Abstract
Glutamate delta 1 (GluD1) and glutamate delta 2 (GluD2) form the delta family of ionotropic glutamate receptors; these proteins plays widespread roles in synaptic architecture, motor behavior, and cognitive function. Though the role of GluD2 at cerebellar parallel fiber-Purkinje cell synapses is well established, attention now turns to the function of GluD receptors in the forebrain. GluD1 regulates synaptic assembly and modulation in multiple higher brain regions, acting as a postsynaptic cell adhesion molecule with effects on both excitatory and inhibitory transmission. Furthermore, variations and mutations in the GRID1 gene, which codes for GluD1, and in genes which code for proteins functionally linked to GluD1, are associated with mental disorders including autism, schizophrenia, bipolar disorder, and major depression. Cerebellin (Cbln) family proteins, the primary binding partners of delta receptors, are secreted C1q-like proteins which also bind presynaptic neurexins (NRXNs), forming a tripartite synaptic bridge. Published research explores this bridge’s function in regions including the striatum, hippocampus, cortex, and cer-ebellum. In this review, we summarize region- and circuit-specific functions and expression patterns for GluD1 and its related proteins, and their implications for behavior and disease.
Introduction
Decades of research on ionotropic glutamate receptors (iGluRs) have mapped out some of the central nervous system’s most fundamental machinery. Exhibiting widespread and diverse expression, these chemically gated ion channels are primarily responsible for excitatory transmission in the brain. Their dysfunction is implicated in a plurality of neuropathic disorders, and their various subtypes and subunits are targets for hundreds of drugs. Though new discoveries continue to unfold, the roles of NMDA, AMPA, and kainate receptors, as well as their metabotropic cousins, the mGluRs, in synaptic transmission and plasticity are now well established. However, the role of glutamate delta receptors in the forebrain awaits broader awareness, and though related structurally to other iGluRs, GluD receptors neither bind glutamate nor function as typical ligand-gated ion channels (Kristensen et al., 2015; Lomeli et al., 1993; Naur et al., 2007).
There are two GluD subtypes: GluD1 and GluD2. The two receptors share 56% sequence homology (Lomeli et al., 1993). Expression of GluD2 is highest in the cerebellum where it regulates motor function (Hepp et al., 2015; Konno et al., 2014; Lomeli et al., 1993; Yuzaki and Aricescu, 2017). GluD1, on the other hand, is enriched throughout the forebrain across myriad neuronal and synaptic subtypes (Hepp et al., 2015; Konno et al., 2014; Nakamoto et al., 2019b); and is implicated in behavioral plasticity and numerous higher-order functions including sociability, memory, and addiction (Liu et al., 2018; Nakamoto et al., 2019a; Yadav et al., 2012; Yadav et al., 2013). Extracellular domains of GluD receptors consist of an amino terminal domain (ATD) and a ligand binding domain (LBD) which bears similarity to the LBD of the NMDA receptor GluN1 subunit, binding D-serine and glycine (Kristensen et al., 2015; Naur et al., 2007). Here, another point of divergence between GluD receptors and other iGluRs should be noted. While the fourfold subunit structures of other iGluRs exhibit “domain-swapping” between ATD and LBD regions, GluD receptors exhibit no such domain swapping (Burada et al., 2020a, b). The transmembrane regions of GluD1 and GluD2 share their 4-fold symmetrical structure with other iGluRs, but in recordings from heterologous expression systems as well as in situ, their ion channel gate does not appear to open upon ligand binding like other iGluRs. As for the C-termini of delta receptors, their potential intracellular signaling cascades are not thoroughly mapped, but they are important for receptor function. For example, the C-terminus of GluD2 was shown essential for cerebellar LTD, and controls phosphorylation at specific AMPA receptor tyrosine residues (Kohda et al., 2013). The C-terminus of GluD1 was also found to be essential for synaptic trafficking, but not surface expression. Significantly, although the C-terminus of GluD1 contains a PDZ-binding motif, it was reported to only bind weakly with PSD95, an excitatory postsynaptic marker which is known to bind such motifs and thereby assist in the trafficking of other ionotropic glutamate receptors (Chung et al., 2004; Cui et al., 2007; Tao et al., 2019). Other proteins, including Shank, PICK1, PSD93 and EMAP; also interact with the C-termini of GluD1 and/or GluD2 (Ly et al., 2002; Roche et al., 1999; Uemura et al., 2004; Yawata et al., 2006).
Though the delta receptors do not appear to function as typical ligand-gated ion channels, the channel pores of GluD1 and GluD2 are functional. A naturally occurring mutation in a mouse strain termed “Lurcher” causes spontaneous channel openings resulting in membrane constitutive currents in GluD2 (GluD2Lc). The GluD2Lc has a single nucleotide change resulting in the A654T mutation in the transmembrane domain 3 of GluD2, the region that forms the conserved activation gate for iGluRs (Zuo et al., 1997). When the effects of D-serine and glycine were tested against GluD2Lc, these agonists were found to inhibit the constitutive currents through GluD2Lc (Naur et al., 2007). It has been proposed that this effect is analogous to receptor desensitization (Hansen et al., 2009). Alanine-scanning mutagenesis revealed that the activation gate is also conserved in GluD1; F655A and other mutants lead to constitutively open channels (Yadav et al., 2011). D-serine and glycine only had modest effects on these mutants, but chimeric receptors partly composed of both GluD1 and GluD2 subunits with the lurcher mutation are sensitive to D-serine inhibition. Although D-serine or glycine do not lead to typical ligand-gated ion channel currents through GluD receptors, D-serine binding to GluD2 is required for long-term depression at the parallel fiber-Purkinje cell synapse (Kakegawa et al., 2011). The effect of D-serine and glycine binding to GluD1 remains unclear. There is also evidence showing ion channel activity of GluD1 and GluD2 in response to mGluR1/5 activation (Ady et al., 2014; Benamer et al., 2017; Dadak et al., 2017). This finding is recently supported by an innovative approach using optopharmacological tools to cause GluD2 pore block and unblock using 535 nm and 380 nm light on a cysteine mutant version. Light-induced GluD2 channel closure reduced currents induced by mGluR1/5 activation in both overexpression system and native cerebellar Purkinje neurons (Lemoine et al., 2020). Another recent study also provides evidence that activation of α1 adrenergic receptors induces ionic currents through GluD1 in dorsal raphe nucleus neurons (Gantz et al., 2020).
While the atypical ion channel activity of GluD receptors awaits further exploration, evidence is accumulating for their synaptogenic function, bringing wider implications for neural circuit and behavioral regulation. Critical for these synaptogenic functions are the GluD amino terminal domains and their interactions with cerebellin family proteins, which we will explore in the following sections.
The GluD tripartite bridge
Following the development of the GluD2 knockout model, it was established that GluD2 ablation results in severe cerebellar synaptic and behavioral phenotypes (Yuzaki and Aricescu, 2017). Situated on the dendrites of Purkinje cells, GluD2 mediates incoming excitatory projections from parallel fibers (PF-PC synapses), while also recruiting postsynaptic components and mediating long-term depression (LTD) (Yuzaki and Aricescu, 2017). Free dendritic spines (spines without presynaptic formation), reduction in total PF-PC synapses, and loss of LTD are well documented following GluD2 knockout, as well as motor deficits typical of cerebellar dysfunction (Kakegawa et al., 2009; Kashiwabuchi et al., 1995).
Cbln1, a member of the C1q-TNF (complement component 1q-tumor necrosis factor) superfamily has also been studied for its role in the cerebellum. C1q, a protein with a key role in an immune process dubbed “complement cascade”, shares its highly conserved globular head region with a host of proteins including the secreted proteins cerebellins (Cblns) (Bao et al., 2005; Cheng et al., 2016; Hirai et al., 2005; Miura et al., 2006; Yuzaki, 2017). Each of the four Cbln proteins shows a distinct expression pattern (Miura et al., 2006). Whereas Cbln3 is only expressed significantly in the cerebellum, the region in which these proteins were first studied, Cbln1, 2, and 4 are selectively expressed throughout the brain, with some overlap (Miura et al., 2006; Otsuka et al., 2016; Seigneur and Südhof, 2017). Cbln1 knockout has been studied in multiple regions, including the cerebellum, hippocampus, and striatum (Kusnoor et al., 2010; Miura et al., 2009; Otsuka et al., 2016). Most interestingly, Cbln1 knockout produces synaptic and behavioral changes, often phenotypically overlapping with GluD2 knockout, providing the first clues of some interaction between the two molecules (Hirai et al., 2005; Miura et al., 2009; Rong et al., 2012).
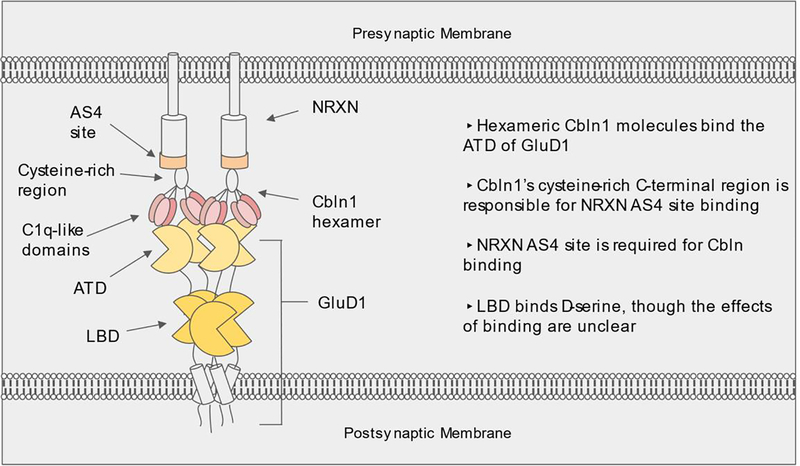
When it was discovered that Cbln1 and GluD2 are binding partners, GluD2’s role as part of a synaptic adhesion complex came into focus. GluD2 is the postsynaptic component in a tripartite trans-synaptic bridge, and GluD1 participates in similar structures. These so-called bridges are composed of presynaptic neurexins (NRXN) which bind with secreted cerebellins (Cbln), which in turn bind to the amino terminal domain (ATD) of their postsynaptic targets, the GluD receptors (Hirai et al., 2005; Matsuda et al., 2010; Uemura et al., 2010). Presynaptic NRXN molecules participating in this bridge require a specific 30-amino acid insert at the AS4 splice region in order to bind with Cbln proteins (Lukacsovich et al., 2019; Matsuda et al., 2010; Uemura et al., 2010) (Figure 1). Neurexin molecules containing this site are hereafter referred to as “AS4+ NRXN”.
Figure 1.
The tripartite synaptic bridge is composed of; (i) AS4-positive NRXN anchored in the presynaptic membrane, (ii) Secreted Cbln hexamers joining the presynaptic (NRXN) and postsynaptic (GluD) bridge components, and (iii) a GluD receptor binding Cbln at its amino terminal domain (ATD).
Structurally, Cbln proteins are composed of a C-terminal C1q-like domain and an N-terminal cysteine-rich region (Figure 1). These proteins form trimers, then homohexamers via disulfide bonds in the N-terminal cysteine-rich region (Bao et al., 2005; Elegheert et al., 2016) (Cheng et al., 2016). As illustrated in Figure 1, Cbln1, 2, and 4 have all been shown to bind both postsynaptic GluD receptors and presynaptic NRXN molecules, although Cbln4 has higher affinity towards presynaptic deleted in colorectal cancer (DCC) protein (Haddick et al., 2014; Joo et al., 2011; Matsuda et al., 2010; Tao et al., 2018; Yasumura et al., 2012). Given the widespread prevalence of various subtypes of NRXNs, Cblns, and GluD receptors throughout the brain, and the modular nature of the tripartite synaptic bridges, a potential synaptic assembly code dictated by the regional and cellular expression and splicing patterns of these proteins can be envisioned.
Expression of GluD receptors and function as synaptic organizers
Expression studies in early 1990s demonstrated that GluD1 was widely expressed in the central nervous system at an early postnatal age (Lomeli et al., 1993; Mayat et al., 1995). Since then, RT-PCR, in-situ hybridization and other techniques have revealed high mRNA and protein expression of GluD1 in many forebrain regions (Hepp et al., 2015; Konno et al., 2014; Nakamoto et al., 2019b; Yadav et al., 2013).
Two 2014 studies published region-by-region expression patterns for GluD1. The first by Konno and colleagues developed a specific antibody to a C-terminal region of GluD1 (Konno et al., 2014). They found high levels of GluD1 in the cerebral cortex, striatum, nucleus accumbens, bed nucleus of stria terminalis, lateral habenula, central amygdala, hippocampus, olfactory bulb, and cerebellar cortex. The widespread expression of GluD1 in the adult brain was corroborated by Hepp et al., who reported a similar GluD1 expression pattern as Konno and others (Hepp et al., 2015). Nakamoto and colleagues more recently published consistent data (Nakamoto et al., 2019b). Forebrain expression of GluD2 has also been reported, though its function has not been studied (Hepp et al., 2015). It is unknown whether GluD1 and GluD2 form heteromeric complexes, but the proteins are often present together in the same postsynaptic site, and may co-immunoprecipitate due to common interactions with scaffolding proteins and synaptic adhesion molecules (Nakamoto et al., 2019b).
Though ionotropic functions for GluD receptors exist, it is appropriate to examine GluD receptors and Cblns alongside postsynaptic assembly molecules like neuroligins. Firstly, GluD protein expression patterns often complement the expression patterns of Cbln proteins (Hepp et al., 2015; Konno et al., 2014; Miura et al., 2006; Otsuka et al., 2016; Seigneur and Südhof, 2017). Secondly, within any given region they are present, GluD receptors appear to designate specific synaptic targets for inputs expressing Cblns, and potentially AS4+ NRXNs. This can be readily observed in the hippocampus, for example, where projections from the entorhinal cortex expressing Cblns form synapses with dendrites of granule cells in the dentate molecular layer and CA pyramidal cells in the stratum lacunosum moleculare (SLM) where GluD1 is expressed (Hepp et al., 2015; Konno et al., 2014; Otsuka et al., 2016).
The classic example of the tripartite synaptic bridge arrangement, however, is observed in the cerebellum, where GluD2 was first studied (Figure 2). At parallel fiber-Purkinje cell (PF-PC) synapses, GluD2 expressed on the dendrites of Purkinje cells binds with Cbln1 secreted from parallel fibers and accumulates postsynaptic scaffolding proteins, regulating LTD and excitatory synapse formation. (Kashiwabuchi et al., 1995; Yuzaki and Aricescu, 2017). Functioning as a synaptic assembly molecule, the presence of GluD2 on PC dendrites also enables these synapses to regenerate following parallel fiber transection, even in adult mice (Ichikawa et al., 2016). GluD1, on the other hand, is expressed on both dendrites and soma of multiple classes of cerebellar interneurons, including stellate, basket, and Golgi cells. GluD1 appears to mediate parallel fiber-interneuron synapses. GluD1 knockout causes a significant reduction in parallel fiber-interneuron soma synapses, interneuron soma size, and total parvalbumin (PV)-positive interneuron count (Konno et al., 2014).
Figure 2:
Schematic showing GluD and Cbln1 function in cerebellum. Cbln1 secreted from parallel fibers synaptogenically engages with GluD1 expressed on cerebellar interneurons and GluD2 expressed on Purkinje cell dendrites.
There are also instances of Cbln isoform segregation where inputs to a specific region from one source express one isoform of Cbln, while inputs from another source express another isoform. One example is seen in the dorsal striatum where Cbln1 characterizes thalamo-striatal projections, while Cbln2 characterizes cortico-striatal projections (Kusnoor et al., 2010; Liu et al., 2020; Seigneur and Sudhof, 2017). Nevertheless, the knowledge of how exactly differential Cbln isoform expression affects neurodevelopment and synaptic specification is still in its infancy. We can, however, gain insight by examining the region-by-region effects of experimentally manipulating Cblns and GluD receptors.
Dorsal striatum: a model for input specificity
The striatum displays rich GluD1 expression, primarily on the dendritic shafts of medium spiny neurons (Liu et al., 2020). GluD1 in the striatum also preferentially colocalizes with vGluT2, a protein present in thalamo-striatal projections, contrasting vGluTl-positive cortico-striatal projections (Liu et al., 2020). The microzonal placement of GluD1 within the striatum varies by species, as was demonstrated by a recent study contrasting mouse and monkey brains. They found GluD1 in the mouse dorsal striatum to favor matrix compartments, whereas GluD1 in monkey striatum favored striosome compartments (Hoover et al., 2020). GluD2 is also present at low levels, but its function is not yet reported (Hepp et al., 2015).
Striatal medium spiny neurons (MSNs) produce no detectable Cbln proteins, though they may be expressed in interneurons (Seigneur and Sudhof, 2017). Cbln proteins are, however, brought into the striatum by afferents which produce them. The parafascicular thalamus (Pf), a main source of thalamo-striatal input, produces Cbln1, 2, and 4. Of these, at least Cbln1 likely mediates Pf-MSN connections via GluD1 (Kusnoor et al., 2010; Liu et al., 2020). Based on reporter model analysis, cortico-striatal inputs appear to be characterized by Cbln2, but its function in these projections is not yet understood (Seigneur and Sudhof, 2017).
Regarding the tripartite bridge’s function in the striatum, clues can be drawn from experimental GluD1 and Cbln knockout. In a striatum-specific conditional GluD1 knockout model, mEPSC (miniature excitatory postsynaptic current) frequency in striatal MSNs is lower (Liu et al., 2020). Thalamo-striatal vGluT2-positive terminals are also reduced. In contrast, vGluT1 levels are unchanged, demonstrating a specific reduction in thalamic inputs. Finally, optogenetically stimulated Pf-MSN synapses show an increased AMPA:NMDA ratio (Liu et al., 2020). In contrast, cortico-striatal synapse function remains unchanged. Behaviorally, higher cocaine-conditioned place-preference was seen in striatal GluD1 KO but not cortico-limbic GluD1 KO (Liu et al., 2018). Furthermore, in a water T maze test measuring an animal’s ability to acquire new spatial memory in response to shifting submerged escape platform location, striatal but not corticolimbic GluD1 deletion alters behavioral flexibility (Liu et al., 2020). The number of preservative errors in which the mouse reaches for the same arm where the platform is originally located are higher in striatal GluD1 KO, and longer training is needed to achieve full reversal of the original learning.
Cbln knockout mice exhibit phenotypes slightly different from GluD1 knockout. Contrasting GluD1 KO, striatal vGluT2 levels remain steady in Cbln1 KO mice (Otsuka et al., 2016; Seigneur and Südhof, 2018). Nevertheless, Kusnoor et al. found all retrograde labeled cells from the Pf to express Cbln1 (Kusnoor et al., 2010). Signeur and Südhof corroborated these findings, reporting thalamo-striatal projections containing Cblns 1, 2, and 4 (Seigneur and Südhof, 2017; Seigneur and Südhof, 2018), so perhaps other Cbln proteins compensate in the case of Cbln1 deletion. Cbln1 was shown, however, to play an essential role in synaptic pruning, with increased MSN dendritic spine density in Cbln1-null mice (Kusnoor et al., 2010). Signeur and Südhof also found NMDA receptor GluN1 subunit levels to increase in the dorsal striatum following Cbln1 KO (Seigneur and Sudhof, 2018). As stated previously, the role of Cbln2 in the striatum is elusive, and its knockout produces none of the synaptic effects seen in Cbln1 KO (Rong et al., 2012). Furthermore, although Cbln2 is able to bind GluD1, it is likely not the primary binding partner of GluD1 in the striatum, as cortico-striatal synapses remain largely unchanged in cases of striatum-specific GluD1 knockout (Liu et al., 2020).
Diseases relevant to the dorsal striatum include autism, schizophrenia, and Parkinson’s disease, the first two of which are genetically correlated with GRID1 variants (Glessner et al., 2009; Griswold et al., 2012; Guo et al., 2007; Nord et al., 2011; Smith et al., 2009; Treutlein et al., 2009). In autism research, investigators continually highlight striatal abnormalities as a characteristic trait of the disorder. As seen in GluD1 KO, many mouse models of ASD (autism spectrum disorder)-associated genes show synaptic and anatomical changes in the striatum (Fuccillo, 2016). GluD1 and Cbln1 join the list of synaptic proteins which genetically correlate with autism, including Shank proteins, contactin associated proteins, and neurexins (Clarke et al., 2012; Jones et al., 2013; Monteiro and Feng, 2017; Peñagarikano et al., 2011; Strauss et al., 2006; Sudhof, 2017; Tromp et al., 2020; Vaags et al., 2012; Yan et al., 2008). Structural changes in the striatum are also reported consistently in autistic human populations, as is striatal hyperconnectivity (Dupong and Di Martino, 2020). How may GluD1 or Cbln1 be contributing to autism pathophysiology?
As mentioned previously, the parafascicular thalamic nucleus has featured prominently in Cbln1 and GluD1 investigation. This nucleus was identified as an autism-relevant region using genetic data-mining techniques; the correlation was corroborated by functional MRI studies (Baldwin et al., 2016). Connections between the parafascicular nucleus and the striatum also degrade significantly in Parkinson’s disease. Interestingly, deep brain stimulation of the Pf has been shown to mitigate symptoms of both Parkinson’s disease and Tourette’s syndrome, the latter genetically correlating with specific mutations in NRXN, as well as with Cbln2 via proximity to translocation breakpoints (Clarke et al., 2012; Smith et al., 2014). Schizophrenia, too, appears to genetically correlate with GRID1 variants (Fallin et al., 2005; Guo et al., 2007). The striatum is a classical pharmacological target for antipsychotics, but whether GluD1 dysfunction in this region contributes to psychosis is unclear.
Nucleus accumbens: relevance to sociability and addiction
Ventral to the aforementioned striatal nuclei is the nucleus accumbens (NAc). As in the dorsal striatum, medium spiny neurons of the NAc abundantly express GluD1, and the region receives innervation from numerous nuclei which produce Cblns. Of these, researchers have highlighted glutamatergic projections originating in the ventral tegmental area (VTA) of the midbrain for their Cbln1-dependent function (Figure 3).
Figure 3:
Schematic illustrating proposed GluD and Cbln involvement in the dorsal and ventral striatum. (a) Cbln2 is present in cortico-striatal projections, but its postsynaptic target and function are not known. (b) Cbln1 is secreted by afferents from the parafascicular thalamus and synapses with GluD1 expressed on the dendrites of striatal medium spiny neurons (MSNs). (c) Cbln1 is produced in glutamatergic neurons in the VTA and affects excitatory transmission in the NAc, though its postsynaptic target is not confirmed.
Neurons originating in the VTA form the primary source of dopaminergic innervation to the NAc. A small number of dopaminergic neuron subclasses in this region have been characterized by Cbln mRNA expression; some express Cbln1, some express Cbln4, others express both (Poulin et al., 2020). A subset of GABAergic VTA neurons, too, show Cbln4 expression (Paul et al., 2019). The region also contains glutamatergic neurons characterized by the presence of vGluT2, many of which terminate in the NAc (Hnasko et al., 2012). While we lack data on Cblns’ functions in dopaminergic and GABAergic VTA neurons, Cbln1’s role in vGluT2+ glutamatergic neurons of the VTA has been studied and demonstrated.
In 2017, Krishnan et al. uncovered the presence of Cbln1 in the vGluT2-positive glutamatergic neurons in the VTA and found it to regulate both synaptic activity and animal behavior. When Cbln1 is deleted from vGluT2-positive neurons, mEPSC frequency in NAc MSNs is reduced and optogenetically stimulated excitatory currents at VTA to NAc synapses are almost completely eliminated (Krishnan et al., 2017). Behaviorally, Cbln1 deletion in the VTA causes decreased animal sociability. In contrast, Cbln1 deletion from dopaminergic neurons did not affect social behavior. Krishnan and colleagues also uncovered a regulatory pathway in which Ubiquitin-protein ligase E3A (UBE3A), a ubiquitin ligase which tags proteins for degradation, is responsible for reducing Cbln1 levels in the VTA. Social deficits and reduced VTA Cbln1 levels were shown to result from pentylenetetrazol-induced seizures, with UBE3A mediating these effects. This carries relevance to human disease, as the gene coding for UBE3A is yet another member of the long list of correlates with autism spectrum disorder (Glessner et al., 2009; Vatsa and Jana, 2018).
As seen in the dorsal striatum, Cbln1 deletion in NAc afferents produces phenotypes significantly different from those produced by deletion of GluD1 at their synaptic destination. For example, basal NAc excitatory transmission is not affected in GluD1-null mice. This may also suggest compensation from GluD2 which is also expressed in the striatum. However, a number of other changes occur in the NAc upon GluD1 deletion. Firstly, GluD1 KO mice show increased preference for cocaine which is proposed to be due to greater maturation of dendritic spines as evidenced by an increase in morphological properties like head and neck diameter (Liu et al., 2018). Dendritic spine density in NAc core MSNs increases more after cocaine exposure in GluD1 KO mice than wild-type. Basal GluN2B subunit-mediated currents are higher in MSNs in GluD1 KO, a trend also seen in the cortex and hippocampus following GluD1 KO (Gupta et al., 2015). This may potentially represent a delay in the developmental switch in NMDA receptor subunits which will be elaborated on in later sections.
Cortex: expression and synaptic effects of GluD1 and Cblns
GluD1 is found in abundance throughout the cortex at varying degrees of density. Found in multiple neuron classes, the receptor is even occasionally co-expressed with GluD2. The retrosplenial cortex exhibits the richest GluD1 expression (Hepp et al., 2015; Konno et al., 2014). Cortical Cbln1 levels are low, and the highest levels are seen in the retrospenial granular cortex (Miura et al., 2006) (Seigneur and Südhof, 2018). Cbln2 and 4, on the other hand, are found throughout the cortex in higher density. Seigneur and Südhof reported that Cbln2 and 4 are expressed each in separate excitatory neuron populations, as well as inhibitory neurons (except PV neurons). Colocalization studies using reporter models demonstrated that out of all labeled neurons in layer 2/3, only 20% colabeled for Cbln2 and Cbln4, whereas 45% labeled for Cbln2 alone and 35% for Cbln4 alone. Thus, Cbln2- and Cbln4-expressing cells are largely segregated possibly both among excitatory and inhibitory neurons (Miura et al., 2006; Seigneur and Südhof, 2017).
Due to strong Cbln expression, the retrosplenial cortex, a posterior cortical region divided between granular (RSG) and dysgranular regions (RDG), has received attention from groups investigating these proteins. Otsuka and colleagues reported strong Cbln1 protein and mRNA expression in the RSG, with mRNA signal particularly strong in layer IV (Otsuka et al., 2016). Regarding other Cblns, Signeur and Südhof found Cbln2 in layers II, III, and V of both the RDG and RSG (Seigneur and Südhof, 2017). Cbln4 was seen in layers II and III of only the RDG. In the retrosplenial cortex, the presynaptic glutamate transporter vGluT1 characterizes cortico-cortical synapses, whereas vGluT2 characterizes thalamo-cortical synapses. In Cbln1/2 and Cbln1/2/4 knockout mice, vGluT1 levels decrease in the RDG, while vGluT2 levels decrease in the RSG (Seigneur and Südhof, 2018). These findings suggest Cbln proteins mediate cortico-cortical synapses in the RDG, and thalamo-cortical synapses in the RSG (Otsuka et al., 2016; Seigneur and Sudhof, 2018).
Different experimental approaches which manipulate GluD1 expression have yielded data which appear contradictory. GluD1 knockout studies revealed functions in the medial prefrontal cortex (mPFC) including regulation of dendritic spine density, NMDA subunit ratios, and total excitatory synapse number. GluD1 KO results in both higher dendritic spine and total synapse count in the mPFC. These changes are accompanied by increased overall excitatory transmission in the mPFC (Gupta et al., 2015). A second model showed overexpression of GluD1 to decrease dendritic spine density up to 75%, while performing GluD1 knockdown with shRNA produced no changes in cortical dendritic spine density or mEPSC frequency in layer 2/3 somatosensory pyramidal neurons. This group used in utero electroporation which produced sparse overexpression or knockdown of GluD1 in single pyramidal cells (Fossati et al., 2019). A third study reported that GluD1 overexpression greatly enhanced mEPSC frequency in the somatosensory cortex, and that GluD1 knockdown significantly decreased excitatory neurotransmission. This group used biolistic transfection on mouse brain slices (Tao et al., 2018). Surely differences in experimental techniques and region sampled can account for such divergent data. Consistent, however, is that GluD1 expression has a major synaptic effect across multiple cortical regions.
Normally, a developmental shift takes place in which GluN2A-containing NMDARs gradually replace those containing subunit GluN2B (Williams et al., 1993). As seen in the straitum, GluD1 deletion prevents this ratio shift in the mPFC (Gupta et al., 2015). Also, cofilin and its regulating kinase LIMK l, which regulate dendritic spine formation, are both found in reduced phosphorylated states in the mPFC of GluD1 KO mice. Application of D-cycloserine and Ro-25–6981, a selective GluN2B inhibitor, partially normalizes GluD1-KO-induced changes in dendritic spine count and LIMKl-cofilin signaling (Gupta et al., 2015).
Seigneur and Südhof also recorded synaptic alterations in the motor cortex following Cbln deletion (Seigneur and Südhof, 2018). Cbln1, Cbln2, and dual Cbln1/2 KO mice were examined, and in all cases, GluA2 subunits increased compared to wild type. Changes in NMDA receptors were also seen in the motor cortex: while GluN1 levels remained steady in both Cbln1 and Cbln2 deletion, Cbln1/2 double deletion caused levels of the subunit to be “dramatically reduced”. Motor cortex GluN2 subunit levels were reportedly increased in Cbln1 and Cbln1/2 KO but remained steady in Cbln2 KO. Overall, our understanding of function of GluD receptors and Cblns in the cortex will be greatly clarified if the functional differences between Cbln1, 2, and 4 are better established. Can the expression of these proteins in largely non-overlapping populations produce differential effects? Or are there differences between the three proteins in their affinity for postsynaptic and presynaptic partners? Perhaps different post-translational modification states of NRXNs (such as heparan sulfate modification, as will discussed be further on), or even of GluD receptors, could contribute to differences in binding affinity for the various Cbln proteins, further defining a synaptic architecture code.
GluD1 and inhibitory synapses
Though discussion has thus far focused on GluD1 and Cbln1 at excitatory synapses, the complex also plays a role in inhibitory synapse formation. A study published in 2012 noted GABAergic presynaptic differentiation in cultured cortical neurons expressing NRXN and exposed to Cbln1 (Ryu et al., 2012). Subsequent research published in 2013 showed reduced levels of hippocampal glutamic acid decarboxylase 67 (GAD67, an inhibitory neuron marker) in GluD1 KO mice (Yadav et al., 2013). Various interneuron classes are also known to express cerebellins, as well as NRXNs containing the AS4 splice site necessary for Cbln binding, suggesting GluD receptor involvement in GABAergic transmission (Nguyen et al., 2016; Seigneur and Südhof, 2017). In one study, Cbln4 was specifically examined in the context of Alzheimer’s Disease (AD); investigators found that overexpression of or treatment with recombinant Cbln4 rescued inhibitory synapses deficits in an AD model and had a protective effect on amyloid beta-induced neuron death (Chacón et al., 2015). Seigneur and Südhof also reported the presence of Cbln4 in multiple inhibitory neuron classes (Seigneur and Südhof, 2017). In 2019, Fossati et al. published information which firmly established a role for GluD1 in inhibitory synapse formation; we will briefly summarize their findings (Fossati et al., 2019).
In layer 2/3 somatosensory pyramidal neurons, GluD1 was found in ~50% of inhibitory synapses, while only present in ~21% of excitatory synapses, usually localized postsynaptically on the periphery of the active zone and colocalized with VGAT (Fossati et al., 2019). Unlike observations from Gupta et al. which recorded increased dendritic spine and excitatory synapse count and neurotransmission in the mPFC of constitutive GluD1 KO mice (Gupta et al., 2015), sparse GluD1 knockdown from single cells with GRID1 shRNA resulted in no such changes in somatosensory layer 2/3. Rather, inhibitory synaptic marker gephyrin decreased upon sparse GluD1 knockdown, a phenotype that sustained into adulthood but was rescued by GluD1 reintroduction. The frequency of mIPSCs was ~35% lower in neurons with GluD1 knockdown. Similarly, sparse overexpression of GluD1 in pyramidal neurons caused an increase in gephyrin levels (Fossati et al., 2019).
With GluD1 clearly taking a key role for inhibitory synapse formation, investigators next verified the involvement of GluD1 ligands (Fossati et al., 2019). It was determined via point mutation experiments in GluD1 that prevent Cbln binding (GluD1R341A/W343A) and D-serine/glycine binding (GluD1R526K) that binding of these ligands is required for GluD1’s inhibitory synaptogenic effect. The intracellular C-terminus of GluD1 is also necessary for inhibitory synapse formation. All the aforementioned experimental conditions resulted in decreased gephyrin cluster density, indicating impaired inhibitory synapse formation. Significantly, a point mutation to prevent ion flow through the receptor’s channel pore (GluD1V617R) had no effect on inhibitory synapse formation. In addition, it was found that Cbln4, but not Cbln2 inactivation causes a significant drop in inhibitory synapse number. Cbln4 is present in somatostatin (SST)+ interneurons (Favuzzi et al., 2019), and these data indicate that SST+ interneurons synapse with cortical pyramidal cells via a Cbln4-GluD1-mediated mechanism. This is consistent with findings that Cbln4 deletion from SST neurons results in reduced presynaptic contact onto pyramidal neurons (Favuzzi et al., 2019). Finally, Fossati and colleagues investigated postsynaptic mechanisms by which GluD1 may trigger inhibitory synapse formation. Two genes whose protein products co-immunoprecipitated with GluD1, ARHGEF12 (rho guanine nucleotide exchange factor 12) and PPP1R12A (protein phosphatase 1 regulatory subunit 12A), were also determined to mediate GluD1 function at inhibitory synapses. Deletion of these genes mimics the effect of GluD1 deletion on inhibitory synapse formation, and double knockouts (GRID1/ARHGEF12 or GRID1/PPP1R12A) exhibited no additional effects on inhibitory synapse formation, suggesting these proteins operate in the same signaling pathway. These two proteins may be involved in non-ionotropic effects of glycine/D-serine binding with GluD1 (Fossati et al., 2019). Considering these data alongside reports of Cbln4 expression in GABAergic neurons across multiple brain regions, the role GluD receptors in inhibitory synapses should be further examined.
Hippocampus: What we know
As in the cortex, Cblns and GluD receptors in the hippocampus likely mediate both excitatory and inhibitory synapses. Firstly, the cornu ammonis (CA) and dentate gyrus show strong GluD1 and sparse GluD2 expression (Hepp et al., 2015; Konno et al., 2014). Secondly, Cblns are present in low density across multiple hippocampal neuron subtypes, but are enriched entorhinal cortex, a major source of afferents to the hippocampus (Miura et al., 2006; Otsuka et al., 2016; Seigneur and Südhof, 2017; Tao et al., 2018). A segregation appears here between Cbln1 and Cbln2: Principal cells of the entorhinal cortex express Cbln1 and Cbln4 but lack Cbln2. In contrast, excitatory and inhibitory cells in the CA1 and CA3 region have sparse Cbln2 and Cbln4 expression with higher cell labeling in the subiculum for Cbln2. With regard to interneurons, one divergence from the cortex is the expression of Cbln4 in PV interneurons. In fact, approximately 50% of Cbln4 labeled cells were positive for PV (Fuccillo et al., 2015; Seigneur and Südhof, 2017). Though the presence and expression pattern of Cblns and GluD receptors in the hippocampus is becoming well documented, their precise function needs further exploration.
Briefly, we will examine evidence for the involvement of GluD receptors and Cbln1 in the perforant pathway. This circuit is composed of projections which arise in the entorhinal cortex and terminate on dentate granule cells and CA pyramidal cells (Bartesaghi and Gessi, 2004). While Cbln1 is not produced at significant levels in principal cells of the dentate gyrus or CA, strong Cbln1 and GluD1 protein signals are detected in the hippocampal SLM and dentate molecular layer where synapses are formed with incoming entorhinal projections, which themselves express Cbln1 mRNA (Hepp et al., 2015; Konno et al., 2014; Nakamoto et al., 2019b; Otsuka et al., 2016). Deletion of all Cblns reduces VGluT1 levels in the hippocampus SLM, indicating a loss of glutamatergic inputs of entorhinal origin (Seigneur and Sudhof, 2018). It may also be noted that Miura et al. reported Cbln2 mRNA in the thalamic reuniens nucleus, another source of glutamatergic input to the hippocampal SLM (Miura et al., 2006; Vertes, 2006).
In addition to perforant pathway synapses, GluD receptors likely mediate inhibitory synapses within the hippocampus. Cblns are present in multiple interneuron subtypes; PV+ neurons have been reported to contain both Cbln1 and 4, while CCK+ express high levels of Cbln2 (Fuccillo et al., 2015; Seigneur and Sudhof, 2017). The role of GluD1 in GABAergic hippocampal synapses has not been confirmed, but the notion is supported by data which show reduced hippocampal GAD67 levels following GluD1 KO (Yadav et al., 2013). Conversely, dendritic spine count and levels of PSD95, an excitatory synapse marker, both increase.
Other hippocampal synaptic changes which follow GluD1 deletion include decreases in GluA1, GluA2, and GluK2 subunits (Yadav et al., 2013). A similar upshift in GluN2B: GluN2A ratio as described in previous sections is also seen (Gupta et al., 2015). GluD1 is also essential for mGluR5-mediated (DHPG-activated) GluA receptor trafficking (Suryavanshi et al., 2016). In addition, GluD1 coimmunoprecipitates with mGluR5 in both hippocampal and striatal sections. Lastly, one group suggested that GluD1 mediates excitatory synapses between CA pyramidal cells. Biolistic transfection was used to induce overexpression of GluD1 in CA1 pyramidal neurons, and CA1 cells transfected with GluD1 showed increased AMPA and NMDA EPSCs compared to neighboring control CA1 cells following Schaffer collateral activation. Authors surmised that presynaptic NRXN 1β was required for this GluD1-induced increase in excitatory transmission. Conversely, cells which underwent GluD1 knockdown with shRNA treatment showed decreased AMPA and NMDA EPSCs (Tao et al., 2018). Though this study implied that Cbln2 mediates excitatory synapses in the hippocampus via GluD1, studies examining Cbln expression show very little Cbln2 expression in excitatory hippocampal cells (Miura et al., 2006; Seigneur and Sudhof, 2017). In a successive study, the same group also provided data supporting the notion of GluD1’s role in hippocampal LTP. When GluD1’s ligand binding domain was genetically replaced with the ligand binding domain from GluK2, LTP was abolished in cells transfected with this chimeric protein (Tao et al., 2019).
In conclusion, GluD receptors and Cblns clearly play synaptic roles in the hippocampus. GluD receptors in this region may designate synaptic targets for Cbln-expressing afferents, or mediate connections within the hippocampus between pyramidal cells, regulating excitatory transmission. The high expression of Cblns in hippocampal interneurons, considered alongside GluD1’s established role in inhibitory signaling in the cortex, also warrants further investigation.
The unresolved NRXN splicing code
Neurexins (NRXNs) 1, 2, and 3 are relatively well-studied synaptic adhesion molecules expressed widely in the brain (Gomez et al., 2021). Though “extended family” members related to the NRXN proteins exists, we are primarily interested in NRXNs 1–3, as they have each been shown to bind with Cbln1, 2, and 4 (Joo et al., 2011). These proteins undergo extensive alternative splicing at six major splice sites, generally referred to as AS1-AS6. In this section we will briefly examine factors which govern NRXN splicing at the AS4 site, the 30-amino acid site responsible for Cbln binding; considering these factors in context with brain-wide Cbln and GluD receptor expression patterns brings to view the broader network in which the tripartite bridge functions (Dai et al., 2019; Seigneur and Sudhof, 2018).
SAM68, SLM1, and SLM2, members of the Signal Transduction and Activation of RNA (STAR) family, are RNA-binding proteins responsible for alternative splicing at NRXN’s AS4 site (Ehrmann et al., 2013; Iijima et al., 2014; Iijima et al., 2011). These proteins induce exon skipping at the AS4 site, producing an AS4-negative NRXN splice variant incapable of binding with Cbln family proteins, both in vivo and in vitro (Ehrmann et al., 2013; Ehrmann et al., 2016; Iijima et al., 2014). The three STAR proteins show unique patterns of distribution in the brain, and also differ in their affinity for various NRXN isoforms. (Iijima et al., 2014). Iijima and colleagues published cerebellar expression patterns for SLM1, SLM2, and SAM68 (Iijima et al., 2011). SLM1 is mostly present in Purkinje cells, while SLM2 remains mostly isolated to interneurons of the internal granular layer and cerebellar cortex. SAM68, on the other hand, is present across multiple cerebellar cell types, but is notably the only STAR protein significantly expressed in granule cells. Here, it regulates alternative splicing of parallel fiber NRXN1, the presynaptic binding partner for Cbln1 at PF-PC synapses (Iijima et al., 2011). Iijima et al. identified an activity-dependent and calcium-dependent process by which SAM68 regulates NRXN1 splicing in parallel fibers; they showed chronic stimulation of granule cells to lead to increased AS4 exclusion, that is to say, fewer transcripts of NRXN1 containing the AS4 site (Iijima et al., 2011). SAM68 undergoes CaMKII-mediated phosphorylation and binds RNA transcripts in depolarized cells, inducing AS4 exon skipping. Outside the cerebellum, the presence of STAR proteins have also been well-described in pyramidal cells and interneurons of the hippocampal formation and the afferent entorhinal cortex. (Ehrmann et al., 2013; Ehrmann et al., 2016; Iijima et al., 2014; Nguyen et al., 2016). In the dentate gyrus, Ding et al. also reported activity-dependent NRXN1 AS4 splicing, though it was attributed to histone modification rather than STAR protein activity (Ding et al., 2017).
Lastly, but certainly not least, are recent unpublished reports by Trotter et al. that hippocampal astrocytes express NRXN almost exclusively in the AS4+ form in vivo, and bind Cbln1-GluD1 complexes in vitro. The same report stated that astrocytic NRXN, but not neuronal neurexin, was highly modified by heparan sulfate, a polysaccharide. These striking data raise a number of possibilities, the first being the unexplored potential involvement of astrocytes in Cbln-GluD complexes. The issue of heparan sulfate modification also brings to mind the possibility of such post-translational modification regulating binding specificity to different Cbln isoforms.
Conclusion
Our knowledge of GluD receptors has begun to reach critical mass, but we have yet to assemble a holistic context. Moving forward, it may be helpful for investigators to examine region-, circuit-, and cell-specific functions of these proteins. These should be considered in the context of neurodevelopment, since Cbln expression patterns change over the course of development. The genetic correlations of GluD receptors, Cblns, and NRXNs with developmental disorders like autism also point to fertile ground for the inquiries of translational researchers. Aspects of how learning and memory, disease states, or drugs of abuse may affect these molecules and lead to dynamic changes in circuitry should also be investigated. We also realize that this system represents only one of many synaptic organizer classes, and how these all work together to organize CNS circuits is yet to be understood. In the meantime, simple questions must be answered to understand differential affinities, downstream signaling, and possibly unique ion pore function in GluD-Cbln complexes. The existence of these proteins also awaits broader awareness in the scientific and medical communities. GluD receptors defy simple categorization, and serve to expand our understanding of biological systems, both small and great.
Highlights:
Glutamate delta receptors have unusual synapse organizer function
The role of GluD receptors in various forebrain circuits is only beginning to emerge and is summarized in this review
Changes in expression and post-translational modifications of GluD receptors and associated proteins can lead to dynamic changes in neural circuits in normal learning and memory as well as in disease states.
Acknowledgments
Funding/Disclosures: This work was supported by grants from the NSF1456818 (SMD), NIH NS104705 (SMD), NIH NS118731 (SMD) and NIH MH116003 (SMD).
Footnotes
Conflict of interest: The authors note no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, Dusart I, Fagni L, Lambolez B, Levenes C, 2014. Type 1 metabotropic glutamate receptors (m G lul) trigger the gating of G lu D 2 delta glutamate receptors. EMBO reports 15, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin PR, Curtis KN, Patriquin MA, Wolf V, Viswanath H, Shaw C, Sakai Y, Salas R, 2016. Identifying diagnostically-relevant resting state brain functional connectivity in the ventral posterior complex via genetic data mining in autism spectrum disorder. Autism Research 9, 553–562. [DOI] [PubMed] [Google Scholar]
- Bao D, Pang Z, Morgan JI, 2005. The structure and proteolytic processing of Cbln1 complexes. Journal of Neurochemistry 95, 618–629. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T, 2004. Parallel activation of field CA2 and dentate gyrus by synaptically elicited perforant path volleys. Hippocampus 14, 948–963. [DOI] [PubMed] [Google Scholar]
- Benamer N, Marti F, Lujan R, Hepp R, Aubier TG, Dupin AAM, Frebourg G, Pons S, Maskos U, Faure P, Hay YA, Lambolez B, Tricoire L, 2017. GluD1, linked to schizophrenia, controls the burst firing of dopamine neurons. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burada AP, Vinnakota R, Kumar J, 2020a. The architecture of GluD2 ionotropic delta glutamate receptor elucidated by cryo-EM. Journal of Structural Biology 211, 107546. [DOI] [PubMed] [Google Scholar]
- Burada AP, Vinnakota R, Kumar J, 2020b. Cryo-EM structures of the ionotropic glutamate receptor GluD1 reveal a non-swapped architecture. Nature structural & molecular biology 27, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón PJ, del Marco Á, Arévalo Á, Domínguez-Giménez P, García-Segura LM, Rodríguez-Tébar A, 2015. Cerebellin 4, a synaptic protein, enhances inhibitory activity and resistance of neurons to amyloid-β toxicity. Neurobiology of aging 36, 1057–1071. [DOI] [PubMed] [Google Scholar]
- Cheng S, Seven AB, Wang J, Skiniotis G, Özkan E, 2016. Conformational plasticity in the transsynaptic neurexin-cerebellin-glutamate receptor adhesion complex. Structure 24, 2163–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau L-F, Huganir RL, 2004. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. Journal of Neuroscience 24, 10248–10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RA, Lee S, Eapen V, 2012. Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Translational Psychiatry 2, e158–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun H-S, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, 2007. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. Journal of Neuroscience 27, 9901–9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadak S, Bouquier N, Goyet E, Fagni L, Levenes C, Perroy J, 2017. mGlu1 receptor canonical signaling pathway contributes to the opening of the orphan GluD2 receptor. Neuropharmacology 115, 92–99. [DOI] [PubMed] [Google Scholar]
- Dai J, Aoto J, Südhof TC, 2019. Alternative splicing of presynaptic neurexins differentially controls postsynaptic NMDA and AMPA receptor responses. Neuron 102, 993–1008. e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Liu S, Tian M, Zhang W, Zhu T, Li D, Wu J, Deng H, Jia Y, Xie W, Xie H, Guan J-S, 2017. Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nature Neuroscience 20, 690–699. [DOI] [PubMed] [Google Scholar]
- Dupong I, Di Martino A, 2020. Hyper-connectivity of the striatum related to restricted and repetitive behaviors’ severity in children with ASD. bioRxiv. [Google Scholar]
- Ehrmann I, Dalgliesh C, Liu Y, Danilenko M, Crosier M, Overman L, Arthur HM, Lindsay S, Clowry GJ, Venables JP, Fort P, Elliott DJ, 2013. The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Neurexin Pre-mRNAs in the Brain. PLoS Genetics 9, e1003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann I, Gazzara MR, Pagliarini V, Dalgliesh C, Kheirollahi-Chadegani M, Xu Y, Cesari E, Danilenko M, Maclennan M, Lowdon K, Vogel T, Keskivali-Bond P, Wells S, Cater H, Fort P, Santibanez-Koref M, Middei S, Sette C, Clowry GJ, Barash Y, Cunningham MO, Elliott DJ, 2016. A SLM2 Feedback Pathway Controls Cortical Network Activity and Mouse Behavior. Cell Reports 17, 3269–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elegheert J, Kakegawa W, Clay JE, Shanks NF, Behiels E, Matsuda K, Kohda K, Miura E, Rossmann M, Mitakidis N, Motohashi J, Chang VT, Siebold C, Greger IH, Nakagawa T, Yuzaki M, Aricescu AR, 2016. Structural basis for integration of GluD receptors within synaptic organizer complexes. Science 353, 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang K-Y, Huganir RL, 2005. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. The American Journal of Human Genetics 77, 918–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E, Deogracias R, Marques-Smith A, Maeso P, Jezequel J, Exposito-Alonso D, Balia M, Kroon T, Hinojosa AJ, Maraver EF, 2019. Distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science 363, 413–417. [DOI] [PubMed] [Google Scholar]
- Fossati M, Assendorp N, Gemin O, Colasse S, Dingli F, Arras G, Loew D, Charrier C, 2019. TransSynaptic Signaling through the Glutamate Receptor Delta-1 Mediates Inhibitory Synapse Formation in Cortical Pyramidal Neurons. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo MV, 2016. Striatal circuits as a common node for autism pathophysiology. Frontiers in neuroscience 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo Marc V., Földy C, Gökce Ö, Rothwell Patrick E., Sun Gordon L., Malenka Robert C., Südhof Thomas C., 2015. Single-Cell mRNA Profiling Reveals Cell-Type-Specific Expression of Neurexin Isoforms. Neuron 87, 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Moussawi K, Hake HS, 2020. Delta glutamate receptor conductance drives excitation of mouse dorsal raphe neurons. eLife 9, e56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, 2009. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459, 569573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Traunmüller L, Scheiffele P, 2021. Neurexins: molecular codes for shaping neuronal synapses. Nature Reviews Neuroscience, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold AJ, Ma D, Cukier HN, Nations LD, Schmidt MA, Chung R-H, Jaworski JM, Salyakina D, Konidari I, Whitehead PL, Wright HH, Abramson RK, Williams SM, Menon R, Martin ER, Haines JL, Gilbert JR, Cuccaro ML, Pericak-Vance MA, 2012. Evaluation of copy number variations reveals novel candidate genes in autism spectrum disorder-associated pathways. Human Molecular Genetics 21, 35133523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S-Z, Huang K, Shi Y-Y, Tang W, Zhou J, Feng G-Y, Zhu S-M, Liu H-J, Chen Y, Sun X-D, He L, 2007. A Case-control association study between the GRID1 gene and schizophrenia in the Chinese Northern Han population. Schizophrenia Research 93, 385–390. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Yadav R, Pavuluri R, Morley BJ, Stairs DJ, Dravid SM, 2015. Essential role of GluD1 in dendritic spine development and GluN2B to GluN2A NMDAR subunit switch in the cortex and hippocampus reveals ability of GluN2B inhibition in correcting hyperconnectivity. Neuropharmacology 93, 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddick PC, Tom I, Luis E, Quiñones G, Wranik BJ, Ramani SR, Stephan J-P, Tessier-Lavigne M, Gonzalez LC, 2014. Defining the ligand specificity of the deleted in colorectal cancer (DCC) receptor. PloS one 9, e84823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, Kastrup JS, Traynelis SF, 2009. Modulation of the dimer interface at ionotropic glutamate-like receptor δ2 by D-serine and extracellular calcium. Journal of Neuroscience 29, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp R, Hay YA, Aguado C, Lujan R, Dauphinot L, Potier MC, Nomura S, Poirel O, El Mestikawy S, Lambolez B, Tricoire L, 2015. Glutamate receptors of the delta family are widely expressed in the adult brain. Brain Structure and Function 220, 2797–2815. [DOI] [PubMed] [Google Scholar]
- Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E, Parris J, Rong Y, Watanabe M, Yuzaki M, 2005. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nature Neuroscience 8, 1534–1541. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH, 2012. Ventral Tegmental Area Glutamate Neurons: Electrophysiological Properties and Projections. Journal of Neuroscience 32, 15076–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover AH, Pavuluri R, Shelkar GP, Dravid SM, Smith Y, Villalba RM, 2020. Ultrastructural localization of glutamate delta 1 (GluD1) receptor immunoreactivity in the mouse and monkey striatum. Journal of Comparative Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa R, Sakimura K, Watanabe M, 2016. GluD2 endows parallel fiber-Purkinje cell synapses with a high regenerative capacity. Journal of Neuroscience 36, 4846–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Iijima Y, Witte H, Scheiffele P, 2014. Neuronal cell type-specific alternative splicing is regulated by the KH domain protein SLM1. The Journal of Cell Biology 204, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Wu K, Witte H, Hanno-Iijima Y, Glatter T, Richard S, Scheiffele P, 2011. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell 147, 1601–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Cadby G, Melton P, Abraham L, Whitehouse A, Moses E, 2013. Genome-wide association study of autistic-like traits in a general population study of young adults. Frontiers in Human Neuroscience 7, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo J-Y, Lee S-J, Uemura T, Yoshida T, Yasumura M, Watanabe M, Mishina M, 2011. Differential interactions of cerebellin precursor protein (Cbln) subtypes and neurexin variants for synapse formation of cortical neurons. Biochemical and biophysical research communications 406, 627–632. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Miyazaki T, Kohda K, Matsuda K, Emi K, Motohashi J, Watanabe M, Yuzaki M, 2009. The N-terminal domain of GluD2 (GluR52) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. Journal of Neuroscience 29, 5738–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, Emi K, Motohashi J, Konno R, Zaitsu K, Yuzaki M, 2011. D-Serine regulates cerebellar LTD and motor coordination through the δ2 glutamate receptor. Nature Neuroscience 14, 603–611. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, 1995. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR52 mutant mice. Cell 81, 245–252. [DOI] [PubMed] [Google Scholar]
- Kohda K, Kakegawa W, Matsuda S, Yamamoto T, Hirano H, Yuzaki M, 2013. The δ2 glutamate receptor gates long-term depression by coordinating interactions between two AMPA receptor phosphorylation sites. Proceedings of the National Academy of Sciences 110, E948–E957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno K, Matsuda K, Nakamoto C, Uchigashima M, Miyazaki T, Yamasaki M, Sakimura K, Yuzaki M, Watanabe M, 2014. Enriched Expression of GluD1 in Higher Brain Regions and Its Involvement in Parallel Fiber-Interneuron Synapse Formation in the Cerebellum. Journal of Neuroscience 34, 7412–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJS, Ozkaynak E, Teng BL, Nagakura I, Mohammad F, Silva MA, Peterson S, Cruz TJ, Kasper EM, Arnaout R, Anderson MP, 2017. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 543, 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Hansen KB, Naur P, Olsen L, Kurtkaya NL, Dravid SM, Kvist T, Yi F, Pohlsgaard J, Clausen RP, Gajhede M, Kastrup JS, Traynelis SF, 2015. Pharmacology and Structural Analysis of Ligand Binding to the Orthosteric Site of Glutamate-Like GluD2 Receptors. Molecular Pharmacology 89, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch AY, 2010. An extra-cerebellar role forCerebellinl: Modulation of dendritic spine density and synapses in striatal medium spiny neurons. The Journal of Comparative Neurology, NA–NA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine D, Mondoloni S, Tange J, Lambolez B, Faure P, Taly A, Tricoire L, Mourot A, 2020. Probing the ionotropic activity of glutamate GluD2 receptor in HEK cells with genetically-engineered photopharmacology. Elife 9, e59026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gandhi PJ, Pavuluri R, Shelkar GP, Dravid SM, 2018. Glutamate delta-1 receptor regulates cocaine-induced plasticity in the nucleus accumbens. Translational Psychiatry 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shelkar GP, Gandhi PJ, Gawande DY, Hoover A, Villalba RM, Pavuluri R, Smith Y, Dravid SM, 2020. Striatal glutamate delta-1 receptor regulates behavioral flexibility and thalamostriatal connectivity. Neurobiology of Disease, 104746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, Wisden W, 1993. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Letters 315, 318–322. [DOI] [PubMed] [Google Scholar]
- Lukacsovich D, Winterer J, Que L, Luo W, Lukacsovich T, Foldy C, 2019. Single-cell RNA-seq reveals developmental origins and ontogenetic stability of neurexin alternative splicing profiles. Cell Reports 27, 3752–3759. e3754. [DOI] [PubMed] [Google Scholar]
- Ly CD, Roche KW, Lee H-K, Wenthold RJ, 2002. Identification of rat EMAP, a S-glutamate receptor binding protein. Biochemical and biophysical research communications 291, 85–90. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, Yuzaki M, 2010. Cbln1 Is a Ligand for an Orphan Glutamate Receptor 2, a Bidirectional Synapse Organizer. Science 328, 363–368. [DOI] [PubMed] [Google Scholar]
- Mayat E, Petralia RS, Wang Y-X, Wenthold RJ, 1995. Immunoprecipitation, immunoblotting, and immunocytochemistry studies suggest that glutamate receptor delta subunits form novel postsynaptic receptor complexes. Journal of Neuroscience 15, 2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura E, Iijima T, Yuzaki M, Watanabe M, 2006. Distinct expression of Cbln family mRNAs in developing and adult mouse brains. European Journal of Neuroscience 24, 750–760. [DOI] [PubMed] [Google Scholar]
- Miura E, Matsuda K, Morgan JI, Yuzaki M, Watanabe M, 2009. Cbln1 accumulates and colocalizes with Cbln3 and GluRS2 at parallel fiber-Purkinje cell synapses in the mouse cerebellum. European Journal of Neuroscience 29, 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Feng G, 2017. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nature Reviews Neuroscience 18, 147. [DOI] [PubMed] [Google Scholar]
- Nakamoto C, Kawamura M, Nakatsukasa E, Natsume R, Takao K, Watanabe M, Abe M, Takeuchi T, Sakimura K, 2019a. GluD1 knockout mice with a pure C57BL/6N background show impaired fear memory, social interaction, and enhanced depressive-like behavior. bioRxiv, 826768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto C, Konno K, Miyazaki T, Nakatsukasa E, Natsume R, Abe M, Kawamura M, Fukazawa Y, Shigemto R, Yamasaki M, 2019b. Expression mapping, quantification, and complex formation of GluD1 and GluD2 glutamate receptors in adult mouse brain. Journal of Comparative Neurology. [DOI] [PubMed] [Google Scholar]
- Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, Vestergaard B, Egebjerg J, Gajhede M, Traynelis SF, 2007. Ionotropic glutamate-like receptor δ2 binds D-serine and glycine. Proceedings of the National Academy of Sciences 104, 14116–14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-M, Schreiner D, Xiao L, Traunmüller L, Bornmann C, Scheiffele P, 2016. An alternative splicing switch shapes neurexin repertoires in principal neurons versus interneurons in the mouse hippocampus. Elife 5, e22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord AS, Roeb W, Dickel DE, Walsh T, Kusenda M, O’connor KL, Malhotra D, McCarthy SE, Stray SM, Taylor SM, 2011. Reduced transcript expression of genes affected by inherited and de novo CNVs in autism. European Journal of Human Genetics 19, 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka S, Konno K, Abe M, Motohashi J, Kohda K, Sakimura K, Watanabe M, Yuzaki M, 2016. Roles of Cbln1 in Non-Motor Functions of Mice. The Journal of Neuroscience 36, 11801–11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul EJ, Tossell K, Ungless MA, 2019. Transcriptional profiling aligned with in situ expression image analysis reveals mosaically expressed molecular markers for GABA neuron sub-groups in the ventral tegmental area. European Journal of Neuroscience 50, 3732–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, 2011. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J-F, Gaertner Z, Moreno-Ramos OA, Awatramani R, 2020. Classification of midbrain dopamine neurons using single-cell gene expression profiling approaches. Trends in Neurosciences 43, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Ly CD, Petralia RS, Wang Y-X, McGee AW, Bredt DS, Wenthold RJ, 1999. Postsynaptic density-93 interacts with the δ2 glutamate receptor subunit at parallel fiber synapses. Journal of Neuroscience 19, 3926–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Wei P, Parris J, Guo H, Pattarini R, Correia K, Li L, Kusnoor SV, Deutch AY, Morgan JI, 2012. Comparison of Cbln1 and Cbln2 functions using transgenic and knockout mice. Journal of Neurochemistry 120, 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu K, Yokoyama M, Yamashita M, Hirano T, 2012. Induction of excitatory and inhibitory presynaptic differentiation by GluD1. Biochemical and biophysical research communications 417, 157–161. [DOI] [PubMed] [Google Scholar]
- Seigneur E, Sudhof TC, 2017. Cerebellins are differentially expressed in selective subsets of neurons throughout the brain. Journal of Comparative Neurology 525, 3286–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur E, Sudhof TC, 2018. Genetic ablation of all cerebellins reveals synapse organizer functions in multiple regions throughout the brain. Journal of Neuroscience 38, 4774–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Spence MA, Flodman P, 2009. Nuclear and mitochondrial genome defects in autisms. Annals of the New York Academy of Sciences 1151, 102–132. [DOI] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Ocampo IH, Wichman T, Bolam P, 2014. The thalamostriatal system in normal and diseased states. Frontiers in systems neuroscience 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH, 2006. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. New England Journal of Medicine 354, 1370–1377. [DOI] [PubMed] [Google Scholar]
- Südhof TC, 2017. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell 171, 745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryavanshi PS, Gupta SC, Yadav R, Kesherwani V, Liu J, Dravid SM, 2016. Glutamate Delta-1 Receptor Regulates Metabotropic Glutamate Receptor 5 Signaling in the Hippocampus. Molecular Pharmacology 90, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Díaz-Alonso J, Sheng N, Nicoll RA, 2018. Postsynaptic 51 glutamate receptor assembles and maintains hippocampal synapses via Cbln2 and neurexin. Proceedings of the National Academy of Sciences 115, E5373–E5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Ma C, Bemben MA, Li KH, Burlingame AL, Zhang M, Nicoll RA, 2019. Mechanisms underlying the synaptic trafficking of the glutamate delta receptor GluD1. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Mühleisen TW, Frank J, Mattheisen M, Herms S, Ludwig KU, Treutlein T, Schmael C, Strohmaier J, Böβhenz KV, 2009. Dissection of phenotype reveals possible association between schizophrenia and Glutamate Receptor Delta 1 (GRID1) gene promoter. Schizophrenia research 111, 123–130. [DOI] [PubMed] [Google Scholar]
- Tromp A, Mowry B, Giacomotto J, 2020. Neurexins in autism and schizophrenia—a review of patient mutations, mouse models and potential future directions. Molecular Psychiatry, 1–14. [DOI] [PubMed] [Google Scholar]
- Uemura T, Lee S-J, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M, 2010. Trans-synaptic interaction of GluRδ2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141, 1068–1079. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M, 2004. Direct interaction of GluRδ2 with Shank scaffold proteins in cerebellar Purkinje cells. Molecular and Cellular Neuroscience 26, 330–341. [DOI] [PubMed] [Google Scholar]
- Vaags Andrea K., Lionel Anath C., Sato D, Goodenberger M, Stein Quinn P., Curran S, Ogilvie C, Ahn Joo W., Drmic I, Senman L, Chrysler C, Thompson A, Russell C, Prasad A, Walker S, Pinto D, Marshall Christian R., Stavropoulos Dimitri J., Zwaigenbaum L, Fernandez Bridget A., Fombonne E, Bolton Patrick F., Collier DA, Hodge Jennelle C., Roberts W, Szatmari P, Scherer Stephen W., 2012. Rare Deletions at the Neurexin 3 Locus in Autism Spectrum Disorder. The American Journal of Human Genetics 90, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsa N, Jana NR, 2018. UBE3A and its link with autism. Frontiers in molecular neuroscience 11, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142, 1–20. [DOI] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB, 1993. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron 10, 267–278. [DOI] [PubMed] [Google Scholar]
- Yadav R, Gupta SC, Hillman BG, Bhatt JM, Stairs DJ, Dravid SM, 2012. Deletion of Glutamate Delta-1 Receptor in Mouse Leads to Aberrant Emotional and Social Behaviors. PloS one 7, e32969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Hillman BG, Gupta SC, Suryavanshi P, Bhatt JM, Pavuluri R, Stairs DJ, Dravid SM, 2013. Deletion of Glutamate Delta-1 Receptor in Mouse Leads to Enhanced Working Memory and Deficit in Fear Conditioning. PloS one 8, e60785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Rimerman R, Scofield MA, Dravid SM, 2011. Mutations in the transmembrane domain M3 generate spontaneously open orphan glutamate delta 1 receptor. Brain Research 1382, 1–8. [DOI] [PubMed] [Google Scholar]
- Yan J, Noltner K, Feng J, Li W, Schroer R, Skinner C, Zeng W, Schwartz CE, Sommer SS, 2008. Neurexin 1α structural variants associated with autism. Neuroscience letters 438, 368–370. [DOI] [PubMed] [Google Scholar]
- Yasumura M, Yoshida T, Lee S-J, Uemura T, Joo J-Y, Mishina M, 2012. Glutamate receptor 51 induces preferentially inhibitory presynaptic differentiation of cortical neurons by interacting with neurexins through cerebellin precursor protein subtypes. Journal of Neurochemistry 121, 705–716. [DOI] [PubMed] [Google Scholar]
- Yawata S, Tsuchida H, Kengaku M, Hirano T, 2006. Membrane-proximal region of glutamate receptor δ2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. Journal of Neuroscience 26, 3626–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzaki M, 2017. The C1q complement family of synaptic organizers: not just complementary. Current opinion in neurobiology 45, 9–15. [DOI] [PubMed] [Google Scholar]
- Yuzaki M, Aricescu AR, 2017. A GluD Coming-Of-Age Story. Trends in Neurosciences 40, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N, 1997. Neurodegeneration in Lurcher mice caused by mutation in δ2 glutamate receptor gene. Nature 388, 769–773. [DOI] [PubMed] [Google Scholar]