Abstract
Objectives:
The AHA/ACC-2017 hypertension guideline recommends an age-independent target blood pressure (BP) of less than 130/80 mmHg. In an elderly cohort without established cardiovascular disease (CVD) at baseline, we determined the impact of this guideline on the prevalence of hypertension and associated CVD risk.
Methods:
Nineteen thousand, one hundred and fourteen participants aged at least 65 years from the ASPirin in Reducing Events in the Elderly (ASPREE) study were grouped by baseline BP: ‘pre-2017 hypertensive’ (BP ≥140/90 mmHg and/or on antihypertensive drugs); ‘reclassified hypertensive’ (normotensive by pre-2017 guidelines; hypertensive by AHA/ACC-2017 guideline), and ‘normotensive’ (BP <130 and <80 mmHg). For each group, we evaluated CVD risk factors, predicted 10-year CVD risk using the Atherosclerotic Cardiovascular Disease (ASCVD) risk equation, and reported observed CVD event rates during a median 4.7–year follow-up.
Results:
Overall, 74.4% (14 213/19 114) were ‘pre-2017 hypertensive’; an additional 12.3% (2354/19 114) were ‘reclassified hypertensive’ by the AHA/ACC-2017 guideline. Of those ‘reclassified hypertensive’, the majority (94.5%) met criteria for antihypertensive treatment although 29% had no other traditional CVD risk factors other than age. Further, a relatively lower mean 10-year predicted CVD risk (18% versus 26%, P<0.001) and lower CVD rates (8.9 versus 12.1/1000 person-years, P=0.01) were observed in ‘reclassified hypertensive’ compared with ‘pre-2017 hypertensive’. Compared with ‘normotensive’, a hazard ratio (95% confidence interval) for CVD events of 1.60 (1.26–2.02) for ‘pre-2017 hypertensive’ and 1.26 (0.93–1.71) for ‘reclassified hypertensive’ was observed.
Conclusion:
Applying current CVD risk calculators in the elderly ‘reclassified hypertensive’, as a result of shifting the BP threshold lower, increases eligibility for antihypertensive treatment but documented CVD rates remain lower than hypertensive patients defined by pre2017 BP thresholds.
Keywords: elderly, guidelines, hypertension, target blood pressure
INTRODUCTION
Elevated blood pressure (BP) is one of the leading risk factors for cardiovascular disease (CVD). An estimated 1.39 billion (~31%) of the world’s adult population aged at least 20 years had hypertension in 2010 [1], and more than 90% of individuals who are free of hypertension at 55 years of age will develop hypertension during their remaining lifespan [2]. Despite availability of effective treatments, the management of hypertension in the elderly population presents unique challenges [3,4].
Hypertension guidelines prior to 2017, as well as the recent 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) guideline, recommended BP (systolic/diastolic) target levels less than 140/90 mmHg based on office BP measurements; the exception being the Eight Joint National Commission (JNC-8) report, which recommended target levels of less than 150/90 mmHg for those individuals 60 years and older [5–7]. The American Heart Association and American College of Cardiology (AHA/ACC) 2017 guidelines for the management of high BP now recommend BP levels below 130/80 mmHg for all individuals, including older adults [8]. This recommendation follows findings from the Systolic Blood Pressure Intervention Trial (SPRINT), which demonstrated improved cardiovascular outcomes in high-risk people with BP targeted to a SBP treatment goal of 120 mmHg compared with a SBP goal of 140 mmHg [9].
We examined the prevalence of hypertension in an elderly cohort free of documented evidence of CVD, who were enrolled from Australia and the United States (US) into a primary prevention trial assessing aspirin’s ability to prolong healthy independent lifespan [10]. In this analysis, we sought to determine to what extent the change in classification of hypertension, by applying the AHA/ACC 2017 guideline, would increase the prevalence of hypertension in the cohort. Further, we quantified the presence of additional CVD risk factors in addition to hypertension, calculated predicted future CVD risk and observed CVD events among these elderly cohort; and compared between individuals reclassified as hypertensive patients by the new guideline and those deemed hypertensive by traditional (pre-2017) hypertension guideline.
METHODS
Study population
We used baseline and follow-up data from participants enrolled in the ASPirin in Reducing Events in the Elderly (ASPREE) study (NCT01038583). The ASPREE study design and primary findings have been previously published [10–12]. In brief, the study recruited 19 114 participants (16 703 in Australia and 2411 in the United States) during 2010–2014, and randomized participants in a double-blind manner to receive enteric-coated aspirin (100 mg daily) or matched placebo, with a median of 4.7 years of follow-up. Two baseline visits were conducted to determine study eligibility. Detailed information about inclusion and exclusion criteria has been previously reported [10]; in brief, ASPREE participants at enrolment were free of documented CVD or dementia, significant physical disability, or any illness expected to limit their life expectancy to less than 5 years. Further, participants with SBP at least 180 mmHg and/or DBP at least 105 mmHg were excluded. In Australia, recruitment was mostly conducted in the general (family) practice setting with the participant’s usual primary care (family) physician. In the United States, recruitment was community-based through academic and clinical trial centers. To achieve a goal of at least 50% minorities in the United States, the ASPREE recruitment protocol was modified in 2011 and recruitment in the United States was then restricted to minorities (e.g. African American, Hispanics) only, and their age of inclusion lowered to 65 years. ASPREE had multiple Institutional Review Board approvals in the United States and Australia.
Baseline measures
Participants were asked during baseline visits about their medical history and were assessed for physical function, lifestyle, and other clinical, anthropometric, and laboratory data. In addition, information about concomitant prescription medications was collected by review with the participant and through their general practice records. BP for each participant was measured three times on one occasion according to established guidelines by trained study staff, and following standardized operating procedures, prior to randomization to aspirin or placebo. The average of the three oscillometric BP measurements taken at least 1 min apart was recorded as the BP for that participant’s visit.
Follow-up and cardiovascular events
Participants were followed for a median of 4.7 years until the aspirin intervention ceased. CVD was a prespecified secondary endpoint, and consisted of fatal coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal stroke, and hospitalization for heart failure. All events were adjudicated by an endpoint committee blinded to treatment allocation.
Hypertension definition and grouping of ASPirin in Reducing Events in the Elderly study participants
We defined hypertension in the ASPREE participants at baseline according to BP and use of antihypertensives, and grouped them into one of three categories. Participants who had mean SBP at least 140 mmHg and/or mean DBP at least 90 mmHg (regardless of whether they were prescribed any antihypertensive), as well as those using any antihypertensive medication (regardless of BP), at baseline were classified as having hypertension. We termed this group ‘pre-2017 hypertensive’ as this definition used the traditional (pre-2017) BP threshold for classification of hypertension, and this group included both participants who were receiving treatment at baseline before the new AHA/ACC 2017 guideline was introduced, as well as treated participants with BP controlled (<140/90 mmHg). Next, we defined a second group of participants as ‘reclassified hypertensive’ if they were not hypertensive according to the pre-2017 definition above but did meet the BP thresholds set in the recent AHA/ACC 2017 guideline. These individuals had mean SBP 130–139 mmHg and/or DBP 80–89 mmHg and were not receiving any antihypertensive medication. Finally, participants with SBP less than 130 mmHg and DBP less than 80 mmHg, and not receiving any antihypertensives were classified as the ‘normotensive’, referent group. Antihypertensive medications were classified according to the WHO Anatomical Therapeutic Chemical (ATC) Classification System [i.e. antihypertensives (C02), diuretics (C03), peripheral vasodilators (C04), beta-blocking agents (C07), calcium channel blockers (C08) and agents acting on the renin–angiotensin system (C09)].
Statistical analyses
We used descriptive analysis (frequency/sample proportions or mean with standard deviations) to summarize the prevalence of hypertension and CVD risk factors among the ASPREE participants. Student t-test, ANOVA or chi-square tests were used to compare the distributions of baseline characteristics or risk factors in different participant subgroups (i.e. pre-2017 hypertensive, reclassified hypertensive, normotensive). Further, we assessed the distribution of risk factors and predicted risk for CVD events by participant subgroups. We categorized the presence of other traditional CVD risk factors (excluding hypertension) into ‘none’, ‘one’, ‘two’ and ‘three or more’; counting the presence of diabetes, hypercholesterolemia, smoking, obesity and reduced renal function. We used the modified Framingham risk score (FRS) [13] and the Atherosclerotic Cardiovascular Disease (ASCVD) risk score [14] at baseline to predict 10-year CVD risk for each participant. The FRS equation incorporates participant age, sex, total cholesterol, high-density lipoprotein (HDL) cholesterol, SBP, diabetes mellitus, antihypertensive medication use and current smoking status among those aged 74 or less years old. The ASCVD risk score includes information on age, sex, ethnicity, total cholesterol, HDL, SBP, diabetes, current smoking status and antihypertensive treatment for participants aged 79 years old or less. For participants with age higher than the maximum age for a risk prediction model, we substituted the maximum age of the respective model. We also conducted a restricted analysis to assess and compare the 10-year predicted risk among participants aged 74 years or less (for FRS) or 79 years or less (for ASCVD) by baseline hypertension status, as per the risk equation models.
We assessed the occurrence of documented CVD events (i.e. rate per 1000 person-years) during the follow-up period by participant groups (‘pre-2017’, ‘reclassified’ and ‘normotensive’). Cumulative incidences based on competing-risk regression models were used to show event risk, which were stratified according to groups based on baseline hypertension status among all ASPREE participants. In addition, a Cox proportional hazards model was used to compare hazards between groups. This analysis was adjusted for key potential confounders, such as age, sex, ethnicity, country of residence and randomized treatment regimen (i.e. aspirin/placebo). Further, given the importance of treating BP to prevent stroke in this age group, we repeated the above analysis for stroke outcomes.
Sensitivity analyses
To test the robustness of our results, we conducted several sensitivity analyses. First, we regrouped the ASPREE participants based on baseline BP level and whether they were prescribed antihypertensive medication or would be recommended to receive antihypertensive medication. This resulted in the following groups: pre-2017 hypertensive on medication (irrespective of BP level), pre-2017 hypertensive not on medication, ‘reclassified hypertensive’ recommended for antihypertensive medication (which includes participants with BP 130–139/80–89 mmHg and ASCVD risk score ≥10%); and participants not requiring treatment with antihypertensive medication (which includes ‘reclassified hypertensive’ not requiring treatment because of ASCVD predicted risk less than 10% and ‘normotensive’ participants). We assessed the ASCVD risk as well as CVD event rate in these re-grouped participants and used a Cox proportional hazards model to compare hazards between groups in relation to those ‘not requiring treatment with antihypertensive medication’.
Next, we regrouped the participants using actual BP and on-treatment status. This resulted in the following groups: BP at least 140/90 mmHg and prescribed antihypertensive; BP at least 140/90 mmHg and not prescribed antihypertensive; SBP130–39 mmHg/DBP 80–89 mmHg and prescribed antihypertensive; SBP130–39 mmHg /DBP 80–89 mmHg and not prescribed antihypertensive; SBP less than 130 mmHg and DBP <80 mmHg and prescribed antihypertensive; and SBP <130 mmHg and DBP <80 mmHg and not prescribed antihypertensive. We assessed the CVD event rate in these re-grouped participants and used a Cox proportional hazards model to compare hazards between groups in relation to those ‘SBP <130 mmHg and DBP <80 mmHg and not prescribed antihypertensive’. All statistical analyses were performed using Stata version 15.1 for Windows (StataCorp LP, College Station, Texas, USA).
RESULTS
Blood pressure
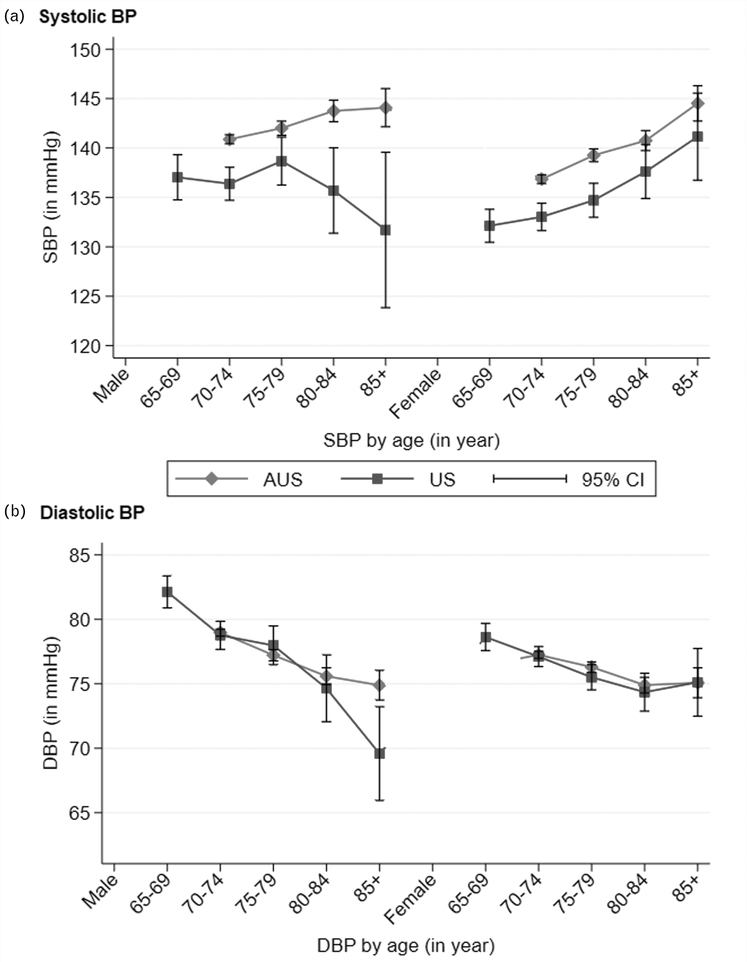
Mean BP (SBP/DBP) at baseline for the cohort was 139 ± 16/77 ± 10mmHg (Australia: 140 ± 16/77 ± 10mmHg, United States: 135 ± 17/77 ± 10mmHg). The distribution of SBP and DBP by age and sex for Australia and United States showed increasing SBP (except in United States men), but decreasing DBP with older age (Fig. 1). The mean SBP was higher in Australian compared with United States participants in all age groups, irrespective of sex. We did not observe any difference in mean DBP between countries by age–sex groups except for a lower DBP in the United States male participants aged 85 years and older.
FIGURE 1.
Distribution of SBP and DBP by age and sex among ASPREE participants from the United States and Australia.
Hypertension prevalence and impact of American College of Cardiology/American Heart Association 2017 hypertension definition
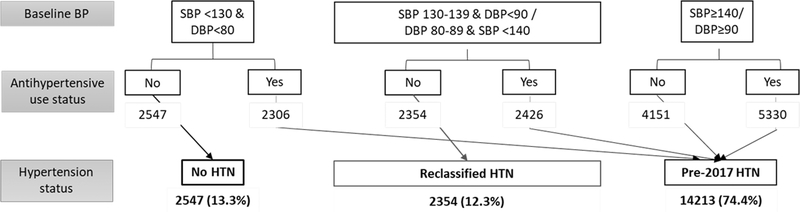
Figure 2 summarizes the ASPREE participants’ hypertension status at baseline based on baseline BP and antihypertensive status. Overall, 74.4% (95% CI: 73.7–75.0) of the study participants had hypertension (United States: 70% and Australia: 75%) based on ‘Pre-2017’ thresholds. Applying the AHA/ACC 2017 hypertension guidelines identified an additional 2354 individuals as hypertensive (‘reclassified hypertensive’), increasing the overall prevalence from 74.4 to 86.7% (95% CI: 86.2–87.2) with slight differences by country (US 83% and Australia 87%). Further, we observed an additional 2426 (~12% of ASPREE participants) elderly hypertensive individuals, who were already prescribed antihypertensives and had BP controlled based on pre-2017 hypertension guideline (i.e. ‘SBP 130–39 and DBP <90 mmHg’/’DBP 80–89 mmHg and SBP <140 mmHg’), but who would no longer be controlled when applying the AHA/ACC 2017 guidelines. We observed higher hypertension prevalence with older age in Australian participants and US women but not for US men, and these patterns held for both hypertension definitions (Supplementary Figure 1a and Figure 1b, http://links.lww.com/HJH/B419). The prevalence of hypertension remained consistently lower in the US participants compared with Australian, irrespective of age and sex. The US minorities aged 65–70 years had relatively higher hypertension prevalence than those aged 70–74 years according to both hypertension definitions (Supplementary Figure 1b, http://links.lww.com/HJH/B419).
FIGURE 2.
ASPREE participants’ hypertension status at baseline based on blood pressure and antihypertensive use.
Characteristics of the ‘reclassified hypertensive’ individuals
The characteristics of participants in the ‘pre-2017 hypertensive’, ‘reclassified hypertensive’ and ‘normotensive’ groups are summarized in Table 1. Among ‘pre-2017 hypertensives’, 71% of participants (Australia 70% and United States 77%) were receiving antihypertensive treatment. ‘Reclassified hypertensive’ participants were younger, with higher proportions being men and reporting active consumption of alcohol, as well as having higher blood lipids (except triglyceride), in relation to ‘pre-2017 hypertensive’ group participants (Table 1). Further, ‘reclassified hypertensive’ participants tended to have lower BMI and were less likely to have diabetes (based on fasting blood glucose levels ≥7mmol/l), as well as lower use of medication for comorbidities, such as dyslipidemia, diabetes and depression in relation to ‘pre-2017 hypertensive’ (Table 1). An additional comparison of baseline characteristics was conducted between these groups of participants by excluding those who were receiving antihypertensive (irrespective of BP) in the pre-2017 hypertensive group (Supplementary Table 1, http://links.lww.com/HJH/B419). The results were similar to the comparison between the ‘pre-2017 hypertensive (untreated)’ and the ‘reclassified hypertensive’ group except there was no statistically significant difference in the presence of comorbidities, such as diabetes, hypercholesterolemia (Supplementary Table 1, http://links.lww.com/HJH/B419). We observed few statistically significant differences in baseline characteristics of those ‘reclassified hypertensive’ and ‘normotensive’: a higher proportion of ‘reclassified hypertensives’ were men, and they had higher BMI, lower HDL, and higher triglycerides (Table 1).
TABLE 1.
Baseline characteristics of the ASPirin in Reducing Events in the Elderly study participants by presence of hypertension including those identified as having hypertension using American Heart Association and American College of Cardiology 2017 guideline
| Overall N = 19114 | Pre-2017 hypertensive N = 14213 | Reclassified hypertensive N = 2354 | Normotensive N = 2547 | |
|---|---|---|---|---|
| Age (year, mean ± SD) | 74.6 ± 4.6 | 74.8 ± 4.7 | 74.1 ± 4.2a | 74.0 ± 4.2 |
| Age category (%) | ||||
| 65–69 | 3.0 | 3.0 | 2.7a | 2.8 |
| 70–74 | 55.4 | 53.5 | 61.1 | 61.1 |
| 75–79 | 26.3 | 26.9 | 24.6 | 24.3 |
| 80–85 | 11.5 | 12.3 | 9.1 | 9.3 |
| 85 and above | 3.8 | 4.3 | 2.5 | 2.5 |
| Men (%) | 43.6 | 44.1 | 47.0a | 37.4b |
| Smoking current (%) | 3.8 | 3.7 | 4.3 | 4.0 |
| Alcohol drink current (%) | 76.6 | 76.0 | 79.2a | 77.5 |
| SBP (mmHg, mean ± SD) | 139 ± 16 | 144 ± 16 | 133 ± 5a | 119 ± 8b |
| DBP (mmHg, mean ± SD) | 77 ± 10 | 79 ± 11 | 77 ± 7a | 69 ± 6b |
| BMI (kg/m2, mean ± SD) | 28.1 ± 4.7 | 28.6 ± 4.8 | 27.2 ± 4.1a | 26.2 ± 4.13b |
| Obese (%) | 29.8 | 33.5 | 21.8a | 16.5b |
| LDL (mmol/l, mean ± SD) | 3.0 ± 0.9 | 3.00 ± 0.88 | 3.20 ± 0.86a | 3.17 ± 0.86 |
| HDL (mmol/l, mean ± SD) | 1.59 ± 0.0.48 | 1.57 ± 0.48 | 1.61 ± 0.46a | 1.67 ± 0.48b |
| Triglyceride (mmol/l, mean ± SD) | 1.33 ± 0.66 | 1.37 ± 0.68 | 1.25 ± 0.63a | 1.18 ± 0.58b |
| Total cholesterol (mmol/l, mean ± SD) | 5.24 ± 0.99 | 5.19 ± 0.99 | 5.39 ± 0.97a | 5.37 ± 0.98 |
| Diabetes (fasting blood glucose ≥7 mmol/l (%) | 5.7 | 6.8 | 2.9a | 2.2 |
| eGFR (ml/min per 1.73 m2) | 73.0 ± 14.2 | 72.1 ± 14.6 | 75.5 ± 12.6a | 76.0 ± 12.7 |
| In past 2 weeks any walk outside home (%) | 95.3 | 95.0 | 96.2a | 96.3 |
| Any difficulty in walking (a distance of 1 mile/1.6 km, %) | 20.1 | 22.1 | 14.9a | 13.7 |
| Family history CVD any (%) | 60.9 | 61.9 | 57.9a | 58.0 |
| Australian participants | 87.4 | 88.2 | 86.4a | 83.9b |
| Medication use: | ||||
| Lipid-lowering drug (%) | 33.8 | 37.2 | 23.9a | 24.2 |
| Diabetes medication (%) | 6.1 | 7.4 | 2.7a | 2.2 |
| Depression medication (%) | 11.2 | 11.5 | 9.8a | 11.3 |
| Regular aspirin use (%) | 11.0 | 11.4 | 9.5a | 9.8 |
| Antihypertensive (%) | 52.6 | 70.8 | 0.0 | 0.0 |
| Presence of comorbidity | ||||
| Hypercholesterolemia (raised total cholesterol and/or on medication) (%) | 65.2 | 67.1 | 60.2a | 59.6 |
| Diabetes (raised blood glucose level and/or on medication) (%) | 8.6 | 10.2 | 4.2a | 3.6 |
| Depression | 13.8 | 13.8 | 13.0 | 14.1 |
| Presence of risk factorsc | ||||
| None | 22.7 (4332) | 20.0 (2837) | 28.8 (678)a | 32.1 (817) |
| One | 51.2 (9794) | 50.5 (7173) | 53.4 (1257)a | 53.5 (1364) |
| Two | 22.2 (4249) | 24.8 (3527) | 16.5 (388)a | 13.1 (334) |
| Three or more | 3.9 (739) | 4.7 (676) | 1.3 (31)a | 1.3 (32) |
Percentages reported for each characteristic are based on the ‘N’ mentioned within that column). CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Significant differences (P < 0.05) observed between ‘pre-2017’ and ‘reclassified’ hypertensive group of participants.
Significant differences (P < 0.05) observed between ‘reclassified hypertensive’ and ‘normotensive’ group participants.
CVD risk factors are based on presence of diabetes, elevated cholesterol/taking cholesterol-lowering drug, active smoker, obese (BMI ≥30 kg/m2,), poor renal function (undergoing renal dialysis or eGFR less than 15 ml/min per 1.73 m2).
Presence of cardiovascular risk factors and predicted future risk
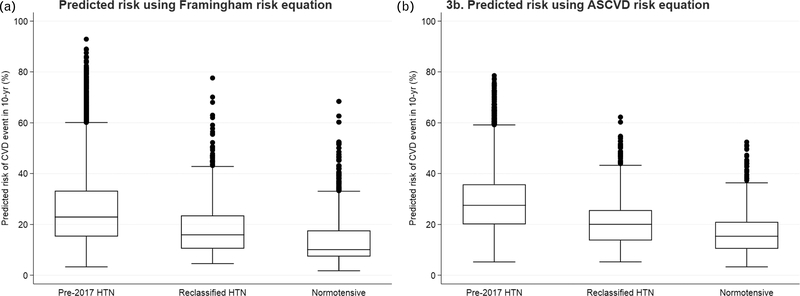
A lower prevalence of CVD risk factors other than hypertension was observed in the ‘reclassified hypertensive’ participants compared with ‘pre-2017 hypertensive’ participants (Table 1). We observed that 29% of the ‘reclassified hypertensive’ participants did not have any other traditional CVD risk factors, compared with 20% of the ‘pre-2017 hypertensive’ participants. Baseline predicted probability of having CVD over the next 10 years was significantly higher in the ‘pre-2017 hypertensive’ group than the ‘reclassified hypertensive’ group based on both Framingham risk scores (mean predicted probability 25.5 versus 18.0%, P<0.001; Fig. 3a) and ASCVD risk scores (mean predicted probability 28.6 versus 20.5%, P< 0.001; Fig. 3b). On the basis of ASCVD predicted risk score of at least 10%, among the ‘reclassified hypertensive’ participants, 94.5% would be recommended for treatment with antihypertensives according to AHA/ACC 2017 guideline. A similar difference in CVD risk prediction and also in the distribution of prevalence for other traditional CVD risk factors were observed among ASPREE participants by baseline hypertension status, when analyses were restricted to participants with age 74 years or less (for FRS) or 79 years or less (for ASCVD) (Supplementary Table 2, http://links.lww.com/HJH/B419).
FIGURE 3.
Distribution of predicted cardiovascular disease event risk (10-year) at baseline among ASPREE participants by baseline hypertension status (pre-2017 hypertensive, reclassified hypertensive and normotensive).
Cardiovascular disease events during follow-up and its association with baseline hypertension status
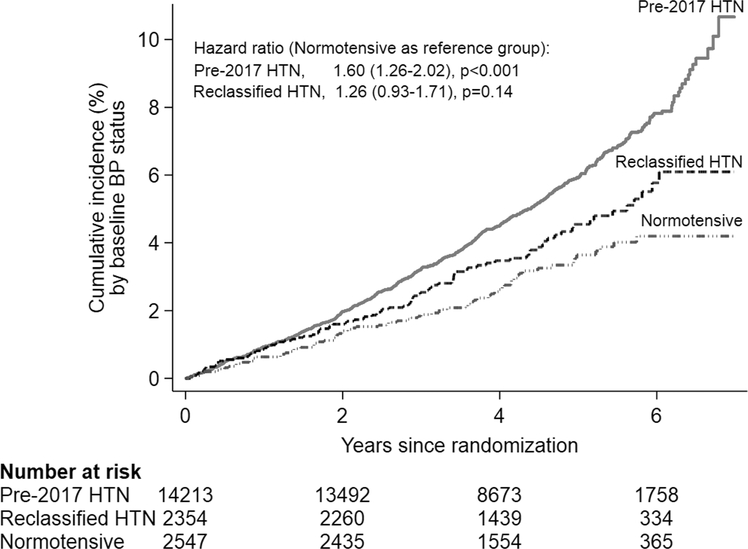
Over a median 4.7 years of follow-up, 922 CVD events (11.0 events per 1000 person-years) occurred among the study participants. Table 2 summarizes the distribution of CVD events and corresponding event rates by baseline hypertension status among ASPREE participants. There was a significantly higher CVD event rate (per 1000 person-years) observed in the ‘pre-2017 hypertensive’ group compared with ‘reclassified hypertensive’ group participants (12.1 versus 8.9 per 1000 person-years; hazard ratio 1.35, 95% CI: 1.09–1.68, P=0.01) and compared with the ‘normotensive’ group (12.1 versus 6.8 per 1000 person-years; hazard ratio 1.79, 95% CI: 1.42–2.27, P<0.001). However, we did not observe a statistically significant difference in the CVD event rates between ‘normotensive’ and ‘reclassified hypertensive’ groups (6.8 versus 8.9 events per 1000 person-years; hazard ratio 1.33, 95% CI 0.98–1.79, P=0.07). Further, on exploration of the key characteristics of the ‘reclassified hypertensive’ and ‘normotensive’ participants who experienced a CVD event, we did not observe any significant differences between them except a difference in BP (Supplementary Table 3, http://links.lww.com/HJH/B419). The cumulative incidence of CVD events by baseline hypertension status are shown in Fig. 4. The risk for CVD events for the ‘pre-2017 hypertensive’ (hazard ratio 1.60, 95% CI 1.26–2.02, P<0.001) and ‘reclassified hypertensive’ (hazard ratio 1.26, 95% CI 0.93–1.71, P=0.14) group in relation to ‘normotensive’ group remained similar when adjusted for possible confounding factors (Fig. 4).
TABLE 2.
Distribution of cardiovascular and stroke event, and corresponding event rate among all ASPirin in Reducing Events in the Elderly study participants by baseline hypertensive status
| Pre-2017 hypertensive (N = 14 213) | Reclassified hypertensive (N = 2354) | Normotensive (N = 2547) | |
|---|---|---|---|
| Cardiovascular disease event (922, 10.97 events per 1000 person-years) | |||
| Number of event (n, %) | 753 (5.3%) | 93 (4.0%)a | 76 (3.0%)a |
| Event rate per 1000 person-year (95% CI) | 12.1 (11.2–13.0) | 8.9 (7.3–11.0)a | 6.8 (5.4–8.5)a |
| Hazard ratio (95% CI) | |||
| Unadjusted | 1.79 (1.42–2.27) P < 0.001 | 1.33 (0.98–1.80) P = 0.07 | 1.00 |
| Adjustedb | 1.60 (1.26–2.02) P < 0.001 | 1.26 (0.93–1.71) P = 0.14 | 1.00 |
| Stroke-only eventc (315, 3.7 events per 1000 person-years) | |||
| Number of event (n, %) | 252 (1.8%) | 36 (1.5%) | 27 (1.1%)a |
| Event rate per 1000 person-years (95% CI) | 4.0 (3.5–4.5) | 3.4 (2.5–4.8) | 2.4 (1.6–3.5)a |
| Hazard ratio (95% CI) | |||
| Unadjusted | 1.67 (1.13–2.49) P = 0.01 | 1.44 (0.87–2.37) P = 0.15 | 1.00 |
| Adjustedb | 1.52 (1.02–2.27) P = 0.04 | 1.39 (0.84–2.29) P = 0.20 | 1.00 |
Significant difference (P < 0.05) observed with ‘Pre-2017 hypertensive’ group.
Significant difference (P < 0.05) observed between ‘reclassified hypertensive’ and ‘normotensive’ groups.
Adjusted for age, sex, ethnicity, current smoking, country of residence, treatment (aspirin/placebo).
Stroke included cases that were adjudicated as ischemic stroke, cases for which stroke type was uncertain after adjudication, and cases of ischemic stroke with haemorrhagic transformation.
FIGURE 4.
Cumulative incidence of cardiovascular disease events.
On restricting CVD events to stroke only, we did not observe a statistically significant difference in stroke event rate between the ‘pre-2017 hypertensive’ and ‘reclassified hypertensive’ groups; and also between ‘reclassified hypertensive’ and ‘normotensive’ groups (Table 2). On adjusted Cox proportional hazard regression model, a higher risk of stroke event (hazard ratio 1.52, 95% CI: 1.02–2.27, P=0.04) was observed in the ‘pre-2017 hypertensive’ group compared with ‘normotensive’ group but no significant difference (hazard ratio 1.39, 95% CI: 0.84–2.29, P=0.20) was observed between ‘reclassified hypertensive’ and ‘normotensive’ groups (Table 2).
Sensitivity analysis based on baseline blood pressure and antihypertensive medication use or recommended for use
Among the ‘pre-2017 hypertensive’ participants, 70.8% were on antihypertensive medication, whereas the rest were not on antihypertensive therapy despite meeting the BP threshold for antihypertensive treatment according to the pre-2017 guidelines. Among those ‘reclassified hypertensive’ participants, 94.5% had a baseline predicted CVD risk of at least 10% based on ASCVD risk score, and would be recommended for antihypertensive medication according to the AHA/ACC 2017 guideline. Overall, based on antihypertensive use or recommendation for antihypertensive treatment, 52.6% of ASPREE participants were ‘pre-2017 hypertensive’ and on medication, 21.7% of participants were ‘pre-2017 hypertensive’ and not on medication, 11.7% of participants were ‘reclassified hypertensive’ and recommended for antihypertensive medication but not on medication, and 14% of participants – either ‘reclassified hypertensive’ or ‘normotensive’ – were not recommended for treatment. We observed a significantly lower predicted CVD risk in the ‘reclassified hypertensive and recommended for medication’ group compared with the ‘pre-2017 hypertensive and on medication’ group (21.2 versus 29.3%, P<0.001) and with the ‘pre-2017 hypertensive and not on medication’ group (21.2 versus 26.8%, P<0.001; Supplementary Table 4, http://links.lww.com/HJH/B419). There was also a significantly lower CVD event observed, compared with the ‘pre-2017 hypertensive and on medication’ group, in the ‘reclassified hypertensive and recommended for medication’ group (9.3 versus 12.5 per 1000 person-years; adjusted hazard ratio 0.75, 95% CI: 0.60–0.94, P=0.01) and in the ‘pre-2017 hypertensive and not on medication’ group (11.0 versus 12.5 per 1000 person-years; adjusted hazard ratio 0.85, 95% CI: 0.72–1.00, P=0.049). We did not observe a statistically significant difference in CVD event between ‘reclassified hypertensive and recommended for medication’ group compared with the ‘pre-2017 hypertensive and not on medication’ group (9.3 versus 11.0 per 1000 person-years; adjusted hazard ratio 0.89, 95% CI: 0.69–1.14, P=0.34). Further, no statistically significant difference in CVD event was observed between the ‘reclassified hypertensive and recommended for medication’ group in relation to the ‘participants not recommended for treatment with antihypertensive medication’ group (i.e. ‘reclassified hypertensive not recommended for treatment’ or ‘normotensive’) (9.3 versus 6.6 per 1000 person-years; adjusted hazard ratio 1.26, 95% CI: 0.93–1.71, P=0.13; Supplementary Table 5, http://links.lww.com/HJH/B419).
Sensitivity analysis based on participants baseline BP and antihypertensive use status revealed a statistically significant increased risk of CVD events among participants with ‘BP at least 140/90 mmHg’ and/or ‘receiving any antihypertensive irrespective of BP’ groups compared with those with ‘BP less than 130 and less than 80 mmHg and not receiving antihypertensive’ group (Supplementary Figure 2, http://links.lww.com/HJH/B419). For stroke-only events, we only observed an increased stroke risk among those with ‘BP at least 140/90 mmHg’ irrespective of antihypertensive treatment status compared with those with ‘BP less than 130 and less than 80 mmHg, and not receiving antihypertensive’. We did not observe any difference in stroke risk between the participants with ‘SBP130–39/DBP 80–89 mmHg and receiving antihypertensive’ (hazard ratio 1.48, 95% CI: 0.90–2.41, P=0.12) and ‘SBP less than 130 mmHg and DBP <80 mmHg, and receiving antihypertensive’ (hazard ratio 1.15, 95% CI: 0.68–1.94, P=0.61) in relation to those ‘BP less than 130 and less than 80 mmHg, and not receiving antihypertensive’ (Supplementary Figure 2, http://links.lww.com/HJH/B419).
DISCUSSION
In this large elderly cohort aged 65 years and older without a history of CVD recruited from Australia or the United States, hypertension was highly prevalent regardless of the BP thresholds used to define it. There was a 12% increase in hypertension prevalence when study participants’ BP was classified according to the AHA/ACC 2017 hypertension guideline. Nearly 30% of these reclassified hypertensive participants (versus 20% among the pre-2017 hypertensive participants) had no other CVD risk factors (i.e. diabetes, hypercholesterolemia, active smoking, obesity or reduced renal function) other than age. Moreover, in the ASPREE cohort, the 10-year predicted CVD risk as well as observed CVD events (over a median 4.7 years) were also significantly lower in the ‘reclassified hypertensive’ participants than in those who were classified as hypertensive using pre-2017 guidelines. This suggests the reclassification of hypertension among the healthy elderly using the combination of lower BP thresholds and CVD risk calculator is less sensitive in identifying individuals with significantly higher CVD risk than those identified using traditional BP thresholds (i.e. ≥140/90 mmHg).
Our findings on hypertension prevalence are consistent with other studies, which have examined the potential impact of the AHA/ACC 2017 guideline on prevalence of hypertension in the United States and Australian general populations [15,16]. A 14% increase in hypertension prevalence was observed when the recent AHA/ACC 2017 hypertension guideline was applied to define hypertension compared with the pre-2017 guideline, among 9623 US participants in NHANES 2011–2014 who were at least 20 years of age [15]. Adopting the AHA/ACC 2017 guideline in Australia is estimated to have an even larger impact, potentially doubling the proportion of hypertensive population aged at least 20 years, from 26 to 51%, based on 2014–2015 Australian health survey data [16].
In our study, 29% of the subset of reclassified hypertensive participants had no other known CVD risk factors (compared with 20% for the pre-2017 hypertensive group) other than age, and their estimated 10-year predicted CVD risk was also significantly lower compared with pre-2017 hypertensive participants using both the Framingham risk equation (18 versus 25%) and the ASCVD risk equation (20 versus 27%). Furthermore, we observed fewer CVD events in the ‘reclassified hypertensive’ participants in comparison with those classified as having hypertension using the pre-2017 BP threshold; and no significant difference observed in CVD and stroke-only events risk in the ‘reclassified hypertensive’ participants in comparison with those classified as having ‘no hypertension’ at baseline. This finding could reflect a healthy volunteer bias and/or because of fewer events between the two much smaller groups in comparison with those in ‘pre-2017 hypertensive’.
Ninety-five percent of the ‘reclassified hypertensive’ participants identified in ASPREE with ‘SBP 130–139 mmHg and DBP less than 90 mmHg’ or ‘DBP 80–89 mmHg and SBP less than 140 mmHg’ and ASCVD predicted risk at least 10% would be recommended for treatment with antihypertensive medication. This is despite one-third of them having no other CVD risk factors with the exception of being 70 years or older at baseline. Although the ‘reclassified hypertensive’ group had higher predicted risk at baseline (i.e. an overall intermediate risk) compared with the ‘normotensive’ group, similar CVD event rates were observed in both groups. Considering age is an integrated part of the CVD risk prediction equations along with absence of risk prediction tools for very elderly (e.g. ≥75 years), the absolute baseline risk is higher in the elderly regardless of having any major comorbidity. These findings highlight that current risk prediction models have limited utility for contemporary elderly cohorts who have survived through an era of rapidly declining CVD event rates, and suggests a need for further research to develop risk calculators, which will better identify those elderly individuals most likely to derive benefits from preventive treatment.
The risks and benefits of a lower threshold for antihypertensive therapy in older persons need to be carefully considered, given the potential impact of initiation of antihypertensive medications in millions of people moving into the eighth and ninth decade of life across the globe. The potential benefits of this strategy were observed in this age group in the SPRINT study, where intensive BP control significantly lowered the rate of CVD events and all-cause mortality [17]. Recently, a meta-regression analysis demonstrated that for each 1 mmHg lowering in mean SBP between the intensive BP control group and the standard BP control group, there was 3% reduction in major adverse CVD events, including mortality [18]. Lower BP may also be related to reducing other aging disorders including cognitive decline and dementia. Recent results from the SPRINT-MIND study suggest that despite failing to prevent the onset of probable dementia over a 5-year period (hazard ratio 0.83; 95% CI 0.67–1.04), the secondary endpoint of development of mild cognitive impairment and probably dementia was significantly reduced (hazard ratio 0.85; 95% CI: 0.74–0.97) in the tighter BP control group [19]. However, it should be noted that SPRINT participants were enrolled on the basis of being at increased risk for CVD based on a history of clinical or subclinical CVD, chronic kidney disease, 10-year Framingham Risk Score at least 15%, or age at least 75 years. There is less evidence for a high degree of absolute benefit of intensive BP control in those with low or intermediate risk of having CVD [20]. This is further supported by a number of recent meta-analyses among the elderly (mean age 64 years), which demonstrated that lowering SBP levels less than 140 mmHg was not associated with any benefit in the setting of primary prevention but might benefit only those with a previous history of heart disease [21,22]. Likewise, the risks associated with broadening the classification of hypertension include those risks associated with poly-pharmacy, medication complexity and pill burden [23]. Adherence to medication, potential for adverse and drug interactions, drug costs and illness perception are all well known risks to individuals receiving additional drug treatments for disease prevention [23].
Our study has important limitations to acknowledge. We used the conventional office BP measurement at study entry (average of three readings obtained at a single examination, taken 1 min apart on an oscillometric device). In clinical practice, this would normally be followed by further visits and, if necessary, by home and/or ambulatory BP measurements to confirm elevated BP readings. Furthermore, participants’ reported prescriptions for antihypertensives was used to classify hypertensive status, and in some cases, antihypertensives could have been used primarily for another indication (e.g. alpha blocker for benign prostatic hyperplasia), or prescribed but not actually ingested. Both might have caused an overestimation of our prevalence rates. Secondly, we used FRS and ASCVD equations to estimate 10-year predicted probability of having CVD events among all participants. Both of these equations were developed within younger cohorts, during a time when rates of CVD among the general population were higher and CVD risk factors, such as smoking were more common and use of statins was less; therefore, use of these risk scores among the elderly are subject to significant limitations. However, on age-restricted sensitivity analysis among participants aged 74 years or less (for FRS) and 79 years or less (for ASCVD), our findings were unchanged. Lastly, we explored the observed CVD events based on presence of hypertension defined from the study participants’ BP level at first baseline visit. We did not adjust the CVD events rate for information, such as antihypertensive drug adherence, duration of BP-lowering treatment or the introduction of new BP-lowering treatment during the study, or the emergence of comorbidities, which independently increase CVD risk, such as diabetes or chronic kidney disease.
Despite these limitations, our analysis from ASPREE highlights that hypertension is highly prevalent among the healthy elderly and that the prevalence expectedly increases when applying the AHA/ACC 2017 guideline. Further, because of higher predicted CVD risk predominantly because of aging, nearly all of these newly classified elderly hypertensive participants would be recommended for antihypertensive treatment. Yet our results suggest that in those older adults free of CVD who are reclassified for antihypertensive treatment as a result of shifting the BP threshold lower, the associated incremental absolute risk of CVD is lower than predicted compared with those defined by the traditional hypertensive threshold of at least 140/90 mmHg. Further, compared with ‘normotensives’, no significant difference was observed in CVD events in the ‘reclassified hypertensives’ over a median 4-year follow-up and also no significant difference observed in characteristics among those who experienced CVD event. These findings highlight the limitations of using lower BP thresholds and existing CVD risk calculators for primary prevention in a healthy older cohort.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the dedicated and skilled staff in Australia and the United States for the conduct of the trial. The authors also are most grateful to the ASPREE participants, who so willingly volunteered for this study, and the general practitioners and medical clinics who supported the participants in the ASPREE study. Bayer AG provided aspirin and matching placebo.
Funding: The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant number U01AG029824); the National Health and Medical Research Council of Australia (grant numbers 334047, 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia).
Conflicts of interest
Bayer AG supplied study drug (aspirin) and matching placebo also produce BP lowering medication (no link with ASPREE’s participants) and had no other role in the trial. E.K.C. has received High Blood Pressure Research Council Australia early career research transition grant to support current work. M.R.N. received travel and consultancy support from Bayer to attend a meeting in Berlin. A.M.T. has received unrelated research support and honoraria/travel expenses from Bayer. C.M.R. is supported on a NHMRC Principal Research Fellowship (1136372). All other authors have no conflict of interest to declare in relation to this study.
Abbreviations:
- AHA/ACC
American Heart Association and American College of Cardiology
- ASCVD
Atherosclerotic Cardiovascular Disease risk score
- ASPREE
ASPirin in Reducing Events in the Elderly study
- BP
blood pressure
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESC/ESH
European Society of Cardiology/European Society of Hypertension guideline
- FRS
Framingham risk score
- HDL
high-density lipoprotein
- HR
hazard ratio
- JNC-8
Eight Joint National Commission
- SD
standard deviation
Footnotes
ASPREE Investigator Group – a complete list of the ASPREE trial investigators is provided in the Supplementary Appendix, http://links.lww.com/HJH/B420.
REFERENCES
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA 2002; 287:1003–1010. [DOI] [PubMed] [Google Scholar]
- 3.Redon J Controversies in blood-pressure goals among the elderly. Nat Rev Cardiol 2017; 14:193. [DOI] [PubMed] [Google Scholar]
- 4.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013ESH/ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 6.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 7.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 9.SPRINT Research Group: Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013; 36:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. , ASPREE Investigator Group. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018; 379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. , ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018; 379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care. Circulation 2008; 117:743. [DOI] [PubMed] [Google Scholar]
- 14.Goff David C, Lloyd-Jones Donald M, Bennett G, Coady S, D’Agostino Ralph B, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation 2014; 129 (25 Suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 15.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings GL, Kingwell BA, Hoare E. Potential implications of the new American hypertension guidelines in Australia. Med J Australia 2018; 209:108–109. [DOI] [PubMed] [Google Scholar]
- 17.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol 2017; 69:486–493. [DOI] [PubMed] [Google Scholar]
- 19.The SPRINT MIND Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: a Randomized Clinical Trial. JAMA 2019; 321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheppard JP, Stevens S, Stevens R, Martin U, Mant J, Hobbs FDR, et al. Benefits and Harms of Antihypertensive Treatment in Low-Risk Patients With Mild Hypertension. JAMA Intern Med 2018; 178:1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstrom M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han M, Chen Q, Liu L, Li Q, Ren Y, Zhao Y, et al. Stage 1 hypertension by the 2017 American College of Cardiology/American Heart Association hypertension guidelines and risk of cardiovascular disease events: systematic review, meta-analysis, and estimation of population etiologic fraction of prospective cohort studies. J Hypertens 2020; 38:573–578. [DOI] [PubMed] [Google Scholar]
- 23.Rajpura J, Nayak R. Medication adherence in a sample of elderly suffering from hypertension: evaluating the influence of illness perceptions, treatment beliefs, and illness burden. J Manage Care Pharm 2014; 20:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.