Abstract
BACKGROUND
Obesity is a well-established risk factor for heart failure (HF). However, implications of pericardial fat on incident HF is unclear.
OBJECTIVES
This study sought to examine the association between pericardial fat volume (PFV) and newly diagnosed HF.
METHODS
This study ascertained PFV using cardiac computed tomography in 6,785 participants (3,584 women and 3,201 men) without pre-existing cardiovascular disease from the MESA (Multi-Ethnic Study of Atherosclerosis). Cox proportional hazards regression was used to evaluate PFV as continuous and dichotomous variable, maximizing the J-statistic: (Sensitivity + Specificity − 1).
RESULTS
In 90,686 person-years (median: 15.7 years; interquartile range: 11.7 to 16.5 years), 385 participants (5.7%; 164 women and 221 men) developed newly diagnosed HF. PFV was lower in women than in men (69 ± 33 cm3 vs. 92 ± 47 cm3; p < 0.001). In multivariable analyses, every 1-SD (42 cm3) increase in PFV was associated with a higher risk of HF in women (hazard ratio [HR]: 1.44; 95% confidence interval [CI]: 1.21 to 1.71; p < 0.001) than in men (HR: 1.13; 95% CI: 1.01 to 1.27; p = 0.03) (interaction p = 0.01). High PFV (≥70 cm3 in women; ≥120 cm3 in men) conferred a 2-fold greater risk of HF in women (HR: 2.06; 95% CI: 1.48 to 2.87; p < 0.001) and a 53% higher risk in men (HR: 1.53; 95% CI: 1.13 to 2.07; p = 0.006). In sex-stratified analyses, greater risk of HF remained robust with additional adjustment for anthropometric indicators of obesity (p ≤0.008), abdominal subcutaneous or visceral fat (p ≤ 0.03) or biomarkers of inflammation and hemodynamic stress (p < 0.001) and was similar among Whites, Blacks, Hispanics, and Chinese (interaction p = 0.24). Elevated PFV predominantly augmented the risk of HF with preserved ejection fraction (p < 0.001) rather than reduced ejection fraction (p = 0.31).
CONCLUSIONS
In this large, community-based, ethnically diverse, prospective cohort study, pericardial fat was associated with an increased risk of HF, particularly HF with preserved ejection fraction, in women and men.
Keywords: adipose tissue, adiposity, heart failure, obesity, pericardial fat
CONDENSED ABSTRACT
In a large, community-based, ethnically diverse, prospective cohort of 45- to 84-year-old participants free of cardiovascular disease at baseline, the amount of pericardial fat was lower in women than in men. After adjustment for established risk factors of heart failure, excess pericardial fat doubled the risk of heart failure in women and conferred about a 50% greater risk in men. This association remained statistically significant even after accounting for anthropometric indicators of obesity, abdominal fat depots, or inflammatory biomarkers and a plasma natriuretic peptide. It was not modified by race and/or ethnicity. High pericardial fat predominantly augmented the risk of heart failure with preserved rather than reduced left ventricular ejection fraction.
Obesity, defined using anthropometric indicators such as body mass index (BMI) and waist circumference, is an established risk factor for heart failure (HF) (1-5). Epicardial fat, located between the myocardium and the visceral pericardium, has potential cardioprotective effects and cardiotoxic implications mediated by proinflammatory and profibrotic cytokines through paracrine and/or vasocrine pathways (6-8) and is associated with coronary atherosclerosis (9). Paracardial mediastinal fat, located external to the parietal pericardium and contiguous with perivascular aortic adipose tissue, is an established location of brown adipose tissue (10) and expresses markers of metabolic activity, but it has a less certain physiopathological significance from a cardiac standpoint (6-8). Nonetheless, in epidemiological studies, the composite of epicardial and paracardial fat, termed as pericardial fat, has been correlated with coronary atherosclerotic plaques (11) and associated with incident myocardial infarction (12). However, the evidence linking pericardial fat depot to the occurrence of HF is limited (13). Therefore, we examined the influence of pericardial fat volume (PFV), noninvasively determined using computed tomography (CT), on the risk of HF in a large, community-based, ethnically diverse, prospective cohort of women and men.
METHODS
STUDY SAMPLE.
The MESA (Multi-Ethnic Study of Atherosclerosis) is a prospective study of 6,814 participants (53% women and 47% men) from 4 different ethnic groups (38% White, 28% African American, 22% Hispanic, and 12% Chinese American), ages 45 to 84 years, without clinical cardiovascular disease (no history of angina, nitroglycerin intake, myocardial infarction, stroke, transient ischemic attack, HF, current atrial fibrillation, or cardiovascular procedures such as angioplasty, coronary artery bypass graft surgery, valve replacement, pacemaker or defibrillator implantation, or any other surgery of the heart or blood vessels) at baseline who were recruited between July 17, 2000, and August 31, 2002, from 6 communities in the United States (Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota) (14). After excluding 3 participants who did not undergo cardiac CT at baseline and 26 participants with suboptimal image quality for PFV measurement, 6,785 participants (3,584 women and 3,201 men) were eligible. An additional 29 participants (0.4% of eligible participants) were excluded due to missing information on newly diagnosed HF during follow-up.
STUDY OVERSIGHT.
The MESA steering committee approved the research proposal, and the Mount Sinai Institutional Review Board designated the study as Not Human Subjects Research because deidentified datasets were used for analyses. All participants provided written informed consent for the overall study. The first author (S.K.) designed the study, had unrestricted access to the data, performed statistical analysis, wrote the first and subsequent drafts of the manuscript, obtained critical inputs from all co-authors, and vouches for the completeness and accuracy of the data and analyses.
EXPOSURE.
We performed noncontrast cardiac CT scans using: 1) electron-beam CT (Imatron C-150 [General Electric Healthcare, Milwaukee, Wisconsin] at the Chicago, Los Angeles, and New York field centers) with voltage of 130 kVp, current of 630 mA, exposure time of 100 ms, slice thickness of 3.0 mm, and prospective electrocardiogram triggered at 80% of the RR interval; or 2) multidetector CT (LightSpeed QXi or Plus [General Electric Healthcare, Waukesha, Wisconsin] or Volume Zoom [Siemens Healthineers, Erlangen, Germany] at the Baltimore, Forsyth County, and St. Paul field centers) with voltage of 120 (LightSpeed systems) or 140 (Volume Zoom system) kVp, current of 139 to 400 mA, exposure time of 330 to 520 ms, 4 concurrent 2.5-mm slices per cardiac cycle, and prospective electrocardiogram triggered at 50% of the RR interval with the exception of the LightSpeed QXi that utilized retrospective gating (12,15,16). Three experienced CT analysts measured PFV around the proximal coronary arteries (left main, left anterior descending, left circumflex, and right coronary arteries) on tomographic slices within 15 mm above and 30 mm below the superior extent of the left main coronary artery with anterior border of the volume defined by the chest wall and posterior border by the aorta and the bronchus (16). We used volume analysis software in Advantage Workstation for Diagnostic Imaging (General Electric, Waukesha, Wisconsin) to manually draw the outer contour of pericardial fat and specified a density range of −190 to −30 Hounsfield units to isolate adipose tissue (Supplemental Figure 1A) (11). We calculated PFV as the sum of all voxels containing fat. Our measurement of PFV around the proximal coronary arteries was highly reproducible; intraclass correlation coefficient was 0.99 for intrareader reliability and 0.89 for inter-reader reliability in a random sample of 80 MESA participants (16).
COVARIATES.
We used standard questionnaires to collect information on demographic variables, medical history, and medication use; performed physical examination; and obtained blood samples to measure biomarkers (Table 1). Measurement and definition of baseline covariates, abdominal subcutaneous and visceral fat volumes in a 30% random subsample of MESA participants (Supplemental Figure 1B), and interim myocardial infarction are shown in Supplemental Table 1.
TABLE 1.
Baseline Characteristics According to Categories of PFV in Women and Men
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Normal PFV (n = 2,130) |
High PFV (n = 1,462) |
P value | Normal PFV (n = 2,483) |
High PFV (n = 710) |
p Value | |
| Age, yrs | 60.2 ± 10.2 | 64.9 ± 9.7 | <0.001 | 61.6 ± 10.3 | 64.5 ± 9.5 | <0.001 |
| Race | <0.001 | <0.001 | ||||
| White | 796 (37) | 664 (39) | 885 (36) | 370 (52) | ||
| Black | 720 (34) | 329 (23) | 737 (30) | 98 (14) | ||
| Hispanic | 379 (18) | 394 (27) | 519 (21) | 197 (28) | ||
| Chinese | 235 (11) | 175 (12) | 342 (14) | 45 (6) | ||
| Body mass index, kg/m2† | 26.7 ± 5.3 | 31.7 ± 6.3 | <0.001 | 26.8 ± 3.9 | 31.4 ± 4.3 | <0.001 |
| Lean | 913 (43) | 172 (12) | 832 (34) | 27 (4) | ||
| Overweight | 737 (35) | 478 (33) | 1,157 (47) | 283 (40) | ||
| Obese | 480 (23) | 812 (56) | 494 (20) | 400 (56) | ||
| Waist circumference , cm | 91.1 ± 13.8 | 105.9 ± 14.9 | <0.001 | 96.3 ± 10.7 | 109.7 ± 11.5 | <0.001 |
| High‡ | 1,167 (55) | 1,300 (89) | 679 (27) | 528 (74) | ||
| Hip circumference, cm | 104.1 ± 11.1 | 112.5 ± 13.6 | <0.001 | 101.8 ± 8.4 | 109.4 ± 9.4 | <0.001 |
| ≥Median (≥104 cm) | 985 (46) | 1,044 (71) | <0.001 | 912 (37) | 484 (68) | <0.001 |
| Waist-to-hip ratio | 0.87 ± 0.08 | 0.94 ± 0.07 | <0.001 | 0.95 ± 0.06 | 1.00 ± 0.06 | <0.001 |
| ≥Median (≥0.94) | 519 (24) | 802 (55) | <0.001 | 1425 (57) | 645 (91) | <0.001 |
| Abdominal computed tomography§ | ||||||
| Subcutaneous fat volume, cm3 | 956 ± 389 (513) | 1,138 ± 421 (290) | <0.001 | 723 ± 302 (666) | 898 ± 341 (147) | <0.001 |
| Visceral fat volume, cm3 | 341 ± 146 (567) | 194 ± 176 (391) | <0.001 | 484 ± 204 (750) | 749 ± 237 (209) | <0.001 |
| Vigorous physical activity|| | 526 (25) | 220 (15) | <0.001 | 1,120 (45) | 268 (38) | <0.001 |
| Cigarette smoking | 0.51 | <0.001 | ||||
| No | 1,267 (60) | 847 (58) | 1,053 (43) | 234 (33) | ||
| Past | 605 (28) | 441 (30) | 1,044 (42) | 386 (54) | ||
| Current | 252 (12) | 168 (12) | 377 (15) | 89 (13) | ||
| Alcohol consumption# | <0.001 | 0.04 | ||||
| No or past | 990 (47) | 834 (58) | 935 (38) | 240 (34) | ||
| Mild to moderate | 1,028 (49) | 559 (39) | 1,390 (56) | 414 (58) | ||
| Heavy | 98 (5) | 53 (4) | 139 (6) | 54 (8) | ||
| Blood pressure, mm Hg | ||||||
| Systolic | 123.7 ± 23.0 | 132.0 ± 22.6 | <0.001 | 124.8 ± 19.3 | 130.3 ± 18.7 | <0.001 |
| Diastolic | 68.9 ± 10.3 | 69.4 ± 9.9 | <0.001 | 74.8 ± 9.4 | 76.1 ± 9.3 | <0.001 |
| Hypertension** | 831 (39) | 843 (58) | <0.001 | 975 (39) | 392 (55) | <0.001 |
| Diabetes mellitus†† | 159 (7) | 250 (17) | <0.001 | 304 (12) | 143 (20) | <0.001 |
| Dyslipidemia‡‡ | 704 (33) | 753 (52) | <0.001 | 1,299 (52) | 486 (68) | <0.001 |
| Biomarkers | ||||||
| C-reactive protein, mg/L | 3.8 ± 5.6 | 5.7 ± 6.5 | <0.001 | 2.7 ± 5.6 | 3.7 ± 5.0 | <0.001 |
| Interleukin-6, pg/mL | 1.4 ± 1.1 | 2.0 ± 1.3 | <0.001 | 1.4 ± 1.1 | 1.9 ± 1.4 | <0.001 |
| Log-NT-proBNP, pg/mL | 4.1 ± 1.0 | 4.2 ± 1.0 | 0.004 | 3.6 ± 1.2 | 3.7 ± 1.2 | 0.001 |
Values are mean ± SD, n (%), or mean ± SD (n). By design, none of the participants had clinically apparent cardiovascular disease at baseline (see text for details).
High PFV value was defined as ≥70 cm3 in women and ≥120 cm3 in men.
The body mass index was calculated as the weight in kilograms (kg) divided by the square of the height in meters (m2). The body mass index was <25 kg/m2 in lean participants, 25 to 29.9 kg/m2 in overweight participants, and ≥30 kg/m2 in obese participants.
High waist circumference was defined as >88 cm in women and >102 cm in men.
Data on abdominal subcutaneous and visceral fat volume computed from a total of 6 axial slices, 2 adjacent slices each at the level of L2-L3, L3-L4, and L4-L5 intervertebral disc spaces, was available on 1,616 (24%) and 1,917 (28%) of 6,785 participants, respectively, on abdominal computed tomography scans performed at 2.7 ± 0.9 years from the baseline examination. Number of participants with available data in each subgroup is shown in parentheses.
Vigorous physical activity was considered as present if a participant reported heavy intensity exercise such as high impact aerobics, fast bicycling, running, jogging, fast swimming, health club machine use, judo, kickboxing, and karate every week.
Mild-to-moderate alcohol consumption was defined as up to 1 drink per day in women and up to 2 drinks per day in men; heavy consumption was defined as >1 drink per day in women and >2 drinks per day in men.
Hypertension was defined as history of treated hypertension or untreated systolic blood pressure of ≥140 mm Hg or untreated diastolic blood pressure of ≥90 mm Hg.
Diabetes mellitus was defined as treatment with insulin or oral hypoglycemic agents or fasting glucose of ≥126 mg/dl.
Dyslipidemia was defined as current lipid-lowering therapy or abnormal fasting lipid profile (total cholesterol of ≥240 mg/dl, low-density lipoprotein cholesterol of ≥160 mg/dl, high-density lipoprotein cholesterol of <40 mg/dl, or triglycerides of ≥200 mg/dl).
Log-NT-proBNP = logarithm-transformed N-terminal pro–B-type natriuretic peptide; PFV = pericardial fat volume.
OUTCOME.
The MESA participants are contacted annually for the development of cardiovascular outcomes and death. At least 2 physicians who were blinded to the exposure status independently reviewed the medical records to ascertain the diagnosis of HF. Disagreements in the adjudication of HF between 2 reviewers were resolved by a third physician reviewer or, if required, by the Endpoint Committee as a whole.
We classified HF as probable, definite, or absent. Probable HF required HF symptoms such as shortness of breath or signs such as edema and HF diagnosed by a physician and patient receiving medical treatment for HF. Definite HF required all features of probable HF and one or more other criteria, such as pulmonary edema or congestion by chest radiography; dilated ventricle or poor left ventricular function by echocardiography or ventriculography; or evidence of left ventricular diastolic dysfunction. We considered participants not meeting any criteria, including just a physician diagnosis of HF without any other evidence, as having no HF.
We used information on left ventricular ejection fraction (LVEF) ascertained at or after the diagnosis of HF and categorized newly diagnosed HF as heart failure with preserved ejection fraction (HFpEF) (LVEF ≥50%), heart failure with mid-range ejection fraction (HFmrEF) (LVEF >40% but <50%), and heart failure with reduced ejection fraction (HFrEF) (LVEF ≤40%) (17,18). We considered newly diagnosed HF without information on LVEF as heart failure with unknown ejection fraction (HFuEF).
STATISTICAL ANALYSIS.
We summarized the distribution of PFV using mean ± SD, median, interquartile range, and range. In primary analyses, we defined HF as probable or definite HF. We conducted receiver-operating curve analyses and categorized PFV as normal or high by means of sex-specific optimal cutoff value determined using Youden statistic (19):
. We calculated means ± SD for continuous and proportions (expressed as percentage) for categorical variables. We compared the distribution of baseline variables between PFV categories using Student’s ttest for continuous and chi-square test for categorical variables. We used Kaplan-Meier estimation method to construct cumulative incidence curves for HF according to PFV categories in women and men and compared the cumulative incidence of HF between groups using the log-rank test. We examined the shape of the association between PFV and newly diagnosed HF using semiparametric generalized additive models to detect nonlinearity by fitting smoothing splines for PFV with 5 degrees of freedom (1 for linear portion of the fit and 4 for nonlinear spline portion) and specified binomial distribution, logit link function, generalized cross-validation smoothing parameter, and alpha of 0.05 as model options. Furthermore, we examined Cox proportional hazards regression models with both linear and transformed PFV terms (logarithm, square root, cube root, quadratic, or cubic) to the risk of HF. Thereafter, we evaluated PFV as continuous (per SD increase) and categorical variables (normal vs. high). To avoid underestimation of true hazards and to provide insight into the attenuating influence of intermediary variables, we considered covariates as likely or not likely in the causal pathway based on a priori hypothesis. We constructed sex-specific and sex-stratified models adjusted for: 1) age (per year increase); 2) age, race (White [referent], Black, Hispanic, Chinese), cigarette smoking (no [referent], past, current), alcohol consumption (no or past [referent], mild-to-moderate, heavy), and vigorous physical activity at baseline (potential confounders not likely in the causal pathway); and 3) all above-mentioned covariates and hypertension, diabetes mellitus, and dyslipidemia at baseline and interim myocardial infarction as a time-dependent covariate during follow-up (variables likely in the causal pathway). We assessed for the correlation between PFV and anthropometric indicators of obesity such as BMI, waist circumference, hip circumference, and waist-to-hip ratio in the overall sample and abdominal fat depots such as subcutaneous and visceral fat volumes in the 30% random subsample using Pearson rank correlation and examined hierarchical statistical models with each anthropometric indicator of obesity or volume of abdominal fat depot included as a covariate.
In secondary analyses, to determine the influence of biomarkers of inflammation (C-reactive protein and interleukin 6) and hemodynamic stress (N-terminal pro–B-type natriuretic peptide) on the association between PFV and incident HF, we included these intermediary variables in multivariable models. To assess for the effect modification of the association between PFV and newly diagnosed HF by baseline covariates, we introduced interaction terms in sexstratified and fully adjusted models and conducted subgroup analyses stratified by covariate levels of interest. To examine the association between PFV and HF subtypes, we constructed cumulative incidence curves for each HF subtype according to high and normal PFV categories and compared the difference between groups using log-rank test. We performed sex-stratified and age-adjusted, partially adjusted, and fully adjusted Cox analyses to examine the association between PFV and incidence of individual subtype of HF where follow-up on other categories of HF were censored at the time of their onset.
In supplementary analyses, to examine the competing influence of all-cause death on the occurrence of HF, we constructed cumulative incidence curves of all-cause death using Kaplan-Meier estimation methods according to normal and high PFV categories, estimated the cumulative incidence rates of HF in normal and high PFV groups using Kaplan-Meier–like method where all-cause death was coded as a competing event, and compared the 2 cumulative incidence functions using the nonparametric Gray test for equality (20) and the 2 subdistribution hazard functions using the parametric multivariable Fine-and-Gray models (21). We adopted a similar approach to account for the competing risk of all-cause death on HF subtypes. For example, in models evaluating PFV and the risk of newly diagnosed HFpEF, we considered other HF subtypes and all-cause death as competing events. To determine the public health impact of high PFV, we calculated the population attributable risk (PAR) for HF with the use of proportion of cases exposed to high PFV (pd) and relative risk (RR) from fully adjusted models as:
(22) Lastly, we examined the association between PFV and definite HF only using the same statistical approach we have detailed but with participants censored at the time of onset of probable but not definite HF.
We computed relative risks, 95% confidence intervals (CIs), and 2-tailed p values. We conducted all analyses using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
In the overall study sample of 6,785 individuals, mean PFV was 80 ± 42 cm3, median was 71 cm3, interquartile range was 49 to 100 cm3, and range was 7 to 405 cm3. Women had lesser amount of PFV than men did (69 ± 33 cm3 vs. 92 ± 47 cm3; p < 0.001) (Supplemental Figure 2). During 90,686 person-years of follow-up (mean: 13.4 ± 4.6 years; median: 15.7 years; interquartile range: 11.7 to 16.5 years; maximum: 17.5 years), 383 participants (5.7%, 164 women and 221 men) developed newly diagnosed HF. Optimal cutoff value (Youden index) to dichotomize PFV as normal or high was 69.8 cm3 (rounded to 70 cm3) in women and 120.6 cm3 (rounded to 120 cm3) for men (Supplemental Figure 3).
BASELINE CHARACTERISTICS.
In both sexes, participants with high PFV were older, had higher BMI, waist circumference, hip circumference, and waist-to-hip ratio, lesser amount of vigorous physical activity, higher prevalence of hypertension, diabetes mellitus and dyslipidemia, and increased levels of biomarkers of inflammation and hemodynamic stress (Table 1). A heterogeneity in the distribution of race/ethnicity, cigarette smoking, and alcohol consumption was also evident between groups.
PERICARDIAL FAT AND THE RISK OF HEART FAILURE.
The cumulative incidence of HF was higher in participants with high compared with normal PFV in both women and men (log-rank p < 0.001) (Central Illustration). In generalized additive models stratified by sex and adjusted for all baseline covariates in fully adjusted models, PFV was linearly associated with newly diagnosed HF (highly significant p < 0.001 for parametric linear trend and insignificant p = 0.22 for nonparametric spline component of PFV). In sex-stratified and fully adjusted Cox models, linear PFV term was highly significant (p < 0.001) but additional logarithm, square-root, cube-root, quadratic, or cubic-transformed PFV terms were statistically insignificant (p $ 0.11 for all). Overall, these results were consistent with a positive linear association rather than a nonlinear J-shaped or U-shaped association between PFV and newly diagnosed HF.
Central Illustration: Cumulative Incidence of Heart Failure by Categories of Pericardial Fat Volume at Baseline.
In MESA (Multi-Ethnic Study of Atherosclerosis), newly diagnosed heart failure (HF) occurred more frequently among participants with high compared with normal pericardial fat volume (PFV) in both women (A) and men (B). Vertical bars represent standard errors. Data shown are truncated at 16 years of follow-up.
In multivariable Cox models adjusting for age and potential confounders (partially adjusted models), every 1-SD increment (42 cm3) in PFV increased the risk of HF by 68% (95% CI: 42% to 98%; p < 0.001) in women, 24% (95% CI: 12% to 37%; p < 0.001) in men, and 34% (95% CI: 23% to 46%; p < 0.001) in the overall sample (Table 2, models 1A, 1B, and 1C, respectively). Additional adjustment for variables in the causal pathway (fully adjusted models) decreased the hazard ratio from 1.68 to 1.44 in women and 1.24 to 1.13 in men, thus explaining 35% and 46% of the elevated risk in women and men, respectively. The association between PFV and newly diagnosed HF was stronger in women than in men (interaction p = 0.01 in fully adjusted models). Similarly, in fully adjusted models evaluating PFV as a categorical variable, compared with normal PFV, high PFV was associated with a 2-fold greater risk (95% CI: 48% to 187%; p < 0.001) of HF in women, 53% (95% CI: 13% to 107%; p = 0.006) in men, and 77% (95% CI: 42% to 120%; p < 0.001) in the overall sample (Table 2, models 2A, 2B, and 2C, respectively).
TABLE 2.
Cox Proportional Hazards Regression Analyses Evaluating the Association between PFV and the Risk of HF
| No. of Events/ No. at Risk (%) |
Follow-up,
Py (Rate/10,000 Py) |
HR (95% CI), p Value | |||
|---|---|---|---|---|---|
| Age-Adjusted | Partially-Adjusted* | Fully-Adjusted† | |||
| Model 1. PFV as a continuous variable (per 1-SD increment)‡ | |||||
| A. Women | 164/3575 (4.6) | 48,907 (33.5) | 1.68 (1.43-1.98), <0.001 | 1.68 (1.42-1.98), <0.001 | 1.44 (1.21-1.71), <0.001 |
| B. Men | 219/3181 (6.9) | 41,778 (52.4) | 1.25 (1.13-1.38), 0.0001 | 1.24 (1.12-1.37), <0.001 | 1.13 (1.01-1.27), 0.03 |
| C. All (sex-stratified) | 383/6756 (5.7) | 90,686 (42.2) | 1.34 (1.24-1.46), <0.001 | 1.34 (1.23-1.46), <0.001 | 1.22 (1.12-1.34), <0.001 |
| Model 2. PFV as a categorical variable | |||||
| A. Women | |||||
| Normal PFV | 59/2120 (2.8) | 30,195 (19.5) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 105/1455 (7.2) | 18,713 (56.1) | 2.15 (1.56-2.97), <0.001 | 2.15 (1.55-2.99), <0.001 | 2.06 (1.48-2.87), <0.001 |
| B. Men | |||||
| Normal PFV | 137/2478 (5.5) | 32,981 (41.5) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 82/703 (12.0) | 8,797 (93.2) | 1.89 (1.44-2.49), <0.001 | 1.91 (1.43-2.54), <0.001 | 1.53 (1.13-2.07), 0.006 |
| C. All (sex-stratified) | |||||
| Normal PFV | 196/4598 (4.3) | 63,176 (31.0) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 187/2158 (8.7) | 27,510 (68.0) | 2.00 (1.63-2.46), <0.001 | 2.04 (1.65-2.52), <0.001 | 1.77 (1.42-2.20), <0.001 |
Adjusted for age (for every 1-year increase), race (White [referent], Black, Hispanic, Chinese), cigarette smoking (no [referent], past, current), alcohol consumption (no or past [referent], mild-to-moderate, heavy), and vigorous physical activity at baseline. Excluded 51 participants (31 women and 20 men) comprising 0.8% of 6,756 participants (1 HF event) because of missing information on covariates in the model.
Adjusted for all the above-mentioned covariates and presence or absence of hypertension, diabetes mellitus, and dyslipidemia at the baseline examination and interim myocardial infarction as a time-dependent variable during follow-up. Excluded 78 participants (46 women and 32 men) comprising 1.2% of 6,756 participants (1 HF event) because of missing information on covariates in the model.
1 SD = 42 cm3.
High PFV value was ≥70 cm3 in women and ≥120 cm3 in men.
CI = confidence interval; HF = heart failure; HR = hazard ratio; Py = person-years; PFV = pericardial fat volume.
INFLUENCE OF ANTHROPOMETRIC INDICATORS OF OBESITY.
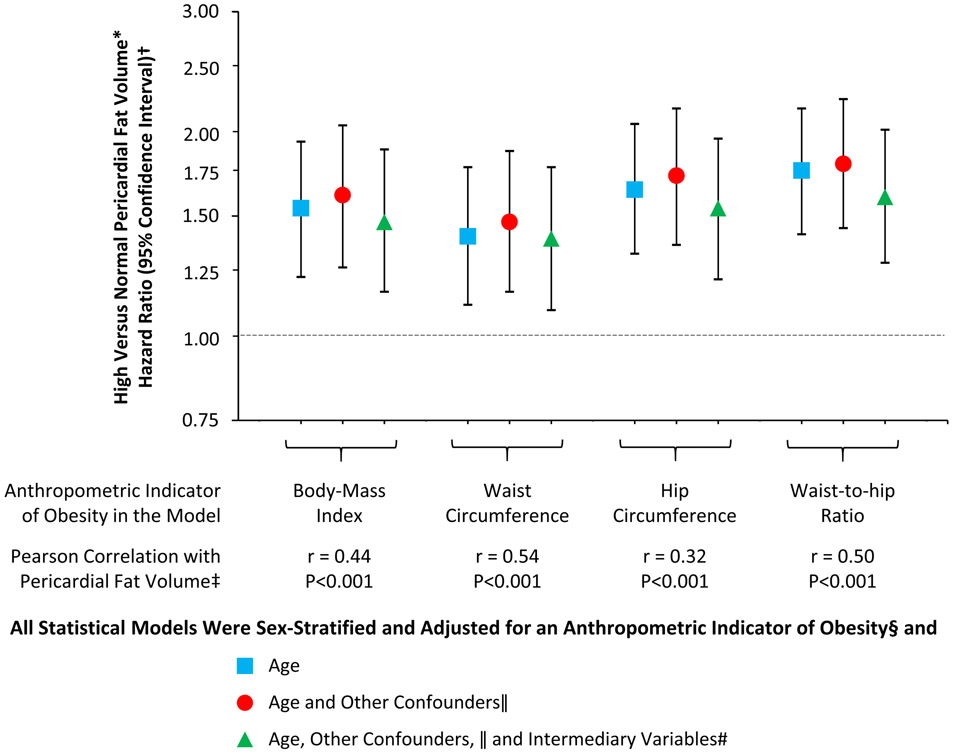
A weak-to-moderate linear correlation was noted between PFV and BMI (r = 0.44; p < 0.001), waist circumference (r = 0.54; p < 0.001), hip circumference (r = 0.32; p < 0.001), and waist-to-hip ratio (r ¼ 0.50; p < 0.001). High PFV was noted in 10%, 29%, and 55% of lean, overweight, and obese participants, respectively; 11% and 50% of participants with normal and high waist circumference, respectively; 19% and 45% of participants with hip circumference <median and ≥median value (104 cm), respectively; and 21% and 43% of participants with waist-to-hip ratio <median and ≥median value (0.94), respectively. In sex-stratified and age-adjusted, partially adjusted, or fully adjusted models where each anthropometric indicator of obesity was individually introduced as a covariate, the association between PFV and newly diagnosed HF remained robust; 95% CIs did not cross the line of unity (Figure 1). Specifically, in sex-stratified and fully adjusted models, compared with normal PFV, high PFV was associated with hazard ratios of 1.47 (95% CI: 1.16 to 1.88; p = 0.002) with BMI in the model, 1.39 (95% CI: 1.09 to 1.77; p = 0.008) with waist circumference, 1.54 (95% CI: 1.21 to 1.95; p < 0.001) with hip circumference, and 1.60 (95% CI: 1.28 to 2.01; p < 0.001) with waist-to-hip ratio.
FIGURE 1. PFV and the Risk of HF Adjusted for Anthropometric Indicators of Obesity.
Elevated pericardial fat volume (PFV) was associated with a greater risk of heart failure (HF) after adjustment for anthropometric indicators of obesity in multivariable models. *High PFV was defined as ≥70 cm3 in women and ≥120 cm3 in men. †Hazard ratios are shown on a logarithmic scale. Vertical bars denote 95% confidence intervals. ‡r denotes correlation coefficient. §Introduced as a continuous variable in statistical models. ||Race, cigarette smoking, alcohol consumption, and vigorous physical activity at baseline. #Hypertension, diabetes mellitus, and dyslipidemia at baseline and interim myocardial infarction during follow-up.
INFLUENCE OF ABDOMINAL SUBCUTANEOUS AND VISCERAL FAT.
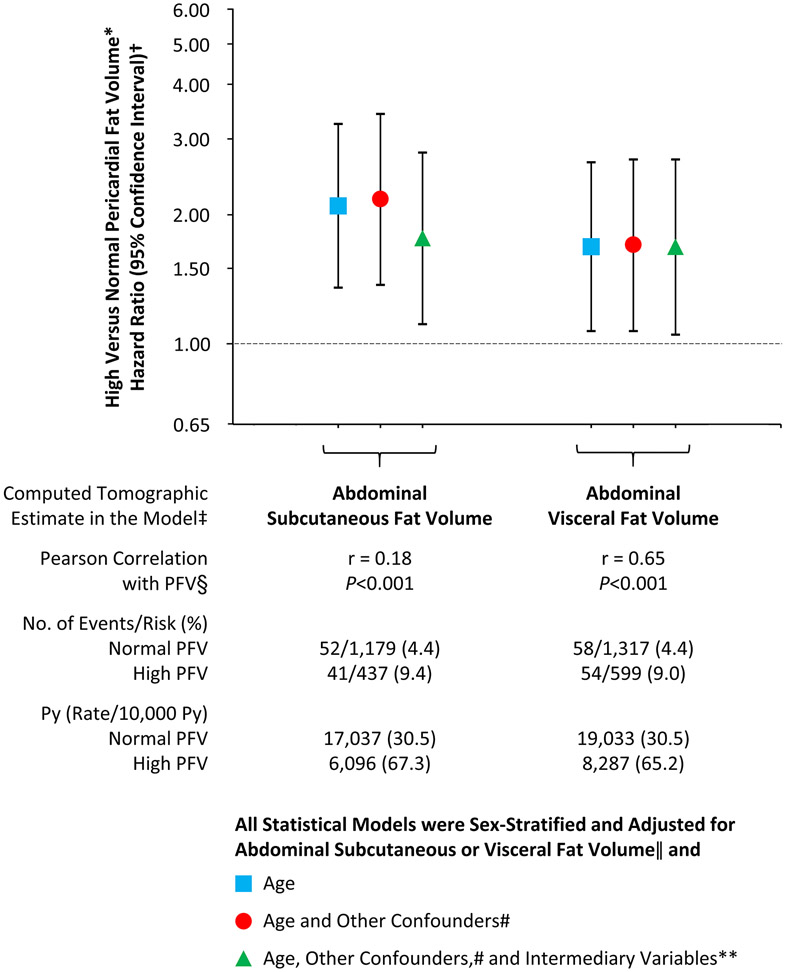
On abdominal CT scans performed at a mean of 2.7 ± 0.9 years and a median of 3.1 years (interquartile range: 1.6 to 3.4 years) from the baseline examination, data on abdominal subcutaneous and visceral fat volumes were available for 1,616 (24%) and 1,917 (28%) of 6,785 participants, respectively. High PFV was evident in 19% and 36% of participants with abdominal subcutaneous fat volume <median and ≥median value (816 cm3), respectively; and 14% and 49% of participants with abdominal visceral fat volume <median and ≥median value (455 cm3), respectively. PFV was poorly correlated with abdominal subcutaneous fat volume (r = 0.18; p < 0.001) but moderately correlated with abdominal visceral fat volume (r = 0.65; p < 0.001). Newly diagnosed HF occurred in 93 of 1,616 participants (5.8%) with data on abdominal subcutaneous fat and 112 of 1,916 participants (5.8%) with data on abdominal visceral fat (information on HF was missing in 1 participant). In sex-stratified and age-adjusted, partially adjusted, and fully adjusted multivariable models where estimates of abdominal subcutaneous or visceral fat volumes were individually introduced as covariates, the association between PFV and newly diagnosed HF remained statistically significant (Figure 2). Particularly, in sex-stratified and fully adjusted models, compared with normal PFV, high PFV was associated with hazard ratios of 1.76 (95% CI: 1.11 to 2.78; p = 0.016) with abdominal subcutaneous fat volume as an additional covariate and 1.68 (95% CI: 1.05 to 2.68; p = 0.030) with abdominal visceral fat volume.
Figure 2. PFV and the Risk of HF Adjusted for Abdominal Fat Depots.
Elevated PFV was associated with a greater risk of HF after adjustment for abdominal fat depots in multivariable models. *High PFV was defined as ≥70 cm3 in women and ≥120 cm3 in men. †Hazard ratios are shown on a logarithmic scale. Vertical bars denote 95% confidence intervals. ‡Abdominal subcutaneous and visceral fat volumes were computed from a total of 6 axial slices, 2 adjacent slices each at the level of L2-L3, L3-L4, and L4-L5 intervertebral disc spaces, in 1,616 (24%) and 1,917 (28%) of 6,785 participants, respectively, on abdominal computed tomographic scans performed at 2.7 ± 0.9 years from the baseline examination. §r denotes correlation coefficient. ||Introduced as a continuous variable in statistical models. #Race, cigarette smoking, alcohol consumption, and vigorous physical activity at baseline. **Hypertension, diabetes mellitus, and dyslipidemia at baseline and interim myocardial infarction during follow-up. Py = person-years of follow-up; other abbreviations as in Figure 1.
INFLUENCE OF BIOMARKERS OF INFLAMMATION AND HEMODYNAMIC STRESS AS INTERMEDIARY VARIABLES.
Information on C-reactive protein, interleukin 6, and N-terminal pro–B-type natriuretic peptide was available in 6,733 (99.2%), 6,593 (97.2%), and 6,762 (99.7%) of 6,785 participants, respectively, at baseline. Additional adjustment for these 3 biomarkers did not substantially change the association between PFV and newly diagnosed HF; specifically, in sex-stratified and full-adjusted models, the hazard ratios were 1.21 (95% CI: 1.10 to 1.33; p < 0.001) for every 1-SD increase in PFV and 1.76 (95% CI: 1.40 to 2.22; p < 0.001) for high compared with normal PFV.
INTERACTION AND SUBGROUP ANALYSES.
The association between PFV and newly diagnosed HF was not modified by age, race, anthropometric indicators of obesity, vigorous physical activity, cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, or dyslipidemia at baseline (interaction p ≥ 0.10 for all) (Figure 3).
Figure 3: PFV and the Risk of HF According to Subgroups.
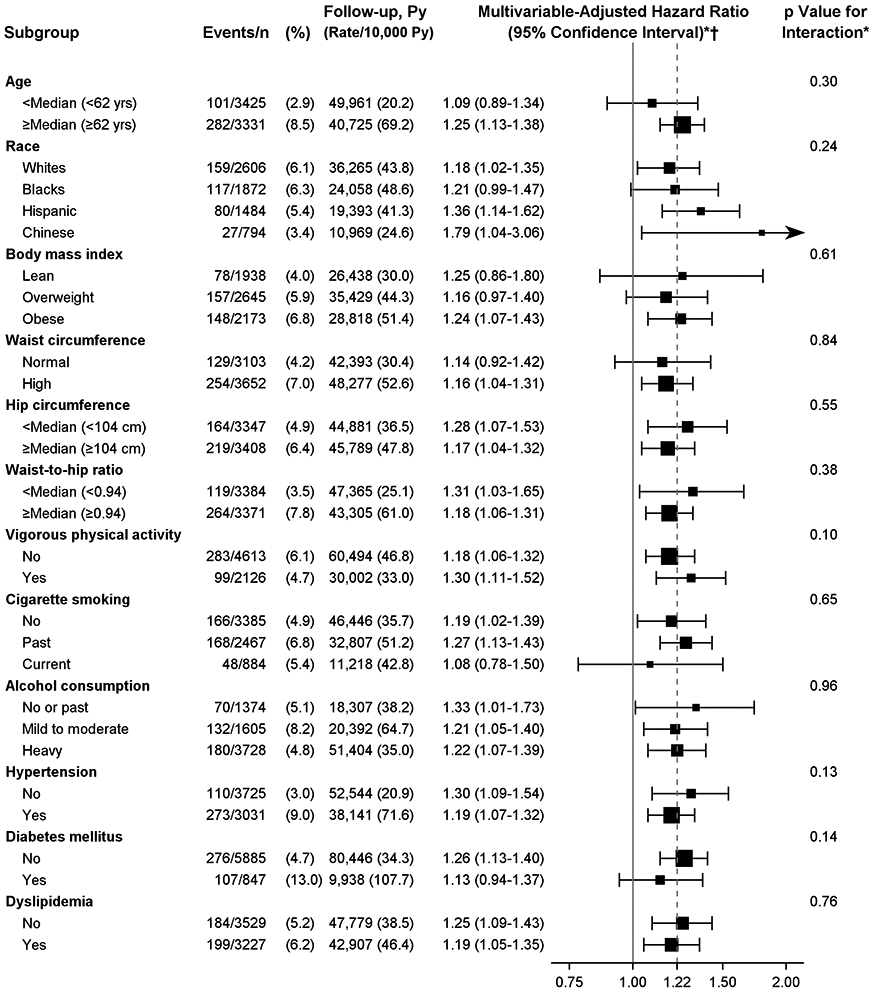
Effect of PFV on the risk of HF was similar in various subgroups. *All analyses were sex-stratified and adjusted for age, race, cigarette smoking, alcohol consumption, vigorous physical activity, hypertension, diabetes mellitus, and dyslipidemia at baseline and interim myocardial infarction during follow-up. †Risk estimates were for every 1-SD increase (42 cm3) in PFV. Size of squares are proportional to the number of events in the specified subgroup. Hazard ratios are shown on a logarithmic scale. Horizontal error bars indicate 95% confidence intervals. n = number at risk; other abbreviations as in Figures 1 and 2.
PERICARDIAL FAT AND THE RISK OF HF SUBTYPES.
Information on LVEF was available in 356 of 385 participants (92.5%) who developed newly diagnosed HF. In the overall sample comprising 6,756 individuals, 167 (2.5%) developed HFpEF, 38 (0.6%) HFmrEF, 151 (2.2%) HFrEF, and 29 (0.4%) HFuEF. High PFV compared with normal PFV was associated with a higher cumulative incidence of HFpEF (logrank p < 0.001), HFmrEF (log-rank p = 0.002), and HFuEF (log-rank p = 0.001) but not HFrEF (log-rank p = 0.10) (Figure 4). In sex-stratified and fully adjusted analyses, every 1-SD increase (42 cm3) in PFV was associated with a 42% greater risk of HFpEF (p < 0.001) and high PFV compared with normal PFV conferred a 2.3-fold greater risk of HFpEF (p < 0.001) (Table 3, models 1A and 1B). However, the association between elevated PFV and newly diagnosed HFrEF did not reach statistical significance in partially adjusted models (p = 0.06) and was further attenuated on additional adjustment for variables in the causal pathway (p ≥ 0.31) (Table 3,models 2Aand2B). Of note, despite small numbers of HFmrEF and HFuEF, high PFV compared with normal PFV was modestly or borderline associated with over a 2-fold greater risk of HFmrEF and HFuEF (p ≤ 0.02 in partially adjusted models and p ≤ 0.05 in fully adjustedmodels) (Table 3, models 3 and 4).
Figure 4: Cumulative Incidence of HF Subtypes by Categories of PFV at Baseline.
High versus normal PFV and the cumulative incidence of (A) heart failure with preserved ejection fraction (HFpEF); (B) heart failure with reduced ejection fraction (HFrEF); (C) heart failure with mid-range ejection fraction (HFmrEF); (D) heart failure with unknown ejection fraction (HFuEF). High PFV was defined as ≥70 cm3 in women and ≥120 cm3 in men. Vertical bars represent standard errors. (C, D) The insets show the same data on an enlarged y-axis. Data shown are truncated at 16 years of follow-up. LVEF = left ventricular ejection fraction; other abbreviations as in Figure 1.
TABLE 3.
PFV and the Risk of HF Subtypes in Sex-Stratified Cox Proportional Hazards Regression Models
| No. of Events/ No. at Risk (%) |
Follow-up,
Py (Rate/10,000 Py) |
HR (95% CI), p Value | |||
|---|---|---|---|---|---|
| Age-Adjusted | Partially-Adjusted* | Fully-Adjusted† | |||
| Model 1. HFpEF (LVEF ≥50%) | |||||
| A. PFV as a continuous variable (per 1-SD increment)‡ | 167/6,756 (2.5%) | 90,686 (18.4) | 1.54 (1.37-1.73), <0.001 | 1.52 (1.35-1.72), <0.001 | 1.42 (1.25-1.62), <0.001 |
| B. PFV as a categorical variable | |||||
| Normal PFV | 71/4,598 (1.5%) | 63,176 (11.2) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 96/2,158 (4.4%) | 27,510 (34.9) | 2.57 (1.87-3.53), <0.001 | 2.55 (1.85-3.53), <0.001 | 2.32 (1.66-3.23), <0.001 |
| Model 2. HFrEF (LVEF ≤40%) | |||||
| A. PFV as a continuous variable (per 1-SD increment)‡ | 151/6,756 (2.2%) | 90,686 (16.7) | 1.13 (0.97-1.30), 0.11 | 1.15 (1.00-1.33), 0.06 | 1.04 (0.89-1.21), 0.65 |
| B. PFV as a categorical variable | |||||
| Normal PFV | 96/4,598 (2.1%) | 63,176 (15.2) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 55/2,158 (2.5%) | 27,510 (20.0) | 1.33 (0.95-1.87), 0.10 | 1.40 (0.99-1.99), 0.06 | 1.20 (0.84-1.73), 0.31 |
| Model 3. HFmrEF (LVEF >40% and <50%) | |||||
| A. PFV as a continuous variable (per 1-SD increment)‡ | 38/6,756 (0.6%) | 90,686 (4.2) | 1.41 (1.11-1.81), 0.006 | 1.44 (1.12-1.85), 0.004 | 1.25 (0.95-1.65), 0.11 |
| B. PFV as a categorical variable | |||||
| Normal PFV | 18/4,598 (0.4%) | 63,176 (2.8) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 20/2,158 (0.9%) | 27,510 (7.3) | 2.51 (1.30-4.84), 0.006 | 2.68 (1.37-5.27), 0.004 | 2.05 (1.02-4.11), 0.04 |
| Model 4. HFuEF | |||||
| A. PFV as a continuous variable (per 1-SD increment)‡ | 27/6,756 (0.4%) | 90,686 (3.0) | 1.35 (0.99-1.84), 0.06 | 1.23 (0.89-1.70), 0.21 | 1.09 (0.77-1.56), 0.62 |
| B. PFV as a categorical variable | |||||
| Normal PFV | 11/4,598 (0.2%) | 63,176 (1.7) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High PFV§ | 16/2,158 (0.7%) | 27,510 (5.8) | 2.91 (1.31-6.45), 0.009 | 2.66 (1.17-6.03), 0.02 | 2.36 (1.01-5.52), 0.05 |
Adjusted for age (for every 1-year increase), race (White [referent], Black, Hispanic, Chinese), cigarette smoking (no [referent], past, current), alcohol consumption (no or past [referent], mild-to-moderate, heavy), and vigorous physical activity at baseline. Excluded 51 participants (31 women and 20 men) comprising 0.8% of 6,756 participants (1 HF event) because of missing information on covariates in the model.
Adjusted for all the above-mentioned covariates and presence or absence of hypertension, diabetes mellitus, and dyslipidemia at the baseline examination and interim myocardial infarction as a time-dependent variable during follow-up. Excluded 78 participants (46 women and 32 men) comprising 1.2% of 6,756 participants (1 HF event) because of missing information on covariates in the model.
1 SD = 42 cm3.
High PFV value was ≥70 cm3 in women and ≥120 cm3 in men.
HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HFuEF = heart failure with unknown ejection fraction; LVEF = left ventricular ejection fraction; other abbreviations as in Tables 1 and 2.
COMPETING RISK ANALYSES.
During follow-up, death due to all causes occurred in 1,547 individuals (689 women and 858men). The cumulative incidence of all-cause death was higher in participants with high compared with normal PFV in both sexes (log-rank p < 0.001) (Supplemental Figure 4). The cumulative incidence of HF with all-cause death as a competing event was greater among high compared with normal PFV in both women and men (Gray p < 0.001) and subdistributional hazards of HF were greater in high versus normal PFV in fully adjusted Fine-and-Gray models in both sexes (p < 0.001). Elevated PFV was significantly associated with newly diagnosed HFpEF after accounting for the competing risk of other HF subtypes and all-cause death(p < 0.001 in Gray test and p < 0.001 in sex-stratified and fully adjusted Fine-and-Gray models). In similar models, elevated PFV was borderline associated with HFmrEF (Gray p = 0.005; Fine-and-Gray p = 0.05) but not with newly diagnosed HFrEF (Gray p = 0.20 and Fine-and-Gray p = 0.42) or HFuEF (Gray p = 0.002; Fine-and-Gray p = 0.10).
POPULATION ATTRIBUTABLE RISK.
Among MESA participants, HF risk attributable to high PFV was 21% (95% CI: 14% to 27%) in the overall sample. It was higher in women (33%; 95% CI: 21% to 42%) than in men (13%; 95% CI: 4% to 19%).
PERICARDIAL FAT AND THE RISK OF DEFINITE HF.
Of the 382 participants with probable or definite HF, 291 participants (76%; 126 women and 165 men) met the criteria for definite HF. All analyses using this more stringent criteria for HF did not materially alter the association between PFV and newly diagnosed HF. For instance, in sex-stratified and fully adjusted models, hazard ratios for definite HF were 1.20 (95% CI: 1.08 to 1.33; p < 0 .001) for every 1-SD increase (42 cm3) in PFV and 1.68 (95% CI: 1.30 to 2.15; p < 0.001) for high compared with normal PFV.
DISCUSSION
PRINCIPAL FINDINGS.
In our large, community-based, ethnically diverse, prospective cohort of 45- to 84-year-old participants free of cardiovascular disease at baseline, higher PFV was associated with a greater risk of HF in both women and men. Increasing amount of PFV was associated with a linear increase in the risk of HF without evidence of a threshold. Although the amount of PFV was lower in women than in men, the relative risk of newly diagnosed HF associated with elevated PFV was higher in women than in men. Hypertension, diabetes mellitus, dyslipidemia, and interim myocardial infarction explained about one-third of the association between PFV and newly diagnosed HF in women and almost one-half in men. High, compared with normal, PFV doubled the risk of HF in women and conferred about a 50% greater risk in men. Although PFV was weakly-to-moderately correlated with anthropometric indicators of obesity and abdominal visceral fat volume, the association between PFV and newly diagnosed HF remained robust when accounting for these variables in multivariable statistical models. The effect of PFV on the occurrence of HF was not attenuated by biomarkers of inflammation and hemodynamic stress nor modified by race and/or ethnicity. The proportion of all cases of HF that is attributable to high PFV among MESA participants was 32% in women, 13% in men, and 21% in the overall sample.
Elevated PFV was strongly associated with newly diagnosed HFpEF and modestly associated with HFmrEF. The absence of a statistically significant association between PFV and newly diagnosed HFrEF in fully adjusted models should be interpreted in the context of a reduced number of events in analyses on HF subtypes and the attenuation of hazards due to the adjustment for variables in the causal pathway. Given the small number of newly diagnosed HFmrEF and HFuEF, multivariable analyses for the occurrence of these HF subtypes should be considered as exploratory or hypothesis-generating approaches.
COMPARISON WITH PREVIOUS STUDIES.
Sex-based differences in the amount of pericardial fat are unclear. One autopsy study of normal hearts has reported that women have lower amount of epicardial fat tissue than men (23); however, other studies have reported greater amount in women (24,25). Women were noted to have thicker epicardial fat tissue on histological specimens from normal hearts (26), but an echocardiographic study showed no significant difference in epicardial fat thickness in the 2 sexes (27). A prior study showed that pericardial fat, but not hepatic fat, was associated with cardiovascular events including HF (13). However, this study did not quantify the risk estimates by considering covariates in or not in the causal pathway, assess for sex-based differences in the association between PFV and newly diagnosed HF, or examine the implications of PFV on HF subtypes.
MECHANISMS.
Excess epicardial fat, perhaps owing to its proximity to the myocardium with no fascia separating the 2 tissues and a common blood supply (8), may contribute to myocardial steatosis (intracellular accumulation of fat; fatty degeneration of the heart) (28) or extend between myocardial bundles and muscle fibers (extracellular infiltration of fat; adiposity of the heart) (29), resulting in myocardial dysfunction. The association of epicardial fat with essential hypertension (30), ventricular hypertrophy (25), diastolic dysfunction (31), and altered hemodynamics (32) may precipitate symptoms and signs of HF (33) and contribute to a distinct obese-HFpEF phenotype (34). Promotion of oxidative stress (35) and proinflammatory mediators (36) may predispose to coronary atherosclerosis and cardiomyopathy by paracrine and/or vasocrine pathways (6-8). In addition, the systemic effects of epicardial fat include increased susceptibility to insulin resistance (37), diabetes mellitus type 2 (38), and metabolic syndrome (39). Modified secretory profile of epicardial fat in diabetics may promote cardiomyocyte dysfunction (40). Although the distinct role of paracardial mediastinal fat to the development of HF is not well elucidated, pericardial fat (comprising both epicardial and paracardial fat depots) is correlated with coronary atherosclerotic plaques (11) and obstructive coronary artery disease (41) and is prospectively associated with myocardial infarction (12), a potent risk factor for HF (42).
STUDY STRENGTHS.
Our study sample was large, community-based, and included similar numbers of women and men from 4 different racial and/or ethnic groups; thus increasing the generalizability of the results of our investigation. Prospective study design; volumetric measurement of pericardial fat rather than ascertainment of its unidimensional thickness; good reproducibility of exposure measurement; outcome adjudication by physician reviewers and an endpoint committee; application of uniform criteria for the diagnosis of HF during the course of the study; very little missing information on covariates of interest; long duration of follow-up; and multivariable adjustment for potential confounders with close attention to correlated variables, intermediary factors, and the competing risk of all-cause death are additional strengths of our investigation.
STUDY LIMITATIONS.
First, our measurement of PFV was limited to the region of the heart encompassing the proximal coronary arteries rather than the entire extent of the heart. This is unlikely to adversely impact the overall findings of our study because our measurement of PFV around proximal coronaries was well-correlated with the total amount of PFV around the heart (correlation coefficient = 0.93; p < 0.001, in a random sample of 10 participants of the Diabetes Heart Study) (16). Second, we could not separately quantify epicardial and paracardial fat repositories around the heart because of challenges with adequately delineating the pericardium separating these 2 fat depots, particularly in individuals with lean or normal BMI. However, because epicardial and paracardial fat volumes were highly correlated (Spearman correlation coefficient = 0.92; p < 0.001, in a random sample of 159 MESA participants) (12), we surmised that the composite of these 2 fat depots, pericardial fat, is an adequate measure for an epidemiological investigation. Our use of this composite variable may have underestimated the magnitude of the effect of epicardial fat on the risk of HF. Third, data on abdominal subcutaneous and visceral fat was available in only 24% and 28%, respectively, of the overall study and that too a few years after the baseline examination. Nonetheless, the presence of a statistically significant association between PFV and newly diagnosed HF in multivariable analyses accounting for abdominal fat depots, even in this relatively small representative but noncontemporary sample, lends credence to the possibility that pericardial fat is not just a surrogate for abdominal fat depot, but that its specific location around the heart may have a causal implication for the development of HF. Fourth, cutoff values to categorize PFV as normal or high in women and men using the Youden J-statistic need further validation in other cohort studies. Lastly, exposure to radiation is always a matter of concern in CT-based estimation of PFV. In this regard, non-radiation-based technologies such as echocardiography, albeit limited to the measurement of pericardial fat thickness, and cardiovascular magnetic resonance imaging for quantification of PFV are good alternatives for future studies.
CONCLUSIONS
In our prospective cohort of middle-age to elderly participants without clinically apparent cardiovascular disease, greater amount of pericardial fat was associated with a higher risk of HF in both women and men and about one-fifth of newly diagnosed HF (approximately one-third in women and over one-tenth in men) was attributable to high PFV. Excess pericardial fat should be considered as a novel risk factor for HF. Our findings must be replicated in cohorts that are not as healthy as MESA participants are. Future studies are warranted to differentiate the relative contribution of epicardial and paracardial fat depots, particularly given known differences in their embryological, anatomic, biochemical, biomolecular, and physiopathological profiles (6-8), to the risk of HF. Effect of lifestyle modification and target therapies to reduce regional fat depot around the coronary arteries and the heart and, in turn, reduce the incidence of HF also need further investigation.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
High pericardial fat volume (≥70 cm3 in women and ≥120 cm3 in men) increases the risk of developing heart failure by approximately 2-fold in women and about 50% in men.
TRANSLATIONAL OUTLOOK:
Future studies are warranted to assess this relationship in other cohorts, differentiate the relative contribution of epicardial and paracardial fat depots to the risk of heart failure, examine the impact of lifestyle modification, and evaluate specific therapies that reduce pericardial fat deposition.
ACKNOWLEDGMENTS
The authors thank all the participants, staff, and other investigators of the MESA (Multi-Ethnic Study of Atherosclerosis) for their valuable contributions.
FUNDING SUPPORT AND AUTHOR DISCLOSURES:
Supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH); and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. Dr. Kenchaiah was partly supported by the intramural research program of the NHLBI, NIH, grant number Z99 HL999999. The funding agencies had no role in the design of the study; collection, management, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- HF
heart failure
- HFmrEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HFuEF
heart failure with unknown ejection fraction
- LVEF
left ventricular ejection fraction
- PFV
pericardial fat volume
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Andreas Kalogeropoulos, MD, MPH, PhD, served as Guest Associate Editor for this paper. Javed Butler, MD, MPH, MBA, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate.
Manuscript received February 18, 2021; revised manuscript received March 30, 2021, accepted April 2, 2021.
REFERENCES
- 1.Kenchaiah S, Evans JC, Levy D et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am 2004;88:1273–94. [DOI] [PubMed] [Google Scholar]
- 3.Lee DS, Massaro JM, Wang TJ et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension 2007;50:869–76. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami H, Bluemke DA, Kronmal R et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;51:1775–83. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 2009;119:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev 2007;8:253–61. [DOI] [PubMed] [Google Scholar]
- 7.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007;153:907–17. [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 2015;11:363–71. [DOI] [PubMed] [Google Scholar]
- 9.Bos D, Shahzad R, van Walsum T et al. Epicardial fat volume is related to atherosclerotic calcification in multiple vessel beds. Eur Heart J Cardiovasc Imaging 2015;16:1264–9. [DOI] [PubMed] [Google Scholar]
- 10.Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013;62:1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Kritchevsky SB, Harris TB et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding J, Hsu FC, Harris TB et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2009;90:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah RV, Anderson A, Ding J et al. Pericardial, But Not Hepatic, Fat by CT Is Associated With CV Outcomes and Structure: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2017;10:1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Nelson JC, Wong ND et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Kritchevsky SB, Hsu FC et al. Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2008;88:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–53. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 22.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol 1995;76:414–8. [DOI] [PubMed] [Google Scholar]
- 24.Reiner L, Mazzoleni A, Rodriguez FL. Statistical analysis of the epicardial fat weight in human hearts. AMA Arch Pathol 1955;60:369–73. [PubMed] [Google Scholar]
- 25.Corradi D, Maestri R, Callegari S et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 2004;13:313–6. [DOI] [PubMed] [Google Scholar]
- 26.Schejbal V. [Epicardial fatty tissue of the right ventricle--morphology, morphometry and functional significance]. Pneumologie 1989;43:490–9. [PubMed] [Google Scholar]
- 27.Iacobellis G, Assael F, Ribaudo MC et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 2003;11:304–10. [DOI] [PubMed] [Google Scholar]
- 28.Laennec RTH. De l'auscultation médiate, ou, Traité du diagnostic des maladies des poumons et du coeur : fondé principalement sur ce nouveau moyen d'exploration. Paris: J.-A. Brosson, et J.-S. Chaudé, 1819. [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HL, Willius FA. Adiposity of the heart - A clinical and pathologic study of one hundred and thirty-six obese patients. Archives of Internal Medicine 1933;52:911–31. [Google Scholar]
- 30.Austys D, Dobrovolskij A, Jablonskiene V, Dobrovolskij V, Valeviciene N, Stukas R. Epicardial Adipose Tissue Accumulation and Essential Hypertension in Non-Obese Adults. Medicina (Kaunas) 2019;55:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iacobellis G, Leonetti F, Singh N, A MS. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol 2007;115:272–3. [DOI] [PubMed] [Google Scholar]
- 32.Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2020;8:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawel-Boehm N, Kronmal R, Eng J et al. Left Ventricular Mass at MRI and Long-term Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2019;293:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 2010;299:H202–9. [DOI] [PubMed] [Google Scholar]
- 36.Mazurek T, Zhang L, Zalewski A et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–6. [DOI] [PubMed] [Google Scholar]
- 37.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab 2005;90:6300–2. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Liu B, Li Y et al. Epicardial fat tissue in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2019;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iacobellis G, Ribaudo MC, Assael F et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 2003;88:5163–8. [DOI] [PubMed] [Google Scholar]
- 40.Greulich S, Maxhera B, Vandenplas G et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012;126:2324–34. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Chen Y, Zhang Y et al. Epicardial Fat Volume Improves the Prediction of Obstructive Coronary Artery Disease Above Traditional Risk Factors and Coronary Calcium Score. Circ Cardiovasc Imaging 2019;12:e008002. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999; 159:1197–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.