Abstract
In light of substantial discoveries in epithelial and hair pigmentation pathophysiology, this review summarizes the current understanding of skin pigmentation mechanisms. Melanocytes are pigment-producing cells and their key regulating transcription factor is the melanocyte specific microphthalmia-associated transcription factor (m-MITF). Ultraviolet (UV) radiation is a unique modulator of skin pigmentation influencing tanning pathways. The delayed tanning pathway occurs as UVB produces keratinocyte DNA damage, causing p53-mediated expression of the pro-opiomelanocortin (POMC) gene that is processed to release α-melanocyte stimulating hormone (α-MSH). α-MSH stimulates the melanocortin 1 receptor (MC1R) on melanocytes, leading to m-MITF expression and melanogenesis. POMC cleavage also releases β-endorphin, which creates a neuro-endocrine pathway that promotes UV seeking behaviors. Mutations along the tanning pathway can affect pigmentation and increase the risk of skin malignancies. MC1R variants have received considerable attention, yet the allele is highly polymorphic with varied phenotypes. Vitiligo presents with depigmented skin lesions due to autoimmune destruction of melanocytes. UVB phototherapy stimulates melanocyte stem cells in the hair bulge to undergo differentiation and upwards migration resulting in perifollicular re-pigmentation of vitiliginous lesions, which is under sophisticated signaling control. Melanocyte stem cells, normally quiescent, undergo cyclic activation/differentiation and downward migration with the hair cycle, providing pigment to hair follicles. Physiologic hair graying results from progressive loss of melanocyte stem cells and can be accelerated by acute stress-induced, sympathetic driven hyperproliferation of the melanocyte stem cells. Ultimately, by reviewing the pathways governing epithelial and follicular pigmentation, numerous areas of future research and potential points of intervention are highlighted.
Keywords: Melanocyte stem cells, perifollicular re-pigmentation, hair graying, tanning, MC1R
Introduction
Disorders of skin pigmentation exist across a broad spectrum and include manifestations of hypopigmentation and hyperpigmentation in a variety of benign and malignant contexts. Ultraviolet (UV) radiation is arguably one of the best-understood carcinogens and carries the dubious distinction of being one the most common carcinogenic exposures for man. UV also functions as a significant modulator of skin pigmentation. The understanding of pathophysiologic mechanisms governing epithelial and hair pigmentation has continued to see significant advances. In this review, we aim to provide a current overview of the understanding of skin pigmentation mechanisms, highlight recent advances, identify future areas of research, and suggest novel points of intervention.
Melanocytes are neural crest-derived, melanin pigment-producing cells that localize to multiple anatomic sites, most prominently the epidermis.1 They are dendritic in shape, found primarily at the epidermal-dermal junction, represent approximately 10% of basal epidermal cells, and connect to the basement membrane by a dense plate structure that shares similarities with hemidesmosomes.2,3 The melanocyte stem cell pool and niche exist in the hair follicle at the hair bulge.4 Melanocyte stem cells and melanoblasts are typically quiescent but undergo cyclical proliferation, differentiation, and migration.5 Differentiated melanocytes of the hair follicle are found in the hair bulb where they provide melanin to the growing hair matrix.5
Melanin is synthesized and accumulated within the melanosome, organelle inside the melanocyte. The two melanin pigment classes are eumelanin, which has a black or brown hue and pheomelanin, which has a yellow to red hue.6 Eumelanin has photoprotective qualities via its ability to absorb and scatter ultraviolet radiation and scavenge reactive oxygen species.7 In contrast, pheomelanin is photosensitizing and produces reactive oxygen species when exposed to UVA.8,9 After melanin is synthesized, pigment containing melanosomes are transported to keratinocytes, internalized, and trafficked to perinuclear locations where they can absorb UV light and protect keratinocyte nucleus from UV-associated radiation damage.
One of the critical transcription factors modulating melanogenesis, melanocyte survival, and proliferation is the melanocyte-specific microphthalmia-associated transcription factor (m-MITF). MITF has numerous activities and many isoforms, which have been reviewed by a number of investigators (Steingrimmsen et al 2004,10 Chelli et al 2009,11 Goding and Arnheiter 201912). As it relates to the present discussion, M-MITF contains a basic region/helix-loop-helix/leucine zipper (b/HLH/LZ) DNA binding and dimerization motif, which mediates formation of homodimer or heterodimer complexes with related b/HLH/LZ transcription factors TFEB, TFEC or TFE3. The basic region in the b/HLH/LZ motif is responsible for DNA binding. MITF binds to DNA as a dimer. Dimeric MITF binds to specific E-box sequences to transcriptionally activate numerous target genes involved in melanogenesis (TYR, TYRP1, TYRP2 and gp100), melanocyte proliferation (CDK2), melanocyte survival (BCL2), and other activities.13,14,15,16
The mitf gene was originally identified on the basis of a striking microopthalmia/hypopigmentation phenotypic combination.17. MITF has a genomic configuration in which a series of alternative promoters produce distinct transcriptional isoforms, the most proximal of which is restricted to melanocyte (m-MITF isoform). The melanocyte specific expression of m-MITF is responsive to signals arising from melanocortin 1 receptor (MC1R), a 7 transmembrane G-protein coupled receptor on the melanocyte that is activated by α-melanocyte-stimulating hormone (α-MSH). Early investigational studies helped illustrate that α-MSH leads to skin darkening and that hypomorphic MC1R variants lead to changes in the coat color of mice as well as brown/blond/red hair color in humans, with fully nonfunctional human alleles producing the “red hair phenotype”.18,19 These findings have contributed to current understanding of the αMSH-MC1R-MITF pathway, which is crucial for ultraviolet radiation induction of skin pigmentation. In this pathway α-MSH binds the MC1R receptor leading to dissociation of the alpha subunit, which activates adenylate cyclase leading to the production of cyclic AMP (cAMP). cAMP activates protein kinase A (PKA), which phosphorylates and activates the transcription factor CREB as well as Salt Inducible Kinase (SIK) where its phosphorylation is inhibitory.20 SIK inhibition releases CRTC translocation to the nucleus where it binds CREB and co-activates the promotor for m-MITF, leading to its transcription and, subsequently, melanogenesis.
Ultraviolet Control of Tanning
The variation in skin pigmentation and ability to tan between individuals represents primarily differences in melanogenesis and melanin distribution rather than differences in the number of melanocytes. There are broad similarities in the number of melanocytes across races, ethnicities, and skin phenotypes.21,22,23 Darkly pigmented skin has been illustrated at baseline to have higher constitutive activation of MITF and melanosome maturation.23 Increases in these factors following ultraviolet radiation occurs across multiple races, but with the most notable differences in magnitude of melanogenesis and melanin distribution throughout the epidermis.23 Red-haired individuals do not typically experience skin darkening after UV exposure. Further, even among people whose skin darkens, within one week of UV exposure there is no appreciable change in number of melanocytes.24 However, in skin with chronic sun exposure, there is a mild increase in melanocyte density.24
Solar ultraviolet radiation contains UVA (~95%) and UVB (~5%). Collectively, they govern two tanning pathways, immediate and delayed tanning. Immediate tanning is transient, thought to be non-photoprotective, driven by UVA, and consists of two dose-dependent phases.25,26 The initial stage is immediate pigment darkening (IPD) and occurs at ~ 1 J/cm2 within minutes and lasts for a few hours.26 The second phase is persistent pigment darkening (PPD) and occurs at greater than 11.0 J/cm2 and lasts for up to a day.26 Both IPD and PPD are thought to be secondary to the dispersion of larger pigment granules and oxidation of existing melanin and are not due to melanogenesis.25
Delayed tanning occurs within days and last up to weeks or months and is due to increased melanogenesis. It is primarily driven by UVB, but there is some ambiguity regarding how "delayed tanning" terminology is used in the current literature. UVA can produce pigment darkening that lasts for multiple months, resulting in some authors describing a delayed tanning process of UVA due to "neo-melanogenesis".26,27,28 Although there is appreciable long-term pigmentation, other investigational studies illustrate that there is significantly less to no production of eumelanin, pheomelanin, melanogenic intermediates, and metabolites in UVA versus UVB only exposure.25,29 These studies have also suggested that tanning caused by UVA might not confer the photoprotective benefits that UVB does.25 Thus, although UVA can produce a longer duration of tan, ultraviolet-induced melanogenesis and its photoprotective qualities are mostly secondary to UVB. Further studies describing delayed tanning should document melanogenic markers when attributing "neo-melanogenesis" to UVA. This will help explain whether the prolonged duration of tanning seen with UVA is a byproduct of reactive oxygen species that affects melanin deeper in the epidermis, true "neo-melanogenesis," or another pathway.
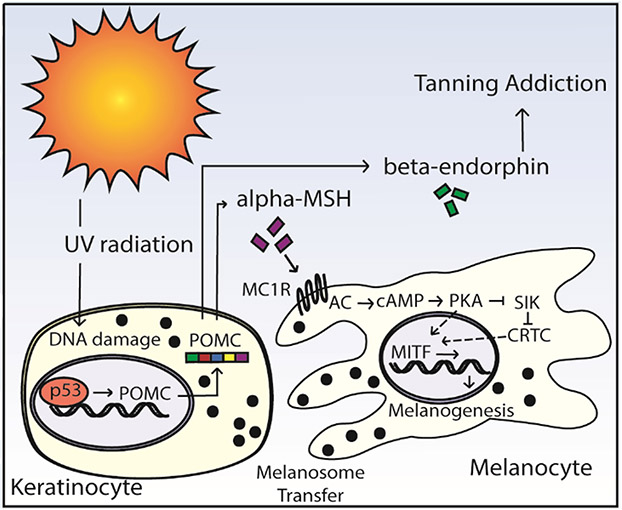
The pathway via which UVB induces delayed tanning is presented in Figure 1. Preferentially in keratinocytes, UVB radiation induces DNA damage which stimulates the expression of p53, that directly leads to transcription of the pro-opiomelanocortin (POMC) gene.30 Post-translational processing of POMC leads to the production of, amongst other products, α-MSH, adrenal corticotropin hormone (ACTH), and the opioid peptide β-endorphin. In a paracrine fashion, α-MSH stimulates MC1R on melanocytes, leading to the expression of m-MITF and melanogenesis as outlined above. Newly synthesized melanosomes are subsequently trafficked and transferred to keratinocytes where they coalesce and reside as perinuclear caps.
Figure 1:
The delayed tanning pathway. UV radiation causes keratinocyte DNA damage and p53 expression. P53 expression leads to transcription of POMC. Processing of POMC produces α-MSH and β-endorphins. α-MSH binds MC1R on melanocytes which leads to the expression of MITF and resultant melanogenesis. As a byproduct of POMC cleavage, β-endorphins can influence tanning addiction.
Although the p53 driven α-MSH/MC1R/m-MITF is the most well-documented UVB induced tanning pathway, keratinocyte p53 expression has been illustrated to induce pigmentation via other routes. The stabilization of p53 and its activation results in the downstream paracrine release of Kit-ligand (Kit-L), which can cause skin hyperpigmentation via the Kit-L/Kit pathway.31,32 This expression of Kit-L due to p53 stabilization has been shown to be induced by both ribosomal mutations,32 and by ablation of casein kinase 1-α which is a part of the β-catenin destruction complex.31 Hyperpigmentation by both pathways has been documented as independent of POMC expression. Moreover, UV irradiation-induced p53 activation results in the downstream expression of endothelin-1, which contributes to melanocyte homeostasis.33 Thus, it is possible that in addition to the p53 driven expression of POMC, UV irradiation-induced hyperpigmentation is facilitated by p53 release of Kit-L and endothelin-1.
Ultraviolet Radiation and Tanning Addiction
A notable byproduct of the cutaneous UVB response is the production of β-endorphin via POMC cleavage. β-endorphin is a known endogenous opioid with affinity for mu-opioid receptors.34,35 Via modified CAGE and DSM-IV addiction criteria, two decades of research have illustrated alarmingly high frequency of tanning addiction in people who routinely tan (39-53%).36,37,38 These results are consistent across indoor and outdoor tanning and, although most pronounced in younger white females, have been confirmed across sexes, adolescents, college, and adult populations.39 Tanning addiction is also associated with other psychiatric disorders, including obsessive compulsive disorder, anxiety, and substance abuse, including alcohol and marijuana.37,39
In 2014, via a mouse model, Fell et al illustrated UVB irradiation-induced plasma β-endorphin elevations and opioid-mediated behaviors that were reversed with naloxone.40 These findings included β-endorphin-driven analgesia, opiate tolerance, dependence upon UV light, and withdrawal symptoms induced by naloxone, which collectively illustrated both peripheral and central nervous system effects of UVB-induced β-endorphin. The findings, coupled with observations that withdrawal symptoms can be induced by naloxone in frequent human tanners,41 shed light on a neuro-endocrine pathway present even in rodents, which is capable of driving tanning addiction. Specifically, the pathway governing tanning addiction and habit-forming qualities of recreational tanning share substantial overlap with the pathway causing opioid dependence. A factor that is often over-looked and under-utilized.
Despite decades of literature and robust data illustrating the extent of tanning addiction and its biologic process, broader recognition and public health interventions have continued to lag. The DSM-V came out in 2013 and currently does not include tanning addiction. Future physician advocacy efforts should aim to help recognize tanning addiction as a valid psychiatric condition to broaden recognition amongst healthcare workers and the public and improve patient care.
Despite the strong correlates between tanning and opiate addiction, health policy has not kept pace with scientific discovery. Currently, only nineteen states have age restrictions of eighteen and older on tanning beds.42 Six states have no restrictions, and the others have varying lower age requirements or require parental permission.42
A similarly straightforward yet underutilized policy intervention is access to sunscreen in primary schools. When the Federal Drug Administration approved sunscreen because it prevents skin cancer, it was classified as a class I drug and requires written permission from a physician to be used in primary schools. In 2017, house bill 282 aimed to repeal the over-the-counter drug ban in primary schools but stalled in Congress. In light of this, states have individually started to address the issue, and to date, ten states have passed bills to waive the requirement of a physician’s note.43 Confounding the importance of childhood access to sunscreen is the unfortunate combination of young adults/adolescents having the lowest rates of sun protection.44 Sun damage is cumulative, and even one severe sunburn in childhood can double an individual’s risk for melanoma.45 It must also be noted that the elimination of requiring a physician’s note places the onus on the child-parent unit to provide and use sunscreen, which, although important, does not address sociodemographic access gaps that exist in society.
In sum, the advanced understanding of the prevalence of tanning addiction, biologic processes governing addiction, comorbid psychiatric and substance disorders, and long term sequela of skin cancer, create a yet to be capitalized vehicle for creating policy interventions. We would urge individuals to use the current information to advance sun protection policies by advocating for more substantial restrictions to tanning beds and allowing access to sunscreen in primary schools, with a long-term goal of having access to free sunscreen routine in schools and public places with high sunlight such as beaches and public parks.
Mutations in the Ultraviolet Controlled Pathway
An advanced understanding of how ultraviolet radiation regulates pigmentation has led to many insights into how defects in this pathway lead to pigmentation disorders. One of the critical topics receiving considerable attention is how polymorphisms in MC1R may increase the risk of melanoma and/or result in the "red hair phenotype" (red hair, pale skin, inability to tan and burn quickly).
In the early 1990s, loss of function of MC1R in mice was identified as resulting in a red-colored coat.46 Subsequently, Valverde et al documented that in a small Irish and British population, 70% of individuals with a red hair phenotype were MC1R variants.19 These findings led to the understanding that with the loss of function of MC1R, α-MSH-induced eumelanogenesis is severely diminished. Correspondingly, the lack of eumelanogensis results in pheomelanin predominance, leading to the red hair phenotype.
As seen within the red hair phenotype, a pheomelanin predominance leads to numerous differences in melanosome makeup, structure, and function. The pigmentation of red hair follicles is due to greater amounts of pheomelanosomes compared to eumelanosomes' dominance in black hair.47 Isolated pheomelanosomes from these follicles tend to be smaller, rounder, and less stable than the larger, elliptical, and more stable eumelanosomes.47 Within the epidermis, lighter skin phenotypes have a greater proportion of pheomelanosomes.48 Melanosomes within lighter skin phenotypes also reside in the lower layers of the epidermis, as opposed to throughout the epidermis in darker skin phenotypes, and are more quickly degraded.49 In addition to structural and location differences, pheomelanin is phototoxic when exposed to UVA inducing free radicals and provides lower photoprotection.8,9,50 Ultimately, the combination of these factors contributes to the attributes seen in the red hair phenotype, but the spectrum of MC1R mutations has not proven to be as straightforward.
In the last twenty-five years, robust research efforts have elucidated genomic variants within MC1R as highly polymorphic, with greater than 100 individual variants currently documented, expressing similarly varied phenotypes. Amongst the most common alleles are D84E, R142H, R151C, I155T, R160W, D294H, V60L, V92M, and R163Q. A meta-analysis in 2008 documented D84E, R142H, R151C, I155T, R160W, R163Q, and D294H associated with melanoma.51 Of these, D84E, R142H, and R151C were also associated with the red hair phenotype. In contrast, V60L and V92M did not appear to be associated with either the red hair phenotype or melanoma.
Distinct variants affect MC1R differently. Thus far, D84E, R151C, and R160W have reduced cell surface expression of MC1R, and of them, R151C and R160W have also been found intracellularly.51,52 R142H and D294H have wild type expression levels but a reduced response to α-MSH.51
Significant variation exists among MC1R and the resultant phenotypes. In the pediatric population, those with cutaneous melanoma have higher rates of MC1R variants than adult populations with cutaneous melanoma, with V60L and D294H having the most influential association.53 Wendt et al documented that in Austria, MC1R variants are an independent risk factor for melanoma in females but not males.54 In a genome-wide association study, MC1R variants more strongly contributed to melanoma in darker-skinned Europeans than lighter-skinned Europeans.55 White et al recently documented that Hispanics in New Mexico have high rates of R163Q variants (47.6%) but this does not lead to an increase in melanoma.56
MC1R variants appear to have a synergistic effect when occurring in patients with the high-risk CDKN2A mutations.57 In this setting, it doubles the risk for melanoma, resulting in a younger age of presentation, and is exacerbated by more than one MC1R variant. Lastly, Taylor et al documented an inverse association with mortality from melanoma in MC1R variants lacking a consensus allele.58
The pathways contributing to the increase in melanoma in MC1R variants are still being examined. In those variants with associated red hair phenotype, decreased ultraviolet protection and increased oxidative stress likely contribute heavily. Animal models have demonstrated a significantly elevated melanoma risk in the red hair background, entirely independent of UV exposure—based upon the observation that introduction of an albino allele (pigment enzyme mutation) reversed the melanoma risk conferred by non-functional MC1R.59 This mechanism appears to be driven by reactive oxygen species. An increased risk for melanoma remains in MC1R variants not associated with red hair. A potential contributing pathway is via defective DNA repair. Ultraviolet mutagenic photoproducts typically induce signature mutations, but the precursor nucleotide adducts (e.g., cyclobutene pyrimidine dimers) are frequently removed by nucleotide excision repair, a process that MC1R has been illustrated to promote.60 Correspondingly, melanomas arising in MC1R variants carry a heavy single-nucleotide mutational burden.61 MC1R also prevents UVB-induced degradation of the tumor suppressor PTEN, a process that is defective in MC1R variants.62 Thus, a multifactorial process with influences from decreased UV protection, increased oxidative stress, defective nucleotide excision repair, and deficient PTEN may contribute to the increased risk of melanoma in MC1R variants.
Although research efforts have advanced the understanding of MC1R mutational effects, the development of interventions remains inadequate. In MC1R deficient mice forskolin, a cAMP agonist, can rescue the downstream MC1R pathway, leading to transcription of m-MITF and subsequent eumelanogenesis,63 and this pigment was seen to be photoprotective (including against UV carcinogenesis) in mice. A similar rescue of pigmentation results from suppression of PDE4D3, a regulator of melanocytic cAMP, in mice.64 However, neither of these agents appear to be capable of penetrating human skin.
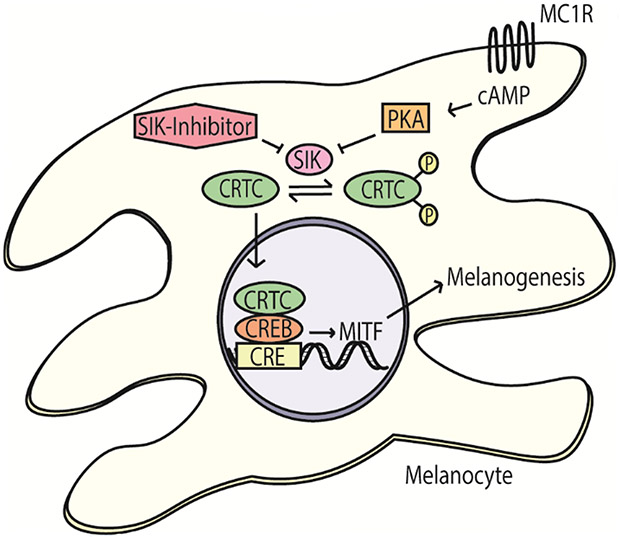
Salt inducible kinase (SIK) inhibitors may provide a vehicle to rescue eumelanogenesis in human skin. In the normal MC1R pathway, cAMP induces PKA, which phosphorylates and represses SIK. In turn, CRTC is no longer phosphorylated by SIK and translocates to the nucleus. In mice, independent of CREB phosphorylation by PKA, CRTC positively regulates the expression of MITF and melanogenesis. In contrast, SIK2 negatively regulates CRTC via phosphorylation,20 which leads to its sequestration within the cytoplasm (Figure 2). In light of this discovery, Mujahid et al illustrated that SIK inhibitors induced the expression of MITF and rescued melanogenesis in red haired mice with an inactive MC1R.65 Subsequently, they developed second-generation SIK inhibitors with epidermal penetration properties. Topical application of these second-generation SIK inhibitors induced eumelanization and resulted in transferring of melanosomes to keratinocytes within human skin explants in vitro. Thus, SIK inhibitors represent a potential route to modulate the most heavily implicated adaptive pigmentation pathway. Careful consideration regarding safety is crucial and further investigation of the role of SIK inhibitors in human applications is underway.
Figure 2:
CRTC regulates the expression of MITF independent of CREB phosphorylation by PKA. CRTC can be sequestered in the cytoplasm via phosphorylation by salt inducible kinase (SIK). SIK inhibitors lead to increased un-phosphorylated CRTC, which can increase MITF expression and melanogenesis.
Another modulator of the ultraviolet-induced pigmentation pathway is the agouti signaling protein (ASIP). ASIP serves as a reverse agonist to the MC1R whereby it inactivates the receptor, even relative to basal ligand-independent signaling. It was first identified in mice where activating mutations produced a predominance of pheomelanin and a resultant reddish to blonde coat.66 Since that time, ASIP has been shown to have many associations with skin pigmentation characteristics in European populations.67,68 Of note, a unique haplotype, (rs1015362[G], rs4911414[T]) (AH), has a strong association with skin sensitivity to the sun, freckling, and red/blonde hair as well as increased risk for cutaneous melanoma and basal cell carcinoma.69,70 These findings are analogous to some MC1R variants and have led to the understanding of ASIP polymorphisms as gain of function mutations.
Further studies have identified AH as significantly increasing the risk for non-melanoma skin cancer, multiple primary melanomas, and an increased hazard of death from melanoma.58,71,72 These and other genomic studies have identified numerous other polymorphisms within the ASIP locus with statistically significantly increased risk for both melanoma and non-melanoma skin cancer.71,73 Of note, the downstream consequences of ASIP mutations and its sequela share many similarities with MC1R loss of function variants. If SIK inhibitors eventually prove to be a viable intervention for MC1R variants, they may also affect pigmentation in the context of ASIP mutations.
A third modulator of the same ultraviolet-associated pigment pathway is the pro-opiomelanocortin (POMC) gene. The expression of the pro-hormone POMC and its post-translational cleavage result in production of the bioactive peptides α-MSH, ACTH, and β-endorphin, amongst others. A complete loss of POMC function in humans results in early-onset obesity, adrenal insufficiency, and red hair/fair skin.74,75 In this population, red hair and fair skin are due to a lack of stimulation of the MC1R by α-MSH. At the same time, obesity occurs secondary to the lack of stimulation by α-MSH of the MC4R, which results in hyperphagia. Adrenal insufficiency occurs due to the lack of ACTH and resultant hypocortisolism. POMC mutations were initially discovered in humans and then confirmed in mice in the late 1990s. Complete loss of function mutations in POMC are rare, requiring either compound heterozygous or homozygous mutations. They present at birth or early childhood, and there are few documented adults. Multiple cases have histories of a sibling dying in infancy due to adrenal insufficiency. Heterozygous mutations in POMC appear to be associated with obesity and not the other sequela. One genetic study found that no POMC single nucleotide polymorphisms are associated with basal cell carcinoma.76,77 A recent trial of Setmelanotide, an MC4R agonist, in two adults with POMC deficiency resulted in remarkable weight loss, improved satiety, as well as skin and hair darkening.78
Vitiligo and the Role of Stem Cells in Perifollicular Re-pigmentation
Vitiligo is a common, under-recognized T-cell driven autoimmune disease. It results in the loss of epidermal melanocytes presenting with depigmented macules and patches. It has significant psychological and social sequela and affects approximately one percent of the population.79 Its pathophysiology is complex and most easily understood through conversion theory, which was initially presented in the 1990s and recently updated.80 Conversion theory explains how biochemical processes, melanocyte cellular stress, environmental influences, genetic predisposition, innate immunity, and adaptive immunity converge to cause the presentation of vitiligo.
Melanocytes in vitiligo are thought to experience increased cellular stress.81 This increased stress can be due to defective melanogenesis, as seen in tyrosinase mutations and chemical leukoderma, or an unfolded protein response due to other mutations.81 The increased stress leads to elevated reactive oxygen species and potentially together with neoantigen formation may trigger an innate immune response. The innate immune system causes the production of pro-inflammatory cytokines and the recruitment of adaptive T-cells, including a predominance of cytotoxic CD8+ T-cells. Within this pathway, an increase in IFN-γ initiates the release of CXCL9 and CXCL10 from keratinocytes which bind CXCR3 on melanocyte-specific CD8+ T-cells.82 The binding of these chemokines helps home CD8+ T-cells to melanocytes, resulting in their destruction and depigmentation of the epidermis.82
Genetic predisposition sits in tangent to the above pathway with notable mutations documented in TYR, XBP1, HLAI, HLAII, NLRP1, and OCA2.83 Although genetic predisposition exists and there is some familial clustering, there is only 23% concordance in monozygotic twins.84
An interesting though controversial question is whether affected vitiligo patients may have lower rates of melanoma and especially non-melanoma skin cancer.85,86 While melanocytes are the epidermis' photoprotective cells and their absence should increase sensitivity to UV damage, vitiligo is ultimately a CD8+ driven process, such that altered melanoma rates might potentially arise secondary to the same autoimmunity. But, autoimmunity should not explain reduced non-melanoma (squamous cell carcinoma) skin cancer rates. One theory is that vitiligo patients experience up-regulation of the tumor suppressor p53, which protects against epithelial malignancies.87 Another hypothesis is that increased superoxide dismutase and glutathione peroxidase in the setting of decreased IL-10 and TGF-β protects from epithelial malignancies.88
The overall goal of therapy in vitiligo is to halt immune destruction and promote re-pigmentation. Standard approaches include topical corticosteroids, topical calcineurin inhibitors, and phototherapy. For disease affecting more than 5% of the body, phototherapy is a backbone of treatment, with topical treatments used in adjunct. Phototherapy historically includes psoralen plus ultraviolet A (PUVA), broadband UVB, narrow-band UVB (nbUVB), and targeted ultraviolet phototherapy. Among these, the current standard of therapy has become nbUVB supported by an updated 2015 Cochrane Review.89
UVB may induce a striking re-pigmentation response which is characterized by perifollicular re-pigmentation. This process was initially observed before an understanding of hair follicle melanocyte stem cells in the clinical setting, based on the appearance that re-pigmentation typically begins at the orifices of hair follicles and extends outwards radially, and that pigment is hard to recover in glabrous (hairless) skin.90
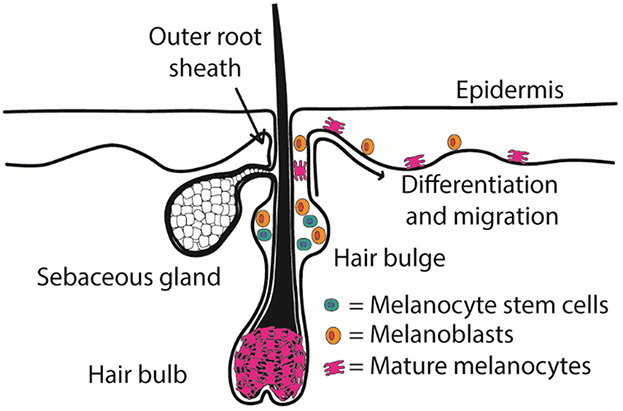
The reservoir for melanocyte stem cells and their niche is in the lower portion of the permanent hair follicle, also called the hair bulge near the insertion of the arrector pili muscle4 (Figure 3). Under the influence of TGF-β, melanocyte stem cells reside here in a quiescent state. However, they undergo cyclic activation, downward migration, and maturation during early anagen of the hair cycle, as discussed below.4,91 In the setting of UVB-induced re-pigmentation, melanocyte stem cells in the hair bulge region become activated and migrate upwards to the supra bulge region. As melanoblasts, these cells begin to differentiate in the supra bulge region and migrate up the outer root sheath towards the epidermis surrounding the hair follicle's orifice. In the epidermis surrounding the orifice, a portion of the melanoblasts become fully differentiated melanocytes, which migrate out to re-pigment the vitiliginous lesions (Figure 3).
Figure 3:
Melanocyte stem cells are located in the hair bulge. Perifollicular re-pigmentation occurs as a portion of quiescent melanocyte stem cells and melanoblasts become activated and migrate to the supra bulge region. From the supra bulge region they undergo further differentiation and migration upwards along the outer root sheath and into the peripheral epidermis.
Deciphering signaling cascades regulating re-pigmentation process may not only provide fundamental insights into pathophysiological mechanisms of vitiligo, but also point to novel therapeutic strategies. Although molecular mechanisms underlying re-pigmentation are not fully understood, several important signaling pathways have been identified and are discussed below. Melanocyte stem cells are maintained in a state of quiescence by TGF-β.91 UVB irradiation results in reduced TGF-β signaling,92 which may serve to lower the threshold for melanocyte activation.
UVB induces expression of Wnt7A in hair follicle stem cells, hair follicle keratinocytes, and epidermal keratinocytes.93 It also causes an increase of Kit-ligand.93 Melanocyte stem cells express Wnt receptors whose activation prevents the degradation of β-catenin, that is subsequently transferred to the nucleus where it serves as a transcriptional co-activator and trigger the differentiation of melanocyte stem cells (likely via MITF induction) into melanoblasts.93 The increase in Kit-ligand serves to support melanoblasts and melanocyte differentiation and survival.4 Interestingly, re-pigmentation can still occur in the absence of Kit-ligand/Kit signaling but not without Wnt.93 This finding suggests that Wnt/β-catenin signaling is critical in re-pigmentation, and the increase in Kit-ligand is supportive.
Another pathway of interest is the endothelin/endothelin receptor B (EDN/EDNRB). Sato-Jin et al showed that EDN/EDNRB signaling markedly up-regulated MITF expression via mitogen-activated protein kinase (MAPK)-p90 ribosomal S6 kinase (RSK)-cAMP response element-binding protein (CREB) pathway and cAMP-protein kinase A (PKA)-CREB pathway, that EDNRB expression was dependent on MITF, and that EDN induced MITF-dependent up-regulation of SILV mRNA (melanosomal structure and melanocyte pigmentation marker) and CDK2 mRNA (melanocyte proliferation marker) in human primary melanocytes.94 These results suggest that a self-reinforcing positive feedback loop between EDN/EDNRB signaling and MITF expression enhances the proliferation and pigmentation of melanocytes. Moreover, Takeo et al demonstrated that Wnt-dependent EDN1/EDNRB signaling cascade boosted proliferation and differentiation of melanocyte stem cells and promoted regeneration of epidermal melanocytes post-wounding.95 EDN, overexpressed in mouse epithelial cells, binds to EDNRB on the surface of melanocyte stem cells and promotes melanocyte regeneration and epidermal pigmentation. Together, these two reports support the notion that EDN/EDNRB signaling is crucial in melanocyte proliferation, regeneration, and re-pigmentation.
In addition to UVB-induced Wnt/β-catenin, Kit-ligand, and EDN1, the MC1R pathway plays a role. MC1R deficient mice exhibit less re-population of melanocytes triggered by UVB.96 Further, in the presence of a functional MC1R, the addition of MC1R stimulators, ACTH or α-MSH analogs, enhances the re-pigmentation abilities, likely illustrating another supportive role.96
In summary, the signaling pathways governing re-pigmentation of vitiliginous lesions are complex. A hypothetical model would be as follows: UVB phototherapy results in the keratinocyte lineage driven increase of Wnt, Kit-ligand, EDN1, ACTH/α-MSH as well as a decrease in TGF-β. The reduction in TGF-β renders melanocyte stem cells more susceptible to activation. In a paracrine fashion, Wnt activates melanocyte stem cells via the Wnt/β-catenin pathway. The stimulation of EDNRB by EDN1 supports activation of melanocyte stem cells. As activated melanoblasts are now in the supra bulge region, they migrate up the outer root sheath towards the epidermis and differentiate towards mature melanocytes. This process may include a balance of melanocyte stem cell migration away from the follicular niche versus maintenance of follicular melanocyte stem cells that preserve hair pigmentation. The process of migration and differentiation downstream of Wnt is supported and enhanced by Kit-ligand/Kit, EDN1/EDNRB, ACTH/MC1R, and α-MSH/MC1R signaling.
Detailed knowledge of the above pathways offers unique points of potential intervention. One area already explored is the modulation of the MC1R pathway by combining nbUVB with afamelanotide, a synthetic analog of α-MSH.97,98 A randomized control trial found that this combined therapy resulted in significantly superior and faster re-pigmentation than nbUVB monotherapy.98 Afamelanotide is administered as an implant, which results in systemic absorption. Thus, typical side effects in the above study were diffuse hyperpigmentation of non-lesional skin and nausea.
Other potential intervention targets could be selected from the above-outlined pathways. Importantly, a possible crucial initiation step of stem cell stimulation has been identified in Wnt/β-catenin as well as multiple supporting pathways. Thus, a future of vitiligo treatment with perifollicular re-pigmentation without phototherapy may eventually be achievable.
Hair Greying and the Role of Stem Cells
As mentioned above, melanocyte stem cells and their niches exist in the hair bulge and the sub bulge areas.4 These areas are located in the lower permanent portion of the hair follicles, near the arrector pili attachment, and share their niches with epithelial stem cells.4,99 Melanocyte stem cells in the bulge are primarily quiescent but undergo cyclic activation and migration.
In early anagen, a subpopulation of quiescent melanoblasts and melanocyte stem cells become activated and begin to surround the secondary hair germ.4,100 As the secondary hair germ begins to grow and migrate downwards, these activated melanoblasts are separated from the niche/sub-bulge area and continue to undergo division and differentiation.4,100 Eventually, these melanoblasts become fully differentiated melanocytes that surround the hair bulb. These mature melanocytes then produce melanosomes that are transferred to keratinocytes, supplying the growing hair with pigmentation.4 Shortly after the start of anagen, activated melanoblasts and melanocyte stem cells in the bulge re-enter a quiescent state and serve as the melanocyte stem cell population for the next hair growth cycle.91 In catagen, the mature melanocytes in the hair bulb undergo apoptosis and are replaced from the stem cell pool in the next hair cycle.101
Similar to re-pigmentation in vitiligo, this cyclic process of melanocyte stem cells producing hair pigmentation is under complex signaling control that is still being elucidated. Several pathways deserve mention. First, the activation of melanocyte stem cells in early anagen and terminal differentiation of melanocytes is dependent on Wnt/β-catenin signaling.100 Wnt is produced by cells adjacent to melanocyte stem cells under paracrine control. Epithelial stem cells undergo cyclic activation, division, migration, and differentiation in parallel with melanocyte stem cells.100 In this same process, epithelial stem cells secrete EDN1 and 2 that bind to the melanocyte lineage EDNRB receptor, promoting proliferation and differentiation.100 At the same time, melanoblast differentiation and eventual pigmentation are supported by Kit-ligand/Kit signaling.102. Notch signaling works alongside the above signals by facilitating proper migration and terminal pigmentation in the hair bulb.103
The entrance and maintenance of melanocyte stem cells and melanoblasts into quiescence is controlled by TGF-β.91 In late anagen, TGF-β levels increase in the niche, halting stem cell differentiation and inducing and maintaining quiescence through a BCL2-dependent pro-survival mechanism.91 In addition to TGF-β, Notch signaling also helps maintain melanocyte stem cells in a quiescent state.103,104
Ultimately, hair greying often occurs through the inability of hair follicles to maintain melanocyte stem cells in their niches. In 2005, Nishimura et al modeled this process in a series of experiments utilizing Bcl2 knockout and Mitf mutant mice.5 Bcl2 knockout mice exhibited hair graying due to the rapid depletion of melanocyte stem cells at postnatal day 8. Mutated Mitfvit mice had a more gradual loss of melanocyte stem cell which was seen to be accompanied by ectopic pigmentation within the bulge region. Pigmented melanocytes within the stem cell niche should be a “forbidden” event, which was thus proposed to represent a mechanism leading to the demise of melanocyte stem cells and their eventual depletion. Similar findings were seen in scalp hair follicles from human subjects, where progressive loss of hair bulge melanoblasts was seen, with younger (20-30-year-olds) having an abundance, middle-aged (40-60-year-olds) having moderate amounts, and older aged (70-90-year-olds) having a near absence. Again, in human aging, “ectopically pigmented” melanocytes were observed within the stem cell niche in association with loss of melanocyte stem cells. These findings led to the understanding of senile hair greying secondary to progressive melanocyte stem cell loss through a mechanism involving an odd form of stem cell differentiation within the niche.
A recent study investigating how acute stress induces hair greying by Zhang et al has significantly advanced these findings.105 Their study was undertaken based on the anecdotal observation that acute stress caused greying. Using mouse models, they illustrated that acute stress caused hair greying through rapid depletion of melanocyte stem cells. They observed this process to be independent of immune destruction or increased stress glucocorticoids. Instead, it was driven by norepinephrine release from sympathetic neurons that terminate around the hair bulge. Free norepinephrine directly bound β2-adrenergic receptors on melanocyte stem cells, leading to activation, differentiation, and ectopic migration of approximately 50% of niche melanocytes stem cells, which is contrasted against the 6% of stem cell activation in regular hair cycling. This hyperproliferation quickly produced depletion of melanocyte stem cells and hair graying during the subsequent hair follicle cycle. Lastly, they outlined that acute stress-induced hair greying could be prevented by blocking the sympathetic release of norepinephrine, preventing the binding of norepinephrine to melanocyte stem cells, or suppressing melanocyte proliferation.
Interestingly, patients who have undergone partial sympathectomy exhibit decreased hair greying on the denervated side.106,107 Thus, the findings of Zhang et al appear to suggest that senile hair greying may result from decades of cumulative stress-associated melanocyte stem cell depletion.105 Their model also documents a novel route of melanocyte stem cell activation via β2-adrenergic receptors independent of epithelial stem cells.105 This raises the question of the extent to which sympathetic activation might play a role in UVB-induced re-pigmentation in vitiligo? As it pertains to hair greying, these advances introduce several potential points of intervention. Based on current understanding, senile greying might be prevented by topically blocking the sympathetic release of norepinephrine or blocking the binding of norepinephrine to melanocyte stem cells. Such approaches would require careful attention to safety and toxicity.
Further, some studies on re-pigmentation illustrate that migrating melanoblasts might occasionally repopulate depleted stem cell niches and potentially produce pigmented hairs. Thus, could the process of senile hair greying become reversible?
Major Open Questions
Through this review, we have documented numerous open questions. One question to answer is whether the UVA-driven delayed tanning process is genuinely due to “neo-melanogenesis.” If so, why does this process not produce the similar photoprotection as UVB (within its context as a mutagen and carcinogen)? Future research efforts comparing UVA and UVB tanning will hopefully elucidate melanogenic markers in each of these tanning responses.
Another open question is whether current understanding of tanning addiction can be levied to promote sun protection policies. We outline neuro-endocrine pathways governing tanning addiction, provide an overview of its extent in the public arena, and review some common-sense sun protection policies. We would encourage this information to support advocacy for more significant sun protection policies such as via recognition of the organic, opioid-related addiction response triggered by UV.
Pertaining to mutations in the UV-induced pigmentation pathway, this review covered current literature on MC1R, ASIP, and POMC variants. Within MC1R, the last robust meta-analysis was published in 2008. Since that time, numerous studies have reported considerable heterogeneity across high-frequency polymorphisms, including geographic location, sex, ethnicity, and age. Thus, another high-quality meta-analysis may help guide the effects of MC1R variants. The question also arises whether SIK inhibitors might offer a therapeutic approach to MC1R and ASIP variants or in other pigmentation contexts. Future studies may thus explore the safety and potential role(s) of SIK inhibitors in human populations.
This review has discussed signaling pathways thought to govern melanocyte stem cell-driven perifollicular re-pigmentation in vitiligo. Based on the recent finding of sympathetic activation of stem cells in acute stress, an interesting question is whether sympathetic activation could play a role in UVB-induced re-pigmentation. This can be tested through a series of experiments modeling re-pigmentation and blocking or stimulating the β2-adrenergic receptors on melanocyte stem cells. Other small molecule strategies (described above) which modulate the state of melanocytes may also warrant examination in the context of vitiligo.
Through our review of hair greying mechanisms and recent discoveries, we highlight multiple potential points of intervention. Can hair greying be impeded by interrupting sympathetic activation of melanocyte stem cells? Future studies could explore to what extent topical therapies might prevent the stimulation and depletion of melanocyte stem cells.
Conclusions and Perspectives
UV radiation is a critical modulator of human pigmentation. Amongst its many byproducts, the increase in β-endorphin can result in UV addiction. This addiction is mediated by a bona-fide neuroendocrine circuit, and numerous studies have illustrated that it meets DSM-IV addiction criteria. Thus, tanning addiction should be viewed as a psychiatric disorder and deserves recognition as such. From an evolutionary perspective, one wonders whether this sun-seeking behavior might have represented a survival advantage that contributed early to the incorporation of “addictive propensities” into the armamentarium of behavioral responses. The likely benefit of UV-seeking behavior would be maintenance of vitamin D production by skin. Future public health advocacy efforts should focus on sun protection policies, with tanning booth restrictions and access to sunscreen in schools as practical examples. Although research discoveries advance incrementally, well-placed policies can affect significant proportions of the population and are under-utilized in the prevention of skin cancer.
Genomic sequence variation among genes within the UV pigmentation control pathway is common, and in our review, we focused on MC1R, ASIP, and POMC. MC1R in particular is highly polymorphic, and is likely to reflect at least some of the variability that contributes to varied UV-tanning responsiveness, with redhaired alleles being associated with essentially disabled UV-tanning. It is notable that a high-quality meta-analysis could help advance the understanding of its effects, especially as personalized genetic risk profiles may become more widespread in tailoring optimal skin cancer prevention approaches. Further, several small molecule approaches toward mitigating high risk skin cancer alleles may provide new options for novel prevention strategies.
nbUVB phototherapy is the backbone of vitiligo treatment and results in perifollicular re-pigmentation of lesions. Perifollicular re-pigmentation occurs from the activation and migration of melanocyte stem cells. Our review focusses on several pathways governing and modulating this process. UVB reduces TGF-β which may lower the threshold for melanocyte stem cell activation, in response to Wnt/β-catenin signaling. EDN/EDNRB, Kit-ligand/Kit, α-MSH/MC1R, and ACTH/MC1R serve to support melanocyte stem cell and melanoblast stimulation, differentiation, and migration. Early results from combining afamelanotide with nbUVB illustrate that modulation of these pathways can have significant therapeutic benefits. Modulating other pathways may also warrant investigation. It must also be noted that further research is required to uncover additional potential pathways and targets for modulation of melanocyte stem cell state. Based on the sympathetic activation of melanocyte stem cells reviewed here, sympathetic influence in UVB-induced re-pigmentation will be interesting to examine.
Lastly, the physiological process of senile hair greying results from the progressive loss of melanocyte stem cells. The cyclic hair growth process results in melanocyte stem cell and melanoblast activation, division, migration, and differentiation. TGF-β/BCL2 initiates and maintains quiescence. Wnt/β-catenin signaling causes stem activation, and EDN/EDNRB, Kit-ligand/Kit, and Notch have varying supportive roles. Acute stress induces the sympathetic activation of β2-adrenergic receptors on melanocyte stem cells to provide a Wnt-independent route of activation and results in their rapid depletion. Thus, the sympathetic release of norepinephrine may significantly influence hair greying, and modulation of this pathway may help to mitigate hair greying. Advances in understanding melanocyte biology, from molecular to organismal levels, have progressed at a remarkable rate, in some instances serving as prime examples of fundamental biologic processes (e.g., cancer prevention and stem cell behaviors). It is hoped further discovery will continue to enlighten basic concepts while offering novel opportunities to improve quality and longevity of human lives.
Acknowledgements:
The authors would like to thank the American Academy of Dermatology Diversity Mentorship Program, which advances mentorship of underrepresented minority medical students. Without the program the presented work would not have been feasible. D.E.F. gratefully acknowledges grant support from the NIH (R01AR043369-24, R01CA222871-03, R01AR072304-04 and P01 CA163222-07) and from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. We apologize for not being able to include every research work of our colleagues due to space constraints. The authors gratefully acknowledge Dr. W. Robert Liu for valuable assistance with manuscript preparation.
Footnotes
Conflict of Interests: D.E.F. has a financial interest in Soltego, a company developing salt inducible kinase inhibitors for topical skin-darkening treatments that might be used for a broad set of human applications. The interests of D.E.F. were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. J.M.Y.F. has no disclosures or conflict of interest to report.
References:
- 1.Boissy RE, Nordlund JJ. Molecular basis of congenital hypopigmentary disorders in humans: a review. Pigment Cell Res. 1997;10(1-2):12–24. [DOI] [PubMed] [Google Scholar]
- 2.Cochran AJ. The incidence of melanocytes in normal human skin. J Invest Dermatol. 1970;55(1):65–70. [DOI] [PubMed] [Google Scholar]
- 3.Tarnowski WM. Ultrastructure of the epidermal melanocyte dense plate. J Invest Dermatol. 1970;55(4):265–268. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416(6883):854–860. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307(5710):720–724. [DOI] [PubMed] [Google Scholar]
- 6.Ozeki H, Ito S, Wakamatsu K, Hirobe T. Chemical characterization of hair melanins in various coat-color mutants of mice. J Invest Dermatol. 1995;105(3):361–366. [DOI] [PubMed] [Google Scholar]
- 7.Wang A, Marino AR, Gasyna Z, Gasyna E, Norris J. Photoprotection by porcine eumelanin against singlet oxygen production. Photochem Photobiol. 2008;84(3):679–682. [DOI] [PubMed] [Google Scholar]
- 8.Wenczl E, Van der Schans GP, Roza L, et al. (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J Invest Dermatol. 1998;111(4):678–682. [DOI] [PubMed] [Google Scholar]
- 9.Panzella L, Szewczyk G, d'Ischia M, Napolitano A, Sarna T. Zinc-induced structural effects enhance oxygen consumption and superoxide generation in synthetic pheomelanins on UVA/visible light irradiation. Photochem Photobiol. 2010;86(4):757–764. [DOI] [PubMed] [Google Scholar]
- 10.Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. [DOI] [PubMed] [Google Scholar]
- 11.Cheli Y, Ohanna M, Ballotti R, Bertolotto C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010;23(1):27–40. [DOI] [PubMed] [Google Scholar]
- 12.Goding CR, Arnheiter H. MITF-the first 25 years. Genes Dev. 2019;33(15-16):983–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aksan I, Goding CR. Targeting the microphthalmia basic helix-loop-helix-leucine zipper transcription factor to a subset of E-box elements in vitro and in vivo. Mol Cell Biol. 1998;18(12):6930–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109(6):707–718. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. The American Journal of Pathology. 2003;163(1):333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Widlund HR, Horstmann MA, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6(6):565–576. [DOI] [PubMed] [Google Scholar]
- 17.Hodgkinson CA, Moore KJ, Nakayama A, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74(2):395–404. [DOI] [PubMed] [Google Scholar]
- 18.Lerner AB, McGuire JS. Effect of Alpha- and Beta-Melanocyte Stimulating Hormones on the Skin Colour of Man. Nature. 1961;189(4760):176–179. [DOI] [PubMed] [Google Scholar]
- 19.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328–330. [DOI] [PubMed] [Google Scholar]
- 20.Horike N, Kumagai A, Shimono Y, et al. Downregulation of SIK2 expression promotes the melanogenic program in mice. Pigment Cell Melanoma Res. 2010;23(6):809–819. [DOI] [PubMed] [Google Scholar]
- 21.Gilchrest BA, Blog FB, Szabo G. Effects of aging and chronic sun exposure on melanocytes in human skin. J Invest Dermatol. 1979;73(2):141–143. [DOI] [PubMed] [Google Scholar]
- 22.Staricco RJ, Pinkus H. Quantitative and qualitative data on the pigment cells of adult human epidermis. J Invest Dermatol. 1957;28(1):33–45. [DOI] [PubMed] [Google Scholar]
- 23.Tadokoro T, Yamaguchi Y, Batzer J, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124(6):1326–1332. [DOI] [PubMed] [Google Scholar]
- 24.Hendi A, Brodland DG, Zitelli JA. Melanocytes in long-standing sun-exposed skin: quantitative analysis using the MART-1 immunostain. Arch Dermatol. 2006;142(7):871–876. [DOI] [PubMed] [Google Scholar]
- 25.Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011;24(1):136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohli I, Sakamaki T, Dong Tian W, Moyal D, Hamzavi IH, Kollias N. The dynamics of pigment reactions of human skin to ultraviolet A radiation. Photodermatol Photoimmunol Photomed. 2019;35(6):387–392. [DOI] [PubMed] [Google Scholar]
- 27.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. 2013;12(1):54–64. [DOI] [PubMed] [Google Scholar]
- 28.Suh KS, Roh HJ, Choi SY, et al. Long-term evaluation of erythema and pigmentation induced by ultraviolet radiations of different wavelengths. Skin Res Technol. 2007;13(2):154–161. [DOI] [PubMed] [Google Scholar]
- 29.Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21(4):487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. [DOI] [PubMed] [Google Scholar]
- 31.Chang CH, Kuo CJ, Ito T, et al. CK1α ablation in keratinocytes induces p53-dependent, sunburn-protective skin hyperpigmentation. Proc Natl Acad Sci U S A. 2017;114(38):E8035–e8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGowan KA, Li JZ, Park CY, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet. 2008;40(8):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyter S, Coleman DJ, Ganguli-Indra G, et al. Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell Melanoma Res. 2013;26(2):247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng LF, Loh HH, Li CH. Beta-Endorphin as a potent analgesic by intravenous injection. Nature. 1976;263(5574):239–240. [DOI] [PubMed] [Google Scholar]
- 35.Schoffelmeer AN, Warden G, Hogenboom F, Mulder AH. Beta-endorphin: a highly selective endogenous opioid agonist for presynaptic mu opioid receptors. J Pharmacol Exp Ther. 1991;258(1):237–242. [PubMed] [Google Scholar]
- 36.Harrington CR, Beswick TC, Leitenberger J, Minhajuddin A, Jacobe HT, Adinoff B. Addictive-like behaviours to ultraviolet light among frequent indoor tanners. Clin Exp Dermatol. 2011;36(1):33–38. [DOI] [PubMed] [Google Scholar]
- 37.Mosher CE, Danoff-Burg S. Addiction to indoor tanning: relation to anxiety, depression, and substance use. Arch Dermatol. 2010;146(4):412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warthan MM, Uchida T, Wagner RF. UV light tanning as a type of substance-related disorder. Arch Dermatol. 2005;141(8):963–966. [DOI] [PubMed] [Google Scholar]
- 39.Miller KA, Piombo SE, Cho J, et al. Prevalence of Tanning Addiction and Behavioral Health Conditions among Ethnically and Racially Diverse Adolescents. J Invest Dermatol. 2018;138(7):1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates addiction to UV light. Cell. 2014;157(7):1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur M, Liguori A, Lang W, Rapp SR, Fleischer AB, Feldman SR. Induction of withdrawal-like symptoms in a small randomized, controlled trial of opioid blockade in frequent tanners. J Am Acad Dermatol. 2006;54(4):709–711. [DOI] [PubMed] [Google Scholar]
- 42.Indoor Tanning Restrictions for Minors ∣ A State-By-State Comparison.
- 43.Relaxing School Sunscreen Restrictions.
- 44.García-Romero MT, Geller AC, Kawachi I. Using behavioral economics to promote healthy behavior toward sun exposure in adolescents and young adults. Prev Med. 2015;81:184–188. [DOI] [PubMed] [Google Scholar]
- 45.Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Ann Epidemiol. 2008;18(8):614–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins LS, Nadeau JH, Johnson KR, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72(6):827–834. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Hong L, Wakamatsu K, et al. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem Photobiol. 2005;81(1):135–144. [DOI] [PubMed] [Google Scholar]
- 48.van Nieuwpoort F, Smit NP, Kolb R, van der Meulen H, Koerten H, Pavel S. Tyrosine-induced melanogenesis shows differences in morphologic and melanogenic preferences of melanosomes from light and dark skin types. J Invest Dermatol. 2004;122(5):1251–1255. [DOI] [PubMed] [Google Scholar]
- 49.Ebanks JP, Koshoffer A, Wickett RR, et al. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J Invest Dermatol. 2011;131(6):1226–1233. [DOI] [PubMed] [Google Scholar]
- 50.Simon JD, Peles DN. The red and the black. Acc Chem Res. 2010;43(11):1452–1460. [DOI] [PubMed] [Google Scholar]
- 51.Raimondi S, Sera F, Gandini S, et al. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer. 2008;122(12):2753–2760. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Malek ZA, Swope VB, Starner RJ, Koikov L, Cassidy P, Leachman S. Melanocortins and the melanocortin 1 receptor, moving translationally towards melanoma prevention. Arch Biochem Biophys. 2014;563:4–12. [DOI] [PubMed] [Google Scholar]
- 53.Pellegrini C, Botta F, Massi D, et al. MC1R variants in childhood and adolescent melanoma: a retrospective pooled analysis of a multicentre cohort. Lancet Child Adolesc Health. 2019;3(5):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wendt J, Mueller C, Rauscher S, Fae I, Fischer G, Okamoto I. Contributions by MC1R Variants to Melanoma Risk in Males and Females. JAMA Dermatol. 2018;154(7):789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adhikari K, Mendoza-Revilla J, Sohail A, et al. A GWAS in Latin Americans highlights the convergent evolution of lighter skin pigmentation in Eurasia. Nat Commun. 2019;10(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White KAM, Dailey YT, Guest DD, et al. MC1R Variation in a New Mexico Population. Cancer Epidemiol Biomarkers Prev. 2019;28(11):1853–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fargnoli MC, Gandini S, Peris K, Maisonneuve P, Raimondi S. MC1R variants increase melanoma risk in families with CDKN2A mutations: a meta-analysis. Eur J Cancer. 2010;46(8):1413–1420. [DOI] [PubMed] [Google Scholar]
- 58.Taylor NJ, Reiner AS, Begg CB, et al. Inherited variation at MC1R and ASIP and association with melanoma-specific survival. Int J Cancer. 2015;136(11):2659–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitra D, Luo X, Morgan A, et al. A UV-independent pathway to melanoma carcinogenesis in the redhair-fairskin background. Nature. 2012;491(7424):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarrett SG, Wolf Horrell EM, Christian PA, et al. PKA-mediated phosphorylation of ATR promotes recruitment of XPA to UV-induced DNA damage. Mol Cell. 2014;54(6):999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Robles-Espinoza CD, Roberts ND, Chen S, et al. Germline MC1R status influences somatic mutation burden in melanoma. Nat Commun. 2016;7:12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao J, Wan L, Hacker E, et al. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol Cell. 2013;51(4):409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D'Orazio JA, Nobuhisa T, Cui R, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443(7109):340–344. [DOI] [PubMed] [Google Scholar]
- 64.Khaled M, Levy C, Fisher DE. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 2010;24(20):2276–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mujahid N, Liang Y, Murakami R, et al. A UV-Independent Topical Small-Molecule Approach for Melanin Production in Human Skin. Cell Rep. 2017;19(11):2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8(1):59–65. [DOI] [PubMed] [Google Scholar]
- 67.Liu F, Visser M, Duffy DL, et al. Genetics of skin color variation in Europeans: genome-wide association studies with functional follow-up. Hum Genet. 2015;134(8):823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaorska K, Zawierucha P, Nowicki M. Prediction of skin color, tanning and freckling from DNA in Polish population: linear regression, random forest and neural network approaches. Hum Genet. 2019;138(6):635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sulem P, Gudbjartsson DF, Stacey SN, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40(7):835–837. [DOI] [PubMed] [Google Scholar]
- 70.Gudbjartsson DF, Sulem P, Stacey SN, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet. 2008;40(7):886–891. [DOI] [PubMed] [Google Scholar]
- 71.Lin W, Qureshi AA, Kraft P, et al. ASIP genetic variants and the number of non-melanoma skin cancers. Cancer Causes Control. 2011;22(3):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helsing P, Nymoen DA, Rootwelt H, et al. MC1R, ASIP, TYR, and TYRP1 gene variants in a population-based series of multiple primary melanomas. Genes Chromosomes Cancer. 2012;51(7):654–661. [DOI] [PubMed] [Google Scholar]
- 73.Maccioni L, Rachakonda PS, Scherer D, et al. Variants at chromosome 20 (ASIP locus) and melanoma risk. Int J Cancer. 2013;132(1):42–54. [DOI] [PubMed] [Google Scholar]
- 74.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–157. [DOI] [PubMed] [Google Scholar]
- 75.Coll AP, Farooqi IS, Challis BG, Yeo GSH, O'Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89(6):2557–2562. [DOI] [PubMed] [Google Scholar]
- 76.Rizzato C, Scherer D, Rudnai P, et al. POMC and TP53 genetic variability and risk of basal cell carcinoma of skin: Interaction between host and genetic factors. J Dermatol Sci. 2011;63(1):47–54. [DOI] [PubMed] [Google Scholar]
- 77.Clark AJL. 60 YEARS OF POMC: The proopiomelanocortin gene: discovery, deletion and disease. J Mol Endocrinol. 2016;56(4):T27–37. [DOI] [PubMed] [Google Scholar]
- 78.Kühnen P, Clément K, Wiegand S, et al. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N Engl J Med. 2016;375(3):240–246. [DOI] [PubMed] [Google Scholar]
- 79.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491. [DOI] [PubMed] [Google Scholar]
- 80.Kundu RV, Mhlaba JM, Rangel SM, Le Poole IC. The convergence theory for vitiligo: A reappraisal. Exp Dermatol. 2019;28(6):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol Rev. 2016;269(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riding RL, Harris JE. The Role of Memory CD8+ T Cells in Vitiligo. J Immunol. 2019;203(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strassner JP, Harris JE. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr Opin Immunol. 2016;43:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16(3):208–214. [DOI] [PubMed] [Google Scholar]
- 85.Teulings HE, Overkamp M, Ceylan E, et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168(1):162–171. [DOI] [PubMed] [Google Scholar]
- 86.Paradisi A, Tabolli S, Didona B, Sobrino L, Russo N, Abeni D. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol. 2014;71(6):1110–1116. [DOI] [PubMed] [Google Scholar]
- 87.Salem MMAEL, Shalbaf M, Gibbons NCJ, Chavan B, Thornton JM, Schallreuter KU. Enhanced DNA binding capacity on up-regulated epidermal wild-type p53 in vitiligo by H2O2-mediated oxidation: a possible repair mechanism for DNA damage. FASEB J. 2009;23(11):3790–3807. [DOI] [PubMed] [Google Scholar]
- 88.Feily A, Pazyar N. Why vitiligo is associated with fewer risk of skin cancer? Providing a molecular mechanism. Arch Dermatol Res. 2011;303(9):623. [DOI] [PubMed] [Google Scholar]
- 89.Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database of Systematic Reviews. 2015(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97(3):410–416. [DOI] [PubMed] [Google Scholar]
- 91.Nishimura EK, Suzuki M, Igras V, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6(2):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar Ultraviolet Irradiation Reduces Collagen in Photoaged Human Skin by Blocking Transforming Growth Factor-β Type II Receptor/Smad Signaling. The American Journal of Pathology. 2004;165(3):741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamada T, Hasegawa S, Inoue Y, et al. Wnt/β-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133(12):2753–2762. [DOI] [PubMed] [Google Scholar]
- 94.Sato-Jin K, Nishimura EK, Akasaka E, et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. Faseb j. 2008;22(4):1155–1168. [DOI] [PubMed] [Google Scholar]
- 95.Takeo M, Lee W, Rabbani P, et al. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016;15(6):1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chou WC, Takeo M, Rabbani P, et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19(7):924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013;149(1):68–73. [DOI] [PubMed] [Google Scholar]
- 98.Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol. 2015;151(1):42–50. [DOI] [PubMed] [Google Scholar]
- 99.Lavker RM, Miller S, Wilson C, et al. Hair follicle stem cells: their location, role in hair cycle, and involvement in skin tumor formation. J Invest Dermatol. 1993;101(1 Suppl):16S–26S. [DOI] [PubMed] [Google Scholar]
- 100.Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145(6):941–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tobin DJ, Hagen E, Botchkarev VA, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? J Invest Dermatol. 1998;111(6):941–947. [DOI] [PubMed] [Google Scholar]
- 102.Botchkareva NV, Khlgatian M, Longley BJ, Botchkarev VA, Gilchrest BA. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 2001;15(3):645–658. [DOI] [PubMed] [Google Scholar]
- 103.Aubin-Houzelstein G, Djian-Zaouche J, Bernex F, et al. Melanoblasts' proper location and timed differentiation depend on Notch/RBP-J signaling in postnatal hair follicles. J Invest Dermatol. 2008;128(11):2686–2695. [DOI] [PubMed] [Google Scholar]
- 104.Moriyama M, Osawa M, Mak S-S, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173(3):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang B, Ma S, Rachmin I, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577(7792):676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lerner AB. Gray hair and sympathectomy. Report of a case. Arch Dermatol. 1966;93(2):235–236. [PubMed] [Google Scholar]
- 107.Ortonne JP, Thivolet J, Guillet R. Graying of hair with age and sympathectomy. Arch Dermatol. 1982;118(11):876–877. [DOI] [PubMed] [Google Scholar]