Abstract
Terminal, or postprocessing, sterilization of composite biomaterials is crucial for their use in wound healing and tissue-engineered devices. Recent research has focused on optimizing traditional biomaterial formulations to create better products for commercial and academic use which incorporate hydrophobic compounds or secondary gel networks. To use a hydrogel in a clinical setting, terminal sterilization is necessary to ensure patient safety. Lyophilization, gamma-irradiation, and ethylene oxide treatment all have negative consequences when applied to alginate scaffolds for clinical use. Here, we aim to find alternative terminal sterilization methods for alginate and alginate-based composite hydrogels which maintain the structure of composite alginate networks for use in biomedical applications. A thorough investigation of the effect of common sterilization methods on swollen alginate-based hydrogels has not been reported and therefore, this work examines autoclaving, ethanol washing, and ultraviolet light as sterilization techniques for alginate and alginate/Pluronic® F68 composite hydrogels. Preservation of structural integrity is evaluated using shear rheology and analysis of water retention, and efficacy of sterilization is determined via bacterial persistence within the hydrogel. Results indicate that ethanol sterilization is the best method of those investigated because ethanol washing results in minimal effects on mechanical properties and water retention and eliminates bacterial persistence. Furthermore, this study suggests that ethanol treatment is an efficacious method for terminally sterilizing interpenetrating networks or other composite hydrogel systems.
Keywords: terminal sterilization, alginate, composite hydrogel, Pluronic® F68, rheology
INTRODUCTION
Commercialization of novel hydrogel wound dressing formulations is critical for the translational potential of these systems. Currently, commercially available wound dressings are commonly hydrogel-based, which are lyophilized to remove all water before packaging. The lyophilized hydrogel matrix is re-swollen upon application to the wound.1 Hydrogel-based wound dressings are clinically successful due to their high water retention and ability to remove excess wound exudate. Recent advances in wound dressing technology suggest that these hydrogels could make excellent drug delivery mechanisms, delivering local antibiotics or other compounds, such as oxygen, to healing wounds.2–8 Alginate is a natural polymer often used in hydrogel-based wound dressing formulations (e.g., Kaltostat®, Sorbsan®, SeaSorb®, Tegaderm™) due to its low cost, ease of manipulation, elasticity, and water retention.9–14 The material properties of alginate hydrogels can be tailored to a given application (e.g., creating gels with Young’s moduli ranging from 0.05 to 100 kPa) by altering the crosslinking density, polymer concentration, and ratio of mannuronate: guluronate monomers in the alginate polymer.4,15–18
In order to create wound dressings and other clinically desirable alginate hydrogels, it is highly desirable to expand the range of material properties accessible with alginate-based materials using alternative approaches. For example, creating interpenetrating networks (IPNs) or semi-IPNs of alginate and other polymers has been explored as a means to better control rheology and gelation behavior of alginate-based biomaterials.19 The grafting of hydrophobic chains onto alginate polymers to create hydrophobically-modified alginate (HMA) has been studied by our group5,20 and others21–24 as a way to enhance mechanical robustness and improve solubility of hydrophobic drugs within alginate matrices. Studies on composite hydrogels of alginate, surfactants (e.g., Pluronic® F68 (F68)), and perfluorocarbons (PFCs) to determine the applicability of these formulations for cell encapsulation and protein or drug delivery have been reported.19,25–28 Use of surfactant additives in biomedical device formulations allows tuning of the matrix properties and can affect protein or drug solubility within the hydrogel.28 When a surfactant, such as F68, is incorporated at concentrations above its critical micelle concentration (CMC), hydrophobic compounds (i.e., proteins, peptides, or small molecules) can be successfully integrated into aqueous hydrogel matrices for applications in delivery of therapeutic agents.29–31
The efficacy of any biomaterial is significantly impacted by the sterility of the material. For in vivo applications, sterilization reduces the risk of infection and inflammation. Lyophilization is commonly used to sterilize conventional alginate materials; for example, those used in commercially available wound dressings (e.g., Kaltostat®). However, lyophilization can damage hydrogel structure, impacting the final mechanical properties and drug release dynamics of the hydrogel.3 This becomes an even greater concern for the more complex alginate-based materials mentioned above, where lyophilization may destroy microstructures that have self-assembled in an aqueous environment (e.g., F68 micelles loaded with hydrophobic drugs, associations between hydrophobic chains in HMA gels, or physical crosslinks in semi-IPNs). Gamma-irradiation and ethylene oxide gas have also been used to sterilize hydrogels.32 However, alginate polymers have been shown to degrade when exposed to gamma-irradiation.33 Additionally, residual ethylene oxide gas is potentially carcinogenic and toxic, so its use in clinical applications is of major concern.32 Thus, it is desirable to explore terminal sterilization methods that can be applied to alginate gels in a wet or swollen state that will preserve the complex network structures present within composite or IPN alginate gels for clinical and biomedical applications.
Common terminal sterilization techniques, namely ultraviolet (UV) irradiation, treatment with an ethanol wash, and autoclaving, are investigated in this work, focusing on the effects on the mechanical properties of alginate and alginate/F68 hydrogels via shear rheometry and equilibrium swelling. Sterilization efficacy is evaluated through measurement of bacterial persistence within the hydrogel post-treatment. From a bioprocessing point of view, the establishment of an effective terminal sterilization technique that does not significantly alter the native properties of a biomaterial may decrease processing costs, reducing the need for sterility throughout the process. Overall, this work investigates the ability to terminally sterilize a composite alginate hydrogel, preserving the existence of any matrix additives while maintaining the desired mechanical properties.
MATERIALS AND METHODS
Materials
Cell culture grade sodium alginate (lot#1353824,%G ≥ 60, Mv ~ 240 kDa determined from intrinsic viscosity in 0.1 M sodium chloride), HEPES buffer, D(+) glucose, sulfuric acid, sodium dichromate dehydrate, silver nitrate, and Escherichia coli (E. coli, DH5α with chloramphenicol resistance) were obtained from Sigma Aldrich (St. Louis, MO). All additional materials were obtained from Thermo-Fisher Scientific (Waltham, MA) unless otherwise noted. A Thermo Scientific Barnstead NANOPure® Infinity water system was used to purify all water to a resistivity of 18 MΩ (nanopure water).
Hydrogel preparation
Alginate stock solutions at 1.5% weight by volume (w/v) were prepared using nanopure water with the addition of glucose and HEPES buffer and stirred for 24 h. When applicable, F68 was added during initial solution preparation. Hydrogels were formed by internal crosslinking by adding CaEDTA and glucono-δ-lactone (GDL) to the alginate solution as previously described.28,34 In this work, the alginate stock solution was diluted to a final concentration of 1% w/v alginate, 10 mM HEPES buffer, and 0.1% w/v glucose with a final concentration of 50 mM for both the CaEDTA and GDL. Hydrogels were formed in 12-well nontissue culture treated plates or Petri dishes (35 mm or 100 mm in diameter) and allowed to crosslink for 24 h and stored within a humidified chamber at 25°C (≥90% humidity). Crosslinked hydrogels were subsequently soaked in a 0.1 M calcium chloride (and 1% F68 when appropriate) for 48 h to ensure complete crosslinking. Before all treatments, hydrogels were dipped in a large beaker of nanopure water five times to rinse off any residual CaCl2 from the surface of the hydrogel.
Ethanol disinfection
Hydrogels were exposed to 250 mL of 70% (v/v) ethanol with 0.1 M (containing 1% F68 when appropriate) in nanopure water for 20 min. Following disinfection, the ethanol-water mixture was discarded and the hydrogels were quickly submerged in 600 mL of nanopure water to remove surface ethanol before analysis.
UV irradiation
Hydrogels were exposed to UV irradiation at a dose of approximately 250 nm for 20 min per side on a raised surface within a Forma class IIa/B3 biological safety cabinet (Thermo Fisher Scientific, Waltham, MA) with a measured dose of 75 to 100 μW/cm2. Hydrogels were flipped using sterile tweezers and placed on new foil for the second 20 min. Absorption of 250 nm UV light by alginate and alginate/F68 solutions was used to estimate transmittance of 250 nm UV light through alginate and alginate/F68 hydrogels. Absorption of both solutions was measured as 0.481 using a UV-penetrable cuvette with a 1 cm path length. Following traditional methods for determining transmittance, absorption (A) was converted to transmittance (T) by T=10(−A). An absorption value of 0.0481 was used because the hydrogels are approximately 1 mm thick, following the relationship between absorption and path length given by the Beer-Lambert law. These calculations result in a transmittance of T=0:895. Given that hydrogel samples were not perfectly uniform in thickness, an approximate transmittance of 85–90% was assumed. This suggests that approximately 10–15% of the 250 nm light was unable to pass through the hydrogel and aid in sterilization.
Autoclave sterilization
Two methods were used to autoclave the hydrogels: (1) a hydrogel was gently patted dry with a Kimwipe® (Kimberly-Clark) and placed within a sterile autoclave bag (Fisherbrand® Instant Sealing Sterilization Pouches, Thermo Fisher Scientific, Waltham, MA), or (2) a hydrogel was placed in a glass crystallizing dish containing 250 mL of 0.1 M (±1% F68) crosslinking solution to ensure that Ca2+ ions do no leach out of the hydrogel during treatment. The autoclave bags and the crystallizing dishes were sterilized on a liquid cycle for 15 min at 250°C and 15 psi in a Tuttnauer® (69 Series) large capacity laboratory autoclave.
Hydrogel swelling and water uptake
Hydrogel swelling studies were performed as previously reported.28,35 For each treatment group (n = 5), the hydrogels were removed from crosslinking solution and treated (ethanol disinfection, UV irradiation, two autoclaving methods, or untreated). Following treatment, hydrogels were weighed, lyophilized for 24 h using a Labconco FreeZone 6 freeze dry system (Kansas City, MO), and reweighed to determine the wet and dry weights (or water content) of the treated hydrogels. The mass of water in the hydrogels, or swelling ratio, was calculated by dividing the difference in mass of a swollen hydrogel (mg,s) and mass of dry polymer (mp) in the hydrogel by the mass of dry polymer:
| (1) |
The relative swelling ratio was determined by dividing all treated samples by the control (untreated) sample. The untreated 1% alginate swelling ratio was used for all alginate samples and the untreated 1% alginate + 1% F68 swelling ratio was used for all of the 1% alginate + 1% F68 samples. Therefore, the relative swelling ratio highlights the change in swelling ratio resulting from the sterilization treatment. A two-tailed heteroscedastic Student’s t-test was used to determine the significance in the data (p ≤ 0.05, n = 5). Data were plotted relative to each formulation (alginate and alginate/F68) and one standard deviation is reported.
Rheological characterization
The mechanical properties of sterilized alginate hydrogels were measured by performing small amplitude oscillatory shear rheology on disk-shaped hydrogels (n = 5). The change in mechanical properties after sterilization was assessed relative to untreated alginate hydrogels (control). Measurements were taken using a 40 mm parallel plate geometry on an AR-G2 stress-controlled rheometer (TA Instruments, New Castle, DE). An oscillatory frequency sweep from 0.01 to 100 Hz at 25°C and at fixed stress of 1 Pa, determined from performing a stress sweep, was used to measure the storage (G′) and loss (G″) moduli. A frequency of 1 Hz was chosen to determine statistical changes resulting from hydrogel treatment methods. Statistical significance was determined at 1 Hz using a two-tailed heteroscedastic Student’s t-test (p ≤ 0.05).
Bacterial persistence
E. coli (DH5α with chloramphenicol resistance) was grown in Lysogeny broth (LB) and an E. coli stock solution in LB broth was made at a concentration of 106 cells/mL. Concentrations were determined using optical density (OD) measurements at 600 nm on a μQuant spectrophotometer (BioTek Instruments, Inc., Richmond, VA) which were correlated with the colony forming unit relationship determined for this strain (cells/mL = 5.5 × OD600 + 0.04, data not shown). For each treatment group (n = 5), hydrogel samples were removed from crosslinking solution, rinsed with 600 mL of nanopure water to remove surface solution, and placed in sterile Petri dishes. Hydrogels were seeded with E. coli through soaking in 9.8 mL of LB containing E. coli (106 cells/mL) for 20 min and then rinsed once again in 600 mL of nanopure water by 20 repeated dunks in replenished fresh water. Given that the pore size in alginate hydrogels ranges from 25 to 300 nm, bacterial infiltration into the network was expected. Microscopy was used to confirm bacterial infiltration into the hydrogel. Hydrogels were then treated with either an ethanol wash or UV irradiation as previously described.
Following sterilization treatment, the samples were rinsed again with nanopure water by 20 repeated submersions, cut into 2.5 cm × 2.5 cm pieces to allow for bacteria release from the gel using a sterile scalpel, and transferred to sterile test tubes. LB (10 mL) and 10 μL of chloramphenicol (34 mg/mL) were added to each tube and the tubes were incubated (37°C, 250 rpm). E. coli concentration was measured after 15, 18, and 24 h to determine the bacterial persistence within the hydrogel. The average cell density ± one standard deviation calculated from four hydrogels is reported for each time point. Statistical differences from the untreated control were determined using a two-tailed homoscedastic Student’s t-test (p ≤ 0.05, n = 5). Statistical similarity to zero was determined using a one-tailed homoscedastic Student’s t-test (p ≥ 0.2, n = 5).
Ethanol clearance
To measure the clearance of residual ethanol from hydrogels after sterilization, the concentration of ethanol in each of the wash solutions used to treat the hydrogel was quantified by the colorimetric reduction of chromium (VI) to chromium (III) by ethanol, as described previously.1 The reduction required the use of a modified form of the Jones’ reagent, which contains a silver nitrate catalyst: 4.5 mol/L sulfuric acid (pH = 4.5), 0.42 mol/L sodium dichromate dehydrate, and 14.7 mmol/L silver nitrate. For each sample group (n = 5), the hydrogels (made from 1.75 mL of alginate solution) were treated with 3 mL of 70% (v/v) ethanol with 0.1 M (±1% F68) for 20 min. The amount of ethanol within the hydrogels post-sterilization was determined by dissolution of the hydrogels in 3 mL 50 mM sodium citrate and quantification of ethanol content before any washes for mass balance analysis later. A second set of hydrogels were washed three times with 3 mL soaks in nanopure water. The first two washes were 5 min each and the third wash was 10 min. To quantify the ethanol in each wash solution and in the dissolved hydrogels, the concentration of ethanol in each wash solution or dissolved alginate solution was determined and subtracted from the original content in the hydrogel. Briefly, 150 μL samples of wash solution or dissolved were mixed with 50 μL of modified Jones’ reagent per well of a 96-well plate (n = 3 for each sample and time point). After 30 s, the optical density at 590 nm was measured with a μQuant microplate spectrophotometer. The amount of ethanol left in the hydrogel after each wash was determined as an average of the optical densities for each hydrogel and the average ± one standard deviation for each group (n = 5) are reported.
Gross changes in hydrogel structure
Hydrogels for visual inspection of macroscopic structural changes (discussed below and shown in Figure 1) were prepared as described above, but cast in a mold designed for wound dressing formation. This mold contains a piece of cotton gauze (CVS® Pharmacy) centered in the space. Liquid alginate fills the mold and holds the gauze in place as the alginate gels via the release of Ca2+ from CaEDTA by GDL. Following hydrogel formation, sections of a large hydrogel were cut into 2-inch square sections. The untreated sample was not treated in any way and the ethanol soaked and autoclaved samples were treated as described above. The lyophilized sample was freeze dried for 36 h in a Labconco® FreeZone 6 freeze dry system to observationally show the destruction to the hydrogel network following the lyophilization procedure.
FIGURE 1.
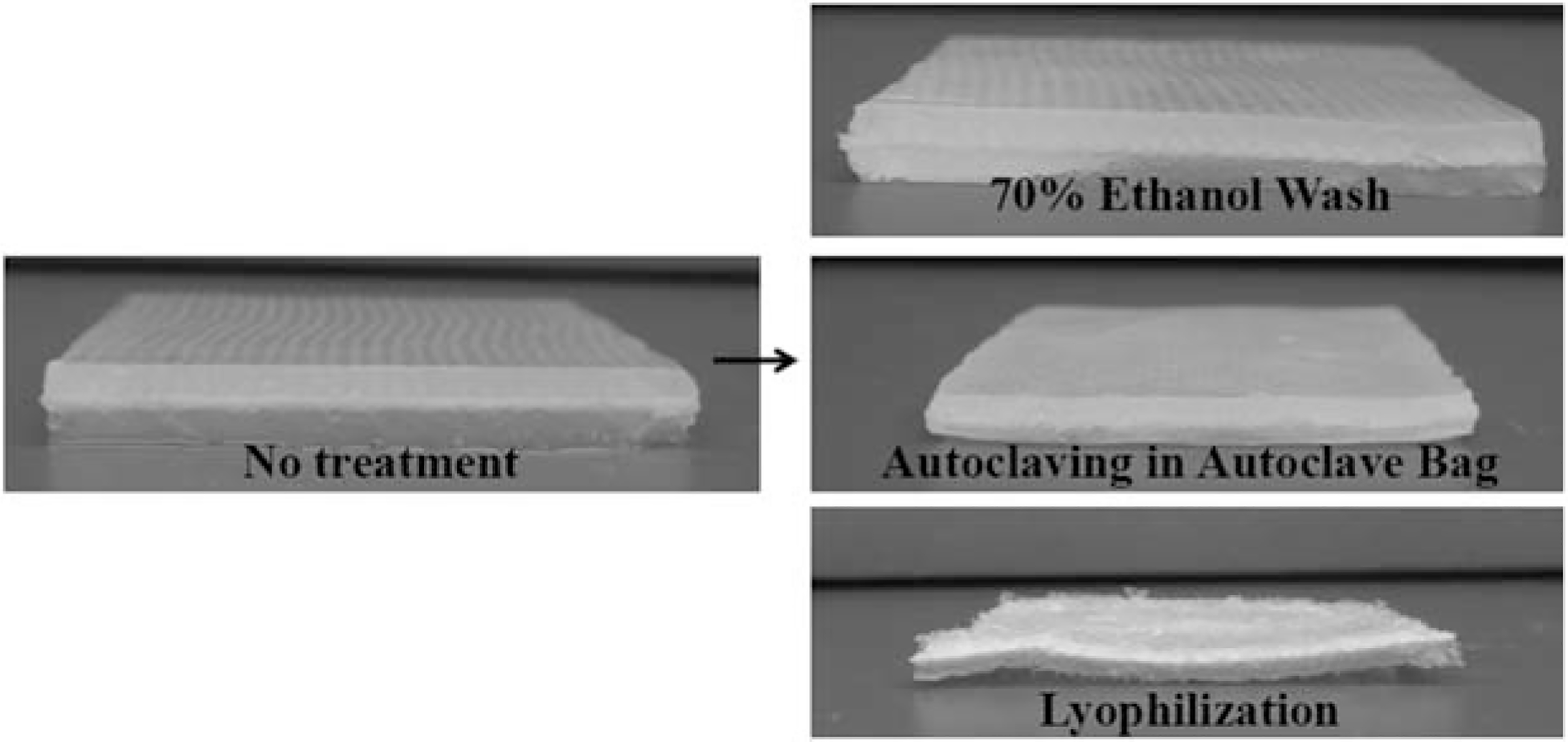
Alginate hydrogels formed over gauze show the progression of the alginate network structure following treatments with 70% ethanol for 20 min, autoclaving in an autoclave bag on the liquid cycle and lyophilization. The ethanol-treated hydrogel is visually and texturally similar to the untreated hydrogel while the autoclaved and lyophilized hydrogels have both visual and structural defects.
RESULTS AND DISCUSSION
For the development of a composite alginate hydrogel for drug delivery or wound healing, changes in mechanical properties can indicate a change in network architecture as well as a change in mechanical robustness. Here, our discussion of the data assumes that the control alginate hydrogel has the ideal properties for the application of interest and therefore a terminal sterilization technique should not change these properties. Visually, Figure 1 shows the macroscopic changes in an alginate hydrogel after various sterilization techniques, indicating that autoclaving causes the hydrogel to shrink and lyophilization causes physical destruction of the hydrogel matrix.
Mechanical properties
Decrease in water retention.
Measuring the water retention yields information on changes in the network architecture through changes in the swelling ratio.36,37 Changes in the swelling ratio (or water reuptake) following a terminal sterilization treatment indicate that the treatment affected the network architecture and crosslinking density. A decrease in the swelling ratio negatively impacts the performance of the hydrogel as a wound dressing (decreases the ability to take up wound exudate) and affects the loading capacity (i.e., of a drug) of the hydrogel.28 Results indicate that both autoclaving protocols lead to significant water loss (Figure 2), while washing with ethanol and UV irradiation do not significantly alter the water content of the hydrogel. The relative swelling ratio shows that the presence of F68 does not alter how the treatments affect water retention within the hydrogel, even though alginate/F68 composite hydrogels contained ~20% less water than alginate hydrogels, which is consistent with previously reported results.28
FIGURE 2.
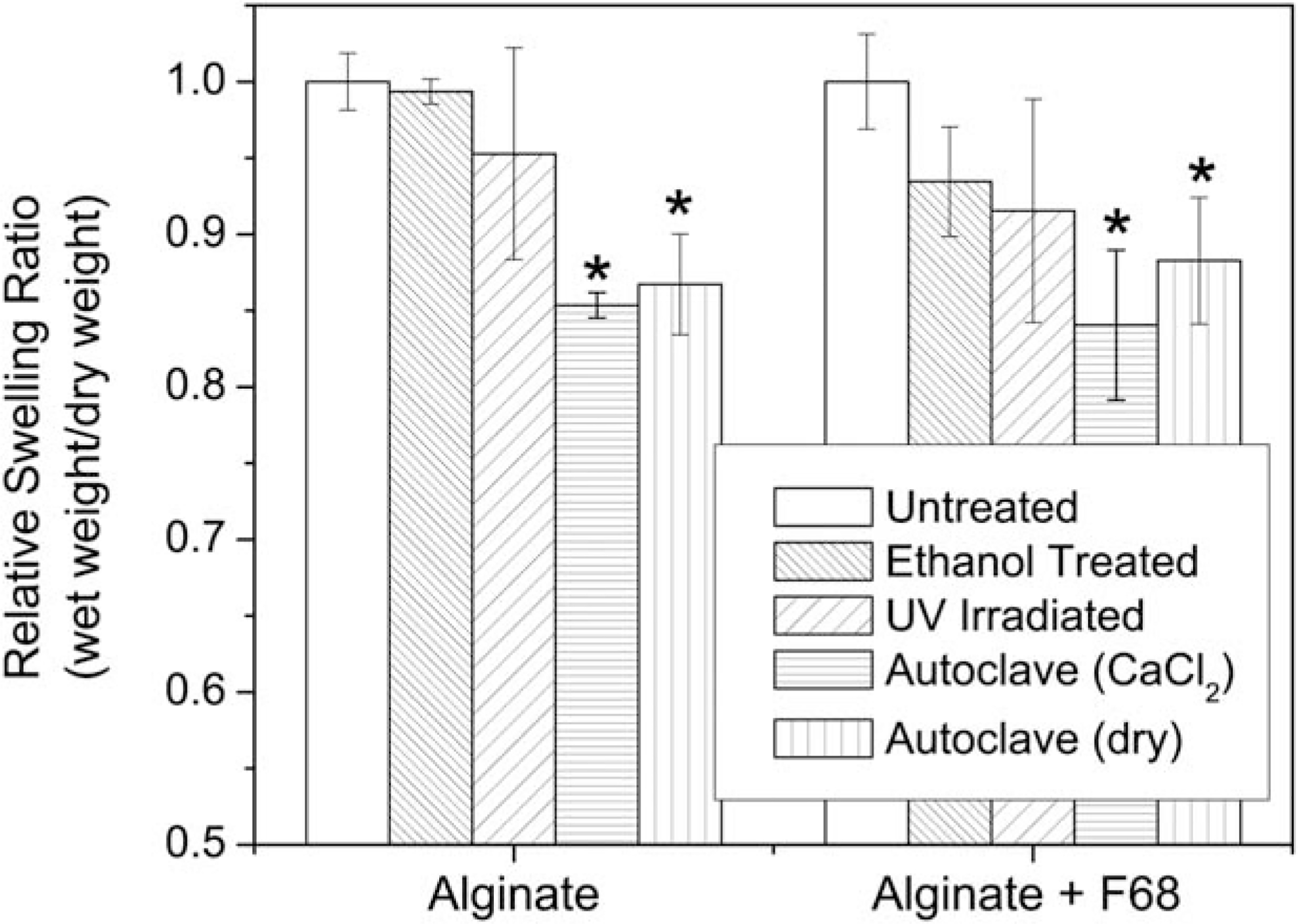
Relative swelling ratios for treated alginate and alginate/F68 composite hydrogels. Results show significant water loss following autoclaving (in 0.1M CaCl2 and in an autoclave bag, dry) for both groups. (*) indicates a significant decrease in the swelling ratio as compared to the untreated hydrogel in each group (p ≤ 0.05, n = 5). Numerical data labels indicate the percent decrease in swelling from the appropriate untreated control for each formulation.
Rheological characterization.
The water loss and disruption of hydrogel structure after autoclaving (Figure 2) translates to a significant change in the mechanical properties (Figures 3 and 4). The average storage and loss moduli are reported in Figure 3 and error in these measurements at 1 Hz is depicted in Figure 4. Evaluation of the change in shear modulus due to sterilization treatment methods suggests that the methods used maintain similar frequency dependence to the untreated control. Representative data showing the average storage and loss moduli for samples containing 1% F68 are shown in Figure 3(a). Data is similar for alginate samples without F68 (full sets of data are not shown). Shear rheology also indicates that autoclaving hydrogels is not a recommended method for terminal sterilization [Figure 3(b)]. All treated hydrogels remain as elastic gels with G′ fairly independent of frequency and roughly an order of magnitude higher than G″ across the frequency range investigated (0.01–100 Hz).
FIGURE 3.
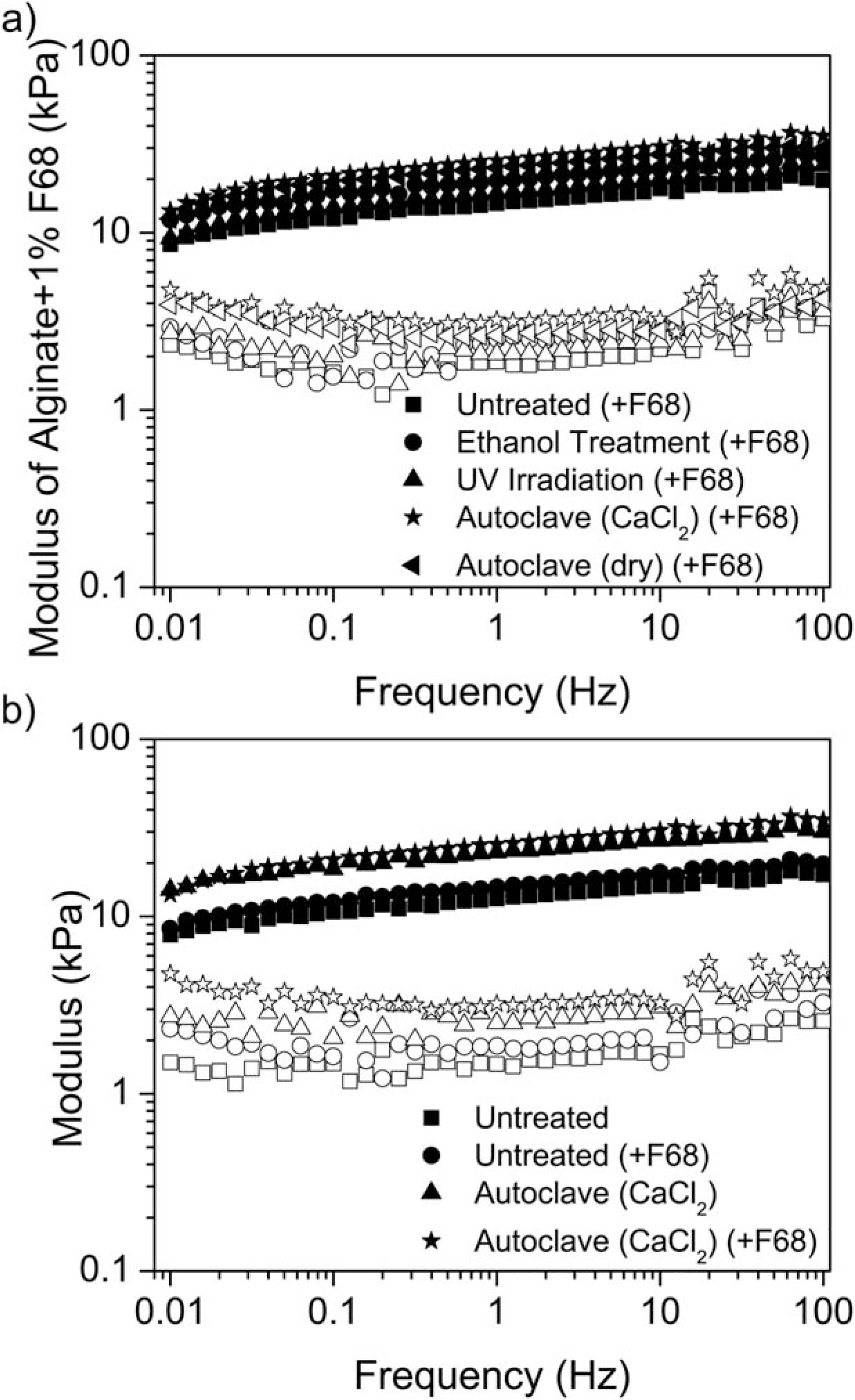
Storage (G′, closed symbols) and loss (G″, open symbols) moduli for alginate hydrogels. a) Representative rheological data showing all samples for alginate + F68 hydrogels over the entire frequency range. b) Rheological data indicates a significant increase in hydrogel stiffness following autoclaving. Autoclaving the hydrogels while submerged in 0.1M CaCl2 significantly increases the stiffness of the hydrogel formulation. A greater difference between the G″ values was measured over the frequency range of 0.01 to 100 Hz for autoclaved hydrogels with F68 (*) and without F68 (Δ) compared to untreated hydrogels (○ and ☐).
FIGURE 4.
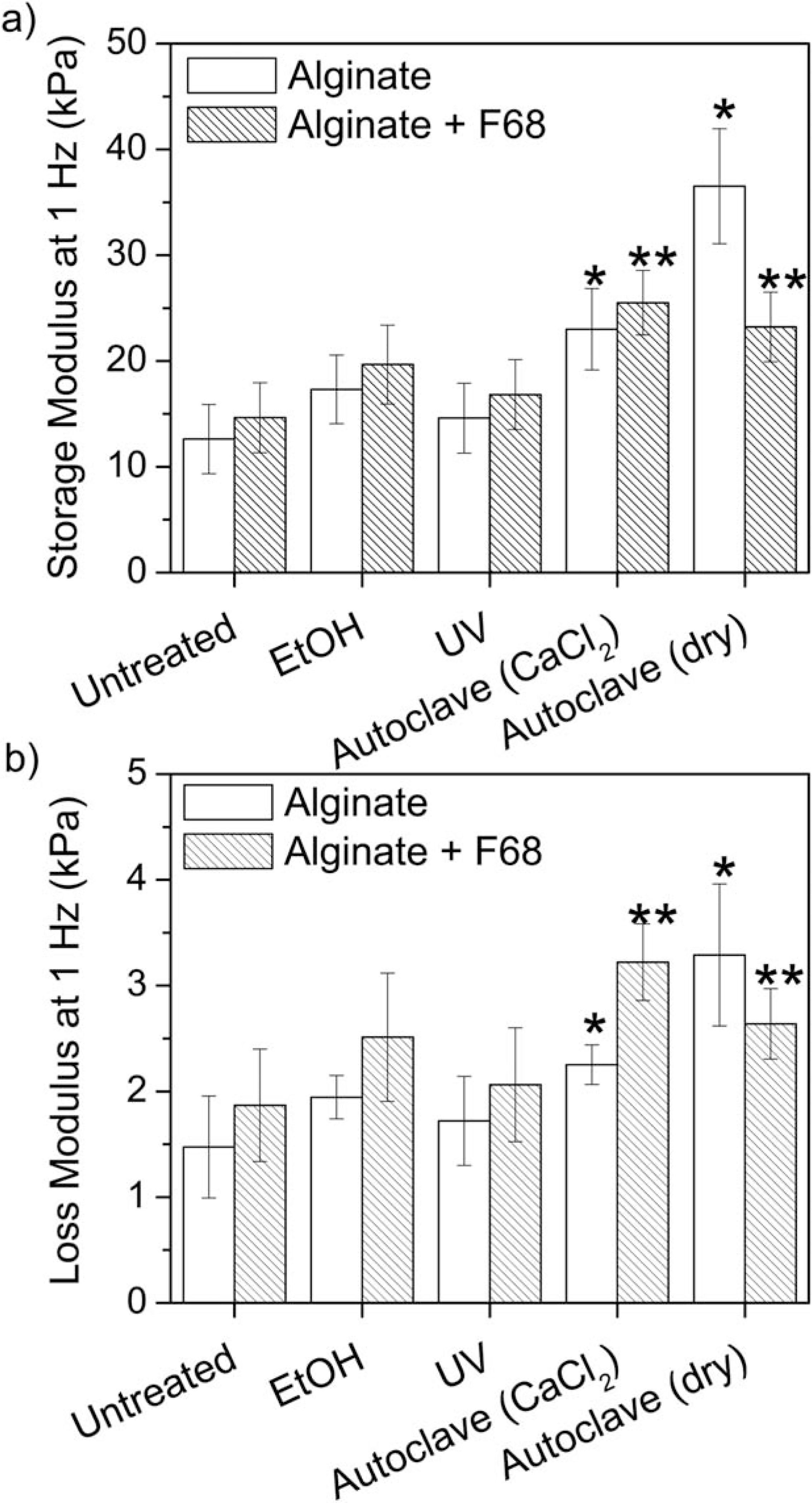
Average value and standard deviation of rheological samples at a frequency of 1 Hz. Results show that autoclaving the alginate hydrogels significantly increased the storage (a) and loss (b) moduli suggesting that autoclaving is not a preferred method for terminal sterilization. (*) indicates a significant difference from the untreated alginate hydrogel and (**) indicates a significant difference from the untreated alginate/F68 composite hydrogel (p ≤ 0.05, n = 5).
Figure 3 shows that autoclaving while submerged in 0.1M CaCl2 increases both G′ and G″, indicating an increase in both the stiffness and viscous response regardless of the presence of F68.28 Furthermore, the increase in modulus for the sample submerged in 0.1M CaCl2 also suggests that the increase is due to a change in network structure and not water loss alone (Figure 2). Similarly, autoclaving in an autoclave bag was found to be significantly different from the untreated hydrogel, while an ethanol wash and UV irradiation were similar to the untreated hydrogel across all frequencies (data not shown). Further analysis of G′ [Figure 4(a)] and G″ [Figure 4(b)] at a frequency of 1 Hz shows that terminal sterilization by autoclaving significantly increases G′ and G″. Additionally, washing with 70% ethanol or UV irradiation did not significantly alter the mechanical properties at 1 Hz regardless of F68 incorporation.
Autoclaving in CaCl2 resulted in an 82% increase in G′ and 53% increase in G″ relative to the untreated control at 1 Hz. With the addition of F68 to create a composite hydrogel, autoclaving in CaCl2 resulted in a 74% increase in G′ and 72% increase in G″ relative to the untreated alginate/F68 composite at 1 Hz. Similarly, autoclaving in an autoclave bag (dry) results in a 189% increase in G′ and 123% increase in G″ relative to the untreated alginate hydrogel (59% and 41% respectively for the alginate/F68 composite).
Analysis of bacterial persistence
Both autoclaving treatments were not evaluated for this analysis because (1) autoclaving guarantees terminal sterilization and (2) this work concluded that autoclaving is not a feasible method due to significant changes in the mechanical properties. Further analysis of both ethanol sterilization and UV irradiation were performed to determine the effectiveness of these methods through exposure to E coli. Hydrogels were submerged in bacterial solutions before treatment and the bacterial persistence within the material was measured and reported (Figure 5). The addition of 1% F68 had no statistically significant effect on the bacterial growth results for untreated and ethanol disinfected samples (Figure 5). The ethanol wash was an effective sterilization treatment because all data were statistically similar to zero (p ≥ 0.99), indicating that no bacteria remained within the hydrogel regardless of F68 concentration (Figure 5).
FIGURE 5.
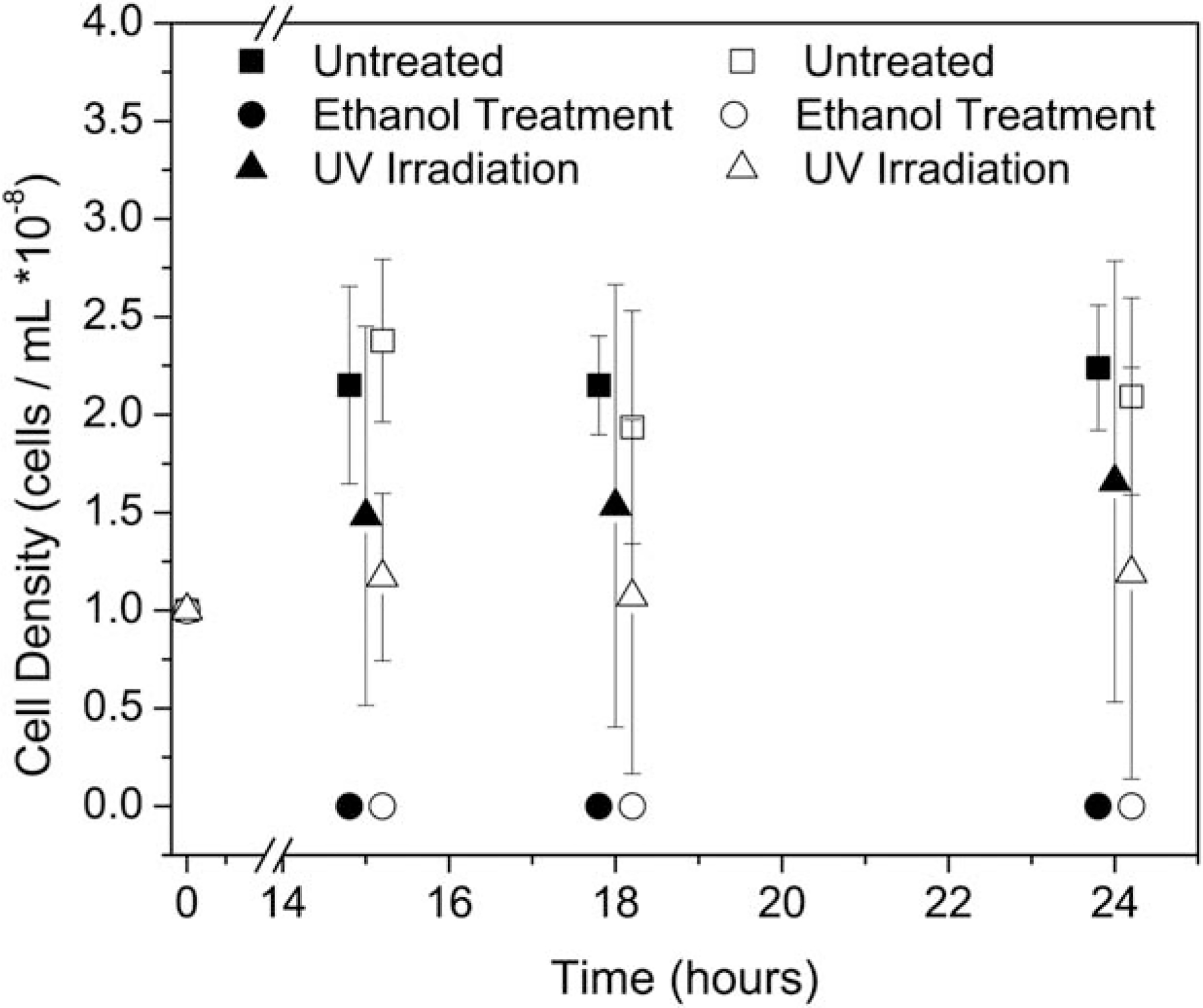
Bacterial persistence within treated alginate (closed) and alginate/F68 composite (open) hydrogels. Ethanol washing removed all bacterial contamination in both hydrogel formulations. In all cases, ethanol treated samples were statistically similar to zero, (p > 0.95, n = 5). The large error bar associated with the UV irradiated samples results from high amounts of variation in the treatment outcome. Some samples resulted in no bacterial persistence, while others were very close to the control.
The UV irradiation treatment was not as effective as the ethanol treatment and the average of six samples was not statistically similar to zero, which would have indicated complete sterilization. Instead, UV-irradiated results suggested that results for each individual sample varied, as noted by the large errors bars. For UV-irradiated samples, the standard deviation was greater than 66% of the actual measured value. This large error is indicative of a heterogeneous sample population. The large observed variability is due in part to alginate absorption in the UV (transmittance of 250 nm light through 1 mm thick 1% alginate hydrogels ± 1% F68 was measured as 85–90%). It is important to note that the transmittance of a hydrogel is dependent upon the absorption of light at a specific wavelength by the material and the thickness of the material. Any increase in hydrogel thickness will decrease the transmittance and therefore limit the effectiveness of the UV-treatment. These challenges limit the overall application of UV-irradiation as a recommendable sterilization option.
While UV-irradiated alginate/F68 hydrogels were statistically different from their untreated control (p ≤ 0.05, n = 5), the UV-irradiated alginate hydrogels were not statistically different than their untreated control. After 24 h, UV irradiated alginate hydrogels exhibited 45% less E. coli/mL than the untreated alginate hydrogels. While this is some improvement, clinical applications require complete removal of all contamination.
Unlike the UV-irradiated hydrogels, all ethanol washed samples had no detectable bacteria residing within the hydrogel as noted by the lack of error bars on the samples (zero cells/mL, n = 5). Results agree with previously reported data which showed that ethanol-treated N-isopropylacrylamide–based hydrogels did not show any measurable growth of E. coli.1
Results suggest that a 20-min ethanol wash is sufficient to remove bacterial contamination from 1.5 mm thick hydrogels, while the UV-irradiation is not consistently effective at eliminating bacterial persistence. The effectiveness of UV-irradiation is highly dependent upon the source of power and UV dosage, and for these experiments, the use of a biological safety cabinet was not sufficient for eliminating the bacteria within the hydrogel.
Therefore, ethanol disinfection is the best method for terminal sterilization of alginate or composite alginate hydrogels when compared to the other methods investigated. Further optimization of these procedures would be necessary for thicker hydrogels or hydrogels with soluble components for clinical implementation. Packaging options which store the hydrogel in a small amount ethanol may be an alternative to soaking the samples before packaging. However, the use of ethanol is a cost-effective practice that is accessible to clinicians, hydrogel manufacturers, and research laboratories. Ethanol can also be used in areas with limited resources, such as triage centers in third-world countries or the battlefield, increasing the applicability of complex, yet effective treatments in difficult situations.
Ethanol clearance
To use ethanol as a terminal sterilization method, clearance of ethanol from the hydrogel is necessary since residual ethanol may impact the biological system for which the hydrogel was designed. Colorimetric data from the reduction of chromium (VI) to chromium (III) by ethanol were fit to a standard curve to determine the ethanol concentration within the hydrogel. The concentration of ethanol in each wash solution was used to determine the amount of ethanol remaining in the hydrogels (Figure 6). This was achieved by using a mass balance where the initial ethanol content was quantified and the amount released into the wash solution was subtracted from the starting ethanol content within the hydrogel. Results in Figure 6 indicate that less than 1% of the liquid in the hydrogel is ethanol after three 5-min washes in water. Results reported in Figure 6 are consistent with ethanol clearance studies that determined that N-isopropylacrylamide–based hydrogels contained negligible levels of ethanol after three washes.1
FIGURE 6.
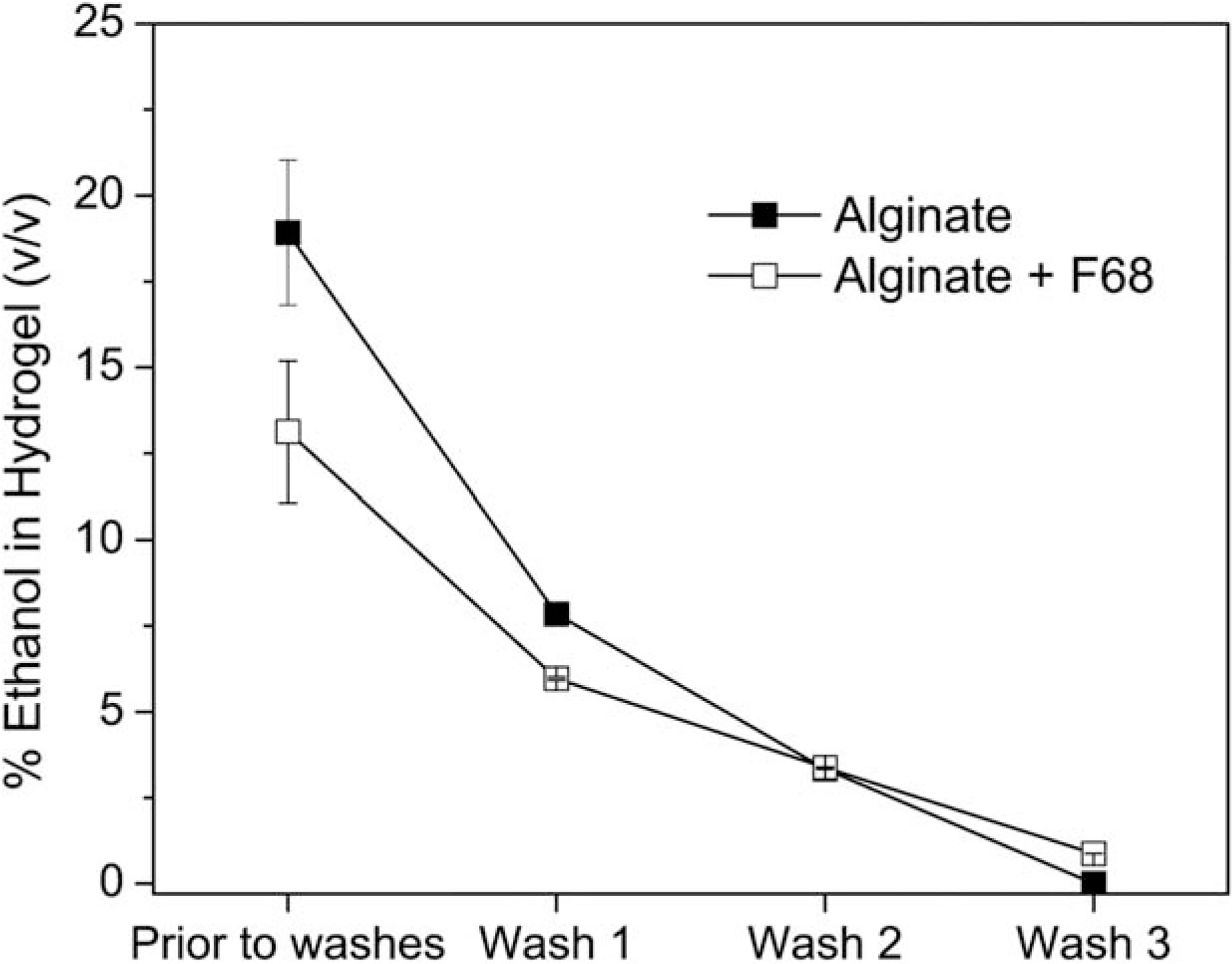
Ethanol clearance from alginate (■) and alginate/F68 composite (☐) hydrogels following soak in 70% ethanol solution for 20 min and three subsequent 5-min washes in water.
CONCLUSION
While effective sterilization is crucial for clinical utilization of tissue engineering devices, maintaining the mechanical integrity of the hydrogel is also critical for proper wound dressing performance. Lyophilization and gamma-irradiation as a terminal sterilization method for IPNs or composite materials is not feasible due to significant negative effects on mechanical function and polymer degradation. Although autoclaving guarantees terminal sterilization, results clearly indicate that this method also comprises mechanical function. UV irradiation did not significantly affect mechanical properties, yet it was not an effective method for terminal sterilization of these 1 mm thick alginate and alginate/F68 composites. Ultimately, we found an ethanol wash to be a practical, effective, low-cost method for terminal sterilization of complex soft biomaterials.
Acknowledgments
Contract grant sponsor: National Science Foundation (NSF)-sponsored Center for Hierarchical Manufacturing; contract grant number: CMMI-0531171
Contract grant sponsor: University of Massachusetts Commercial Ventures and Intellectual Property Office
Contract grant sponsor: NSF-funded Materials Research Science and Engineering Center (MRSEC) on Polymers; contract grant number: DMR-0213695
Contract grant sponsor: National Research Service Award from the National Institutes of Health (to W.L.S.); contract grant number: T32 GM08515
Contract grant sponsor: NSF-sponsored Institute for Cellular Engineering IGERT program; contract grant number: DGE-0654128 (to W.L.S.)
Contract grant sponsor: NSF-sponsored Institute for Cellular Engineering IGERT program (to J.C.W.); contract grant number: DGE-0654128
Contract grant sponsor: University of Massachusetts Commonwealth Honors College (to S.D.H)
Contract grant sponsor: University of Massachusetts Amherst Institute for Cellular Engineering NSF Research Experience for Undergraduates (to A.C.H.); contract grant number: EEC-1005083
REFERENCES
- 1.Huebsch N, Gilbert M, Healy KE. Analysis of sterilization protocols for peptide-modified hydrogels. J Biomed Mater Res Part B: Appl Biomater 2005;74:440–447. [DOI] [PubMed] [Google Scholar]
- 2.Tønnesen HH, Karlsen J. Alginate in Drug Delivery Systems. Drug Dev Ind Pharm 2002;28:621–630. [DOI] [PubMed] [Google Scholar]
- 3.Lai HL, Abu’Khalil A, Craig DQM. The preparation and characterisation of drug-loaded alginate and chitosan sponges. Int J Pharm 2003;251:175–181. [DOI] [PubMed] [Google Scholar]
- 4.Augst AD, Kong HJ, Mooney DJ. Alginate Hydrogels as Biomaterials. Macromolecular Bioscience 2006;6(8):623–633. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary S, Reck JM, Bhatia SR. Modified Alginate for Biomedical Applications. MRS Symp. Proc. 1140E – Advances in Material Design for Regenerative Medicine, Drug Delivery, and Targeting/Imaging, Shastri VP, Lendlein A, Liu L, Mitragotri S, Mikos A, editors. 2009. [Google Scholar]
- 6.Choudhary S, Reck J, Bhatia SR. Hydrophobically Modified Alginate for Drug Delivery Systems. Proceedings of the IEEE: 35th Annual Northeast Bioengineering Conference, 2009. pp 18–19. [Google Scholar]
- 7.Lin HY, Wang HW. The influence of operating parameters on the drug release and antibacterial performances of alginate fibrous dressings prepared by wet spinning. Biomatter 2012;2:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawar HV, Tetteh J, Boateng JS. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf B 2013;102: 102–110. [DOI] [PubMed] [Google Scholar]
- 9.Gilchrist T, Martin AM. Wound treatment with sobsan—An alginate fiber dressing. Biomaterials 1983;4:317–320. [DOI] [PubMed] [Google Scholar]
- 10.Doyle JW, Roth TP, Smith RM, Li YQ, Dunn RM. Effect of calcium alginate on cellular wound healing processes modeled in vitro. J Biomed Mater Res 1996;32:561–568. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Nishimura Y, Tanihara M, Suzuki K, Nakamura T, Shimizu Y, Yamawaki Y, Kakimaru Y. Evaluation of a novel alginate gel dressing: Cytotoxicity to fibroblasts in vitro and foreign-body reaction in pig skin in vivo. J Biomed Mater Res 1998;39: 317–322. [DOI] [PubMed] [Google Scholar]
- 12.Hilton JR, Williams DT, Beuker B, Miller DR, Harding KG. Wound Dressings in Diabetic Foot Disease. Clin Infect Dis 2004;39:S100–S103. [DOI] [PubMed] [Google Scholar]
- 13.Qin YM. The characterization of alginate wound dressings with different fiber and textile structures. J Appl Polym Sci 2006;100: 2516–2520. [Google Scholar]
- 14.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: A review. J Pharm Sci 2008;97:2892–2923. [DOI] [PubMed] [Google Scholar]
- 15.Lee KY, Rowley JA, Eiselt P, Moy EM, Bouhadir KH, Mooney DJ. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 2000;33:4291–4294. [Google Scholar]
- 16.Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 2004;5:1720–1727. [DOI] [PubMed] [Google Scholar]
- 17.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005;26:2455–2465. [DOI] [PubMed] [Google Scholar]
- 18.Saarai A, Kasparkova V, Sedlacek T, Saha P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J Mech Behav Biomed Mater 2013;18:152–166. [DOI] [PubMed] [Google Scholar]
- 19.Choudhary S, White JC, Stoppel WL, Roberts SC, Bhatia SR. Gelation behavior of polysaccharide-based interpenetrating polymer network (IPN) hydrogels. Rheol Acta 2011;50:39–52. [Google Scholar]
- 20.Choudhary S, Bhatia SR. Rheology and nanostructure of hydrophobically modified alginate (HMA) gels and solutions. Carbohydr Polym 2012;87:524–530. [DOI] [PubMed] [Google Scholar]
- 21.Galant C, Kjøniksen A-L, Nguyen GTM, Knudsen KD, Nyström B. Altering associations in aqueous solutions of a hydrophobically modified alginate in the presence of β-cyclodextrin monomers. J Phys Chem B 2005;110:190–195. [DOI] [PubMed] [Google Scholar]
- 22.Yao B, Ni C, Xiong C, Zhu C, Huang B. Hydrophobic modification of sodium alginate and its application in drug controlled release. Bioprocess Biosyst Eng 2010;33:457–463. [DOI] [PubMed] [Google Scholar]
- 23.Yang JS, Jiang B, He W, Xia YM. Hydrophobically modified alginate for emulsion of oil in water. Carbohydr Polym 2012;87: 1503–1506. [Google Scholar]
- 24.Li Q, Liu CG, Huang ZH, Xue FF. Preparation and characterization of nanoparticles based on hydrophobic alginate derivative as carriers for sustained release of vitamin D3. J Agric Food Chem 2011;59:1962–1967. [DOI] [PubMed] [Google Scholar]
- 25.Fraker CA, Mendez AJ, Stabler CL. Complementary methods for the determination of dissolved oxygen content in perfluorocarbon emulsions and other solutions. J Phys Chem B 2011;115: 10547–10552. [DOI] [PubMed] [Google Scholar]
- 26.Gattás-Asfura KM, Fraker CA, Stabler CL. Perfluorinated alginate for cellular encapsulation. J Biomed Mater Res Part A 2012;100: 1963–1971. [DOI] [PubMed] [Google Scholar]
- 27.White JC, Stoppel WL, Roberts SC, Bhatia SR. Addition of perfluorocarbons to alginate hydrogels significantly impacts molecular transport and fracture stress. J Biomed Mater Res Part A 2013; 101:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoppel WL, White JC, Horava SD, Bhatia SR, Roberts SC. Transport of biological molecules in surfactant–alginate composite hydrogels. Acta Biomater 2011;7:3988–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh KT, Bronich TK, Kabanov AV. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic((R)) block copolymers. J Controlled Release 2004;94:411–422. [DOI] [PubMed] [Google Scholar]
- 30.Mei L, Sun HF, Song CX. Local delivery of modified paclitaxel-loaded poly(epsilon-caprolactone)/pluronic F68 nanoparticles for long-term inhibition of hyperplasia. J Pharm Sci 2009;98: 2040–2050. [DOI] [PubMed] [Google Scholar]
- 31.Sahu A, Kasoju N, Goswami P, Bora U. Encapsulation of curcumin in pluronic block copolymer micelles for drug delivery applications. J Biomater Appl 2011;25:619–639. [DOI] [PubMed] [Google Scholar]
- 32.Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, Donovan D. Effects of sterilization on poly(ethylene glycol) hydrogels. J Biomed Mater Res Part A 2008;87:608–617. [DOI] [PubMed] [Google Scholar]
- 33.Lee DW, Choi WS, Byun MW, Park HJ, Yu Y-M, Lee CM. Effect of γ-irradiation on degradation of alginate. J Agric Food Chem 2003; 51:4819–4823. [DOI] [PubMed] [Google Scholar]
- 34.Draget KI, Ostgaard K, Smidsrod O. Homogeneous alginate gels - A technical approach. Carbohydr Polym 1990;14:159–178. [Google Scholar]
- 35.Peppas NA, Moynihan HJ, Lucht LM. The structure of highly crosslinked poly(2-hydroxyethyl methacrylate) hydrogels. J Biomed Mater Res 1985;19:397–411. [DOI] [PubMed] [Google Scholar]
- 36.Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Controlled Release 1987;5:37–42. [PubMed] [Google Scholar]
- 37.Brannon-Peppas L, Peppas NA. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem Eng Sci 1991;46:715–722. [Google Scholar]


