Abstract
Background and Objectives.
Many medications have cognitive impairment, memory loss, amnesia, or dementia as side effects (“cognitive side effects” hereafter), but little is known about trends in the prevalence of these medications or their implications for population-level cognitive impairment.
Research Design and Methods.
This study uses the nine most recent two-year cycles (1999-2000 to 2015-2016) of the National Health and Nutrition Examination Survey to assess trends in utilization of medications with cognitive side effects for adults aged 60 and older (n=16,937). It then investigates the association between cognitive function and the utilization of medications with cognitive side effects (n=3,177). Cognitive function is measured using several objective cognitive assessments, as well as self-reported confusion and memory loss.
Results.
Between 1999-2000 and 2015-2016, the prevalence of older adults taking one, two, and at least three medications with cognitive side effects increased by 10.5% (p=0.129), 55% (p<0.001), and 279% (p<0.001), respectively. Compared to individuals taking no medications with cognitive side effects, those taking at least three medications with cognitive side effects score 0.22 to 0.32 standard deviations lower in word learning and recall (p<0.01), animal fluency (p<0.01), digit symbol substitution (p<0.001), the average standardized score of the three assessments (p<0.001); and are 1.5 to 3 times more likely to report incidents of confusion or memory loss.
Discussion and Implications.
Exposure to medications with cognitive side effects is associated with increased risk of cognitive impairment. These findings highlight the need for cognitive function screenings among patients consuming medications with cognitive side effects. They also highlight one important implication of the recent rise in polypharmacy.
Keywords: Prescription medications, side effects, cognitive impairment, polypharmacy
I. Introduction
Adults aged 65 and older represent the fastest-growing population in the United States and their numbers are expected to nearly double by 2060, creating urgency around the prevention and treatment of aging-related health conditions (Federal Interagency Forum on Aging-Related Statistics (US), 2016). Cognitive impairment has emerged as a significant public health concern for older adults as it leads to loss of independence, worsened quality of life, and increased disability, which in turn have consequences for individuals, families, and government programs (Hurd, Martorell, Delavande, Mullen, & Langa, 2013; Langa et al., 2008; Seeher, Low, Reppermund, & Brodaty, 2013). In 2002, more than ten million U.S. adults aged 70 and older lived with dementia or milder cognitive impairments without dementia, with an expected doubling by 2050 (B L Plassman et al., 2007; Brenda L Plassman et al., 2008). Although the prevalence of cognitive impairment has declined gradually in the past decades due to better control of some key risk factors (Langa et al., 2017; Sheffield & Peek, 2011), substantial growth in the absolute number of older adults living with cognitive impairment continues to expand the scope of this public health concern.
Given growing concern about cognitive impairment, previous studies have attempted to identify risk factors for cognitive impairment, such as age, socio-demographic status, chronic conditions, and health behaviors (Livingston et al., 2017). Despite these efforts, little is known about the consequences of using prescription medications with cognitive side effects on cognitive function. This is a potentially significant omission. Prescription medications have become increasingly common among older adults. In 2011-2012, 90% reported using at least one medication and 40% took five or more in the past month, compared to 82% and 22% in 1999-2000, respectively (Kantor, Rehm, Haas, Chan, & Giovannucci, 2015). While there is evidence that pharmaceutical innovations improve health, a growing concern has emphasized the adverse effect of commonly used medications on health, especially under conditions of polypharmacy. Particularly, older adults taking multiple medications concurrently are two times more likely to experience adverse drug events and four times more likely to be hospitalized due to adverse drug events, compared to those taking fewer medications (Bourgeois, Shannon, Valim, & Mandl, 2010; Marcum et al., 2012; Nguyen, Fouts, Kotabe, & Lo, 2006).
Prior studies on medications with cognitive side effects have produced contradictory results over various kinds of medication. Studies have found that benzodiazepines, lorazepam, and oxybutynin significantly increase the incidence of amnestic and non-amnestic cognitive impairments, and the dose-response relationship persists even after controlling for potential confounders (Tannenbaum, Paquette, Hilmer, Holroyd-Leduc, & Carnahan, 2012). H(1)-antihistamine agents and tricyclic antidepressants may have a more limited effect: evidence indicates only that they induce non-amnestic deficits in attention and information processing (Tannenbaum et al., 2012). Other studies have found that benzodiazepines, tricyclic antidepressants, first-generation antihistamines, and bladder antimuscarinics are associated with an increased risk of Alzheimer’s disease, raising concerns that the cognitive side effects of medications are irreversible and long-lasting (de Gage et al., 2014; Gray et al., 2015). Yet, a handful of studies have reported a trivial and potentially non-causal increase in cognitive deficits as a result of using benzodiazepine (Gray et al., 2016; Imfeld, Bodmer, Jick, & Meier, 2015).
Although prior studies have provided some evidence for an association between cognitive impairment and medications with cognitive side effects, further investigation of this topic is warranted. It possible that cognitive side effects of such medications are much more pronounced in naturalistic and larger population settings. Most studies have been clinical in nature, exploring the effects of a single class of medication, or using relatively small samples. For this reason, little is known about how frequently such medications are used in the larger adult population or about how the use of such medications has changed. Moreover, little is known about how many adults consume multiple such medications and the consequences of such combinations for cognitive health. Even if much of the evidence suggests that the risks associated with a single medication are small, the total impact of medications with cognitive side effects on population-level cognitive health could be much larger, especially in a context of significant polypharmacy.
This study improves the previous literature by using a nationally representative survey and a database of all medications that have been previously linked to cognitive side effects. Our study has two aims:
What are the trends in the utilization of medications with cognitive side effects from 1999 to 2016 among adults aged 60 and older?
What is the relationship between cognitive function and utilization of medications with cognitive side effects for older adults?
II. Design and Methods
Data
This study uses the National Health and Nutrition Examination Survey (NHANES), a nationally representative survey of the civilian noninstitutionalized U.S. population. The survey consists of an interview that documents respondents’ socio-demographic characteristics, health conditions, cognitive functioning, and medications taken in the last 30 days, followed by a physical examination. NHANES data is obtained using a complex and multistage probability sampling design to represent the general population but with an oversampling of Black, Hispanic, and persons aged 60 and older. The survey non-response rate is, on average, 22%. All analyses use survey weight to produce nationally-representative estimates and to avoid potential non-response bias. This study first relies on data from all nine most recent two-year cycles (1999-2000 to 2015-2016) to assess trends in the utilization of medication with cognitive side effects for older adults aged 60 and above (n=16,937). We then use data from the two-year cycles in 2011-2012 and 2013-2014 to investigate the association between cognitive function and utilization of medications with cognitive side effects (n=3,177). Information on cognitive function is only available in these years.
Cognitive Function Measurements
This study measures cognitive function using a series of self-reports and assessments in 2011-2012 and 2013-2014. Non-response among those administered the cognitive function questions ranges from 0.1% to 5% among 3,177 respondents. Self-reported measures of cognitive function include two questions that have been previously used to differentiate persons with various levels of cognitive impairment (Aigbogun, Stellhorn, Krasa, & Kostic, 2017). The first question asks if, during the past 12 months, a respondent has experienced confusion or memory loss that is happening more often or is getting worse. Response categories were yes or no, and we create a binary variable indicating those who gave an affirmative response. The second question asks if, during the past seven days, a respondent had trouble remembering where they put things, like their keys or wallet. Possible responses include “never,” “about once,” “two or three times,” “nearly every day,” or “several times a day.” Those who had trouble remembering at least two times are classified as having cognitive impairment.
The objective cognitive assessments consist of three tests, including word learning and recall, animal fluency, and digit symbol substitution. These assessments remain unchanged in both survey cycles. The word leaning and recall assessment has been successfully implemented in major epidemiological studies in various ethnic and cultural contexts to investigate immediate and delayed learning ability for new verbal information (Fillenbaum et al., 2008; Prince et al., 2003). The test is comprised of three trials and one delayed recall challenge. In each trial, respondents are asked to read out loud each of the ten unrelated words, one at a time, as they are presented on a computer. Following the presentation, respondents are asked to recall, in no particular order, as many of the ten words as possible. The order of the words presented to respondents changes in each trial. The delayed word recall challenge takes place after the animal fluency and digit symbol substitution tests are completed (approximately 8-10 minutes following the start of the word learning trials). Each correct word is worth one point, and the maximum score is ten. Overall, 3,123 respondents completed this test.
The animal fluency assessment examines verbal fluency that is independent of formal educational attainment (Prince et al., 2003). The test has been proven to differentiate persons with normal cognition from those with mild cognitive impairment and more-severe impairment such as Alzheimer’s disease (Henry, Crawford, & Phillips, 2004). Respondents are instructed to name as many animals as they can in one minute. Each distinct animal is worth one point. The total observed score ranges from 1 to 40. 3,107 respondents completed this assessment.
Finally, the digit symbol substitution assessment is adopted from the Wechsler Adult Intelligence Scale and is used to assess processing speed, sustained attention, and working memory (Dumont & Willis, 2008). The test is conducted on a sheet of paper that contains a key at the top with nine numbers, each paired with a symbol. Respondents are asked to copy the corresponding symbols to 133 boxes underneath adjoining numbers. Each correct symbol is worth one point, and the total observed score ranges from 0 to 105. 3,012 respondents completed this assessment.
Using the total scores, we constructed two sets of outcome variables for each assessment. The first set of variables are the standardized scores for each assessment in a given year. The second set of variables are indicators for whether a respondent is in the top 25% of the distribution of an assessment in a given year. Finally, we constructed two composite variables for those who completed all three assessments to represent global cognitive function (n=2,935), an average standardized score of the three assessments and a binary indicator of whether a person’s standardized scores were in the top 25% for at least two assessments.
Although these measures cannot substitute for clinical diagnoses of cognitive impairment, they do provide meaningful information to study the association between cognitive impairment and medications with cognitive side effect.
Prescription Medications
NHANES documents the use of medications within the last month. Respondents who took any medications in the last month were asked to show interviewers the medication containers of all medications they had taken. If respondents could not show a container, they were asked to verbally report the medication’s name. When interviewers entered complete medication names into a computer, 96% of entries resulted in automatic matches with an existing drug. The drug database used for the match was obtained from Lexicon Plus, a proprietary database of Cerner Multum that provides, on an annual basis, a comprehensive list of all prescription and some non-prescription medications available in the U.S. market.
Prescription Medications with Cognitive Impairment Side Effects
We use the Micromedex database to identify medications with cognitive side effects. Prior studies have independently established the accuracy and reliability of adverse effects listed in Micromedex (Cheng, Guglielmo, Maselli, & Auerbach, 2010). The database is based on several sources: the U.S. Food and Drug Administration’s black box warnings, MedWatch, post-marketing surveillance, clinical trials, and comprehensive literature reviews. In our study, medications with cognitive side effects were identified using a keyword search including the following words: cognitive impairment, cognitive decline, memory loss, amnesia, and dementia. Using these words, we identified 102 medications. It is important to note that this number does not represent the full set of medications with such side effects in the U.S market, but rather the number of medications with cognitive side effects that are consumed by respondents aged 60 and older in the NHANES from 1999-2000 to 2015-2016 (see Supplementary Table 1). We include all medications with cognitive side effects, irrespective of any reported frequency of those side effects as reported in Micromedex. This decision likely underestimates the association between cognitive function and the utilization of medications with cognitive side effects, though it is possible that small clinical trials underestimate the prevalence of side-effects among those who take the drug. Using the reported number of medications with cognitive side effects, we constructed a variable that indicates whether in the past 30 days, a respondent took no medications with cognitive side-effects (the reference category in the regression models), one medication, two medications, or at least three medications with cognitive side effects.
Other Variables
Depression.
The models control for the presence of depression, which can confound the relationship between medications and cognitive impairment. Late-life depression has been found to increase the risk of dementia by twofold (Cherbuin, Kim, & Anstey, 2015). Furthermore, many medications with cognitive side effects, such as benzodiazepines, are prescribed to treat anxiety and insomnia, which can be prodromal symptoms of dementia. The NHANES includes a nine-item depression-screening instrument from the Patient Health Questionnaire. Respondents were asked the frequency with which, in the past two weeks, they had little interest in doing things; felt down, depressed, or hopeless; had trouble sleeping or slept too much; felt tired or had little energy; had poor appetite or overeating; felt bad about themselves; had trouble concentrating on things; moved or spoke slowly or too fast; and thought they would be better off dead. Responses to these questions were “not at all,” “several days,” “more than half the days,” and “nearly every day,” and were assigned a corresponding value from 0 to 3. The nine items were then combined to create a summary score ranging from 0 to 27. Following recommendations from previous studies, we classified respondents as likely having major depression if their score is 10 or higher (Kroenke, Spitzer, & Williams, 2001).
Obesity.
Previous studies have consistently reported an association between obesity and cognitive deficits (Beydoun, Beydoun, & Wang, 2008). Moreover, obesity has been found to be a risk factor for other chronic conditions that require pharmaceutical treatment (Luppino et al., 2010; Samper-Ternent & Al Snih, 2012). Using measured height and weight, we calculated respondent BMI and created an indicator for whether the person was obese.
Chronic conditions.
Many medications with cognitive side effects are intended for the treatment of chronic conditions. We control for comorbidities in all analysis models, as they are associated with an increased risk of cognitive decline (Livingston et al., 2017). Respondents were asked if they had ever been told by a health professional that they had asthma, arthritis, cancer, congestive health failure, coronary heart diseases, heart attack, angina, emphysema, bronchitis, stroke, hypertension, or diabetes. While these variables are self-reported and may be subject to measurement error, they capture the presence of these conditions on the respondent’s lifetime and not just at the time of the interview. Inclusivity of this sort is important for our models. Studies that find an association between health conditions and cognitive impairment usually measure health conditions in mid-life rather than late-life (Rosengren, Skoog, Gustafson, & Wilhelmsen, 2005; Whitmer, Sidney, Selby, Johnston, & Yaffe, 2005). Each of these chronic conditions is introduced in the models as a binary variable.
Other covariates.
In all models we control for socio-demographic variables that are potentially associated with one’s cognitive function and use of medications, such as age, gender, marital status (married/living with a partner: reference category, widowed/divorced/separated, or never married), educational attainment (less than high school: reference category, high school graduate, some college or two-year degree, college graduate or higher), whether the person’s household income is under the federal poverty threshold, and whether the person has any health insurance. We also include a dummy variable for year to account for any trend in the outcome.
Statistical Analysis
To adjust for complex and multistage sampling weighted prevalence estimates of medications with cognitive side effects in each year were calculated using Taylor linearization methods. The statistical significance of trends of medications with cognitive side effects was assessed using logistic regression. Weighted multivariate linear least-squared and logistic regression models were used to investigate the association between cognitive function and the use of medications with cognitive side effects, controlling for potential confounders. We impute missing data for all control variables using the STATA module for multiple imputation with chained equations. Twenty imputed datasets were generated and were used in all analyses. Most control variables have a small number of missing cases (<1%), except for depression and poverty status, which have up to 12% missing cases.
III. Results
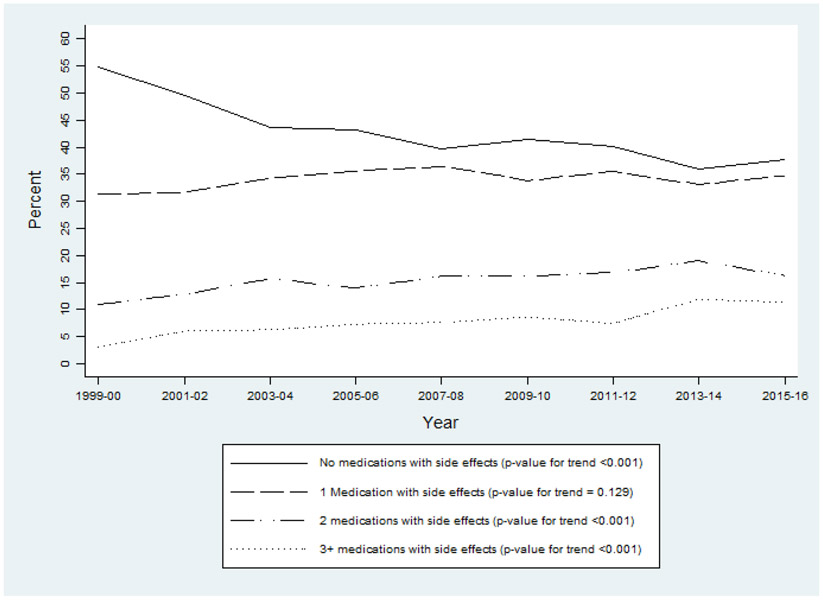
1. Trend in utilization of medications with cognitive side effects
In Figure 1, we first present the trend in utilization of medications with cognitive side effects from 1999-2000 to 2015-2016 for adults aged 60 and older. In 1999-2000, approximately 55% of U.S. older adults did not consume any medications with cognitive side effects. In 2015-2016, this estimate declined to 38% (p-value for trend <0.001). Further investigations reveal that the prevalence of older adults taking one medication with such side effects increased modestly from 31.4% to 34.7% over the same period of time (p-value for trend = 0.129). The largest increase was concentrated among those who consume two or more such medications. Relatively to 1999-2000, the prevalence of older adults taking two and at least three medications with cognitive side effects in 2015-2016 went up by 55% (p-value for trend <0.001) and 279% (p-value for trend <0.001), respectively. Compared to trends of utilization of medications in general among older adults, which increased by 7% between 1999-2000 and 2011-2012 (Kantor et al., 2015), we observe a much larger relative increase in the utilization of medications with cognitive side effects.
Figure 1.
Weighted Prevalence of U.S. Adults Aged 60+ Taking Medications with Cognitive Side Effects. Data Source: NHANES 1999-2000 to 2015-2016.
Supplementary Table 2 lists the 25 medications with the largest change over time in absolute prevalence. A large proportion of the total increase in the prevalence of medications with cognitive side effects can be attributed to certain medications or classes of medication: medications that treat high cholesterol and reduce risks of heart attack or stroke, such as Simvastatin (+8.54%), Atorvastatin (+7.63%), Pravastatin (+4.67%), Rosuvastatin (+4.54%), and Lovastatin (+1.92%); gastrointestinal agents, such as Omeprazole (+5.94%), Esomeprazole (+3.82), and Pantoprazole (+2.53%); and central nervous agents, such as Gabapentin (+3.79%), Pregabalin (+1.70%), Zolpidem (+1.47%), Fluoxetine (+1.44%), Alprazolam (+1.26%), Clonazepam (+1.14%), and Baclofen (+0.87%). A majority of these medications are intended for treatment and control of health conditions that have been associated with an increased risk of cognitive impairment such as hypercholesterolemia (Whitmer et al., 2005), depression (Cherbuin et al., 2015), or cardiovascular diseases (Luchsinger et al., 2005).
2. Descriptive Statistics
Panel A of Table 1 presents descriptive statistics for cognitive function measurements by the number of medications with cognitive side effects. Consistently across all cognitive assessments, those who take more medications with cognitive side effects score lower on cognitive assessments and are more likely to report confusion, memory loss, and trouble remembering. For example, those who do not consume any medications with cognitive side effects score on average 25.51 on the summary measure of the three assessments, while those taking at least three medications with cognitive side effects score on average 23.4. Similarly, 30% of respondents who take at least three medications with cognitive side effects report having confusion, memory loss, or trouble remembering, compared to less than 16% of those who do not consume medications with cognitive side effects.
Table 1.
Descriptive Statistics of Cognitive Function, Medication Utilization, and Control Variables Among U.S. Adults Aged 60+. Data Source: NHANES 2011-2012 and 2013-2014.
| All respondents |
Number of medications with cognitive side effects taken by respondents | P-value a | ||||
|---|---|---|---|---|---|---|
| None | 1 medication | 2 medications | 3+ medications | |||
| Panel A: Cognitive function b | ||||||
| Cognitive functioning assessments (mean score) | ||||||
| Delayed word recall (N=3,123) | 6.25 | 6.37 | 6.14 | 5.97 | 5.78 | p=0.009 |
| Animal fluency (N=3,107) | 18.12 | 18.62 | 17.75 | 17.58 | 16.71 | p=0.002 |
| Digit symbol substitution (N=3,012) | 52.14 | 54.33 | 52.10 | 49.90 | 46.91 | p<0.001 |
| Average score of three tests (N=2,935) | 25.51 | 26.53 | 25.49 | 24.54 | 23.40 | p<0.001 |
| Self-reporting cognitive function (proportion) | ||||||
| Had confusion/memory loss last 12mo (N=3,175) | 0.14 | 0.09 | 0.11 | 0.18 | 0.30 | p<0.001 |
| Had trouble remembering last 7 days (N=3,171) | 0.20 | 0.16 | 0.17 | 0.26 | 0.30 | p=0.006 |
| Panel B: Medication utilization, socio-demographic characteristics and health conditions c | ||||||
| Whether taking drugs WITH cognitive side effects (proportion) | ||||||
| None | 0.38 | |||||
| 1 drug | 0.34 | |||||
| 2 drugs | 0.18 | |||||
| 3+ drugs | 0.10 | |||||
| Whether taking drugs WITHOUT cognitive side effects (proportion) | p<0.001 | |||||
| None | 0.18 | 0.34 | 0.12 | 0.06 | 0.03 | |
| 1 drug | 0.18 | 0.26 | 0.16 | 0.11 | 0.05 | |
| 2 drugs | 0.17 | 0.16 | 0.19 | 0.21 | 0.07 | |
| 3+ drugs | 0.47 | 0.24 | 0.52 | 0.62 | 0.85 | |
| Race & ethnicity (proportion) | p=0.036 | |||||
| Non-Hispanic White | 0.79 | 0.76 | 0.79 | 0.81 | 0.83 | |
| Hispanic | 0.07 | 0.09 | 0.06 | 0.07 | 0.06 | |
| Non-Hispanic Black | 0.09 | 0.09 | 0.09 | 0.08 | 0.07 | |
| Non-Hispanic others | 0.05 | 0.06 | 0.05 | 0.04 | 0.04 | |
| Female (proportion) | 0.55 | 0.54 | 0.51 | 0.58 | 0.63 | p=0.015 |
| Age (proportion) | p<0.001 | |||||
| 60-69 | 0.55 | 0.61 | 0.52 | 0.49 | 0.56 | |
| 70-79 | 0.30 | 0.27 | 0.33 | 0.32 | 0.26 | |
| 80+ | 0.15 | 0.12 | 0.16 | 0.19 | 0.19 | |
| Marital status (proportion) | p=0.093 | |||||
| Married or living with partner | 0.64 | 0.62 | 0.66 | 0.66 | 0.62 | |
| Widowed, divorced, separated | 0.32 | 0.32 | 0.30 | 0.32 | 0.34 | |
| Never married | 0.04 | 0.06 | 0.04 | 0.03 | 0.04 | |
| Education (proportion) | p=0.019 | |||||
| Less than high school | 0.17 | 0.14 | 0.17 | 0.21 | 0.19 | |
| High school graduate | 0.22 | 0.21 | 0.23 | 0.23 | 0.21 | |
| Some college or AA degree | 0.31 | 0.32 | 0.31 | 0.29 | 0.34 | |
| College graduate or above | 0.30 | 0.34 | 0.29 | 0.27 | 0.26 | |
| Poverty (proportion) | p=0.385 | |||||
| <100% poverty threshold | 0.10 | 0.10 | 0.09 | 0.11 | 0.11 | |
| Chronic conditions (proportion) | ||||||
| Asthma | 0.14 | 0.12 | 0.12 | 0.15 | 0.30 | p<0.001 |
| Arthritis | 0.50 | 0.38 | 0.48 | 0.65 | 0.75 | p<0.001 |
| Cancer | 0.24 | 0.22 | 0.24 | 0.25 | 0.28 | p=0.354 |
| Congestive heart failure | 0.07 | 0.04 | 0.07 | 0.12 | 0.11 | p<0.001 |
| Coronary heart disease | 0.09 | 0.02 | 0.12 | 0.15 | 0.16 | p<0.001 |
| Heart attack | 0.08 | 0.03 | 0.09 | 0.15 | 0.12 | p<0.001 |
| Angina | 0.06 | 0.02 | 0.05 | 0.11 | 0.13 | p<0.001 |
| Emphysema | 0.05 | 0.03 | 0.03 | 0.08 | 0.09 | p=0.002 |
| Bronchitis | 0.08 | 0.06 | 0.07 | 0.11 | 0.10 | p=0.169 |
| Stroke | 0.07 | 0.05 | 0.07 | 0.08 | 0.09 | p=0.028 |
| Hypertension | 0.40 | 0.39 | 0.41 | 0.37 | 0.42 | p=0.688 |
| Diabetes | 0.21 | 0.10 | 0.26 | 0.28 | 0.30 | p<0.001 |
| Mental Distress (K6 >= 13) (proportion) | 0.08 | 0.04 | 0.07 | 0.10 | 0.19 | p<0.001 |
| Obese (BMI >= 30) (proportion) | 0.38 | 0.32 | 0.37 | 0.43 | 0.50 | p=0.002 |
P-value indicates if means are significantly different across respondents who take none, one, two, or at least three medications with cognitive side effects, based on linear least-squared regression and survey weights.
Although this study includes 3,177 adults aged 60 and older, the sample size for each cognitive measure may vary due to missing data.
All medication utilization, socio-demographic and health conditions estimates are based on a full sample of 3,177 adults aged 60 and older, of which 1,252 did not consume any medications with cognitive side effects, 1,028 used one medication with cognitive side effects, 584 used two medications with cognitive side effects, and 313 used at least three medications with side effects in the past month.
Panel B of Table 1 describes differences in socio-demographic characteristics and health conditions by the number of medications with cognitive side effects. Compared to respondents who do not consume medications with cognitive side effects, those who take at least three medications with such side effects are more likely to also consume medications without cognitive side effects (p<0.001), more likely to be White (p=0.036), female (p=0.015), older (p<0.001), less likely to have a college degree or higher (p=0.019), and are more likely to report health conditions such as asthma, arthritis, congestive heart failure, coronary heart disease, heart attack, angina, emphysema, stroke, diabetes, obesity, and depression.
3. Association between cognitive function and medications with side effects
In Table 2, we demonstrate the association between cognitive impairment and the number of medications with cognitive side effects consumed by respondents aged 60 and older. We find that compared to respondents who do not consume medications with cognitive side effects, those who take at least three medications score 0.22 to 0.32 standard deviation lower in word learning and recall (p<0.01), animal fluency (p>0.01), digit symbol substitution (p<0.001), and the summary score (p<0.001). Similarly, taking at least three medications with side effects reduces the probability of being in the top 25% of the distribution by 40-50 percentage points across all three measurements (p<0.05) and the probability of scoring in the top 25% by 57% for at least two out of three assessments (p<0.05). We find a similar relationship between self-reported cognitive function and medications with cognitive side effects. Particularly, taking two or at least three medications with cognitive side effects increase the likelihood of reporting confusion or memory loss in the past year, or trouble remembering in the last seven days, by 1.5 to 3 times compared to the reference group. Taking numerous such medications is critical: The association between medications with cognitive side effects and cognitive function is small and not statistically significant for those taking fewer than three medications (for cognitive assessment outcomes) and fewer than two medications (for self-reporting outcomes). Nonetheless, we observe a dose-response relationship such that the association between medications with cognitive side effects and cognitive function increases over each category of additional medication.
Table 2.
OLS and Logistic Regressions of Cognitive Function on Medications with Cognitive Side Effects for Adults Aged 60+. Data Source: NHANES 2011-2012 and 2013-2014.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome: | Word learning and recalls | Animal Fluency | Digit Symbol Substitution | Composite Measurement | Self-reported cognitive impairment |
|||||
| Standardized score |
Whether in top 25 percent |
Standardized score |
Whether in top 25 percent |
Standardized score |
Whether in top 25 percent |
Average standardized score |
Whether 2+ tests in top 25 percent |
Had confusion or memory loss last 12 months |
Had trouble remembering last 7 days |
|
| Coef. (SE) | OR (SE) | Coef. (SE) | OR (SE) | Coef. (SE) | OR (SE) | Coef. (SE) | OR (SE) | OR (SE) | OR (SE) | |
| Whether taking drugs WITH cognitive side effects | ||||||||||
| None (reference) | ||||||||||
| 1 drug (0/1) | −0.015 (0.051) |
1.047 (0.156) |
−0.036 (0.061) |
0.888 (0.116) |
−0.011 (0.043) |
0.820 (0.144) |
−0.020 (0.038) |
0.885 (0.171) |
1.139 (0.195) |
1.024 (0.172) |
| 2 drugs (0/1) | −0.096 (0.050) |
0.852 (0.126) |
−0.012 (0.080) |
0.952 (0.161) |
−0.043 (0.054) |
0.741 (0.130) |
−0.049 (0.047) |
0.835 (0.142) |
1.729** (0.300) |
1.569* (0.302) |
| 3+ drugs (0/1) | −0.240** (0.085) |
0.581* (0.152) |
−0.223** (0.079) |
0.506* (0.139) |
−0.317*** (0.062) |
0.399** (0.111) |
−0.240*** (0.062) |
0.430* (0.140) |
3.024*** (0.728) |
1.697* (0.341) |
| Race & ethnicity | ||||||||||
| Non-Hispanic White (reference) | ||||||||||
| Hispanic (0/1) | −0.261*** (0.052) |
0.609** (0.095) |
−0.288*** (0.060) |
0.474*** (0.063) |
−0.650*** (0.050) |
0.325*** (0.043) |
−0.390*** (0.046) |
0.383*** (0.056) |
1.289 (0.265) |
1.643** (0.271) |
| Non-Hispanic Black (0/1) | −0.200*** (0.054) |
0.699* (0.102) |
−0.535*** (0.052) |
0.333*** (0.044) |
−0.660*** (0.045) |
0.194*** (0.038) |
−0.440*** (0.039) |
0.286*** (0.048) |
0.706* (0.105) |
0.966 (0.142) |
| Non-Hispanic others (0/1) | 0.064 (0.077) |
1.044 (0.317) |
−0.513*** (0.105) |
0.305*** (0.097) |
−0.173** (0.060) |
0.819 (0.212) |
−0.202*** (0.053) |
0.590 (0.164) |
1.274 (0.251) |
0.906 (0.242) |
| Female (0/1) | 0.315*** (0.032) |
2.361*** (0.207) |
−0.006 (0.054) |
0.917 (0.101) |
0.310*** (0.035) |
2.449*** (0.418) |
0.204*** (0.029) |
1.965*** (0.311) |
0.944 (0.126) |
1.454** (0.177) |
| Age (continuous) | −0.047*** (0.004) |
0.905*** (0.009) |
−0.045*** (0.004) |
0.912*** (0.008) |
−0.055*** (0.003) |
0.872*** (0.010) |
−0.049*** (0.003) |
0.866*** (0.010) |
1.053*** (0.011) |
1.033** (0.011) |
| Marital status | ||||||||||
| Married or living with partner (reference) | ||||||||||
| Widowed, divorced, separated (0/1) | −0.072 (0.045) |
0.783 (0.107) |
−0.001 (0.048) |
0.783 (0.107) |
−0.078 (0.043) |
0.827 (0.138) |
−0.040 (0.034) |
0.867 (0.124) |
0.943 (0.148) |
1.020 (0.151) |
| Never married (0/1) | −0.067 (0.096) |
0.764 (0.191) |
−0.063 (0.126) |
0.764 (0.191) |
−0.089 (0.100) |
0.932 (0.278) |
−0.055 (0.088) |
0.806 (0.241) |
1.124 (0.379) |
0.530 (0.172) |
| Educational attainment | ||||||||||
| Less than high school (reference) | ||||||||||
| High school graduate (0/1) | 0.098 (0.065) |
1.618* (0.370) |
0.140** (0.049) |
1.618* (0.370) |
0 414*** (0.041) |
2.902*** (0.816) |
0.205*** (0.036) |
2.435** (0.692) |
0.732 (0.149) |
1.105 (0.253) |
| Some college or AA degree | 0.274*** (0.058) |
3.054*** (0.576) |
0.462*** (0.046) |
3.054*** (0.576) |
0.673*** (0.043) |
5.335*** (1.251) |
0.454*** (0.036) |
5.656*** (1.290) |
0.786 (0.140) |
0.919 (0.171) |
| College graduate or above | 0.306** (0.087) |
5.027*** (0.903) |
0.781*** (0.078) |
5.027*** (0.903) |
0.858*** (0.053) |
10.344*** (3.243) |
0.646*** (0.057) |
9.709*** (2.456) |
0.783 (0.167) |
0.827 (0.177) |
| Poverty threshold < 100% (0/1) | −0.072 (0.054) |
1.047 (0.175) |
−0.088 (0.060) |
1.047 (0.175) |
−0.246*** (0.054) |
0.581* (0.120) |
−0.135** (0.045) |
0.782 (0.155) |
1.410 (0.250) |
0.918 (0.141) |
| Has any health insurance (0/1) | 0.146* (0.066) |
1.647 (0.538) |
0.127 (0.111) |
1.647 (0.538) |
0.222** (0.068) |
1.446 (0.378) |
0.172** (0.059) |
1.963* (0.500) |
0.853 (0.246) |
0.910 (0.217) |
| Mental Distress (PHQ9 >= 10) (0/1) | −0.138* (0.067) |
0.869 (0.174) |
−0.250** (0.071) |
0.869 (0.174) |
−0.216* (0.085) |
0.744 (0.227) |
−0.211*** (0.055) |
0.926 (0.215) |
4.956*** (1.073) |
2.687*** (0.493) |
| Obese (BMI >= 30) (0/1) | 0.092* (0.043) |
1.173 (0.146) |
0.084 (0.052) |
1.173 (0.146) |
0.066 (0.041) |
1.041 (0.143) |
0.074 (0.038) |
1.083 (0.144) |
0.829 (0.138) |
0.774 (0.117) |
| Year fixed effect (2013-2014 vs. 2011-2012) | 0.071 (0.047) |
0.932 (0.101) |
−0.082 (0.051) |
0.932 (0.101) |
−0.089* (0.036) |
1.000 (0.142) |
−0.036 (0.032) |
0.843 (0.123) |
0.930 (0.121) |
0.836 (0.111) |
| Chronic conditions a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Number of observations | 3123 | 3123 | 3107 | 3107 | 3012 | 3012 | 2935 | 2935 | 3175 | 3171 |
Notes:
p<0.05
p<0.01
p<0.01. Standard errors are in parenthesis. All analyses are weighted using survey weight.
Chronic conditions include a set of binary indicators for whether a person has ever been told by a health professional that they have asthma, arthritis, cancer, congestive health failure, coronary heart diseases, heart attack, angina, emphysema, bronchitis, stroke, diabetes, or hypertension.
4. Sensitivity analysis
In a sensitivity analysis, we introduce a categorical variable for the number of medications without cognitive side effects into the models. If the relationship between medications with cognitive side effects and cognitive function is driven by unobserved heterogeneity in health, the relationship between medications without cognitive side effects and cognitive function should be equally significant as that between medications with cognitive side effects and cognitive function. Table 3 shows no significant relationship between medications without cognitive side effects and cognitive impairment, except for the digit symbol substitution outcome in Model 3, and in this case the coefficient is smaller than the coefficient for three or more medications with side effects. In these models, the association between medications with side effects and cognitive function is still statistically significant for those taking at least three medications with side effects.
Table 3:
Sensitivity Analysis for OLS and Logistic Regressions of Cognitive Function on Medications with Cognitive Side Effects for Adults Aged 60+. Data Source: NHANES 2011-2012 and 2013-2014.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Word learning and recalls | Animal Fluency | Digit Symbol Substitution | Composite Measurement | Self-reported cognitive impairment |
||||||
| Outcome: | Standardized score |
Whether in top 25 percent |
Standardized score |
Whether in top 25 percent |
Standardized score |
Whether in top 25 percent |
Standardized score |
Whether 2+ tests in top 25 percent |
Had confusion or memory loss last 12 months |
Had trouble remembering last 7 days |
| Coef. (SE) | OR (SE) | Coef. (SE) | OR (SE) | Coef. (SE) | OR (SE) | Coef. (SE) | OR (SE) | OR (SE) | OR (SE) | |
| Whether taking drugs WITH cognitive side effects | ||||||||||
| None (reference) | ||||||||||
| 1 drug (0/1) | −0.004 (0.052) |
1.045 (0.155) |
−0.026 (0.068) |
0.924 (0.142) |
0.016 (0.048) |
0.905 (0.172) |
−0.003 (0.043) |
0.889 (0.200) |
1.098 (0.190) |
0.989 (0.168) |
| 2 drugs (0/1) | −0.082 (0.049) |
0.850 (0.124) |
−0.001 (0.081) |
0.998 (0.181) |
−0.013 (0.054) |
0.825 (0.153) |
−0.029 (0.047) |
0.837 (0.146) |
1.651** (0.265) |
1.501* (0.280) |
| 3+ drugs (0/1) | −0.225* (0.086) |
0.581* (0.152) |
−0.206* (0.087) |
0.544* (0.159) |
−0.266*** (0.063) |
0.485* (0.137) |
−0.212** (0.063) |
0.441* (0.159) |
2.834*** (0.727) |
1.616* (0.300) |
| Whether taking drugs WITHOUT cogiitive side effects | ||||||||||
| None (reference) | ||||||||||
| 1 drug (0/1) | 0.039 (0.066) |
1.044 (0.180) |
−0.114 (0.087) |
0.786 (0.172) |
−0.078 (0.076) |
0.801 (0.180) |
−0.056 (0.056) |
0.987 (0.278) |
1.280 (0.298) |
0.938 (0.242) |
| 2 drugs (0/1) | −0.045 (0.060) |
1.021 (0.175) |
−0.044 (0.084) |
0.878 (0.175) |
−0.049 (0.066) |
0.849 (0.185) |
−0.052 (0.055) |
1.075 (0.255) |
1.130 (0.294) |
1.159 (0.270) |
| 3+ drugs (0/1) | −0.031 (0.045) |
1.019 (0.157) |
−0.082 (0.081) |
0.772 (0.165) |
−0.156* (0.062) |
0.571* (0.117) |
−0.091 (0.046) |
0.953 (0.243) |
1.298 (0.305) |
1.134 (0.221) |
| Race & ethnicity | ||||||||||
| Non-Hispanic White (reference) | ||||||||||
| Hispanic (0/1) | −0.261*** (0.052) |
0.610** (0.095) |
−0.293*** (0.058) |
0.467*** (0.061) |
−0.657*** (0.050) |
0.315*** (0.041) |
−0.394*** (0.045) |
0.383*** (0.056) |
1.306 (0.264) |
1.643** (0.271) |
| Non-Hispanic Black (0/1) | −0 199*** (0.054) |
0.699* (0.100) |
−0.534*** (0.052) |
0.335*** (0.044) |
−0.655*** (0.045) |
0195*** (0.038) |
−0.438*** (0.039) |
0.287*** (0.047) |
0.704* (0.105) |
0.966 (0.142) |
| Non-Hispanic others (0/1) | 0.062 (0.076) |
1.045 (0.320) |
−0.513*** (0.104) |
0.305*** (0.097) |
−0.173** (0.060) |
0.821 (0.213) |
−0.203*** (0.053) |
0.593 (0.163) |
1.278 (0.248) |
0.906 (0.242) |
| Female (0/1) | 0.316*** (0.032) |
2.361*** (0.206) |
−0.005 (0.053) |
0.919 (0.100) |
0.312*** (0.035) |
2 493*** (0.425) |
0.205*** (0.029) |
1.965*** (0.313) |
0.941 (0.127) |
1.454** (0.177) |
| Age (continuous) | −0.047*** (0.004) |
0.905*** (0.009) |
−0.045*** (0.004) |
0 914*** (0.008) |
−0.054*** (0.003) |
0.875*** (0.011) |
−0.048*** (0.003) |
0.867*** (0.010) |
1.051*** (0.011) |
1.033** (0.011) |
| Marital status | ||||||||||
| Married or living with partner (reference) | ||||||||||
| Widowed, divorced, separated (0/1) | −0.072 (0.045) |
0.877 (0.131) |
−0.003 (0.048) |
0.780 (0.105) |
−0.080 (0.044) |
0.823 (0.138) |
−0.041 (0.034) |
0.868 (0.123) |
0.948 (0.149) |
1.021 (0.150) |
| Never married (0/1) | −0.067 (0.096) |
0.893 (0.264) |
−0.066 (0.128) |
0.755 (0.190) |
−0.093 (0.099) |
0.930 (0.280) |
−0.057 (0.089) |
0.807 (0.239) |
1.131 (0.382) |
0.529 (0.172) |
| Educational attainment | ||||||||||
| Less than high school (reference) | ||||||||||
| High school graduate (0/1) | 0.098 (0.065) |
1.336 (0.246) |
0.143** (0.049) |
1.625* (0.370) |
0 415*** (0.040) |
2.908*** (0.812) |
0.206*** (0.036) |
2.431** (0.685) |
0.729 (0.148) |
1.108 (0.253) |
| Some college or AA degree | 0.276*** (0.058) |
2 357*** (0.456) |
0.461*** (0.046) |
3.048*** (0.576) |
0.672*** (0.044) |
5.334*** (1.256) |
0 454*** (0.036) |
5.635*** (1.289) |
0.789 (0.140) |
0.916 (0.168) |
| College graduate or above | 0.305** (0.087) |
2.412*** (0.558) |
0.780*** (0.077) |
5.023*** (0.894) |
0.855*** (0.053) |
10.379*** (3.225) |
0.645*** (0.057) |
9.685*** (2.481) |
0.786 (0.168) |
0.834 (0.175) |
| Poverty threshold < 100% (0/1) | −0.074 (0.053) |
0.900 (0.147) |
−0.082 (0.060) |
1.061 (0.181) |
−0.243*** (0.054) |
0.587* (0.120) |
−0.133** (0.045) |
0.784 (0.153) |
1.394 (0.246) |
0.922 (0.139) |
| Has any health insurance (0/1) | 0.145* (0.063) |
1.643* (0.347) |
0.135 (0.112) |
1.685 (0.562) |
0.231** (0.070) |
1.493 (0.394) |
0.177** (0.059) |
1.969* (0.509) |
0.836 (0.241) |
0.909 (0.210) |
| Mental Distress (PHQ9 >= 10) (0/1) | −0.135 (0.067) |
0.883 (0.189) |
−0.252** (0.072) |
0.867 (0.174) |
−0.214* (0.086) |
0.753 (0.237) |
−0.210*** (0.055) |
0.928 (0.216) |
4 957*** (1.074) |
2.665*** (0.485) |
| Obese (BMI >= 30) (0/1) | 0.096* (0.042) |
0.994 (0.117) |
0.087 (0.054) |
1.192 (0.153) |
0.076 (0.043) |
1.088 (0.151) |
0.080* (0.039) |
1.089 (0.146) |
0.820 (0.131) |
0.764 (0.112) |
| Year fixed effect (2013-2014 vs. 2011-2012) | 0.073 (0.046) |
0.822 (0.118) |
−0.083 (0.051) |
0.929 (0.100) |
−0.089* (0.036) |
1.006 (0.146) |
−0.036 (0.032) |
0.843 (0.120) |
0.932 (0.121) |
0.832 (0.110) |
| Chronic conditions a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Number of observations | 3123 | 3123 | 3107 | 3107 | 3012 | 3012 | 2935 | 2935 | 3175 | 3171 |
Notes:
p<0.05
p<0.01
p<0.01. Standard errors are in parenthesis. All analyses are weighted using survey weight.
Chronic conditions include a set of binary indicators for whether a person has ever been told by a health professional that they have asthma, arthritis, cancer, congestive health failure, coronary heart diseases, heart attack, angina, emphysema, bronchitis, stroke, diabetes, or hypertension.
IV. Discussion and Implications
This study is the first to assess trends in the utilization of prescription medications with cognitive impairment, memory loss, amnesia, and dementia side effects. We find that between 1999-2000 and 2015-2016, the prevalence of older adults taking one, two, or at least three medications with cognitive side effects increased by 10.5%, 55%, and 279%, respectively. Most of the increase in utilization of medications with cognitive side effects was attributed to an increase in the consumption of medications that treat hypercholesterolemia, depression and anxiety, or cardiovascular diseases. We then find that taking three or more such medications is associated with cognitive deficits, compared to those not taking medications with cognitive side effects. The relationship persists even after controlling for socio-demographic characteristics and past and current health conditions.
This study found a rapidly growing prevalence of taking three or more medications with cognitive side effects, as well as broad consequences of such medications for a variety of cognitive outcomes. The role of medications in the cognitive performance of older adults has likely been underappreciated. Although there are numerous guidelines for the diagnosis and treatment of chronic physical diseases (Bingley et al., 2001; Chobanian et al., 2003; Criner et al., 2015; Wender et al., 2013), there are currently no guidelines for the screening of cognitive impairment. Part of this may reflect the limited clinical benefits of such screenings. Following a review of the literature, the U.S. Preventive Services Task Force (2015) concluded that there was insufficient evidence on the benefits of screening for cognitive impairment. Yet a large number of older adults report worrisome cognitive impairments, and medication use may play an increasingly important role in their experience. In tandem with a lack of clinical guidelines for screening cognitive impairment, the growing intensity of diagnosis and treatment for chronic physical diseases may contribute significantly to cognitive impairment among older adults. Future studies should consider the benefits of screening for cognitive impairment particularly among patients who utilize medications with cognitive side effects.
The results also highlight the impact of polypharmacy. The most significant side effects documented in this study are limited to those taking three of more medications. Although most people who take medication with such a side effect take only one or two, polypharmacy is increasingly common, and in our study about 10% of adults are taking three or more medications with side effects. Kantor et al. (2015) found that the prevalence of older adults taking at least five medications increase by 81.8% from 1999-2000 to 2011-2012. This current study found that the prevalence of older adults taking at least three medications with cognitive side effects has increased considerably more, by 279% since 1999-2000. Polypharmacy may present unique risks for side effects, amplifying the effects of each of the medications in a set (Qato et al., 2018). For instance, taking multiple medications is a risk factor for dementia and delirium, as well as other adverse events (Jyrkkä, Enlund, Lavikainen, Sulkava, & Hartikainen, 2011; Martin, Stones, Young, & Bédard, 2000). As the pharmaceutical treatment of chronic disease is common, future research should further investigate its spillover effects to other illnesses.
Limitations
While this study improves the previous literature by using nationally representative data and including a comprehensive list of all medications with cognitive side effects, it faces several limitations. First, we are not able to establish a causal relationship between cognitive function and medications with cognitive side effects. Due to the cross-sectional nature of the NHANES, it is challenging to determine whether medications with cognitive side effects cause cognitive impairment or if cognitive impairment leads to the onset of other health conditions that require pharmaceutical treatment. Second, the medications in this study not only have cognitive side effects but also other side effects that may indirectly influence cognitive function. We address both of these issues by controlling for a comprehensive list of current and past health conditions, including conditions that may influence a respondent’s cognitive function, but there are nonetheless potentially unobserved conditions that are influential. Third, although Micromedex is reliable when it comes to identifying adverse effects of medications, it is possible that there are medications with cognitive side effects that are not included in the database. Moreover, since NHANES only collects data on outpatient and over-the-counter medications, we lack information on medications administered to inpatients at hospitals. To address some forms of unobserved heterogeneity, we follow another similar study by Qato, Ozenberger, & Olfson (2018) and include in some of our models the number of medications without any known cognitive side effects. We find that there is almost no association between medications without such side effects and cognitive function. This suggests both that unobserved heterogeneity with respect to health is unlikely to explain the results and that there are few medications with cognitive side effects that have not been correctly identified by Micromedex.
Conclusion
This study demonstrated a strong relationship between taking multiple medications with cognitive side effects and cognitive functioning. Approximately 10% of older adults take three or more such medications, and this percentage is likely to increase more in the future. The investigation of cognitive side effects is an important frontier for future research.
Supplementary Material
Supplementary Table 1: Medications with Cognitive Side Effects Consumed by U.S. Adults Aged 60+ in NHANES from 1999-2000 to 2015-2016
Supplementary Table 2: Top 25 Medications with the Largest Change in Utilization Among U.S. Adults Aged 60+ from 1999-2000 to 2015-2016. Data Source: NHANES 1999-2000 to 2015-2016.
Reference List
- Aigbogun MS, Stellhorn R, Krasa H, & Kostic D (2017). Severity of memory impairment in the elderly: Association with health care resource use and functional limitations in the United States. Alzheimer’s & Dementia (Amsterdam, Netherlands), 8, 51–59. 10.1016/j.dadm.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun H, & Wang Y (2008). Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obesity Reviews, 9(3), 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingley PJ, Bonifacio E, Ziegler A-G, Schatz DA, Atkinson MA, & Eisenbarth GS (2001). Proposed guidelines on screening for risk of type 1 diabetes. Diabetes Care, 24(2), 398–398. [DOI] [PubMed] [Google Scholar]
- Bourgeois FT, Shannon MW, Valim C, & Mandl KD (2010). Adverse drug events in the outpatient setting: an 11-year national anal ysis. Pharmacoepidemiology and Drug Safety, 19(9), 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CM, Guglielmo BJ, Maselli J, & Auerbach AD (2010). Coverage of FDA medication boxed warnings in commonly used drug information resources. Archives of Internal Medicine, 170(9), 831–833. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Kim S, & Anstey KJ (2015). Dementia risk estimates associated with measures of depression: a systematic review and meta-analysis. BMJ Open, 5(12), e008853. 10.1136/bmjopen-2015-008853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, … Wright JT Jr (2003). Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension, 42(6), 1206–1252. [DOI] [PubMed] [Google Scholar]
- Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, … Camp PG (2015). Prevention of acute exacerbations of COPD: American college of chest physicians and Canadian thoracic society guideline. Chest, 147(4), 894–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gage SB, Moride Y, Ducruet T, Kurth T, Verdoux H, Tournier M, … Bégaud B (2014). Benzodiazepine use and risk of Alzheimer’s disease: case-control study. Bmj, 349, g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont R, & Willis JO (2008). Wechsler Adult Intelligence Scale–Third Edition. Encyclopedia of Special Education, 2129–2130. [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics (US). (2016). Older Americans 2016: Key indicators of well-being. Government Printing Office. [Google Scholar]
- Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC, … Welsh-Bohmer KA (2008). Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): the first twenty years. Alzheimer’s & Dementia, 4(2), 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, … Larson EB (2015). Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Internal Medicine, 175(3), 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Dublin S, Yu O, Walker R, Anderson M, Hubbard RA, … Larson EB (2016). Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. Bmj, 352, i90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, & Phillips LH (2004). Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia, 42(9), 1212–1222. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, & Langa KM (2013). Monetary Costs of Dementia in the United States. New England Journal of Medicine, 368(14), 1326–1334. 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld P, Bodmer M, Jick SS, & Meier CR (2015). Benzodiazepine use and risk of developing alzheimer’s disease or vascular dementia: A case–control analysis. Drug Safety, 38(10), 909–919. [DOI] [PubMed] [Google Scholar]
- Jyrkkä J, Enlund H, Lavikainen P, Sulkava R, & Hartikainen S (2011). Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiology and Drug Safety, 20(5), 514–522. [DOI] [PubMed] [Google Scholar]
- Kantor ED, Rehm CD, Haas JS, Chan AT, & Giovannucci EL (2015). Trends in Prescription Drug Use Among Adults in the United States From 1999-2012. JAMA, 314(17), 1818–1830. 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, & Weir DR (2017). A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Internal Medicine, 177(1), 51–58. 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, & Rosen AB (2008). Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 4(2), 134–144. 10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, … Cohen-Mansfield J (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734. [DOI] [PubMed] [Google Scholar]
- Luchsinger J, Reitz C, Honig LS, Tang M-X, Shea S, & Mayeux R (2005). Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology, 65(4), 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, & Zitman FG (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry, 67(3), 220–229. [DOI] [PubMed] [Google Scholar]
- Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, & Pugh MJV (2012). Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. Journal of the American Geriatrics Society, 60(1), 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NJ, Stones MJ, Young JE, & Bédard M (2000). Development of delirium: a prospective cohort study in a community hospital. International Psychogeriatrics, 12(1), 117–127. [DOI] [PubMed] [Google Scholar]
- Nguyen JK, Fouts MM, Kotabe SE, & Lo E (2006). Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. The American Journal of Geriatric Pharmacotherapy, 4(1), 36–41. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB (2007). Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology, 29(1–2), 125–132. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman Brenda L, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148(6), 427–434. 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Acosta D, Chiu H, Scazufca M, Varghese M, & 10/66 Dementia Research Group. (2003). Dementia diagnosis in developing countries: a cross-cultural validation study. The Lancet, 361(9361), 909–917. [DOI] [PubMed] [Google Scholar]
- Qato DM, Ozenberger K, & Olfson M (2018). Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. Jama, 319(22), 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A, Skoog I, Gustafson D, & Wilhelmsen L (2005). Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Archives of Internal Medicine, 165(3), 321–326. [DOI] [PubMed] [Google Scholar]
- Samper-Ternent R, & Al Snih S (2012). Obesity in older adults: epidemiology and implications for disability and disease. Reviews in Clinical Gerontology, 22(1), 10–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittker J, & Karandinos G (2010). Methuselah’s medicine: Pharmaceutical innovation and mortality in the United States, 1960–2000. Social Science & Medicine, 70(7), 961–968. [DOI] [PubMed] [Google Scholar]
- Seeher K, Low L-F, Reppermund S, & Brodaty H (2013). Predictors and outcomes for caregivers of people with mild cognitive impairment: A systematic literature review. Alzheimer’s & Dementia, 9(3), 346–355. 10.1016/j.jalz.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Sheffield KM, & Peek MK (2011). Changes in the prevalence of cognitive impairment among older Americans, 1993–2004: overall trends and differences by race/ethnicity. American Journal of Epidemiology, 174(3), 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, & Carnahan R (2012). A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs & Aging, 29(8), 639–658. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. (2015). Screening for Cognitive Impairment in Older Adults: Recommendation Statement. US Preventive Services Task Force. [Google Scholar]
- Wender R, Fontham ET, Barrera E Jr, Colditz GA, Church TR, Ettinger DS, … Kelsey DK (2013). American Cancer Society lung cancer screening guidelines. CA: A Cancer Journal for Clinicians, 63(2), 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, & Yaffe K (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 64(2), 277–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Medications with Cognitive Side Effects Consumed by U.S. Adults Aged 60+ in NHANES from 1999-2000 to 2015-2016
Supplementary Table 2: Top 25 Medications with the Largest Change in Utilization Among U.S. Adults Aged 60+ from 1999-2000 to 2015-2016. Data Source: NHANES 1999-2000 to 2015-2016.