Abstract
Graphene and its derivatives have continued to garner worldwide interest due to their unique characteristics. Having expanded into biomedical applications, there have been efforts to employ their exceptional properties for the regeneration of different tissues, particularly bone. This article presents a comprehensive review on the usage of graphene-based materials for bone regenerative engineering. The graphene family of materials (GFMs) are used either alone or in combination with other biomaterials in the form of fillers in composites, coatings for both scaffolds and implants, or vehicles for the delivery of various signaling and therapeutic agents. The applications of the GFMs in each of these diverse areas are discussed and emphasis is placed on the characteristics of the GFMs that have implications in this regard. In tandem and of importance, this article evaluates the safety and biocompatibility of the GFMs and carefully elucidates how various factors influence the biocompatibility and biodegradability of this new class of nanomaterials. In conclusion, the challenges and opportunities regarding the use of the GFMs in regenerative engineering applications are discussed, and future perspectives for the developments in this field are proposed.
Keywords: biocompatibility, biomaterials, bones, graphene, tissue engineering
1. Introduction
Musculoskeletal conditions account for more than 50% of all chronic conditions in people over the age of 50 in developed countries and are among the most disabling and costly conditions suffered.[1] Bone grafting procedures are the second most frequent tissue transplantation, after blood transfusion, with over two million procedures taking place annually worldwide.[2] These procedures involve the treatment of segmental bone loss caused by trauma, tumor excision, infection, nonunions, osteonecrosis, or developmental deformities for long bone, spinal fusion, craniomaxillofacial, foot and ankle, dental, and joint reconstruction.[3]
The current gold standard for treatment is using autografts (bone grafts taken from a donor site to a recipient site during surgery); however, limited supply, prolonged operation times, risk of donor site infection, and morbidity are drawbacks. Allografts (bone grafts from human donors) are an alternative, although they are also associated with complications of disease transmission, infection, and immunogenicity.[2,4] As a result, there has been a growing interest and numerous efforts to develop synthetic bone graft substitutes to reduce the usage of autografts and allografts in surgeries. The current most common synthetic bone graft substitutes are calcium sulfates, calcium phosphate (CaP) ceramics, CaP cements, bioactive glass, or combinations thereof.[5] Despite some attained success, however, the treatment outcome of using these synthetic substitutes is inferior to using autologous bone grafts[6] and there is still a need for an effective bone graft substitute that is able to produce successful clinical outcomes in all subspecialties of orthopedic surgery.
The limitations associated with the current treatments imply that a shift in paradigm is required to achieve successful bone regeneration. Regenerative engineering is defined as the convergence of advanced materials sciences, stem cell sciences, physics, developmental biology, and clinical translation for the regeneration of complex tissues and organ systems.[7] This trans-disciplinary approach is founded on combining the latest advancements made in each of these disparate fields with a clinical perspective to develop highly translational technologies to regenerate complex tissues and organs. Within these various fields, advanced materials science and engineering are integral to the development of intelligent biomaterials that can modulate cellular functions and harness the body’s innate regenerative potential.[7a,c,8]
Since its discovery in 2004, graphene and its derivatives have continued to fascinate scientists worldwide. Having been awarded the Nobel Prize in Physics only six years after its discovery, this “wonder material” has raised great expectations for versatile applications such as electronics, heat transport, field emission, sensors, composites, and energy. More recently, there have been efforts to employ graphene-based materials for the regeneration of tissues, particularly bone. In this review, we assess the current literature on graphene-based biomaterials for bone regeneration and explore the potential of this remarkable nanomaterial for bone regenerative engineering.
2. The Graphene Family
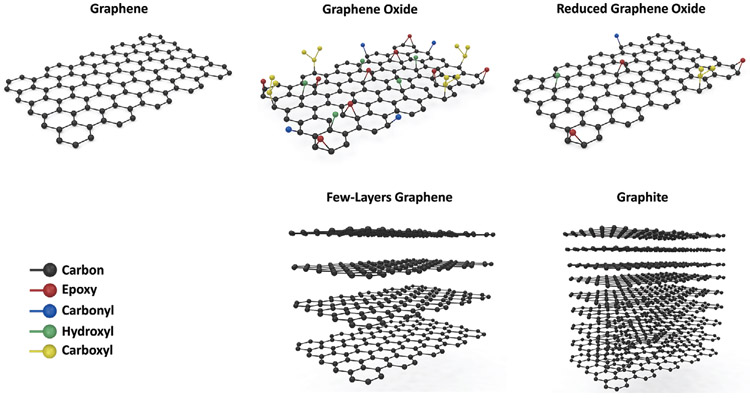
Graphene is one of the allotropes of carbon, which consists of a one-atom-thick 2D sheet of sp2 hybridized carbon atoms that are tightly packed into rings of six forming a honeycomb lattice (Figure 1).[9] Each carbon atom has three in-plane σ-bonds that are responsible for the perfect planar nature of graphene, and one out-of-plane π-bond that can interact with neighboring atoms.[10] This unique structure has endowed graphene with extraordinary mechanical, electrical, thermal, and optical properties, many of which exceed those obtained in any other material, with some reaching theoretically predicted limits.[11]
Figure 1.
Molecular structure of various members of the graphene family of materials (GFMs).
The stability of the σ-bonds that form the hexagonal lattice and oppose a variety of in-plane deformations account for the impressive mechanical properties of graphene.[12] Its Young’s modulus and intrinsic strength have been reported to be as high as 1 TPa and 130 GPa, respectively, and its breaking strength is 200 times greater than steel.[13] Graphene has a large specific surface area (theoretically calculated 2630 m2 g−1 for a single graphene sheet)[14] and an extremely high thermal conductivity (reported 5000 W m−1 K−1 for a suspended single-layer graphene at room temperature.[15] Moreover, it has exceptionally high electronic quality (electron mobilities of 250 000 cm2 V−1 s−1 at room temperature),[16] is impermeable to gases,[17] and absorbs 2.3% of incident white light despite being only one atom thick.[18]
In spite of its remarkable properties, biomedical applications of pristine graphene have been relatively limited due to the cost and difficulty of scalable high-quality synthesis, poor dispersibility in aqueous media and physiological fluids, and concerns regarding its biocompatibility and toxicity.[11,19] This has led to other members of the graphene family to be explored for bioapplications (Figure 1).
At present, the most common form used in biomedical applications is graphene oxide (GO).[19b] GO is the oxidized form of graphene, which is produced by the oxidation and exfoliation of graphite into a single layer. The sheet of carbon atoms contains various oxygen functional groups, which are mainly hydroxyl and epoxy groups on the basal plane, and smaller amounts of carboxy, carbonyl, phenol, lactone, and quinone groups at the edges. The oxygen functional groups introduce hydrophilicity to the otherwise hydrophobic graphene structure, rendering GO an amphiphile with superior dispersibility in many solvents including water.[20] They also provide chemical handles that allow for the properties of GO to be manipulated and tailored for various applications, further offering a vast array of opportunities.[21] The presence of these functional groups, however, act as structural defects in the perfect planar structure of graphene and compromise the electrical, mechanical, and thermal properties.[22] Ultimately, however, because of its accessibility, ease of synthesis, solution processability, and versatile tunable properties, GO is considered an attractive cost-efficient alternative for the large-scale production of graphene-based single sheets.[20]
The other variants of the graphene family of materials (GFMs) include reduced graphene oxide (rGO), multilayer graphene, graphene nanoribbons, and various functionalized members of the GFMs.[23] These variants differ in layer number, surface chemistry, lateral dimensions, purity, composition, and defect density.[24] For instance, rGO is generated by treating GO under reducing conditions. This reduces the oxygen content and material hydrophilicity, however partially restores electrical conductivity for electrochemical applications.[25]
3. Graphene-Based Biomaterials in Bone Regeneration
Bone has the intrinsic ability to heal itself after injury. However, in complex clinical conditions where bone is required in large quantities or the regenerative process is compromised, this ability can be impaired.[26] With the worldwide increase in the incidence of bone disorders and conditions, it is imperative to devise strategies to overcome the current limitations and develop bone graft substitutes that can successfully regenerate bone in all capacities. The success of this approach relies on providing the biochemical and mechanical stimuli in an accurate spatiotemporal manner to promote osteogenesis and osseointegration with the host.[27]
An ideal bone graft should be I) biocompatible and support normal cellular activity without eliciting any local or systemic toxic effects to the host tissue; II) osteoconductive and allow the bone cells to adhere, proliferate, and grow on its surface; III) osteoinductive and induce osteogenesis by recruiting progenitor cells and stimulating their differentiation into bone cells; IV) mechanically competent to match the properties of host bone and effectively withstand and transfer loads; V) porous with appropriate interconnected pore sizes for cell and tissue in-growth; and VI) biodegradable with a controlled degradation rate matching the growth of new bone.[28]
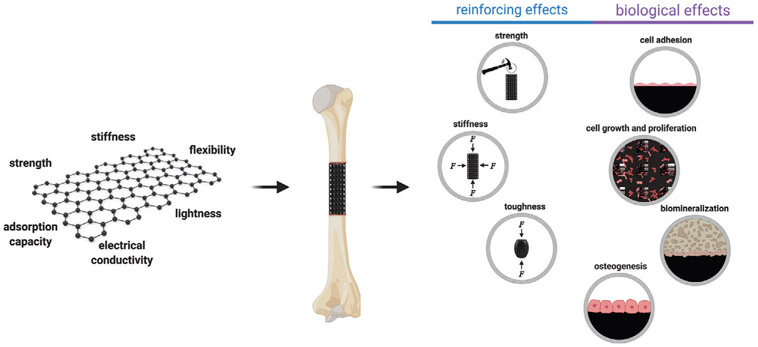
Owing to their unique characteristics, the GFMs have emerged as a new generation of nanomaterials for regenerative engineering different tissues,[29] and particularly bone (Figure 2). Various strategies have been developed to employ the GFMs and their characteristics toward the production or modification of biomaterials through methods including blending, embedding, chemical or physical crosslinking, and surface coating. The inclusion of the GFMs into bone biomaterials, in general, leads to improvements in the mechanical properties of the constructs and biological characteristics, which are necessary for tissue formation and regeneration (Figure 3).
Figure 2.
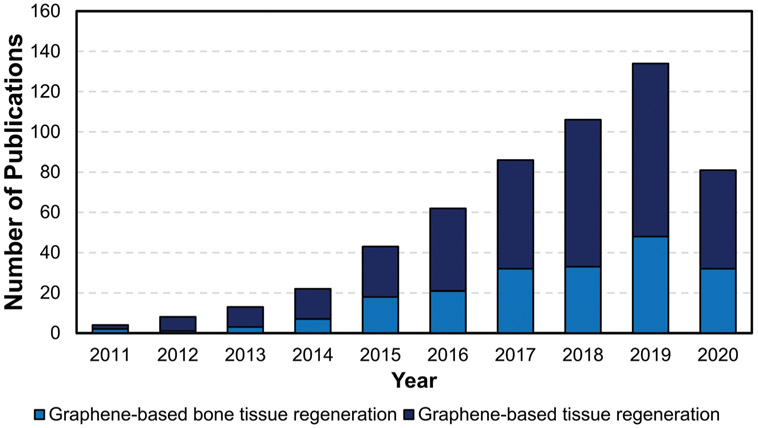
Publication trend in the field of graphene-based tissue regeneration and graphene-based bone tissue regeneration (data obtained from PubMed; search strings: “graphene” and “tissue regeneration” or “bone regeneration”).
Figure 3.
Applications of graphene-based materials in bone regenerative engineering.
3.1. The Rationale for Using Graphene and Its Derivatives
3.1.1. Reinforcing Effects
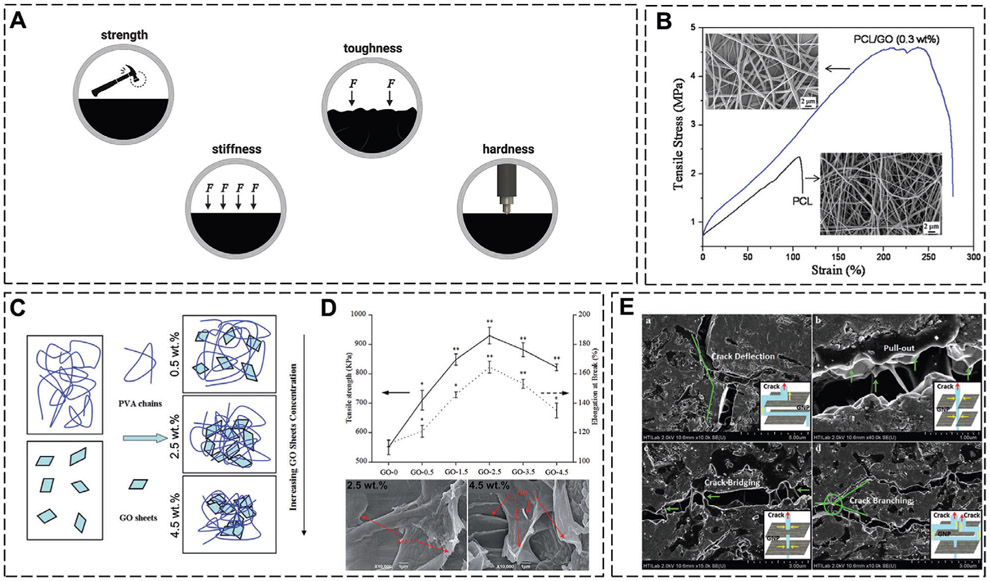
The GFMs have been employed to physically reinforce a wide range of biomaterials for bone applications such as bone cements,[30] pellets,[31] porous scaffolds,[32] membranes,[33] electrospun nanofibers,[34] and hydrogels.[35] Due to their strong mechanical properties, the presence of the GFMs, even at small amounts, in these structures can significantly enhance their mechanical strength, stiffness, and toughness (Figure 4A) and thus produce biomaterials that are more mechanically competent for load-bearing applications.
Figure 4.
A) The mechanical improvements imparted by GFM incorporation. B) Tensile stress–strain curves and SEM images of PCL and PCL/GO (0.3 wt%) nanofibrous membranes. C) Schematic representation of the dispersion of GO sheets within a PVA matrix at various GO loading concentrations. D) Tensile strength (solid line, left axis) and elongation at break (dotted line, right axis) of PVA/GO matrices at various loading concentrations of GO, and SEM images of the tensile fracture surface of the two PVA/GO compositions. E) The various toughening mechanisms in hydroxyapatite-GFMs. B) Reproduced with permission.[33] Copyright 2011, IOP Publishing Ltd. C,D) Reproduced with permission.[37] Copyright 2015, The Royal Society of Chemistry. E) Reproduced with permission.[38a] Copyright 2018, Taylor & Francis.
Although the addition of graphene in itself predominantly reinforces biomaterials, the greatest improvements are usually observed at specific concentrations of graphene loading. This threshold and specific concentration depends on the fabrication method, the matrix, the graphene variant in use, and the interactions of the two. The interactions between the GFM and the matrix facilitate the transfer of load. For example, although the modulus of GO (reported to be up 500 MPa[36]) is about a fraction of that of graphene, the presence of the functional groups in GO enables greater interactions with the matrix for a more efficient load transfer.[12]
The GFMs can be used as reinforcing agents in various polymeric matrices. When GO was incorporated into porous poly(vinyl alcohol)/GO (PVA/GO) scaffolds that were fabricated using selective laser sintering, the greatest mechanical enhancements were observed at specifically 2.5 wt% GO. At this concentration, the GO sheets were homogeneously dispersed in the PVA matrix and strong hydrogen bonds had formed between the two, facilitating efficient load transfer and resulting in a 60%, 152%, and 69% improvement in the compressive strength, Young’s modulus, and tensile strength values to 240.49 kPa, 2.47 MPa, and 929.54 kPa, respectively. Upon increasing the GO concentration beyond this threshold, the GO sheets restacked with each other and formed irreversible agglomerates that weakened the scaffolds and diminished the mechanical enhancements (Figure 4C,D).[37] The threshold concentration for incorporating GO nanoplatelets (GONPs) into polycaprolactone (PCL) membranes prepared via solution-casting was much lower at 0.3 wt%. At this concentration, the GONPs increased the Young’s modulus, tensile strength, and energy at break by 66%, 95%, and 416% to ≈ 230 MPa, 25.1 MPa, and 101.1 MJ m−3, respectively (Figure 4B). The reinforcing effects were ascribed to the uniform dispersion of the GONPs in the polymer matrix, the intrinsic high strength and stiffness of the GONPs, and their abundant surface area and functionality, which generated strong interfacial interactions with the polymer chains for effective load transfer. GONPs, in particular, also have a flexible wrinkled structure that can crumple in the polymer matrix under load and create voids that reduce the Poisson’s ratio of the matrix and increase the overall strength and toughness of the composites.[33] Electrospun nanofibrous scaffolds under tensile load similarly display an improvement in their tensile strength and Young’s modulus upon GO incorporation.[34a,b]
Graphene can also improve the mechanical properties of brittle materials such as CaPs or bioactive glass that generally suffer from a low fracture toughness. The high surface area of the GFMs and their strong interfacial bonding with the matrix strengthens these composites by enabling effective load transfer from the matrix to the GFM for stress absorption. This strengthening of the matrix and structural refinement is also responsible for the increased hardness and toughness of the composites. The toughening mechanisms that play a role in these composites are I) crack tip deflection, where the crack deviates away from the GFMs seeking a lower energy path; II) GFM necking and crack bridging, where the GFMs restrict further widening of the crack; III) crack branching, which consumes more energy and thus restricts further crack propagation; and IV) GFM pull-out, which dissipates energy because of interfacial friction with the matrix and leads to toughening (Figure 4E).[31,32,38]
3.1.2. Biological Effects
Promoting Cell Adhesion and Growth:
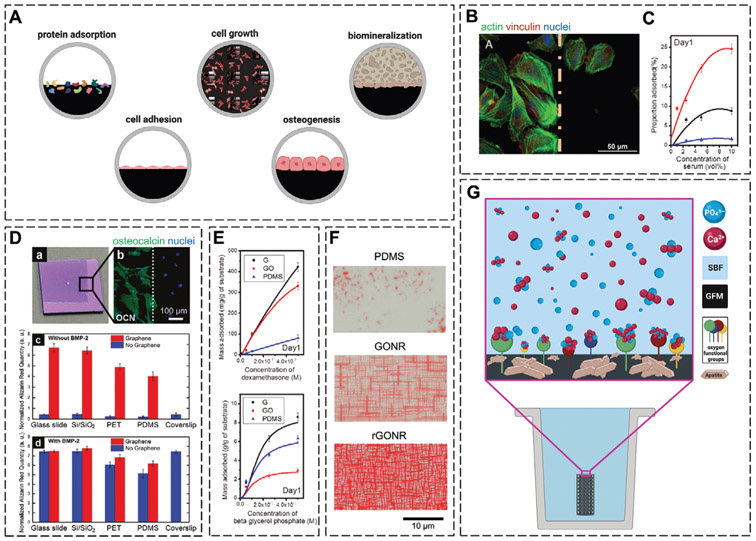
The ability of graphene to promote the adherence and proliferation of mesenchymal stromal/stem cells (MSCs) was initially demonstrated in 2010.[39] The study revealed that not only are graphene-coated substrates nontoxic to MSCs and human osteoblasts, but that they can serve as substrates for cell culture to stimulate the adherence and proliferation of cells (Figure 5B), an important criterion in developing scaffolds for tissue regeneration. Since then, many studies have documented the capabilities of the GFMs in promoting the adhesion, growth, and proliferation of various cell types. These studies typically involve using the GFMs as coatings for various substrates or as fillers in composite structures.
Figure 5.
A) The biological improvements imparted by GFM incorporation. B) Osteoblasts cultured on the border of graphene and Si/SiO2 substrates in medium supplemented with 15% FBS. C) Loading capacity of serum onto polydimethylsiloxane (PDMS), graphene, and GO substrates after 1 day. D-a,b) Optical image of a partially graphene-coated Si/SiO2 chip, showing bone cell formation only on the graphene-coated area. c,d) Alizarin Red quantification of MSCs on substrates with/without graphene and in the absence and presence of BMP2. E) Loading capacity of dexamethasone and β-glycerolphosphate onto PDMS, graphene, and GO substrates after 1 day. F) Osteogenic differentiation of MSCs monitored by Alizarin Red staining after 1 week on PDMS, and patterned GO and rGO nanogrids. G) Biomineralization mechanism. B) Reproduced with permission.[41a] Copyright 2012, Wiley-VCH GmbH. C,E) Reproduced with permission.[40] Copyright 2011, American Chemical Society. D) Reproduced with permission.[42] Copyright 2011, American Chemical Society. F) Reproduced with permission.[44] Copyright 2013, Elsevier.
The capability of GFM-based substrates to promote cell adhesion, morphology, and growth partly originates from their strong capacity to adsorb proteins. The adsorbed proteins create a layer that mediates the adhesion, spreading, and growth of cells. In graphene, the π-electron cloud interacts with the inner hydrophobic core of proteins to facilitate protein adsorption, and in GO, the oxygen functional groups enable binding through electrostatic interactions. In vitro, both graphene and GO adsorb high densities of serum proteins, consisting of many different extracellular proteins and adhesion molecules, that in turn mediate cell adhesion through the formation of focal adhesions (Figure 5C).[40]
Intriguingly, a few studies have shown the ability of GFM-based substrates to support cell adhesion in the absence of serum proteins. The cell–substrate interactions, in this case, are nonspecific, either van der Waals or hydrogen bonding or charged interactions between the polar groups of GO and the cell surface molecules. A comparison of the behavior of anchorage-dependent Saos-2 osteoblastic cells on graphene substrates in the presence or absence of fetal bovine serum (FBS) revealed a similar number of adhered cells, irrespective of FBS presence. Interestingly however, the cells cultured in the absence of FBS were larger in size. According to the study, in the absence of FBS, the cells spread as much as possible to search and find areas that are suitable for attachment. Their spreading and enlargement enables a greater number of, albeit weak, nonspecific adhesions to form, thus helping to sustain cell viability.[41]
Osteoinstructive Effects:
A pioneering study in 2011 showed that coating various substrates with graphene induces osteogenesis in human MSCs. In osteogenic medium, graphene coating remarkably accelerated cellular differentiation, at a rate comparable to differentiation under the influence of bone morphogenetic protein-2 (BMP2) (Figure 5D).[42] Since then, a number of studies have been conducted to elucidate the mechanisms behind the osteoinstructive effects of graphene. One proposed mechanism is the strong noncovalent π–π binding capabilities of graphene that adsorb the proteins and osteogenic supplements that are present in osteogenic media, namely, dexamethasone (DEX) and β-glycerolphosphate (Figure 5E). Through binding these supplements, graphene creates preconcentration platforms that facilitate substrate–cell interactions and enhance and accelerate osteogenic differentiation. Additionally, the −OH groups in GO can further adsorb ascorbic acid through hydrogen bonding.[40,43] Lastly, the introduction of topographical features onto GO or rGO sheets can further play a role and elevate osteogenic differentiation by both enhancing the adsorption of osteogenic inducers, mainly ascorbic acid, and inducing physical stresses (Figure 5F).[44]
The molecular signaling pathways by which graphene enhances stem cell differentiation have only begun to be investigated. At present, it seems that GO can activate the Wnt signaling pathway by preventing the proteasomal degradation of β-catenin.[45] GO can also facilitate the phosphorylation of Erk1/2 and increase the expression of BMP2.[46] The increase in substrate stiffness observed in GO-incorporated matrices may also play a role in promoting osteogenic differentiation.[47] However, further studies are required to better understand the interplay of graphene-based substrates and mechanosensory pathways, and their influence on stem cell differentiation.
Biomineralization Capacity:
The biomineralization capacity of a biomaterial, i.e., its ability to form apatite precipitation on its surface during immersion in simulated body fluid (SBF), is an indicator of its in vivo bone bioactivity and ability to integrate with living bone. In this method, the biomaterials are immersed in a supersaturated solution comprising ions at concentrations similar to that of human blood plasma. The apatite layer that is formed on the surface is then used to predict the bone-bonding and bone-forming ability of the biomaterial in vivo.[48]
The GFMs have been shown to enhance the biomineralization capacity of biomaterials by facilitating the formation of bone-like hydroxyapatite (HAp) and through promoting the nucleation and crystallization of apatite particles. The oxygen functional groups in GO provide binding sites to adsorb Ca2+ ions from the supersaturated solution through electrostatic interactions. Apatite nucleation takes place with many nanosized crystals forming. These precipitates then serve as nucleation centers that attract further Ca2+ ions to adsorb and to contribute to the growth of the HAp crystals (Figure 5G).[49] The biomineralization capacity of the GFMs is particularly beneficial when they are used as coatings for implants, where they improve osseointegration with the host bone and the implant’s stability and load-bearing capabilities.
3.2. Applications of Graphene-Based Biomaterials
The mechanical and biological improvements imparted by the GFMs have led to their utilization in a wide range of biomaterials. As such, graphene-based materials have been used either alone or in combination with other biomaterials in the form of fillers in composite structures, coatings for both scaffolds and implants, or vehicles for the delivery of various signaling and therapeutic agents.
3.2.1. As Scaffolds for Bone Regeneration
Scaffolds are temporary 3D templates that provide the cells with an appropriate environment for tissue formation and regeneration.[50] Graphene-based scaffolds are mainly prepared by combining the graphenic component with other biomaterials to improve their features (Figure 6, Table 1). However, there have been a few studies using the GFMs to fabricate standalone 3D structures. For instance, porous graphene foams were prepared by growing graphene onto 3D nickel scaffolds, and subsequently removing the nickel through FeCl3 etching. These 3D graphenic foam constructs served as substrates to grow human MSCs and were shown to promote MSC attachment, viability, and spontaneous osteogenesis through increased osteocalcin (OCN) and osteopontin (OPN) expression.[51] Multilayered graphene hydrogel membranes were also fabricated as bulk films for guided bone regeneration. In vitro culture of the membranes with rat bone marrow MSCs (BMSCs) promoted cell attachment, proliferation, and enhanced and accelerated osteogenesis. The membranes were subsequently implanted into rat calvarial defects as barriers to prevent the in-growth of connective tissue and allow new bone to form. Implantation promoted substantial new bone formation at a faster rate compared to conventional titanium (Ti) membranes that are clinically used. The newly formed bone exhibited a uniform mature lamellar structure that was found to be substantially greater in quantity and quality compared to the Ti membrane groups. Additionally, these membranes were implanted subcutaneously in rats to evaluate the host tissue response and biocompatibility. The membranes produced minimal fibrous capsule formation and a mild host tissue response with no obvious toxic side effects, demonstrating the potential of these membranes for guided bone regeneration therapy.[52]
Figure 6.
Advantages of using graphene-based biomaterials as scaffolds for bone regeneration.
Table 1.
Graphene-based materials as scaffolds for bone regeneration.
| Scaffold | Highlights | Refs. |
|---|---|---|
| Multilayered graphene hydrogel membranes | The membranes accelerated and enhanced osteogenesis in MSCs by increasing ALP activity and OCN protein expression in vitro. In vivo implantation of the membranes in rat calvarial defects for guided bone regeneration promoted osteogenesis and accelerated mineralization, allowing mature lamellar bone to form. |
[52] |
| PLGA/graphene nanoplate films | GO incorporation enhanced the adhesion, proliferation, and osteogenic differentiation of MSCs via increased ALP activity, calcium mineral deposition, and expression of COL1A1, OPN, BMP2, OCN, and RUNX2 genes possibly through activating the PI3K/Akt/GSK-3β/β-catenin pathway. In vivo, the composite films promoted greater alveolar bone regeneration after 8 weeks in SD rats. |
[45b] |
| Collagen/GO sponges | Covalent conjugation of GO flakes onto collagen sponges enhanced MSC proliferation and osteogenic differentiation by increasing calcium deposition and the expression of RUNX2, ALP, and OPN genes through the ROCK pathway. | [47] |
| Chitosan/GO freeze-dried scaffolds | Incorporation of 3 wt% GO into chitosan scaffolds significantly increased new bone formation by both promoting the early and late stages of bone regeneration. The increased infiltration of cells into the scaffolds and early expression of RUNX2 and BMP facilitated the differentiation of osteoprogenitors, whereas the elevated expression of OPN and OCN at later stages enhanced new bone formation. | [57] |
| 3D printed PCL/graphene scaffolds | The addition of graphene into PCL scaffolds increased protein adsorption, and MSC adhesion and proliferation. In vivo, the composite scaffolds enhanced and accelerated bone formation and remodeling. |
[58d] |
| PLLA/graphene nanofibrous scaffolds | Incorporation of 1 or 3 wt% of graphene into PLLA nanofibrous scaffolds promoted osteogenic differentiation in MSCs through increased ALP activity, calcium deposition, and type I collagen production. Upon implantation in muscle pouches of nude mice, 3 wt% PLLA/graphene promoted vascularized bone formation and substantially increased type I collagen production. |
[60b] |
| Poly(ethylene glycol) diacrylate/GO cryogels | GO inclusion increased matrix mineralization and the expression of RUNX2, OCN, COL1, and ALP in MSCs. Implantation of the cryogels into calvarial defects of Balb/c-nude immunodeficient mice increased the bone regeneration volume and surface and led to greater collagen matrix production. |
[63] |
| GO-coated collagen sponges | GO coating improved the resistance of the scaffolds to degradation and their adsorption of proteins and calcium ions. In canine extraction sockets, GO coating promoted new bone formation and in canine periodontal class II furcation defects, the coating promoted alveolar bone healing and periodontal ligament-like tissue and cementum-like tissue formation. |
[55,66] |
| rGO–HAp composites | The addition of rGO increased MSC osteogenic differentiation through increasing ALP activity, calcium mineral deposition, and expressions of OPN and OCN proteins. In rabbit calvarial defects, rGO inclusion led to greater new bone formation. | [69] |
The more common approach in utilizing the GFMs for bone tissue regeneration has been to combine them with other biomaterials, synthetic or natural, through a variety of methods. When graphene nanoplates were incorporated into poly(lactic-co-glycolic acid) (PLGA) films fabricated through solution-casting, the composite films promoted greater adhesion, proliferation, and osteogenic differentiation of rat BMSCs. There was greater alkaline phosphatase (ALP) activity, calcium mineral deposition, and expression of osteogenic genes such as collagen type 1 alpha 1 (COL1A1), OPN, BMP2, OCN, and runt-related transcription factor-2 (RUNX2) due to the addition of the graphene nanoplates.[45b]
Collagen is one of the most widely used natural biopolymers in biomedical applications. Collagen-based scaffolds are biocompatible, biodegradable, and biologically active and exhibit low immunogenicity; however, they suffer from inadequate mechanical strength making them inapt for bone applications.[53] To overcome mechanical inadequacy, GO flakes were covalently conjugated onto 3D collagen sponges via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) crosslinking. The addition of GO increased the elastic modulus of the collagen scaffolds by more than 2.5-fold to ≈38.7 kPa. GO incorporation further increased the proliferation and osteogenesis of human MSCs via promoting matrix mineralization and the expressions of RUNX2, ALP, and OPN genes. The enhanced osteogenic differentiation was found to be mediated through the adhesion of the MSCs to the stiffer GO–collagen substrates and the Rho-associated kinase (ROCK) mechanotransduction pathway. Incorporating the GO flakes noncovalently onto the collagen sponges also improved the proliferation and osteogenic differentiation of the human MSCs, but had no significant effect on the elastic moduli of the structures.[47] In another study, GO was introduced into collagen scaffolds to improve their biomineralization capacity. Soaking the collagen–GO scaffolds in SBF for 7 days resulted in a homogenous distribution of bone-like apatite with a Ca/P ratio that was similar to natural bone. This led to significantly greater bone formation in rat cranial defects with a twofold increase in bone volume and density after 12 weeks.[54] Coating GO onto collagen sponges similarly promoted greater new bone formation in rat cranial defects. Interestingly, the residual scaffold area in the GO-coated collagen sponges was 3.6-fold lower than that of collagen sponges, suggesting that the presence of GO in the collagen scaffolds stimulated scaffold degradation.[55]
GO has also been combined with natural biopolymers other than collagen. Inclusion of GO at 0.25% (w/v) in chitosan/gelatin scaffolds prepared through freeze-drying promoted osteogenic differentiation of C3H10T1/2 mouse MSCs through upregulating RUNX2, the master transcription factor of osteogenesis, and osteoblast marker genes such as ALP, COL1, and OCN. When implanted in rat tibial defects, the chitosan/gelatin/GO scaffolds initiated bone healing and formation in as early as 2 weeks and increased collagen deposition compared to the chitosan/gelatin scaffolds.[56] The osteogenic capacity of chitosan/GO (0.5 and 3 wt%) scaffolds was assessed in vivo in critical-sized mice calvarial defects for up to 18 weeks. GO incorporation, especially at 3 wt%, promoted significantly greater new bone formation in the defects. The proregenerative effects of GO were found to be imparted from 72 h postsurgery when the presence of GO improved cell infiltration into the scaffolds and initiated BMP and RUNX2 expression. The expression of these early osteogenic markers increased up until week 4 upon which scaffold resorption and bone restoration started to take place and the cells begun to express osterix (OSX) and deposit calcium phosphate crystals. After 8 weeks, the scaffolds had mineralized and late osteogenic markers typical for bone matrix such as OPN and OCN were elevated. This led to significantly greater new bone formation at 18 weeks postimplantation that was characteristic of collagen-enriched woven bone.[57]
In addition to the different materials that the GFMs have been combined with, there are various fabrication techniques to produce composite graphene-based structures. 3D printing is a state-of-the-art technology that enables the fabrication of complex structures through a layer-by-layer deposition process. The GFMs have been incorporated into various inks to produce composite 3D printed structures with improved surface roughness, hydrophilicity, and mechanical properties, which can better support protein adsorption and cell adhesion, proliferation, and osteogenic differentiation.[58] The inclusion of small amounts of graphene (0.78 wt%) into 3D printed PCL/graphene scaffolds improved bone formation in calvarial defects of male Wistar rats. The produced scaffolds were electrically active and found to be most effective upon in vivo microcurrent stimulation. There was accelerated and improved new bone tissue formation and remodeling taking place with organized and mature bone forming after 2 months.[58d] Alternatively, 3D printed scaffolds with higher contents of graphene (>32 wt%) have been fabricated, which have also shown to be both mechanically stable and electrically conductive for tissue regeneration purposes.[59]
Graphene and its derivatives have further been employed to enhance the performance of nanofibrous scaffolds. The incorporation of the GFMs into nanofibrous structures reduces fiber diameter and improves the porosity, hydrophilicity, thermal stability, mechanical properties, and biological activity of the matrices.[34a,b,60] The osteogenic effects of graphene and carbon nanotubes were compared by incorporating them into poly-l-lactide acid (PLLA) nanofibrous scaffolds fabricated through thermal-induced phase separation. The inclusion of both graphene and carbon nanotubes at 1 and 3 wt% increased ALP activity, calcium deposition, and type I collagen production in mouse BMSCs, with graphene demonstrating stronger osteoinstructive effects. In vivo implantation of the BMSC-seeded scaffolds in muscle pouches of nude mice revealed the ability of both carbon nanomaterials to promote vascularized bone formation, with the 3 wt% PLLA/graphene scaffolds, in particular, enhancing the synthesis of type I collagen. Of note, there was no obvious necrosis, inflammatory response, or fibrous membrane formation elicited in any of the groups.[60b] When GO was incorporated into electrospun poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/GO nanofibrous scaffolds, the composite nanofibers significantly increased the expression of osteogenic genes such as RUNX2, ALP, OCN, OPN, BMP2, and BMP4 in rat BMSCs. Implantation of the scaffolds into rat calvarial defects significantly increased bone volume and density in the composite groups at both 4 and 8 weeks. By 8 weeks, the defect had almost fully regenerated and more mature organized woven bone and cortical-like lamellar bone had formed, with some evidence of blood vessels and bone marrow space. Moreover, there were no visible signs of inflammation or a severe immunological response in any of the groups.[34b]
Hydrogels also display improvements in their physicochemical and biological properties upon GO incorporation. The inclusion of GO into chitosan/glycerophosphate thermosensitive hydrogels improved their protein adsorption, swelling behavior, and biomineralization capacity, as well as their ability to promote osteogenic differentiation in MSCs.[61] In injectable thermosensitive hydrogels of poly(polyethylene glycol citrate-co-N-isopropylacrylamide) and gelatin (PPCNg), the addition of GO maintained the thermoresponsive behavior of the hydrogels and increased ALP activity and osteogenic gene expression in mesenchymal progenitor immortalized mouse adipose-derived cells (iMADs). Subcutaneous injection of the composite hydrogels in BMP9-transduced iMADs in athymic nude mice resulted in well-mineralized, highly vascularized trabecular bone that was significantly more mature and denser than the PPCNg hydrogels.[62] Incorporation of GO into poly(ethylene glycol) diacrylate (PEGDA)-based cryogels similarly promoted osteogenic differentiation in human tonsil-derived MSCs, which was measured by the increased deposition of calcium and higher expressions of RUNX2, OCN, COL1, and ALP genes. The higher concentrations of GO (0.5 and 1 mg mL−1) within the cryogels were accompanied with a loss in cell viability to less than 60% and thus the concentrations of 5 and 10 μg mL−1 were determined optimal. Implantation of these MSC-seeded cryogels into calvarial defects of Balb/c-nude immunodeficient mice revealed greater bone regeneration and collagen I production in the PEGDA–GO groups.[63] In another study, low oxygen content graphene nanoparticles were mixed with adipose-derived stem cells (ADSCs) and implanted into a unicortical rat tibial bone defect using agarose as an inert scaffold. After 45 days, the defects treated with the nanoparticles + ADSCs showed marked healing and new mineralized bone formation with more evidence of vasculature, implying the possible osteoinductive and osteoconductive effects of graphene on stem cell-driven bone formation.[64]
In addition to their use in bone biomaterials, graphene-based materials have been employed in dental scaffolds. The use of the GFMs in dental scaffolds influences the osteogenic and odontoblastic differentiation of dental pulp stem cells and periodontal ligament stem cells, making them suitable for use in dental tissue regeneration.[65] Implantation of GO-coated collagen sponges into the tooth extraction sockets of beagle dogs significantly promoted new bone formation in as early as 2 weeks. Further implantation of these scaffolds in periodontal class II furcation defects in beagle dogs corroboratively demonstrated the capacity of GO to improve alveolar bone healing and periodontal attachment repair.[55,66] Additionally, PLGA/graphene nanoplate films were shown to induce greater alveolar bone regeneration after 8 weeks in Sprague-Dawley rats,[45b] highlighting the promising use of graphene-based composites for dental applications.
Graphene Inorganics:
A large body of work on the use of the GFMs for bone regenerative engineering has been to combine them with osteoinstructive ceramics such as CaPs, including HAp, tricalcium phosphates (TCP), biphasic calcium phosphates, calcium sulfates, and bioactive glass. These materials are extensively used in various bone graft substitutes; however, in general, suffer from their brittleness, poor ductility, and high insolubility. Many studies have investigated using the GFMs to improve the mechanical and biological properties of these inorganics. The addition of the GFMs has shown to improve their stiffness, fracture toughness, and hardness.
HAp is the most commonly used CaP in orthopedic and dental applications. HAp is the main inorganic component of bone tissue that is well-recognized for its biocompatible, bioactive, osteoconductive, and in some cases, osteoinductive properties.[28b,67] Due to these properties and ease of fabrication, GFM–HAp composites are the most widely studied area in graphene-inorganic composites. GFM–HAp composites are fabricated through various techniques, including in situ synthesis methods, biomimetic mineralization, mechanical mixing, hydrothermal synthesis, and chemical vapor deposition, and are further densified into 3D structures through several sintering methods such as hot-press sintering, spark plasma sintering, and selective laser sintering.[38a,68]
The methods by which GFM–HAp composites are synthesized heavily affects their microstructural characteristics, mechanical properties, and biological activity. One study compared GO–HAp composites that were fabricated by in situ sol–gel synthesis to those created through biomimetic mineralization via SBF incubation. In situ GO–HAp synthesis resulted in spindle-like HAp nanoparticles that were decorated randomly and uniformly on the surface area and edges of the GO sheets. The high specific surface area and oxygen functional groups of GO led to the formation of strong interactions between the HAp nanoparticles and GO. Conversely, when the composites were fabricated through biomimetic treatment, a layer of amorphous CaP formed on the GO sheets, with the CaP particles being nanosphere-like and not nanocrystalline. These microstructural differences between the composites subsequently had different effects on the proliferation and osteogenic differentiation of MSCs. Whereas the GO–HAp composites prepared via biomimetic treatment sustained cell viability and proliferation, the in situ synthesized composites promoted greater ALP activity. These diverse effects demonstrate the different implications that GFM–HAp composites have, which can be harnessed depending on the intented application.[49a] Reduced graphite oxide–HAp (rGtO–HAp) nanocomposites synthesized via liquid precipitation had nanorod-like HAp grains that displayed oriented nucleation and growth on the rGtO sheets. The (300) plane of the HAp crystals formed a coherent interfacial bond with the graphene wall and the (002) plane built a strong interface with the cross-section of the rGtO sheets. When the nanocomposites where consolidated into pellets with spark plasma sintering, these strong and coherent interfacial bonds enhanced the densification of the composites and precluded the growth of the HAp grains, and thereby enhanced the stiffness, toughness, and hardness of the composites.[31a]
The GFMs have further been combined with preassembled HAp powder to prepare composite structures. rGO–HAp composites were fabricated by mixing rGO and HAp at a 1:1 ratio in deionized (DI) water, followed by vigorous vortexing and airdrying. The resultant composites synergistically enhanced ALP activity, calcium deposition, and the expressions of OPN and OCN in MC3T3-E1 preosteoblastic cells. Implantation of the composites as bone graft pastes into rabbit calvarial defects significantly increased new bone tissue formation.[69] Using rGO as a coating for HAp particles can also markedly improve the osteogenic differentiation of human MSCs.[70]
Various 3D composite scaffolds comprising HAp and the GFMs have been evaluated for bone applications. rGO-loaded porous HAp scaffolds promoted greater new bone formation and integration with host bone in a rat femoral defect. The repaired femur displayed greater mechanical strength and a higher breaking strength signifying the efficiency of the reparative process.[71] Porous scaffolds of rGO with various amounts of nano-HAp (20, 40, and 80 wt%) were fabricated via temperature-driven selfassembly. The composite scaffolds of 20 wt% rGO–nano-HAp significantly enhanced the proliferation and osteogenic differentiation of rat BMSCs compared to rGO and the other composite scaffolds. Implantation of the 20 wt% composite scaffolds into rabbit calvarial defects enhanced collagen deposition and mineralization, resulting in the quicker formation of a new integrated and mature bone matrix.[72] In another study, graphene–HAp hydrogels were prepared via self-assembly and through entrapping colloidal HAp nanoparticles into the 3D graphene network. The resulting hydrogels had a uniform deposition of HAp nanoparticles and were highly porous, strong, electrically conductive, and biocompatible, making them suitable nanocomposites for bone regeneration.[73]
GO–HAp composites have also been used collectively in combination with polymeric scaffolds. To improve the interfacial bonding between polyetheretherketone (PEEK) and HAp, GO was used as an interfacial phase. The high specific surface area and out-of-plane π bonds of GO facilitated π–π stacking interactions with the benzene rings in PEEK, whereas the negative charge of the oxygen functional groups allowed adsorption of the positively charged calcium atoms in HAp. This reduced the agglomeration of HAp nanoparticles in the polymer matrix, thereby improving the compressive strength and stiffness of the scaffolds.[74] Highly crystalline HAp nanowhiskers were synthesized at GO surfaces via microwave-assisted biomimetic mineralization, and dispersed into polylactic acid (PLA) matrices for mechanical enhancement. The strong interactions of GO–HAp, along with the high density of the oxygen functional groups, led to excellent GO–HAp exfoliation and uniform dispersion in the PLA matrices, even at high filler contents of 30 wt% GO–HAp. This resulted in composite films with higher tensile strength and toughness values of 105 MPa and 2.9 MJ m−3, respectively.[75] GO inclusion in electrospun PLGA/GO/HAp nanofibrous scaffolds reduced fiber diameter and improved the hydrophilicity, protein adsorption, and tensile strength. The PLGA/GO/HAp scaffolds further promoted the proliferation and osteogenic differentiation of MC3T3-E1 cells compared to PLGA/HAp and PLGA/GO matrices, demonstrating their efficacy as scaffolds for bone regeneration.[76] The addition of GO to electrospun PCL/silicate-doped nano-HAp scaffolds was also shown to increase protein adsorption, and the adhesion, spreading, proliferation, and ALP activity of human osteosarcoma Saos-2 cells.[77]
Other types of CaPs have also been combined with the GFMs. When GO was coated onto β-TCP disks, it significantly enhanced the viability, ALP activity, and expression of osteogenic genes (RUNX2, OPN, OCN, and COL1) in human bone marrow stromal cells in vitro and significantly increased the rate of new bone formation in rabbit critical-sized calvarial defects in vivo.[45a] The inclusion of GO into Pluronic F-127-coated GO–CaP nanoparticles enhanced the osteogenic differentiation of human MSCs by increasing the expressions of OCN and ALP and calcium mineral deposition.[78]
The GFMs have been combined with calcium silicates, one of the promising bioactive materials for bone regeneration. rGO addition to calcium silicate composites fabricated hydrothermally followed by hot isostatic pressing increased their stiffness, hardness, and fracture toughness through toughening mechanisms of crack bridging, crack branching, crack deflection, and rGO pull-out. At higher concentrations, however, the addition of rGO hampered grain densification causing greater porosity in the composites, which together with the greater agglomeration of the rGO sheets reduced the overall density and mechanical properties of the structures. The incorporation of rGO also enhanced the attachment, proliferation, and ALP activity of human osteoblast cells.[31b] When various ratios of graphene (0.25, 0.5, and 1.0 wt%) were incorporated into calcium silicate cements, it was revealed that although 1 wt% hindered apatite precipitation, it improved the mechanical and biological performance of the cements. At 1 wt%, there was greater proliferation and osteogenic differentiation in human MSCs through increased COL1 secretion, ALP activity, calcium deposition, and the expressions of OPN and OCN proteins.[79]
3.2.2. As Coatings for Implants
Graphene and its derivatives have been used to coat and modulate the surface behavior of various orthopedic implants (Table 2). Coating with the GFMs or compositions of the GFMs and other materials improves the durability and mechanical properties, producing implants that are better equipped to withstand the load-bearing environment of bone tissue. Surface features such as roughness, wettability, biomineralization capacity, and protein and cell adsorption can be enhanced, which contribute to an improved bone–implant interface. Additionally, the osteoconductive and osteoinductive properties of the GFMs support new bone formation and osseointegration with the host bone, leading to more efficacious bone implants (Figure 7).
Table 2.
Graphene-based materials as coatings for implants for bone regeneration.
| Implant | Highlights | Refs. |
|---|---|---|
| Graphene-coated CoCrMo | Graphene was uniformly distributed and tightly attached to the surface, and its presence as a coating promoted the growth and proliferation of MSCs. | [81] |
| GO-coated quartz | GO coating improved the biomineralization capacity of the substrates and improved ALP activity and OCN protein secretion in preosteoblastic cells. | [80] |
| GO-coated Ti6Al4V | GO coating increased surface roughness and hydrophilicity, and promoted the adhesion, proliferation, and osteogenic differentiation of MSCs via promoting ALP activity, calcium deposition, and the expressions of ALP, BMP2, COL1A1, OCN, and RUNX2 genes. | [84] |
| GO-coated NiTi | GO coating improved protein adsorption, and the adhesion, spreading, proliferation, and osteogenic differentiation of preosteoblastic cells. GO coating also elicited strong antibacterial activities. | [86] |
| GO/HAp-coated Mg | GO/HAp coating increased the hydrophilicity and biomineralization capacity of the implants and improved their biodegradation behavior. | [89] |
| GO/HAp-coated Ti | GO/HAp coating adhered strongly to Ti and improved its corrosion resistance and biomineralization capacity. The coating also promoted the proliferation of osteoblast-like cells. | [88] |
Figure 7.
Advantages of using graphene-based biomaterials as coatings for implants for bone regeneration.
A multitude of implants such as Ti alloys, cobalt chromium molybdenum (CoCrMo), nitinol (NiTi), and quartz have been coated with the GFMs or GFM-based composites. When GO was coated onto quartz substra, it enhanced the biomineralization capacity of the implants and increased ALP activity and OCN secretion in MC3T3-E1 cells.[80] Graphene coating on CoCrMo alloys also promoted the viability and proliferation of mouse BMSCs while proving to be stable and tightly attached to the substrate.[81] GO-coated porcine bone granules were used in a sheep model of mandibular bone regeneration. While the addition of GO did not appear to improve the efficacy of new bone formation, it did demonstrate good biocompatibility with the surrounding soft and hard tissue.[82]
Ti alloys have been widely used as implants for a variety of bone defects and conditions.[83] Coating Ti6Al4V alloys with GO improved their hydrophilicity and surface roughness and facilitated the growth, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. In vitro, GO coating increased ALP activity, calcium mineral deposition, and the expression of osteoblast markers such as ALP, BMP2, COL1A1, OCN, and RUNX2.[84] GO coating on Ti membranes for guided bone regeneration significantly stimulated ALP activity in MC3T3-E1 cells and led to greater new bone formation after 8 weeks in a rat critical-sized calvarial bone defect in vivo.[85] Coating of NiTi alloys with GO or gelatin functionalized with GO improved protein adsorption, and the attachment, growth, and osteogenic differentiation of MC3T3-E1 cells.[86] One study fabricated graphene directly onto NiTi alloys by chemical vapor deposition and found the growth of the graphene layer to be independent of Ni but rather correlated with the formation of the TiC phase. The formed graphene layer increased the osteogenic differentiation of MSCs through increasing the expressions of RUNX2, OCN, OPN, and BMP2.[87]
GO has also been combined with inorganic materials as coatings to synergistically enhance the surface properties of implants. The addition of GO into HAp coatings on Ti substrates increased the coating adhesion and implant-coating bonding strength. Compared to pure HAp coatings, the composite GO/HAp coatings prevented the formation of surface cracks and improved the corrosion resistance of the implants. Moreover, the addition of GO induced apatite layer formation, increased the crystallinity of the apatite particles, and improved the proliferation of osteoblast-like MG63 cells.[88] Coating magnesium implants with GO/HAp coatings similarly improved implant surface features such as wettability, degradability, and apatite layer formation.[89] Various ratios of graphene (0.5, 1.5, and 4 wt%) were incorporated into calcium silicate coatings that were vacuum plasma sprayed onto Ti6Al4V substrates. At 1.5 wt%, the graphene sheets were homogeneously distributed within the matrix, improving the wetting behavior and mechanical properties of the implants. The high wear resistance observed in the composite coatings was attributed to the uniform dispersion of the graphene sheets, their interfacial bonding with calcium silicate, and the improvements in implant microhardness. Subsequent implantation of these implants in a rabbit femur condyle defect stimulated new bone formation; however, no significant difference was found in the bone–implant contact ratio when compared to pure calcium silicate coatings.[90]
3.2.3. As Vehicles for Controlled Delivery
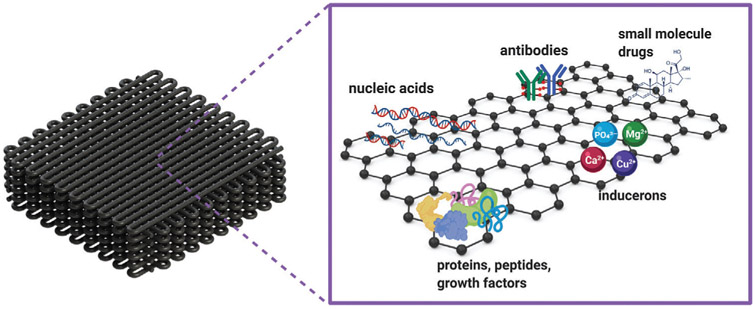
Graphene and its derivatives can be used for the loading and controlled delivery of signaling and therapeutic agents from biomaterials (Figure 8, Table 3). The high specific surface area and aromatic structure of pristine graphene allow for the high-density loading of hydrophobic drugs via π–π stacking, and electrostatic and hydrophobic interactions.[25] The presence of oxygen functional groups in GO further provides a vast range of chemical reactions and functionalization opportunities that can be exploited to tune the properties of GO.[20,25] GO is in fact considered an amphiphile due to its hydrophilic edges and more hydrophobic basal plane.[91] Therefore, in addition to the noncovalent interactions of the unmodified hydrophobic areas of graphene, the polar functional groups enable covalent bonding, hydrogen bonding, and polar interactions.[19a,25] The carboxylate groups also introduce a pH-responsive behavior that can be used for the delivery of drugs with pH-dependent solubility.[92]
Figure 8.
Applications of graphene-based materials as drug delivery vehicles for bone regeneration.
Table 3.
Graphene-based materials as delivery vehicles for bone regeneration.
| System | Highlights | Refs. |
|---|---|---|
| BMP2 mixed with GO powder in PBS | GO retained BMP2 upon subcutaneous injections in mice, resulting in localized and controlled bone formation at the injection sites. | [97] |
| BMP2-loaded GO-coated Ti | GO coating enabled a higher adsorption and sustained release of BMP2 while preserving its structure and bioactivity. This resulted in greater osteogenic differentiation of MSCs and increased expressions of RUNX2, ALP, and OCN genes in vitro, and greater in vivo bone regeneration in mouse calvarial defects. | [93a,b] |
| BMP2-loaded GO flakes suspended in fibrin gel | BMP2 delivery using GO reduced the effective dose for bone regeneration by 50% compared to that of fibrin gel + BMP2. | [93c] |
| DEX-loaded rGO-coated Ti | DEX delivery using rGO coating improved the osteogenesis of MSCs via increased ALP activity, calcium deposition, and expressions of RUNX2, OPN, COL1, and OCN genes. | [99] |
| DEX-loaded GO- and rGO-coated Ti implants | GO coating enabled a higher loading of DEX compared to rGO coating, resulting in greater osteogenic differentiation of MSCs and enhanced ALP activity, calcium deposition, and OPN and OCN expression. | [100] |
| Vancomycin-loaded rGO/HAp scaffolds | The rGO/HAp scaffolds enabled an initial burst release followed by an extended release that achieved prolonged antibacterial activity in vitro. The drug-loaded scaffolds were able to control the infection and promote bone regeneration in a rabbit infected radial bone defect model. | [102] |
| GO–Cu nanocomposite-coated CaP scaffolds | The GO–Cu nanocomposites enabled a sustained release of Cu2+ ions, which promoted osteogenesis in MSCs in vitro and vascularized bone formation in rat calvarial defects in vivo. | [46] |
| GO–Sr nanocomposites crosslinked to collagen scaffolds | The GO–Sr nanocomposites demonstrated a sustained release of Sr2+ ions, which promoted osteogenesis and angiogenic activities in vitro and in vivo. In a rat calvarial defect model, there was significantly greater vascularized bone regeneration and greater expression levels of RUNX2, OCN, OPN, and CD31-positive vessels. | [106] |
| GO functionalized with Ca2+, PO43−, Li+, Mg2+, K+, and Na+ | GO functionalized with Ca2+ and PO43− ions induced osteogenesis in MSCs in vitro and de novo bone formation in a mouse subcutaneous model when injected with bone marrow stromal cells. | [97] |
Among the different growth factors that have been investigated for controlled delivery using the GFMs, BMP2 is the most widely studied. BMP2 is a potent osteoinductive growth factor that is clinically approved for inducing bone formation and thus has naturally been extensively evaluated for delivery using the GFMs. The capabilities of GO as a carrier for the loading and delivery of BMP2 was investigated in a series of studies by La et al.[93] GO coating on Ti substrates enabled the loading of large doses of BMP2 with a sustained release profile while preserving the structure and bioactivity of the growth factor. This translated into enhanced osteogenic differentiation of human BMSCs in vitro and greater new bone formation in mouse calvarial defects in vivo.[93a] The GO-coated Ti was further used for the co-delivery of BMP2 and substance P, a peptide involved in MSC homing and recruitment to injury sites.[94] Dual delivery of the molecules resulted in significantly greater bone formation in mouse calvarial defects, which was attributed to the capability of substance P to recruit the MSCs and BMP2 to induce osteogenic differentiation in the recruited cells.[93b] In a subsequent study, BMP2-loaded GO flakes were suspended in fibrin gels (GO/F) to assess whether GO could reduce the effective BMP2 dose required for regeneration, so as to avoid the undesirable side effects caused by using high doses of BMP2. Delivery of BMP2 with GO resulted in enhanced osteogenic differentiation of human BMSCs in vitro compared to delivery with fibrin gel alone (F). When implanted in mouse calvarial defects, a half-dose of BMP2 delivered with GO/F resulted in bone regeneration similar to that resulting from a full dose of BMP2 delivered by F. This higher efficacy in bone regeneration was likely due to the high loading, sustained release, higher structural stability, and higher bioactivity of the BMP2 that was delivered by GO/F.[93c]
Other studies using GO for BMP2 delivery have used composite electrospun scaffolds or microspheres and have shown that the inclusion of GO facilitates the adsorption of BMP2, which in turn promotes the attachment, proliferation, and osteogenic differentiation of progenitor cells.[95] One study circumvented the lack of functional groups in graphene for growth factor functionalization by modifying the surface of 3D graphene foams with heparin-dopamine molecules. The modification significantly improved the binding ability, loading, and sustained delivery of BMP2. As such, the BMP2-loaded treated graphene foams increased ALP activity, calcium deposition, and the expression of osteogenic genes such as RUNX2, bone sialoprotein (BSP), and OCN.[96] We have shown that mixing GO powder with BMP2 through simply pipetting is sufficient to retain the BMP2 in its bioactive form for transcutaneous injections. When GO+BMP2 was injected subcutaneously in mice, de novo bone was formed at the site of injection in a localized and controlled manner, underscoring the growth factor binding capabilities of GO.[97]
DEX is a synthetic glucocorticoid that has excellent binding capabilities to graphene through the π–π stacking of its aromatic rings with the basal plane of graphene. Moreover, it is a hydrophobic drug that can be stably loaded onto graphene-based substrates to improve long-term osteogenesis and osseointegration, especially in implants.[40,98] DEX loading onto rGO-coated Ti alloys improved ALP activity and calcium deposition, and up-regulated the expressions of RUNX2, OPN, OCN, and COL1 genes.[99] More recently, it was shown that GO-coated Ti implants adsorb a higher amount of DEX compared to their rGO-coated counterparts, and can thus promote greater osteogenic differentiation in BMSCs.[100]
Antibiotics can also be delivered using graphene-based scaffolds. Infected bone defects are a serious orthopedic challenge that are often a result of severe open fractures. The segmental damage to bone tissue, surrounding soft tissue and blood supply, together with the propagation of bacterial infection, are all contributing factors that limit bone regeneration.[101] The effective antibacterial agent, vancomycin, was incorporated into 3D porous scaffolds that were composed via the self-assembly of rGO and HAp. The π–π stacking between graphene and vancomycin enabled an initial burst release of vancomycin for an efficient initial eradication of the infection that was followed by a sustained drug release to ensure long-term antibacterial effects. In a rabbit radius model of infected bone defects, implantation of the vancomycin-releasing scaffolds lowered infection markers, including elevated white blood cell count and C-reactive protein, reduced tissue inflammation, and improved wound healing. Suppressing infection successfully promoted bone regeneration in the radius bone as there was greater new bone formation, bone volume, and bone mineral density in the treated defects.[102] In another study, BMP2 and vancomycin were encapsulated in gelatin microspheres that were then anchored onto GO–Ti scaffolds for a dual effect. This delivery method enabled the sustained release of both drugs to efficiently promote bone regeneration and prevent bacterial infection.[103]
An interesting approach in using the GFMs for controlled delivery has been to functionalize them with inorganic signaling agents such as osteoinstructive inducerons to augment bone regeneration.[104] Copper (Cu)-decorated rGO nanoparticles embedded into PCL scaffolds enabled a steady release of Cu2+ ions compared to PCL–Cu scaffolds, which led to improvements in the biological activity of the matrices by imparting osteogenic and angiogenic effects.[105] GO–Cu nanocomposites coated onto porous CaP scaffolds similarly displayed a long-term sustained release of Cu2+ ions. GO–Cu coating increased osteogenesis in rat BMSCs in vitro and promoted vascularized bone formation in a rat critical-sized calvarial defect in vivo.[46] GO–strontium (GO–Sr) nanocomposites have also been fabricated and loaded onto collagen scaffolds to achieve a sustained release of Sr2+ ions. These scaffolds promoted osteogenesis in MSCs and endothelial migration and tube formation in human umbilical vein endothelial cells (HUVECs) in vitro. In vivo implantation in rat calvarial defects led to significantly greater new bone and blood vessel formation in the defects, with higher expression levels of RUNX2, OCN, OPN, and CD31-positive vessels.[106] Incorporation of rGO–Sr nanoparticles into PCL scaffolds similarly improved osteogenesis by increasing the proliferation and calcium deposition of preosteoblastic MC3T3E-1 cells.[107]
In addition to creating nanocomposites of the GFMs with various ions, intrinsically osteoinductive nanomaterials can be prepared by functionalizing GO to produce osteomimetic phosphate graphenes (PGs). The PGs contain covalently bound polyphosphate chains that are balanced with counterions (Ca2+, K+, Li+, Mg2+, or Na+), which are released over time and act as signaling molecules. Calcium phosphate graphene (CaPG), in particular, induced osteogenesis in both osteogenic media and regular growth media in vitro through promoting ALP activity, calcium deposition, and the expression of osteogenic genes. Furthermore, and when combined with bone marrow stromal cells, CaPG induced de novo osteogenesis ectopically in the subcutaneous space of mice. The new bone was produced by the contributions of both host and donor cells. There was calcium mineral deposition, ALP-positive osteoblasts, and TRAP-positive osteoclasts throughout the implant, indicating the active resorption and remodeling of the implants that was taking place.[97,108]
4. Biocompatibility and Biodegradability Considerations of Graphene and Its Derivatives
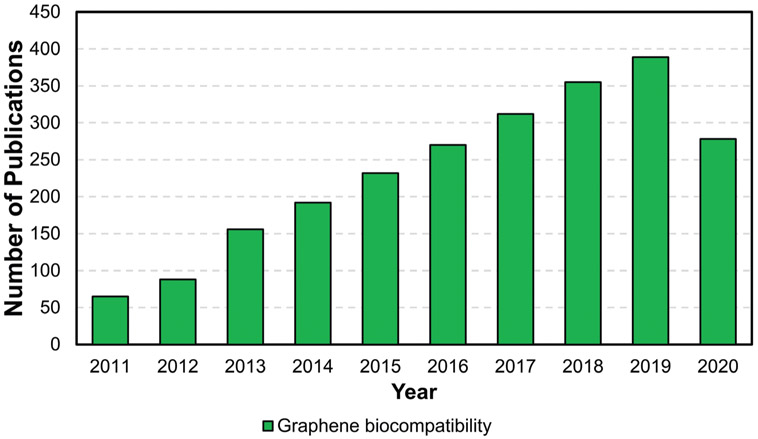
For materials to be safely used in clinical settings, their biocompatibility, biodistribution, and biodegradation must be carefully assessed. As the number of publications on the use of graphene-based biomaterials for tissue regeneration continues to grow (Figure 2), investigative studies into the safety and biocompatibility of this new class of nanomaterials is also increasing (Figure 9). Given the importance of this topic, the second half of this review focuses on surveying the current available literature on the biological interactions of the GFMs with living organisms and biological systems. We assess the different factors that impact these interactions and that play a role in determining the biocompatibility and biodegradation of the GFMs. A summary of these factors is summarized in Figure 10.
Figure 9.
Publication trend on the biocompatibility of graphene-based materials (data obtained from PubMed; search strings: “graphene,” “biocompatibility”).
Figure 10.
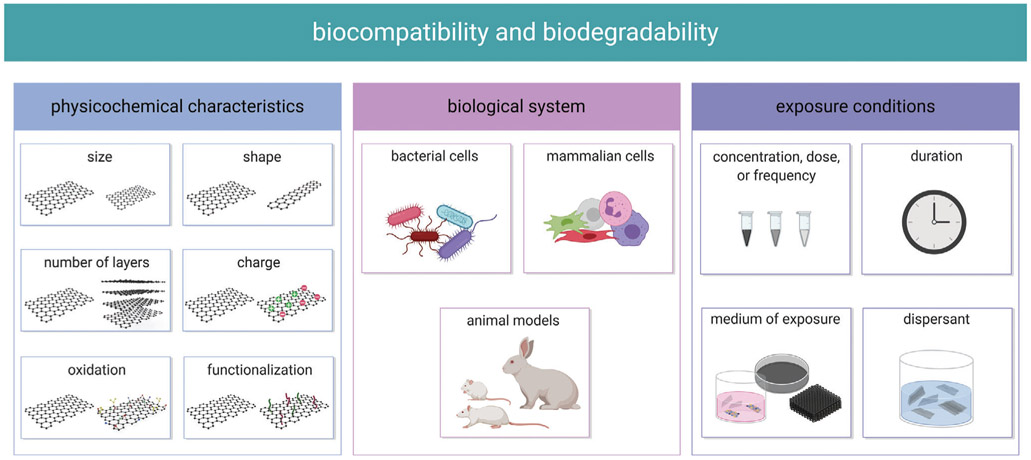
Factors influencing the biocompatibility and biodegradability of the GFMs.
4.1. Biocompatibility Considerations
The biocompatibility of the GFMs is governed by their inherent physicochemical characteristics and the conditions in which they are exposed to the biological system of study. Physiochemical properties such as size, shape, and surface chemistry may vary depending on the raw materials used, or the manufacturing and purification methods employed (Figure 10). The biological system of study itself is important in that it can affect the outcome. Dose and duration of exposure inherently are factors that influence the biocompatibility and safety profile of a material. Further, the medium of exposure may alter the physicochemical properties of the GFMs and thus alter the biological interactions. These factors collectively determine the biocompatibility of the GFMs, and although the precise role of each factor is not yet fully understood, we are beginning to elucidate their effects.
4.1.1. In Vitro Biocompatibility: Bacterial Cells
The GFMs are recognized as a promising class of antibacterial agents that exhibit strong biocidal effects. They can be used either alone as single components, in combination with other antibacterial agents such as silver, or composited within other materials.[109] A three-step antibacterial mechanism was proposed for the GFMs similar to that of carbon nanotubes. First, bacterial cells adhere and deposit onto the GFMs. Second and upon direct contact, the sharp edges of the GFMs physically damage the cell membranes and destruct their integrity, leading to leakage of the intracellular contents and bacterial cell death.[110] Third, the GFMs induce oxidative stress toward bacterial cells through oxidizing vital cellular components via reactive oxygen species (ROS)-dependent and ROS independent pathways.[110b,111] In general, the more the GFMs and bacterial cells interact, the more the first step of cell deposition occurs, allowing steps two and three to take place, likely in synergy with one another.[110b] This process is regulated by the physicochemical properties of the GFM, the concentration, time, and means of exposure, and the bacteria strain that is used in the tests.
Physicochemical properties such as lateral size, number of layers, shape, surface chemistry and modifications, and dispersibility influence the antibacterial activity of the GFMs. Under similar concentration and incubation conditions, GO dispersions demonstrate the highest antibacterial activity toward Escherichia coli, sequentially followed by rGO, graphite, and graphite oxide. Whereas GO wrapped around E. coli bacteria in individual sheets, rGO formed large aggregates that trapped the bacteria within. This could be attributed to the greater dispersibility of GO secondary to its functional groups that enabled greater direct contact interactions with the bacteria leading to a continuous decrease in cell viability. Although due to its higher conductivity, rGO had the highest oxidation capacity, its initial cell deposition was lower than that of GO; hence, direct interactions did not form to allow oxidation stress production. Thus, it was suggested that in the first 2 h, membrane stress plays a more important role, whereas after the bacterial cells are covered by the GFMs, oxidative stress becomes more prominent.[110b] A subsequent study toward Pseudomonas aeruginosa strains similarly showed that GO and rGO exert stronger antibacterial effects than graphite oxide and graphite.[111a] However, when GO or rGO were integrated in agar plates, rGO showed stronger antibacterial activity against Bacillus subtilis and P. aeruginosa strains.[112] rGO nanowalls were also found to be more toxic than GO nanowalls due to their sharper edges and better charge transfer capabilities.[110a] On the effect of size, one study reported that GO sheets that are larger have stronger biocidal properties since they can interact with and cover cell surfaces more easily and inhibit their actions.[113] Despite these invaluable studies, however, there are discrepancies on the effects of the physicochemical features of the GFMs and further studies are necessary to clarify these effects.
The concentration and duration of exposure influence the antibacterial activity of the GFMs. Increasing the concentration or time of exposure decreases bacterial viability and, as mentioned, may influence the mechanism of action. Importantly, the conditions in which bacteria are exposed to the GFMs largely impact the outcome of the study. While GO dispersions in saline and DI water exerted strong antibacterial activities, dispersions in nutrient broths enhanced bacterial cell growth. This was attributed to the noncovalent adsorptions of certain nutrient broth adsorbates onto the basal planes of GO, which masked these important action sites and deactivated the antibacterial activities of GO.[114] The morphology of the sheets may also influence the biological interactions. For instance, GO films with wrinkled morphologies were a robust platform to kill bacteria. The nanogrooves trapped the bacteria of matching sizes and prompted greater direct interactions and antibacterial effects.[115]
The bacterial strains that are investigated and their characteristics largely influence the study outcome. Although their precise role has yet to be determined, the size of the bacterial cell and the thickness of the outer wall/membrane have been identified as some of the influential factors.
The exposure conditions of the GFMs and bacteria need to be considered when interpreting results. This is especially important when the GFMs are combined with other materials, as the substrate may influence the conditions in which the bacteria are exposed to the GFMs and the antibacterial efficacy of the GFMs. Graphene’s antibacterial activity was shown to depend on its ability to disrupt the charge transfer of bacterial membranes. When monolayer graphene was coated onto conductor or semiconductor surfaces, a circuit with the bacteria formed that transferred electrons from the bacterial membrane through graphene to the underlying substrate. This continuous process interfered with the electron transport requirements for bacterial respiration and rapidly depleted ATP levels, which led to cell death. Conversely, when graphene was coated on insulator substrates, the electron transfer circuit did not form and the bacteria survived.[116] When GO was incorporated into polyvinyl-N-carbazole, it presented better antibacterial activities than pristine GO, which was attributed to the increased dispersion of GO within the polymer and the greater surface area contact with the bacteria.[117] Combining GO with Pluronic-127 also significantly enhanced the antibacterial activity of GO by forming stable nanoassemblies that populated around the bacteria and increased their interactions.[118]
In summary, the antibacterial properties of the GFMs offer great potential for developing biomedical technologies, especially for biomaterials for tissue regeneration. Combining the GFMs with other materials may maintain, reduce, or increase the overall antibacterial effects of the GFMs and requires further investigation. Understanding the mechanisms and factors involved in the antibacterial activity of the GFMs is critical for designing biomaterials to combat infections. Lastly, it is imperative to evaluate the antibacterial activity of biomaterials in conjugation with their in vitro and in vivo biocompatibility, as the latter is essential to their performance.
4.1.2. In Vitro Biocompatibility: Mammalian Cells
The in vitro biocompatibility of the GFMs has been investigated with several different cell types, including those of the immune system such as neutrophils and macrophages, fibroblasts, stem cells, and various cell lines. These studies typically encompass exposing the cells to specific concentrations of the GFMs for set durations, and while they cannot account for the high complexity of in vivo systems, they provide excellent information on the biological interactions and mechanisms involved in highly controlled experimental settings.
As with any material, the in vitro biocompatibility of the GFMs is dose- and time-dependent. Most studies show that at high concentrations, the GFMs adversely affect cell viability. The cytotoxic ranges vary in between studies depending on the GFM variant in use and the conditions of exposure, however, generally concentrations below 5–10 μg mL−1 do not induce a significant loss in cell viability. The cytotoxic effects of the GFMs at these higher concentrations is mainly through provoking an increase in oxidative stress, impairing the cell membrane and cytoskeleton, and causing DNA damage.[119] At a cellular level, graphene induces cytotoxicity through increasing intracellular ROS levels and depleting the mitochondrial membrane potential, which in turn trigger apoptosis through the mitogen-activated protein kinases (MAPKs) and transforming growth factor (TGF)-β-related signaling pathways.[119c] Through an oxidative stress-mediated mechanism, lipid peroxidation, gluthathione oxidation, and cytoplasmic Ca2+ levels increase, which contribute to the depolarization of the mitochondrial membrane potential and ultimately cell death. At a molecular level, these interactions alter the expression of genes involved in cell adhesion, nucleosome assembly, cellular component biogenesis, protein folding, stress response, and DNA damage.[119a] Also, at the molecular level and at high concentrations, GO induces mutagenesis by interacting with the genomic DNA and interfering with its synthesis and replication leading to cell cycle arrest.[119d] Morphologically, high concentrations of the GFMs alter cytoskeletal assembly and architecture and severely compromise the plasma membrane integrity, which can lead to higher membrane permeability and Ca2+ influx.[119a,b]
The physicochemical properties of the GFMs greatly influence their interactions with cells. Several studies have shown a strong correlation between the size of the GFMs and their cytocompatibility. In A549 cells, exposure to smaller-sized GO (160 nm) induced greater oxidative stress and loss of viability compared to larger-sized GO (780 nm).[120] In HUVECs, both GO and rGO of smaller size (400 nm) caused greater oxidative stress and viability loss compared to their counterparts of larger sizes (800 nm).[121] Larger micrometer-sized GO sheets exhibited cytotoxicity in human fibroblasts at doses as high as 50 μg mL−1 and above.[122] Conversely, one study showed that large GO (750–1300 nm) was significantly more toxic to J774.A1 macrophages than small (50–350 nm) and intermediate GO (350–750 nm), ranked as small < intermediate < large. Additionally, the large GO induced the expression of proinflammatory genes such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, through activating toll-like receptors and the proinflammatory nuclear factor-κB (NF-κB) signaling pathways.[123] In human immune cells, there was no significant difference in cell viability between small (100–500 nm) and large GO (1–10 μm). There was, however, a clear size-dependent impact on cell activation and gene expression. In human peripheral blood mononuclear cells at a molecular level, the small GO was able to modulate the expression of a large number of genes involved in the innate and adaptive immune responses, which could potentially have implications for immunomodulatory applications such as cancer immunotherapy.[124]
The size-dependent response of the cells to the GFMs is most likely governed by their differential cellular interactions and size-dependent uptake mechanisms. Protein-coated GO adhered to cells and internalized through energy-dependent processes in as little as 30 min. Smaller sheets (420 nm) entered mainly through clathrin-mediated endocytosis while larger ones (860 nm) used both clathrin-mediated endocytosis and phagocytosis.[125] GO sheets of 350 nm and 2 μm were internalized by macrophages to the same extent but by different uptake mechanisms. The 350 nm GO was wrapped by the filopodia before entry and remained almost unvaried in shape after internalization. The 2 μm GO, on the other hand, oriented nearly perpendicularly to the membrane and underwent shape change and wrinkling before eventually being sequestered by lysozymes. Furthermore, the micrometer-sized GO induced a stronger inflammatory response in the macrophages. Interestingly, the internalization of both GO sizes was negligible in non-phagocyte cells.[126] One study tested a range of single-layer rGONP sizes against human MSCs and found that the most likely mechanisms involved in the greater cytotoxicity of smaller NPs (11 nm) at ≈1 μg mL−1 was their ability to penetrate into the nucleus of the cells and to induce genotoxic effects such as DNA fragmentation and chromosomal aberrations. Larger NPs (3.8 μm), conversely, did not cause significant genotoxicity to the cells, even at high concentrations, and only exerted cytotoxic effects at concentrations above 100 μg mL−1.[127]
The surface properties and oxidation of the GFMs largely dictate their interactions with the cells. Pristine graphene was found to be more cytotoxic than carboxyl functionalized graphene (G-COOH). Whereas the hydrophobic pristine graphene accumulated on the cell membrane at high concentrations, destabilized cytoskeletal alignment and deformed the cells, the more hydrophilic G-COOH was internalized at lower concentrations and did not seem to affect cell function. Moreover, pristine graphene induced cell death by inducing intracellular ROS generation and not by physical damage to the plasma membrane.[128] In hydroxylated graphene (G-OH) also, the presence of the hydroxyl groups improved hydrophilicity and supported stem cell proliferation.[129] Thus, it seems that the density of the oxygen functional groups, through its influence on intracellular ROS levels, plays an important role in mediating cytotoxicity,[121,130] and can be used as a means to tune the biocompatibility of the GFMs.
The biocompatibility of the GFMs can further be improved by modulating their surface properties and through surface functionalization with biocompatible macromolecules. GO and rGO were functionalized with poly(ethylene glycol) (PEG) and bovine serum albumin (BSA). Both molecules greatly attenuated the loss in cell viability and genotoxic effects of the materials, with GO–PEG displaying the greatest improvements.[131] The in vitro biocompatibility of various GO derivatives including GO aminated GO (GO–NH2), poly(acrylamide)-functionalized GO (GO–PAM), poly(acrylic acid)-functionalized GO (GO–PAA), and GO–PEG were investigated. While GO was easily recognized and phagocytosed by macrophages, surface functionalization reduced its recognition by the cells. Functionalizing with PEG or PAA had the greatest effects on ameliorating GO-induced membrane damage, with GO–PAA inducing the least amount of toxicity.[119b] Polyvinylpyrrolidone (PVP) coating of GO also reduced its immunogenicity against human immune cells. PVP–GO delayed apoptosis in T lymphocytes and delayed the differentiation and maturation of dendritic cells. In addition, PVP coating lowered the phagocytosis of GO by macrophages and significantly improved cellular metabolic activity and physiological activity.[132]
In conclusion, many factors influence the in vitro biocompatibility of the GFMs. Some factors such as size, surface chemistry, and functionalization have been identified, although their precise role has yet to be established. The discrepancies in the available studies are partly due to the variations in study designs and experimental setups. For instance, it is now known that the composition of the media used in cell assays influences the interactions of the GFMs with cells. High serum concentrations in the media can adsorb onto GO and provide a coating that masks their cellular interactions and mitigates their cytotoxic effects, while low concentrations enable more direct interactions and physical damage to the cell membrane.[119b,133] The cell type used in the assays can also influence the study as some cells are more susceptible to graphenic nanostructures.[134] Additionally, the manufacturing method and purification steps can influence the cytotoxicity of the GFMs.[135] It is also important to note that many studies evaluate the in vitro biocompatibility of the GFMs at extremely high concentrations and assess the cellular responses at short time points that may not necessarily be relevant for regenerative engineering applications, where cells are typically exposed to lower amounts and during longer time periods. In these conditions, the studies are more mechanistic than toxicological. Nevertheless, they are significant and provide much needed insight into the interactions of cells and the GFMs.
4.1.3. In Vivo Biocompatibility
The in vivo biocompatibility, biodistribution, and biopersistence of the GFMs is determined not only by their physicochemical characteristics, their exposure time, and the animal of study, but also importantly by the method, route, and dose of administration. To evaluate in vivo biocompatibility and toxicity, the GFMs are mainly suspended in a dispersant and administered into the body intravenously, intraperitoneally, intragastrically, intratracheally, subcutaneously, or directly into the organ of study.
Naturally, the dose and frequency of GFM administration affects their in vivo biocompatibility and biodistribution. The survival rate of Balb/c mice receiving intratail vein injections of GO at 0.05, 1, 5, 10, and 20 mg kg−1 was 100%, 80%, 60%, 20%, and 0%, respectively. All deaths occurred on the first day. GO accumulated mostly in the lung, liver, and spleen in a dose-dependent manner and induced pathological damages to the lung and liver. In the lungs, high doses of GO caused fibrosis, inflammation, and significant occlusion of lung blood vessels with platelet thrombi.[119b] The high uptake and long-term retention of GO in the lungs were also demonstrated in Kunming mice receiving IV injections of radiolabeled GO at 1 and 10 mg kg−1. GO exhibited a relatively long blood circulation half-life of 5.3 ± 1.2 h and was shown to clear from the bloodstream within 48 h for distribution to the vital organs. Accumulation was predominantly in the lungs, followed by the reticuloendothelial system, such as the liver and spleen. While there were no toxicological effects on the liver, spleen, and kidneys at both doses, 10 mg kg−1 GO did result in significant pathological changes to the lungs, due to high-level deposition and slow clearance. Of note, GO showed a rapid renal excretion within 12 h.[136] Additionally, dose-dependent pulmonary toxicity of GO was shown in Kunming mice where injection of a high dose of ≈14 mg kg−1 caused mortality in 4/9 mice due to their major airways being blocked by GO agglomeration.[122] The toxicity of the GFMs can be modulated by changing the exposure frequency. CD-1 mice exposed to seven repeated doses of 0.3 mg kg−1 GO had greater GO accumulation and more severe toxicity compared to those receiving a single dose of 2.1 mg kg−1.[137] Additionally, repeated administration increases the exposure levels and leads to greater material accumulation and retention in the organs.[137,138] Interestingly, the speed at which the injections are performed impacts the toxicity of the material. GO that was injected at slower rates had better animal survival rates, even at high doses of injection.[139]
The physicochemical properties of the GFMs can influence their biodistribution and biocompatibility in vivo. A few studies have shown that GO that is larger and thicker in size remains in the body for a longer time period due to slower excretion mechanisms, and induces more damage to the vital organs.[136,137,140] Moreover, the degree of GO oxidation influences in vivo compatibility and pharmacokinetics. A reduction in the degree of oxidation in GO expedited immune cell infiltration, uptake, and clearance following both subcutaneous and intraperitoneal (IP) implantation in C57BL/6J mice.[141] Pulmonary toxicity and inflammation following intratracheal exposure were also greater in GO than rGO and in GO compared to pristine graphene,[142] suggesting that the biocompatibility of the GFMs is strongly correlated to their oxidation. A long-term study on the toxicity, biodistribution, and immune response of FLG and FLG-COOH in Swiss albino mice found that irrespective of surface chemistry and carboxylation, both materials injected at 20 mg kg−1 accumulated in the lungs, liver, spleen, and kidneys and caused severe pathological changes including acute to chronic inflammation in these organs.[143]
Modifying the surface of the GFMs, through either covalent or noncovalent conjugation, is an effective method to improve biodistribution and biocompatibility. PEGylation is a well-established method that enhances the therapeutic efficacy of nanomaterials by prolonging their blood circulation time.[144] The long-term in vivo toxicology of GO–PEG was systemically examined through intravenous (IV) administration of radionuclide-labeled GO at 20 mg kg−1 in Balb/c mice. Within an hour of IV administration, GO–PEG distributed to many different organs and mainly accumulated in the reticuloendothelial system such as the liver and spleen. The GO–PEG levels decreased gradually in most organs within a few days, possibly through renal and fecal clearance mechanisms. Over the course of 90 days, the GO–PEG did not induce any obvious hepatic, renal, or hematological toxicity in any of the mice.[145] PEGylation of FLG injected at 20 mg kg−1 similarly diminished its toxic side effects and possibly enhanced its biodegradation in Swiss albino mice.[143] One study showed that surface functionalization with PAA had a greater impact on improving the biocompatibility of GO compared to PEGylation.[119b] Dextran, an FDA-cleared natural polymer, has also been used to coat GONPs and improve their biodistribution and biocompatibility. Dextran-coated GONPs were intravenously administered to Wistar rats at doses between 1 and 500 mg kg−1 body weight. The coating improved the maximum tolerable dose of GONPs to 50–125 mg kg−1, and no mortalities occurred at doses below 125 mg kg−1. The GONP-dextran had a half-life within 30 min, distributed to the liver, heart, lungs, kidneys, and brain within 1 day of injections, and was excreted mostly through feces. Further, the GONP-dextran < 125 mg kg−1 had no significant effects on the respiratory, cardiovascular, or hematological parameters of the rats and cleared from the major organs mostly by day 30. Doses above 125 mg kg−1 caused mortality in 9/25 of the animals within ≈2 min of injection, possibly due to pulmonary congestion.[139]
The choice of dispersant can affect the stability and distribution of the GFMs in the body. Graphene was intratracheally administered to C57BL/6 mice in either water (as aggregated) or the block copolymer Pluronic (as dispersed). While aggregated graphene persisted in the airways and induced a local fibrotic response, Pluronic-dispersed graphene distributed homogeneously in the lungs and induced a modest acute lung inflammation that was not fibrogenic.[142a] The importance of the dispersion was also shown when GO was suspended in the surfactant Tween 80 and injected intravenously in Balb/c mice. GO, despite having no acute adverse effects, predominantly accumulated in the lungs and liver. However, when it was suspended in 1% Tween 80, its biodistribution was altered and its blood circulation mobility was improved. Histological analyses demonstrated that GO suspended in Tween 80 had reduced retention in the lungs and greater accumulation in the liver. Additionally, neither material significantly altered the hematological indexes and blood constituents.[146]
The pharmacokinetics, biodistribution, and biopersistence of the GFMs are closely related to their administration route. While most studies have investigated the biocompatibility of the GFMs following IV injections, administration through other routes may yield different outcomes. The in vivo biocompatibility of GO and less oxidized GO were evaluated in murine sites that are typically relevant for medical devices. IV administration at either 2 or 20 mg kg−1 resulted in lung occlusions within 1 min, likely due to particle aggregation and/or swelling. On the other hand, injections at 20 mg kg−1 into the subcutaneous or IP space revealed moderate compatibility. At both tissue sites, GO and less oxidized GO elicited a foreign body response consistent with a typical foreign body reaction, with evidence of clearance and cell uptake.[141] Both IV and IP administration of 10 mg kg−1 rGO–PEG led to their absorption from the blood circulation to the major organs (liver, brain, kidneys, and spleen) through different distribution profiles.[138] Compared to oral feeding, IP administration of GO–PEG derivatives at 4 mg kg−1 resulted in a greater biodistribution and organ accumulation. GO–PEG derivatives administered orally to Balb/c mice had rather limited intestinal adsorption and were rapidly excreted, whereas IP administration caused a high accumulation and persistence of the GO–PEG in the reticuloendothelial system including the liver and spleen. Despite their long-term retention until 90 days, however, toxicity to the treated animals was insignificant.[147] Long-term continuous oral exposure of maternal mice and their offspring to GO can, however, lead to abnormal development of the offspring, a consequential finding, especially for developing graphene-based implants for long-term use.[148] With regards to pulmonary safety, a single instillation of graphene NPs at 2.5 and 5 mg kg−1 resulted in lung accumulations after 28 days and a subchronic inflammatory response.[149] Similarly, pharyngeal aspiration of multilayered graphene platelets led to large material deposit accumulations in the lungs. Despite their biopersistence, however, there was minimal inflammation after 6 weeks.[150] The pulmonary safety of the GFMs and the considerations regarding their biodegradation in this administration route requires further investigation. The ocular safety of the GFMs for ophthalmic applications has also been assessed. Japanese white rabbits were injected intravitreally into the eyes with GO at 0.1, 0.2, and 0.3 mg. GO caused no changes to the eyeball appearance, intraocular pressure, eyesight, or histological morphology of the retina suggesting their safety.[151]
In summary, there is insufficient knowledge on the in vivo behavior of the GFMs. The in vivo circulation time, hemocompatibility, biopersistance, biodegradation, and fate of the byproducts are all areas that warrant detailed investigations. The interactions of the GFMs with the immune system, in particular, are not clearly understood. Surface functionalization and coating with copolymers and surfactants could potentially improve the biocompatibility and biodistribution of the different GFMs, while maintaining their excellent capabilities as drug delivery systems. However, the effects of these molecules on the clearance and biodegradation of the GFMs needs to be evaluated. Additionally, most studies assess the safety of the GFMs in short time periods, and not much is known on the long-term safety of these nanomaterials. For use in biomaterials for tissue regeneration, this would be of paramount importance.
4.2. Biodegradability Considerations
Biodegradable materials are those that can transform from an initial, stable structure into soluble products that can be resorbed or processed by the body. The long-term safety of the GFMs for clinical applications relies on their capacity to be biodegraded or readily excreted and eliminated from the body.[152] As with any new material, understanding the fate and biopersistance of the GFMs and their degradation products is integral for progression toward clinical applications.
There is evidence to suggest that cells of the immune system, specifically neutrophils and macrophages, can mediate the biodegradation of GO[153] and that the degradation products pose no substantial risk to cells.[153b,154] Indeed, GO can undergo biodegradation through both major in vivo degradation pathways, namely hydrolytic and enzymatic degradation.
In water, GO is proposed to be dynamic, constantly changing its chemical structure, and even undergoing C─C bond cleavage. Prolonged exposure can eventually degrade GO flakes into humic acid-like structures, which are regarded as the end degradation products of organic matter.[154,155] The degradation of multilayer graphene was reported in the presence of hydrogen peroxide (H2O2), a naturally occurring ubiquitous compound in the body. Incubation of multilayered graphene with H2O2 resulted in the generation of small holes that were randomly distributed within the structure. These small defects were initially created by the random attack of H2O2 on the C─C bonds, however upon production, enabled H2O2 to progressively attack more C─C bonds, especially around the initial defects, which contributed to the production of larger holes and accelerated the degradation of graphene.[156]
Several studies have reported the enzymatic degradation of the GFMs, using enzymes such as myeloperoxidase (MPO), horseradish peroxidase (HRP), and lignin peroxidase (LiP). These enzymes can catalyze the degradation of GO in the presence of low concentrations of H2O2. In one study, the capacity of MPO to degrade GO was shown to depend on the oxidation and colloidal stability of GO. GO that was highly aggregated had negligible degradation upon MPO exposure and underwent almost no structural changes. However, GO that had a higher percentage of carboxylic groups and was more colloidally stable and dispersible, degraded completely in 24 h. The greater degradation of the colloidally stable GO was suggested to be mediated by its greater oxidation, which contributes to the cleavage of hydroxyl and epoxide groups and C─C and C═C bonds by MPO, creating holes in the lattice structure that lead to the eventual degradation of GO into residual carbonaceous fragments.[157] Single- or few-layer graphene degraded by similar mechanisms in vitro, either in the presence of recombinant human MPO or using activated neutrophils, further suggesting the biodegradable potential of pristine graphene for biomedical applications.[158] In addition to MPO, HRP has also been investigated for catalyzing the biodegradation of the GFMs. HRP was shown to catalyze the oxidation of GO and cause holes to form on its basal plane; however, under the same conditions, it failed to oxidize rGO. This was attributed to the epoxy and hydroxyl groups of GO and its flexibility, which upon the binding of HRP allowed it to be more dynamic and active.[159] Exposure of 3D graphene foams to HRP/H2O2 followed a two-stage biodegradation mechanism. First, small fragments were disintegrated from the scaffold. Next, the small fragments were decomposed into smaller fragments until only single-layer graphene was left, which then biodegraded into carbonaceous materials.[160]
The biodegradability of the GFMs can also be modulated through functionalization with various molecules. To improve its biodegradation, the surface of GO was functionalized with two well-known substrates of HRP, coumarin, and catechol. The two molecules were covalently conjugated to enhance the catalytic activity of peroxidases. Functionalization enhanced HRP-mediated biodegradation through improving GO–HRP interactions or enhancing enzymatic activity.[161] Oxygen plasma treatment of graphene foams expedited biodegradation through creating more sites for HRP/H2O2 attack. By introducing −OH groups to the structure, defect sites were created, which provided more surfaces for peroxidase activity.[160] Surface functionalization with PEG or BSA, on the other hand, impeded the HRP-induced biodegradation of the GO sheets. This was circumvented by conjugating the PEG molecules through a cleavable disulfide bond, allowing the enzymatic degradation to take place.[131]
5. Conclusion and Future Perspectives
Bone regeneration is a dynamic and complex physiological process that involves the interplay of numerous cells and signaling molecules in a well-orchestrated spatiotemporal sequence.[26] Biomaterials have been developed to foster the endogenous regenerative potential in cases where healing and repair are impaired in an effort to restore the function of the damaged tissue.[162]
Since the encouraging studies demonstrating the osteoinstructive effects of graphene in 2011,[40,42] various researchers have investigated the usage of the GFMs for developing bone biomaterials with enhanced mechanical and biological properties. The excellent mechanical properties of the GFMs (high strength, stiffness, and flexibility) and their favorable physicochemical characteristics can be used toward physically reinforcing scaffolds and implants and producing biomaterials that are better equipped to withstand the load-bearing environment of bone tissue. Mechanical competency and compatibility with the native bone are not only important for withstanding the loads from the surrounding environment but also necessary for providing stability and support for the regenerative process to take place and new tissues to form.[28b] Additionally, and of significance, using the GFMs in bone biomaterials improves their biological performance. Through supporting cell adhesion, spreading, morphology, and growth, and enhancing and accelerating osteogenesis, the GFMs have found applications in various scaffolds, implants, and composites. The addition of the GFMs also facilitates biomineralization and the formation of bone-like apatite on the surface, which improves the bone-bonding and bone-forming abilities of biomaterials and further enhances their bone regenerative potential. Lastly, utilizing the GFMs in biomaterials enables various therapeutic and signaling agents to be functionalized and delivered, which in turn offers a plethora of opportunities to modulate and engineer the biomaterials for distinct biological activities or to further enhance bone regeneration.
Despite the many versatile properties of the GFMs that have been used in bone regenerative engineering applications, there are also features that have not been as widely investigated.[29] For instance, the electrical properties of graphene can be harnessed to produce electroconductive scaffolds, a prospect that has been extensively explored for regenerating muscle or neural tissues, but that can also be applied to electrically stimulate bone regeneration.[163] Moreover, there is evidence to suggest that the GFMs can stimulate angiogenic activity,[79,164] a finding that is especially encouraging for fostering vascularized bone regeneration in larger defects. The advances made in using the GFMs as delivery vehicles is a significant development in the wide-scale use of these nanomaterials. Future advances may include exploring the capacity of graphene-based biomaterials to deliver biomolecules such as nucleic acids (RNA, mRNA, miRNA, and DNA), enzymes, lipid membranes, and various bioactive factors that are secreted by cells.[8a,22,165] Lastly, graphene quantum dots can be used in combination with scaffolds as bioimaging agents to offer better insight into in vivo regenerative processes.[166]
As research into the usage of graphene-based materials for bone regeneration rapidly expands and evolves, there are a number of considerations that require special attention. It is imperative to have a thorough understanding on the interactions of the GFMs with biological systems. The mechanisms through which the GFMs impart their biological effects is still unknown. The reinforcing effects of the GFMs for various structures has been studied; however, the biological effects of the GFMs are equally, if not more, important to successfully develop biomaterials for clinical use. Detailed investigations are required to better understand how the GFMs influence cell behavior. The high adsorption capacity of the GFMs is now known to play a role in the superior biological performance of GFM-based biomaterials. It is plausible that additional features imparted by incorporating the GFMs (e.g., changes in stiffness, surface roughness, or topographical features) may also have an impact and should be thoroughly examined.
Moreover, the long-term safety of graphenic material is yet to be established and little is known on their cellular uptake mechanism and intracellular fate. Studies have demonstrated the various parameters that influence the interactions of the GFMs with biological systems, including the physicochemical characteristics of the GFMs (size, shape, surface chemistry, and functionalization), the physiological milieu, and the dose and duration of exposure. However, further in vitro and in vivo studies are required to elucidate the precise role of each of these parameters. Additionally, there is insufficient knowledge on the biodegradability and biodistribution of the GFMs, and their adverse acute and chronic effects on the body.
The concerns surrounding the biocompatibility and biodegradation of this new class of materials may somewhat be due to the conflicting findings of different research groups. As discussed, variations in the physicochemical properties of the GFMs heavily affects their safety profile. The discrepancies across different studies may be partly due to the different characteristics of the GFM variant in use, which confounds comparing and contrasting results. This confusion is also partly due to the terminology that is used in papers to describe the graphenic derivatives. Researchers should diligently define and describe their graphenic material according to standardized nomenclature and strive to characterize the properties of their material.[23,167] Standardizing the reporting format will expedite our understanding on the relation between the physicochemical characteristics of the GFMs and the biological interactions. The conditions of exposure and the physiological milieu heavily impact the outcomes, and thus it is crucial for researchers to characterize and describe their testing conditions. Special attention should be given to selecting the biological assays for determining the biocompatibility and cytocompatibility of graphene-based biomaterials. The high surface area and the unique adsorption and optical properties of the GFMs at the nanoscale may be incompatible with conventionally used assays and obscure findings. The testing methods should be chosen with caution, designed with proper experimental controls, and preferably validated with alternative methods and assays.[21,168] Although research in this field is recent, the guidelines proposed here and in other excellent reviews will aid in advancing and expanding our knowledge of the field.
Acknowledgements
The authors would like to acknowledge funds from the NIH DP1 AR068147 and NSF/EFRI #1332329. C.T.L. is a recipient of the National Medal of Technology and Innovation. L.D. is supported by the General Electric (GE) Fellowship for Innovation. Figures were created with the assistance of BioRender.com.
Biography

Leila Daneshmandi is a Ph.D. candidate in biomedical engineering and a General Electric fellow for Innovation at the University of Connecticut. She earned her B.S. degree in biomedical engineering from Amirkabir University of Technology (Tehran Polytechnic). Her doctoral research under the supervision of Dr. Cato T. Laurencin focuses on the development and evaluation of graphene-based biomaterials for the regenerative engineering of bone tissue.

Mohammed Barajaa is currently pursuing his Ph.D. degree in the Department of Biomedical Engineering at the University of Connecticut. He earned his B.S.E. in biomedical engineering from the University of Hartford and earned his M.S. in biomedical engineering from the University of Connecticut. He is now working on developing a novel in vivo platform for complex tissue regeneration in a nonregenerative animal, alongside developing an integrated graft system for complex tissue regenerative engineering. He is working under the supervision of Dr. Cato T. Laurencin.

Cato T. Laurencin is a designated university professor at the University of Connecticut. He is a professor of chemical and biomolecular engineering, materials science and engineering, and biomedical engineering, and the Albert and Wilda Van Dusen distinguished endowed professor of orthopaedic surgery. He is the chief executive officer of the Connecticut Convergence Institute for Translation in Regenerative Engineering at the University of Connecticut. He earned a B.S.E. in chemical engineering from Princeton University, his M.D., Magna Cum Laude, from the Harvard Medical School, and his Ph.D. in biochemical engineering/biotechnology from the Massachusetts Institute of Technology.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Leila Daneshmandi, Connecticut Convergence Institute for Translation in Regenerative Engineering, UConn Health, Farmington, CT 06030, USA; Raymond and Beverly Sackler Center for Biomedical Biological, Physical and Engineering Sciences, UConn Health, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Orthopaedic Surgery, UConn Health, Farmington, CT 06030, USA.
Mohammed Barajaa, Connecticut Convergence Institute for Translation in Regenerative Engineering, UConn Health, Farmington, CT 06030, USA; Raymond and Beverly Sackler Center for Biomedical Biological, Physical and Engineering Sciences, UConn Health, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Orthopaedic Surgery, UConn Health, Farmington, CT 06030, USA.
Armin Tahmasbi Rad, Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA; Institute of Materials Science, University of Connecticut, Storrs, CT 06269, USA.
Stefanie A. Sydlik, Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213, USA; Department of Biomedical Engineering, Carnegie Mellon University, Pittsburgh, PA 15213, USA
Cato T. Laurencin, Connecticut Convergence Institute for Translation in Regenerative, Engineering, UConn Health, Farmington, CT 06030, USA; Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, UConn Health, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Orthopaedic Surgery, UConn Health, Farmington, CT 06030, USA; Institute of Materials Science, University of Connecticut, Storrs, CT 06269, USA; Department of Materials Science and Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Chemical and Biomolecular Engineering, University of Connecticut, Storrs, CT 06269, USA
References
- [1].Katagiri W, Watanabe J, Toyama N, Osugi M, Sakaguchi K, Hibi H, Implant Dent. 2017, 26, 607. [DOI] [PubMed] [Google Scholar]
- [2].Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, Logroscino G, J. Mater. Sci.: Mater. Med 2014, 25, 2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Pneumaticos SG, Triantafyllopoulos GK, Basdra EK, Papavassiliou AG, J. Cell. Mol. Med. 2010, 14, 2561; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Soucacos PN, Kokkalis ZT, Piagkou M, Johnson EO, Injury 2013, 44, S70. [DOI] [PubMed] [Google Scholar]
- [4].Calori G, Mazza E, Colombo M, Ripamonti C, Injury 2011, 42, S56. [DOI] [PubMed] [Google Scholar]
- [5].Zwingenberger S, Nich C, Valladares RD, Yao Z, Stiehler M, Goodman SB, BioDrugs 2012, 26, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Wang W, Yeung KW, Bioact. Mater 2017, 2, 224; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Koons GL, Diba M, Mikos AG, Nat. Rev. Mater 2020, 5, 584; [Google Scholar]; c) Sohn H-S, Oh J-K, Biomater. Res 2019, 23, 9; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset A-M, Benkirane-Jessel N, Bornert F, Offner D, J. Tissue Eng 2018, 9, 2041731418776819; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lobb DC, DeGeorge BR Jr, Chhabra AB, J. Hand Surg 2019, 44, 497. [DOI] [PubMed] [Google Scholar]
- [7].a) Laurencin CT, Khan Y, Am. Assoc. Adv. Sci 2012; [Google Scholar]; b) Laurencin CT, Khan Y, Regenerative Engineering, CRC Press, Boca Raton, FL: 2013; [Google Scholar]; c) Laurencin CT, Nair LS, Regener. Biomater 2016, 3, 123; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Tang X, Daneshmandi L, Awale G, Nair LS, Laurencin CT, Regener. Eng. Transl. Med 2019, 5, 233; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Barajaa MA, Nair LS, Laurencin CT, Sci. Rep 2020, 10, 11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daneshmandi L, Shah S, Jafari T, Bhattacharjee M, Momah D, Saveh-Shemshaki N, Lo KW-H, Laurencin CT, Trends Biotechnol. 2020, 10.1016/j.tibtech.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Daneshmandi L, Laurencin CT, J. Biomed. Mater. Res., Part A 2020, 108, 1045. [DOI] [PubMed] [Google Scholar]
- [9].a) Allen MJ, Tung VC, Kaner RB, Chem. Rev 2010, 110, 132; [DOI] [PubMed] [Google Scholar]; b) Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S, Prog. Mater. Sci. 2011, 56, 1178. [Google Scholar]
- [10].Shin SR, Li Y-C, Jang HL, Khoshakhlagh P, Akbari M, Nasajpour A, Zhang YS, Tamayol A, Khademhosseini A, Adv. Drug Delivery Rev 2016, 105, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Novoselov KS, Fal V, Colombo L, Gellert P, Schwab M, Kim K, Nature 2012, 490, 192. [DOI] [PubMed] [Google Scholar]
- [12].Papageorgiou DG, Kinloch IA, Young RJ, Prog. Mater. Sci 2017, 90, 75. [Google Scholar]
- [13].a) Lee C, Wei X, Kysar JW, Hone J, Science 2008, 321, 385; [DOI] [PubMed] [Google Scholar]; b) Kuila T, Bose S, Mishra AK, Khanra P, Kim NH, Lee JH, Prog. Mater. Sci 2012, 57, 1061. [Google Scholar]
- [14].Stoller MD, Park S, Zhu Y, An J, Ruoff RS, Nano Lett. 2008, 8, 3498. [DOI] [PubMed] [Google Scholar]
- [15].Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau CN, Nano Lett. 2008, 8, 902. [DOI] [PubMed] [Google Scholar]
- [16].Orlita M, Faugeras C, Plochocka P, Neugebauer P, Martinez G, Maude DK, Barra A-L, Sprinkle M, Berger C, De Heer WA, Phys. Rev. Lett 2008, 101, 267601. [DOI] [PubMed] [Google Scholar]
- [17].Bunch JS, Verbridge SS, Alden JS, Van Der Zande AM, Parpia JM, Craighead HG, McEuen PL, Nano Lett. 2008, 8, 2458. [DOI] [PubMed] [Google Scholar]
- [18].Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NM, Geim AK, Science 2008, 320, 1308. [DOI] [PubMed] [Google Scholar]
- [19].a) Liu J, Cui L, Losic D, Acta Biomater. 2013, 9, 9243; [DOI] [PubMed] [Google Scholar]; b) McCallion C, Burthem J, Rees-Unwin K, Golovanov A, Pluen A, Eur. J. Pharm. Biopharm 2016, 104, 235. [DOI] [PubMed] [Google Scholar]
- [20].Chen D, Feng H, Li J, Chem. Rev 2012, 112, 6027. [DOI] [PubMed] [Google Scholar]
- [21].Holt BD, Wright ZM, Arnold AM, Sydlik SA, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol 2017, 9, e1437. [DOI] [PubMed] [Google Scholar]
- [22].Cheng C, Li S, Thomas A, Kotov NA, Haag R, Chem. Rev. 2017, 117, 1826. [DOI] [PubMed] [Google Scholar]
- [23].Bianco A, Cheng H-M, Enoki T, Gogotsi Y, Hurt RH, Koratkar N, Kyotani T, Monthioux M, Park CR, Tascon JMD, Zhang J, Carbon 2013, 65, 1.6. 10.1016/j.carbon.2013.08.038. [DOI] [Google Scholar]
- [24].Ding X, Liu H, Fan Y, Adv. Healthcare Mater. 2015, 4, 1451. [DOI] [PubMed] [Google Scholar]
- [25].Goenka S, Sant V, Sant S, J. Controlled Release 2014, 173, 75. [DOI] [PubMed] [Google Scholar]
- [26].Dimitriou R, Jones E, McGonagle D, Giannoudis PV, BMC Med. 2011, 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Orciani M, Fini M, Di Primio R, Mattioli-Belmonte M, Front. Bioeng. Biotechnol 2017, 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Bose S, Roy M, Bandyopadhyay A, Trends Biotechnol. 2012, 30, 546; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gao C, Peng S, Feng P, Shuai C, Bone Res. 2017, 5, 17059; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ogueri KS, Jafari T, Ivirico JLE, Laurencin CT, Regener. Eng. Transl. Med 2019, 5, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laurencin CT, Daneshmandi L, Int. J. Ceram. Eng. Sci 2020, 2, 140. [Google Scholar]
- [30].a) Gonçalves G, Cruz SM, Ramalho A, Grácio J, Marques PA, Nanoscale 2012, 4, 2937; [DOI] [PubMed] [Google Scholar]; b) Paz E, Ballesteros Y, Abenojar J, Del Real J, Dunne N, Materials 2019, 12, 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a) Liu Y, Huang J, Li H, J. Mater. Chem. B 2013, 1, 1826; [DOI] [PubMed] [Google Scholar]; b) Mehrali M, Moghaddam E, Shirazi SFS, Baradaran S, Mehrali M, Latibari ST, Metselaar HSC, Kadri NA, Zandi K, Osman NAA, ACS Appl. Mater. Interfaces 2014, 6, 3947. [DOI] [PubMed] [Google Scholar]
- [32].a) Gao C, Liu T, Shuai C, Peng S, Sci. Rep. 2015, 4, 4712; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nair M, Nancy D, Krishnan AG, Anjusree G, Vadukumpully S, Nair SV, Nanotechnology 2015, 26, 161001; [DOI] [PubMed] [Google Scholar]; c) Boga JC, Miguel SP, de Melo-Diogo D, Mendonça AG, Louro RO, Correia IJ, Colloids Surf., B 2018, 165, 207; [DOI] [PubMed] [Google Scholar]; d) Kaur T, Thirugnanam A, Pramanik K, Mater. Today Commun 2017, 12, 34. [Google Scholar]
- [33].Wan C, Chen B, Biomed. Mater 2011, 6, 055010. [DOI] [PubMed] [Google Scholar]
- [34].a) Shao W, He J, Sang F, Wang Q, Chen L, Cui S, Ding B, Mater. Sci. Eng., C 2016, 62, 823; [DOI] [PubMed] [Google Scholar]; b) Zhou T, Li G, Lin S, Tian T, Ma Q, Zhang Q, Shi S, Xue C, Ma W, Cai X, ACS Appl. Mater. Interfaces 2017, 9, 42589; [DOI] [PubMed] [Google Scholar]; c) Mohammadi S, Shafiei SS, Asadi-Eydivand M, Ardeshir M, Solati-Hashjin M, J. Bioact. Compat. Polym 2017, 32, 325. [Google Scholar]
- [35].a) Shin SR, Aghaei-Ghareh-Bolagh B, Dang TT, Topkaya SN, Gao X, Yang SY, Jung SM, Oh JH, Dokmeci MR, Tang X, Adv. Mater 2013, 25, 6385; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yu P, Bao R-Y, Shi X-J, Yang W, Yang M-B, Carbohydr. Polym 2017, 155, 507. [DOI] [PubMed] [Google Scholar]
- [36].a) Suk JW, Piner RD, An J, Ruoff RS, ACS Nano 2010, 4, 6557; [DOI] [PubMed] [Google Scholar]; b) Liu L, Zhang J, Zhao J, Liu F, Nanoscale 2012, 4, 5910. [DOI] [PubMed] [Google Scholar]
- [37].Shuai C, Feng P, Gao C, Shuai X, Xiao T, Peng S, RSC Adv. 2015, 5, 25416. [Google Scholar]
- [38].a) Basirun WJ, Nasiri-Tabrizi B, Baradaran S, Crit. Rev. Solid State Mater. Sci 2018, 43, 177; [Google Scholar]; b) Baradaran S, Moghaddam E, Nasiri-Tabrizi B, Basirun WJ, Mehrali M, Sookhakian M, Hamdi M, Alias Y, Mater. Sci. Eng., C 2015, 49, 656. [DOI] [PubMed] [Google Scholar]
- [39].Kalbacova M, Broz A, Kong J, Kalbac M, Carbon 2010, 48, 4323. [Google Scholar]
- [40].Lee WC, Lim CHY, Shi H, Tang LA, Wang Y, Lim CT, Loh KP, ACS Nano 2011, 5, 7334. [DOI] [PubMed] [Google Scholar]
- [41].a) Kalbacova M, Broz A, Kalbac M, J. Biomed. Mater. Res., Part A 2012, 100A, 3001; [DOI] [PubMed] [Google Scholar]; b) Verdanova M, Broz A, Kalbac M, Kalbacova M, Phys. Status Solidi B 2012, 249, 2503. [Google Scholar]
- [42].Nayak TR, Andersen H, Makam VS, Khaw C, Bae S, Xu X, Ee P-LR, Ahn J-H, Hong BH, Pastorin G, ACS Nano 2011, 5, 4670. [DOI] [PubMed] [Google Scholar]
- [43].a) Qi W, Yuan W, Yan J, Wang H, J. Mater. Chem. B 2014, 2, 5461; [DOI] [PubMed] [Google Scholar]; b) Kim J, Kim HD, Park J, Lee E.-s., Kim E, Lee SS, Yang J-K, Lee Y-S, Hwang NS, Biomater. Res 2018, 22, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Akhavan O, Ghaderi E, Shahsavar M, Carbon 2013, 59, 200. [Google Scholar]
- [45].a) Wu C, Xia L, Han P, Xu M, Fang B, Wang J, Chang J, Xiao Y, Carbon 2015, 93, 116; [Google Scholar]; b) Wu X, Zheng S, Ye Y, Wu Y, Lin K, Su J, Biomater. Sci. 2018, 6, 1147. [DOI] [PubMed] [Google Scholar]
- [46].Zhang W, Chang Q, Xu L, Li G, Yang G, Ding X, Wang X, Cui D, Jiang X, Adv. Healthcare Mater. 2016, 5, 1299. [DOI] [PubMed] [Google Scholar]
- [47].Kang S, Park JB, Lee T-J, Ryu S, Bhang SH, La W-G, Noh M-K, Hong BH, Kim B-S, Carbon 2015, 83, 162. [Google Scholar]
- [48].Kokubo T, Takadama H, Biomaterials 2006, 27, 2907. [DOI] [PubMed] [Google Scholar]
- [49].a) Raucci M, Giugliano D, Longo A, Zeppetelli S, Carotenuto G, Ambrosio L, J. Tissue Eng. Regener. Med 2017, 11, 2204; [DOI] [PubMed] [Google Scholar]; b) Wen T, Wu X, Liu M, Xing Z, Wang X, Xu A-W, Dalton Trans. 2014, 43, 7464. [DOI] [PubMed] [Google Scholar]
- [50].Barajaa MA, Nair LS, Laurencin CT, Regener. Eng. Transl. Med 2019. 10.1007/s40883-019-00132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Crowder SW, Prasai D, Rath R, Balikov DA, Bae H, Bolotin KI, Sung H-J, Nanoscale 2013, 5, 4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].a) Lu J, He YS, Cheng C, Wang Y, Qiu L, Li D, Zou D, Adv. Funct. Mater 2013, 23, 3494; [Google Scholar]; b) Lu J, Cheng C, He YS, Lyu C, Wang Y, Yu J, Qiu L, Zou D, Li D, Adv. Mater 2016, 28, 4025. [DOI] [PubMed] [Google Scholar]
- [53].Sarker B, Hum J, Nazhat SN, Boccaccini AR, Adv. Healthcare Mater 2015, 4, 176. [DOI] [PubMed] [Google Scholar]
- [54].Zhou C, Liu S, Li J, Guo K, Yuan Q, Zhong A, Yang J, Wang J, Sun J, Wang Z, ACS Appl. Mater. Interfaces 2018, 10, 44080. [DOI] [PubMed] [Google Scholar]
- [55].Kawamoto K, Miyaji H, Nishida E, Miyata S, Kato A, Tateyama A, Furihata T, Shitomi K, Iwanaga T, Sugaya T, Int. J. Nanomed 2018, 13, 2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Saravanan S, Chawla A, Vairamani M, Sastry T, Subramanian K, Selvamurugan N, Int. J. Biol. Macromol 2017, 104, 1975. [DOI] [PubMed] [Google Scholar]
- [57].a) Hermenean A, Codreanu A, Herman H, Balta C, Rosu M, Mihali CV, Ivan A, Dinescu S, Ionita M, Costache M, Sci. Rep 2017, 7, 16641; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dinescu S, Ionita M, Ignat S-R, Costache M, Hermenean A, Int. J. Mol. Sci 2019, 20, 5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].a) Wang W, Huang B, Byun JJ, Bartolo P, J. Mech. Behav. Biomed. Mater 2019, 93, 52; [DOI] [PubMed] [Google Scholar]; b) Palmieri V, Lattanzi W, Perini G, Augello A, Papi M, De Spirito M, 2D Mater. 2020, 7, 022004; [Google Scholar]; c) Choe G, Oh S, Seok JM, Park SA, Lee JY, Nanoscale 2019, 11, 23275; [DOI] [PubMed] [Google Scholar]; d) Wang W, Junior JRP, Nalesso PRL, Musson D, Cornish J, Mendonça F, Caetano GF, Bártolo P, Mater. Sci. Eng., C 2019, 100, 759. [DOI] [PubMed] [Google Scholar]
- [59].Jakus AE, Secor EB, Rutz AL, Jordan SW, Hersam MC, Shah RN, ACS Nano 2015, 9, 4636. [DOI] [PubMed] [Google Scholar]
- [60].a) Nalvuran H, Elçin AE, Elçin YM, Int. J. Biol. Macromol 2018, 114, 77; [DOI] [PubMed] [Google Scholar]; b) Duan S, Yang X, Mei F, Tang Y, Li X, Shi Y, Mao J, Zhang H, Cai Q, J. Biomed. Mater. Res., Part A 2015, 103, 1424; [DOI] [PubMed] [Google Scholar]; c) Song J, Gao H, Zhu G, Cao X, Shi X, Wang Y, Carbon 2015, 95, 1039. [Google Scholar]
- [61].Saravanan S, Vimalraj S, Anuradha D, Biomed. Pharmacother 2018, 107, 908. [DOI] [PubMed] [Google Scholar]
- [62].Zhao C, Zeng Z, Qazvini NT, Yu X, Zhang R, Yan S, Shu Y, Zhu Y, Duan C, Bishop E, ACS Biomater. Sci. Eng 2018, 4, 2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim HD, Kim J, Koh RH, Shim J, Lee J-C, Kim T-I, Hwang NS, ACS Biomater. Sci. Eng 2017, 3, 2470. [DOI] [PubMed] [Google Scholar]
- [64].Elkhenany H, Bourdo S, Hecht S, Donnell R, Gerard D, Abdelwahed R, Lafont A, Alghazali K, Watanabe F, Biris AS, Nanomedicine 2017, 13, 2117. [DOI] [PubMed] [Google Scholar]
- [65].a) Xie H, Cao T, Gomes JV, Neto AHC, Rosa V, Carbon 2015, 93, 266; [Google Scholar]; b) Rosa V, Xie H, Dubey N, Madanagopal TT, Rajan SS, Morin JLP, Islam I, Neto AHC, Dent. Mater 2016, 32, 1019; [DOI] [PubMed] [Google Scholar]; c) Xie H, Chua M, Islam I, Bentini R, Cao T, Viana-Gomes JC, Neto AHC, Rosa V, Dent. Mater 2017, 33, e13. [DOI] [PubMed] [Google Scholar]
- [66].Nishida E, Miyaji H, Kato A, Takita H, Iwanaga T, Momose T, Ogawa K, Murakami S, Sugaya T, Kawanami M, Int. J. Nanomed 2016, 11, 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N, Int. J. Biol. Macromol 2010, 47, 1. [DOI] [PubMed] [Google Scholar]
- [68].Li M, Xiong P, Yan F, Li S, Ren C, Yin Z, Li A, Li H, Ji X, Zheng Y, Cheng Y, Bioact. Mater 2018, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].a) Shin YC, Lee JH, Jin OS, Kang SH, Hong SW, Kim B, Park J-C, Han D-W, Carbon 2015, 95, 1051; [Google Scholar]; b) Lee JH, Shin YC, Lee S-M, Jin OS, Kang SH, Hong SW, Jeong C-M, Huh JB, Han D-W, Sci. Rep 2015, 5, 18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lee JH, Shin YC, Jin OS, Kang SH, Hwang Y-S, Park J-C, Hong SW, Han D-W, Nanoscale 2015, 7, 11642. [DOI] [PubMed] [Google Scholar]
- [71].Zhou K, Yu P, Shi X, Ling T, Zeng W, Chen A, Yang W, Zhou Z, ACS Nano 2019, 13, 9595. [DOI] [PubMed] [Google Scholar]
- [72].Nie W, Peng C, Zhou X, Chen L, Wang W, Zhang Y, Ma PX, He C, Carbon 2017, 116, 325. [Google Scholar]
- [73].Xie X, Hu K, Fang D, Shang L, Tran SD, Cerruti M, Nanoscale 2015, 7, 7992. [DOI] [PubMed] [Google Scholar]
- [74].Peng S, Feng P, Wu P, Huang W, Yang Y, Guo W, Gao C, Shuai C, Sci. Rep 2017, 7, 46604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen C, Sun X, Pan W, Hou Y, Liu R, Jiang X, Zhang L, ACS Sustainable Chem. Eng 2018, 6, 3862. [Google Scholar]
- [76].Fu C, Bai H, Zhu J, Niu Z, Wang Y, Li J, Yang X, Bai Y, PLoS One 2017, 12, e0188352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dalgic AD, Alshemary AZ, Tezcaner A, Keskin D, Evis Z, J. Biomater. Appl 2018, 32, 1392. [DOI] [PubMed] [Google Scholar]
- [78].Tatavarty R, Ding H, Lu G, Taylor RJ, Bi X, Chem. Commun 2014, 50, 8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shie M-Y, Chiang W-H, Chen I-WP, Liu W-Y, Chen Y-W, Mater. Sci. Eng., C 2017, 73, 726. [DOI] [PubMed] [Google Scholar]
- [80].Zhao C, Lu X, Zanden C, Liu J, Biomed. Mater 2015, 10, 015019. [DOI] [PubMed] [Google Scholar]
- [81].Zhang Q, Li K, Yan J, Wang Z, Wu Q, Bi L, Yang M, Han Y, Biochem. Biophys. Res. Commun 2018, 497, 1011. [DOI] [PubMed] [Google Scholar]
- [82].Ettorre V, De Marco P, Zara S, Perrotti V, Scarano A, Di Crescenzo A, Petrini M, Hadad C, Bosco D, Zavan B, Carbon 2016, 103, 291. [Google Scholar]
- [83].a) Rad AT, Novin M, Solati-Hashjin M, Vali H, Faghihi S, Colloids Surf., B 2013, 103, 200; [DOI] [PubMed] [Google Scholar]; b) Rad AT, Solati-Hashjin M, Osman NAA, Faghihi S, Ceram. Int 2014, 40, 12681. [Google Scholar]
- [84].Li K, Yan J, Wang C, Bi L, Zhang Q, Han Y, Biochem. Biophys. Res. Commun 2017, 489, 187. [DOI] [PubMed] [Google Scholar]
- [85].Park KO, Lee JH, Park JH, Shin YC, Huh JB, Bae J-H, Kang SH, Hong SW, Kim B, Yang DJ, Appl. Spectrosc. Rev 2016, 51, 540. [Google Scholar]
- [86].Zhao C, Pandit S, Fu Y, Mijakovic I, Jesorka A, Liu J, RSC Adv. 2016, 6, 38124. [Google Scholar]
- [87].Li J, Wang G, Geng H, Zhu H, Zhang M, Di Z, Liu X, Chu PK, Wang X, ACS Appl. Mater. Interfaces 2015, 7, 19876. [DOI] [PubMed] [Google Scholar]
- [88].a) Li M, Liu Q, Jia Z, Xu X, Cheng Y, Zheng Y, Xi T, Wei S, Carbon 2014, 67, 185; [Google Scholar]; b) Zeng Y, Pei X, Yang S, Qin H, Cai H, Hu S, Sui L, Wan Q, Wang J, Surf. Coat. Technol 2016, 286, 72. [Google Scholar]
- [89].Santos C, Piedade C, Uggowitzer P, Montemor M, Carmezim M, Appl. Surf. Sci 2015, 345, 387. [Google Scholar]
- [90].Xie Y, Li H, Ding C, Zheng X, Li K, Int. J. Nanomed 2015, 10, 3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kim J, Cote LJ, Kim F, Yuan W, Shull KR, Huang J, J. Am. Chem. Soc 2010, 132, 8180. [DOI] [PubMed] [Google Scholar]
- [92].Chung C, Kim Y-K, Shin D, Ryoo S-R, Hong BH, Min D-H, Acc. Chem. Res 2013, 46, 2211. [DOI] [PubMed] [Google Scholar]
- [93].a) La WG, Park S, Yoon HH, Jeong GJ, Lee TJ, Bhang SH, Han JY, Char K, Kim BS, Small 2013, 9, 4051; [DOI] [PubMed] [Google Scholar]; b) La W-G, Jin M, Park S, Yoon H-H, Jeong G-J, Bhang SH, Park H, Char K, Kim B-S, Int. J. Nanomed 2014, 9, 107; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) La W-G, Jung M-J, Yoon J-K, Bhang SH, Jang H-K, Lee T-J, Yoon H-H, Shin J-Y, Kim B-S, Carbon 2014, 78, 428. [Google Scholar]
- [94].Hong H-S, Yoon KJ, Son Y, Arch. Pharmacal Res 2011, 34, 2003. [DOI] [PubMed] [Google Scholar]
- [95].a) Fu C, Yang X, Tan S, Song L, Sci. Rep 2017, 7, 12549; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu J, Zheng A, Liu Y, Jiao D, Zeng D, Wang X, Cao L, Jiang X, Int. J. Nanomed 2019, 14, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yao Q, Liu Y, Sun H, J. Tissue Eng. Regener. Med 2018, 12, 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Arnold AM, Holt BD, Daneshmandi L, Laurencin CT, Sydlik SA, Proc. Natl. Acad. Sci. USA 2019, 116, 4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hasani-Sadrabadi MM, Hajrezaei SP, Emami SH, Bahlakeh G, Daneshmandi L, Dashtimoghadam E, Seyedjafari E, Jacob KI, Tayebi L, Nanomedicine 2015, 11, 1809. [DOI] [PubMed] [Google Scholar]
- [99].Jung HS, Lee T, Kwon IK, Kim HS, Hahn SK, Lee CS, ACS Appl. Mater. Interfaces 2015, 7, 9598. [DOI] [PubMed] [Google Scholar]
- [100].Ren N, Li J, Qiu J, Yan M, Liu H, Ji D, Huang J, Yu J, Liu H, Dent. Mater 2017, 33, 525. [DOI] [PubMed] [Google Scholar]
- [101].Lu H, Liu Y, Guo J, Wu H, Wang J, Wu G, Int. J. Mol. Sci 2016, 17, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Weng W, Nie W, Zhou Q, Zhou X, Cao L, Ji F, Cui J, He C, Su J, RSC Adv. 2017, 7, 2753. [Google Scholar]
- [103].Han L, Sun H, Tang P, Li P, Xie C, Wang M, Wang K, Weng J, Tan H, Ren F, Biomater. Sci 2018, 6, 538. [DOI] [PubMed] [Google Scholar]
- [104].O’Neill E, Awale G, Daneshmandi L, Umerah O, Lo KW-H, Drug Discovery Today 2018, 23, 879. [DOI] [PubMed] [Google Scholar]
- [105].Jaidev L, Kumar S, Chatterjee K, Colloids Surf., B 2017, 159, 293. [DOI] [PubMed] [Google Scholar]
- [106].Chen Y, Zheng Z, Zhou R, Zhang H, Chen C, Xiong Z, Liu K, Wang X, ACS Appl. Mater. Interfaces 2019, 11, 15986. [DOI] [PubMed] [Google Scholar]
- [107].Kumar S, Chatterjee K, Nanoscale 2015, 7, 2023. [DOI] [PubMed] [Google Scholar]
- [108].Arnold AM, Holt BD, Sydlik SA, Laurencin CT, Daneshmandi L, Google Patents, 2020. [Google Scholar]
- [109].Han W, Wu Z, Li Y, Wang Y, Chem. Eng. J. 2018. [Google Scholar]
- [110].a) Akhavan O, Ghaderi E, ACS Nano 2010, 4, 5731; [DOI] [PubMed] [Google Scholar]; b) Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y, ACS Nano 2011, 5, 6971. [DOI] [PubMed] [Google Scholar]
- [111].a) Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim J-H, Int. J. Nanomed 2012, 7, 5901; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Krishnamoorthy K, Veerapandian M, Zhang L-H, Yun K, Kim SJ, J. Phys. Chem. C 2012, 116, 17280. [Google Scholar]
- [112].Mokkapati V, Pandit S, Kim J, Martensson A, Lovmar M, Westerlund F, Mijakovic I, R. Soc. Open Sci 2018, 5, 181083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Liu S, Hu M, Zeng TH, Wu R, Jiang R, Wei J, Wang L, Kong J, Chen Y, Langmuir 2012, 28, 12364. [DOI] [PubMed] [Google Scholar]
- [114].Hui L, Piao J-G, Auletta J, Hu K, Zhu Y, Meyer T, Liu H, Yang L, ACS Appl. Mater. Interfaces 2014, 6, 13183. [DOI] [PubMed] [Google Scholar]
- [115].Zou F, Zhou H, Jeong DY, Kwon J, Eom SU, Park TJ, Hong SW, Lee J, ACS Appl. Mater. Interfaces 2017, 9, 1343. [DOI] [PubMed] [Google Scholar]
- [116].Li J, Wang G, Zhu H, Zhang M, Zheng X, Di Z, Liu X, Wang X, Sci. Rep 2015, 4, 4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Carpio IEM, Santos CM, Wei X, Rodrigues DF, Nanoscale 2012, 4, 4746. [DOI] [PubMed] [Google Scholar]
- [118].Karahan HE, Wei L, Goh K, Wiraja C, Liu Z, Xu C, Jiang R, Wei J, Chen Y, Small 2016, 12, 951. [DOI] [PubMed] [Google Scholar]
- [119].a) Sasidharan A, Swaroop S, Chandran P, Nair S, Koyakutty M, Nanomedicine 2016, 12, 1347; [DOI] [PubMed] [Google Scholar]; b) Xu M, Zhu J, Wang F, Xiong Y, Wu Y, Wang Q, Weng J, Zhang Z, Chen W, Liu S, ACS Nano 2016, 10, 3267; [DOI] [PubMed] [Google Scholar]; c) Li Y, Liu Y, Fu Y, Wei T, Le Guyader L, Gao G, Liu R-S, Chang Y-Z, Chen C, Biomaterials 2012, 33, 402; [DOI] [PubMed] [Google Scholar]; d) Liu Y, Luo Y, Wu J, Wang Y, Yang X, Yang R, Wang B, Yang J, Zhang N, Sci. Rep 2013, 3, 3469; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Zhang X, Wei C, Li Y, Li Y, Chen G, He Y, Yi C, Wang C, Yu D, J. Biomed. Mater. Res., Part A 2020, 108, 614. [DOI] [PubMed] [Google Scholar]
- [120].Chang Y, Yang S-T, Liu J-H, Dong E, Wang Y, Cao A, Liu Y, Wang H, Toxicol. Lett 2011, 200, 201. [DOI] [PubMed] [Google Scholar]
- [121].Das S, Singh S, Singh V, Joung D, Dowding JM, Reid D, Anderson J, Zhai L, Khondaker SI, Self WT, Part. Part. Syst. Charact 2013, 30, 148. [Google Scholar]
- [122].Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, Cui D, Nanoscale Res. Lett 2011, 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ma J, Liu R, Wang X, Liu Q, Chen Y, Valle RP, Zuo YY, Xia T, Liu S, ACS Nano 2015, 9, 10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Orecchioni M, Jasim DA, Pescatori M, Manetti R, Fozza C, Sgarrella F, Bedognetti D, Bianco A, Kostarelos K, Delogu LG, Adv. Healthcare Mater 2016, 5, 276. [DOI] [PubMed] [Google Scholar]
- [125].Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, Sun Y-P, Yan B, ACS Appl. Mater. Interfaces 2012, 4, 2259. [DOI] [PubMed] [Google Scholar]
- [126].Yue H, Wei W, Yue Z, Wang B, Luo N, Gao Y, Ma D, Ma G, Su Z, Biomaterials 2012, 33, 4013. [DOI] [PubMed] [Google Scholar]
- [127].Akhavan O, Ghaderi E, Akhavan A, Biomaterials 2012, 33, 8017. [DOI] [PubMed] [Google Scholar]
- [128].a) Sasidharan A, Panchakarla L, Chandran P, Menon D, Nair S, Rao C, Koyakutty M, Nanoscale 2011, 3, 2461; [DOI] [PubMed] [Google Scholar]; b) Sasidharan A, Panchakarla LS, Sadanandan AR, Ashokan A, Chandran P, Girish CM, Menon D, Nair SV, Rao C, Koyakutty M, Small 2012, 8, 1251. [DOI] [PubMed] [Google Scholar]
- [129].Sun J, Deng Y, Li J, Wang G, He P, Tian S, Bu X, Di Z, Yang S, Ding G, ACS Appl. Mater. Interfaces 2016, 8, 10226. [DOI] [PubMed] [Google Scholar]
- [130].a) Pinto AM, Gonçalves C, Sousa DM, Ferreira AR, Moreira JA, Gonçalves IC, Magalhães FD, Carbon 2016, 99, 318; [Google Scholar]; b) Zhang W, Yan L, Li M, Zhao R, Yang X, Ji T, Gu Z, Yin J-J, Gao X, Nie G, Toxicol. Lett 2015, 237, 61. [DOI] [PubMed] [Google Scholar]
- [131].Li Y, Feng L, Shi X, Wang X, Yang Y, Yang K, Liu T, Yang G, Liu Z, Small 2014, 10, 1544. [DOI] [PubMed] [Google Scholar]
- [132].Zhi X, Fang H, Bao C, Shen G, Zhang J, Wang K, Guo S, Wan T, Cui D, Biomaterials 2013, 34, 5254. [DOI] [PubMed] [Google Scholar]
- [133].Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, Fan C, Huang Q, ACS Nano 2011, 5, 3693. [DOI] [PubMed] [Google Scholar]
- [134].a) Talukdar Y, Rashkow JT, Lalwani G, Kanakia S, Sitharaman B, Biomaterials 2014, 35, 4863; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liao K-H, Lin Y-S, Macosko CW, Haynes CL, ACS Appl. Mater. Interfaces 2011, 3, 2607. [DOI] [PubMed] [Google Scholar]
- [135].a) Ali-Boucetta H, Bitounis D, Raveendran-Nair R, Servant A, Van den Bossche J, Kostarelos K, Adv. Healthcare Mater 2013, 2, 433; [DOI] [PubMed] [Google Scholar]; b) Chng ELK, Chua CK, Pumera M, Nanoscale 2014, 6, 10792. [DOI] [PubMed] [Google Scholar]
- [136].Zhang X, Yin J, Peng C, Hu W, Zhu Z, Li W, Fan C, Huang Q, Carbon 2011, 49, 986. [Google Scholar]
- [137].Liu J-H, Wang T, Wang H, Gu Y, Xu Y, Tang H, Jia G, Liu Y, Toxicol. Res 2015, 4, 83. [Google Scholar]
- [138].Syama S, Paul W, Sabareeswaran A, Mohanan PV, Biomaterials 2017, 131, 121. [DOI] [PubMed] [Google Scholar]
- [139].Kanakia S, Toussaint JD, Chowdhury SM, Tembulkar T, Lee S, Jiang Y-P, Lin RZ, Shroyer KR, Moore W, Sitharaman B, Biomaterials 2014, 35, 7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].a) Jasim DA, Boutin H, Fairclough M, Menard-Moyon C, Prenant C, Bianco A, Kostarelos K, Appl. Mater. Today 2016, 4, 24; [Google Scholar]; b) Cho YC, Pak PJ, Joo YH, Lee H-S, Chung N, Sci. Rep 2016, 6, 38884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sydlik SA, Jhunjhunwala S, Webber MJ, Anderson DG, Langer R, ACS Nano 2015, 9, 3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].a) Duch MC, Budinger GS, Liang YT, Soberanes S, Urich D, Chiarella SE, Campochiaro LA, Gonzalez A, Chandel NS, Hersam MC, Nano Lett. 2011, 11, 5201; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bengtson S, Knudsen KB, Kyjovska ZO, Berthing T, Skaug V, Levin M, Koponen IK, Shivayogimath A, Booth TJ, Alonso B, PLoS One 2017, 12, e0178355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sasidharan A, Swaroop S, Koduri CK, Girish CM, Chandran P, Panchakarla L, Somasundaram VH, Gowd GS, Nair S, Koyakutty M, Carbon 2015, 95, 511. [Google Scholar]
- [144].Mishra P, Nayak B, Dey R, Asian J Pharm. Sci 2016, 11, 337. [Google Scholar]
- [145].Yang K, Wan J, Zhang S, Zhang Y, Lee S-T, Liu Z, ACS Nano 2011, 5, 516. [DOI] [PubMed] [Google Scholar]
- [146].Qu G, Wang X, Liu Q, Liu R, Yin N, Ma J, Chen L, He J, Liu S, Jiang G, J. Environ. Sci 2013, 25, 873. [DOI] [PubMed] [Google Scholar]
- [147].Yang K, Gong H, Shi X, Wan J, Zhang Y, Liu Z, Biomaterials 2013, 34, 2787. [DOI] [PubMed] [Google Scholar]
- [148].Fu C, Liu T, Li L, Liu H, Liang Q, Meng X, Biomaterials 2015, 40, 23. [DOI] [PubMed] [Google Scholar]
- [149].Park E-J, Lee G-H, Han BS, Lee B-S, Lee S, Cho M-H, Kim J-H, Kim D-W, Arch. Toxicol 2015, 89, 1557. [DOI] [PubMed] [Google Scholar]
- [150].Schinwald A, Murphy F, Askounis A, Koutsos V, Sefiane K, Donaldson K, Campbell CJ, Nanotoxicology 2014, 8, 824. [DOI] [PubMed] [Google Scholar]
- [151].Yan L, Wang Y, Xu X, Zeng C, Hou J, Lin M, Xu J, Sun F, Huang X, Dai L, Chem. Res. Toxicol 2012, 25, 1265. [DOI] [PubMed] [Google Scholar]
- [152].Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B, Biochim. Biophys. Acta, Gen. Subj 2011, 1810, 361. [DOI] [PubMed] [Google Scholar]
- [153].a) Girish CM, Sasidharan A, Gowd GS, Nair S, Koyakutty M, Adv. Healthcare Mater 2013, 2, 1489; [DOI] [PubMed] [Google Scholar]; b) Mukherjee SP, Gliga AR, Lazzaretto B, Brandner B, Fielden M, Vogt C, Newman L, Rodrigues AF, Shao W, Fournier PM, Nanoscale 2018, 10, 1180. [DOI] [PubMed] [Google Scholar]
- [154].Holt BD, Arnold AM, Sydlik SA, Adv. Healthcare Mater 2016, 5, 3056. [DOI] [PubMed] [Google Scholar]
- [155].a) Dimiev AM, Ceriotti G, Behabtu N, Zakhidov D, Pasquali M, Saito R, Tour JM, ACS Nano 2013, 7, 2773; [DOI] [PubMed] [Google Scholar]; b) Wright Z, Arnold A, Holt B, Eckhart K, Sydlik S, Regener. Eng. Transl. Med 2019, 5, 190. [Google Scholar]
- [156].Xing W, Lalwani G, Rusakova I, Sitharaman B, Part. Part. Syst. Charact 2014, 31, 745. [Google Scholar]
- [157].Kurapati R, Russier J, Squillaci MA, Treossi E, Ménard-Moyon C, Del Rio-Castillo AE, Vazquez E, Samorì P, Palermo V, Bianco A, Small 2015, 11, 3985. [DOI] [PubMed] [Google Scholar]
- [158].Kurapati R, Mukherjee SP, Martín C, Bepete G, Vázquez E, Pénicaud A, Fadeel B, Bianco A, Angew. Chem., Int. Ed 2018, 57, 11722. [DOI] [PubMed] [Google Scholar]
- [159].Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, Klein-Seetharaman J, Kagan VE, Star A, ACS Nano 2011, 5, 2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Loeblein M, Perry G, Tsang SH, Xiao W, Collard D, Coquet P, Sakai Y, Teo EHT, Adv. Healthcare Mater 2016, 5, 1177. [DOI] [PubMed] [Google Scholar]
- [161].Kurapati R, Bonachera F, Russier J, Sureshbabu AR, Ménard-Moyon C, Kostarelos K, Bianco A, 2D Mater. 2017, 5, 015020. [Google Scholar]
- [162].Winkler T, Sass F, Duda G, Schmidt-Bleek K, Bone Jt. Res 2018, 7, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].a) Xiong K, Wu T, Fan Q, Chen L, Yan M, ACS Appl. Mater. Interfaces 2017, 9, 44356; [DOI] [PubMed] [Google Scholar]; b) Shuai C, Zeng Z, Yang Y, Qi F, Peng S, Yang W, He C, Wang G, Qian G, Mater. Des 2020, 190, 108564; [Google Scholar]; c) Yao Q, Liu H, Lin X, Ma L, Zheng X, Liu Y, Huang P, Yu S, Zhang W, Lin M, J. Biomed. Nanotechnol 2019, 15, 602. [DOI] [PubMed] [Google Scholar]
- [164].a) Mukherjee S, Sriram P, Barui AK, Nethi SK, Veeriah V, Chatterjee S, Suresh KI, Patra CR, Adv. Healthcare Mater 2015, 4, 1722; [DOI] [PubMed] [Google Scholar]; b) Park J, Kim YS, Ryu S, Kang WS, Park S, Han J, Jeong HC, Hong BH, Ahn Y, Kim BS, Adv. Funct. Mater 2015, 25, 2590. [Google Scholar]
- [165].a) Wang Y, Hu X, Dai J, Wang J, Tan Y, Yang X, Yang S, Yuan Q, Zhang Y, J. Mater. Chem. B 2017, 5, 6794; [DOI] [PubMed] [Google Scholar]; b) Dou C, Ding N, Luo F, Hou T, Cao Z, Bai Y, Liu C, Xu J, Dong S, Adv. Sci 2018, 5, 1700578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Luo PG, Sahu S, Yang S-T, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L, Sun Y-P, J. Mater. Chem. B 2013, 1, 2116. [DOI] [PubMed] [Google Scholar]
- [167].Reina G, González-Domínguez JM, Criado A, Vázquez E, Bianco A, Prato M, Chem. Soc. Rev 2017, 46, 4400. [DOI] [PubMed] [Google Scholar]
- [168].Sanchez VC, Jachak A, Hurt RH, Kane AB, Chem. Res. Toxicol 2012, 25, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]