Abstract
Patients with cancer experience a higher burden of SARS-CoV-2 infection, disease severity, complications, and mortality, than the general population. SARS-CoV-2 mRNA vaccines are highly effective in the general population; however, few data are available on their efficacy in patients with cancer. Using a prospective cohort, we assessed the seroconversion rates and anti-SARS-CoV-2 spike protein antibody titers following the first and second dose of BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in patients with cancer in US and Europe from January to April 2021. Among 131 patients, most (94%) achieved seroconversion after receipt of two vaccine doses. Seroconversion rates and antibody titers in patients with hematological malignancy were significantly lower than those with solid tumors. None of the patients with history of anti-CD-20 antibody in the 6 months before vaccination developed antibody response. Antibody titers were highest for clinical surveillance or endocrine therapy groups and lowest for cytotoxic chemotherapy or monoclonal antibody groups.
Keywords: COVID-19, vaccine, immune response, malignancy, tumor, pandemic, seroconversion, antibody, oncology, anti-cancer treatment
Graphical abstract
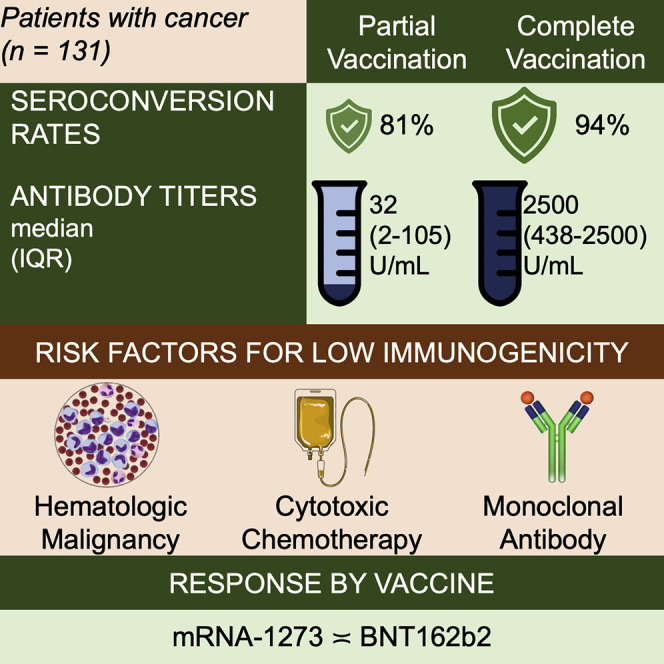
Addeo et al. show patients with cancer have poor antibody response after one dose and excellent antibody response at 3 weeks after two doses with mRNA COVID-19 vaccines. A subset of immunocompromised patients (i.e., those receiving anti-CD20), are at high risk for not developing antibodies post-vaccination.
Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic has spread throughout the world with over 161 million confirmed cases globally and more than 3 million deaths as of May 2021 (https://covid19.who.int/). Unprecedented global effort has been made to develop different SARS-CoV-2 vaccines using technologies based on messenger RNA (mRNA), synthetic long viral peptides, plasmid DNA, and inactivated, attenuated, or genetically modified viruses, including BNT162b2 (Pfizer-BioNTech) (Polack et al., 2020), mRNA-1273 (Moderna) (Baden et al., 2020), AZD1222 (Oxford/AstraZeneca) (Voysey et al., 2021), Ad26.COV2.S (Johnson & Jonhson) (Sadoff et al., 2021), Sputnik V (Gamaleya) (Logunov et al., 2021), and BBIBP-CorV (Sinopharm) (Xia et al., 2021)). Efficacy ranges between 60% and 94% with excellent safety profile in the general population. However, scarce experimental data about safety and efficacy of vaccine have been reported on patients with cancer, as those on active therapy were excluded from SARS-CoV-2 vaccine clinical trials (Friese et al., 2021).
Compared with the general population, patients with cancer are more likely to be at high risk of serious COVID-19-related complications and mortality (Bakouny et al., 2020; Grivas et al., 2021; Kuderer et al., 2020), hence having information about efficacy of vaccine and optimal timing in relation to anti-cancer therapy to promote an effective immunity in this population remains crucial.
Here, we report results from an international collaborative prospective cohort study assessing short-term humoral immune response (seroconversion rates and antibody titers) by measuring anti-SARS-CoV-2 spike protein (S) immunoglobulin G (IgG) antibody titer as a surrogate after two doses of mRNA vaccines (mRNA-1273 and BNT162b2) in two different cohorts of patients with solid and hematological malignancies. To put our study findings in the context of the existing literature, we also present data from available studies (published or pre-print) examining anti-S IgG antibody response rates in patients with cancer who received SARS-CoV-2 vaccines.
Results
Study cohort
We enrolled a total of 140 patients with cancer who received either BNT162b2 or mRNA-1273 vaccine at one of the enrolling sites. Among these patients, 131 were SARS-CoV-2 naive as determined by a negative anti-SARS-CoV-2 nucleocapsid (N) protein IgG test at baseline, and thus included in the immunogenicity analysis. Study cohort characteristics are listed in Table 1 . The median follow-up time was 50 (interquartile range [IQR]: 49–55) days, which is equivalent to 22 (22–24) days after receipt of a second vaccine dose. The median (IQR) age at vaccination was 63 (55–69) years and the racial/ethnic distribution of patients was: non-Hispanic white (80%), Hispanic (18%), and black (2%). There was an almost equal proportion of males (55%) and females (45%) at both sites. Most malignancies were solid tumors (81%), with breast (33%) and urological (19%) cancer being the most common solid tumor types. Twenty-five (19%) patients had hematological malignancy. Approximately, one-third did not receive anti-cancer therapy within 6 months before COVD-19 vaccination. The most common anti-cancer therapy received by this cohort of patients was cytotoxic chemotherapy (23%), followed by endocrine therapy (15%), monoclonal antibody therapy (13%), kinase inhibitor therapy (11%), and immunotherapy (11%).
Table 1.
Clinical characteristics of the study cohort
| N | 131 |
| Age, years, median (IQR) | 63 (55–69) |
| Sex | |
| Male | 72 (55%) |
| Female | 59 (45%) |
| Race | |
| Non-Hispanic white | 105 (80%) |
| Hispanic | 23 (18%) |
| Black | 3 (2%) |
| Type of malignancy | |
| Solid malignancies | 106 (81%) |
| Breast | 27 |
| Urological | 20 |
| Gynecological | 3 |
| Skin cancersa | 7 |
| Thoracic malignancy | 18 |
| Gastrointestinal | 16 |
| Head and neck cancer | 3 |
| Brain | 8 |
| Connective tissue | 4 |
| Hematological malignancies | 25 (19%) |
| Acute lymphoblastic leukemia | 1 |
| Chronic myeloid leukemia | 1 |
| Chronic lymphocytic leukemia | 1 |
| Diffuse large B cell lymphoma | 6 |
| Follicular lymphoma | 2 |
| MALT lymphoma | 2 |
| T cell lymphoma/mycosis fungoides | 2 |
| Hodgkin's lymphoma | 4 |
| Polycythemia vera | 1 |
| Myeloma | 5 |
| Type of anti-cancer treatmentb(within 6 months before vaccination) | |
| Clinical surveillance | 49 (37%) |
| Cytotoxic chemotherapy | 30 (23%) |
| Immunotherapy | 14 (11%) |
| Endocrine therapy | 19 (15%) |
| Anti-CD-20 antibody | 4 (3%) |
| Anti-CD-38 antibody | 1 (1%) |
| Anti-HER antibody | 2 (2%) |
| Anti-VEGF antibody | 6 (5%) |
| RANKL antibody | 4 (3%) |
| Kinase inhibitor | 15 (11%) |
| Unknownc | 1 (1%) |
| SARS-CoV-2 vaccine | |
| BNT162b2 | 38 (29%) |
| mRNA-1273 | 93 (71%) |
| Days between first vaccine dose and final outcome measurement, median (range) | 50 (49–55) |
| Days between second vaccine dose and final outcome measurement, median (range) | 24 (22–24) |
Six melanoma, one Merkel cell.
Twelve patients received more than one anti-cancer treatment.
Patient enrolled in a double-blinded placebo-controlled trial.
Serological outcomes
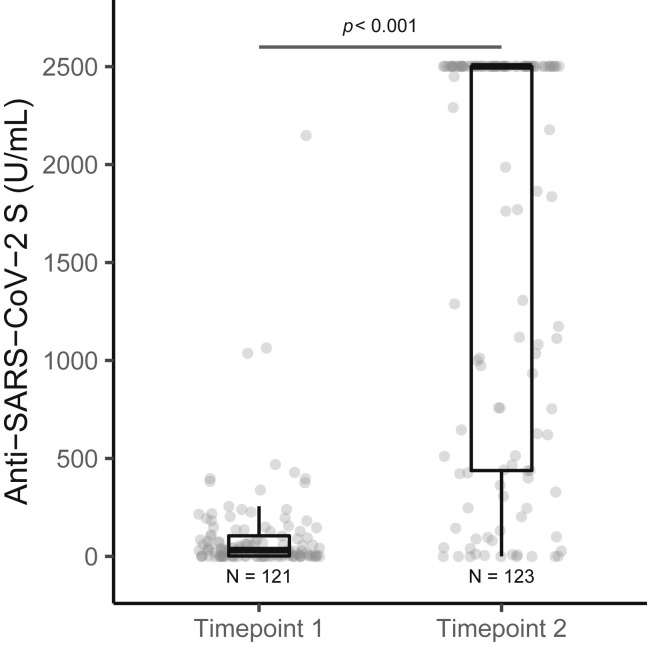
Overall, a high rate of seroconversion (anti-S IgG) (94%) was observed in our cohort of patients with cancer who received complete mRNA vaccination series. Seroconversion rate at time point 1 (after the first vaccine dose) was significantly lower compared with time point 2 (after the second vaccine dose), p < 0.001 (Figure 1 ). The seroconversion rates and antibody titers were significantly lower after the first vaccine dose compared with those after the second dose in all subgroups (Table 2 ). Antibody titers were significantly higher in females compared with males, but no other significant differences in seroconversion rates by age, sex, or race were noted. We did not observe statistically significant difference between the seroconversion rates (93% versus 95%, p = 0.678) and antibody titers (median, IQR: 1,232 [258–2,500] versus 2,500 [442–2,500], p = 0.254) after completion of vaccination series between BNT162b2 and mRNA-1273 vaccines, respectively (Figure S1).
Figure 1.
Differences in anti-SARS-CoV-2 S (anti-S) IgG titers following partial and complete vaccination
Anti-S antibody titers (U/mL) were significantly lower at time point 1 (post first vaccination dose) compared with time point 2 (post second vaccination dose). Number of patient samples assessed at time point 1 (121) and time point 2 (123). Boxplot showing median (horizontal bar), the 25th and 75th quartiles, and the error bars depicting largest and smallest values. Differences were assessed by Kruskal-Wallis test.
Table 2.
Serological outcomes after SARS-CoV-2 mRNA vaccination
| Seropositive |
Titer (U/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Time point 1 |
Time point 2 |
Time point 1 |
Time point 2 |
|||||
| n (%) | p value | n (%) | p value | Median (IQR) | p value | Median (IQR) | p value | |
| Overall | 98/121 (81%) | 116/123 (94%) | 0.002a,b | 32 (2–105) | 2,500 (438–2,500) | <0.001a,b | ||
| mRNA vaccine | ||||||||
| BNT162b2 | 24/29 (83%) | 1 | 28/30 (93%) | 0.678 | 29 (2–103) | 0.668 | 1,232 (258–2,500) | 0.254 |
| mRNA-1273 | 74/92 (80%) | 88/93 (95%) | 34 (3–106) | 2,500 (442–2,500) | ||||
| Age, years | ||||||||
| Younger than 65 | 54/64 (84%) | 0.359 | 64/66 (97%) | 0.248 | 34 (3–118) | 0.479 | 2,500 (506–2,500) | 0.254 |
| 65 and older | 44/57 (77%) | 52/57 (91%) | 31 (1–96) | 2,177 (401–2,500) | ||||
| Sex | ||||||||
| Male | 53/69 (77%) | 0.243 | 64/69 (93%) | 0.465 | 18 (1–74) | 0.09 | 1,762 (364–2,500) | 0.048b |
| Female | 45/52 (87%) | 52/54 (96%) | 44 (8–148) | 2,500 (840–2,500) | ||||
| Race/ethnicity | ||||||||
| Non-Hispanic white | 79/100 (79%) | 0.13 | 96/102 (94%) | 0.156 | 32 (2–106) | 0.688 | 2,500 (438–2,500) | 0.793 |
| Hispanic | 18/19 (95%) | 18/18 (100%) | 32 (5–125) | 2,396 (755–2,500) | ||||
| Black | 1/2 (50%) | 2/3 (67%) | 29 (15–44) | 1,770 (885–2,136) | ||||
| Type of malignancy | ||||||||
| Solid tumor | 80/96 (83%) | 0.252 | 99/101 (98%) | 0.002b | 44 (4–137) | 0.018b | 2,500 (514–2,500) | 0.029b |
| Hematological malignancy | 18/25 (72%) | 17/22 (77%) | 6 (0–33) | 832 (24–2,500) | ||||
| Anti-cancer therapy | 0.015b | <0.001b | 0.002b | 0.001b | ||||
| Clinical surveillance | 38/44 (86%) | 44/45 (98%) | 60 (5–185) | 2,500 (934–2,500) | ||||
| Cytotoxic | 20/29 (69%) | 28/30 (93%) | 4 (0–18) | 611 (160–1,956) | ||||
| Immunotherapy | 11/13 (85%) | 13/14 (93%) | 21 (4–43) | 1,116 (627–2,500) | ||||
| Endocrine therapy | 15/16 (94%) | 18/18 (100%) | 66 (30–137) | 2,500 (2,500–2,500) | ||||
| Anti-CD-20 antibody | 0/4 (0%) | 0/4 (0%) | <0.4 | <0.4 | ||||
| Anti-CD-38 antibody | 1/1 (100%) | 1/1 (100%) | 1 | 203 | ||||
| Anti-HER antibody | 2/2 (100%) | 1/1 (100%) | 18 (11–25) | 2,500 | ||||
| Anti-VEGF antibody | 4/5 (80%) | 5/5 (100%) | 3 (1–77) | 329 (82–2,500) | ||||
| RANKL antibody | 3/4 (75%) | 3/3 (100%) | 35 (21–64) | 2,500 (1,301–2,500) | ||||
| Kinase inhibitor | 13/15 (87%) | 12/13 (92%) | 51 (6–78) | 2,500 (439–2,500) | ||||
Time point 1, antibody measurement after partial vaccination (post first vaccine dose); time point 2, antibody measurement after complete vaccination (post second vaccine dose).
Comparison between two time points.
Statistically significant at α = 0.05.
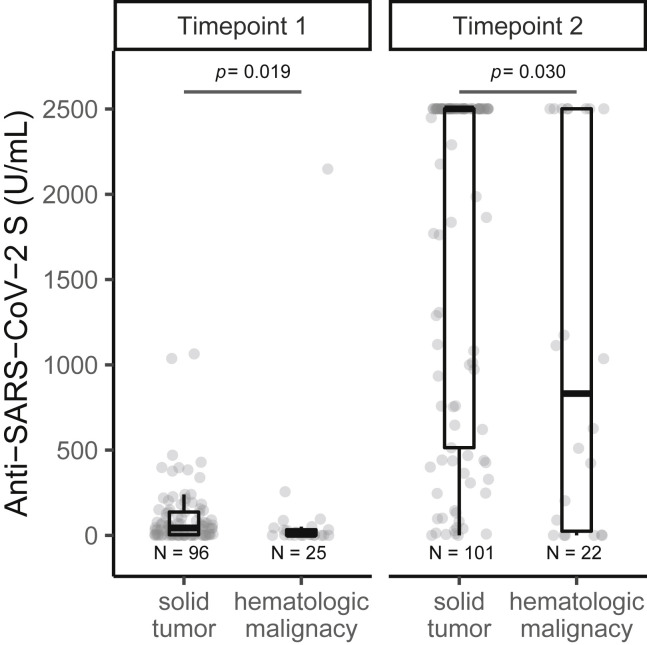
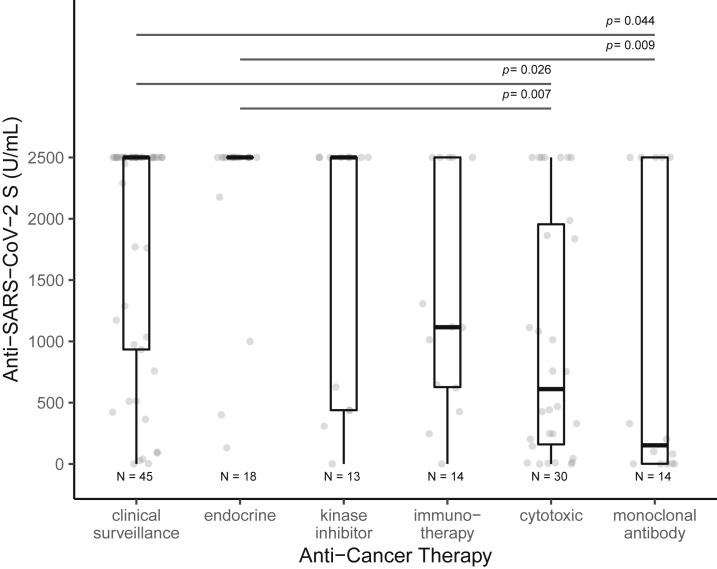
Patients with hematological malignancy had significantly lower rates of seroconversion (77% versus 98%, p = 0.002) and antibody titers (median, IQR: 832 [24––2,500] versus > 2,500 [514–2,500], p = 0.029) at time point 2 compared with those with solid tumors (Figure 2 ). Significant difference in antibody response was noted between the various anti-cancer treatment modalities (Figure 3 ). Patients receiving no therapy (i.e., clinical surveillance) or endocrine therapy had the best outcomes, with high seroconversion rates (98%–100%) and excellent median antibody titer (>2,500 U/mL), which was the upper limit of titer detection after completing vaccination series. Compared with those on clinical surveillance (median, IQR: 152 [2–2,500]), significantly lower levels of antibody titer were observed for those who received cytotoxic chemotherapy (611 [160–1,956], p = 0.019) and monoclonal antibody therapy (152 [2–2,500], p = 0.029) within 6 months before first vaccine dose (Table 2). None of the four patients receiving anti-CD-20 antibody showed seroconversion.
Figure 2.
Differences in anti-SARS-CoV-2 S (anti-S) IgG titers following partial and complete vaccination, stratified by type of cancer
Anti-S antibody titers (U/mL) were significantly lower in patients with hematological malignancy compared with those with solid tumor, at time point 1 (post first vaccination dose) and at time point 2 (post second vaccination dose). Boxplot showing median (horizontal bar), the 25th and 75th quartiles, and the error bars depicting largest and smallest values. Differences assessed by Kruskal-Wallis test.
Figure 3.
Differences in anti-SARS-CoV-2 S (anti-S) IgG titers following complete vaccination, stratified by anti-cancer treatment modality
Anti-S antibody titers (U/mL) after complete vaccination were significantly different among anti-cancer treatment groups. Significantly lower levels of antibody titers were observed for those on cytotoxic chemotherapy within 6 months before vaccination compared with those on clinical surveillance or endocrine therapy. Patients receiving monoclonal antibody treatment had the lowest antibody titers, and the difference was statistically significant when compared with antibody titers in those receiving endocrine therapy. Boxplots are shown and differences measured by Kruskal-Wallis test with Dunn's post-hoc test, corrected by the Benjamini-Hochberg method.
Trajectories of anti-S IgG for individual patients over the study time showed a drastic increase in antibody titers from partial to complete vaccination (Figure S2). None of the patients on the study tested positive for anti-N IgG while on the study, so no breakthrough SARS-CoV-2 infections during the study time period were noted in this cohort.
Patients without antibody response after two vaccine doses
A total of seven patients (6%) did not develop any antibodies at time point 2 after completing two doses of mRNA vaccines. A disproportionately higher proportion of the patients with no antibody response had hematological malignancy (5/7 [71%]) and all but one patient (6/7 [86%]) with non-response were either on cytotoxic chemotherapy or rituximab therapy within 6 months before vaccination.
Antibody response in patients with prior SARS-CoV-2 exposure
We examined antibody response after the first and second doses of vaccines in the subset of patients with prior SARS-CoV-2 infection who were excluded from the overall vaccine immunogenicity analysis (Table S1). Of these nine patients, six had received mRNA-1273 and three had received BNT-162b2. Most of the patients were older than 55 years (median, IQR: 56 years [56–69 years]), were female (67%), non-Hispanic white (78%), and had solid tumors (67%). We observed that pre-vaccination anti-S titer was low (132 [55–389]) in these patients but showed robust response after the first dose (2,238 [696–2,500]) and second dose (2,500 [1,376–2,500]), although statistical testing was not performed due to the small numbers (Figure S3).
Discussion
We present results of an international collaborative prospective cohort study at two cancer centers in the US and Switzerland assessing the humoral immune response using anti-S IgG as a surrogate in patients with solid and hematological malignancies who received mRNA vaccines. Although the seroconversion rates were low at 3–4 weeks after the first dose, the seroconversion rate was consistently high (94%) in the overall cohort at 3–4 weeks after receiving the second dose of the mRNA vaccine. Patients with hematological malignancy had significantly reduced humoral response compared with those with solid tumors. In fact, a subset of patients (e.g., those receiving anti-CD-20 antibody) did not develop any antibody response even after receiving two doses. In a small subset of patients with previous SARS-CoV-2 exposure, we also noted an increase in anti-S IgG antibody level from pre-vaccination to post-vaccination.
Given the high pressure posed by the pandemic and by evidence that patients with cancers are highly vulnerable to COVID-19 (Kuderer et al., 2020; Wang et al., 2021; Westblade et al., 2020), widespread vaccination campaign of patients with cancer has quickly taken off across the globe. While this strategy should be praised and promoted, little is known on the efficacy of vaccines in patients with cancer and about the impact that their anti-cancer treatments might have on the vaccine efficacy. Limited data on the level of seroconversion in patients with cancer after COVID-19 vaccination is summarized in Table 3 . Notably the anti-S IgG seroconversion rates were lower or less pronounced in patients with hematological conditions, in particular in patients treated with highly immune suppressive therapy, such as stem cell transplantation, anti-CD20 therapy, or chimeric antigen receptor-T cell therapy (Thakkar et al., 2021b). Small cohort studies have reported low seroconversion rates after a single dose of mRNA vaccination in the UK and France or while examining specific groups of immunocompromised patients (e.g., chronic lymphocytic leukemia, multiple myeloma) (Barrière et al., 2021; Monin et al., 2021). Within our cohort of 131 patients, the overall seropositivity rate was 81% after the first dose and up to 94% at 3–4 weeks after the second dose. No difference in seroconversion rates between the two vaccines were noted. Although not significant, there was a trend in higher antibody titers following mRN-1273 compared with BNT162b2, but this could be due to small sample size. However, the seroconversion rate was numerically lower in patients with hematological malignancy, 72% after partial vaccination and up to 77% after complete vaccination. None of the patients receiving anti-CD-20 therapy (0%, 4/4) produced any anti-S IgG antibodies despite receiving two doses of vaccine. Other treatments, including endocrine therapy or immunotherapy (immune checkpoint inhibitors) had no discernable impact on the seropositivity rates, with an overall seroconversion rate ranging from 90% to 95% in published studies that measured response at a minimum of 3 weeks after completion of vaccination series. As previously shown in other studies, to properly appreciate seroconversion rate, the timing of sampling is essential (Barrière et al., 2021; Bird et al., 2021; Monin et al., 2021; Palich et al., 2021). Testing for antibody levels at 3 weeks after only the first dose of vaccine provided only partial information, making it difficult to interpret or infer vaccine efficacy. On the contrary, waiting 3–4 weeks after the second dose for antibody measurement, as we did in our study, could provide more reliable information on the seroconversion rate and antibody titer level, thus offering a more comprehensive picture. We commend the studies that examined immunogenicity using an anti-S IgG, neutralization assay and T cell repertoire simultaneously (Monin et al., 2021), which provides more nuanced picture about the vaccination response.
Table 3.
Studies on Anti-SARS-CoV-2 spike IgG seroconversion after partial or complete vaccination in patients with cancer
| Study | Country | Cancer type | No. of patients assessed in the study | Vaccine | No. of vaccine doses received before antibody measurement | Days between the latest vaccine dose and antibody measurement | Anti-spike IgG antibody test platform | Seroconversion (number of patients, [%]) |
|---|---|---|---|---|---|---|---|---|
| Palich et al., 2021 | France | solid cancer | 95 | BNT162b2 | 1 | 21 | Abbott | 52 (55) |
| Monin et al., 2021 | UK | both | 100 | BNT162b2 | 1 | 21 | ELISA (in-house) | 29 (29) |
| 24 | BNT162b2 | 2 | 14 | 21 (87.5) | ||||
| Herishanu et al., 2021 | Israel | chronic lymphocytic leukemia | 167 | BNT162b2 | 2 | 0 | Elecsys | 66 (39.5) |
| Agha et al., 2021 | US | hematological malignancy | 67 | mRNA-1273 BNT162b2 | 2 | N/A | Beckman Coulter | 31 (46.3) |
| Bird et al., 2021 | UK | myeloma | 93 | BNT162b2 AZD1222 | 1 | 21 | Ortho Clinical Diagnostics Total Antibody Test | 65 (70) |
| Terpos et al., 2021 | Greece | myeloma | 44 | BNT162b2 | 1 | 21 | cPass NAbs Detection Kit | 9 (20.6) |
| Barrière et al., 2021 | France | solid cancer | 122 | BNT162b2 | 1 | 21–28 | Elecsys | 58 (47.5) |
| 42 | 2 | 15–27 | 40 (95.2) | |||||
| Thakkar et al., 2021a | US | both | 200 | BNT162b2 mRNA-1273 | 2 | 14 | Abbott | 109 (95) 58 (94) |
| AD26.COV2.S | 1 | 7 | 17 (85) | |||||
| Massarweh et al., 2021 | Israel | solid cancer | 102 | BNT162b2 | 2 | >19 | Abbott | 92 (90) |
| Addeo Shah et al. (this study) | Switzerland, US | both | 29 | BNT162b2 | 1 | 21 | Elecsys | 24 (83) |
| 30 | 2 | 29 | 28 (93) | |||||
| 92 | mRNA-1273 | 1 | 28 | 74 (80) | ||||
| 93 | 2 | 22 | 88 (95) |
N/A, not applicable.
Our data confirm the efficacy of the vaccine in triggering the humoral immune response in patients with cancer. On the other hand, it also reinforces the potential concern of inadequate protection in immunocompromised patients, especially those receiving anti-CD20 treatment, namely rituximab. There have been many publications highlighting the potential immunosuppressive activity of anti-CD20 therapy. Rituximab is a chimeric human-mouse monoclonal antibody used in the treatment of hematological malignancies and autoimmune diseases (https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5367s5388lbl.pdf; https://www.ema.europa.eu/en/medicines/human/EPAR/mabthera). It reacts specifically with the CD20 antigen expressed on more than 95% of normal and malignant B cells, inducing complement-mediated and antibody-dependent cellular cytotoxicity. Rituximab could indeed cause a rapid depletion of pre-B cells and mature B cells, which remain at low or undetectable levels for 2–6 months before returning to pretreatment levels, generally within 12 months. Growing evidence supports that rituximab might influence T cell immunity as well. Rituximab may cause immunosuppression through several mechanisms, such as delayed onset cytopenia, neutropenia in particular, if administered for long periods. It comes with no surprise that, in our study, patients receiving anti-CD-20 therapy did not develop any antibody titers for IgG-S. The optimal approach for vaccinating and monitoring this subset of patients at high risk for non-response to SARS-CoV-2 vaccines remains unclear. Although a possible strategy might be to withhold immunosuppressive treatment, such as anti-CD-20, until after the two doses of vaccines have been administered, when possible, a more evidence-based strategy would be preferable. For instance, the health authority in France has issued a statement suggesting a third dose of vaccine, 3–4 weeks after the second dose in immunocompromised patients, but data on implementation and outcomes of adopting such a strategy have not been published as yet. In addition, we observed that patients with prior SARS-CoV-2 exposure had low levels on anti-S antibody at baseline and showed a robust response after partial and complete vaccination. Despite small numbers, this signals vaccination benefit in patients with a history of COVID-19 and should be examined in a larger study.
Studying an international prospective cohort of vaccinated patients with cancer, we present data across diverse age groups, cancer types, cancer treatment types, which are representative of the patient populations cared for at our cancer centers. This provides a comprehensive assessment of immunogenicity after one and two doses of SARS-CoV-2 mRNA vaccines in patients with solid and hematological malignancy. Secondly, our results are consistent, irrespective of the vaccine type and the patient characteristics across centers, and in line with existing literature on seropositivity rates in similar populations. We assessed anti-N IgG at all the same pre-specified time points as anti-S IgG to ensure that no asymptomatic infection was overlooked. Furthermore, we reported response at 3–4 weeks after vaccination completion, a long duration of follow-up in vaccinated patients with malignancy.
Despite these strengths, there remain limitations due to the lack of corresponding data on cellular immunity for these patients. We acknowledge that this is an important component of the comprehensive examination of post-vaccine immune repertoire, so cell-mediated immune response analyses from this cohort are underway. A second potential limitation might be the utilization of anti-S IgG assay as a surrogate for COVID-19 immunity in lieu of neutralizing antibodies against SAS-CoV-2 virus; however, it is a reasonable scientific expectation that anti-spike antibody titers would be highly correlated with neutralizing antibody activity. Thus, given its high sensitivity, specificity, agreement with other platforms, low cost and labor requirement, technical ease, and faster turn-around time, we chose anti-S IgG assay for this study, which can allow validation of these results in different population-based vaccine response studies (Alvim et al., 2020; Mazzini et al., 2021). The upper limit of antibody titer measurements was capped at 2,500 U/mL, so the differences between various groups in our study could potentially be larger than observed here. Furthermore, we did not have a centralized laboratory for analysis of antibodies; however, this test has been validated in multiple studies and we did not identify a signal for center level differences in our results. Accurate surrogates for protection in the clinical setting remain to be established. Finally, due to our geographical location and time constraints, the cohort has inadequate representation of certain minority patients (e.g., black, Asian, etc.) (Schmidt et al., 2020), individual cancer types, and cancer treatments. The findings based on a small number of minority patients were not statistically significant and need to be interpreted with caution. We hope that this gap in knowledge will be addressed through a larger multi-national collaborative effort to validate and expand on our study findings.
To summarize, our study documents that the vast majority of patients with cancer develop positive anti-SARS-CoV-2 spike antibody response at 3 weeks post-completion of mRNA vaccination series, hence administration of both doses is recommended. Our results stress the importance of identifying patients at high risk of non-response post-vaccination, so alternate protection strategy can be developed.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Serum sample | Patients recruited in this study | In this study |
| Critical commercial assays | ||
| Elecsys® Anti-SARS-CoV-2 Nucleocapsid | Roche | Catalog number 7304 |
| Elecsys® Anti-SARS-CoV-2 Spike | Roche | Catalog number 3608 |
| Deposited data | ||
| Computer code | Github | https://github.com/pankil-shah/cancer_cell_covid_vaccine |
| Software and algorithms | ||
| R 4.0.5 | https://www.r-project.org/ | https://www.r-project.org/ |
| Other | ||
| Clinical data | Electronic medical record | Study ID |
Resource availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dimpy Shah, shahdp@uthscsa.edu.
Materials availability
This study did not generate new unique reagents.
Data and code availability
The published article includes all data generated and analyzed during this study. Data will be made available freely from the corresponding authors upon request. The utilized computer code has been deposited in GitHub (https://github.com/pankil-shah/cancer_cell_covid_vaccine). All analyses were conducted with built-in and freely available R packages.
Experimental model and subject details
Patient data collection
This study was approved by institutional review boards at each institution. We performed a prospective observational cohort study on patients with cancer who received mRNA-1273 or BNT162b2 vaccine at University Hospital of Geneva (HUG) and Mays Cancer Center at University of Texas Health San Antonio MD Anderson (MCC) between January 29, 2021, and April 24, 2021. Vaccination series was administered as per the manufacturer guidelines (gap between first and second dose was 21 days for mRNA-1273 and 28 days for BNT162b2). Participants were enrolled in the study by signing an informed consent. The inclusion criteria consisted of adult patients (age 18 years or older), eligible to receive COVID-19 vaccination, diagnosed with any malignancy with the exception of early-stage squamous cell skin cancer, early-stage basal cell skin carcinoma and non-invasive pathology such as Ductal Carcinoma in-situ (DCIS). Patients who were currently receiving anti-cancer treatment or had received active treatment within the last 5 years, were eligible. Exclusion criteria included a laboratory confirmed diagnosis of SARS-CoV-2 exposure either by polymerase chain reaction or serology, previous enrollment in a COVID-19 vaccine trial, pregnancy or breastfeeding, and unable to comply with study-related procedures. Clinical characteristics were collected by clinical chart review at each center using same definitions. Blood samples are collected at the time of the first vaccine dose (baseline), at the time of the second vaccine dose which was equivalent to 3 weeks after first dose of BNT162b2 and 4 weeks after first dose of mRNA-1273 (time point 1) and at 3 weeks after second dose of mRNA-1273 or 4 weeks after second dose of BNT162b2 (time point 2). Here, we are reporting on all available serum samples from baseline, time point 1, and time point 2. These samples were tested for both anti-SARS-CoV-2 spike (S) IgG and nucleocapsid (N) IgG titers. The current study has two primary outcomes: 1) rates of seroconversion to the SARS-CoV-2 S protein; and 2) anti-S antibody titer levels in patients with cancer following first and second dose of vaccination with BNT162b2 or mRNA-1273.
Method details
Anti-SARS-CoV-2 spike IgG and nucleocapsid IgG assays
Blood samples collected using standard sampling tubes were directly centrifuged, and serum was stored at −80C until batch analysis in US and Europe, respectively. The immunogenicity of mRNA vaccines was assessed by Elecsys Anti-SARS-CoV-2 S immunoassay for the in vitro quantitative determination of antibodies (including IgG) to the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) in human serum and plasma (Elecsys Anti-SARS-CoV-2 S. Package Insert, 2020-09, V1.0; Material Numbers 09289267190 and 09289275190). The assay uses a recombinant protein representing the RBD of the S antigen in a one-step double-antigen sandwich (DAGS) assay format, which favors detection of high affinity antibodies against SARS-CoV-2. The test is intended as an aid to assess the adaptive humoral immune response to the SARS-CoV-2 S protein. Briefly, patient samples are incubated with a mix of biotinylated and ruthenylated RBD antigen. After addition of streptavidin-coated microparticles, the DAGS complexes bind to the solid phase via interaction of biotin and streptavidin. The reagent mixture is transferred to the measuring cell, where the microparticles are magnetically captured onto the surface of the electrode. Unbound substances are subsequently removed. Electrochemiluminescence is then induced by applying a voltage and measured with a photomultiplier. The signal yield increases with the antibody titer. Using internal Roche standard for anti-SARS-CoV-2-S consisting of monoclonal antibodies, 1 nM antibodies correspond to 20 U/mL of the Elecsys Anti-SARS-CoV-2 S assay. The cutoff value for this assay is 0.8 U/mL with <0.8 U/mL values reported as negative, and the maximum value is 2500 U/mL. This threshold resulted in a sensitivity of 98.8% (95% CI: 98.1–99.3%) in 1,610 samples from a cohort of 402 symptomatic patients with PCR confirmed SARS-CoV-2 infection and a specificity of 99.98% (95% CI: 99.91–100%) in a cohort of 5991 samples from pre-pandemic routine diagnostics and blood donors (Elecsys Anti-SARS-CoV-2 S. Package Insert, 2020-09, V1.0; Material Numbers 09289267190 and 09289275190). Total antibodies against the N antigen of SARS-CoV-2 were measured on a Cobas e801 analyzer (Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturer's instructions. Results are reported as numeric values in form of a cut-off index (signal sample/cutoff or signal calibrator ratio) and are considered as positive when equal to or above 1.
Quantification and statistical analysis
After excluding patients with previous SARS-CoV-2 exposure based on positive anti-N IgG test at baseline, all remaining eligible patients with available samples and data were included in the immunogenicity analyses. For the primary analysis, we assessed seroconversion rates (number of patients with positive anti-SARS-CoV-2 S IgG antibody divided by the number of patients assessed) at time point 1 (post first vaccine dose) and time point 2 (post second vaccine dose). The differences in seroconversion rates by number of vaccine doses, age, sex, race/ethnicity, vaccine type, cancer type, and anti-cancer treatment modality were compared by Fisher exact test, corrected by Benjamini-Hochberg method. We also compared differences in anti-S antibody titers by number of vaccine doses, age, sex, race/ethnicity, vaccine type, cancer type, and anti-cancer treatment modality using Kruskal-Wallis Rank-Sum test with Dunn's post-hoc test, corrected by Benjamini-Hochberg method. We also present the change in antibody response from pre-vaccination to post-vaccination in the subset of patients with prior SARS-CoV-2 exposure that were excluded from the overall immunogenicity analysis; however, statistical analysis was not performed. Statistics were computed in R, version 4.0.5 (R Core Team, 2021).
Acknowledgments
This project has been funded in whole or in part with federal funds from National Cancer Institute grant P30 CA054174 (Mays Cancer Center at University of Texas Health San Antonio MD Anderson); and grant MRSG-16-152-01-CCE the American Cancer Society and Hope Funds for Cancer Research. A “Research & Development Program” grant from Geneva University Hospitals awarded to N.B. funded part of this study. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We thank Sebastien Bugeia, Garance Gutknecht, and Anna Battagin from HUG, and the University of Texas Health Biospecimen and Translational Genomics Core Laboratory for support with the enrollment and follow-up of participants and processing of specimens. We also thank Brandon Wing, Nazneen Ali, and Adrian Tan from UTHSA for their health informatic support.
Author contributions
A.A., P.K.S., and D.P.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.A. and P.K.S. contributed equally. K.L., N.M., and D.P.S. are senior authors. Concept and design, A.A., P.K.S., V.K., R.M., R.J.L., K.L., and D.P.S.; acquisition, analysis, or interpretation of data, A.A., P.K.S., R.D.H., N.B., N.M., S.F., P.-F.S., M.D.M., P.-Y.D., B.A., D.P., J.W., R.J.L., K.L., and D.P.S.; statistical analysis, P.K.S. and D.P.S.; drafting of the manuscript, A.A., P.K.S., and D.P.S.; critical revision of the manuscript for important intellectual content, all co-authors; funding acquisition, A.A., N.M., P.D., R.M., and D.P.S.; administrative, technical, or material support, R.D.H., B.A., D.P., J.W., R.J.L., and M.D.M.; supervision, A.A., N.M., P.K.S., J.W., R.J.L., K.L., and D.P.S.
Declaration of interests
A.A. reported receiving personal fees for attending advisory from Bristol-Myers Squibb, AstraZeneca, Roche, Pfizer, Merck Sharp and Dohme, Astella, Eli Lilly, and Boehringer Ingelheim and receiving fees for speaking bureau for Eli Lilly, AstraZeneca, Merck Sharp and Dohme for work performed outside of this study. P.S. reported receiving a grant from the Biomedical Advanced Research and Development Authority outside of this work. I.L.-G. reported receiving personal fees for attending advisory from AstraZeneca. N.M. is a founder and minority shareholder of MaxiVAX SA, a private biotech company based in Geneva, Switzerland, working on personalized cancer immunotherapy and infectious disease vaccines, with no impact on the current manuscript. R.M. reported receiving research support from Celgene, Incyte, Abbvie, Samus, Genotech, Promedior, and CTI; and consulting fees from Novartis, Sierra Onc, LaJolla, and Pharma. D.S. reported receiving a grant from the Biomedical Advanced Research and Development Authority outside of this work. All other co-authors reported no competing interests.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of study participants. We worked to ensure ethnic or other types of diversity in the recruitment of study participants.
Published: August 9, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ccell.2021.06.009.
Supplemental information
References
- Agha M., Blake M., Chilleo C., Wells A., Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021 doi: 10.1101/2021.04.06.21254949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvim R.G.F., Lima T.M., Rodrigues D.A.S., Marsili F.F., Bozza V.B.T., Higa L.M., Monteiro F.L., Abreu D.P.B., Leitão I.C., Carvalho R.S., et al. An affordable anti-SARS-COV-2 spike protein ELISA test for early detection of IgG seroconversion suited for large-scale surveillance studies in low-income countries. medRxiv. 2020 doi: 10.1101/2020.07.13.20152884. [DOI] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl. J. Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouny Z., Hawley J.E., Choueiri T.K., Peters S., Rini B.I., Warner J.L., Painter C.A. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., Petit E., Leysalle A., Raimondi V., Carles M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021;S0923-7534:01183–01184. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird S., Panopoulou A., Shea R.L., Tsui M., Saso R., Sud A., West S., Smith K., Barwood J., Kaczmarek E., et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021 doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese C.R., Choueiri T.K., Duma N., Farmakiotis D., Grivas P., Rini B.I., Shah D.P., Thompson M.A., Pergam S.A., Mishra S., et al. Care without a compass: including patients with cancer in COVID-19 studies. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas P., Khaki A.R., Wise-Draper T.M., French B., Hennessy C., Hsu C.Y., Shyr Y., Li X., Choueiri T.K., Painter C.A., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., Morales Moshiashvili M., Ziv-Baran T., Shorer Y., Scarfo L., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021 doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.-Y., Desai A., de Lima Lopes G., Jr., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarweh A., Eliakim-Raz N., Stemmer A., Levy-Barda A., Yust-Katz S., Zer A., Benouaich-Amiel A., Ben-Zvi H., Moskovits N., Brenner B., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L., Martinuzzi D., Hyseni I., Benincasa L., Molesti E., Casa E., Lapini G., Piu P., Trombetta C.M., Marchi S., et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J. Immunological Methods. 2021;489:112937. doi: 10.1016/j.jim.2020.112937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palich R., Veyri M., Marot S., Vozy A., Gligorov J., Maingon P., Marcelin A.G., Spano J.P. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann. Oncol. 2021 doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. New Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021 https://www.R-project.org/ [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. New Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A.L., Bakouny Z., Bhalla S., Steinharter J.A., Tremblay D.A., Awad M.M., Kessler A.J., Haddad R.I., Evans M., Busser F., et al. Cancer care disparities during the COVID-19 pandemic: COVID-19 and cancer outcomes study. Cancer Cell. 2020;38:769–770. doi: 10.1016/j.ccell.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Trougakos I.P., Gavriatopoulou M., Papassotiriou I., Sklirou A.D., Ntanasis-Stathopoulos I., Papanagnou E.-D.D., Fotiou D., Kastritis E., Dimopoulos M.A. Low neutralizing antibody responses against SARS-CoV-2 in elderly myeloma patients after the first BNT162b2 vaccine dose. Blood. 2021 doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar A., Gonzalez-Lugo J.D., Goradia N., Gali R., Shapiro L.C., Pradhan K., Rahman S., Kim S.Y., Ko B., Sica R.A., et al. Seroconversion rates following COVID-19 vaccination amongst patients with cancer. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar A., Pradhan K., Jindal S., Cui Z., Rockwell B., Shah A.P., Packer S., Sica R.A., Sparano J., Goldstein D.Y., et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat. Cancer. 2021;2:392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westblade L.F., Brar G., Pinheiro L.C., Paidoussis D., Rajan M., Martin P., Goyal P., Sepulveda J.L., Zhang L., George G., et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer cell. 2020;38:661–671.e662. doi: 10.1016/j.ccell.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data generated and analyzed during this study. Data will be made available freely from the corresponding authors upon request. The utilized computer code has been deposited in GitHub (https://github.com/pankil-shah/cancer_cell_covid_vaccine). All analyses were conducted with built-in and freely available R packages.