study cohort with a mean enrollment of 116 participants per site, ranging from 6 to 280.
Fig. 1.
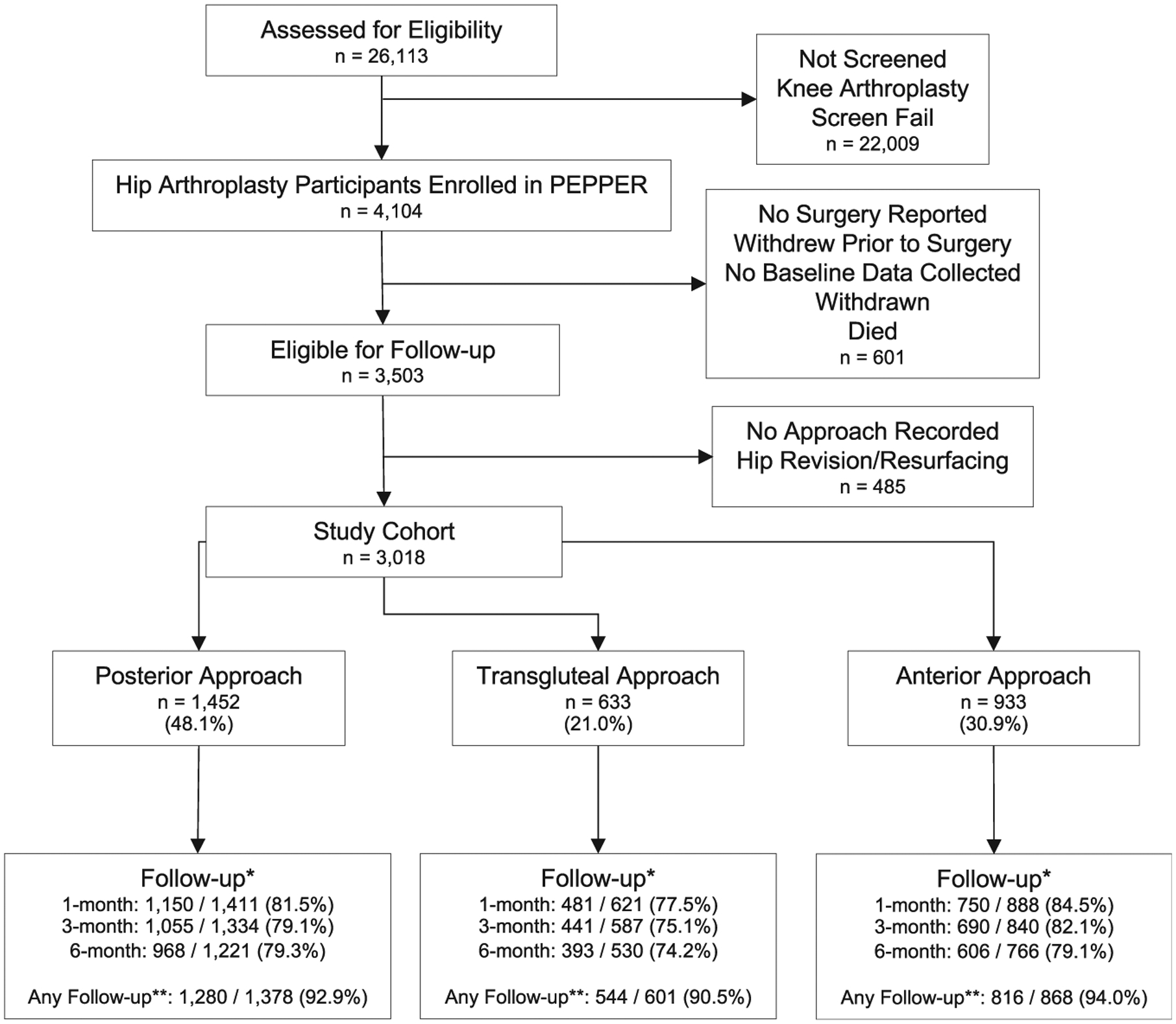
Study cohort selection. * Specific time point follow-up rates were calculated using the following formula:
**Any follow-up was calculated using the following formula:
PEPPER, Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement.
Descriptive Data
Table 1 provides baseline demographic information based on surgical approach. Participants undergoing an anterior approach generally had a lower BMI, were more likely to be white and non-Hispanic, and were less likely to drink alcohol than participants undergoing the transgluteal or posterior approaches. Participants undergoing a transgluteal approach were less likely to be a college graduate, currently working, and more likely to have one or more comorbidities. Participants undergoing a posterior THA were generally younger than participants undergoing the other approaches.
Table 1.
Participant Demographics.
| Baseline Characteristic | Posterior | Transgluteal | Anterior | All | P Valuea |
|---|---|---|---|---|---|
| N (% of total) | 1452 (48.1) | 633 (20.1) | 933 (30.9) | 3017 | |
| Age | |||||
| Age (mean) | 60.7 | 62.1 | 62.1 | 61.4 | .004 |
| BMI | |||||
| BMI (mean) | 30.9 | 31.3 | 29.8 | 30.6 | <.001 |
| Sex | |||||
| Male (%) | 48.1 | 35.5 | 48.1 | 45.5 | <.001 |
| Female (%) | 51.9 | 64.5 | 51.9 | 54.5 | |
| Race | |||||
| White (%) | 82.5 | 76.2 | 86.9 | 82.6 | <.001 |
| Black (%) | 12.9 | 22.2 | 10.1 | 14.0 | |
| Other/multiple (%) | 4.6 | 1.6 | 3.0 | 3.5 | |
| Ethnicity | |||||
| Not Hispanic (%) | 97.6 | 97.4 | 99.0 | 98.0 | <.025 |
| Hispanic or Latino (%) | 2.4 | 2.6 | 1.0 | 2.0 | |
| Education | |||||
| Less than college (%) | 54.5 | 62.1 | 52.4 | 55.5 | <.001 |
| College graduate (%) | 45.5 | 37.9 | 47.6 | 44.5 | |
| Work | |||||
| Working (%) | 43.7 | 38.2 | 44.6 | 42.8 | .003 |
| Unemployed looking (%) | 2.4 | 0.8 | 1.3 | 1.7 | |
| Sick of leave or disability (%) | 19.4 | 18.8 | 16.9 | 18.5 | |
| Student, homemaker, retired (%) | 34.4 | 41.4 | 37.1 | 36.7 | |
| Alcohol | |||||
| Never (%) | 31.0 | 30.5 | 25.8 | 29.3 | .007 |
| Monthly or less (%) | 24.0 | 25.9 | 21.9 | 23.8 | |
| 2–4 Times a mo (%) | 15.2 | 16.4 | 16.1 | 15.7 | |
| 2–3 Times a wk (%) | 15.4 | 16.1 | 19.5 | 16.8 | |
| ≥4 Times a wk (%) | 15.3 | 11.7 | 17.1 | 15.1 | |
| Smoke | |||||
| Never (%) | 53.4 | 52.8 | 47.5 | 51.5 | .025 |
| Current (%) | 6.7 | 8.7 | 8.6 | 7.7 | |
| Former (%) | 39.8 | 38.4 | 43.9 | 40.8 | |
| Comorbidity count | .009 | ||||
| 0 (%) | 66.1 | 59.5 | 65.3 | 64.5 | |
| 1 (%) | 25.1 | 37.5 | 23.7 | 25.2 | |
| 2+ (%) | 8.8 | 13.0 | 11.0 | 10.3 |
BMI, body mass index.
Significance testing based on χ-square comparisons for categorical variables and analysis of variance for continuous variables.
Patient-Reported Outcomes
Compared to baseline scores, we observed statistically significant and clinically important improvements in mean patient-reported function, physical health, and pain at 6 months within each approach cohort (Fig. 2). For example, by 6 months, the mean adjusted HOOS Jr improved by 37.8 (95% confidence interval [CI], 36.8–38.8) in the posterior cohort, 35.9 (95% CI, 34.3–37.6) in the transgluteal cohort, and 37.2 (95% CI, 35.9–38.5) in the anterior cohort (Table 2).
Fig. 2.
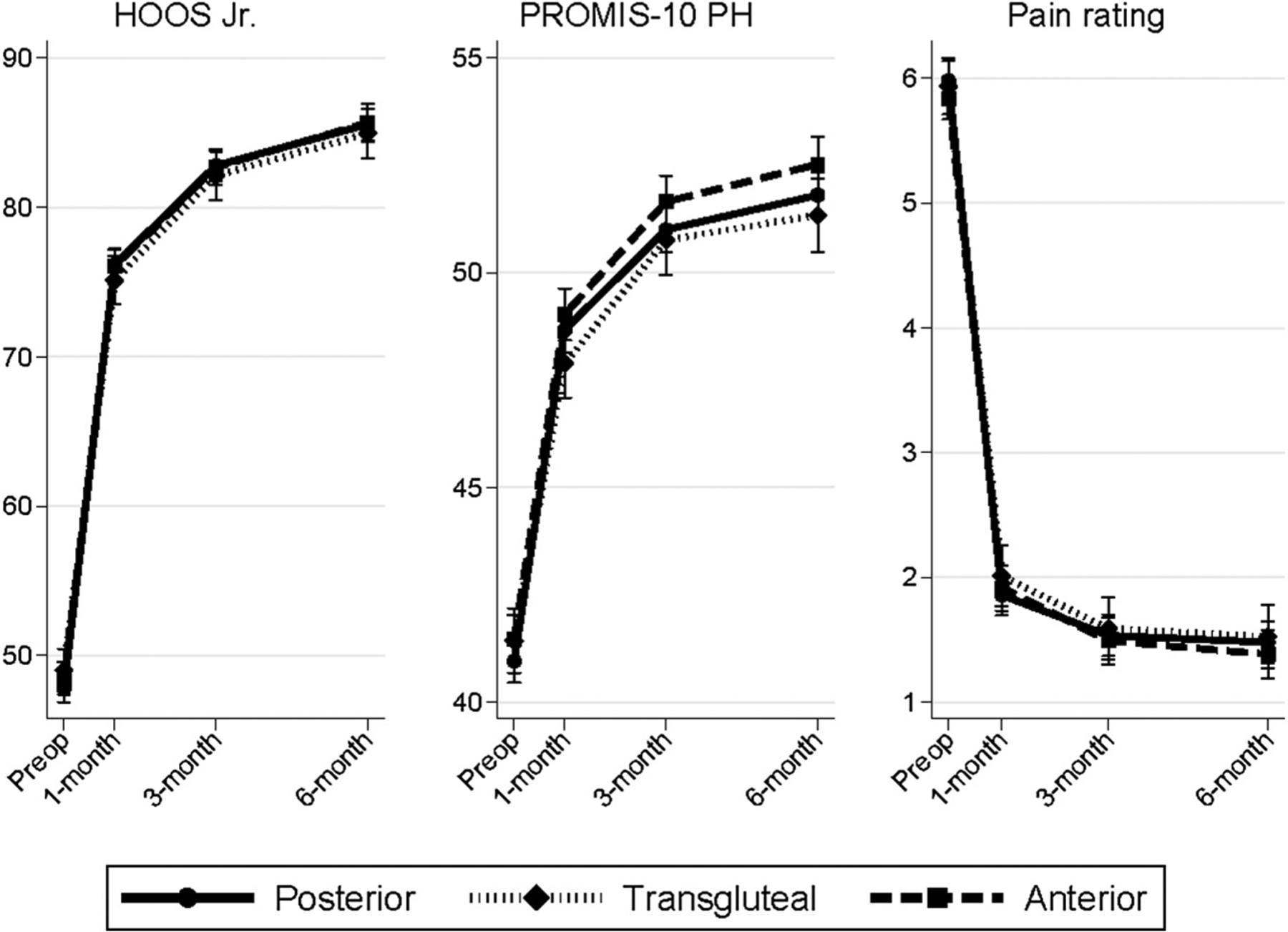
Patient-reported outcomes following total hip arthroplasty. Estimates are adjusted for age, sex, race, ethnicity, education, work status, alcohol use, smoking, and comorbidity. Preop, preoperative; HOOS Jr, the brief Hip disability and Osteoarthritis Outcome Score; PROMIS-PH, Patient-Reported Outcomes Measurement Information System Physical Health Summary; NPRS, Numeric Pain Rating Scale.
Table 2.
Unadjusted and Adjusted Mean Patient-Reported Outcomes.
| Total Hip Arthroplasty | Posterior | Transgluteal | Anterior | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | 95% CI | N | Mean | 95% CI | N | Mean | 95% CI | |||
| HOOS Jr | Unadjusted | Baseline | 1435 | 47.4 | 15.6–73.5 | 609 | 48.3 | 20.8–73.5 | 911 | 48 | 20.8–70.4 |
| 1 mo | 1044 | 76.7 | 53.0–100 | 420 | 75.7 | 53.0–100 | 695 | 76.5 | 53.0–100 | ||
| 3 mo | 1000 | 83.3 | 58.9–100 | 418 | 82.6 | 58.9–100 | 659 | 83.2 | 56.0–100 | ||
| 6 mo | 930 | 86.3 | 58.9–100 | 371 | 84.9 | 58.9–100 | 582 | 86.3 | 58.9–100 | ||
| Adjusted | Baseline | 1404 | 47.8 | 46.9–48.7 | 562 | 49.0 | 47.6–50.4 | 886 | 48.5 | 47.4–49.5 | |
| 1 mo | 1404 | 76.2 | 75.1–77.2 | 562 | 75.1 | 73.5–76.7 | 886 | 76.0 | 74.9–77.2 | ||
| 3 mo | 1404 | 82.8 | 81.8–83.8 | 562 | 82.1 | 80.5–83.8 | 886 | 82.7 | 81.5–83.9 | ||
| 6 mo | 1404 | 85.6 | 84.5–86.6 | 562 | 85.0 | 83.3–86.6 | 886 | 85.7 | 84.4–86.9 | ||
| PROMIS-PH | Unadjusted | Baseline | 1446 | 40.8 | 29.6–54.1 | 627 | 40.8 | 29.6–54.1 | 929 | 41.2 | 29.6–54.1 |
| 1 mo | 1128 | 49.0 | 34.9–61.9 | 473 | 47.6 | 34.9–61.9 | 735 | 49.4 | 37.4–61.9 | ||
| 3 mo | 1029 | 51.4 | 37.4–67.7 | 432 | 50.8 | 37.4–61.9 | 682 | 52.0 | 37.4–67.7 | ||
| 6 mo | 951 | 52.0 | 37.4–67.7 | 384 | 51.1 | 34.9–67.7 | 593 | 52.9 | 37.4–67.7 | ||
| Adjusted | Baseline | 1414 | 41.0 | 40.5–41.4 | 581 | 41.4 | 40.7–42.2 | 901 | 41.5 | 40.9–42.0 | |
| 1 mo | 1414 | 48.7 | 48.1–49.2 | 581 | 47.9 | 47.1–48.7 | 901 | 49.0 | 48.4–49.6 | ||
| 3 mo | 1414 | 51 | 50.4–51.5 | 581 | 50.8 | 49.9–51.6 | 901 | 51.7 | 51.0–52.3 | ||
| 6 mo | 1414 | 51.8 | 51.2–52.4 | 581 | 51.3 | 50.5–52.2 | 901 | 52.5 | 51.9–53.2 | ||
| NPRS | Unadjusted | Baseline | 1452 | 6.0 | 1.0–10.0 | 633 | 6.1 | 1.0–10.0 | 933 | 5.9 | 2.0–10.0 |
| 1 mo | 1142 | 1.9 | 0.0–6.0 | 480 | 1.9 | 0.0–6.5 | 743 | 1.9 | 0.0–6.0 | ||
| 3 mo | 1048 | 1.4 | 0.0–6.0 | 440 | 1.5 | 0.0–6.0 | 686 | 1.4 | 0.0–5.0 | ||
| 6 mo | 961 | 1.5 | 0.0–6.0 | 389 | 1.4 | 0.0–6.0 | 603 | 1.3 | 0.0–5.0 | ||
| Adjusted | Baseline | 1419 | 6.0 | 5.8–6.1 | 585 | 5.9 | 5.7–6.2 | 905 | 5.8 | 5.7–6.0 | |
| 1 mo | 1419 | 1.9 | 1.7–2.0 | 585 | 2.0 | 1.8–2.3 | 905 | 2.0 | 1.7–2.1 | ||
| 3 mo | 1419 | 1.5 | 1.4–1.7 | 585 | 1.6 | 1.3–1.8 | 905 | 1.5 | 1.3–1.7 | ||
| 6 mo | 1419 | 1.5 | 1.3–1.6 | 585 | 1.5 | 1.3–1.8 | 905 | 1.4 | 1.2–1.6 | ||
Missing surveys or surveys with missing responses were dropped from the unadjusted data. Adjusted scores include imputed values to account for survey and individual survey item missingness.
CI, confidence interval; HOOS Jr, the brief Hip disability and Osteoarthritis Outcome Score; PROMIS-PH, Patient-Reported Outcomes Measurement Information System Physical Health Summary; NPRS; Numeric Pain Rating Scale.
At 1 month, mean adjusted HOOS Jr score improved by 28.3 points (95% CI, 27.4–29.3) in the posterior cohort and by 26.1 points (95% CI, 24.6–27.7) in the transgluteal cohort, a 2.2-point smaller improvement for the transgluteal cohort (95% CI, 0.40–4.06; P = .017). These slight differences persisted at 3 and 6 months, although the observed differences at 6 months were not statistically significant. Similarly, PROMIS-PH score improvement at 1 month was 1.3 points less (95% CI, 0.48–2.04; P = .002) in the transgluteal cohort compared to the posterior cohort. No significant between-group differences were observed in NPRS improvement. No significant differences were observed in the anterior cohort compared to the posterior cohort in any adjusted PRO measure at any time point (Appendix B).
At 6 months, 95.9% of our study cohort achieved a MCID in HOOS Jr, 78.7% achieved MCID in PROMIS-PH scores, and 80.1% achieved MCID in NPRS scores after THA compared to their preoperative values, irrespective of surgical approach. There were no significant differences in the proportion of participants who achieved MCID in each measure based on surgical approach (Fig. 3).
Fig. 3.
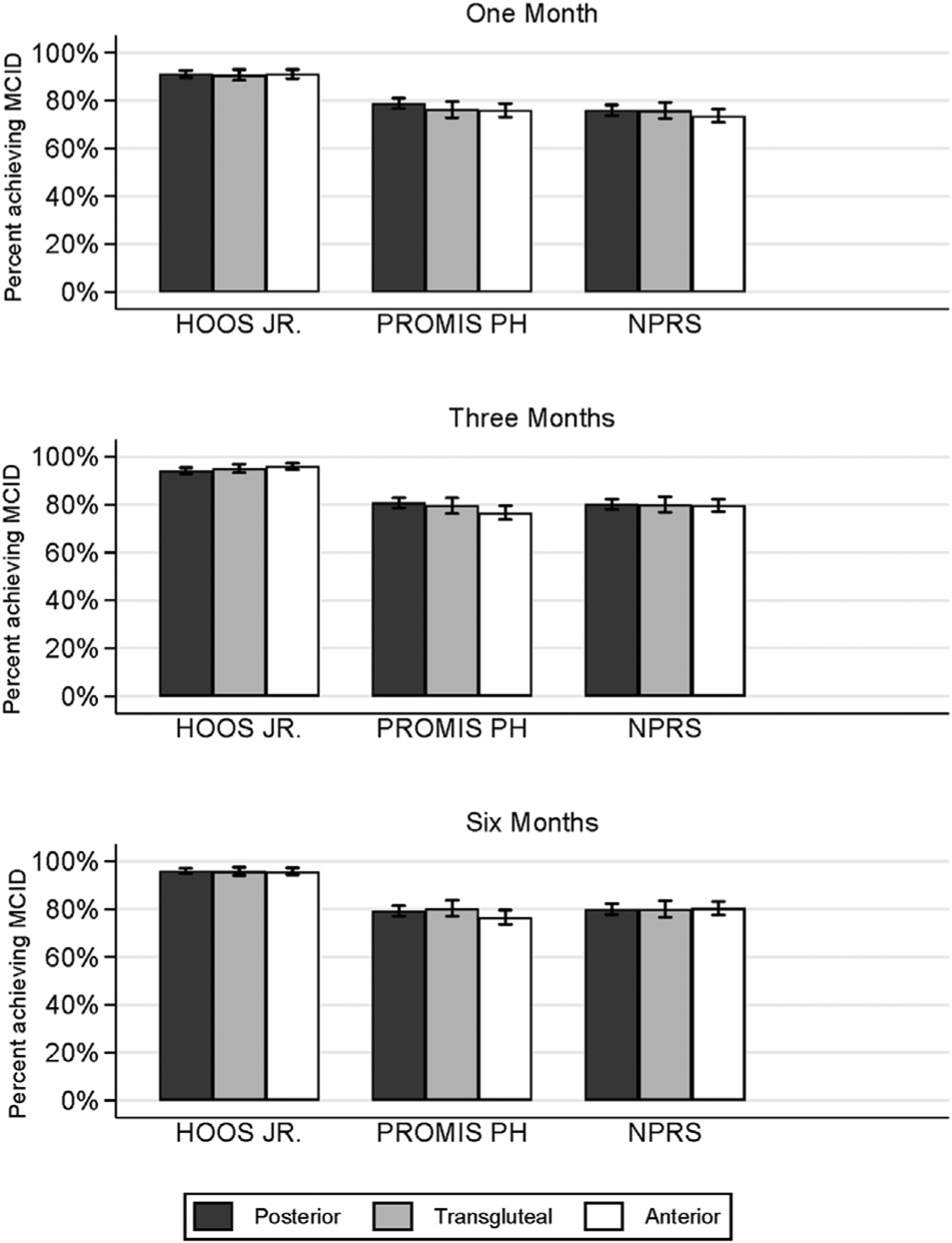
Proportion of participants achieving MCID at 1, 3, and 6 months. Calculated using unadjusted differences in each PRO between baseline and given month. MCID, minimum clinically important difference; PRO, patient-reported outcome.
To ensure that nonresponse had no effect on our results, we performed additional “sensitivity” analyses by limiting our analysis to the subset of participants who had passed through the 6-month follow-up window with or without completing the 6-month follow-up survey and separately for those who had completed the 6-month follow-up survey. These subanalyses (not shown) did not differ from the primary analysis.
Discharge Disposition
Following surgery, routine discharge to home or home health agency was greater in the posterior (93.0%) and anterior groups (94.1%) compared to the transgluteal group (86.6%; χ-squared comparison: P ≤ .001; Appendix C).
Discussion
Our analysis of prospectively collected outcomes from 3018 patients who underwent THA enrolled at 26 medical centers demonstrated substantial but similar improvements in patient-reported function, global health, and pain in each of the 3 main categories of surgical approach. Collecting follow-up data at 1 month allowed for assessment of early recovery, and patients undergoing transgluteal approaches reported a decreased rate of home discharge and experienced lesser improvements in early PROs.
Proponents of anterior approaches point to evidence of better implant positioning [30,31], fewer postoperative dislocations [32,33], increased frequency of home discharge [34], less narcotic use [31,34,35], and reduced length of stay [31,34,36,37], when compared to other approaches. However, others have found higher complications or blood loss when using anterior approaches [36,38,39]. Thus, there is no clear consensus in the literature concerning the ideal surgical approach to THA. While previous comparisons between posterior, transgluteal, and anterior approaches have produced varied results, many are consistent with our findings of no significant differences in patient-reported function and pain [7,36,40–48]. Rosenlund et al [49] found no difference in improvement in HOOS at 3-, 6-, and 12-month follow-up after randomization to transgluteal or posterior approaches. Similarly, Zomar et al [50] found no difference in patient-reported pain or function at 2, 6, and 12weeks after surgery in a randomized trial comparing anterior and transgluteal approaches. Restrepo et al [45] reported that anterior approaches were associated with improved Western Ontario and McMaster Universities Osteoarthritis Index scores 1 year postoperatively compared to transgluteal approaches in a randomized trial, but there were no differences at 2 years. Other studies suggest that anterior approaches result in better immediate functional and pain recovery in comparison to the transgluteal and posterior approaches [7,11,37,41,44–46,51–54], which we may not have captured given that our first follow-up window fell at 1 month postoperatively.
This study has several limitations. First, although our data are prospectively collected and statistically controlled, our study is nonrandomized with respect to surgical approaches. The choice of surgical approach was left to the discretion of the surgeon which may introduce confounding. We cannot account for surgeon experience, preferences, or training. Given the individual variation in more specific approach by surgeon, the classification into 3 broad categories might not fully capture the detail in technique used for each case, especially given the pragmatic nature of the PEPPER trial, where perioperative protocols are not specified. Follow-up rates for PRO collection were high; nevertheless, the impact of those lost to follow-up is not fully known. We did not assess outcomes in the immediate postoperative period (before 30 days) or longer term (beyond 6 months). This study includes a broad patient population, and further studies are necessary to understand more subtle differences between outcomes that may exist in the case of particular diagnoses or patient characteristics. Due to the ongoing nature of the PEPPER study, primary safety outcomes like readmission or complications are suppressed until the end of the study and could not be included in this analysis. The comparisons of discharge disposition are potentially biased due to possible concordance of surgical techniques and established discharge pathways at specific hospitals. Finally, data from this study were collected at medical centers with well-developed research infrastructures, which may limit the generalizability of the findings to various practices in the broader orthopedic community.
Conclusions
This study reports similar improvement in PROs at 1, 3, and 6 months after THA using a posterior, transgluteal, or anterior surgical approaches. Likewise, a similar proportion of patients achieved a MCID in all outcomes measured across the approach cohorts. Further studies are necessary to assess longer-term outcomes. Nevertheless, compared to posterior and anterior approaches, transgluteal approaches had a statistically smaller early improvement in function and physical health, no difference in the proportion of patients achieving MCID, and a lower rate of home discharge. Based on our reporting of PROs, the choice of surgical approach for THA remains appropriately determined through patient-provider discussion of patient preferences and the technical expertise of the surgeon.
Acknowledgment
Collaborators:
The PEPPER Trial Investigators.
Funding/Support:
The research reported in this article was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (PCS-1402-09328) for the PEPPER trial, and through an Agency for Healthcare Research and Quality Award (R01HS024714) for the analysis. The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of PCORI, AHRQ, or their Board of Governors or Methodology Committees.
Appendix. Members of the PEPPER Investigators
James A. Browne, MD; Charles M. Davis III, MD, PhD; Navin D. Fernando, MD; Kevin B. Fricka, MD; Richard J. Friedman, MD; Kevin L. Garvin, MD; Richard Iorio, MD; Michael S. Kain, MD; Stephen L. Kates, MD; Carol A. Lambourne, PhD; Brent A. Lanting, MD, MSc; Carlos J. Lavernia, MD; Brock A. Lindsey, MD; William J. Maloney, MD; Robert M. Molloy, MD; Michael A. Mont, MD; Wayne E. Moschetti, MD, MS; James Nace, DO, MPT; Charles L. Nelson, MD; Kevin I. Perry, MD; James D. Slover, MD; Mark J. Spangehl, MD; Lawrence M. Specht, MD; Scott M. Sporer, MD; Robert S. Sterling, MD; Lucian C. Warth, MD
Appendix A.
PEPPER and Approach Cohort Enrollment Criteria.
| Inclusion Criteria |
|
| Exclusion Criteria |
|
PEPPER, Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement.
Appendix B.
Regression Models for Total Hip Arthroplasty.
| Covariates | HOOS | PROMIS-PH | NPRS | |||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Surgical approach (ref = posterior) | 0 | 0 | 0 | |||
| Transgluteal | 1.7 | .040a | 0.9 | .051 | −0.1 | .316 |
| Anterior | 0.3 | .661 | 0.2 | .636 | −0.1 | .405 |
| Follow-up (ref = baseline) | 0 | 0 | 0 | |||
| 1 mo | 28.3 | .000c | 7.7 | .000c | −4.1 | .000c |
| 3 mo | 35 | .000c | 10.1 | .000c | −4.4 | .000c |
| 6 mo | 37.8 | .000c | 10.9 | .000c | −4.5 | .000c |
| Approach interaction (ref = posterior) | 0 | 0 | 0 | |||
| Transgluteal, 1 mo | −2.2 | .01a | −1.3 | .002b | 0.2 | .125 |
| 3 mo | −1.9 | .048a | −0.7 | .081 | 0.1 | .455 |
| 6 mo | −1.8 | .063 | −1.0 | .026a | 0.1 | .514 |
| Anterior, 1 mo | −0.8 | .331 | −0.1 | .679 | 0.2 | .073 |
| 3 mo | −0.8 | .346 | 0.1 | .679 | 0.1 | .397 |
| 6 mo | −0.5 | .508 | 0.2 | .558 | 0 | .71 |
| Year | 0.1 | .000c | 0 | .001b | −0.0 | .002b |
| Sex (ref = male) | 0 | 0 | 0 | |||
| female | −1.5 | .000c | −1.4 | .000c | 0.2 | .000c |
| Body mass index | −0.1 | .033a | −0.2 | .000c | 0 | .102 |
| Race (ref = white) | 0 | 0 | 0 | |||
| Black | −3.7 | .000c | −1.2 | .001c | 0.7 | .000c |
| Other or multiple | −0.2 | .887 | 0.2 | .8 | 0 | .935 |
| Hispanic (ref = No) | 0 | 0 | 0 | |||
| Yes | −5.9 | .000c | −1.6 | .057 | 0.7 | .001b |
| Education (ref = less than college) | 0 | 0 | 0 | |||
| College or higher | 2.1 | .000c | 0.8 | .000c | −0.3 | .000c |
| Work status (ref = working) | 0 | 0 | 0 | |||
| Unemployed | −0.2 | .901 | −1.8 | .043a | 0.2 | .35 |
| Sick or disabled | −5.9 | .000c | −5.0 | .000c | 0.9 | .000c |
| Student, homemaker, retired | −0.8 | .138 | −0.9 | .002b | 0 | .633 |
| Alcohol use (ref = never) | 0 | 0 | 0 | |||
| Monthly or less | 0.9 | .139 | 0.5 | .082 | −0.2 | .010b |
| 2–4 Times a month | 0.7 | .271 | 1 | .006b | −0.1 | .174 |
| 2–3 Times a week | 1.2 | .078 | 1.7 | .000c | −0.2 | .009b |
| ≥4 Times a week | 2 | .004b | 1.8 | .000c | −0.4 | .000c |
| Smoking status (ref = never) | 0 | 0 | 0 | |||
| Current | −5.7 | .000c | −2.9 | .000c | 0.7 | .000c |
| Former | −2.3 | .000c | −1.4 | .000c | 0.2 | .000c |
| Comorbidity (ref = no) | 0 | 0 | 0 | |||
| COPD | −2.3 | .028a | −2.0 | .000c | 0.4 | .007b |
| Paralysis | −1.2 | .663 | 0.9 | .527 | −0.7 | .049a |
| Heart attack | −0.3 | .805 | −1.4 | .020a | 0 | .945 |
| Carotid artery disease | −3.5 | .097 | −1.6 | .174 | 0.1 | .703 |
| Stroke | −1.9 | .177 | −2.0 | .008b | 0.3 | .145 |
| Rheumatoid arthritis | −2.5 | .001c | −2.4 | .000c | 0.4 | .000c |
| Diabetes | −0.7 | .282 | −1.0 | .004b | 0.1 | .211 |
| Cancer | 0.8 | .305 | −0.2 | .585 | −0.2 | .018a |
| Liver disease | −1.2 | .457 | −0.6 | .459 | 0.1 | .711 |
| Peripheral vascular disease | −0.2 | .927 | 0.3 | .713 | −0.2 | .361 |
| Kidney disease | −2.0 | .116 | −2.3 | .001b | 0.3 | .094 |
| Ulcer disease | −4.3 | .005b | −1.8 | .024a | 0.8 | .000c |
| HIV or AIDS | 3.7 | .042a | 1.8 | .074 | −0.1 | .7 |
| Constant | 46.4 | .000c | 45.6 | .000c | 6.1 | .000c |
| Variance parameters | ||||||
| Center (se) | 1.1 | −.66 | 0.4 | −.23 | 0.06 | −.023 |
| Participant (se) | 67.8 | −3.26 | 24.9 | −.91 | 1.03 | −.06 |
| Residual (se) | 137 | −2.54 | 26.6 | −.47 | 2.91 | −.05 |
Based on multivariable mixed effects regression with robust standard errors clustering for observations within individual patients and patients nested within hospitals. HOOS Jr, the brief Hip disability and Osteoarthritis Outcomes Score; PROMIS-PH, Patient-Reported Outcomes Measurement Information System Physical Health Summary; NPRS, Numeric Pain Rating Scale; ref, reference; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome.
P < .05.
<0.01.
<0.001.
Appendix C.
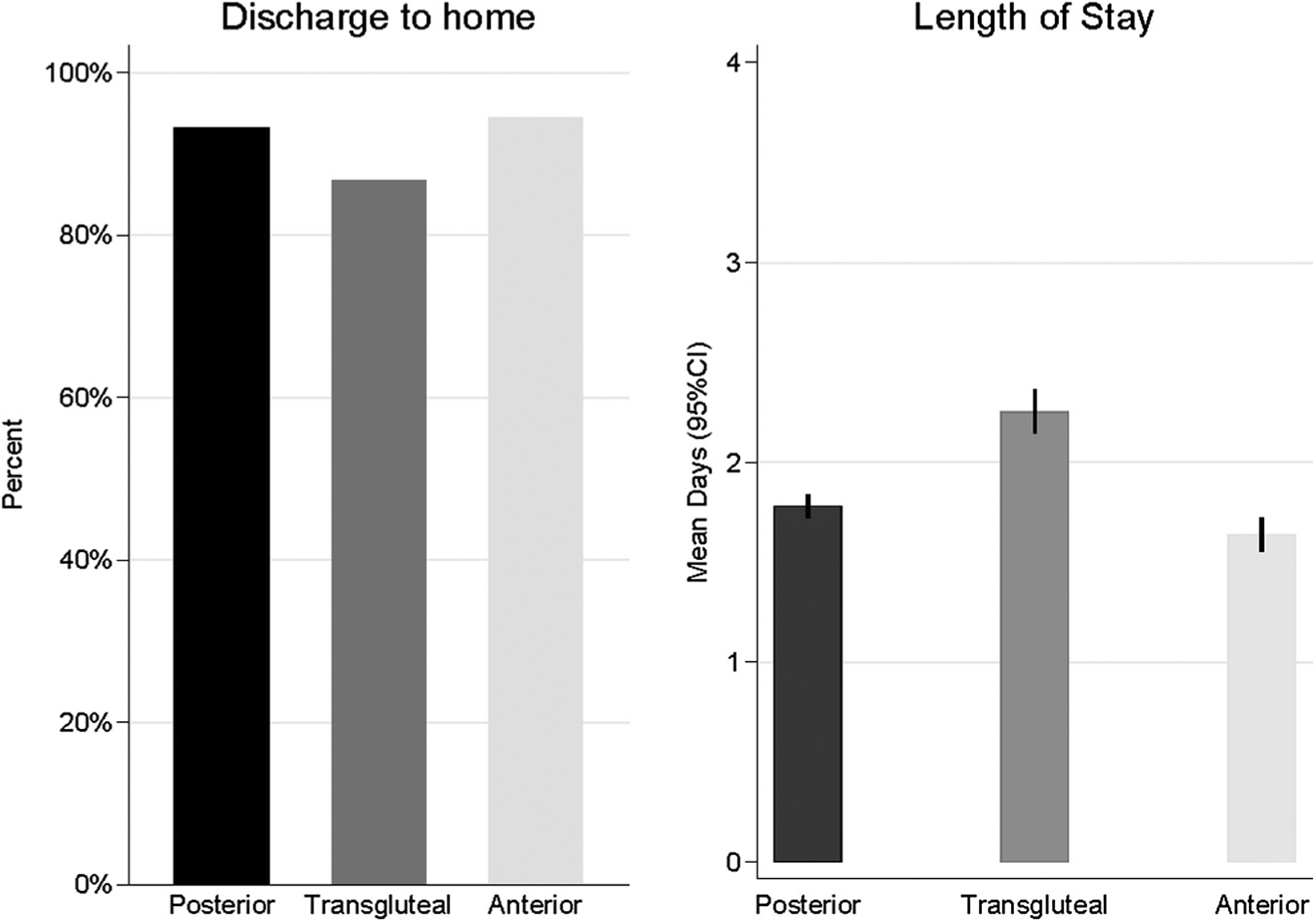
Length of Stay and Discharge Disposition for Hip Cohort. CI, confidence interval.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2019.10.017.
The authors from the PEPPER Investigators are listed in Appendix.
References
- [1].Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA 1996;275:858–65. [PubMed] [Google Scholar]
- [2].Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- [3].Waddell J, Johnson K, Hein W, Raabe J, FitzGerald G, Turibio F. Orthopaedic practice in total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY). Am J Orthop (Belle Mead NJ) 2010;39(9 Suppl):5–13. [PubMed] [Google Scholar]
- [4].Moretti VM, Post ZD. Surgical approaches for total hip arthroplasty. Indian J Orthop 2017;51:368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hardinge K The direct lateral approach to the hip. J Bone Joint Surg Br 1982;64:17–9. [DOI] [PubMed] [Google Scholar]
- [6].Mulliken BD, Rorabeck CH, Bourne RB, Nayak N. A modified direct lateral approach in total hip arthroplasty: a comprehensive review. J Arthroplasty 1998;13:737–47. [DOI] [PubMed] [Google Scholar]
- [7].Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty 2015;30:419–34. [DOI] [PubMed] [Google Scholar]
- [8].Kennon RE, Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am 2003;85-A:39–48. [DOI] [PubMed] [Google Scholar]
- [9].Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res 2013;471:3409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amlie E, Havelin LI, Furnes O, Baste V, Nordsletten L, Hovik O, et al. Worse patient-reported outcome after lateral approach than after anterior and posterolateral approach in primary hip arthroplasty. A cross-sectional questionnaire study of 1,476 patients 1–3 years after surgery. Acta Orthop 2014;85:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peters RM, van Beers L, van Steenbergen LN, Wolkenfelt J, Ettema HB, Ten Have B, et al. Similar superior patient-reported outcome measures for anterior and posterolateral approaches after total hip arthroplasty: postoperative patient-reported outcome measure improvement after 3 months in 12,774 primary total hip arthroplasties using the anterior, anterolateral, straight lateral, or posterolateral approach. J Arthroplasty 2018;33: 1786–93. [DOI] [PubMed] [Google Scholar]
- [12].Comparative Effectiveness of Pulmonary Embolism Prevention after Hip and Knee Replacement (PEPPER). http://www.muschealth.org/pepper/index.html [accessed 02.07.2019]. [DOI] [PMC free article] [PubMed]
- [13].Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res 2016;474:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hung M, Bounsanga J, Voss MW, Saltzman CL. Establishing minimum clinically important difference values for the Patient-Reported Outcomes Measurement Information System Physical Function, hip disability and osteoarthritis outcome score for joint reconstruction, and knee injury and osteoarthritis outcome score for joint reconstruction in orthopaedics. World J Orthop 2018;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lyman S, Lee YY, McLawhorn AS, Islam W, MacLean CH. What are the minimal and substantial improvements in the HOOS and KOOS and JR versions after total joint replacement? Clin Orthop Relat Res 2018;476:2432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hung M, Saltzman CL, Greene T, Voss MW, Bounsanga J, Gu Y, et al. Evaluating instrument responsiveness in joint function: the HOOS JR, the KOOS JR, and the PROMIS PF CAT. J Orthop Res 2018;36:1178–84. [DOI] [PubMed] [Google Scholar]
- [17].Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [18].statix.com [accessed 17.09.19].
- [19].Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 2011;32:970–86. [DOI] [PubMed] [Google Scholar]
- [20].Berbari EF, Osmon DR, Lahr B, Eckel-Passow JE, Tsaras G, Hanssen AD, et al. The Mayo prosthetic joint infection risk score: implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol 2012;33:774–81. [DOI] [PubMed] [Google Scholar]
- [21].Mesko NW, Bachmann KR, Kovacevic D, LoGrasso ME, O’Rourke C, Froimson MI. Thirty-day readmission following total hip and knee arthroplasty - a preliminary single institution predictive model. J Arthroplasty 2014;29:1532–8. [DOI] [PubMed] [Google Scholar]
- [22].Oldmeadow LB, McBurney H, Robertson VJ. Predicting risk of extended inpatient rehabilitation after hip or knee arthroplasty. J Arthroplasty 2003;18: 775–9. [DOI] [PubMed] [Google Scholar]
- [23].Wuerz TH, Kent DM, Malchau H, Rubash HE. A nomogram to predict major complications after hip and knee arthroplasty. J Arthroplasty 2014;29: 1457–62. [DOI] [PubMed] [Google Scholar]
- [24].Wuerz TH, Regenbogen SE, Ehrenfeld JM, Malchau H, Rubash HE, Gawande AA, et al. The Surgical Apgar Score in hip and knee arthroplasty. Clin Orthop Relat Res 2011;469:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain 2011;152:2287–93. [DOI] [PubMed] [Google Scholar]
- [26].Edelstein AI, Kwasny MJ, Suleiman LI, Khakhkhar RH, Moore MA, Beal MD, et al. Can the American College of Surgeons risk calculator predict 30-day complications after knee and hip arthroplasty? J Arthroplasty 2015;30(9 Suppl):5–10. [DOI] [PubMed] [Google Scholar]
- [27].Manning DW, Edelstein AI, Alvi HM. Risk prediction tools for hip and knee arthroplasty. J Am Acad Orthop Surg 2016;24:19–27. [DOI] [PubMed] [Google Scholar]
- [28].Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- [29].Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage 2014;22:918–27. [DOI] [PubMed] [Google Scholar]
- [30].Lin TJ, Bendich I, Ha AS, Keeney BJ, Moschetti WE, Tomek IM. A comparison of radiographic outcomes after total hip arthroplasty between the posterior approach and direct anterior approach with intraoperative fluoroscopy. J Arthroplasty 2017;32:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schweppe ML, Seyler TM, Plate JF, Swenson RD, Lang JE. Does surgical approach in total hip arthroplasty affect rehabilitation, discharge disposition, and readmission rate? Surg Technol Int 2013;23:219–27. [PubMed] [Google Scholar]
- [32].Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty 2008;23:266–72. [DOI] [PubMed] [Google Scholar]
- [33].Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res 2004:164–73. [DOI] [PubMed] [Google Scholar]
- [34].Zawadsky MW, Paulus MC, Murray PJ, Johansen MA. Early outcome comparison between the direct anterior approach and the mini-incision posterior approach for primary total hip arthroplasty: 150 consecutive cases. J Arthroplasty 2014;29:1256–60. [DOI] [PubMed] [Google Scholar]
- [35].Miller LE, Gondusky JS, Bhattacharyya S, Kamath AF, Boettner F, Wright J. Does surgical approach affect outcomes in total hip arthroplasty through 90 days of follow-up? A systematic review with meta-analysis. J Arthroplasty 2018;33: 1296–302. [DOI] [PubMed] [Google Scholar]
- [36].Graves SC, Dropkin BM, Keeney BJ, Lurie JD, Tomek IM. Does surgical approach affect patient-reported function after primary THA? Clin Orthop Relat Res 2016;474:971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty 2013;28:849–54. [DOI] [PubMed] [Google Scholar]
- [38].Christensen CP, Karthikeyan T, Jacobs CA. Greater prevalence of wound complications requiring reoperation with direct anterior approach total hip arthroplasty. J Arthroplasty 2014;29:1839–41. [DOI] [PubMed] [Google Scholar]
- [39].Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res 2011;469:503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maffiuletti NA, Impellizzeri FM, Widler K, Bizzini M, Kain MS, Munzinger U, et al. Spatiotemporal parameters of gait after total hip replacement: anterior versus posterior approach. Orthop Clin North Am 2009;40:407–15. [DOI] [PubMed] [Google Scholar]
- [41].Meermans G, Konan S, Das R, Volpin A, Haddad FS. The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J 2017;99-b:732–40. [DOI] [PubMed] [Google Scholar]
- [42].Spaans AJ, van den Hout JA, Bolder SB. High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop 2012;83:342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Taunton MJ, Mason JB, Odum SM, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty 2014;29(9 Suppl):169–72. [DOI] [PubMed] [Google Scholar]
- [44].Rodriguez JA, Deshmukh AJ, Rathod PA, Greiz ML, Deshmane PP, Hepinstall MS, et al. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res 2014;472:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty 2010;25: 671–679.e1. [DOI] [PubMed] [Google Scholar]
- [46].Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty 2013;28:1634–8. [DOI] [PubMed] [Google Scholar]
- [47].Goebel S, Steinert AF, Schillinger J, Eulert J, Broscheit J, Rudert M, et al. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop 2012;36:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sibia US, Turner TR, MacDonald JH, King PJ. The impact of surgical technique on patient reported outcome measures and early complications after total hip arthroplasty. J Arthroplasty 2017;32:1171–5. [DOI] [PubMed] [Google Scholar]
- [49].Rosenlund S, Broeng L, Holsgaard-Larsen A, Jensen C, Overgaard S. Patient-reported outcome after total hip arthroplasty: comparison between lateral and posterior approach. Acta Orthop 2017;88:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zomar BO, Bryant D, Hunter S, Howard JL, Vasarhelyi EM, Lanting BA. A randomised trial comparing spatio-temporal gait parameters after total hip arthroplasty between the direct anterior and direct lateral surgical approaches. Hip Int 2018;28:478–84. [DOI] [PubMed] [Google Scholar]
- [51].Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am 2009;91(Suppl 6):107–20. [DOI] [PubMed] [Google Scholar]
- [52].Mayr E, Nogler M, Benedetti MG, Kessler O, Reinthaler A, Krismer M, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech (Bristol, Avon) 2009;24:812–8. [DOI] [PubMed] [Google Scholar]
- [53].Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty 2009;24:698–704. [DOI] [PubMed] [Google Scholar]
- [54].Barrett WP, Turner SE, Murphy JA, Flener JL, Alton TB. Prospective, randomized study of direct anterior approach vs posterolateral approach total hip arthroplasty: a concise 5-year follow-up evaluation. J Arthroplasty 2019;34: 1139–42. [DOI] [PubMed] [Google Scholar]


