Abstract
This cross-sectional study assesses national data for commercially insured and Medicare Advantage enrollees to analyze the use of levothyroxine in the US over time.
Levothyroxine is one of the most commonly prescribed drugs in the US, with approximately 7% of the population estimated to have an active prescription.1,2 For nonpregnant adults with subclinical hypothyroidism (thyrotropin level elevated but ≤10 mIU/L and normal free thyroxine [FT4] levels), evidence consistently demonstrates no clinically relevant benefits of levothyroxine replacement for quality of life or thyroid-related symptoms.3,4 To better understand the use of levothyroxine in the US over time, we analyzed national data for commercially insured and Medicare Advantage enrollees.
Methods
We conducted a retrospective analysis of deidentified administrative claims data linked with laboratory results from OptumLabs Data Warehouse, which includes commercially insured and Medicare Advantage enrollees throughout the US.5 The study was deemed not to require Mayo Clinic Institutional Review Board review owing to the use of deidentified data in accordance with the Code of Federal Regulations, 45 CFR 46.102. The cohort included adults (≥18 years of age) who newly filled levothyroxine prescriptions between January 1, 2008, and December 31, 2018, and who had a thyrotropin level measured within 3 months prior to levothyroxine initiation. We excluded people with a history of thyroid surgery, thyroid cancer, or central hypothyroidism and pregnant women. We used descriptive statistics to characterize trends in thyrotropin levels and categories of thyroid function measured prior to levothyroxine initiation. In a subsample with thyrotropin and thyroxine levels available, thyroid function was defined as overt hypothyroidism (elevated thyrotropin and low free or total thyroxine [FT4 or T4] levels), subclinical hypothyroidism (elevated thyrotropin and normal FT4 or T4 levels), and normal thyroid function (normal thyrotropin and FT4 or T4 levels).6 We also separately examined mild subclinical hypothyroidism (thyrotropin level of 4.5 mIU/L to <10 mIU/L with normal FT4 or T4), moderate subclinical hypothyroidism (thyrotropin level of 10-19.9 mIU/L), and severe subclinical hypothyroidism (thyrotropin level > 19.9 mIU/L). Pearson correlation test (2-tailed approach; level of significance of .05) was conducted to test the linear associations between thyrotropin and year, and if the proportion of patients in different categories of thyroid function has changed over time. Microsoft Excel 2010 (Microsoft Corp) and SAS, version 9.4 (SAS Institute Inc) were used to conduct the analyses. Data analysis was performed June through March 2021.
Results
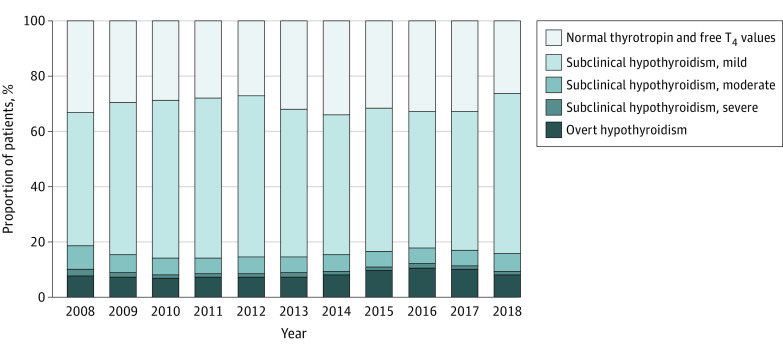
Between 2008 and 2018, 110 842 patients newly initiated levothyroxine treatment (Table). The median thyrotropin level at treatment initiation did not significantly change: 5.8 mIU/L in 2008 to 5.3 mIU/L in 2018 (P = .79). In a subset of 58 706 patients with thyrotropin and FT4 or T4 levels available, levothyroxine was initiated for overt hypothyroidism (4948 [8.4%]), subclinical hypothyroidism (35 814 [61.0%]), and normal thyroid levels (17 944 [30.5%]). From 2008 to 2018, the proportion of adults with overt hypothyroidism increased (7.6% to 8.4%; P = .02); the proportion with subclinical hypothyroidism did not change (59.3% to 65.7%; P = .36); and the proportion with normal thyroid function did not change (32.9% to 26.2%; P = .84). Among patients with subclinical hypothyroidism, the proportion with mild subclinical hypothyroidism (48.2% to 57.9%; P = .73) and moderate subclinical hypothyroidism (8.5% to 6.4%; P = .16) did not change significantly, and the proportion with severe subclinical hypothyroidism decreased (2.5% to 1.3%; P = .02) (Figure).
Table. Characteristics of Commercially Insured and Medicare Advantage Adults Initiating Levothyroxine Therapy Between 2008 and 2018.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total (n = 110 842) | 2008 (n = 6152) | 2018 (n = 9331) | |
| Levothyroxine dose, mcg | |||
| ≤50 | 86 558 (78.1) | 4524 (73.5) | 7802 (83.6) |
| 51-100 | 18 932 (17.1) | 1291 (21.0) | 1222 (13.1) |
| 101-150 | 4497 (4.1) | 281 (4.6) | 266 (2.9) |
| 151-200 | 828 (0.7) | 53 (0.9) | 37 (0.4) |
| >200 | 27 (0.0) | NRa | NRa |
| Baseline thyrotropin category, mIU/L | |||
| 0.3-4.4 | 31 494 (28.4) | 1688 (27.4) | 2386 (25.6) |
| 4.5-9.9 | 66 109 (59.6) | 3476 (56.5) | 5809 (62.3) |
| 10-19.9 | 7923 (7.1) | 578 (9.4) | 685 (7.3) |
| >19.9 | 5316 (4.8) | 410 (6.7) | 451 (4.8) |
| Baseline thyrotropin, mIU/L | |||
| Mean (SD) | 8.5 (21.5) | 9.2 (17.7) | 8.4 (18.7) |
| Median (IQR) | 5.3 (4.1-7.0) | 5.8 (4.1-7.9) | 5.3 (4.4-7.1) |
| Range | 0.3-993.6 | 0.3-370.8 | 0.3-570.1 |
| Age, y | |||
| Mean (SD) | 46.1 (12.7) | 46.1 (11.6) | 47.2 (14.6) |
| Median (IQR) | 47 (36.0-56.0) | 47 (38.0-55.0) | 48 (36.0-58.0) |
| Age group, y | |||
| <35 | 23 392 (21.1) | 1105 (18.0) | 2091 (22.4) |
| 35-44 | 24 636 (22.2) | 1519 (24.7) | 1930 (20.7) |
| 45-54 | 30 899 (27.9) | 1892 (30.8) | 2227 (23.9) |
| 55-64 | 26 537 (23.9) | 1431 (23.3) | 2067 (22.2) |
| ≥65 | 5378 (4.9) | 205 (3.3) | 1016 (10.9) |
| Sex | |||
| Female | 81 189 (73.2) | 4675 (76.0) | 6613 (70.9) |
| Male | 29 653 (26.8) | 1477 (24.0) | 2718 (29.1) |
| Race/ethnicity | |||
| Asian | 8525 (7.7) | 333 (5.4) | 703 (7.5) |
| Black | 12 019 (10.8) | 769 (12.5) | 923 (9.9) |
| Hispanic | 15 780 (14.2) | 695 (11.3) | 1526 (16.4) |
| White | 70 696 (63.8) | 4179 (67.9) | 5476 (58.7) |
| Unknown | 3822 (3.4) | 176 (2.9) | 703 (7.5) |
| Census region | |||
| Midwest | 11 881 (10.7) | 581 (9.4) | 954 (10.2) |
| Northeast | 8809 (7.9) | 438 (7.1) | 798 (8.6) |
| South | 66 643 (60.1) | 4157 (67.6) | 5314 (56.9) |
| West | 23 412 (21.1) | 974 (15.8) | 2244 (24.0) |
| Unknown | 97 (0.1) | NRa | 21 (0.2) |
| Household income, $ | |||
| <40 000 | 14 408 (13.0) | 606 (9.9) | 1456 (15.6) |
| 40 000-74 999 | 25 549 (23.0) | 1257 (20.4) | 2022 (21.7) |
| 75 000-124 999 | 31 170 (28.1) | 1551 (25.2) | 2206 (23.6) |
| 125 000-199 999 | 18 871 (17.0) | 1052 (17.1) | 1440 (15.4) |
| ≥200 000 | 13 124 (11.8) | 753 (12.2) | 999 (10.7) |
| Unknown | 7720 (7.0) | 933 (15.2) | 1208 (12.9) |
| Specialty of prescriber | |||
| Endocrinology | 12 276 (11.1) | 554 (9.0) | 1155 (12.4) |
| Primary care | 66 509 (60.0) | 4160 (67.6) | 4434 (47.5) |
| Other | 24 042 (21.7) | 1041 (16.9) | 2406 (25.8) |
| Missing/unknown | 8015 (7.2) | 397 (6.5) | 1336 (14.3) |
| Charlson group | |||
| 0 | 91 166 (82.2) | 4992 (81.1) | 7441 (79.7) |
| 1 | 12 642 (11.4) | 753 (12.2) | 1061 (11.4) |
| 2 | 3989 (3.6) | 249 (4.0) | 405 (4.3) |
| ≥3 | 3045 (2.7) | 158 (2.6) | 424 (4.5) |
Abbreviations: IQR, interquartile range; NR, not reported.
Cannot include No. when population size is less than 11 to protect patient confidentiality.
Figure. Trend of Levothyroxine Use Between 2008 and 2018 by Thyroid Function Categories.
T4 indicates thyroxine.
Discussion
We found that levothyroxine treatment was commonly initiated for mildly increased thyrotropin levels, and this did not change significantly over time. Among patients for whom full thyroid function test results were available, 60% initiated levothyroxine for treatment of subclinical hypothyroidism (mostly for mild subclinical hypothyroidism) and 30% for normal thyroid function, without significant change in these patterns over time. Frequent initiation of levothyroxine in these patients is at odds with evidence demonstrating no significant association of levothyroxine replacement with measures of health-related quality of life, thyroid-related symptoms, depressive symptoms, fatigue, or cognitive function.3,4
Our study has some limitations. The study included commercially insured patients, who may be more likely to use levothyroxine, and thus findings may not generalize to other populations. We could not assess symptoms or other potential compelling reasons for levothyroxine initiation.
In conclusion, these results suggest substantial overuse of levothyroxine during the entire duration of the study, suggesting opportunities to improve care.
References
- 1.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. doi: 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JS, Rohde S, Sangaralingham L, et al. Generic and brand-name thyroid hormone drug use among commercially insured and Medicare beneficiaries, 2007 through 2016. J Clin Endocrinol Metab. 2019;104(6):2305-2314. doi: 10.1210/jc.2018-02197 [DOI] [PubMed] [Google Scholar]
- 3.Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006 [DOI] [PubMed] [Google Scholar]
- 4.Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320(13):1349-1359. doi: 10.1001/jama.2018.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33(7):1187-1194. doi: 10.1377/hlthaff.2014.0038 [DOI] [PubMed] [Google Scholar]
- 6.Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670-1751. doi: 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]