Abstract
Objective:
This investigation used magnetoencephalography (MEG) to identify the neurophysiological mechanisms contributing to the altered cognition seen in adults with cerebral palsy (CP).
Methods:
Adults with CP (GMFCS levels I-IV) and demographically-matched controls completed a Sternberg-type working memory task during MEG. Secondarily, they completed the National Institutes of Health (NIH) cognitive toolbox. Beamforming was used to image the significant MEG oscillatory responses and the resulting images were examined using statistical parametric mapping to identify cortical activity that differed between groups.
Results:
Both groups had a left-lateralized decrease in alpha–beta (11–16 Hz) power across the occipital, temporal, and prefrontal cortices during encoding, as well as an increase in alpha (9–13 Hz) power across the occipital cortices during maintenance. The strength of alpha–beta oscillations in the prefrontal cortices were weaker in those with CP during encoding. Weaker alpha–beta oscillation within the prefrontal cortex was associated with poorer performance on the NIH toolbox and a higher GMFCS level.
Conclusions:
Alpha-beta aberrations may impact the basic encoding of information in adults with CP, which impacts their overall cognition. Altered alpha–beta oscillation might be connected with gross motor function.
Significance:
This experimental work highlights the aberrant alpha–beta during encoding as possible neurophysiological mechanism of the cognitive deficiencies.
Keywords: Alpha, Beta, Encoding, Magnetoencephalography, Maintenance
1. Introduction.
Cerebral palsy (CP) is the most common pediatric-onset neuromuscular disorder, and is identified with lifelong disability (Christensen et al., 2014, Rosenbaum et al., 2007). The initial definition of CP described the disorder as a nonprogressive permanent brain lesion in the developing brain that impacts the motor system, with no mention of non-motor symptoms (Morris, 2007). However, it is now widely acknowledged that cognitive impairments often accompany motor impairments in individuals with CP (Bax et al., 2005), with about half of all individuals with CP exhibiting cognitive deficits (Novak et al., 2012).
Working memory is generally described as a cognitive process by which information is temporarily stored and/or transformed for use toward a current goal. Working memory includes three distinct sub-phases: encoding, maintenance, and retrieval operations. Briefly, encoding involves loading information into memory, maintenance refers to the active storage and rehearsal of information, and retrieval is the active recall of information (Baddeley, 1992a, 2000, Baddeley et al., 2011, D’Esposito, 2007, Heinrichs-Graham and Wilson, 2015). Several behavioral studies have identified working memory impairments in children and adults with CP (Bottcher et al., 2010, Di Lieto et al., 2017, Goble et al., 2012, Jenks et al., 2009, Jenks et al., 2007, Jenks et al., 2012, Toomela, 2012). Specifically, working memory deficits in children and adults with CP have been suggested to impact arithmetic (Jenks et al., 2009, Jenks et al., 2007, Jenks et al., 2012, Pueyo et al., 2009), proprioception (Goble et al., 2012), as well as social and learning problems (Bottcher et al., 2010, Di Lieto et al., 2017). Despite the mounting evidence for working memory impairments seen in individuals with CP, the neurophysiological underpinnings of these deficits remains unknown.
Numerous functional magnetic resonance imaging (fMRI) have determined that the dorsolateral prefrontal cortices (DLPFC), parietal-occipital areas, and the left supramarginal gyrus comprise key networks for verbal working memory processing (c.f., Cabeza and Nyberg, 2000). Electroencephalography and magnetoencephalography (MEG) studies have extended these findings by quantifying the spatiotemporal cortical dynamics of verbal working memory in healthy participants (Bonnefond and Jensen, 2012, Heinrichs-Graham and Wilson, 2015, Wilson et al., 2016). These investigations have identified an initial decrease in alpha–beta (9–16 Hz) cortical oscillations bilaterally in the occipital cortices during early encoding, which then spreads anterior across the middle temporal, supramarginal, and DLPFC areas of the left hemisphere during the encoding and early maintenance phases. Such left hemispheric oscillatory activity is thought to reflect the initial processing/decoding of verbal information and the loading and rehearsal of such information during working memory processing (Embury et al., 2018, Embury et al., 2019, Heinrichs-Graham and Wilson, 2015, Proskovec et al., 2016, Proskovec et al., 2019, Wiesman et al., 2016, Wilson et al., 2017). These studies have also identified a strong increase in alpha (8–14 Hz) activity in parieto-occipital cortices during later maintenance. This increase has been directly linked with the inhibition of incoming task-irrelevant visual information during the maintenance phase (Bonnefond and Jensen, 2012, Jensen et al., 2002, Tuladhar et al., 2007). Potentially, alterations in these extensively studied working memory cortical dynamics may be partially connected with the cognitive impairments seen in adults with CP.
In the current study, we used MEG to evaluate if the cortical dynamics serving working memory are altered in adults with CP compared to demographically similar healthy controls. Given the numerous behavioral studies indicating that adults with CP have various degrees of cognitive impairment (Bottcher et al., 2010, Di Lieto et al., 2017, Goble et al., 2012, Jenks et al., 2009, Jenks et al., 2007, Jenks et al., 2012, Toomela, 2012), we hypothesized that the cortical oscillations associated with working memory would be altered in adults with CP compared with the controls. Secondarily, we hypothesized that these atypical oscillations would be associated with declines in cognitive performance as reflected by the Cognitive Battery of the NIH Toolbox.
2. Methods
2.1. Participants
Adults with CP (N = 17; Age = 32.9 ± 2.2 yrs.; spastic diplegic N = 14, spastic hemiplegic 3) and controls (N = 19; Age = 33.0 ± 2. 3 yrs.) participated in this investigation. The adults with CP had gross motor function classification score (GMFCS) levels that ranged between I-IV, where level I indicates a mild gait disturbance and IV indicates larger mobility challenges that often results in the dependence on the use of powered mobility in most settings. All participants had sufficient visual and cognitive abilities to complete the task as determined through a practice session immediately prior to the recording. Each adult provided written consent to participate in the investigation. The protocol for this investigation was approved by the University of Nebraska Medical Center’s Institutional Review Board and in compliance with the Code of Ethics of the World Medical Association.
2.2. Working memory task experiment
Participants performed a four-load, letter-based version of a Sternberg-type working memory task during MEG (Sternberg, 1966). Throughout the task, the participants focused on a fixation cross centrally located within a 2 × 3 grid on the screen (Fig. 1). Each trial began with an empty grid for 1300 ms. The grid was then filled with four consonants for 2000 ms, signifying the encoding period. Dollar signs were shown within the grid locations not occupied by consonants. The consonants then disappeared from the grid indicating the start of the maintenance period. Lastly, following the 3000 ms maintenance period, a single “probe” consonant appeared for 900 ms during the retrieval period. The participants respond with their right hand via a button pad as to whether the probe letter was one of the four letters previously presented. A button press with the second digit indicated “yes”, while a button press with the third digit indicated “no”. Each trial lasted 7200 ms, (including the 1300 ms pre-stimulus fixation), and each participant completed 128 trials. Our laboratory has validated the use of this four load working memory task in previous work (Proskovec et al., 2019).
Fig. 1.
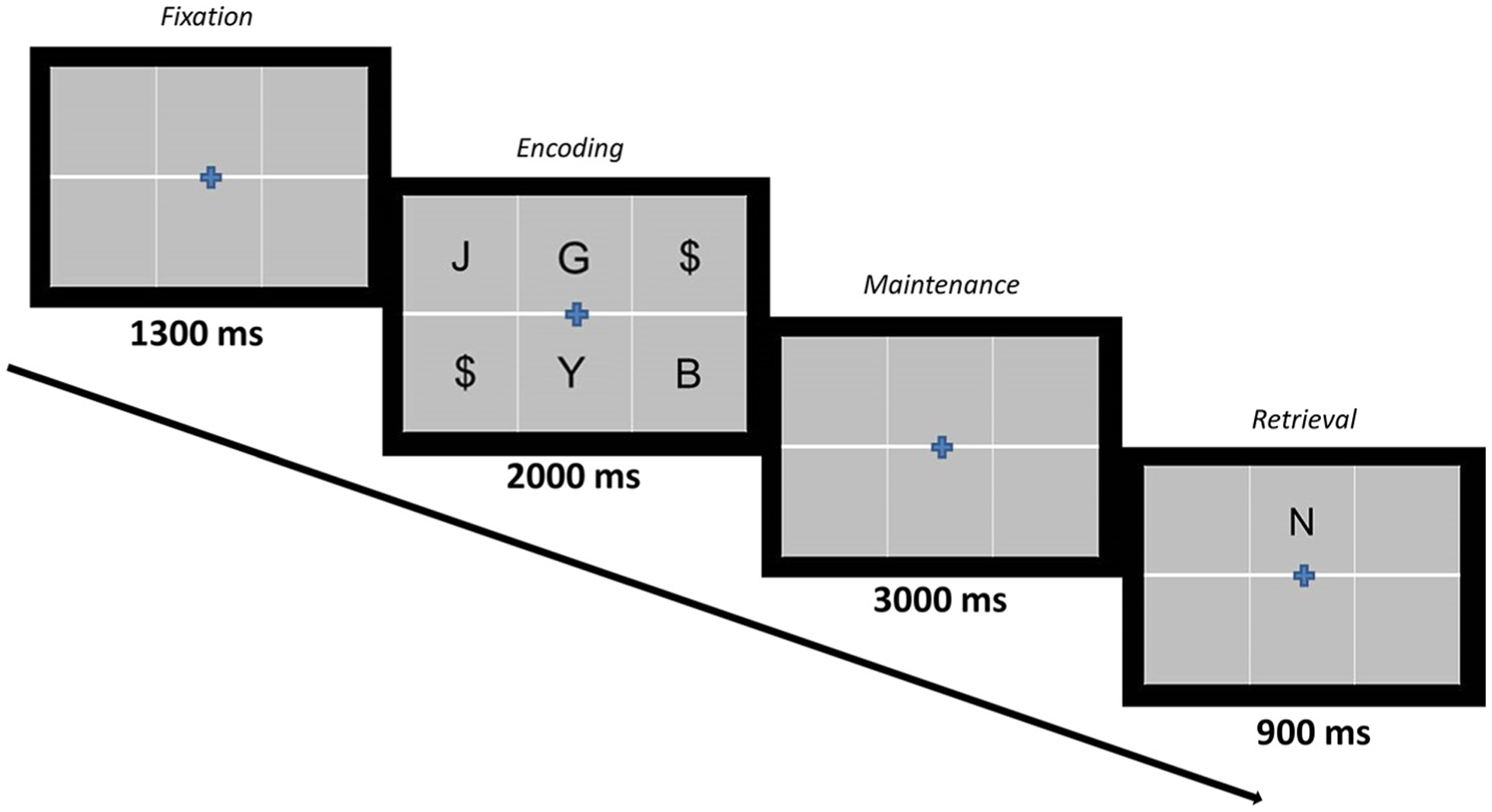
Verbal Working Memory Task Paradigm. Each trial began with the presentation of a fixation cross that was within an empty grid for 1300 ms, followed by the appearance of four consonants within the grid for 2000 ms (encoding), then an empty grid for 3000 ms (maintenance), and lastly the probe letter for 900 ms (retrieval). During retrieval, participants responded via a button pad with their right hand as to whether the probe was a letter in the previous encoding set.
2.3. MEG data acquisition and coregistration with MRI
A more detailed description of the MEG methods employed in this investigation can be found in (Proskovec et al., 2019, Wiesman and Wilson, 2020). An Elekta MEG system (Helsinki, Finland) was used to collect the neuromagnetic responses at 1 kHz (acquisition bandwidth of 0.1–330 Hz). The respective neuromagnetic data was corrected for head motion and the signal space separation method with a temporal extension was used for noise reduction (Taulu and Simola, 2006, Taulu et al., 2005). Four coils were attached to the participant’s head and fed a unique frequency labeled current (e.g., 322 Hz) during the MEG data acquisition; these were used for head localization throughout the recording. The location of the coils and digitized scalp surface points (Fastrak, Polhemus, Colchester, VT, USA) were also used to transform the data into a standard space in order to co-register the MEG data with the high-resolution T1-weighted axial images, which were obtained with a 64-channel head/neck coil and a Siemens Prisma 3 T scanner (TR: 1720 ms; TE: 2.48 ms; flip angle = 8 deg; FOV: 230 mm; slice thickness: 1 mm; in-plane resolution: 0.9 × 0.9 × 1.0 mm).
2.4. MEG time-frequency transformation and statistics
The neuromagnetic time series was divided into epochs of 7200 ms in duration, with 0 ms defined as the onset of the encoding grid and the baseline being −400 to 0 ms. The raw sensor-level data were inspected for artifacts that were induced by eye blinks, heartbeats, and/or muscle, and signal space projection was used to remove such artifacts (Uusitalo and Ilmoniemi, 1997). A fixed threshold method was then applied whereby trials that had an amplitude greater than 1495 fT between successive data points were rejected. Artifact-free epochs for each sensor were transformed into the time–frequency domain using complex demodulation and averaged over trials (Kovach and Gander, 2016). In brevity, the complex demodulation approach involves applying a heterodyning procedure to the Fourier transformed MEG signal through a series of complex sinusoids that have increasing carrier frequencies. A 4 Hz low pass finite impulse response filter (frequency step of 2 Hz and a time-step of 25 ms) was subsequently applied to the complex signals to prevent spectral leakage. The resulting time–frequency data were then normalized per time–frequency bin using the mean power for the specific frequency bin during the baseline. The time–frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. This involved performing paired-sample t-tests against baseline on each pixel of the spectrograms, and then controlling for multiple comparisons through a cluster based non-parametric permutation approach using 1000 permutations (Ernst, 2004, Maris and Oostenveld, 2007). Note that the determination of the time–frequency windows to image was based on statistical analysis of spectrograms across the whole array of gradiometers and all participants (i.e., collapsed across both groups). In other words, no group comparisons were performed at the sensor level. For a more detailed description of our methodology, see (Wiesman and Wilson, 2020).
2.5. MEG imaging and statistics
The source power across the entire brain volume was determined with the dynamic imaging of coherent sources (DICS) beamforming algorithm (Gross et al., 2001, Hillebrand et al., 2005). The source power in these images (4.0 × 4.0 × 4.0 mm resolution) was normalized per subject using a separately averaged pre-stimulus noise period of equal duration and bandwidth (Hillebrand and Barnes, 2005, Hillebrand et al., 2005, Van Veen et al., 1997). To investigate the effect of group on the oscillatory sources serving working memory, we averaged each participant’s source-level oscillatory activity (pseudo-t values) across time separately for the encoding and maintenance windows (i.e., one averaged map for encoding, one averaged map for maintenance). Whole-brain, voxel-wise independent-sample t-tests between groups were then computed separately for the encoding and maintenance periods, and a cluster-correction was applied to the resulting statistical parametric maps (SPM). The peak voxel was then identified in each image (i.e., encoding and maintenance) and the individual pseudo-t values were extracted for correlational analyses with the behavioral data and the cognitive testing results based on the NIH Toolbox. For a more detailed description of our imaging methodology, see (Wiesman and Wilson, 2020).
2.6. Neuropsychological testing and behavioral measures
Prior to the MEG experiment, each participant underwent the National Institutes of Health (NIH) Toolbox Cognition Battery for 18+ year-olds (Weintraub et al., 2013). The NIH Toolbox Cognition Battery includes the cognitive constructs of attention, executive function, episodic memory, working memory, language, and processing speed. Construct and composite scores are automatically computed as part of this test battery.
In addition, the response selection during the MEG experiment was used to quantify behavioral performance. The time the participant took to respond as to whether the probe letter was one of the four letters previously presented letters was defined as the reaction time. Accuracy was defined as the number of correct trials divided by the total number of trials, and this was multiplied by 100 per participant to convert to a percentage for reporting. Lastly, Spearman rank-order correlations were utilized to determine potential relationships between demographic, behavioral, and neuronal outcome metrics.
3. Results.
3.1. Behavioral results
Of the 36 participants who completed the task, two adults with CP and two controls were excluded due to excessive head motion and/or other MEG artifacts, while two additional controls were excluded due to poor accuracy. These latter participants scored less than three standard deviations below the mean for each group (mean ± 1 SD; CP: 84.9 ± 10.0%; controls: 92.3 ± 8.8%). The remaining 13 adults with CP (Age = 34.2 ± 0.6 yrs.) and 16 controls (Age = 34.7 ± 0.7 yrs.) did not differ on age, sex, and other demographic factors. Compared with the controls, the adults with CP were slower (CP: 1000.8 ± 78.8 ms; controls: 712.6 ± 30.4 ms; P = 0.001) and less accurate on the working memory task (CP: 81.9 + 4.0% ms; controls: 92.2 + 2.0 % P = 0.049). Furthermore, the adults with CP had lower demographically normalized composite scores on the NIH Toolbox Cognitive battery when compared with the controls (CP: 45.2 ± 10.5; controls: 54.4 ± 8.9; P = 0.02). Note that both correct and incorrect trials were included for the MEG analyses, and we controlled for the total number of accepted epochs per group to avoid differences in the signal-to-noise ratio (CP: 90.3 ± 14.5 trials; controls: 98.4 ± 12.5 trials). The range of trails accepted for the participants with CP and controls was 60–107 trials and 63–109 trials, respectively.
3.2. Sensor level results
Statistical analysis of the time–frequency spectrograms revealed a significant cluster of decreased alpha–beta (11–16 Hz) oscillatory activity that began 200 ms after encoding onset and was sustained throughout the remainder of the encoding time window until dissipating shortly after the onset of maintenance at about 2200 ms (P < 0.001, corrected; Fig. 2). During the maintenance phase, there emerged a narrower band of increased alpha (9–13 Hz) activity (P < 0.001, corrected; Fig. 2). This alpha increase began around 2600 ms (i.e., 600 ms into the maintenance period) and persisted throughout the remainder of the maintenance period and terminated shortly into the retrieval period. These two oscillatory responses were observed in a large number of posterior gradiometers, located bilaterally near the parietal and occipital cortices. For illustrative purposes, we show the results from a peak sensor located near the parieto-occipital region across groups and separately for controls and the adults with CP (Fig. 2). However, note that the statistical analysis of the sensor-level spectrogram data was done sensor-by-sensor across both groups (i.e., paired t-tests against baseline per time–frequency bin following by permutation testing). Qualitative, inspection of the time–frequency components shown in Fig. 2 suggest that the strength of the alpha–beta encoding response and the alpha maintenance response were notably weaker for the adults with CP when compared with the controls.
Fig. 2.
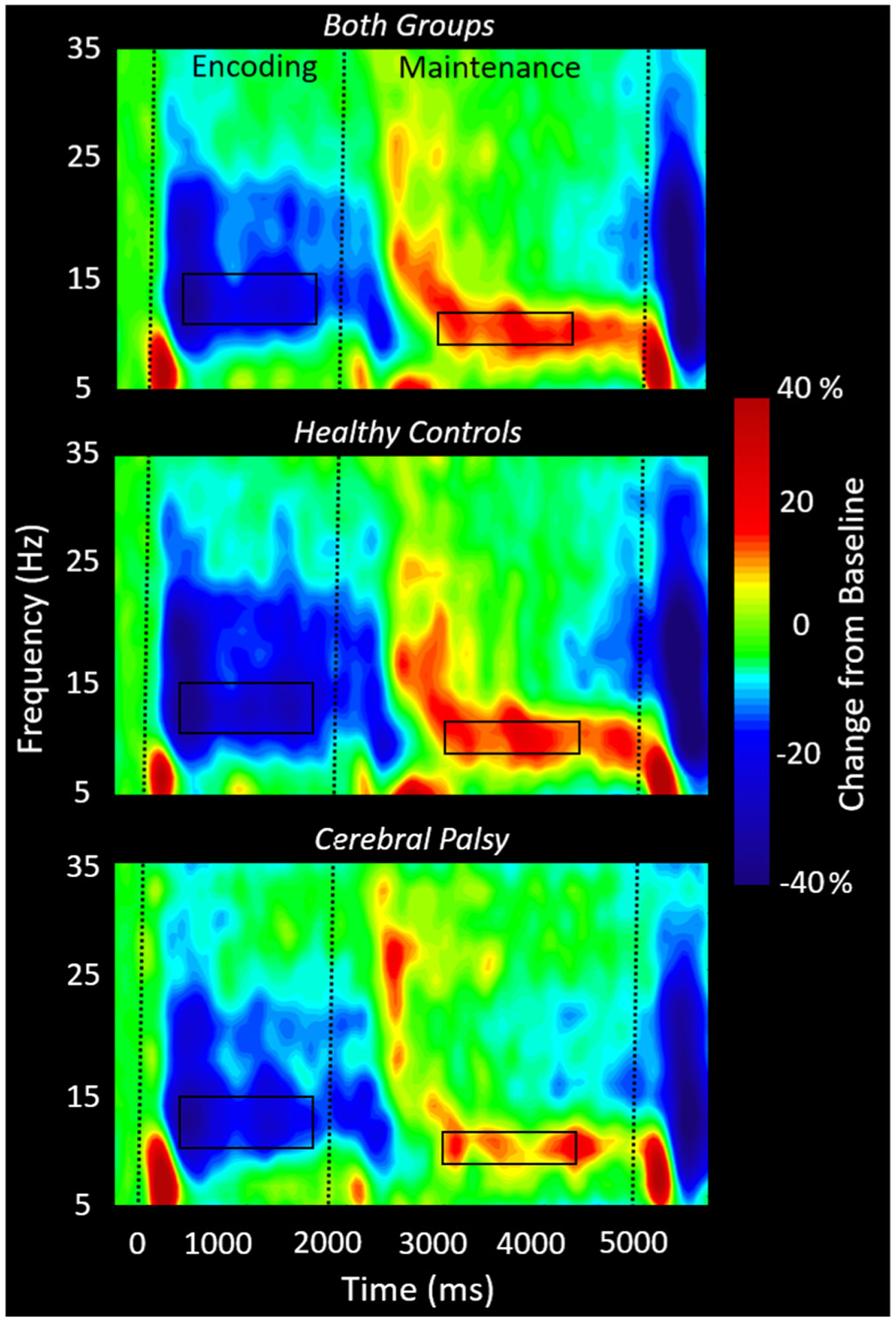
Group-averaged spectrograms from the same gradiometer sensor (located near the right parietal-occipital junction) are shown collapsed across groups (top) for the controls (middle) and adults with cerebral palsy (CP) (bottom). The time–frequency spectrogram displays time (ms) on the x-axis and frequency (Hz) on the y-axis. Percent power change was computed using the mean power in the baseline period (−400 to 0 ms) per bin. As shown in the respective figures, a significant decrease in alpha–beta (11–16 Hz) power can be discerned throughout the encoding and into early maintenance, followed by a significant alpha (9–13 Hz) increase throughout mid to late maintenance. Visual inspection of the spectrograms suggests that adults with CP had weaker oscillatory activity in the alpha–beta and alpha range compared to controls in the encoding and maintenance time windows, respectively. Time-frequency windows for source imaging (beamforming) were derived from statistical analysis of the sensor-level spectrogram data across all participants and these windows are outlined by the black boxes.
3.3. Encoding source level results
To evaluate the dynamics of oscillatory changes in the alpha–beta range, we applied a beamformer to the following windows throughout the encoding period using a common baseline of equal duration and bandwidth (i.e., −400 to 0 ms, 11–16 Hz: 200–600 ms, 600–1000 ms, 1000–1400 ms, 1400–1800 ms). The beamformer images across all participants revealed a decrease in alpha–beta power in left hemispheric brain regions throughout the encoding period. The response initially emerged in the occipital cortices during early encoding and extended to the left temporal and inferior frontal regions throughout the remainder of encoding (Fig. 3).
Fig. 3.
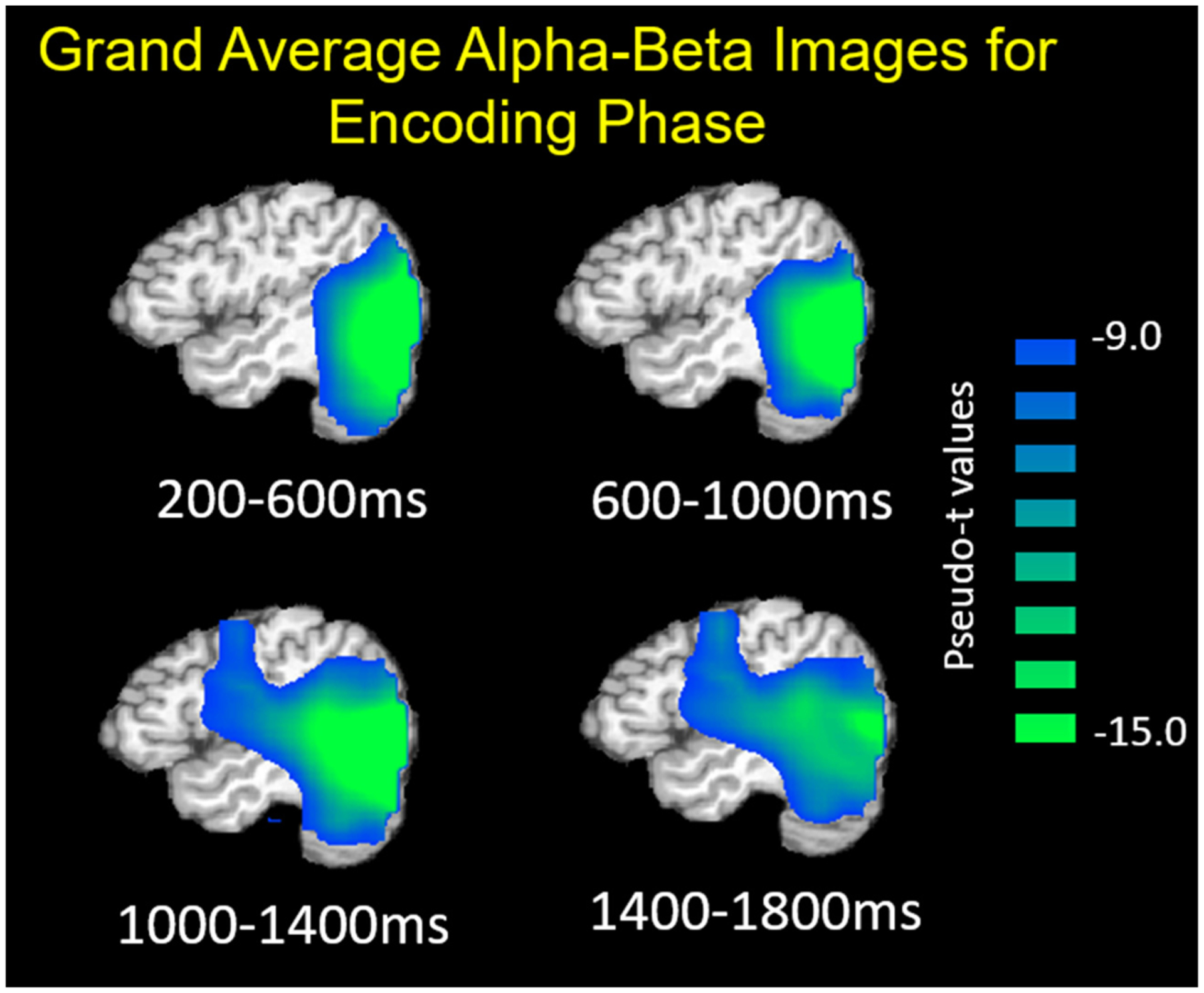
Grand-averaged beamformer images (pseudo-t) during the encoding period showing left hemispheric alpha–beta (11–16 Hz) oscillatory dynamics. A strong decrease in alpha–beta activity relative to baseline (−400 to 0 ms) can be seen at the beginning of encoding in the occipital cortices, stretching anterior to the temporal and prefrontal regions during later encoding.
To investigate the effect of group on the neural oscillations during encoding, we averaged each participant’s whole-brain alpha–beta oscillatory maps (pseudo-t values) across the encoding and transition time window (200–1800 ms). Independent sample t-tests were then used to compute statistical parametric maps showing the group differences. These images were thresholded and a cluster-correction (see methods) was applied to the suprathresholded voxels to reduce the risk of false positives due to multiple comparisons. These images revealed that the alpha–beta oscillatory response during encoding was weaker in the prefrontal cortex in the adults with CP compared with the controls during encoding (P < 0.001; Fig. 4A).
Fig. 4.
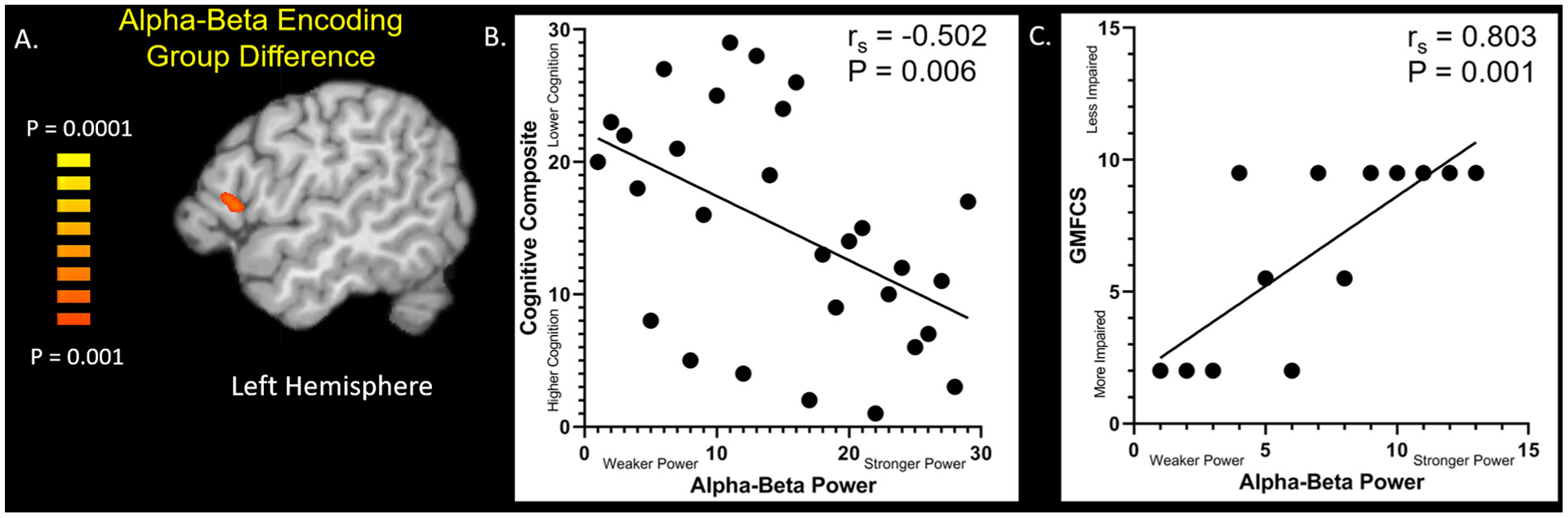
Alpha-Beta Oscillatory Group Difference. (A) Significant group differences (P < 0.001, corrected) during the encoding period were observed in the prefrontal cortices. (B) The strength of the alpha–beta power seen in the prefrontal cortices had a negative rank order relationship with the composite score for the National Institutes of Health (NIH) Toolbox Cognitive Battery (rs = −0.502; P = 0.006). This relationship suggests that those with weaker alpha–beta oscillations tended to have lower scores on the NIH Toolbox cognitive assessment. (C) In the adults with CP, the strength of the alpha–beta power seen in the prefrontal cortices had a positive rank-order correlation with the Gross Motor Function Classification Score level (rs = 0.803; P = 0.001). Adults that had a weaker alpha–beta response had greater mobility deficits.
3.4. Maintenance source level results
Similar to our approach for encoding, we initially imaged the dynamics of alpha changes during maintenance by applying a beamformer to the following time windows using a common baseline of equal duration and bandwidth (i.e., −400 to 0 ms, 9–13 Hz: 2200–2600 ms, 2600–3000 ms, 3000–3400 ms, 3400–3800 ms, 3800–4200 ms, 4200–4600 ms, and 4600–5000 ms). The average maps indicated that across both groups there was an increase in alpha power during the maintenance period within the bilateral occipital cortices (Fig. 5).
Fig. 5.
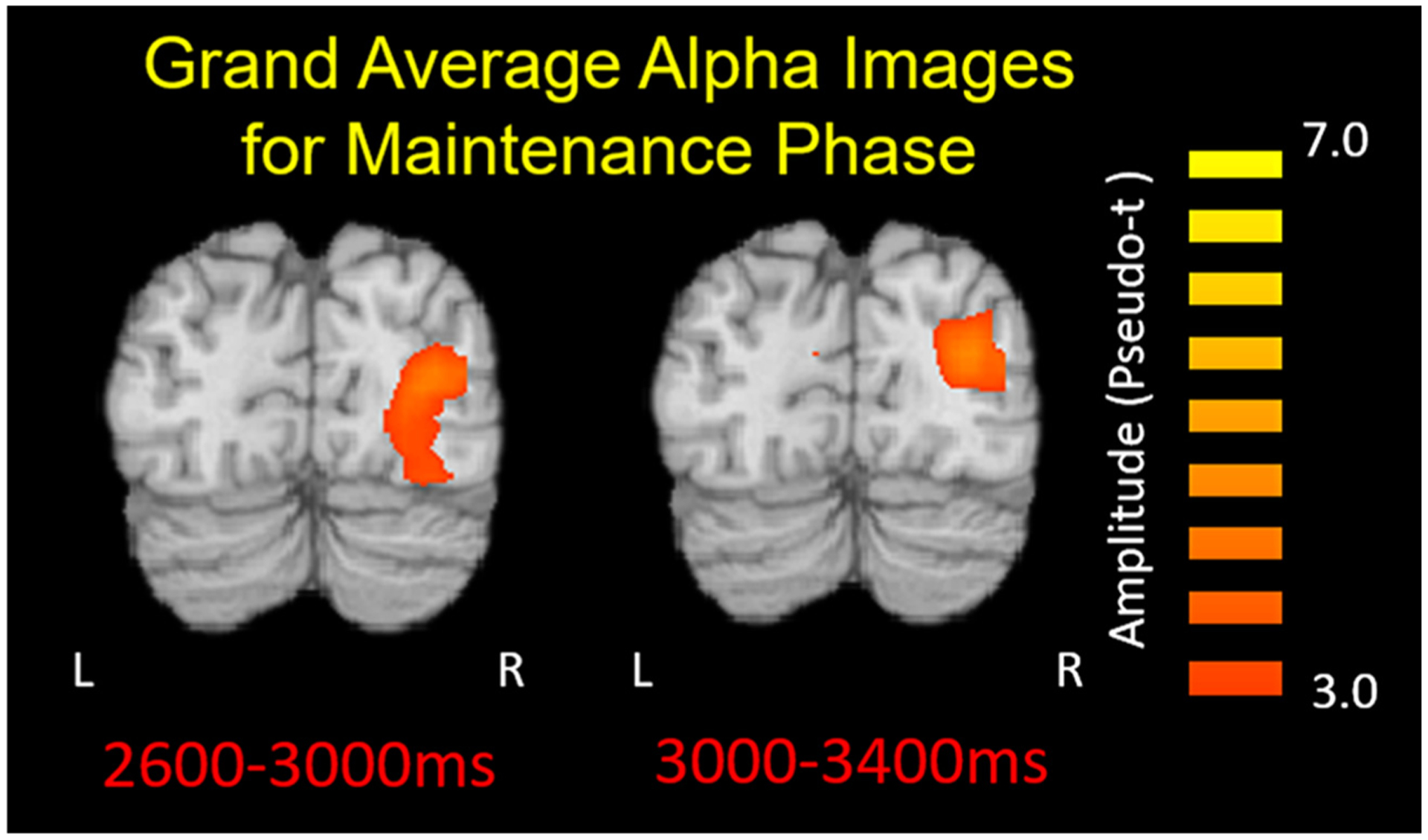
Grand-averaged beamformer images (pseudo-t) of the occipital alpha (9–13 Hz) response is displayed for a portion of the maintenance stage (2600–3800 ms). The current consensus is that the increased alpha power reflects neural activity involved in the inhibition of incoming visual information that could disturb maintenance of the recently encoded information.
As per group effects, similar to our approach above, we again averaged each participant’s whole-brain maps (pseudo-t values) across the maintenance time window (2200–5000 ms) and conducted voxel-wise independent sample t-tests on the output images to generate statistical parametric maps reflecting group differences in maintenance activity. These images revealed that there were no significant group differences were for alpha oscillations during maintenance (P > 0.05).
3.5. Neuro-behavioral results and correlations
Across groups, there was a significant positive rank-order relationship between the strength of prefrontal alpha–beta oscillations during encoding and the NIH Toolbox Cognitive Battery composite score (Fig. 4B; rs = −0.502, P = 0.006). This relationship indicates that those that had a weaker alpha–beta response tended to have a lower composite score. In the adults with CP, there was also a strong positive relationship between the strength of the alpha–beta response during encoding and the participant’s GMFCS value (Fig. 4C; rs = 0.803, P = 0.001), which indicates that adults with CP who have a weaker alpha–beta response tended to also have higher GMFCS scores (i.e., greater mobility impairment). All other correlations with the behavioral data were not significant (P > 0.05).
4. Discussion
In the current study, we utilized MEG to investigate the neural dynamics serving working memory in adults with CP. Both groups demonstrated a strong decrease in alpha–beta power during encoding that began in the occipital regions and spread to the left temporal and prefrontal cortices. During maintenance, strong neural responses in a narrower alpha band emerged in the posterior parietal and occipital cortices and was largely sustained until retrieval. In regards to group effects, we found that adults with CP had weaker alpha–beta oscillatory responses during encoding in the prefrontal cortices, while neural oscillatory responses during the maintenance phase did not differ between groups. Below, we discuss the implications of these findings for verbal working memory performance and possible therapeutic impacts.
For the entire group of participants, those that had a stronger decrease in the left-lateralized prefrontal alpha–beta oscillatory response during the encoding phase also tended to perform better on the NIH Toolbox Cognitive Battery. This correlation implies that disruptions in the prefrontal alpha–beta cortical oscillation during encoding might be connected with declines in cognitive performance. The alpha–beta power decrease seen in the temporal and prefrontal regions during encoding has been interpreted in the context of a widely popular Baddeley Model of working memory (Baddeley, 1992a, 1992b, 2000, Baddeley et al., 2011). Specifically, neural activity in these regions has been connected with the theoretical phonological loop and central executive constructs of this model (Baddeley, 1992a, D’Esposito, 2007, Heinrichs-Graham and Wilson, 2015, Proskovec et al., 2016, Rottschy et al., 2012). The phonological loop represents both vocal and sub-vocal rehearsal mechanisms, and is thought to have a key role in maintaining the encoded information (Baddeley, 1992b, 2000). Essentially, these studies imply that those with weaker prefrontal cortical oscillations during the encoding stage tend to have cascading disruptions on the phonological loop.
A key finding of this study was that the adults with CP had weaker alpha–beta oscillations during encoding in the prefrontal cortex. Such prefrontal oscillations during working memory encoding may reflect executive involvement in the loading of verbal information into left superior temporal and supramarginal memory buffers for temporary storage and rehearsal memory representations. Thus, the reduced oscillatory responses seen in adults with CP may indicate executive dysfunction during encoding, which is at least partially supported by the deficient performance observed in this group relative to controls. However, future neurophysiological studies are needed to decipher the precise role of these oscillations in working memory processing. That said, these overall findings are supported by previous clinical studies that have suggested individuals with CP have impairments in short-term memory processing during executive function tasks and that these deficits are related to the integrity of frontal and temporal cortices (Crichton et al., 2020, White and Christ, 2005). Together, our results imply that the neurophysiological nexus for the noted cognitive impairments in the clinical literature may partly be related to aberrations in the cortical oscillations involved in encoding information. Alterations in the strength of the alpha–beta prefrontal cortical oscillations may provide insight on the neurophysiological underpinnings that are driving the success of therapeutics that aim to improve cognition of adults with CP.
Another key finding of this study was that adults with CP that had higher GMFCS levels (e.g., greater mobility impairments) also tended to have weaker alpha–beta cortical oscillations during encoding. Associations between motor and cognitive abilities have been previously shown in healthy individuals (Diamond, 2000, Rigoli et al., 2012) and children with CP (Al-Nemr and Abdelazeim, 2018, Delacy and Reid, 2016, Gabis et al., 2015, Sigurdardottir et al., 2008). Some have explained this relationship by showing that overlapping regions of the prefrontal cortex serve specific motor and cognitive tasks when performed separately (Diamond, 2000), while others have shown that cognitive and motor performance are correlated (Al-Nemr and Abdelazeim, 2018, Rigoli et al., 2012). Pathologically speaking, the extent of brain injury may partially explain the relationship between GMFCS and working memory for our study, as more diffuse brain injuries are generally associated with more severe motor (Arnfield et al., 2013, Arrigoni et al., 2016, Lee et al., 2017, Lee et al., 2011, Meyns et al., 2016, Reid et al., 2015) and cognitive impairments (Himmelmann and Uvebrant, 2011, Krägeloh-Mann et al., 2002, Rai et al., 2013). An alternative explanation may be that individuals with CP that have greater mobility impairments tend to have fewer opportunities for interacting and exploring their environment (Harbourne and Berger, 2019, Logan et al., 2016). Hence, we suspect that the lack of rich environmental experiences may place individuals with CP at a disadvantage for the sustainment and development of higher cognitive functions like working memory.
During maintenance, both groups displayed an increase in alpha power within the bilateral occipital cortices. Several studies have suggested that this occipital alpha response reflects the inhibition of incoming information that could degrade the mental representations of the recently encoded information (Bonnefond and Jensen, 2012, Bonnefond and Jensen, 2013, Händel et al., 2011, Payne et al., 2013). Our results suggest that the strength of alpha oscillations during the maintenance stage was similar for both groups, indicating that those with CP were able to adequately inhibit incoming visual information from disturbing the encoded information. However, we believe caution is warranted here, as based on our results those with CP likely had deficient encoding and this reduction in information fidelity may mean that alpha oscillations seen during the maintenance period are artificially similar between the two groups because the adults with CP have less information to maintain. This premise is at least partially supported by the behavioral results, which showed that the adults with CP were less accurate on the working memory task relative to controls. Alternatively, it could be possible that the adults with CP have intact maintenance abilities, as reflected by no alpha power differences during this time period, and poorer accuracy may be explained by aberrations during the encoding period.
5. Conclusions
Although CP is predominantly thought of as a motor centric disorder, our results imply that the initial perinatal brain insults incurred by these individuals appear to also have other effects that impact the brain networks underlying high-order cognition. This premise was supported by the outcomes of our investigation that identified adults with CP have aberrations in alpha–beta oscillatory activity in the prefrontal cortex during the encoding phase of a working memory paradigm. These encoding aberrations likely have a direct impact on what information is maintained and retrieved, thus impacting overall performance. Our results point to the prefrontal alpha–beta cortical oscillations as a probable neurophysiological mechanism that contributes to the diminished cognition reported in the literature for adults with CP. Moreover, alterations in the strength of the alpha–beta oscillations provide means for gauging the success of therapeutics that aim to improve the cognition of adults with CP. Lastly, the cortical abnormalities identified here appear to be partial linked with mobility. These results highlight the possibility that physical therapy strategies that improve the mobility of adults with CP may have corollary cognitive improvements.
highlights.
Adults with cerebral palsy (CP) and controls completed a working memory task during MEG.
Prefrontal alpha–beta cortical oscillations were weaker in adults with CP during encoding.
Weaker alpha–beta oscillation may be the neurophysiological nexus of the reduced cognition seen in adults with CP.
Funding
This work was partially supported by the National Institutes of Health (1R01HD086245; R01HD101833) and a Promotion of Doctoral Studies I Scholarship from the Foundation for Physical Therapy Research.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Nemr A, Abdelazeim F. Relationship of cognitive functions and gross motor abilities in children with spastic diplegic cerebral palsy. Appl Neuropsychol Child 2018;7(3):268–76. [DOI] [PubMed] [Google Scholar]
- Arnfield E, Guzzetta A, Boyd R. Relationship between brain structure on magnetic resonance imaging and motor outcomes in children with cerebral palsy: a systematic review. Res Dev Disabil 2013;34(7):2234–50. [DOI] [PubMed] [Google Scholar]
- Arrigoni F, Peruzzo D, Gagliardi C, Maghini C, Colombo P, Iammarrone FS, et al. Whole-brain DTI assessment of white matter damage in children with bilateral cerebral palsy: evidence of involvement beyond the primary target of the anoxic insult. AJNR Am J Neurorad 2016;37(7):1347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A Working memory. Science 1992a;255(5044):556–9. [DOI] [PubMed] [Google Scholar]
- Baddeley A Working memory: the interface between memory and cognition. J Cog Neurosci 1992b;4(3):281–8. [DOI] [PubMed] [Google Scholar]
- Baddeley A The episodic buffer: a new component of working memory?. Trends Cogn Sci 2000;4(11):417–23. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Allen RJ, Hitch GJ. Binding in visual working memory: the role of the episodic buffer. Neuropsych 2011;49(6):1393–400. [DOI] [PubMed] [Google Scholar]
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol 2005;47(8):571–6. [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol 2012;22(20):1969–74. [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. The role of gamma and alpha oscillations for blocking out distraction. Commun Int Biol 2013;6(1). e22702–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher L, Flachs EM, Uldall P. Attentional and executive impairments in children with spastic cerebral palsy. Dev Med Child Neurol 2010;52(2):e42–7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000;12(1):1–47. [DOI] [PubMed] [Google Scholar]
- Christensen D, Van Naarden BK, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 2014;56(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton A, Ditchfield M, Gwini S, Wallen M, Thorley M, Bracken J, et al. Brain magnetic resonance imaging is a predictor of bimanual performance and executive function in children with unilateral cerebral palsy. Dev Med Child Neurol 2020. 10.1111/dmcn.14462. [DOI] [PubMed] [Google Scholar]
- D’Esposito M From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 2007;362(1481):761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacy MJ, Reid SM. Australian Cerebral Palsy Register G. Profile of associated impairments at age 5 years in Australia by cerebral palsy subtype and Gross Motor Function Classification System level for birth years 1996 to 2005. Dev Med Child Neurol 2016;58(Suppl 2):50–6. [DOI] [PubMed] [Google Scholar]
- Di Lieto MC, Brovedani P, Pecini C, Chilosi AM, Belmonti V, Fabbro F, et al. Spastic diplegia in preterm-born children: executive function impairment and neuroanatomical correlates. Res Dev Disabil 2017;61:116–26. [DOI] [PubMed] [Google Scholar]
- Diamond A Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Devel 2000;71(1):44–56. [DOI] [PubMed] [Google Scholar]
- Embury CM, Wiesman AI, Proskovec AL, Heinrichs-Graham E, McDermott TJ, Lord GH, et al. Altered brain dynamics in patients with type 1 diabetes during working memory processing. Diabetes 2018;67(6):1140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury CM, Wiesman AI, Proskovec AL, Mills MS, Heinrichs-Graham E, Wang YP, et al. Neural dynamics of verbal working memory processing in children and adolescents. Neuroimage 2019;185:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: a basis for exact inference. Stat Science 2004;19 (4):676–85. [Google Scholar]
- Gabis LV, Tsubary NM, Leon O, Ashkenasi A, Shefer S. Assessment of abilities and comorbidities in children with cerebral palsy. J Child Neurol 2015;30 (12):1640–5. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Aaron MB, Warschausky S, Kaufman JN, Hurvitz EA. The influence of spatial working memory on ipsilateral remembered proprioceptive matching in adults with cerebral palsy. Exp Brain Res 2012;223(2):259–69. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A 2001;98(2):694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Händel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cog Neurosci 2011;23(9):2494–502. [DOI] [PubMed] [Google Scholar]
- Harbourne RT, Berger SE. Embodied cognition in practice: exploring effects of a motor-based problem-solving intervention. Phys Ther 2019;99(6):786–96. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex 2015;69:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR. Beamformer analysis of MEG data. Int Rev Neurobiol 2005;68:149–71. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 2005;25 (2):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelmann K, Uvebrant P. Function and neuroimaging in cerebral palsy: a population-based study. Dev Med Child Neurol 2011;53(6):516–21. [DOI] [PubMed] [Google Scholar]
- Jenks KM, de Moor J, van Lieshout EC. Arithmetic difficulties in children with cerebral palsy are related to executive function and working memory. J Child Psychol Psychiatry 2009;50(7):824–33. [DOI] [PubMed] [Google Scholar]
- Jenks KM, de Moor J, van Lieshout EC, Maathuis KG, Keus I, Gorter JW. The effect of cerebral palsy on arithmetic accuracy is mediated by working memory, intelligence, early numeracy, and instruction time. Dev Neuropsychol 2007;32 (3):861–79. [DOI] [PubMed] [Google Scholar]
- Jenks KM, van Lieshout EC, de Moor JM. Cognitive correlates of mathematical achievement in children with cerebral palsy and typically developing children. Br J Educ Psychol 2012;82:120–35. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex 2002;12(8):877–82. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Gander PE. The demodulated band transform. J Neurosci Methods 2016;261:135–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krägeloh-Mann I, Helber A, Mader I, Staudt M, Wolff M, Groenendaal F, et al. Bilateral lesions of thalamus and basal ganglia: origin and outcome. Dev Med Child Neurol 2002;44(7):477–84. [DOI] [PubMed] [Google Scholar]
- Lee D, Pae C, Lee JD, Park ES, Cho S-R, Um M-H, et al. Analysis of structure-function network decoupling in the brain systems of spastic diplegic cerebral palsy. Hum Brain Mapp 2017;38(10):5292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 2011;134:1199–210. [DOI] [PubMed] [Google Scholar]
- Logan SW, Ross SM, Schreiber MA, Feldner HA, Lobo MA, Catena MA, et al. Why we move: social mobility behaviors of non-disabled and disabled children across childcare contexts. Front Pub Health 2016;4:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. J Neurosci Methods 2007;164(1):177–90. [DOI] [PubMed] [Google Scholar]
- Meyns P, Van Gestel L, Leunissen I, De Cock P, Sunaert S, Feys H, et al. Macrostructural and microstructural brain lesions relate to gait pathology in children with cerebral palsy. Neurorehab Neural Repair 2016;30(9):817–33. [DOI] [PubMed] [Google Scholar]
- Morris C. Definition and classification of cerebral palsy: a historical perspective. Dev Med Child Neurol Supplement 2007;109:3–7. [DOI] [PubMed] [Google Scholar]
- Novak I, Hines M, Goldsmith S, Barclay R. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics 2012;130(5):e1285–312. [DOI] [PubMed] [Google Scholar]
- Payne L, Guillory S, Sekuler R. Attention-modulated alpha-band oscillations protect against intrusion of irrelevant information. J Cog Neurosci 2013;25(9):1463–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Hum Brain Mapp 2016;37(6):2348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Load modulates the alpha and beta oscillatory dynamics serving verbal working memory. Neuroimage 2019;184:256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo R, Junqué C, Vendrell P, Narberhaus A, Segarra D. Neuropsychologic impairment in bilateral cerebral palsy. Ped Neurol 2009;40(1):19–26. [DOI] [PubMed] [Google Scholar]
- Rai Y, Chaturvedi S, Paliwal VK, Goyal P, Chourasia A, Singh Rathore RK, et al. DTI correlates of cognition in term children with spastic diplegic cerebral palsy. Eur J Paediatr Neurol 2013;17(3):294–301. [DOI] [PubMed] [Google Scholar]
- Reid SM, Ditchfield MR, Bracken J, Reddihough DS. Relationship between characteristics on magnetic resonance imaging and motor outcomes in children with cerebral palsy and white matter injury. Res Devel Disabil 2015;45–46:178–87. [DOI] [PubMed] [Google Scholar]
- Rigoli D, Piek JP, Kane R, Oosterlaan J. An examination of the relationship between motor coordination and executive functions in adolescents. Dev Med Child Neurol 2012;54(11):1025–31. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007;109:8–14. [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 2012;60(1):830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir S, Eiriksdottir A, Gunnarsdottir E, Meintema M, Arnadottir U, Vik T. Cognitive profile in young Icelandic children with cerebral palsy. Dev Med Child Neurol 2008;50(5):357–62. [DOI] [PubMed] [Google Scholar]
- Sternberg S High-speed scanning in human memory. Science 1966;153 (3736):652–4. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 2006;51 (7):1759–68. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method. IEEE Trans Signal Proc 2005;53(9):3359–72. [Google Scholar]
- Toomela A Short-term memory in young adults with spastic diplegic cerebral palsy. Devel Neuropsych 2012;37(4):317–32. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp 2007;28(8):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Compu 1997;35(2):135–40. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 1997;44(9):867–80. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80(11 Suppl 3):S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Christ SE. Executive control of learning and memory in children with bilateral spastic cerebral palsy. J Int Neuropsych Soc 2005;11(7):920–4. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, McDermott TJ, Santamaria PM, Gendelman HE, Wilson TW. Quiet connections: reduced fronto-temporal connectivity in nondemented Parkinson’s Disease during working memory encoding. Hum Brain Mapp 2016;37(9):3224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW. Attention modulates the gating of primary somatosensory oscillations. Neuroimage 2020;211:116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Proskovec AL, McDermott TJ. Neuroimaging with magnetoencephalography: a dynamic view of brain pathophysiology. Transl Res 2016;175:17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Proskovec AL, Heinrichs-Graham E, O’Neill J, Robertson KR, Fox HS, et al. Aberrant neuronal dynamics during working memory operations in the aging HIV-infected brain. Sci Rep 2017;7:41568. [DOI] [PMC free article] [PubMed] [Google Scholar]


