Abstract
Metabolic liver diseases (MLD) are the second most common indication for liver transplantation (LT) in children. This is based on the fact that the majority of enzymes involved in various metabolic pathways are present within the liver and LT can cure or at least control the disease manifestation. LT is also performed in metabolic disorders for end-stage liver disease, its sequelae including hepatocellular cancer. It is also performed for preventing metabolic crisis’, arresting progression of neurological dysfunction with a potential to reverse symptoms in some cases and for preventing damage to end organs like kidneys as in the case of primary hyperoxalosis and methyl malonic acidemia. Pathological findings in explant liver with patients with metabolic disease include unremarkable liver to steatosis, cholestasis, inflammation, variable amount of fibrosis, and cirrhosis. The outcome of LT in metabolic disorders is excellent except for patients with mitochondrial disorders where significant extrahepatic involvement leads to poor outcomes and hence considered a contraindication for LT. A major advantage of LT is that in the post-operative period most patients can discontinue the special formula which they were having prior to the transplant and this increases their well-being and improves growth parameters. Auxiliary partial orthotopic LT has been described for patients with noncirrhotic MLD where a segmental graft is implanted in an orthotopic position after partial resection of the native liver. The retained native liver can be the potential target for future gene therapy when it becomes a clinical reality.
Keywords: Liver transplantation, Metabolic liver disease, Tyrosinemia, Wilson disease, Glycogen storage diseases, Urea cycle disorders, Pathology, Auxiliary liver transplant
Core Tip: Metabolic disorders are important cause of morbidity and mortality in children. Their clinical presentations are varied and include end-stage liver disease, hepatocellular cancer, renal tubular acidosis, seizures, encephalopathy, myopathy etc. Liver transplantation (LT) is a curative option in many metabolic disorders. LT is contraindicated in mitochondrial disorders with significant extrahepatic involvement. A combined liver kidney transplant is needed in disorders where the underlying defects significantly damages both the organs. The outcome of LT is excellent in metabolic disorders. Auxiliary partial orthotopic LT is an attractive option as it provides the defective enzymes keeping the native liver intact and may hold an option of withdrawing immunosuppression in case gene therapy can be offered in future.
INTRODUCTION
Metabolic disorders are an important indication for pediatric liver transplantation (LT)[1]. The advent of advanced next-generation sequencing techniques has led to more metabolic disorders being diagnosed, and hence their clinical profile is widening[2]. The balance between various biochemical reactions comprises a definite pathway for synthesis and catabolism of various metabolites, which is maintained in a very sophisticated way in the human body, and the liver is where a majority of these reactions are carried out. An error or defect of a single step in such a pathway could induce catastrophic results in the forms of inborn errors of metabolism, which in turn affect multiple organ systems and present with protean clinical manifestations. Liver involvement in metabolic disorders can vary ranging from normal architecture to steatosis, advanced fibrosis and cirrhosis with or without hepatocellular cancer[3]. Pediatric LT either offers a cure or offers symptom control in liver-based metabolic disorders[4]. Often, an auxiliary LT with a smaller liver graft may be sufficient to normalize the defective phenotype[5]. Pediatric metabolic diseases can be broadly divided into three groups (Table 1). The status of LT in the algorithmic management of each of these four groups is different. This review aims to provide insights into paediatric metabolic disease and highlight the evolving role of LT in its management. We also review pathological findings in paediatric metabolic liver diseases (MLD) undergoing transplants.
Table 1.
Metabolic disorders can be classified into the following three broad groups based on a liver transplantation perspective
|
Category
|
Description
|
Examples
|
| A | Disorders with enzyme defect only in the liver and LT is done predominantly for ESLD and its related complications | Tyrosinemia (HT-1); A1AT deficiency; Galactosemia (type 1 Galactosemia); WD; Hereditary fructosemia; GSD 3 and 4; CDG; LSD |
| B | Disorders with enzyme defects limited to the liver. These patients rarely have ESLD; LT is performed for extrahepatic organ involvement | UCD; Porphyrias; GSD type 1: PH, FH; Crigler-Najjar syndrome |
| C | Disorders having enzyme defect in the liver and extrahepatic tissue and LT only partially corrects underlying metabolic disease and alleviates symptoms of extrahepatic organ involvement | MMA; PA; MSUD; MH |
ESLD: End-stage liver disease; HT-1: Hereditary tyrosinemia type 1; A1AT: Alpha-1 antitrypsin; WD: Wilson disease; GSD: Glycogen storage disorder; CDG: Congenital disorder of glycosylation; LSD: Lysosomal storage disorders; UCD: Urea cycle disorders; PH: Primary hyperoxaluria; FH: Familial hypercholesterolemia; LT: Liver transplantation; MMA: Methyl malonic acidemia; PA: Propionic acidemia; MSUD: Maple syrup urine Disease; MH: Mitochondrial hepatopathies.
CATEGORIES OF METABOLIC DISORDERS
Metabolic disorders can be classified into the following three broad groups based on a LT perspective (Table 1).
GROUP A
Disorders with enzyme defects only in the liver, and LT is done predominantly for end-stage liver disease (ESLD) and its related complications.
Tyrosinemia
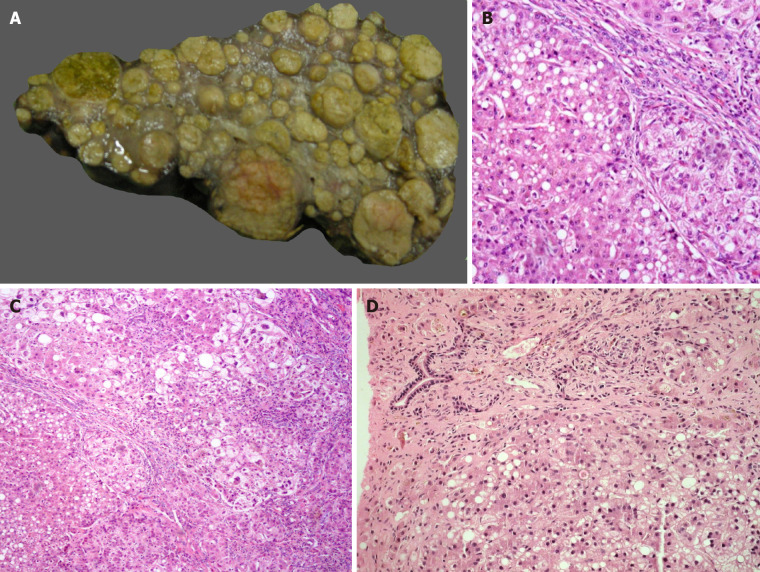
Hereditary tyrosinemia type 1 (HT-1) is an autosomal recessive (AR) disorder caused by a deficiency of fumaryl acetoacetate hydrolase enzyme with a prevalence of about 1 in 100000 new-borns in the general population[6]. The metabolic block induces accumulation of highly reactive intermediate metabolites such as malyl-and fumarylacetoacetate, which are toxic and mutagenic to the hepatocytes. The clinical presentation is heterogenous and includes acute liver failure (ALF), cirrhosis with or without decompensation, hepatocellular carcinoma (HCC), neurologic crisis, and renal tubular acidosis leading to florid rickets and growth failure[6]. Nitro tetrazolium blue choride (NTBC) is useful for metabolic control in HT-1, but this agent does not fully abate the incidence of HCC[7]. A strict dietary control with phenylalanine and tyrosine-free formula needs to be followed along with NTBC, creating a huge financial burden for patients, especially those from the developing countries. LT is indicated in HCC, decompensated cirrhosis, patients non-compliant with medical therapy, and ALF. Explanted livers with tyrosinemia are usually enlarged. The most distinctive macroscopic feature in tyrosinemia is the striking nodularity of the liver and the variegated colors of the nodules ranging from the yellowish lipid-filled nodules to the deep green of cholestatic nodules (Figure 1A)[8]. Light microscopic findings include portal/periportal fibrosis with bridging and nodularity of varying sizes, steatosis, hepatocellular ballooning, bilirubinostasis, and pseudoacini formation (Figure 1B and C)[9]. Liver cell dysplasia, both low and high grade, is frequently observed, and the distinction between dysplastic nodules and HCC may be difficult[9]. Large cell change is also reported. HCC is usually well or moderately differentiated in tyrosinemia[10]. The long-term outcome for LT recipients with HT-1 is excellent, and ranges from 85%-100% across series[11]. There is however, a minimal risk of recurrence of HCC in the transplanted liver, and a serial surveillance is indicated. The urine succinyl acetone may continue to be detected in the post-LT period, but the long-term significance of this causing a renal impairment remains uncertain[6]. Hence a renal-sparing immunosuppression is used in the LT recipients with tyrosinemia.
Figure 1.
Metabolic liver disease. A: Explant liver specimen of a case of tyrosinemia with nodules of varying sizes; B: Tyrosinemia liver with steatosis [hematoxylin and eosin (HE staining)]; C: Tyrosinemia liver with cirrhosis, hepatocellular ballooning, and fatty change (HE staining); D: Galactosemia with bridging fibrosis, bile ductular reaction, fatty change and rosetting (HE staining).
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin (A1AT) deficiency is a common inherited metabolic liver disease in the western hemisphere with an estimated incidence of 1:1600 to 1:3500 cases[12]. SERPINA1, the gene encoding A1AT, has an AR inheritance with codominant expression[13]. The liver injury is caused by aggregates of misfolded protein in the hepatocyte whereas the lung injury is caused by unopposed action of neutrophil elastase[13]. Hence the progression of lung pathology can be prevented with enzyme replacement, but the liver dysfunction does not have a standard medical therapy[14]. Serum levels of A1AT can help in diagnosis of A1AT deficiency, as they are very low in those who are homozygous. The commonest presentation is as infantile cholestasis, and it can mimic biliary atresia both clinically and radiologically. Liver biopsy shows eosinophilic intracytoplasmic globules in hepatocytes, which are periodic acid-schiff (PAS) positive and diastase resistant, and genetic analysis confirms the diagnosis[13]. LT is indicated for ESLD and also for those with HCC[13,15]. The A1AT levels become normal in the post-LT period but the lung functions may continue to deteriorate, especially after the 2nd decade of life. A higher incidence of hepatic artery thrombosis is noted as the blood vessel integrity is defective due to deficiency of A1AT, and the vessel wall can be disrupted during clamping[16]. The post-LT outcomes are excellent with over 90% of recipients living beyond 20 years[17]. Liver impairment resulting from A1AT deficiency may directly contribute to renal abnormalities resembling IgAN.
Galactosemia (type 1 galactosemia)
It is a common metabolic disorder caused by the defect in the enzyme galactose 1-phosphate uridyl transferase (GALT). The incidence is 1 in 45000 births[10]. The metabolites galactitol and galactose-1-phosphate are hepatotoxic and infants present with ALF; nevertheless, there is always an underlying liver scarring[18]. The diagnosis was classically suggested by the detection of urinary reducing sugars (nonglucose), which have no sensitivity or specificity, and may be positive with any cause of liver failure. GALT activity can be measured in erythrocytes, however the test must be done before the child receives blood transfusion[10]. Genetic assay is confirmatory. Missense mutations in GALT gene are associated with low to undetectable enzymatic activity, resulting in the most profound symptoms. Liver biopsy in galactosemia demonstrates panlobular steatosis, bilirubinostasis, bile ductular proliferation, portal/periportal and sinusoidal fibrosis, and hepatocyte necrosis and apoptosis (Figure 1D)[19]. An early recognition of this condition with a prompt withdrawal of lactose/galactose exposure dramatically improves the clinical status and the liver function. Hence despite being a common metabolic disorder, LT is rarely indicated, and is performed only in progressive liver dysfunction despite a galactose-free diet and in HCC[20].
Wilson disease
Wilson disease (WD) is an AR disorder causing ALF, acute-on-chronic liver failure, or cirrhosis in children with the average prevalence of 1 in 30000 individuals worldwide[10]. The genetic defect in ATP7B leads to defective copper excretion from liver leading to its toxic accumulation in liver and various extrahepatic tissues such as brain, kidney and joints[21]. It is one of the few metabolic disorders causing liver disease where a definitive medical therapy in the form of chelation can be offered. It is well-known that a fraction of patients with even advanced liver disease due to WD can reverse hepatic fibrosis with medical treatment[20]. LT should be offered in WD presenting with ALF when the Dhawan score is > or = 11 as this predicts a mortality of more than 97%[22] (Table 2).
Table 2.
The New Wilson (Dhawan) score for prediction of outcome in Wilson disease
|
Score
|
Bilirubin mg/dL
|
INR
|
AST (IU/L)
|
WBC (109/L)
|
Albumin (g/dL)
|
| 0 | < 5.85 | < 1.29 | < 100 | < 6.7 | 4.5 |
| 1 | 5.86-8.77 | 1.3-1.6 | 101-150 | 6.8-8.3 | 3.4-4.4 |
| 2 | 8.78-11.69 | 1.7-1.9 | 151-200 | 8.4-10.3 | 2.5-3.3 |
| 3 | 11.7-17.54 | 2.0-2.4 | 201-300 | 10.4-15.3 | 2.1-2.4 |
| 4 | > 17.55 | > 2.5 | > 300 | > 15.3 | < 2.1 |
A score more than or equal to 11 indicates a high mortality without liver transplantation. These patients benefit most from an urgent liver transplantation. INR: International normalized ratio; WBC: White blood cell.
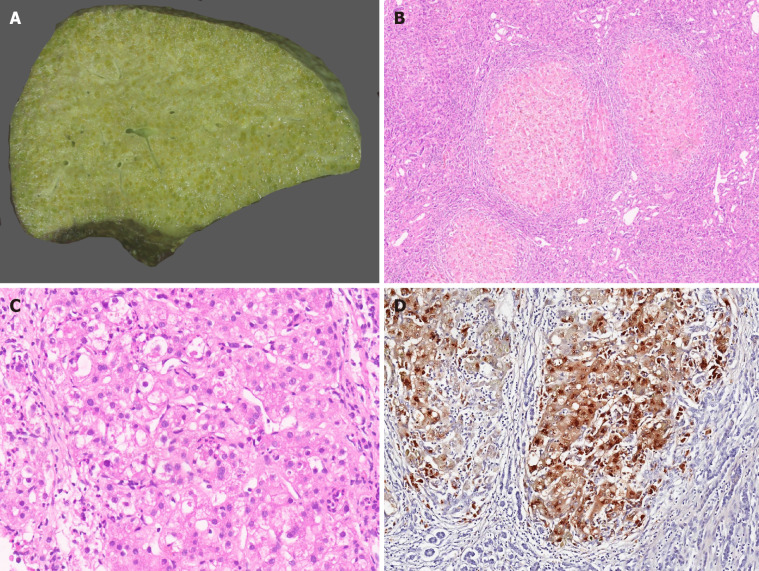
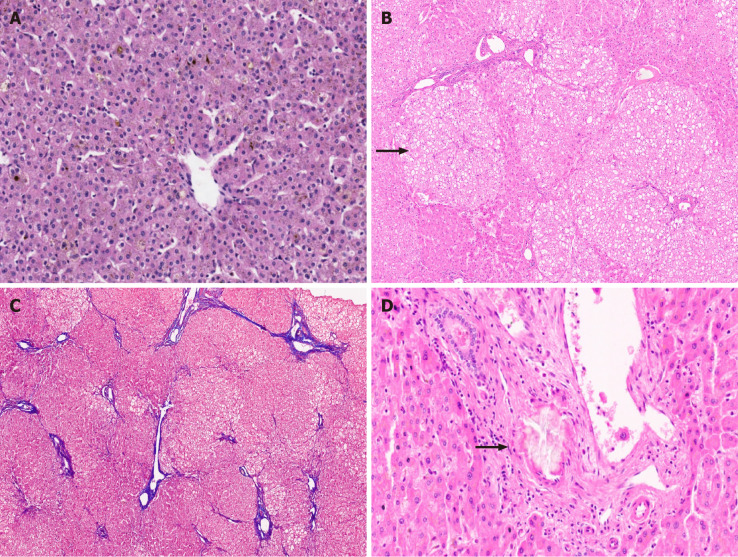
Patients with decompensated cirrhosis having hepatic encephalopathy, those with episodes of massive variceal bleed and synthetic failure not responding to chelation therapy or those worsening on chelation therapy should also be offered LT. In the LDLT settings, an asymptomatic heterozygous family donor can safely donate their liver. In the post-LT period, chelation therapy or dietary restrictions need not be continued. Post-LT survival for WD is excellent with 5-year survival of up to 90%[23]. Interestingly, WD patients transplanted for ALF have a similar outcome to those transplanted for an ESLD[24]. Though a controversial indication, patients with neurological symptoms in the absence of overt liver disease may be given an option for LT if there is no response to chelators. Changes in the brain were reported to reverse in the post-LT period[25]. Therapeutic plasmapheresis (TPE) may be beneficial in WD as it rapidly removes copper from circulation in significant amounts which further reduces hemolysis and progression of renal failure[26-28]. TPE also removes large molecular weight toxins and other factors that may cause hepatic encephalopathy and is often used as a bridge to LT[29]. TPE is recommended as Class 1C and category I indication in fulminant liver failure due to WD[30]. A few recent reports showed that TPE combined with chelating agents improved ALF and eliminated the need for LT[31]. Explanted livers in WD are cirrhotic. Micronodular cirrhosis is most commonly seen (Figure 2A and B). A few explants show mixed nodular cirrhosis. The spectrum of histopathological features in WD is very broad and includes macrovesicular steatosis, portal and/or lobular inflammation, glycogenated nuclei, variable necrosis and apoptosis, cholestasis, and hepatocellular ballooning with Mallory-Denk bodies (Figure 2C). Neutrophilic satellitosis may also be visualized[10]. Other hepatocellular features include slight or moderate hepatocellular anisocytosis and anisokaryosis. Copper and copper associated protein deposits are identified in hepatocytes (Figure 2D). Copper accumulation can be very heterogeneous in cirrhotic liver with some nodules showing diffuse stainable copper while others show little to no stainable copper. Macrophages can also demonstrate copper granules in extensive hepatocellular necrosis.
Figure 2.
Wilson disease. A: Explant hepatectomy specimen in a case of Wilson disease (WD) with marked cholestasis; B: Micronodular cirrhosis in a case of WD [hematoxylin and eosin (HE staining)]; C: WD with hepatocellular ballooning, Mallory Denk bodies, fatty change and neutrophilic satellitosis (HE staining); and D: WD with marked copper deposition in hepatocytes and macrophages (Rhodanine staining).
Hereditary fructosemia
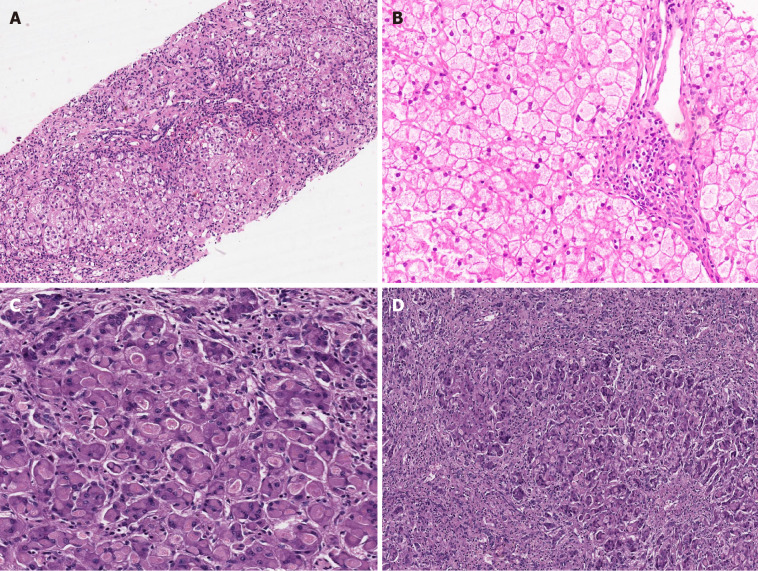
It is an AR disorder caused by deficiency of aldolase B enzyme. Patients usually become symptomatic at the time of weaning with hypoglycemia, lactic acidosis, and ketosis. Hereditary fructosemia is estimated to occur in 1 in 20000 live births[32]. It is one of the causes of infantile ALF and can also lead to cirrhosis in early childhood[32]. Prompt recognition and withholding of fructose in diet will reverse the symptoms and liver disease. Many patients develop an inherent aversion to sweet/sugary food as a secondary adaptation to prevent the accumulation of toxic metabolites. LT is offered for synthetic failure, portal hypertension, malignancies, and growth retardation. Biopsy findings include neonatal giant cell hepatitis, macrovesicular steatosis, portal/periportal fibrosis, cirrhosis, ductular proliferation, acinar transformation of hepatocytes, bilirubinostasis, and sinusoidal collagenisation (Figure 3A)[33].
Figure 3.
Metabolic liver disease. A: Hereditary Fructosemia with bridging fibrosis [hematoxylin and eosin (HE staining)]; B: Glycogen storage disorder (GSD) type III displaying pale enlarged hepatocytes with thickened borders and portal fibrosis (HE); C: GSD type IV liver eosinophilic ground glass cytoplasmic inclusions (HE); D: Explant liver in case of GSD type IV with extensive fibrosis (HE).
Glycogen storage disorder III and IV
Among the glycogen storage disorder types (GSDs), type I, III, IV-, VI and IX are the hepatic glycogenoses. Apart from type 1 GSD, others may lead to progressive liver disease, cirrhosis, and decompensation. Type III GSD involves the striated muscles in 85% of cases, and cardiac morbidity needs ruling out before an LT[34,35]. The liver disease in type III GSD is less severe when compared to type 4 in which cirrhosis with decompensation is universal before 5 years of age[35]. Muscle involvement is also seen in GSD IV and infantile presentations such as spinal muscular atrophy are known. Synthetic failure or portal hypertension, which are the primary indications for LT in GSD III and IV, do not occur in GSD type I. On gross inspection, the liver in GSD is typically enlarged, smooth, and pale in appearance. The liver in most GSDs including type III demonstrates pale enlarged hepatocytes with thickened borders containing abundant glycogen, which is digested with diastase (Figure 3B)[10]. Fibrosis with thin connecting septa can also be noted, as also is steatosis. Hepatocellular adenomas, frank cirrhosis, and even HCC, can develop in GSD type III. Liver in GSD type IV shows large, round or oval-shaped eosinophilic ground glass cytoplasmic inclusions, often surrounded by an artefactual halo in the majority of hepatocytes, which are especially prominent in the periportal zone (Figure 3C)[36]. The inclusions are deeply stained with PAS stain. Further diastase treatment clears the normal glycogen but not the abnormal amylopectin-like material in the inclusions[10]. Fibrosis, which can progress to cirrhosis, is a frequent finding in GSD type IV (Figure 3D).
Congenital disorder of glycosylation
Congenital disorder of glycosylation (CDG) has an incidence of 1 in 10000 in European countries and is known to have multisystemic manifestations including coagulation defects, protein-losing enteropathy, neurological symptoms, cardiac dysfunction, immune deficiency, etc., depending on the various subtypes described[37]. The CDGs causing ESLD are mannose phosphate isomerase deficiency (MPI-CDG), coiled-coil domain-containing 115 (CCDC115-CDG), transmembrane protein 199 andATP6AP1-linked immunodeficiency[38,39]. In spite of oral mannose therapy, MPI-CDG can progress to cirrhosis. LT is indicated for ESLD in CDG and can improve its extrahepatic manifestations such as exercise intolerance and protein-losing enteropathy[38,39]. LT has also been performed for CCDC115-CDG and ATP6AP1-CDG but not in other types of CDG where liver dysfunction is rarely seen, but could theoretically improve other systemic manifestations. Pathological findings are non-specific in CDG. Variable fibrosis and fatty change have been described[40].
Lysosomal storage disorders
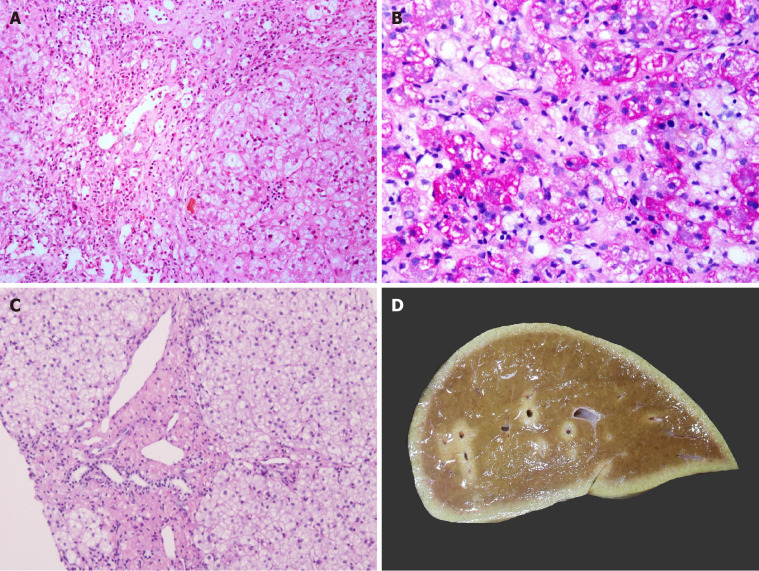
Characterised by multisystemic involvement, lysosomal storage disorders (LSD) are also known to present with cirrhosis, portal hypertension (also contributed by massive splenomegaly), and HCC[41-44]. LSD where LT may be offered include non-neuronopathic Gaucher’s disease, Niemann-Pick disease types B, C and lysosomal acid lipase deficiency with variable outcomes[45-48]. Patients with LSD need to continue enzyme replacement therapy in the post-LT period to control their extrahepatic symptoms and prevent disease recurrence in the graft. Gaucher’s is the most common genetic LSD with estimated incidence around 1 in 50000 to 100000 live births. The diagnostic histological features in Gaucher’s disease are produced by sphingolipid-engorged Kupffer cells and macrophages in sinusoids and portal areas. These cells are large with an eccentric nucleus and eosinophilic, corrugated (“crinkled-paper”) cytoplasm[33]. These cells may completely block the sinusoidal spaces disrupting and atrophying the hepatocyte plates. Eventually, bridging, scarring, and cirrhosis may develop. The liver in Niemann-Pick disease types A and B is estimated to affect 1 in 250000 individuals. Niemann-Pick disease accumulates large macrophages with foamy cytoplasm within hepatic sinusoids with fibrosis (Figure 4A and B)[10]. One may also observe vacuoles in hepatocytes. Cholesteryl ester storage disease shows scarring, fine vacuolation of hepatocytes, and foamy portal macrophages and Kupffer cells with tan-colored cytoplasm (Figure 4C)[49].
Figure 4.
Metabolic liver disease. A: Niemann Pick disease with marked fibrosis [hematoxylin and eosin (HE staining)]; B: Pale-staining storage cell clusters as compared to deeply stained glycogen containing hepatocytes [Periodic acid Schiff (PAS) staining]; C: Cholesteryl ester storage disease liver with granular microphages in portal tract and finely vacuolated hepatocytes (HE staining); and D: Largely unremarkable explant liver in a patient with urea cycle defect.
Other disorders
Transaldolase deficiency, Zellweger spectrum disorders, which are peroxisomal defects, and pyruvate kinase deficiency are the other disorders where LT has been offered for ESLD[50-52]. The overall incidence of Zellweger spectrum disorders is estimated to be around 1/50000. Morphological features in liver in Zellweger spectrum disorders are mostly nonspecific and include disarray of hepatic plates with enlarged hepatocytes, cholestasis, hypoplasia of interlobular bile ducts, portal inflammation, hemosiderosis, fibrosis, and cirrhosis.
GROUP B
This group includes disorders with enzyme defects limited to the liver. These patients rarely have ESLD; LT is instead performed for extrahepatic organ involvement.
Urea cycle disorders
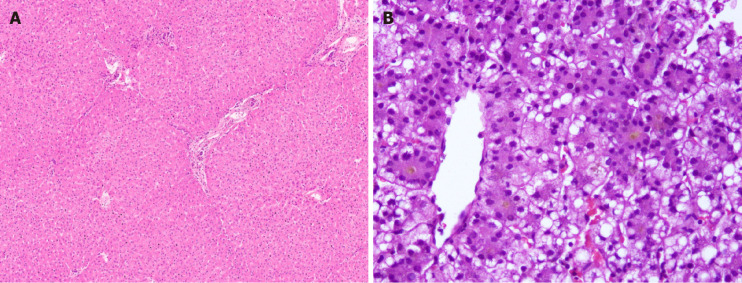
The urea cycle operates in the liver to detoxify the nitrogenous wastes in the body. Urea cycle disorders (UCDs) are inherited in AR pattern [except ornithine transcarbamylase deficiency (OTC), which is X-linked recessive]. LT is offered in severe metabolic defects, elevated ammonia more than 300 umol/L and refractoriness to medical therapy[53]. LT has a definite role in arresting progression of neurological damage by preventing hyperammonemia and occurrence of metabolic crisis[54]. The incidence of UCD is 1 in 30000 live births[54]. These children do not require to continue their low-protein special formula in the post-LT period. Children when transplanted in time can have normal neuro development, and those having brain involvement may potentially have their injury reversed to achieve milestones[54]. Rarely UCDs can have hepatic involvement in form of ALF (OTC deficiency), cirrhosis (arginosuccinic aciduria and hyperammonemia-homocitrullinemia syndrome), or HCC (citrin deficiency)[55]. When present, an acute metabolic crisis should be treated before LT. Ammonia-scavenging agents should not be stopped as a sudden withdrawal can precipitate a metabolic crisis[56]. Hemodialysis may need to be offered when the serum ammonia is > 300 umol/dL in the pre-LT period. Ideally, the serum ammonia should be maintained < 80 umol/L immediately prior to LT[56]. Studies based on the United Network for Organ Sharing database show a post-LT survival for UCD of 89% at 5 years and 87% at 10 years[57]. The reported morphological findings vary from normal histology to variable steatosis, cholestasis, variable portal/periportal fibrosis, early cirrhotic transformation, glycogen accumulation with enlarged pale hepatocytes, and ultrastructural changes with no specific findings for a particular UCD disorder[58]. We have observed largely unremarkable liver explants with no significant morphological features, patchy steatosis, and/or bilirubinostasis in USD (Figures 4D and 5A). One case of arginase-1 deficiency showed glycogen accumulation with clusters of pale enlarged hepatocytes, mild portal inflammation, and focal bridging fibrosis (Figure 5B and C).
Figure 5.
Metabolic liver disease. A: Canalicular bilirubinostasis in case with urea cycle defect [hematoxylin and eosin (HE staining)]; B: Explanted liver in Arginase-1 (ARG-1) deficiency displaying nodules of pale enlarged hepatocytes (arrow, HE staining, B); C: Focal bridging fibrosis in a case of ARG-1 deficiency [Masson trichrome staining]; D: Portal vessel with oxalate crystals in primary hyperoxaluria type 1 (arrow, HE staining).
Porphyrias
Porphyrias are a group of disorders associated with metabolism of heme with a prevalence of 1 in 20000. The major clinical manifestations are either neurovisceral or cutaneous photosensitivity. Among the seven known types, all are curable by LT except congenital erythropoietic porphyria[59]. Among the hepatic porphyrias, only erythropoietic protoporphyrias are known to be associated with ESLD[59]. There is a higher incidence of HCC in these patients. Parenteral heme therapy is known to abort neurological crisis but does not help in reversing neuronal injury. Timely LT prevents the onset and/or progression of neuronal injury[59,60] and also helps to abate the autonomic dysfunction, which otherwise may lead to sudden death. A few anesthetic agents such as tramadol, ketamine, and thiopentone and a few other drugs such as azole antifungals, cotrimoxazole, and carbapenems should be avoided in these patients. The porphyrin levels need to be maintained by plasmapheresis or by heme infusion. The outcome following LT is excellent but with a differential favorable prognosis in those with mild-to-moderate neurological symptoms than those with severe symptoms prior to LT[61]. Liver shows black discoloration macroscopically in erythropoietic protoporphyria[10]. Microscopic examination in erythropoietic protoporphyria shows focal accumulation of a dense, dark-brown pigment in canaliculi, interlobular bile ducts, connective tissue, and Kupffer cells.
GSD type I: Type I GSD is an AR disorder caused by deficiency of glucose-6-phosphatase enzyme in 1 in 100000 live births, and there are two subtypes, 1a and 1b, with the latter having neutropenia or neutrophil dysfunction[62]. The liver function remains normal in these patients, but they are prone to develop hepatic adenomas and HCC (on a non-cirrhotic background). Poor metabolic control with recurrent hypoglycemia can irreversibly injure the brain. They also are prone to chronic kidney disease, hypertriglyceridemia, and osteoporosis. LT is indicated in those with poor metabolic control despite a complex carbohydrate diet (e.g., uncooked corn starch), those developing multiple and growing hepatic adenomas or HCC and growth retardation[63]. A simultaneous liver-kidney transplant needs to be offered in co-existing end-stage kidney disease with or without hepatic neoplasms. Apart from achieving metabolic control, LT in GSD Ib improves the neutropenia and/or neutrophil dysfunction in 50%-66% cases[64]. In those, undergoing an isolated LT, renal-sparing immunosuppression needs to be administered.
Primary hyperoxaluria
Type 1 primary hyperoxaluria (PH) is an AR disorder with a prevalence of 1-3 per 100000 people, characterized by systemic deposition of oxalate crystals. The enzyme alanine glyoxylate aminotransferase is primarily expressed in liver, and its deficiency leads to systemic oxalosis and end-stage renal disease, cardiomyopathy, and fractures, and bone marrow suppression ensues[65]. Patients develop systemic hypertension due to the renal pathology and a relative kidney disease along with systemic arterial involvement causing its non-compliance. LT along with kidney transplant is the only curative option available. Patients having a GFR more than 50 mL/m2/min may undergo an isolated LT. A simultaneous liver-kidney transplant is offered upfront in deceased donor liver transplant, as the transplanted organs have immune privilege with reduced chance of rejections. Also, it is done in living donor-related donor with organ harvested from two different individuals when the GFR > 15 mL/m2/min, children having large abdominal cavity, and GFR < 15 mL/m2/min with no features of systemic oxalosis. A sequential liver-kidney transplant is done predominantly in a living donor-related transplant when there is GFR < 15 mL/m2/min with severe systemic hyperoxalosis, a single liver donor (sequential harvesting after 6 wk), and infants (due to a relatively small abdominal cavity), which can accept only a graft that can be reduced in size, i.e., liver but not kidneys, which is done at a later age[66]. Heterozygous donors need to be screened for high urine oxalate even if their renal function is normal. They can be liver donors but not kidney donors as there is a risk that the contralateral kidney may develop renal failure on a long-term basis. A cardiac evaluation is essential prior to transplant surgery to ensure that the ejection fraction is more than 40%-45%. In low ejection fraction, it is also recommended to do aggressive hemodialysis 4-6 wk prior to LT to decrease the systemic oxalate load, which may improve the cardiac contractility[56]. An increased risk of bleeding episodes in the post-LT period can be attributed to the platelet dysfunction and renal pathology needing support. Liver explants in PH may show oxalate crystals in vessels (Figure 5D). The role of LT in PH type 2 remains unclear, and isolated renal transplant may be an option[67]. A novel Food and Drug Administration-approved small interfering ribonucleic acid called lumasiran decreases hepatic oxalate production by inhibiting the enzyme glycolate oxidase and hence has been found to reduce systemic oxalate load, thereby, decreasing renal excretion of oxalates.
Familial hypercholesterolemia
Familial hypercholesterolemia (FH) is an autosomal dominant disorder characterized by defective low-density lipoprotein cholesterol receptors in the liver. The incidence of homozygous state is 1 in 1 million live births[10]. This persistently elevates serum cholesterol leading to complications such as early atherosclerosis in childhood, which can lead to myocardial infarction[68]. It is, however, more common in a homozygous or a compound heterozygous mutation. LT is curative and is recommended before the development of atherosclerosis in the pediatric age group. In the post-LT period, the cholesterol levels fall to normal within a week’s time. LT by improving the dyslipidemia is known to prevent the progression to coronary artery disease[69,70]. Pathological findings in liver include accumulation of lipid in hepatocytes and Kupffer cells.
Crigler-Najjar syndrome
Crigler-Najjar syndrome (CNS) is an AR disorder caused by a defect in the UGT1A1 gene, the product of which (uridine diphosphate glucuronyl transferase) is responsible for the conjugation of bilirubin before its excretion. With an estimated incidence of one per 750000-1000000 live births, CNS is considered an “ultra-rare orphan disease”[71]. Type 1 is characterized by a complete absence of the enzyme whereas type 2 has 10% of the functioning enzyme[71]. In type 1 CNS, the exceptionally high unconjugated bilirubin leads to irreversible brain injury in the basal ganglia, hippocampus, subthalamic nuclei and cranial nerves (kernicterus)[71]. Type 2 CNS usually responds to phenobarbitone therapy, however it is not effective in type 1 where phototherapy may help lower bilirubin levels. But as age progresses, phototherapy becomes less effective due to a larger body surface area and thickening of the skin, and bilirubin can rise dangerously during intercurrent illness[72]. Plasmapheresis has also been reported to be a useful tool in CNS when a rapid decrease in bilirubin serum concentration is required[73]. Plasmapheresis has been recommended as a useful treatment for extreme acute unconjugated hyperbilirubinemia in children with CNS when phototherapy is transiently impaired for any adverse complication or during nonavailability of heme oxygenase inhibitors[74]. Moreover, due to the need for prolonged phototherapy sessions, unlike his age-matched peers, the child’s quality of life (QOL) is hampered; LT remains the only curative option in such a scenario. LT prevents further neurological injury and, in some cases, may reverse the changes[75,76]. Long-term outcomes are excellent and comparable between auxiliary and orthotopic LT[77]. Even though the blood-brain barrier is impermeable by 2 years of age, an unconjugated bilirubin more than 17 mg/dL can continue to cause neurological damage. Hence, a poorly controlled type 2 CNS may also become an indication for LT. Physiotherapy and tendon release procedures may help to reduce the neurological deficit in the post-LT period. Liver explants in CNS show no specific finding or mild bilirubinostasis (Figure 6A).
Figure 6.
Metabolic liver disease. A: Crigler Najjar explant liver with no significant morphological findings [Hematoxylin and eosin (HE) staining]; B: Mitochondrial hepatopathy with mixed steatosis and bilirubinostasis HE staining).
GROUP C
Disorders having enzyme defect in the liver and extrahepatic tissue with LT done either for extrahepatic organ involvement and/or for ESLD.
Organic acidemias
Methyl malonic acidemia: Methyl malonic acidemia (MMA) is an AR disorder caused by deficiency of adenosyl cobalamine or methyl malonyl-coA mutase. It occurs at a rate of approximately 1 in 50000 live births[78]. In severe cases, patients can present with recurrent metabolic crises in early infancy, which leads to progressive encephalopathy, coma, and death. It can also lead to metabolic cerebrovascular accidents and around 60% can have renal involvement evolving into chronic kidney disease[78,79]. Other complications are optic atrophy and pancreatitis. LT provides the defective enzyme and helps avoid the life-threatening metabolic crises and improves the QOL[79]. The levels of methyl malonic acid come down dramatically after LT but may remain higher than normal[79,80]. Hence progressive renal dysfunction, cognitive deficits, metabolic strokes, or optic atrophy be not be completely prevented after a successful LT[81,82]. Isolated reports of normalized cognition are however available in literature. A combined liver kidney transplant is offered when an end-stage kidney dysfunction has already set in at diagnosis[82,83]. Isolated kidney transplant may provide 18% of the mutase enzyme, but it would improve only the elevated metabolites secondary to renal failure rather than achieving control to prevent metabolic crises[81].
Propionic academia
Propionic acidemia (PA) is an AR disorder caused by the deficiency of propionyl-CoA carboxylase, and the resulting toxic metabolites damage primarily the central nervous system and myocytes[78]. The prevalence is 1 in 100000 of the general population[78]. Medical therapy includes oral antibiotics to control propionic acid production by the gut bacteria and special nutritional formulae. However, patients may still present with metabolic decompensation and growth retardation. LT provides a good control of the metabolism, decreases the toxic metabolic burden and improves the QOL[84]. Despite metabolic control, because of the deficient enzyme in brain and in cardiac muscle, the injury in these organs is usually irreversible, and annual monitoring with clinical examinations and investigations is warranted[84,85]. Improvement in development and cognition has been reported in the post-LT period[86]. Isolated reports reveal reversal of cardiomyopathy after LT. The peri and post-operative lactate levels have been found to indicate good metabolic control followed by serum ammonia in both MMA and PA[87]. The patient should be started on low protein at 0.5 g/kg/d and then gradually built up to 2.5 to 3 g/kg/d by the end of first week so that metabolic adaption happens over this period of time[86]. These patients have a relatively higher incidence of hepatic artery thrombosis when compared to other indications of LT, but a recent analysis has challenged that association[88]. In a recent series, 5-year patient survival for PA liver transplant recipients was 89.5%[89].
Maple syrup urine disease
Maple syrup urine disease (MSUD) is a group AR disorder characterized by defective metabolism of branched chain amino acids including leucine, isoleucine, and valine. It affects 1 in 185000 infants worldwide[90]. The commonest and most severe is the classical MSUD (type 1) caused by defective branched chain keto acid dehydrogenase (BCKAD)[90]. Lethargy, poor feeding, and intermittent apnea along with maple syrup odor of urine or cerumen is the earliest clue in a newborn[90]. Recurrent seizures and cognitive dysfunction start early and leads to early mortality. The E3 type (type 5) is a defect in dihydroprolamine reductase, which results in mitochondrial dysfunction and is the only type presenting with liver dysfunction along with other neurological manifestations. Acute encephalopathy is caused by elevated levels of leucine and isocaproic acid. Liver is the site of 9%-13% of BCKAD enzyme, and LT is found to prevent further recurrence of metabolic crisis and arrest the neurological progression. However, reversal of neurological deficit is not seen except in a few[91]. In a large series involving 37 patients on long-term follow up, the patient survival was found to be 100%[92]. The levels of toxic amino acids normalize within hours of the graft implantation but usually do not return to a physiologically normal value[93]. Hence LT prevents further occurrence of life-threatening metabolic crisis in MSUD[92]. Special formula does not need to be continued in the post-LT period.
Primary mitochondrial hepatopathies
Mitochondrial disorders can happen due to mutations in either nuclear or mitochondrial deoxyribonucleic acid. Even though liver is involved in mitochondrial disorders, it is usually as a part of the multisystemic involvement[94]. It affects 1 in 20000 children under 16 years of age[94]. The spectrum of hepatic presentations includes isolated fatty liver, ALF, chronic cholestasis and cirrhosis[95]. Morphologically, microvesicular and macrovesicular steatosis, cytoplasmic and canalicular bilirubinostasis (Figure 6B), and giant cell transformation are identified. In addition, portal fibrosis and cirrhosis are also reported. The disorders can be grossly classified into disorders of single respiratory chain defects (e.g., BCSIL and SCO1) and multiple complex defects (thymidine kinase 2/TK2, deoxy guanosine kinase/DGUOK, and polymerase gamma/POLG)[95,96]. The common systems involved are brain, cardiac and skeletal muscles, hematological, renal tubules, intestines, and inner ear as these have maximum energy turnover. LT in mitochondrial disorders has always been an area of controversy and initially was listed as contraindication for the same, but current recommendations for LT are for a primary hepatic involvement without apparent extrahepatic organ involvement, and the same policy is followed at the authors’ institute[97,98]. A baseline screen includes a magnetic resonance imaging brain with magnetic resonance spectroscopy, BERA, 2D echocardiography, urine evaluation for tubulopathy, and bone marrow examination (in significant anemia or pancytopenia). The parents should be made aware of the fact that LT will correct only the hepatic manifestations, and the child may have onset of other system involvement as age progresses. These may include pulmonary hypertension, progressive neurological dysfunction, and cardiomyopathy, which are the common causes of death. This is of particular importance in DGUOK, TK2, and POLG defects where the ESLD may overshadow the underlying neurological and other system involvement[96]. Intra- and post-operative use of propofol or Ringer lactate should be preferentially avoided, and sevoflurane is the preferred agent[99]. Rare reports of successful LT for this condition have been reported in literature[100].
Auxiliary LT
Auxiliary LT is a technique by which a healthy grafted liver is implanted on the native liver without fully removing it. When the implantation is done after removing a part of the native liver, it is called auxiliary partial orthotopic LT (APOLT) and when done without manipulating the native liver being in a non-anatomical location is called heterotopic auxiliary LT (HALT)[101]. The HALT technique was initially described in a 15-year-old girl with ALF caused by WD, as it was noted that manipulation of native liver raised intracranial pressure and hence recipient hepatectomy was not done. Even with the aforementioned advantage, certain significant drawbacks with this technique include graft congestion being of primary concern in the long run[102,103]. In the right lobe HALT, the congestion is caused by raised caval pressure whereas in left lobe HALT it occurs due to venous stretching. Also since the portal flow is shared between the native and graft, this along with graft congestion can lead to early graft dysfunction[103]. There is also a higher risk of abdominal compartment syndrome and prolonged post-operative ventilation. Hence, APOLT is preferred more to HALT as it rectifies the above drawbacks, in spite of being technically more challenging, and is currently an accepted technique for the management of ALF. It allows time for the native liver to regenerate and then the patient can be off immunosupressants[103]. The role of APOLT in MLDs is to provide the defective enzymes, and it has shown excellent results for selected metabolic indications such as CNS, PA, and UCD[5,103,104]. Rarely has it been done for WD with a normal liver and neurological dysfunction[105]. The authors have one of the largest experiences of performing APOLT in non-cirrhotic MLDs till date where the post LT outcome was comparable to OLT[5]. OLT is offered for babies less than 6 mo and weighing less than 5 kg for the aforementioned disorders, and APOLT is offered for children more than 6 mo of age[5]. A right lobe graft is preferred in organic acidemias (for a larger volume) whereas left (usually a left lateral segment) APOLT is sufficient in CNS as it is proven that even a 2%-5% of hepatocyte mass with normal UGT1A1 gene is enough for normal conjugation of bilirubin[103,104]. The advantages include an intact native liver with a possibility of gene therapy in future (when immunosuppression can be stopped) and it also helps in recovery till the graft function is stable[101]. There is a risk of portal steal phenomenon due to relatively lower stiffness of the native liver, which is prevented by various flow modulation techniques[106]. Heterozygous carriers can be used as donors except in OTC deficiency unless a normal OTC enzyme level is established[101]. The long-term outcomes after APOLT are excellent in the author’s experience[107].
Domino LT
Liver from a patient with MLD is donated to another with a defect of different enzyme or an ESLD[108]. This is done with the expectation that the enzyme deficiency in the donor liver is negligible for the recipient, which would be expressed by the extrahepatic tissues. The potential domino liver donors include patients suffering from MSUD, familial amyloid polyneuropathy and possibly FH[109-112]. Livers from patients with PH should not be used for domino LT (DLT) as the liver continues to produce oxalates and may result in renal failure in the recipient[97]. Livers of patients with MSUD caused by a defect in BCKADH are used as the allograft in DLT. This is because only 9%-13% of the BCKADH is produced from the liver and hence there are no reports of de novo recurrence of MSUD in DLT recipient as the rest 87%-91% of the enzymes are synthesized from extrahepatic sources. In a recently reported series on DLT describing 21 recipients of MSUD livers, the long-term survival after a median time period of 6.4 years was 100%[113]. Hence DLT should be encouraged as an excellent alternative to deceased donor LT. Recently, Govil et al[114] proposed a novel concept, utilizing domino auxiliary LT to use the normally discarded liver from a patient with a liver-based monogenic metabolic disorder as a domino graft in a patient with another such disorder.
CONCLUSION
LT is a curative option for metabolic monogenic disorders. In many cases, it prevents the progression of extrahepatic organ injury and may potentially reverse them. Life-threatening multisystem organ involvement in MLD is an absolute contraindication to LT.
Footnotes
Conflict-of-interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Manuscript source: Invited manuscript
Peer-review started: February 28, 2021
First decision: May 5, 2021
Article in press: June 3, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ganschow R S-Editor: Zhang L L-Editor: A P-Editor: Yuan YY
Contributor Information
Jagadeesh Menon, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Mukul Vij, Department of Pathology, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India. mukul.vij.path@gmail.com.
Deepti Sachan, Department of Transfusion Medicine, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Ashwin Rammohan, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Naresh Shanmugam, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Ilankumaran Kaliamoorthy, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Mohamed Rela, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
References
- 1.Kim JS, Kim KM, Oh SH, Kim HJ, Cho JM, Yoo HW, Namgoong JM, Kim DY, Kim KH, Hwang S, Lee SG. Liver transplantation for metabolic liver disease: experience at a living donor dominant liver transplantation center. Pediatr Gastroenterol Hepatol Nutr. 2015;18:48–54. doi: 10.5223/pghn.2015.18.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam S, Sood V. Metabolic Liver Disease: When to Suspect and How to Diagnose? Indian J Pediatr. 2016;83:1321–1333. doi: 10.1007/s12098-016-2097-z. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira CR, Cassiman D, Blau N. Clinical and biochemical footprints of inherited metabolic diseases. II. Metabolic liver diseases. Mol Genet Metab. 2019;127:117–121. doi: 10.1016/j.ymgme.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham YH, Miloh T. Liver Transplantation in Children. Clin Liver Dis. 2018;22:807–821. doi: 10.1016/j.cld.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam NP, Valamparampil JJ, Reddy MS, Al Said KJ, Al-Thihli K, Al-Hashmi N, Al-Jishi E, Isa HMA, Jalan AB, Rela M. Auxiliary Partial Orthotopic Liver Transplantation for Monogenic Metabolic Liver Diseases: Single-Centre Experience. JIMD Rep. 2019;45:29–36. doi: 10.1007/8904_2018_137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121–126. doi: 10.1002/ajmg.c.30092. [DOI] [PubMed] [Google Scholar]
- 7.Larochelle J, Alvarez F, Bussières JF, Chevalier I, Dallaire L, Dubois J, Faucher F, Fenyves D, Goodyer P, Grenier A, Holme E, Laframboise R, Lambert M, Lindstedt S, Maranda B, Melançon S, Merouani A, Mitchell J, Parizeault G, Pelletier L, Phan V, Rinaldo P, Scott CR, Scriver C, Mitchell GA. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol Genet Metab. 2012;107:49–54. doi: 10.1016/j.ymgme.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe R. Liver transplant pathology in pediatric metabolic disorders. Pediatr Dev Pathol. 1998;1:102–117. doi: 10.1007/s100249900013. [DOI] [PubMed] [Google Scholar]
- 9.Russo PA, Mitchell GA, Tanguay RM. Tyrosinemia: a review. Pediatr Dev Pathol. 2001;4:212–221. doi: 10.1007/s100240010146. [DOI] [PubMed] [Google Scholar]
- 10.Quaglia A, Roberts EA, Torbenson M. Developmental and Inherited Liver Disease. In: Burt AD, Ferrell LD, Hubscher SG. MacSween’s Pathology of the Liver. 7th ed. Philadelphia: Elsevier, 2018: 111-274. [Google Scholar]
- 11.Fagiuoli S, Daina E, D'Antiga L, Colledan M, Remuzzi G. Monogenic diseases that can be cured by liver transplantation. J Hepatol. 2013;59:595–612. doi: 10.1016/j.jhep.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell EL, Khan Z. Liver Disease in Alpha-1 Antitrypsin Deficiency: Current Approaches and Future Directions. Curr Pathobiol Rep. 2017;5:243–252. doi: 10.1007/s40139-017-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanan P, Mistry PK. Update on Alpha-1 Antitrypsin Deficiency in Liver Disease. Clin Liver Dis (Hoboken) 2020;15:228–235. doi: 10.1002/cld.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey EJ, Iyer VN, Nelson DR, Nguyen JH, Krowka MJ. Outcomes for recipients of liver transplantation for alpha-1-antitrypsin deficiency–related cirrhosis. Liver Transpl. 2013;19:1370–1376. doi: 10.1002/lt.23744. [DOI] [PubMed] [Google Scholar]
- 15.Antoury C, Lopez R, Zein N, Stoller JK, Alkhouri N. Alpha-1 antitrypsin deficiency and the risk of hepatocellular carcinoma in end-stage liver disease. World J Hepatol. 2015;7:1427–1432. doi: 10.4254/wjh.v7.i10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prachalias AA, Kalife M, Francavilla R, Muiesan P, Dhawan A, Baker A, Hadzic D, Mieli-Vergani G, Rela M, Heaton ND. Liver transplantation for alpha-1-antitrypsin deficiency in children. Transpl Int. 2000;13:207–210. doi: 10.1007/s001470050688. [DOI] [PubMed] [Google Scholar]
- 17.Guillaud O, Jacquemin E, Couchonnal E, Vanlemmens C, Francoz C, Chouik Y, Conti F, Duvoux C, Hilleret MN, Kamar N, Houssel-Debry P, Neau-Cransac M, Pageaux GP, Gonzales E, Ackermann O, Gugenheim J, Lachaux A, Ruiz M, Radenne S, Debray D, Lacaille F, McLin V, Duclos-Vallée JC, Samuel D, Coilly A, Dumortier J. Long term results of liver transplantation for alpha-1 antitrypsin deficiency. Dig Liver Dis. 2021;53:606–611. doi: 10.1016/j.dld.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Ishak KG. Inherited metabolic diseases of the liver. Clin Liver Dis. 2002;6:455–479, viii. doi: 10.1016/s1089-3261(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 19.Welling L, Bernstein LE, Berry GT, Burlina AB, Eyskens F, Gautschi M, Grünewald S, Gubbels CS, Knerr I, Labrune P, van der Lee JH, MacDonald A, Murphy E, Portnoi PA, Õunap K, Potter NL, Rubio-Gozalbo ME, Spencer JB, Timmers I, Treacy EP, Van Calcar SC, Waisbren SE, Bosch AM Galactosemia Network (GalNet) International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. J Inherit Metab Dis. 2017;40:171–176. doi: 10.1007/s10545-016-9990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto G, Herfarth C, Senninger N, Feist G, Post S, Gmelin K. Hepatic transplantation in galactosemia. Transplantation. 1989;47:902–903. doi: 10.1097/00007890-198905000-00033. [DOI] [PubMed] [Google Scholar]
- 21.Socha P, Janczyk W, Dhawan A, Baumann U, D'Antiga L, Tanner S, Iorio R, Vajro P, Houwen R, Fischler B, Dezsofi A, Hadzic N, Hierro L, Jahnel J, McLin V, Nobili V, Smets F, Verkade HJ, Debray D. Wilson's Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:334–344. doi: 10.1097/MPG.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 22.Dhawan A, Taylor RM, Cheeseman P, De Silva P, Katsiyiannakis L, Mieli-Vergani G. Wilson's disease in children: 37-year experience and revised King's score for liver transplantation. Liver Transpl. 2005;11:441–448. doi: 10.1002/lt.20352. [DOI] [PubMed] [Google Scholar]
- 23.Arnon R, Annunziato R, Schilsky M, Miloh T, Willis A, Sturdevant M, Sakworawich A, Suchy F, Kerkar N. Liver transplantation for children with Wilson disease: comparison of outcomes between children and adults. Clin Transplant. 2011;25:E52–E60. doi: 10.1111/j.1399-0012.2010.01327.x. [DOI] [PubMed] [Google Scholar]
- 24.Schilsky ML. Liver transplantation for Wilson's disease. Ann N Y Acad Sci. 2014;1315:45–49. doi: 10.1111/nyas.12454. [DOI] [PubMed] [Google Scholar]
- 25.Peedikayil MC, Al Ashgar HI, Al Mousa A, Al Sebayel M, Al Kahtani K, Alkhail FA. Liver transplantation in Wilson's disease: Single center experience from Saudi Arabia. World J Hepatol. 2013;5:127–132. doi: 10.4254/wjh.v5.i3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hursitoglu M, Kara O, Cikrikcioglu MA, Celepkulu T, Aydin S, Tukek T. Clinical improvement of a patient with severe Wilson's disease after a single session of therapeutic plasma exchange. J Clin Apher. 2009;24:25–27. doi: 10.1002/jca.20186. [DOI] [PubMed] [Google Scholar]
- 27.Morgan SM, Zantek ND. Therapeutic plasma exchange for fulminant hepatic failure secondary to Wilson's disease. J Clin Apher. 2012;27:282–286. doi: 10.1002/jca.21239. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds HV, Talekar CR, Bellapart J, Leggett BA, Boots RJ. Copper removal strategies for Wilson's disease crisis in the ICU. Anaesth Intensive Care. 2014;42:253–257. [PubMed] [Google Scholar]
- 29.Jhang JS, Schilsky ML, Lefkowitch JH, Schwartz J. Therapeutic plasmapheresis as a bridge to liver transplantation in fulminant Wilson disease. J Clin Apher. 2007;22:10–14. doi: 10.1002/jca.20118. [DOI] [PubMed] [Google Scholar]
- 30.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu Y, Zantek ND, Dunbar NM, Schwartz GEJ. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34:171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 31.Motobayashi M, Fukuyama T, Nakayama Y, Sano K, Noda S, Hidaka Y, Amano Y, Ikeda S, Koike K, Inaba Y. Successful treatment of fulminant Wilson's disease without liver transplantation. Pediatr Int. 2014;56:429–432. doi: 10.1111/ped.12291. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Byers HM, Diaz-Kuan A, Vos MB, Hall PL, Tortorelli S, Singh R, Wallenstein MB, Allain M, Dimmock DP, Farrell RM, McCandless S, Gambello MJ. Acute liver failure in neonates with undiagnosed hereditary fructose intolerance due to exposure from widely available infant formulas. Mol Genet Metab. 2018;123:428–432. doi: 10.1016/j.ymgme.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Jevon GP, Dimmick JE. Histopathologic approach to metabolic liver disease: Part 2. Pediatr Dev Pathol. 1998;1:261–269. doi: 10.1007/s100249900038. [DOI] [PubMed] [Google Scholar]
- 34.Kishnani PS, Austin SL, Arn P, Bali DS, Boney A, Case LE, Chung WK, Desai DM, El-Gharbawy A, Haller R, Smit GP, Smith AD, Hobson-Webb LD, Wechsler SB, Weinstein DA, Watson MS ACMG. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446–463. doi: 10.1097/GIM.0b013e3181e655b6. [DOI] [PubMed] [Google Scholar]
- 35.Matern D, Starzl TE, Arnaout W, Barnard J, Bynon JS, Dhawan A, Emond J, Haagsma EB, Hug G, Lachaux A, Smit GP, Chen YT. Liver transplantation for glycogen storage disease types I, III, and IV. Eur J Pediatr. 1999;158 Suppl 2:S43–S48. doi: 10.1007/pl00014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jevon GP, Dimmick JE. Histopathologic approach to metabolic liver disease: Part 1. Pediatr Dev Pathol. 1998;1:179–199. doi: 10.1007/s100249900026. [DOI] [PubMed] [Google Scholar]
- 37.Marques-da-Silva D, Dos Reis Ferreira V, Monticelli M, Janeiro P, Videira PA, Witters P, Jaeken J, Cassiman D. Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J Inherit Metab Dis. 2017;40:195–207. doi: 10.1007/s10545-016-0012-4. [DOI] [PubMed] [Google Scholar]
- 38.Janssen MC, de Kleine RH, van den Berg AP, Heijdra Y, van Scherpenzeel M, Lefeber DJ, Morava E. Successful liver transplantation and long-term follow-up in a patient with MPI-CDG. Pediatrics. 2014;134:e279–e283. doi: 10.1542/peds.2013-2732. [DOI] [PubMed] [Google Scholar]
- 39.Verheijen J, Tahata S, Kozicz T, Witters P, Morava E. Therapeutic approaches in Congenital Disorders of Glycosylation (CDG) involving N-linked glycosylation: an update. Genet Med. 2020;22:268–279. doi: 10.1038/s41436-019-0647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Socha P, Vajro P, Lefeber D, Adamowicz M, Tanner S. Search for rare liver diseases: the case of glycosylation defects mimicking Wilson Disease. Clin Res Hepatol Gastroenterol. 2014;38:403–406. doi: 10.1016/j.clinre.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Carubbi F, Cappellini MD, Fargion S, Fracanzani AL, Nascimbeni F. Liver involvement in Gaucher disease: A practical review for the hepatologist and the gastroenterologist. Dig Liver Dis. 2020;52:368–373. doi: 10.1016/j.dld.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Lidove O, Sedel F, Charlotte F, Froissart R, Vanier MT. Cirrhosis and liver failure: expanding phenotype of Acid sphingomyelinase-deficient niemann-pick disease in adulthood. JIMD Rep. 2015;15:117–121. doi: 10.1007/8904_2014_306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pastores GM, Hughes DA. Lysosomal Acid Lipase Deficiency: Therapeutic Options. Drug Des Devel Ther. 2020;14:591–601. doi: 10.2147/DDDT.S149264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein DL, Hülkova H, Bialer MG, Desnick RJ. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J Hepatol. 2013;58:1230–1243. doi: 10.1016/j.jhep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Ayto RM, Hughes DA, Jeevaratnam P, Rolles K, Burroughs AK, Mistry PK, Mehta AB, Pastores GM. Long-term outcomes of liver transplantation in type 1 Gaucher disease. Am J Transplant. 2010;10:1934–1939. doi: 10.1111/j.1600-6143.2010.03168.x. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Luo Y, Xia L, Qiu B, Zhou T, Feng M, Xue F, Chen X, Han L, Zhang J, Xia Q. The Effects of Liver Transplantation in Children With Niemann-Pick Disease Type B. Liver Transpl. 2019;25:1233–1240. doi: 10.1002/lt.25457. [DOI] [PubMed] [Google Scholar]
- 47.Yamada N, Inui A, Sanada Y, Ihara Y, Urahashi T, Fukuda A, Sakamoto S, Kasahara M, Yoshizawa A, Okamoto S, Okajima H, Fujisawa T, Mizuta K. Pediatric liver transplantation for neonatal-onset Niemann-Pick disease type C: Japanese multicenter experience. Pediatr Transplant. 2019;23:e13462. doi: 10.1111/petr.13462. [DOI] [PubMed] [Google Scholar]
- 48.Bernstein DL, Lobritto S, Iuga A, Remotti H, Schiano T, Fiel MI, Balwani M. Lysosomal acid lipase deficiency allograft recurrence and liver failure- clinical outcomes of 18 liver transplantation patients. Mol Genet Metab. 2018;124:11–19. doi: 10.1016/j.ymgme.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Botero V, Garcia VH, Gomez-Duarte C, Aristizabal AM, Arrunategui AM, Echeverri GJ, Pachajoa H. Lysosomal Acid Lipase Deficiency, a Rare Pathology: The First Pediatric Patient Reported in Colombia. Am J Case Rep. 2018;19:669–672. doi: 10.12659/AJCR.908808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tylki-Szymanska A, Wamelink MM, Stradomska TJ, Salomons GS, Taybert J, Dąbrowska-Leonik N, Rurarz M. Clinical and molecular characteristics of two transaldolase-deficient patients. Eur J Pediatr. 2014;173:1679–1682. doi: 10.1007/s00431-014-2261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baes M, Van Veldhoven PP. Hepatic dysfunction in peroxisomal disorders. Biochim Biophys Acta. 2016;1863:956–970. doi: 10.1016/j.bbamcr.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 52.Chartier ME, Hart L, Paganelli M, Ahmed N, Bilodeau M, Alvarez F. Successful Liver Transplants for Liver Failure Associated With Pyruvate Kinase Deficiency. Pediatrics. 2018;141 Suppl 5:S385–S389. doi: 10.1542/peds.2016-3896. [DOI] [PubMed] [Google Scholar]
- 53.Leonard JV, McKiernan PJ. The role of liver transplantation in urea cycle disorders. Mol Genet Metab. 2004;81 Suppl 1:S74–S78. doi: 10.1016/j.ymgme.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 54.Kim IK, Niemi AK, Krueger C, Bonham CA, Concepcion W, Cowan TM, Enns GM, Esquivel CO. Liver transplantation for urea cycle disorders in pediatric patients: a single-center experience. Pediatr Transplant. 2013;17:158–167. doi: 10.1111/petr.12041. [DOI] [PubMed] [Google Scholar]
- 55.Bigot A, Tchan MC, Thoreau B, Blasco H, Maillot F. Liver involvement in urea cycle disorders: a review of the literature. J Inherit Metab Dis. 2017;40:757–769. doi: 10.1007/s10545-017-0088-5. [DOI] [PubMed] [Google Scholar]
- 56.Shanmugam N, Dhawan A. Pediatric Liver Intensive Care. Springer, Singapore. 2019; 1-215. [Google Scholar]
- 57.Perito ER, Rhee S, Roberts JP, Rosenthal P. Pediatric liver transplantation for urea cycle disorders and organic acidemias: United Network for Organ Sharing data for 2002-2012. Liver Transpl. 2014;20:89–99. doi: 10.1002/lt.23765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaplito-Lee J, Chow CW, Boneh A. Histopathological findings in livers of patients with urea cycle disorders. Mol Genet Metab. 2013;108:161–165. doi: 10.1016/j.ymgme.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Seth AK, Badminton MN, Mirza D, Russell S, Elias E. Liver transplantation for porphyria: who, when, and how? Liver Transpl. 2007;13:1219–1227. doi: 10.1002/lt.21261. [DOI] [PubMed] [Google Scholar]
- 60.Singal AK, Parker C, Bowden C, Thapar M, Liu L, McGuire BM. Liver transplantation in the management of porphyria. Hepatology. 2014;60:1082–1089. doi: 10.1002/hep.27086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lissing M, Nowak G, Adam R, Karam V, Boyd A, Gouya L, Meersseman W, Melum E, Ołdakowska-Jedynak U, Reiter FP, Colmenero J, Sanchez R, Herden U, Langendonk J, Ventura P, Isoniemi H, Boillot O, Braun F, Perrodin S, Mowlem E, Wahlin S European Liver and Intestine Transplant Association. Liver Transplantation for Acute Intermittent Porphyria. Liver Transpl. 2021;27:491–501. doi: 10.1002/lt.25959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. doi: 10.1038/gim.2014.128. [DOI] [PubMed] [Google Scholar]
- 63.Boers SJ, Visser G, Smit PG, Fuchs SA. Liver transplantation in glycogen storage disease type I. Orphanet J Rare Dis. 2014;9:47. doi: 10.1186/1750-1172-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasahara M, Horikawa R, Sakamoto S, Shigeta T, Tanaka H, Fukuda A, Abe K, Yoshii K, Naiki Y, Kosaki R, Nakagawa A. Living donor liver transplantation for glycogen storage disease type Ib. Liver Transpl. 2009;15:1867–1871. doi: 10.1002/lt.21929. [DOI] [PubMed] [Google Scholar]
- 65.Bhasin B, Ürekli HM, Atta MG. Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol. 2015;4:235–244. doi: 10.5527/wjn.v4.i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narasimhan G, Govil S, Rajalingam R, Venkataraman C, Shanmugam NP, Rela M. Preserving double equipoise in living donor liver-kidney transplantation for primary hyperoxaluria type 1. Liver Transpl. 2015;21:1324–1326. doi: 10.1002/lt.24167. [DOI] [PubMed] [Google Scholar]
- 67.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. 2009;75:1264–1271. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis. 2018;277:483–492. doi: 10.1016/j.atherosclerosis.2018.06.859. [DOI] [PubMed] [Google Scholar]
- 69.Gulsoy Kirnap N, Kirnap M, Bascil Tutuncu N, Moray G, Haberal M. The curative treatment of familial hypercholesterolemia: Liver transplantation. Clin Transplant. 2019;33:e13730. doi: 10.1111/ctr.13730. [DOI] [PubMed] [Google Scholar]
- 70.Ishigaki Y, Kawagishi N, Hasegawa Y, Sawada S, Katagiri H, Satomi S, Oikawa S. Liver Transplantation for Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb. 2019;26:121–127. doi: 10.5551/jat.RV17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dhawan A, Lawlor MW, Mazariegos GV, McKiernan P, Squires JE, Strauss KA, Gupta D, James E, Prasad S. Disease burden of Crigler-Najjar syndrome: Systematic review and future perspectives. J Gastroenterol Hepatol. 2020;35:530–543. doi: 10.1111/jgh.14853. [DOI] [PubMed] [Google Scholar]
- 72.Pett S, Mowat AP. Crigler-Najjar syndrome types I and II. Clinical experience--King's College Hospital 1972-1978. Phenobarbitone, phototherapy and liver transplantation. Mol Aspects Med. 1987;9:473–482. doi: 10.1016/0098-2997(87)90009-4. [DOI] [PubMed] [Google Scholar]
- 73.Sellier AL, Labrune P, Kwon T, Boudjemline AM, Deschènes G, Gajdos V. Successful plasmapheresis for acute and severe unconjugated hyperbilirubinemia in a child with crigler najjar type I syndrome. JIMD Rep. 2012;2:33–36. doi: 10.1007/8904_2011_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, Morton DH. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur J Pediatr. 2006;165:306–319. doi: 10.1007/s00431-005-0055-2. [DOI] [PubMed] [Google Scholar]
- 75.Ozçay F, Alehan F, Sevmiş S, Karakayali H, Moray G, Torgay A, Arslan G, Haberal M. Living related liver transplantation in Crigler-Najjar syndrome type 1. Transplant Proc. 2009;41:2875–2877. doi: 10.1016/j.transproceed.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 76.Tu ZH, Shang DS, Jiang JC, Zhang W, Zhang M, Wang WL, Lou HY, Zheng SS. Liver transplantation in Crigler-Najjar syndrome type I disease. Hepatobiliary Pancreat Dis Int. 2012;11:545–548. doi: 10.1016/s1499-3872(12)60222-7. [DOI] [PubMed] [Google Scholar]
- 77.Sze YK, Dhawan A, Taylor RM, Bansal S, Mieli-Vergani G, Rela M, Heaton N. Pediatric liver transplantation for metabolic liver disease: experience at King's College Hospital. Transplantation. 2009;87:87–93. doi: 10.1097/TP.0b013e31818bc0c4. [DOI] [PubMed] [Google Scholar]
- 78.Baumgartner MR, Hörster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, Huemer M, Hochuli M, Assoun M, Ballhausen D, Burlina A, Fowler B, Grünert SC, Grünewald S, Honzik T, Merinero B, Pérez-Cerdá C, Scholl-Bürgi S, Skovby F, Wijburg F, MacDonald A, Martinelli D, Sass JO, Valayannopoulos V, Chakrapani A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alkhunaizi AM, Al-Sannaa N. Renal Involvement in Methylmalonic Aciduria. Kidney Int Rep. 2017;2:956–960. doi: 10.1016/j.ekir.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kasahara M, Horikawa R, Tagawa M, Uemoto S, Yokoyama S, Shibata Y, Kawano T, Kuroda T, Honna T, Tanaka K, Saeki M. Current role of liver transplantation for methylmalonic acidemia: a review of the literature. Pediatr Transplant. 2006;10:943–947. doi: 10.1111/j.1399-3046.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 81.Jiang YZ, Sun LY. The Value of Liver Transplantation for Methylmalonic Acidemia. Front Pediatr. 2019;7:87. doi: 10.3389/fped.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yap S, Vara R, Morais A. Post-transplantation Outcomes in Patients with PA or MMA: A Review of the Literature. Adv Ther. 2020;37:1866–1896. doi: 10.1007/s12325-020-01305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noone D, Riedl M, Atkison P, Avitzur Y, Sharma AP, Filler G, Siriwardena K, Prasad C. Kidney disease and organ transplantation in methylmalonic acidaemia. Pediatr Transplant. 2019;23:e13407. doi: 10.1111/petr.13407. [DOI] [PubMed] [Google Scholar]
- 84.Vara R, Turner C, Mundy H, Heaton ND, Rela M, Mieli-Vergani G, Champion M, Hadzic N. Liver transplantation for propionic acidemia in children. Liver Transpl. 2011;17:661–667. doi: 10.1002/lt.22279. [DOI] [PubMed] [Google Scholar]
- 85.Curnock R, Heaton ND, Vilca-Melendez H, Dhawan A, Hadzic N, Vara R. Liver Transplantation in Children With Propionic Acidemia: Medium-Term Outcomes. Liver Transpl. 2020;26:419–430. doi: 10.1002/lt.25679. [DOI] [PubMed] [Google Scholar]
- 86.Critelli K, McKiernan P, Vockley J, Mazariegos G, Squires RH, Soltys K, Squires JE. Liver Transplantation for Propionic Acidemia and Methylmalonic Acidemia: Perioperative Management and Clinical Outcomes. Liver Transpl. 2018;24:1260–1270. doi: 10.1002/lt.25304. [DOI] [PubMed] [Google Scholar]
- 87.Alexopoulos SP, Matsuoka L, Hafberg E, Morgan T, Thurm C, Hall M, Godown J. Liver Transplantation for Propionic Acidemia: A Multicenter-linked Database Analysis. J Pediatr Gastroenterol Nutr. 2020;70:178–182. doi: 10.1097/MPG.0000000000002534. [DOI] [PubMed] [Google Scholar]
- 88.Quintero J, Molera C, Juamperez J, Redecillas S, Meavilla S, Nuñez R, García C, Del Toro M, Garcia Á, Ortega J, Segarra Ó, de Carpi JM, Bilbao I, Charco R. The Role of Liver Transplantation in Propionic Acidemia. Liver Transpl. 2018;24:1736–1745. doi: 10.1002/lt.25344. [DOI] [PubMed] [Google Scholar]
- 89.Zhou GP, Jiang YZ, Wu SS, Kong YY, Sun LY, Zhu ZJ. Liver Transplantation for Propionic Acidemia: Evidence from A Systematic Review and Meta-analysis. Transplantation. 2020 doi: 10.1097/TP.0000000000003501. [DOI] [PubMed] [Google Scholar]
- 90.Blackburn PR, Gass JM, Vairo FPE, Farnham KM, Atwal HK, Macklin S, Klee EW, Atwal PS. Maple syrup urine disease: mechanisms and management. Appl Clin Genet. 2017;10:57–66. doi: 10.2147/TACG.S125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Díaz VM, Camarena C, de la Vega Á, Martínez-Pardo M, Díaz C, López M, Hernández F, Andrés A, Jara P. Liver transplantation for classical maple syrup urine disease: long-term follow-up. J Pediatr Gastroenterol Nutr. 2014;59:636–639. doi: 10.1097/MPG.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 92.Mazariegos GV, Morton DH, Sindhi R, Soltys K, Nayyar N, Bond G, Shellmer D, Shneider B, Vockley J, Strauss KA. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J Pediatr. 2012;160:116–21.e1. doi: 10.1016/j.jpeds.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wendel U, Saudubray JM, Bodner A, Schadewaldt P. Liver transplantation in maple syrup urine disease. Eur J Pediatr. 1999;158 Suppl 2:S60–S64. doi: 10.1007/pl00014324. [DOI] [PubMed] [Google Scholar]
- 94.Lee WS, Sokol RJ. Mitochondrial hepatopathies: advances in genetics and pathogenesis. Hepatology. 2007;45:1555–1565. doi: 10.1002/hep.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee WS, Sokol RJ. Liver disease in mitochondrial disorders. Semin Liver Dis. 2007;27:259–273. doi: 10.1055/s-2007-985071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bandyopadhyay SK, Dutta A. Mitochondrial hepatopathies. J Assoc Physicians India. 2005;53:973–978. [PubMed] [Google Scholar]
- 97.Squires RH, Ng V, Romero R, Ekong U, Hardikar W, Emre S, Mazariegos GV. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology. 2014;60:362–398. doi: 10.1002/hep.27191. [DOI] [PubMed] [Google Scholar]
- 98.Lane M, Boczonadi V, Bachtari S, Gomez-Duran A, Langer T, Griffiths A, Kleinle S, Dineiger C, Abicht A, Holinski-Feder E, Schara U, Gerner P, Horvath R. Mitochondrial dysfunction in liver failure requiring transplantation. J Inherit Metab Dis. 2016;39:427–436. doi: 10.1007/s10545-016-9927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niezgoda J, Morgan PG. Anesthetic considerations in patients with mitochondrial defects. Paediatr Anaesth. 2013;23:785–793. doi: 10.1111/pan.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimura M, Kuranobu N, Ogawa-Tominaga M, Akiyama N, Sugiyama Y, Ebihara T, Fushimi T, Ichimoto K, Matsunaga A, Tsuruoka T, Kishita Y, Umetsu S, Inui A, Fujisawa T, Tanikawa K, Ito R, Fukuda A, Murakami J, Kaji S, Kasahara M, Shiraki K, Ohtake A, Okazaki Y, Murayama K. Clinical and molecular basis of hepatocerebral mitochondrial DNA depletion syndrome in Japan: evaluation of outcomes after liver transplantation. Orphanet J Rare Dis. 2020;15:169. doi: 10.1186/s13023-020-01441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Hoek B, de Boer J, Boudjema K, Williams R, Corsmit O, Terpstra OT. Auxiliary vs orthotopic liver transplantation for acute liver failure. EURALT Study Group. European Auxiliary Liver Transplant Registry. J Hepatol. 1999;30:699–705. doi: 10.1016/s0168-8278(99)80202-5. [DOI] [PubMed] [Google Scholar]
- 102.Rammohan A, Reddy MS, Line PD, Rela M. Heterotopic liver transplantation: Temporary solution, permanent problem? Am J Transplant. 2021;21:903–904. doi: 10.1111/ajt.16271. [DOI] [PubMed] [Google Scholar]
- 103.Rela M, Battula N, Madanur M, Mieli-Vergani G, Dhawan A, Champion M, Raiman J, Heaton N. Auxiliary liver transplantation for propionic acidemia: a 10-year follow-up. Am J Transplant. 2007;7:2200–2203. doi: 10.1111/j.1600-6143.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- 104.Rela M, Muiesan P, Vilca-Melendez H, Dhawan A, Baker A, Mieli-Vergani G, Heaton ND. Auxiliary partial orthotopic liver transplantation for Crigler-Najjar syndrome type I. Ann Surg. 1999;229:565–569. doi: 10.1097/00000658-199904000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haberal M, Akdur A, Moray G, Boyacioglu S, Torgay A, Arslan G, Ozdemir BH. Auxiliary Partial Orthotopic Living Liver Transplant for Wilson Disease. Exp Clin Transplant. 2017;15:182–184. doi: 10.6002/ect.mesot2016.P64. [DOI] [PubMed] [Google Scholar]
- 106.Rela M, Bharathan A, Palaniappan K, Cherian PT, Reddy MS. Portal flow modulation in auxiliary partial orthotopic liver transplantation. Pediatr Transplant. 2015;19:255–260. doi: 10.1111/petr.12436. [DOI] [PubMed] [Google Scholar]
- 107.Rammohan A, Reddy MS, Narasimhan G, Rajalingam R, Kaliamoorthy I, Shanmugam N, Rela M. Auxiliary Partial Orthotopic Liver Transplantation for Selected Noncirrhotic Metabolic Liver Disease. Liver Transpl. 2019;25:111–118. doi: 10.1002/lt.25352. [DOI] [PubMed] [Google Scholar]
- 108.Celik N, Squires JE, Soltys K, Vockley J, Shellmer DA, Chang W, Strauss K, McKiernan P, Ganoza A, Sindhi R, Bond G, Mazariegos G, Khanna A. Domino liver transplantation for select metabolic disorders: Expanding the living donor pool. JIMD Rep. 2019;48:83–89. doi: 10.1002/jmd2.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohan N, Karkra S, Rastogi A, Vohra V, Soin AS. Living donor liver transplantation in maple syrup urine disease - Case series and world's youngest domino liver donor and recipient. Pediatr Transplant. 2016;20:395–400. doi: 10.1111/petr.12666. [DOI] [PubMed] [Google Scholar]
- 110.Hemming AW, Cattral MS, Chari RS, Greig PD, Lilly LB, Ashby P, Levy GA. Domino liver transplantation for familial amyloid polyneuropathy. Liver Transpl Surg. 1998;4:236–238. doi: 10.1002/lt.500040303. [DOI] [PubMed] [Google Scholar]
- 111.Liu C, Niu DM, Loong CC, Hsia CY, Tsou MY, Tsai HL, Wei C. Domino liver graft from a patient with homozygous familial hypercholesterolemia. Pediatr Transplant. 2010;14:E30–E33. doi: 10.1111/j.1399-3046.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 112.Kitchens WH. Domino liver transplantation: indications, techniques, and outcomes. Transplant Rev (Orlando) 2011;25:167–177. doi: 10.1016/j.trre.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Celik N, Kelly B, Soltys K, Squires JE, Vockley J, Shellmer DA, Strauss K, McKiernan P, Ganoza A, Sindhi R, Bond G, Mazariegos G, Khanna A. Technique and outcome of domino liver transplantation from patients with maple syrup urine disease: Expanding the donor pool for live donor liver transplantation. Clin Transplant. 2019;33:e13721. doi: 10.1111/ctr.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Govil S, Shanmugam NP, Reddy MS, Narasimhan G, Rela M. A metabolic chimera: Two defective genotypes make a normal phenotype. Liver Transpl. 2015;21:1453–1454. doi: 10.1002/lt.24202. [DOI] [PubMed] [Google Scholar]