Hepatocellular carcinoma (HCC) is the most common liver cancer, accounting for over 80%–90% of primary liver cancers. HCC is also a leading cause of cancer-specific death, estimated to be the fourth most common cause of cancer-related mortality worldwide.(1) Cholangiocarcinoma (CCA) is the second-most common primary hepatic malignancy after HCC. Although the overall incidence of CCA has increased over recent decades, the 5-year overall survival (OS) remains less than 10%.(2) Potentially curative surgical resection or liver transplantation are options for the small subset of patients with early-stage disease. However, most patients with primary liver cancer present with advanced stage disease not amenable to surgical options. Systemic therapies for advanced stage liver cancer have limited efficacy. Hence, there is an essential need for the development of effective medical therapies.
Immuno-oncology has transformed cancer treatment over the past decade. Immune checkpoints are essential in the maintenance of self-tolerance under physiologic conditions.(3) Tumors co-opt the antitumor immune response by activating immune checkpoints such as programmed death-1 (PD-1) and its ligand PD-L1, as well as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Immune checkpoint inhibition (ICI), using monoclonal antibodies targeting PD-1/PD-L1 and CTLA-4, unleashes preexisting immunity, especially effector CD8+ T cells.(3) ICI therapies have had substantial benefit with durable responses for a subset of patients. However, most patients do not respond to ICI monotherapy. The tumor immune microenvironment (TIME) affects response to immunotherapies. TIMEs are broadly classified into T cell infiltrated-excluded, infiltrated-inflamed, or infiltrated with tertiary lymphoid structures.(4) Hence, an enhanced understanding of the immunobiology of TIME will be essential in the development of next-generation immunotherapies.(4)
Immune Ecosystem of Human Liver Cancer
Under physiologic conditions, the liver has a complex microenvironment with constant exposure to gut-derived antigens from dietary and microbial products.(5) Accordingly, the liver has intrinsic immune tolerogenicity that enables suppression of inappropriate inflammatory responses. Hence, the liver is an immune modulating organ that is poised to respond to deleterious stimuli while maintaining immuno-tolerance.(6) This closely regulated hepatic immune tolerogenic network is deranged in chronic inflammatory liver disease, facilitating liver tumor development. The TIME of HCC and CCA is complex with diverse populations of innate and adaptive immune cells that affect cancer immune evasion, response to immunotherapy, and patient survival. Recent studies using single-cell analysis have begun to uncover the dynamics of immune cell subsets in tumor ecosystems.
ADAPTIVE AND INNATE LYMPHOCYTES IN LIVER CANCER
Antitumor Lymphocytes
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are antitumor immune cells that play an integral role in cancer immune surveillance and eradication of tumor cells. CD8+ T lymphocytes are the primary effector tumor infiltrating lymphocyte (TIL) subset in liver cancer.(6) Moreover, an increase in CD8+ TILs is associated with improved overall patient survival in both HCC and CCA.(7,8) NK cells are innate lymphocytes with potent cytolytic function that can eradicate tumor cells without prior sensitization.(9) A decrease in NK cell number or impairment of function occurs in a variety of malignancies including liver cancer. Several factors contribute to NK cell dysfunction in HCC, including up-regulation of the inhibitory receptor NKG2A and increased production of immunosuppressive cytokines such as transforming growth factor β (TGFβ) and IL-10.(10,11) Additionally, an increased infiltration of CD11b−CD27− cells, an immature and inactive phenotype of NK cells with poor cytolytic activity, occurs in HCC. Infiltration of this subset is associated with human HCC progression.(12) In contrast, high expression of the activating NK cell receptor, natural killer group 2D, correlates with improved disease-free and overall patient survival in CCA.(13) Preclinical studies using NK cell–based therapies such as infusion of ex vivo expanded human NK cells into CCA xenograft mice have demonstrated an antitumor effect in CCA.(14)
Dysregulation of lipid metabolism in nonalcoholic fatty liver disease (NAFLD) alters the adaptive immune response and promotes hepatocarcinogenesis.(15) In preclinical models of NAFLD, lipid dysregulation causes selective loss of CD4+ T lymphocytes but not CD8+ T lymphocytes. Moreover, fewer CD4+ T lymphocytes are found in patients with alcohol-associated and nonalcoholic steatohepatitis than in patients with viral hepatitis, implying that impairment of the adaptive immune response is essential in the progression from NAFLD to hepatocarcinogenesis.(15)
Immunosuppressive Lymphocytes
Accumulation of regulatory T cells (Tregs), lymphocytes with a highly immunosuppressive nature that suppress CTLs, occurs in HCC. Increased tumor infiltration of Tregs is associated with CD8+ T cell dysfunction, HCC invasiveness as well as progression, and poor patient outcomes.(16,17) Tregs are also associated with poor recurrence-free survival in CCA.(18) Moreover, Tregs can attenuate the effectiveness of potential antitumor immunotherapies. In a mouse model of HCC, IL-12 therapy resulted in tumor stabilization or regression in 40% of animals. Activation of immunosuppressive mechanisms including increased abundance of Tregs was noted in nonresponder mice.(19) TGFβ has been implicated in promoting Treg production and differentiation with consequent CD8+ T cell repression.(20) Accordingly, TGFβ inhibition using a specific inhibitor, SM-16, decreased Treg infiltration with resultant HCC tumor regression.(20)
Dynamics of Adaptive and Innate Lymphocytes in Liver Cancer
Single-cell transcriptomic analysis of tumor-infiltrating lymphocytes has become a powerful tool to dissect the role of these cells in the highly complex TIME of various malignancies. Single-cell RNA sequencing of 5,063 single T cells from 6 patients with HCC identified 11 T cell subsets including five CD8+ subsets and six CD4+ subsets.(21) Tregs and exhausted CD8+ T cells were preferentially enriched in HCC tumors. A signature gene, layilin (LAYN), was up-regulated on activated CD8+ T cells as well as Tregs, and was associated with more repressive and stable Tregs.(21) Further functional analysis demonstrated a regulatory function of LAYN with inhibition of interferon-gamma (IFN-γ) production and consequent repression of CD8+ T-cell function.(21)
Intratumoral heterogeneity is linked to poor patient outcomes, particularly in patients with tumors that are refractory to molecularly targeted chemotherapeutics. Single-cell RNA sequencing analysis of tumors from 19 patients with primary HCC and intrahepatic CCA found an association between tumor transcriptomic diversity and OS in liver cancer.(22) T-cell dysfunction as indicated by lower cytolytic activity was noted in tumors with higher heterogeneity, suggesting that assessment of the tumor ecosystem may help guide response to immune therapy. Increased expression of tumor-derived vascular endothelial growth factor (VEGF) was related to TIME polarization including T-cell dysfunction, providing a mechanistic rationale for the combination of anti-VEGF and ICI in liver cancer.
IMMUNOSUPPRESSIVE MYELOID CELLS IN LIVER CANCER
Macrophages
Tumors can co-opt myeloid cells in the TIME to modulate immune evasion and promote cancer growth.(23) Hence, macrophages and dendritic cells (DCs), which can have antitumor functions, may be skewed by tumors to an immunosuppressive, pro-tumor phenotype (Fig. 1). The presence of immunosuppressive tumor-associated myeloid cells, such as tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), influences patient survival. Accordingly, the presence of TAMs is linked to poor outcomes in patients with CCA.(18) An extensive immunogenomic analysis of 10,000 tumors across 33 diverse cancer types revealed six immune subtypes of cancer based on differences in macrophage or lymphocyte signatures.(24) HCCs (n = 6) were classified under the lymphocyte-depleted subtype with a prominent macrophage signature characterized by a Th1-suppressed and high M2 macrophage response. A high TAM state correlates with poor patient outcomes in HCC based on the Cancer Genome Atlas (TCGA) HCC (LIHC) analysis.(25)
FIG. 1.
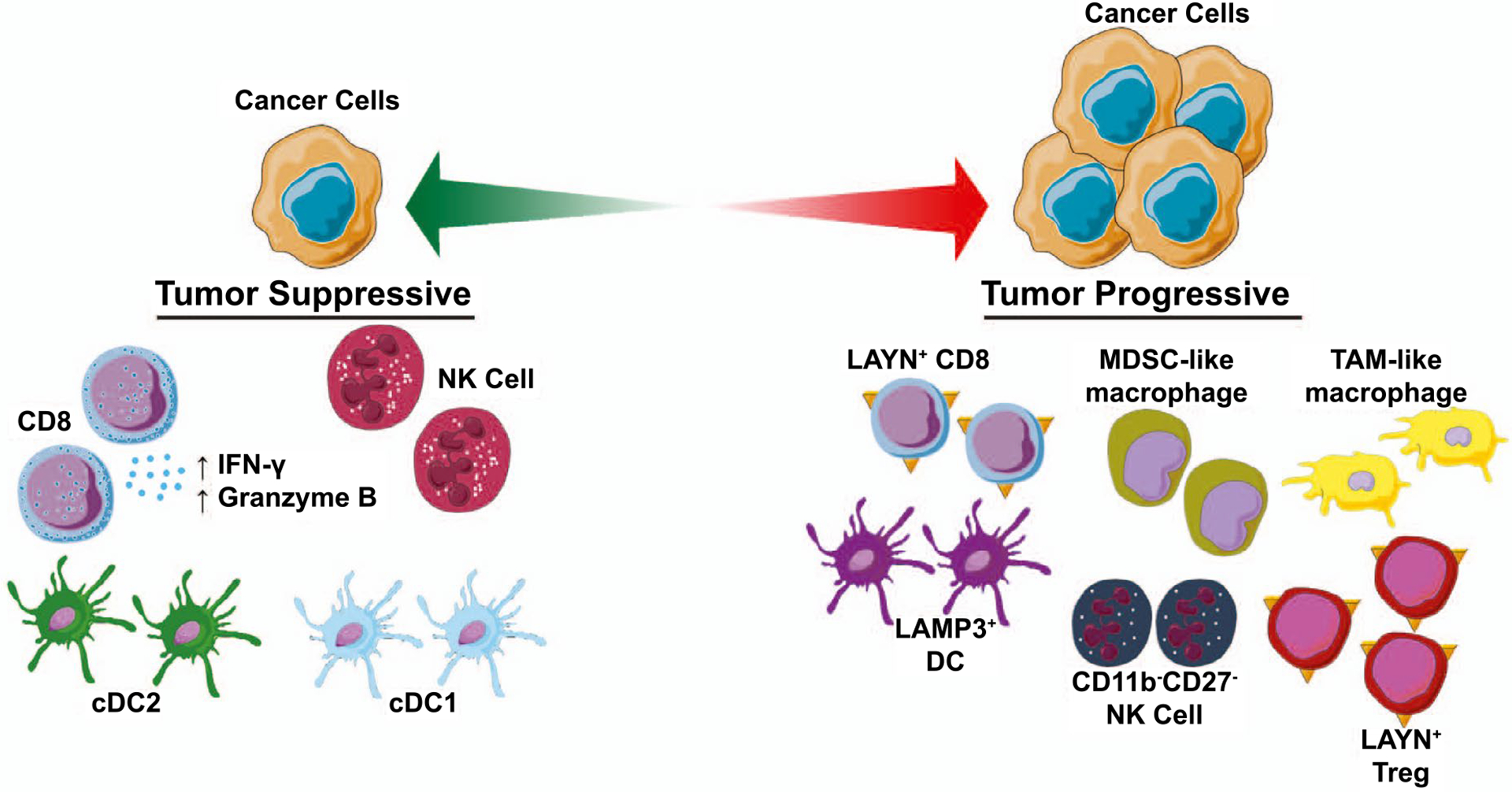
The immune ecosystem of human liver cancer. A tumor-suppressive immune ecosystem is characterized by the presence of immune-stimulatory cell types such as CD8+ T lymphocytes, NK cells, and DCs as well as increased IFN-γ and granzyme B production. A tumor-progressive immune ecosystem is characterized by the presence of immunosuppressive MDSC-like macrophages, TAM-like macrophages, LAMP3+ DCs, LAYN+ regulatory T cells (Tregs), and dysfunctional LAYN+ CD8 T cells.
Transcriptome profiling of 75,000 individual CD45+ cells from 16 patients with HCC revealed the dynamic nature of various immune cells in HCC.(25) Two distinct states of tumor-enriched macrophages were identified: MDSC-like macrophages and TAM-like macrophages. MDSC-like cells had a high expression of S100A family genes FCN1 and VCAN, and low expression of HLA-related genes. In contrast, the gene signature found on TAM-like macrophages resembled a previously described signature in lung cancer TAMs with high expression of APOE, C1QA, C1QB, and TREM2. Moreover, TAM-like cells in HCC had high expression of two additional genes: SLC40A1, which encodes ferroportin, and GPNMB, which encodes type 1 membrane glycoprotein.(25) Based on the TCGA-LIHC analysis, both GPNMB and SLC40A1 were linked to poor patient prognosis. Functional assessment demonstrated an inflammatory role of GPNMB and SLC40A1 in TAM-like cells in the HCC TIME.
A few studies have explored the mechanistic basis of TAM-mediated liver cancer progression. Hepatocyte mitochondrial dysfunction and oxidative stress foster CCA carcinogenesis through activation of tumor necrosis factor (TNF)–producing Kupffer cells (KCs), resident hepatic macrophages.(26) KC-derived TNF lead to JNK-mediated cholangiocyte proliferation and oncogenic transformation, whereas KC depletion reduced premalignant CCA lesions. Canonical Wnt signaling drives cell proliferation, and is activated in liver cancer. In CCA, Wnt signaling is activated by TAMs with consequent tumor progression.(27) Accordingly, TAM depletion or inhibition of Wnt signaling reduced CCA proliferation and augmented apoptosis, with resultant tumor regression. There is also evidence that CCAs may commandeer macrophages to foster a tumor-supportive immune niche. Cellular spheroids generated from CCA cells educated macrophages to a TAM phenotype with invasive properties. These in vitro educated TAMs had a similar molecular phenotype to macrophages isolated from human resected CCA specimens.(28) These studies provide insight into the mechanistic underpinnings of TAM-mediated liver cancer progression, although further work is necessary to elucidate the role of TAMs and their interactions with other elements of the liver cancer TIME.
Dendritic Cells
DCs are antigen-presenting cells that activate the adaptive immune response by migrating to tumor-draining lymph nodes after acquiring tumor antigens and activating T cells. DCs are broadly categorized as conventional DCs (cDCs), which are highly phagocytic antigen-presenting cells or plasmacytoid DCs, which are not phagocytic and require activation.(29) Flow cytometry and immunohistochemical-based studies have demonstrated a correlation between mature cDCs and CD4+/CD8+ T-cell infiltration in CCA, and a decrease in the number of TNFα-producing cDCs in both HCC and CCA tumors.(30,31) Based on single-cell analysis, three subsets of DCs enriched in HCC are cDC1, cDC2, and LAMP3+ DCs; the latter is a more mature form of DCs that migrate from tumors to lymph nodes.(25) LAMP3+ DC signature is associated with exhausted CD8+ T cell and Treg signature, suggesting that these DCs may be linked to T-cell dysfunction.(25) Accordingly, although cDCs with maturation features are typically associated with an enhanced CD8+ T-cell activation, these results signify the complex and dynamic nature of the TIME and the multiple mechanisms by which tumors can co-opt the TIME to evade immune surveillance.
Myeloid-Derived Suppressor Cells
MDSCs are immature myeloid cells that are pathologically activated in the setting of chronic inflammation. MDSCs have potent immunosuppressive properties and inhibit CTLs as well as NK cells through production of arginase, inducible nitric oxide synthase, indoleamine 2,3-dioxygenase, reactive oxygen species, TGFβ, and IL-10.(32) The frequency of MDSCs is significantly increased in human HCC and CCA, and their accumulation in the peripheral blood of patients with HCC is associated with decreased overall survival.(33,34) MDSCs exert their immunosuppressive effects in liver cancer through induction of Tregs as well as attenuation of T-cell and NK-cell function.(33,35,36) In preclinical tumors models, phosphodiesterase 5 (PDE5) inhibition diminishes tumor-immunosuppressive mechanisms through inhibition of MDSC function.(37) Adoptive cell transfer of cytokine-induced killer cells (CIKs) in mice bearing subcutaneous HCC tumors increased infiltration of MDSCs, reducing the antitumor effect of CIK immunotherapy.(38) Treatment with a PDE5 inhibitor enhanced antitumor efficacy of CIK immunotherapy by inhibiting MDSC accumulation and function.(38)
DYSBIOSIS OF GUT MICROBIOTA AND LIVER CARCINOGENESIS
Under physiologic conditions, the liver is constantly exposed to the gut microbiome through the portal circulation. Consequently, chronic liver disease is associated with alteration in the gut microbiota or dysbiosis as well as increased translocation of intestinal bacteria. Impairment of the bile acid metabolism–microbiota crosstalk promotes inflammation and can contribute to the development of liver cancer.(39) Accumulation of gut-derived endotoxin or lipopolysaccharide (LPS) occurs in animal models of carcinogen-induced hepatocarcinogenesis.(40) Accordingly, antibiotic mediated LPS reduction or ablation of its receptor, toll-like receptor 4 (TLR4), had an antitumor effect. Reconstitution of TLR4-expressing myeloid cells in TLR4-deficient mice restored carcinogen-induced hepatic inflammation and proliferation. Similarly, TLR4 and the intestinal microbiota fostered HCC progression, whereas gut sterilization significantly reduced HCC tumor burden, particularly in the later stages of hepatocarcinogenesis (Fig. 2).(41)
FIG. 2.
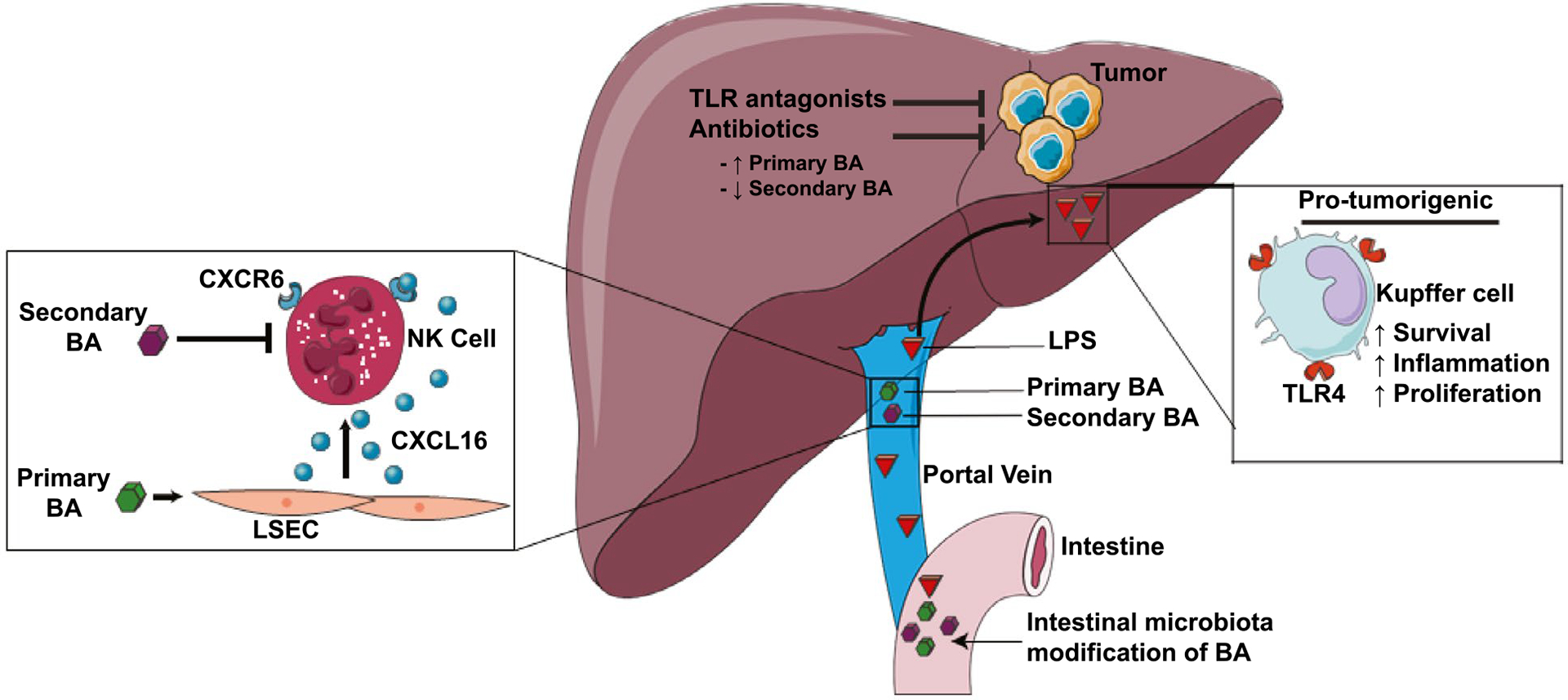
Dysbiosis of gut microbiota and liver carcinogenesis. Intestinal microbiota modify bile acids (BA) and shift the balance between primary BA and secondary BA. Primary BA can regulate chemokine levels and hence influence accumulation of anti-tumor natural killer T cells (NKT). Intestinal microbiota also foster HCC progression via enhanced lipopolysaccharide (LPS)-toll-like receptor 4 (TLR4) signaling, whereas antibiotics or TLR antagonists reduce HCC tumor burden. Abbreviations: BA, bile acid; CXCL16, chemokine (C-X-C motif) ligand 16; CXCR6, cysteine-X-cysteine receptor 6; and LSEC, liver sinusoidal endothelial cell.
The commensal microbiome influences T-cell response and immune checkpoint inhibitor efficacy in melanoma.(42) Alteration of commensal gut bacteria induced an antitumor effect in a mouse model of primary liver cancer through a selective increase in hepatic CXCR6+ natural killer T (NKT) cells.(43) Vancomycin-mediated elimination of gram positive bacteria increased the conversion of primary bile acids to secondary bile acids. This increased expression of C-X-C motif chemokine ligand 16 on liver sinusoidal endothelial cells with consequent augmentation of antitumor immunity through NKT cell recruitment (Fig. 2). The finding that alteration of the commensal gut bacteria has a liver-selective antitumor effect indicates that therapies modulating the intestinal microbiome may be a potential treatment option for liver cancer.
Immunotherapy in Liver Cancer
TARGETING IMMUNE CHECKPOINTS IN HCC
Single-Agent Activity of Antibodies Targeting CTLA-4
The anti-CTLA-4 antibody, tremelimumab, was evaluated in a single-arm phase 2 study of 21 patients with advanced HCC, Child-Pugh A or B, and hepatitis C.(44) Grade 3 aspartate aminotransferase and alanine aminotransferase elevations were noted in 45% and 25% of patients, respectively, but were most often noted to occur early after the first dose and not associated with a parallel decline in liver function. The objective response rate (ORR) was 17% in the 17 patients who were evaluable for response, and another 58.8% of patients had stable disease as their best response. In an intention-to-treat analysis including all 21 patients, the median time to progression was 6.48 months (95% confidence interval [CI] 3.95–9.14) and median OS was 8.2 months (95% CI 4.64–21.34).
Single-Agent Activity of Antibodies Targeting PD-1
Nivolumab, a fully human immunoglobulin G4 monoclonal anti-PD-1 antibody, was first tested for its safety and efficacy in a phase 1/2 study of over 250 patients with advanced HCC, CheckMate 040.(45) The ORR per RECIST 1.1 criteria was 20%; responses occurred in all cohorts, independent of etiology or previous exposure to sorafenib. A favorable safety profile was confirmed in the expansion cohorts with grade 3 and 4 treatment-related adverse events (AEs) at 19%, consisting mostly of transient laboratory abnormalities. Nivolumab was subsequently granted accelerated approval by the Food and Drug Administration (FDA) for the treatment of patients with advanced HCC who had previously been treated with sorafenib in September 2017. The safety and tolerability of single-agent anti-PD-1 therapy noted with nivolumab was duplicated in Keynote 224, a phase 2 study of pembrolizumab in patients with advanced HCC; this study showed an ORR of 17% (95% CI 11–26) in 104 patients.(46) This study used a detailed and centralized review of hepatic events and reported a 3% rate of hepatic immune-mediated events.(46) Camrelizumab, another anti-PD-1 therapy, was assessed in a multicenter, open-label, randomized phase 2 trial in China (n = 303).(47) The ORR with camrelizumab was 14.7% (95% CI 10.3–20.2), and grade 3 or 4 treatment-related AEs occurred in 22% of the 217 patients who received camrelizumab. Several other single-agent ICI targeting the PD-1/PD-L1 axis, including atezolizumab (NCT04157985), durvalumab (NCT03847428), toripalimab (NCT03949231) and tislelizumab (NCT03412773), are currently under investigation in phase 3 trials of HCC.
Nivolumab and pembrolizumab have also been evaluated in phase 3 trials. Keynote 240 was a randomized phase 3 study of pembrolizumab versus best supportive care in patients who failed sorafenib, with co-primary endpoints of progression-free survival (PFS) and OS.(48) Despite a numerically superior median OS for pembrolizumab versus placebo (13.9 [11.6–16] months vs. 10.6 [8.3–13.5] months; P = 0.0238), the trial did not reach statistical significance, as the prerequired P value to reach significance was 0.0174. In CheckMate 459, nivolumab was compared with sorafenib in systemic therapy–naïve patients with advanced HCC.(49) The median OS was 16.4 (13.9–18.4) months versus 14.7 months (11.9–17.2), respectively, with a hazard ratio (HR) of 0.85 (0.72–1.02). When considering subsequent therapies, 20% of patients on the sorafenib arm received a checkpoint inhibitor in second line, and another 11% received investigational agents, some of which were immune-oncology agents. Grade 3–4 treatment– related AEs were noted in 22% of patients on the nivolumab arm and 49% of patients on the sorafenib arm. There was clinically meaningful difference in quality-of-life outcomes favoring nivolumab based on the FACT-hep disease-specific questionnaire. In summary, despite the fact that single-agent anti PD-1 agents manifested consistent ORRs between 14% and 20% with durable responses in phase 1/2 trials, the phase 3 studies of both nivolumab in first line and pembrolizumab in second line failed to show statistically significant improvements in OS. The rapidly evolving landscape of available systemic therapies for HCC being used in a sequential manner and some statistical design limitations may have affected the outcomes of these trials. Nonetheless, despite the evidence of clinical benefit in some patients, it is plausible that the single-agent activity was not sufficient to show significant improvements in median OS in an unselected population. As a result, the development of biomarkers to improved patient selection and the pursuit of combination therapies present possible solutions to harness the full potential of checkpoint inhibition in HCC.
Emerging Biomarkers for Single-Agent Anti-PD-1 Antibodies
In patients with advanced HCC treated with single-agent anti-PD-1 therapy, several potential biomarkers may be associated with improved survival and response. In CheckMate 040, patients with advanced HCC received nivolumab monotherapy, regardless of PD-L1 status. Although response was noted in both PD-L1 positive and negative patients, the response rate in patients with increased tumor cell PD-L1 expression was significantly higher (complete response [CR] + partial response [PR] versus stable disease [SD]; P = 0.00009).(50) Moreover, CD3 expression was also significantly associated with response (CR + PR vs. SD; P = 0.05). In the subset of patients with evaluable tumor samples (n = 37), several inflammatory signatures such as the IFN-γ-related mRNA profile and T-cell exhaustion signature had a significant correlation with improved response and OS with anti-PD-1 therapy.(50,51) In contrast, Wnt/β-catenin mutations such as activating catenin beta-1 (CTNNB1) or inactivating AXIN1 mutations are associated with poor outcomes in patients with HCC treated with anti-PD-1.(52) In summary, this continues to be an area of active investigation; it is possible that a composite of multiple biomarkers may be useful in improving patient selection.
COMBINATORIAL APPROACHES WITH IMMUNE CHECKPOINT INHIBITORS
Targeting PD-1/PD-L1 Plus VEGF Axis
Anti-VEGF therapy decreases the activity of MDSCs and Tregs, enhances PD-L1 expression, and increases CTL infiltration.(53) Accordingly, single-agent VEGF inhibition has shown modest activity in HCC.(54) Moreover, preclinical studies have demonstrated a synergistic antitumor effect with combinatorial blockade of PD-1 and the VEGF axis.(55) Imbrave150 (NCT03434379) is a landmark phase 3 clinical trial comparing the PD-L1 inhibitor atezolizumab plus bevacizumab (n = 336), a VEGF inhibitor, with sorafenib (n = 165) in treatment-naïve patients with unresectable HCC.(56) Trial inclusion criteria limited eligibility to patients who were Child-Pugh A. Primary analysis data demonstrated that with a median follow-up of 8.6 months, there was a 42% reduction in risk of death with the combination group, with an OS HR of 0.58 (95% CI: 0.42–0.79; P = 0.0006). Median PFS was 6.8 in combination versus 4.3 months in sorafenib (HR 0.59; 95% CI: 0.47–0.76; P < 0.0001). Grade 3–4 AEs were reported in 57% of patients receiving the combination and 55% of patients receiving sorafenib. Although these are potentially paradigm-shifting results, as this is the first study to show superiority of a first-line systemic therapy over sorafenib, mature data and longer follow-up are needed. Moreover, safety in a real-world population will also need to be established, and further studies will be necessary to determine whether this combination is safe in patients with more advanced liver disease who have portal hypertension and an increased bleeding risk.
Lenvatinib, a multikinase inhibitor that inhibits VEGF receptor (VEGFR) 1–3, is FDA-approved as first-line therapy in patients with unresectable HCC based on the multicenter, randomized, open-label, noninferiority REFLECT trial.(57) The combination of pembrolizumab and lenvatinib was associated with an ORR of 36.7%, with a median PFS of 9.7 months in unresectable HCC.(58) Grade 3–4 treatment-related AEs were noted in 73% of patients, with 4 (13%) patients experiencing fatal AEs. A phase 3 study of lenvatinib plus pembrolizumab versus lenvatinib alone in patients with advanced unresectable HCC is currently ongoing (LEAP-002; NCT03713593) (Table 1).
TABLE 1.
Ongoing Clinical Trials of Immunotherapy Targeting the Tumor Microenvironment in Patient With Liver Cancer
| Intervention | Target | Trial Type | Trial Description | |
|---|---|---|---|---|
| Immune checkpoint Inhibition plus tyrosine kinase inhibition | ||||
| HCC | ||||
| Atezolizumab + bevacizumab | PD-L1; VEGF | Phase 3, randomized, open label | Advanced or metastatic, treatment-naïve HCC; Child-Pugh A (480 pts) | NCT03434379 |
| Durvalumab + bevacizumab | PD-L1; VEGF | Phase 2, randomized, open label, multicenter | Advanced HCC (433 pts) | NCT02519348 |
| HLX10 + HLX04 | PD-1; VEGF | Phase 2, single arm, open label, multicenter | Advanced HCC with progression or intolerant toxicity after standard treatment; BCLC stage B/C (150 pts) | NCT03973112 |
| Sintilimab + IBI305 | PD-1; VEGF | Phase 1b, singe arm, open label | Advanced HCC; BCLC stage B/C; Child-Pugh score ≤ 7 (45 pts) | NCT04072679 |
| Phase 2/3, randomized, open label, multicenter | First-line treatment of advanced HCC; BCLC stage B/C; Child-Pugh score ≤ 7 (566 pts) | NCT03794440 | ||
| Nivolumab + sorafenib | PD-1; multiple kinases | Phase 3, randomized, open label, multicenter | Advanced, treatment-naïve HCC; Child-Pugh A (1,723 pts) | NCT02576509 |
| Phase 2, nonrandomized, multicenter | Advanced, treatment-naïve HCC (40 pts) | NCT03439891 | ||
| Nivolumab + lenvatinib | PD-1; multiple kinases | Phase 2, single arm, open label, multicenter | Advanced HCC; Child-Pugh ≤ 6 (50 pts) | NCT03841201 |
| Phase 1b, nonrandomized, open label | Advanced HCC; Child-Pugh A (30 pts) | NCT03418922 | ||
| Nivolumab + cabozantinib | PD-L1; VEGFR2/c-MET | Phase 1/2, randomized, open label | Advanced HCC with or without chronic viral hepatitis; Child-Pugh B (1,097 pts) | NCT01658878 |
| Nivolumab + regorafenib | PD-1; VEGFR2/TIE2 | Phase l/2a, single arm, open label | Advanced HCC with progression after sorafenib (69 pts) | NCT04170556 |
| Phase 2, single arm, open label | Chemotherapy-naïve, unresectable or metastatic HCC; Child-Pugh A (42 pts) | NCT04310709 | ||
| Pembrolizumab + sorafenib | PD-1; multiple kinases | Phase 1b/2, single arm, open label | Advanced or metastatic liver cancer; Child-Pugh A (27 pts) | NCT03211416 |
| Pembrolizumab + lenvatinib | PD-1; multiple kinases | Phase 2, single arm, nonrandomized | Advanced hepatobiliary malignancies including HCC and BTC (50 pts) | NCT03895970 |
| Phase 1b, open label | Advanced HCC; BCLC stage B/C (150 pts) | NCT03006926 | ||
| Phase 3, randomized controlled, multicenter | Advanced, treatment-naïve HCC; BCLC stage B/C (750 pts) | NCT03713593 | ||
| Pembrolizumab + regorafenib | PD-1; VEGFR2/TIE2 | Phase 1b, nonrandomized, open label | Advanced, treatment-naïve HCC; BCLC stage B/C (57 pts) | NCT03347292 |
| Camrelizumab + apatinib | PD-1; VEGFR2 | Phase 2, single arm, open label | Advanced-stage HCC; BCLC stage B/C (40 pts); | NCT04014101 |
| phase 2, single arm, open label | unresectable HCC (30 pts) | NCT03793725 | ||
| Phase 3, randomized, open label, multicenter | Advanced, treatment-naïve HCC; BCLC stage B/C (510 pts) | NCT03764293 | ||
| Phase 2 , single arm, open label | Advanced HCC; Child-Pugh A (190 pts) | NCT03463876 | ||
| Sintilimab + lenvatinib | PD-1; multiple kinases | Phase 2, single arm, open label | Local advanced HCC; BCLC stage B/C; Child-Pugh score ≤ 7 (56 pts) | NCT04042805 |
| Sintilimab + anlotinib | PD-1; multiple kinases | Phase 2, single arm, open label | Advanced HCC; BCLC stage B/C; Child-Pugh A (20 pts) | NCT04052152 |
| Toripalimab + axitinib | PD-1; multiple kinases | Phase 2, single arm, open label, single center | Advanced hepatobiliary malignancies including HCC and CCA (60 pts) | NCT04010071 |
| Toripalimab + sorafenib | PD-1; multiple kinases | Phase 1/2, single arm, open label | Unresectable HCC with portal vein tumor thrombus; Child-Pugh score ≤ 7 (39 pts) | NCT04069949 |
| Tislelizumab + regorafenib | PD-1; VEGFR2/TIE2 | Phase 2, randomized, open label | Advanced HCC (125 pts) | NCT04183088 |
| AK105 + anlotinib | PD-1; multiple kinases | Phase 1b/2, open label, multicenter | Unresectable HCC; BCLC stage B/C; Child-Pugh score ≤ 7 (30 pts) | NCT04172571 |
| Durvalumab + tivozanib | PD-L1; VEGFR1–3 | Phase 1b/2, open label | Advanced, treatment-naïve HCC; Child-Pugh A (42 pts) | NCT03970616 |
| Durvalumab + cabozantinib | PD-L1; VEGFR2/c-MET | Phase 1b, single arm, open label | Advanced gastroesophageal cancer including HCC (30 pts) | NCT03539822 |
| Atezoiizumab + cabozantinib | PD-L1; VEGFR2/c-MET | Phase 3, randomized controlled | Advanced, treatment-naïve HCC; BCLC stage B/C (740 pts) | NCT03755791 |
| CCA | ||||
| Pembrolizumab + lenvatinib | PD-1; multiple kinases | Phase 2, single arm, open label | Advanced, refractory, primary liver cancer or BTC (50 pts) | NCT03895970 |
| Phase 2, single arm, open label | Advanced refractory solid tumors including BTC; Child-Pugh score 5–6 (180 pts) | NCT03797326 | ||
| Pembrolizumab + ramucirumab | PD-1; VEGFR2 | Phase 1, single arm, open label | Advanced refractory, biopsiable cancers including BTC (155 pts) | NCT02443324 |
| Camrelizumab + apatinib | PD-1; VEGFR2 | Phase 2, nonrandomized, open label | Advanced primary liver cancer including BTC; Child-Pugh A or B (152 pts) | NCT03092895 |
| Sintilimab + anlotinib + gemcitabine + cisplatin | PD-1; multiple kinases; chemotherapy | Phase 2a, randomized controlled, multicenter | Unresectable or metastatic BTC (80 pts) | NCT04300959 |
| TQB2450 + anlotinib | PD-L1; multiple kinases | Phase 1b/2, single arm, open label | Advanced, refractory BTC or HCC (60 pts) | NCT03825705 |
| Avelumab + regorafenib | PD-L1; VEGFR2/TIE2 | Phase 1/2, nonrandomized, open label | Advanced, refractory digestive tumors, not MMR-deficient (212 pts) | NCT03475953 |
| Immune microenvironment-targeted therapy | ||||
| HCC | ||||
| Nivolumab + galunisertib | PD-1; TGF-β inhibitor | Phase 1b/2, nonrandomized, single arm, open label | Advanced refractory solid tumors including HCC (75 pts) | NCT02423343 |
| Nivolumab + BMS-986205 | PD-1; IDO1 Inhibitor | Phase 1/2, single arm, open label | Advanced HCC; Child-Pugh A (23 pts) | NCT03695250 |
| Nivolumab + cabiralizumab | PD-1; CSF1R | Phase 2, randomized controlled | Advanced HCC; Child-Pugh score ≤ 7 (74 pts) | NCT04050462 |
| CCA | ||||
| Pembrolizumab + sargramostim | PD-1; GM-CSF | Phase 2, single arm, open label | Advanced biliary cancers (42 pts) | NCT02703714 |
| Nivolumab + cabiralizumab | PD-1; CSF1R | Phase 2, randomized, open label | Resectable, biopsiable BTC (16 pts) | NCT03768531 |
| Nivolumab + entinostat | PD-1; histone deacety-lase inhibitor | Phase 2, nonrandomized, open label | Advanced, treatment-naïve CCA or PDAC (54 pts) | NCT03250273 |
| ABBV-181 + ABBV-368 | PD-1; CD40 agonist | Phase 1, nonrandomized, open label | Advanced solid cancers including CCA (170 pts) | NCT03071757 |
Note: A www.ClinicalTrials.gov search was performed using the terms “liver cancer,” “hepatocellular carcinoma,” “liver neoplasm” for HCC; “biliary tract cancer,” “cholangiocarcinoma,” “biliary carcinoma,” “bile duct,” or “biliary tract” for CCA; and “hepatobiliary malignant tumors” for both HCC and CCA. The search identified immunotherapy trials with a status of “Recruiting,” “Not yet recruiting,” “Active, not recruiting,” or “Enrolling by invitation,” and trials without the inclusion of a specific liver cancer cohort or without adequate information available were excluded. Search was updated as of March 30, 2020.
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CD40, cluster of differentiation 40; CSF1R, colony stimulating factor 1 receptor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IDO1, indoleamine 2,3-dioxygenase 1; and PDAC, pancreatic ductal adenocarcinoma.
Regorafenib, a multikinase inhibitor with activity against kinases involved in angiogenesis (VEGFR 1–3) and oncogenesis (RET, c-RAF/RAF-1), is FDA-approved as a second-line agent for patients with advanced HCC who have failed sorafenib treatment.(59) Cabozantinib is another tyrosine kinase inhibitor (TKI) that is approved as a second-line and third-line agent for advanced HCC.(60) Combinatorial regimens of these TKIs plus ICI are currently under investigation in several ongoing clinical trials (Table 1).
Although these combinatorial regimens have significant antitumor potential, their efficacy may be limited by AEs. Unlike bevacizumab, which specifically targets VEGF, TKIs targeting the VEGF axis are multikinase inhibitors with a greater potential for off-target effects and higher rate of AEs. A meta-analysis of bevacizumab versus VEGFR TKIs in patients with metastatic colorectal cancer demonstrated higher rates of AEs group (diarrhea, fatigue, thrombocytopenia, neutropenia, and hypertension) with VEGFR TKIs compared with bevacizumab.(61) We eagerly await the results of ongoing clinical trials in advanced HCC to determine the efficacy as well as safety of these combinatorial regimens.
Targeting PD-1/PD-L1 Plus CTLA-4
The safety and efficacy of combination ipilimumab, an anti-CTLA-4 antibody, and nivolumab was assessed in sorafenib-treated patients with advanced HCC in a CheckMate 040 cohort.(62) Based on an ORR of 32% and median OS of 23 months, the FDA granted accelerated approval to combination ipilimumab 3 mg/Kg and nivolumab 1 mg/Kg in March 2020 for treatment of advanced HCC previously treated with sorafenib. This combination did have a relatively high rate of grade 3–4 treatment-related AEs at 53%, and 57.1% of patients with immune-mediated events required steroids. Several ongoing clinical trials are investigating the safety and efficacy of dual immune checkpoint inhibition in human liver cancer (Table 2).
TABLE 2.
Ongoing Clinical Trials of Dual Immune Checkpoint Inhibition in Patients With Liver Cancer
| Intervention | Target | Trial Type | Trial Description | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Dual immune checkpoint inhibition | ||||
| HCC | ||||
| Nivolumab + ipilimumab | PD-1; CTLA-4 | Phase 3, randomized, multicenter | Advanced, treatment-naïve HCC; Child-Pugh score 5 or 6 (1,084 pts) | NCT04039607 |
| Phase 1/2, randomized, open label | Advanced HCC; Child-Pugh B (1,097 pts) | NCT01658878 | ||
| Durvalumab + tremelimumab | PD-L1; CTLA-4 | Phase 3, randomized, open label, multicenter | Treatment-naïve, unresectable HCC; BCLC stage B/C (1,310 pts) | NCT03298451 |
| Phase 2, randomized, open label, multicenter | Advanced HCC (433 pts) | NCT02519348 | ||
| Phase 2, two arms, open label | Intermediate-stage HCC (30 pts) | NCT03638141 | ||
| Nivolumab + relatlimab | PD-1; LAG-3 | Phase 1/2a, randomized, open label | Advanced, treatment-naïve solid tumors including HCC (1,500 pts) | NCT01968109 |
| TSR-042 + TSR-022 | PD-1; TIM-3 | Phase 2, single arm, open label | Advanced or metastatic HCC; BCLC stage B/C (42 pts) | NCT03680508 |
| CCA | ||||
| Nivolumab + ipilimumab | PD-L1; CTLA-4 | Phase 2, single arm, open label | Advanced, refractory solid tumors including BTC (707 pts) | NCT02834013 |
| Phase 2, randomized, open label | Unresectable, treatment-naïve BTC; Child-Pugh A (64 pts) | NCT03101566 | ||
| Durvalumab + tremelimumab | PD-L1; CTLA-4 | Phase 1, nonrandomized, open label | Advanced, refractory, biopsiable solid tumors including BTC (269 pts) | NCT01938612 |
Note: A www.ClinicalTrials.gov search was performed using the terms “liver cancer,” “hepatocellular carcinoma,” “liver neoplasm” for HCC; “biliary tract cancer,” “cholangiocarcinoma,” “biliary carcinoma,” “bile duct,” or “biliary tract” for CCA; and “hepatobiliary malignant tumors” for both HCC and CCA. The search identified immunotherapy trials with a status of “Recruiting,” “Not yet recruiting,” “Active, not recruiting,” or “Enrolling by invitation,” and trials without the inclusion of a specific liver cancer cohort or without adequate information available were excluded. Search was updated as of March 30, 2020.
Abbreviations: LAG-3, lymphocyte-activation gene 3; TIM-3, T-cell immunoglobulin mucin-3.
IMMUNOTHERAPY BEYOND CHECKPOINT INHIBITORS
Cell-based immunotherapies such as adoptive cell transfer with TILs or CIKs have potent antitumor effects with little cytotoxicity to normal cells. The efficacy and safety of activated CIKs was assessed in the adjuvant setting in 230 patients with HCC who had undergone surgical resection or radiofrequency ablation (RFA) or percutaneous ethanol injection.(63) Median PFS was 44 months in the immunotherapy group versus 30 months in the control group; OS was not reached in either group, but OS was longer in the CIK treatment group (HR: 0.21; 95% CI: 0.06–0.75; P = 0.008). Although overall AEs were significantly higher in the CIK group (62% vs. 41%; P = 0.002), the serious AE rate was similar (7.8% vs. 3.5%; P = 0.15).
Despite these encouraging results, isolating tumor-specific T cells from patients with cancer remains a challenge. Gene-modified T-cell therapy can overcome this limitation by engineering T cells targeting specific tumor antigens. These approaches include tumor antigen–specific T-cell receptor and chimeric antigen receptor technology, and are currently being evaluated in patients with solid tumors, including liver cancer (Table 3).
TABLE 3.
Ongoing Clinical Trials of Cell-Based Therapy in Patients With Liver Cancer
| Intervention | Trial Type | Trial Description | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Cell-based therapy | |||
| HCC | |||
| NK cells | Phase 1/2, nonrandomized | Advanced HCC; Child-Pugh A/B (200 pts) | NCT04162158 |
| iNKT cells + CD8 T cells | Phase 1/2, single-arm, open label | Advanced solid tumor including HCC (40 pts) | NCT03093688 |
| iNKT cells + TACE | Phase 2, randomized, open label | Advanced HCC; BCLC stage C (144 pts) | NCT04011033 |
| CIKs | Phase 1, single arm, open label | Advanced solid tumor including HCC (24 pts) | NCT04282044 |
| Phase 2, randomized, single center | Advanced liver cancer; Child-Pugh score ≤ 7 (80 pts) | NCT03146637 | |
| CIKs + TACE | Phase 2, randomized, open label, multicenter | Intermediate stage HCC undergoing TACE; Child-Pugh B (78 pts) | NCT02856815 |
| CIKs + RFA | Phase 3, randomized .open label | Recurrent HCC (210 pts) | NCT02678013 |
| CIKs + CD3-MUC-1 + Cryotherapy | phase 2, randomized, single center | Advanced liver cancer, BCLC stage C; Child-Pugh score ≤ 7 (90 pts) | NCT03484962 |
| Phase 1, single arm, open label | Advanced GPC3-positive HCC; Child-Pugh score < 7 (30 pts) | NCT03198546 | |
| Anti-GPC3 CAR-T | Phase 1, single arm, open label | Advanced GPC3-positive HCC; Child-Pugh score < 7 (20 pts) | NCT04121273 |
| Phase 1, single arm, open label | Advanced GPC3-positive HCC; BCLC stage B/C (15 pts) | NCT03884751 | |
| Phase 1, sequential assignment, open label | Advanced GPC3-positive HCC; BCLC stage B/C (36 pts) | NCT03980288 | |
| Phase 1, single arm, open label | Advanced GPC3-positive HCC; Child-Pugh score < 7(14 pts) | NCT02905188 | |
| Anti-EpCAM CAR-T | Phase 1, single arm, open label | EpCAM positive solid tumor including HCC (60 pts) | NCT03013712 |
| Anti-EGFR vlll CAR-T/TCR-T | Phase 1/2, single arm, open label, multicenter | EGFR positive solid tumor including HCC (50 pts) | NCT03941626 |
| Anti-CD147-targeted CAR-T | Phase 1, single arm, open label | Advanced HCC; Child-Pugh score ≤ 7 (34 pts) | NCT03993743 |
| Anti-NKG2D-based CAR T | Phase 1, single arm, open label | NKG2D positive relapsed/refractory solid tumor including HCC (10 pts) | NCT04270461 |
| Autologous AFP-specific TCR T cells | Phase 1, single arm, open label | AFP positive HCC; Child-Pugh score ≤ 6 (24 pts) | NCT03132792 |
| Phase 1, single arm, open label | AFP positive, unresectable HCC; BCLC stage B/C; Child-Pugh score ≤ 6 (9 pts) | NCT03971747 | |
| NY-ESO-1-specific TCR T cells | Phase 1, nonrandomized, open label | Advanced solid tumor including HCC (22 pts) | NCT02869217 |
| Phase 1, single arm, open label | NY-ESO-1 expressing advanced cancer including HCC (43 pts) | NCT01967823 | |
| CCA | |||
| Autologous CIKs + RFA | Phase 2/3, nonrandomized, single blind | Unresected cholangiocarcinoma without extrahepatic metastasis (50 pts) | NCT02482454 |
| Autologous CD8 T cells + pembrolizumab | Phase 1, single arm, open label | Advanced GI malignancies including CCA (40 pts) | NCT02757391 |
| Autologous Tern cells + RFA/chemotherapy | Phase 2, randomized, open label | ICC after radical resection (20 pts) | NCT03820310 |
| Autologous transfer TIL | Phase 2, single arm, open label | Unresectable, refractory BTC (59 pts) | NCT03801083 |
| Autologous MUC-1 CAR T | Phase 1/2, single arm, open label | MUC-1 positive ICC (9 pts) | NCT03633773 |
| MSLN-specific TCR T cells | Phase 1/2, single arm, open label | Advanced mesothelin-positive cancer including CCA (70 pts) | NCT03907852 |
Note: A www.ClinicalTrials.gov search was performed using the terms “liver cancer,” “hepatocellular carcinoma,” and “liver neoplasm” for HCC; “biliary tract cancer,” “cholangiocarcinoma,” “biliary carcinoma,” “bile duct,” or “biliary tract” for CCA; and “hepatobiliary malignant tumors” for both HCC and CCA. The search identified immunotherapy trials with a status of “Recruiting,” “Not yet recruiting,” “Active, not recruiting,” or “Enrolling by invitation,” and trials without the inclusion of a specific liver cancer cohort or without adequate information available were excluded. Search was updated as of March 30, 2020.
Abbreviations: AFP, alpha-fetoprotein; CAR-T, chimeric antigen receptor T cells; CD3, cluster of differentiation 3; CD8, cluster of differentiation 8; CD147, cluster of differentiation 147; EGFR, epidermal growth factor receptor; EpCAM, epithelial cell adhesion molecule; GPC3, glypican 3; HBV, hepatitis virus B; iNKT, invariant natural killer T cells; GMUC-1, mucin 1; NKG2D, killer cell lectin like receptor K1; NY-ESO-1, New York esophageal squamous cell carcinoma 1; TACE, transarterial chemoembolization; Tcm, csentral memory T; TCR, T cell receptor; TIL, tumor infiltrating lymphocytes.
IMMUNOTHERAPY IN EARLIER STAGES OF DISEASE
Immunotherapy in the neoadjuvant setting when a higher endogenous tumor antigen burden is present in the primary tumor can augment T-cell priming, and has the potential to eliminate micrometastasis, which would be the source of postsurgical tumor recurrence.(64) Several ongoing advanced phase trials assessing the safety and efficacy of ICI in the adjuvant/neoadjuvant setting are underway in advanced liver cancer (Table 4).
TABLE 4.
Ongoing Clinical Trials of Neoadjuvant/Adjuvant Immunotherapy in Patients With Liver Cancer
| Intervention | Target | Trial Type | Trial Description | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Neoadjuvant Immunotherapy | ||||
| HCC | ||||
| Pembrolizumab | PD-1 | Phase 1, single arm, open label | Adjuvant or neoadjuvant treatment of resectable HCC (45 pts) | NCT04224480 |
| Phase 1, single arm, open label | Adjuvant or neoadjuvant therapy with curative treatment such as resection or RFA; Child-Pugh A (50 pts) | NCT03337841 | ||
| Cemiplimab | PD-1 | Phase 2, randomized, multi-cohort | Resectable HCC (94 pts) | NCT03916627 |
| Nivolumab + ipilimumab | PD-1; CTLA-4 | Phase 2, single arm, open label | HCC with potential for curative surgical resection; Child-Pugh A (40 pts) | NCT03510871 |
| Phase 2, randomized, open-label | HCC with potential for curative surgical resection (45 pts) | NCT03222076 | ||
| Phase 2, single-arm, open-label | Resectable HCC; Child-Pugh A (32 pts) | NCT03682276 | ||
| Nivolumab + BMS-813160/BMS-986253 | PD-1; CCR2/5-inhibitor or IL-8 | Phase 2, randomized, open label | Resectable NSCLC/HCC (50 pts) | NCT04123379 |
| Camrelizumab + apatinib | PD-1; VEGFR2 | Phase 1/2, single arm, open label, multicenter | Downstaging/bridging of HCC before liver transplant; Child-Pugh A or B (120 pts) | NCT04035876 |
| Phase 2, single arm, open label | Perioperative treatment of HCC (20 pts) | NCT04297202 | ||
| Sintilimab + TACE | PD-1; transarterial chemoembolization | Phase 2, single arm, open label | Early and intermediate stage HCC not suitable for primary resection; BCLC stage A/B (61 pts) | NCT04174781 |
| CCA | ||||
| Nivolumab + cabiralizumab | PD-1; CSF1R | Phase 2, randomized, open label | Resectable BTC (16 pts) | NCT03768531 |
| Adjuvant Immunotherapy | ||||
| HCC | ||||
| Nivolumab | PD-1 | Phase 2, single arm, open label, multicenter | Advanced HCC treated by electroporation (NIVOLEP) with curative intent; Child-Pugh A (50 pts) | NCT03630640 |
| Phase 3, randomized, double blinded | HCC at high risk of recurrence after curative hepatic resection or ablation; Child-Pugh score 5 or 6 (530 pts) | NCT03383458 | ||
| Pembrolizumab | PD-1 | Phase 3, randomized, double blinded, two-arm | HCC after surgical resection or local ablation; Child-Pugh A (950 pts) | NCT03867084 |
| Toripalimab | PD-1 | Phase 2/3, randomized-controlled, double-blind | Local advanced HCC after curative hepatic resection; Child-Pugh A (402 pts) | NCT03859128 |
| Nivolumab + cabozantinib | PD-1; VEGFR2/c-MET | Phase 1, single arm, open label | Local advanced HCC after resection (15 pts) | NCT03299946 |
| Nivolumab + PI 101 | PD-1; ropeginterferon alfa-2b | Phase 1/2, randomized, open label | After curative surgery of HBV-related HCC (72 pts) | NCT04233840 |
| Durvalumab + bevacizumab | PD-L1; VEGF | Phase 3, randomized-controlled, multicenter | HCC at high risk of recurrence after curative hepatic resection or ablation; Child-Pugh score of 5 or 6 (888 pts) | NCT03847428 |
| Atezolizumab + bevacizumab | PD-L1; VEGF | Phase 3, randomized, open label, multicenter | HCC at high risk of recurrence after curative hepatic resection or ablation; Child-Pugh A (662 pts) | NCT04102098 |
| HBV-specific TCR T cells | HBV antigen | Phase 1, single-arm, open label | Hepatitis B virus (HBV) related HCC after liver transplantation (10 pts) | NCT02686372 |
| Phase 1, single-arm, open label | Recurrent, HBV-related HCC after liver transplantation (10 pts) | NCT02719782 | ||
| Phase 1, single arm, open label, single center | HBV related HCC post hepatectomy or radiofrequency ablation; BCLC stage C (10 pts) | NCT03899415 | ||
| Phase 1/2, open label, single arm, multicenter | Recurrent, HBV-related HCC after liver transplantation (72 pts) | NCT03634683 |
Note: A www.ClinicalTrials.gov search was performed using the terms “liver cancer,” “hepatocellular carcinoma,” and “liver neoplasm” for HCC; “biliary tract cancer,” “cholangiocarcinoma,” “biliary carcinoma,” “bile duct,” or “biliary tract” for CCA; “hepatobiliary malignant tumors” for both HCC and CCA. The search identified immunotherapy trials with a status of “Recruiting,” “Not yet recruiting,” “Active, not recruiting,” or “Enrolling by invitation,” and trials without the inclusion of a specific liver cancer cohort or without adequate information available were excluded. Search was updated as of March 30, 2020.
Abbreviations: BTC, biliary tract cancer; CCR2/5, C-C chemokine receptor types 2/5; HBV, hepatitis virus B; NSCLC, non-small-cell lung carcinoma.
Locoregional therapy such as RFA, transarterial chemoembolization, radioembolization, or external radiation therapy is an option for treatment of unresectable HCC limited to the liver. The combination of locoregional therapy with ICI has garnered interest based on the premise that locoregional modalities such as RFA may mediate immunogenic cell death and release of antigens that promote a peripheral immune response.(65) This peripheral immune stimulation can theoretically be augmented by ICI. In a cohort of 32 patients with HCC, the anti-CTLA-4 antibody tremelimumab was administered in patients who underwent RFA. PR was noted in 5 of 19 evaluable patients, and increased intratumoral CD8+ T-cell accumulation was noted in patients with response.(65) The combination of ICI and locoregional therapy is currently under active investigation in several clinical trials of liver cancer (Table 5).
TABLE 5.
Ongoing Clinical Trials of Immune Checkpoint Inhibition in Combination With Locoregional Therapy in Liver Cancer
| Intervention | Trial Type | Trial description | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Immune checkpoint Inhibition plus locoregional therapy | |||
| HCC | |||
| Nivolumab (anti-PD-1) + Y90 | Phase 2, nonrandomized, open label, single center | Advanced HCC; Child-Pugh A (40 pts) | NCT03033446 |
| Phase 1/2, one arm, open label | Advanced HCC; Child-Pugh A or B (35 pts) | NCT02837029 | |
| Nivolumab (anti-PD-1) + SIRT | Phase 2, single arm, open label, multicenter | HCC candidates for locoregional therapy (40 pts) | NCT03380130 |
| Phase 2/3, randomized, two arm | Intermediate stage HCC; Child-Pugh B or C (522 pts) | NCT04268888 | |
| Nivolumab (anti-PD-1) + TACE | Phase 1, nonrandomized, open label | Advanced HCC; Child-Pugh A (14 pts) | NCT03143270 |
| Phase 2, single arm, open label | Intermediate stage HCC; Child-Pugh A (49 pts) | NCT03572582 | |
| Pembrolizumab (anti-PD-1) + RFA/MWA | Phase 2, one arm, open label | Early-stage HCC; Child-Pugh score ≤6 (30 pts) | NCT03753659 |
| Pembrolizumab (anti-PD-1) + SBRT | Phase 2, single arm, open label | Advanced HCC after treatment with sorafenib; Child-Pugh A (30 pts) | NCT03316872 |
| Pembrolizumab (anti-PD-1) + TACE | Phase 1b, one arm, open label | Unresectable HCC; Child-Pugh score < 7 (26 pts) | NCT03397654 |
| Sintilimab (anti-PD-1) + TACE ± MWA | Phase 1, single arm, open label | Unresectable HCC; BCLC stage B/C (45 pts) | NCT04220944 |
| Toriplimab (anti-PD-1) + RFA/MWA | Phase 1/2, randomized, open label | Unresectable HCC; Child-Pugh A or B (120 pts) | NCT03864211 |
| Durvalumab (anti-PD-L1) + TACE | Phase 3, randomized controlled, multicenter | Locoregional HCC; Child-Pugh A (600 pts) | NCT03778957 |
| Durvalumab (anti-PD-L1) + tremelimumab (anti-CTLA-4) + TAC/RFA | Phase 2, nonrandomized, open label | Unresectable, refractory HCC or BTC; Child-Pugh A/B (90 pts) | NCT02821754 |
| Phase 2, single arm, open label | Unresectable HCC or BTC; Child-Pugh A (70 pts) | NCT03482102 | |
| Phase 2, nonrandomized, open label | |||
| Intermediate stage of HCC (30 pts) | NCT03638141 | ||
| CCA | |||
| Pembrolizumab (anti-PD-1) + SBRT | Phase 2, randomized, open label | Unresectable, treatment-naïve ICC, eligible for radiotherapy; Child-Pugh A (184 pts) | NCT03898895 |
| Durvalumab (anti-PD-L1) + tremelimumab (anti-CTLA-4) + TACE/RFA/cryoablation | Phase 2, nonrandomized, open label | Unresectable, refractory HCC or BTC (90 pts) | NCT02821754 |
| Phase 2, single arm, open label | Unresectable HCC or BTC (70 pts) | NCT03482102 | |
Note: A www.ClinicalTrials.gov search was performed using the terms “liver cancer,” “hepatocellular carcinoma,” and “liver neoplasm” for HCC; “biliary tract cancer,” “cholangiocarcinoma,” “biliary carcinoma,” “bile duct,” or “biliary tract” for CCA; and “hepatobiliary malignant tumors” for both HCC and CCA. The search identified immunotherapy trials with a status of “Recruiting,” “Not yet recruiting,” “Active, not recruiting,” or “Enrolling by invitation,” and trials without the inclusion of a specific liver cancer cohort or without adequate information available were excluded. Search was updated as of March 30, 2020.
Abbreviations: MWA, microwave ablation; TACE, transarterial chemoembolization; SBRT, stereotactic body radiotherapy.
IMMUNE CHECKPOINT INHIBITORS IN BILIARY CANCERS
The efficacy of ICI monotherapy in biliary tract cancers (BTCs) has been disappointing thus far based on interim results from the KEYNOTE-158 trial, an ongoing phase 2, single-arm, open-label trial of pembrolizumab in advanced cancers, including a cohort of BTC. Based on the interim analysis, the ORR was 5.8% in 104 patients with BTC and the duration of response ranged from 6.2 to over 15 months.(66) The ORR was slightly higher in a cohort of patients with BTC with PD-L1-positive tumors. However, there was no significant difference in the OS (7.2 vs. 9.6 months). Limited data indicate the potential benefit of ICI monotherapy in patients with BTC with microsatellite instability due to mismatch repair deficiency (MSI-high/MMR-deficient). In a prospective trial of 86 patients with advanced MSI-high/MMR-deficient tumors, the disease control rate in patients with BTC (n = 4) was 100%; 1 patient had complete response (CR) and 3 had stable disease.(67) Based on its efficacy in this cohort of patients, pembrolizumab was granted accelerated FDA approval in MSI-high/MMR-deficient tumors including BTC.
In a phase 2 study of patients with advanced BTC, nivolumab resulted in PR in 10 (22%) and SD in 17 (37.8%) of 45 response-evaluable patients.(68) All patients who responded had microsatellite stable tumors. In a phase 1 clinical trial of the anti-PD-L1 antibody durvalumab with (n = 65) or without tremelimumab (n = 42) in patients with advanced BTC, the combination had PR in 7 patients compared with only 2 patients in the durvalumab monotherapy group.(69) Grade 3 or 4 AEs were noted in 23% of patients in the combination arm, and one death related to drug-induced liver injury was reported.
Although single-agent ICI in HCC has shown a trend toward improvement in survival and quality of life, based on the currently available data, the efficacy of single-agent ICI in BTC has been subpar. CCAs are desmoplastic tumors with an abundant tumor-immune microenvironment characterized by immunosuppressive TAMs and MDSCs.(70) An effective approach will likely use a combination of immunotherapies targeting the innate and adaptive immune response in CCA. Combination immunotherapy strategies using ICI are currently under investigation in multiple ongoing clinical trials (Tables 1–5).
Future Perspectives
Recent efforts have begun to uncover the complex hepatic immune network in chronic inflammatory liver diseases as well as liver tumors. Further studies using single cell–based approaches, such as single-cell transcriptomics and multiplexed imaging, are required to illustrate the complex cellular and phenotypic heterogeneity of the liver cancer ecosystem. The information gleaned from these studies will be imperative in the development of successful treatment approaches. The intricate liver cancer TIME with diverse immune cell subsets and extensive tumor-immune cell crosstalk also underscores the need for combination therapeutic approaches targeting these components. Accordingly, the first successful phase 3 trial of systemic therapy in HCC demonstrating survival benefit compared with sorafenib harnessed immune checkpoint inhibition with VEGF inhibition. More studies are needed to identify effective combination therapies for patients with advanced-stage liver cancer. Additionally, immunotherapy holds significant potential in earlier stages of disease, and several trials investigating the effectiveness of immune-based approaches in the neoadjuvant and adjuvant setting are ongoing. Finally, development of biomarkers that can effectively predict immunotherapy response will be essential in identifying the optimal therapeutic target and selecting the appropriate therapy for each individual patient.
Acknowledgments
Supported by the National Institute of Health (1K08CA236874-01[SR] and U01AA027681 [ABE]), Mayo Center for Cell Signaling in Gastroenterology (Pilot & Feasibility Award P30DK084567), American Gastroenterology Association Research Scholar Award, the Satter Family Liver Cancer Award, Hepatobiliary Cancer SPORE (P50 CA210964) Career Enhancement Program, and the Mayo Foundation.
Potential conflict of interest:
Dr. El-Khoueiry advises and received grants from AstraZeneca and Merck. He advises Bayer, Bristol Myers Squibb, EISAI, Exelixis, Agenus, Gilead, and Roche-Genentech. He received grants from Astex.
Abbreviations:
- AE
adverse event
- AFP
alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- CCA
cholangiocarcinoma
- cDC
conventional DCs
- CI
confidence interval
- CIK
cytokine-induced killer cell
- CR
complete response
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CTL
cytotoxic T lymphocyte
- DCs
dendritic cells
- FDA
Food and Drug Administration
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ICI
immune checkpoint inhibition
- KC
Kupffer cell
- LAYN
layilin
- MDSC
myeloid-derived suppressor cell
- MSI
microsatellite instability
- NK
natural killer
- ORR
objective response rate
- OS
overall survival
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- PFS
progression-free survival
- PR
partial response
- RFA
radiofrequency ablation
- SD
stable disease
- TAM
tumor-associated macrophage
- TGFβ
transforming growth factor β
- TIL
tumor-infiltrating lymphocyte
- TIME
tumor immune microenvironment
- TKI
tyrosine kinase inhibitor
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor
- Treg
regulatory T cells
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
REFERENCES
- 1).Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Everhart JE, Ruhl CE. Burden of digestive diseases in the United States. III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134–1144. [DOI] [PubMed] [Google Scholar]
- 3).Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ringelhan M, Pfister D, O’Connor T, Pikarsky E. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222–232. [DOI] [PubMed] [Google Scholar]
- 6).Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014;59:1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017;66:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16:7–19. [DOI] [PubMed] [Google Scholar]
- 10).Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017;6:e1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Sui Q, Zhang J, Sun X, Zhang C, Han Q, Tian Z. NK cells are the crucial antitumor mediators when STAT3-mediated immunosuppression is blocked in hepatocellular carcinoma. J Immunol 2014;193:2016–2023. [DOI] [PubMed] [Google Scholar]
- 12).Zhang QF, Yin WW, Xia Y, Xia Y, Yi YY, He QF, et al. Liver-infiltrating CD11b(−)CD27(−) NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell Mol Immunol 2017;14:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Tsukagoshi M, Wada S, Yokobori T, Altan B, Ishii N, Watanabe A, et al. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci 2016;107:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Jung IH, Kim DH, Yoo DK, Baek SY, Jeong SH, Jung DE et al. In vivo study of natural killer (NK) cell cytotoxicity against cholangiocarcinoma in a nude mouse model. In Vivo 2018;32:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007;132:2328–2339. [DOI] [PubMed] [Google Scholar]
- 17).Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586–2593. [DOI] [PubMed] [Google Scholar]
- 18).Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer 2018;118:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Zabala M, Lasarte JJ, Perret C, Sola J, Berraondo P, Alfaro M, et al. Induction of immunosuppressive molecules and regulatory T cells counteracts the antitumor effect of interleukin-12-based gene therapy in a transgenic mouse model of liver cancer. J Hepatol 2007;47:807–815. [DOI] [PubMed] [Google Scholar]
- 20).Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan J, et al. TGF-β regulates hepatocellular carcinoma progression by inducing Treg cell polarization. Cell Physiol Biochem 2015;35:1623–1632. [DOI] [PubMed] [Google Scholar]
- 21).Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 2017;169:1342–1356.e16. [DOI] [PubMed] [Google Scholar]
- 22).Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 2019;36:418–430.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer 2016;16:447–462. [DOI] [PubMed] [Google Scholar]
- 24).Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity 2019;51:411–412. [DOI] [PubMed] [Google Scholar]
- 25).Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 2019;179:829–845.e20. [DOI] [PubMed] [Google Scholar]
- 26).Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, et al. Kupffer cell-derived TNF triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 2017;31:771–789.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 2015;125:1269–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol 2015;62:198–207. [DOI] [PubMed] [Google Scholar]
- 29).Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol 2012;13:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Martin-Sierra C, Martins R, Laranjeira P, Abrantes AM, Oliveira RC, Tralhão JG et al. Functional impairment of circulating fcepsilonRI(+) monocytes and myeloid dendritic cells in hepatocellular carcinoma and cholangiocarcinoma patients. Cytometry B Clin Cytom 2019;96:490–495. [DOI] [PubMed] [Google Scholar]
- 31).Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol 2004;35:881–886. [DOI] [PubMed] [Google Scholar]
- 32).Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res 2017;5:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135: 234–243. [DOI] [PubMed] [Google Scholar]
- 34).Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The role of myeloid-derived suppressor cells in patients with solid tumors: a meta-analysis. PLoS One 2016;11:e0164514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009;50:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res 2013;73:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006;203:2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Yu SJ, Ma C, Heinrich B, Brown ZJ, Sandhu M, Zhang Q, et al. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J Hepatol 2019;70:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 2018;15:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong Wi, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 2010;52:1322–1333. [DOI] [PubMed] [Google Scholar]
- 41).Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012;21:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81–88. [DOI] [PubMed] [Google Scholar]
- 45).El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 47).Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571–580. [DOI] [PubMed] [Google Scholar]
- 48).Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim HY, et al. Results of KEYNOTE- 240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma. J Clin Oncol 2019;37:4004. [Google Scholar]
- 49).Yau T, Park JW, Finn RS, Cheng A-l, Mathurin P, Edeline J, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol 2019;30:v874–v875. [Google Scholar]
- 50).Melero I, Neely J, Sangro B, Finn R, Abou-Alfa GK, Cheng A-L, et al. Abstract 2675: Assessment of inflammation biomarkers in relation to clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma in CheckMate 040. Clin Res 2019;79:2675. [Google Scholar]
- 51).Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res 2019;25:2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol 2018;9:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013;172:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Cheng AL, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol 2019;30:ix186–ix187. [Google Scholar]
- 57).Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 58).Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, et al. Abstract CT061: A Phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Cancer Res 2019;79:CT061. [Google Scholar]
- 59).Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 60).Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo B-Y, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Lin Z, Yang Y, Huang Y, Liang J, Lu F, Lao X. Vascular endothelial growth factor receptor tyrosine kinase inhibitors versus bevacizumab in metastatic colorectal cancer: a systematic review and meta-analysis. Mol Clin Oncol 2015;3:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol 2019;37:4012. [Google Scholar]
- 63).Lee JH, Lee JH, Lim YS, Yeon JE, Song T-J, Yoon SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015;148:1383–1391.e6. [DOI] [PubMed] [Google Scholar]
- 64).Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367:eaax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Ueno M, Chung HC, Nagrial A, Marabelle A, Kelley Rk, Xu L, et al. Pembrolizumab for advanced biliary adenocarcinoma: results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol 2018;29:viii210. [Google Scholar]
- 67).Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Kim RD, Kim DW, Alese OB, Li D, Shah N, Schell MJ, et al. A phase II study of nivolumab in patients with advanced refractory biliary tract cancers (BTC). J Clin Oncol 2019;37:4097. [Google Scholar]
- 69).Ioka T, Ueno M, Oh D-Y, Fujiwara Y, Chen J-S, Doki Y, et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). J Clin Oncol 2019;37:387. [Google Scholar]
- 70).Loeuillard E, Conboy CB, Gores GJ, Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Rep 2019;1:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]


