ABSTRACT
Acinetobacter spp. have become of increased clinical importance as studies have shown the antimicrobial resistant potential of these species. Efflux pumps can lead to reduced susceptibility to a variety of antibiotics and are present in large number across Acinetobacter spp. There are six families of efflux pumps that have been shown to be of clinical relevance: the major facilitator superfamily (MFS), small multidrug resistance (SMR) family, ATP-binding cassette (ABC) family, multidrug and toxic compound extrusion (MATE) family, proteobacterial antimicrobial compound efflux (PACE) family, and the resistance-nodulation-division (RND) family. Much work has been done for understanding and characterizing the roles these efflux pumps play in relation to antimicrobial resistance and the physiology of these bacteria. RND efflux pumps, with their expansive substrate profiles, are a major component of Acinetobacter spp. antimicrobial resistance. New discoveries over the last decade have shed light on the complex regulation of these efflux pumps, leading to greater understanding and the potential of slowing the reduced susceptibility seen in these bacterial species.
KEYWORDS: Acinetobacter, multidrug resistance, efflux pumps, regulation, ABC transporters, AdeABC, AdeFGH, AdeIJK, MATE, PACE, RND, SMR
INTRODUCTION
In 2017, the World Health Organization released a report on organisms that need to be prioritized in terms of an increase in research and development for novel treatment methods (1). The report listed carbapenem-resistant Acinetobacter baumannii as the top-priority pathogen. This is due to carbapenem-resistant A. baumannii infections becoming increasingly commonplace and, when found, incredibly difficult to treat due to these isolates tending to be resistant to all but antibiotics of last resort, such as colistin and tigecycline (2).
A. baumannii is a Gram-negative organism that has become a nuisance in clinical settings due to its ability to persist within the clinical environment and cause hospital-acquired infections (HAIs) (3). This Gram-negative coccobacillus, a member of the gammaproteobacteria, is part of a biochemically indistinguishable four-member group known as the Acinetobacter-baumannii/calcoaceticus complex, composed of Acinetobacter baumannii, Acinetobacter calcoaceticus, Acinetobacter pittii, and Acinetobacter nosocomialis. The biochemical similarity between the species led to early confusion in characterization and determination of the clinical relevance of each species. The differences in the four species became clearer as molecular tools became more readily available and it is now known that A. baumannii is the most clinically relevant member of the complex, as it accounts for the majority of infections caused by Acinetobacter spp. (4). A. nosocomialis and A. pittii are also becoming an increasingly more common cause of HAIs, but not to the extent of the closely related A. baumannii (4). There is some evidence to suggest that members of the genus Acinetobacter can be found as commensal skin organisms, but A. baumannii is rarely found as part of a healthy individual’s microbiome (3).
A. baumannii has the ability to cause a variety of infections, including but not limited to skin and soft tissue infections, ventilator-associated pneumonia (VAP), catheter-associated urinary tract infections, meningitis, and bacteremia (4). Acinetobacter spp. infections are seen most often in intensive care units (4). This is because the main route of infection for A. baumannii is via invasive procedures or clinical equipment such as ventilators and catheters (4). In the United States, the most common site of infection is the respiratory tract leading to VAP (2). These infections, though problematic in their own right, are complicated by the fact that around 60% of HAIs caused by A. baumannii are multidrug resistant (1). This means fewer treatment options are available to clinicians in treating many of the infections caused by A. baumannii and assessing the course of effective antibiotic treatment as early in the infection process as possible has been shown to be critical for decreasing mortality (3). There are some excellent reviews summarizing the clinical relevance of A. baumannii and the reader is directed toward these for more in-depth analysis (2–4).
A. baumannii’s ability to persist in hospital environments has been established by several studies. For example, A. baumannii was found to be present in nearly a quarter of the air samples taken from rooms in which patients with carbapenem-resistant A. baumannii infections were housed (5, 6). This suggests that transmission via aerosolization within clinical settings is possible. There have also been cases of community-acquired pneumonia caused by A. baumannii in areas with more tropical and humid environments, which also suggests the possibility of transmission via aerosolization may exist (5, 6). Further, A. baumannii can also be transmitted via hospital personnel, as was shown by one study which found that the gloves and gowns of caregivers tested positive for A. baumannii after 39% of encounters with patients infected with multidrug-resistant (MDR) A. baumannii (7). Adding to this ability to persist within clinical settings is the desiccation resistance displayed by Acinetobacter spp. A study of A. baumannii planktonic and biofilm cultures desiccated on plastic surfaces showed mean survival times of 17 and 47 days, respectively (8). This desiccation resistance appears to be linked to changes in the capsule of A. baumannii (9). A. baumannii is not the only member of the genus to display this quality, as A. pittii isolates were likewise shown to survive desiccation for 43 days (10).
Mortality rates of A. baumannii infections vary greatly, with carbapenem-resistant isolates having a reported mortality rate between 16 and 76% (2). Mortality rates are greatly affected by the underlying conditions led to the patient being hospitalized before becoming infected by A. baumannii within the clinical setting and are therefore more difficult to quantify (3). Another major factor contributing to the variability in mortality rates is the difficulty in early identification of an effective treatment plan, since Acinetobacter spp. habitually exhibit an extensive drug resistance (XDR) phenotype (3). XDR phenotypes are described as resistance to all but the last-resort antibiotics such as the polymyxins and tigecycline (3). In fact, this XDR phenotype globally has come to make up greater than 60% of A. baumannii strains (3).
INTRINSIC AND EXTRINSIC RESISTANCE MECHANISMS IN ACINETOBACTER SPP.
Antibiotic resistance mechanisms of Acinetobacter spp., particularly those of A. baumannii, have been covered extensively in previously published reviews (2, 11, 12). Antibiotic resistance of Acinetobacter spp. results from a combination of intrinsic and extrinsic (or acquired) mechanisms. Most Acinetobacter spp. are nonpathogenic but remain an important reservoir of antibiotic resistance, containing carbapenemases and extended-spectrum beta-lactamases among others (13). A. baumannii encodes both native and acquired OXA carbapenemases and metallo-beta-lactamases (MBL) that greatly increase its resistance to carbapenems and beta-lactams as a whole (14). Arguably Acinetobacter’s most effective intrinsic beta-lactamase is a class C cephalosporinase, of which a large variety of alleles have been found (15–17). These beta-lactamases, named ADC for Acinetobacter-derived cephalosporinase, have been shown to confer resistance to an array of beta-lactams, including carbapenems (15–17). Additionally, the effective MBLs, including VIM, IMP, SIM, and NDM, have all been found in clinical isolates of A. baumannii (14). Of particular interest is the NDM MBL in whose dissemination A. baumannii plays a key role (3). The evidence for blaNDM-type genes originating from A. baumannii is continuously mounting, with full-length blaNDM-type genes found both chromosomally and borne on plasmids in Acinetobacter spp., compared to the usually truncated versions found in Enterobacteriaceae (3).
One factor that contributes to the ability of Acinetobacter spp. to readily acquire resistance genes is that they are easily transformed due to their natural competence (18). This ability to incorporate foreign DNA has led to a large associate genome and a small core genome, which in turn allows for greater adaptation to a large variety of environments, including the abiotic clinical environment (19). This ability to take up and incorporate foreign DNA and plasmids has greatly added to the ability to adapt to the hostile clinical environment (2). The genome of A. baumannii contains large resistance islands (RI); the largest found to date contains 88 open reading frames which, as differences in GC content indicate, were acquired from other organisms (19). These large RIs allow A. baumannii to survive the antibiotic onslaught experienced in the hospital setting, while the metabolic flexibility in its genome allows it to thrive in the ecological niches that become available as other more susceptible bacteria are removed.
The intrinsic resistance of A. baumannii results from its low membrane permeability and constitutive efflux pump expression (20). Its low membrane permeability is mostly due to the low number and small size of porins in its outer membrane, which lead to considerably slower uptake of antimicrobials compared to other Gram-negative organisms (3). The bacterium also encodes a large number of efflux pumps; of particular interest are the members of the resistance-nodulation-division (RND) efflux family, which have broad substrate specificity and are able to efflux a vast variety of antibiotics from the periplasm before the antibiotics ever fully enter the cell (20).
INTRODUCTION TO EFFLUX PUMPS
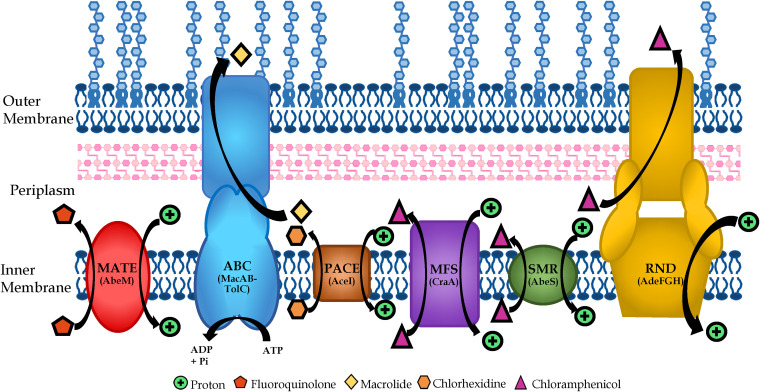
Energy-dependent efflux pumps have also been suggested to play a major role in a bacterium’s intrinsic resistance to antibiotic activity. However, there is mounting evidence that efflux pumps are involved in a myriad of activities, from nutrient balancing to alleviating stresses on the cell to pathogenesis to toxin excretion to heavy metal balancing (21–23), such that antibiotic efflux is not their primary function. Nevertheless, the role of efflux pumps in antibiotic susceptibility is perhaps the most important function attributed to them. Antibiotics are rendered wholly ineffective if the bacterium can excrete them before they are able to reach their desired target. To date, six different classes of efflux pumps have been described and Acinetobacter spp. have been shown to contain all six classes of multidrug efflux pumps (Fig. 1). These pumps in Acinetobacter spp. contribute to their reduced susceptibility to many classes of antibiotics (Table 1). The data shown in Table 1 is for efflux pumps characterized to date in Acinetobacter spp. but, as can be seen in Fig. 2, A. baumannii contains large numbers of each type of efflux pumps with many remaining to be characterized.
FIG 1.
Schematic of various efflux families and their location in the Gram-negative cell membrane. Efflux pumps that span the inner membrane are of the multidrug and toxic compound extrusion family (MATE), ATP-binding cassette family (ABC), proteobacterial antimicrobial compound efflux family (PACE), major facilitator superfamily (MFS), and small multidrug resistance family (SMR). Efflux pumps that span both the inner and outer membrane belong to the resistance-nodulation-division family (RND) and, as pictured, some ABC transporters are able to do so as well. Substrates are examples to indicate direction of transport.
TABLE 1.
Examples of efflux pumps and the role they play in reducing susceptibility to various compounds and the Acinetobacter spp. in which they were first described
| Efflux family | Example | Reduced susceptibility | Acinetobacter sp. | Reference(s) |
|---|---|---|---|---|
| MFS | CraA | Chloramphenicol | A. baumannii | 25 |
| AmvA | Erythromycin | A. baumannii | 26 | |
| AbaF | Fosfomycin | A. baumannii | 28 | |
| AbaQ | Quinolones | A. baumannii | 29 | |
| TetA | Tigecycline | A. baumannii | 30 | |
| CmlA | Chloramphenicol | A. baumannii | 31 | |
| MATE | AbeM | Fluoroquinolones | A. baumannii | 51 |
| ABC | A1S_0536 | Erythromycin | A. baumannii | 21 |
| A1S_1535 | Gentamicin, chloramphenicol | A. baumannii | 21 | |
| MacAB-TolC | Potentially macrolides and tigecycline | A. baumannii | 47 | |
| SMR | AbeS | Chloramphenicol, fluoroquinolones, novobiocin, erythromycin | A. baumannii | 34 |
| QacE | Quaternary ammonia compounds | A. baumannii | 39 – 41 | |
| PACE | AceI | Chlorhexidine | A. baumannii | 53 |
| A1S_1503 | Acriflavine | A. baumannii | 54 | |
| RND | AdeABC | Aminoglycosides, trimethoprim, chloramphenicol, fluoroquinolones, tetracyclines, pentamide | A. baumannii | 61, 69 |
| AdeDE | Amikacin, ceftazidime, chloramphenicol, ciprofloxacin, erythromycin, meropenem, rifampin, tetracycline | A. pittii | 77 | |
| AdeFGH | Chloramphenicol, trimethoprim, tetracycline-tigecycline, clindamycin; fluoroquinolones | A. baumannii | 78 | |
| AdeIJK | β-lactams, chloramphenicol, tetracycline, erythromycin, lincosamides, fluoroquinolones, fusidic acid, novobiocin, rifampin, trimethoprim | A. baumannii | 85 | |
| AdeXYZ | NA | A. baylyi | 90 | |
| CzcABCD | Heavy metals such as copper | A. baumannii | 23 | |
| AbeD | Ceftriaxone, gentamicin, rifampin, tobramycin, benzalkonium chloride | A. baumannii | 92 | |
| ArpAB | Amikacin, tobramycin | A. baumannii | 93 | |
| AcrAB | Colistin, tobramycin, acriflavin | A. nosocomialis | 95 |
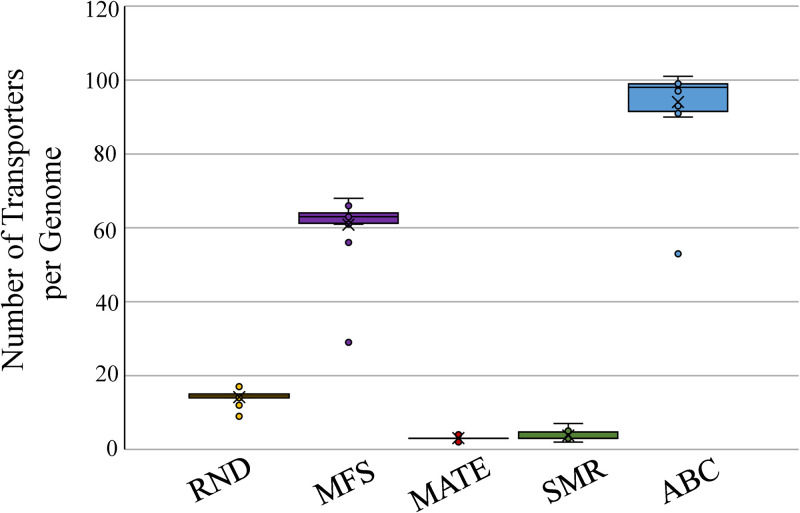
FIG 2.
The Transport Database 2.0 (102) was used to examine the proliferation of various efflux pump families in A. baumannii genomes (n = 16). The PACE family of transporters can currently not be found in the database and therefore has been excluded.
EFFLUX PUMPS IN ACINETOBACTER SPP
Major facilitator superfamily.
The major facilitator superfamily (MFS) is the largest and most diverse superfamily of secondary transporters known to date (24). Members of this superfamily of transporters are ubiquitous across all domains of life. These transporters can be separated into three subcategories: (i) symporters that transport two or more substrates in the same direction using one of the substrates’ electrochemical gradients to do so; (ii) antiporters that transport two or more substrates in opposite directions; and (iii) uniporters that transport one substrate along its concentration gradient. Each transporter individually is quite specific to its substrate, but the MFS superfamily incorporates a broad range of efflux substrates. MFS transporters have been shown to have essentially the same 3D structure, although proteins within the MFS superfamily show a variable number of transmembrane segments, with some members having 12 and others 14 transmembrane segments (24). This similarity in structure suggests that this broad range of specific substrates is specific to a few key residues present in the protein (24).
Acinetobacter spp. so far have not been studied in as much detail when it comes to MFS transporters. Within A. baumannii specifically, there are three MFS transporters relevant to multidrug resistance that are present in all known strains. The first is CraA, chloramphenicol resistance Acinetobacter, which has been shown to increase A. baumannii chloramphenicol susceptibility by up to 128-fold when deleted (Table 1) (25). It is currently not known if craA expression is constitutive and how relevant it is in clinical isolates of A. baumannii. The second MFS transporter in A. baumannii involved in multidrug resistance is AmvA, which mainly effluxes a variety of dyes and disinfectants but has also been shown to affect the susceptibility of A. baumannii to erythromycin (Table 1) (26). Expression of AmvA is regulated by AmvR, a TetR-type regulator (27). AbaF is another example of an MFS transporter involved in reducing the susceptibility to antimicrobials, in this case fosfomycin (Table 1) (28). The final MFS transporter involved in antimicrobial efflux is AbaQ, which is able to transport quinolones (29). There are on average 61 different MFS transporters (Fig. 2) in A. baumannii genomes and further study of these members of the MFS transporters would aid in elucidating the clinical relevance of these transporters.
It should be noted that many A. baumannii clinical isolates have been found to contain Tet-type efflux pumps which belong to the MFS transporters (11). These Tet transporters are acquired and not intrinsic to A. baumannii and confer a decrease in susceptibility to tetracyclines (11); for example, TetA has been shown to play a role in reducing tigecycline susceptibility in A. baumannii (Table 1) (30). Additionally, genomic islands within A. baumannii isolates have been found to contain a variety of cmlA genes that encode chloramphenicol efflux pumps and thus further the reduced susceptibility seen in these isolates (Table 1) (31).
SMALL MULTIDRUG RESISTANCE FAMILY
The small multidrug resistance (SMR) family of efflux pumps is composed of small proteins with four transmembrane α-helical domains (32). SMR proteins are integral inner membrane proteins that most likely work as oligomers, though this has only been shown for some members of this family (33). Many members of the SMR family are involved in the efflux of lipophilic compounds, such as quaternary ammonium compounds (QAC) commonly used as antiseptics and detergents, and therefore play a role in QAC resistance due to their ability to transport these compounds out of the cytosol (33). These proteins transport their substrates via an electrochemical proton gradient (32). A1S_0710, an SMR family transporter in A. baumannii, has been shown to reduce motility and virulence when deleted and may therefore be of greater importance for its physiological role rather than its antimicrobial resistance role (21).
AbeS, the only characterized member of the SMR family that is commonly found in A. baumannii, has been shown to play a minor role in reducing the susceptibility of A. baumannii to chloramphenicol, fluoroquinolones, erythromycin, and novobicin (Table 1) (34). AbeS has also been shown to play a role in resistance to dyes and detergents.
In addition, an analysis of available genomic data from A. baumannii strains shows that qacE, encoding a quaternary ammonium compound (QAC) pump, is present in 40% of strains (35). QacE, usually encoded on an integron (11), has been shown to be responsible for reduced susceptibility to QACs in Klebsiella pneumonia, Klebsiella aerogenes, and Proteus mirabilis (36–38). The presence of QacE in A. baumannii has been correlated with reduced susceptibility to benzalkonium chloride, chlorhexidine, and cetrimide (Table 1) (39–41). There are on average 4 SMR efflux pumps (Fig. 2) encoded in A. baumannii genomes but, with the exception of AbeS, they remain uncharacterized.
ATP-BINDING CASSETTE FAMILY
The ATP-binding cassette (ABC) family of transporters utilizes the free energy released by hydrolysis of ATP to ADP to facilitate the transport of its substrates across a lipid membrane in or out of the cell. All ABC transporters contain four protein domains, consisting of two membrane-spanning domains and two ATP-hydrolyzing domains (42). These domains can be found in one single long protein or can be separated into multiple proteins (42). ABC transporters are ubiquitous in all domains of life and are found to have high conservation of sequence (43). These transporters can be grouped into three subcategories based on function: (i) importers, involved in nutrient uptake; (ii) exporters, involved in secretion of various substrates; and (iii) other, not involved in transport and aid in mRNA translation and DNA repair.
ABC transporters have been shown to be involved in multidrug efflux in several species of bacteria, such as Lactococcus lactis, Bacillus subtilis, and Vibrio cholerae (43), although their clinical relevance in most Gram-negative bacteria remains to be established. Although Acinetobacter spp. contain multiple ABC transporters, an average of 94 for A. baumannii (Fig. 2), within their genomes, little is known about their functions within these species. A study examining 6 different ABC permeases within A. baumannii found two, A1S_0027 and A1S_1057, to have no significant effect on antimicrobial resistance; two, A1S_1242 and A1S_2622, to have a moderate effect, and two, A1S_0536 and A1S_1535, to lead to resistance to erythromycin and to gentamicin and chloramphenicol, respectively (Table 1) (21). Deletion of A1S_1242 and A1S_2622 were shown to lead to reduced motility and virulence, compared to the parent strain ATCC 17978 (21). Further investigation into these transporters and the 88 others encoded in the genome is required to determine whether these transporters aid in the multidrug-resistant phenotype commonly seen in Acinetobacter spp.
MacAB-TolC, though poorly understood in Acinetobacter spp., is known to play a role in antibiotic resistance in other species and is named for conferring macrolide resistance (Table 1) (44). It is structurally different than most classically described ABC transporters in that it is part of the mechanotransducer ABC superfamily, meaning it is able to use cytosolic ATP hydrolysis to move substrates from the periplasmic space outward through TolC (45). TolC is an outer membrane protein often associated with RND-type efflux families and allows for the substrates to be moved out of the outer membrane into the extracellular space (45, 46). In A. baumannii, it has been shown to be regulated by the BaeSR two-component system (TCS) and potentially plays a role in tigecycline resistance (47, 48).
MULTIDRUG AND TOXIC COMPOUND EXTRUSION FAMILY
Due to the presence of 12 transmembrane helices, NorM from Vibrio parahaemolyticus and YdhE from Escherichia coli were originally thought to be a part of the MFS family of efflux pumps. However, as a result of the lack of sequence homology to any of the members within the MFS family, these two proteins became the first members of the multidrug and toxic compound extrusion (MATE) family of efflux pumps (49). Since then, members of the MATE family of efflux pumps have been found in all three domains of life, the Archaea, the Eukarya, and the Eubacteria. Bacterial MATE transporters have been found to efflux cationic drugs in exchange for H+ or Na+ molecules (50). They have also been shown to efflux compounds such as berberine, acriflavine, and norfloxacin (50).
In A. baumannii, the MATE transporter AbeM was observed to play a role in reduced susceptibility to the fluoroquinolones norfloxacin and ciprofloxacin, as well as to ofloxacin, though to a lesser extent (Table 1) (51). These three fluoroquinolones differ with respect to their hydrophobicity, with the first two being more hydrophilic and the latter being a more hydrophobic molecule; this was suggested to be the reason for the observed differences in reduced susceptibility to these antibiotics resulting from the activity of the AbeM pump (51). AbeM, which functions as a proton antiporter (51), was also shown to lead to minor but reproducible decreases in susceptibility to triclosan, trimethoprim, chloramphenicol, and some aminoglycosides (51). It should be noted that, to date, the work performed on susceptibility profiles were performed by expression of this efflux pump in E. coli and not A. baumannii strains. Yet another study showed that ppGpp levels affect the expression of abeM along with the expression of two efflux pumps of the resistance-nodulation-division efflux family, AdeIJK and AdeABC (52), thus suggesting a possible cross talk in the regulation of pumps belonging to two distinct families.
Though not believed to be a major cause of A. baumannii multidrug resistance, MATE transporters such as AbeM nonetheless add to the vast repertoire of defenses that A. baumannii is able to utilize against antibiotics. Deletion of A1S_3371, a MATE efflux pump, leads to reduced motility and virulence compared to ATCC 17978, its parent strain (21). There are 3 MATE family efflux pumps found in A. baumannii and only AbeM is characterized in relation to antimicrobial resistance; therefore, there is still much to learn about MATE transporters in A. baumannii.
PROTEOBACTERIAL ANTIMICROBIAL COMPOUND EFFLUX FAMILY
In 2013 the Paulsen group in Australia described a novel pump in Acinetobacter spp. capable of efflux of active chlorhexidine, a widely used biocide (53). Homologs of AceI, for Acinetobacter chlorhexidine efflux, were found to be present in other bacterial species but especially common in proteobacterial species, leading to the first new family of efflux pumps in 15 years, now known as the proteobacterial antimicrobial compound efflux (PACE) family (54). Members of the PACE family are commonly found encoded within the core genome of a species, suggesting these efflux pumps are perhaps involved in more than the efflux of biocides (54). The efflux of short-chain diamines has been attributed to AceI in A. baumannii (55). AceI has been shown to be positively regulated by a LysR-type regulator AceR in response to the presence of chlorhexidine (56). This regulation is complex, in that AceR interferes with the ability of RNA polymerase to transcribe aceI (57). However, binding of chlorhexidine to AceR can impair its ability to bind to the DNA, allowing for RNA polymerase binding and therefore transcription of aceI (57).
The mechanism of action of PACE pumps is still not clear, as the structural information remains unavailable for any members of the PACE family. PACE pumps are predicted to contain four transmembrane alpha-helices that are arranged into two tandem bacterial transmembrane pair (BTP) domains (58). These proteins tend to be rather small and are most likely acting as oligomers in the inner membrane, though the oligomerization has yet to be proven (58). It has also been shown that the transport across the inner membrane requires proton coupling (55).
Chlorhexidine is a bis-biguanide antimicrobial commonly used as an antiseptic in clinical settings, as well as in common household items such as mouthwashes or soaps (53). Other members of the PACE family have been shown to efflux a variety of biocide substrates, but AceI itself displays greater specificity to chlorhexidine (Table 1) (54). A second PACE protein found within the A. baumannii ATCC 17978 genome appears to result in acriflavine resistance, where acriflavine is a commonly used topical antiseptic (Table 1) (54). Acinetobacter spp. adaptability and the apparent broad specificity of PACE proteins have no doubt led to the development of resistance against these biocides and only further study will show how clinically relevant this new family of efflux pumps will become.
RESISTANCE-NODULATION-DIVISION FAMILY
The resistance-nodulation-division (RND) family of efflux pumps is ubiquitous in all forms of life. In Gram-negative bacteria, the tripartite RND complex can span across both the inner and outer membrane of the bacterium. RND efflux pumps are arguably most relevant clinically in Gram-negative bacteria. Their tripartite structure sets RND family of efflux pumps apart from the others which are only able to facilitate transport across one membrane. RND efflux complexes are composed of an outer membrane protein (OMP), an inner membrane protein (RND) and a periplasmic adapter protein (PAP, also known as the membrane fusion protein or MFP) that connects the OMP to the RND. The RND protein of the complex is responsible for the efflux using proton motive force. (59) These pumps show a broad specificity for substrates that are primarily effluxed from the periplasm, though increasing evidence suggests the capability of direct transport from the cytoplasm of some substrates (11).
RND efflux pumps have been implicated as the cause of the extensive intrinsic multidrug resistance seen in many Gram-negative bacteria (22). The ability of these efflux pumps to recognize a wide variety of substrates allows for the efflux of nearly all classes of antibiotics (11). Their upregulation due to mutations in their regulators is commonly seen in clinical isolates (60). This has been suggested to be due to the use of antibiotics in clinical settings exerting selective pressure on the bacteria to upregulate its defenses to the antibiotics, but there is also increasing awareness that the physiological functions of these pumps may be clinically relevant, further leading to their upregulation (60).
In Acinetobacter spp., the biological functions of these RND efflux pumps are still unknown and require further study. Some of the cell processes that RND efflux pumps have been shown to be involved in are nitrosative stress relief, oxidative stress relief, and virulence (60). The role of RND efflux pumps in multidrug resistance of A. baumannii is, however, becoming increasingly well studied, with six of these efflux pumps having been characterized to various degrees to date. There appear to be, on average, 14 RND efflux pump-encoding operons in the A. baumannii genomes, though this can vary from strain to strain (Fig. 2) (11). Below, we describe RND pumps that have been characterized to date in Acinetobacter spp., along with their transcriptional regulation mechanism(s).
AdeABC.
The first RND efflux pump to be characterized within Acinetobacter spp. was AdeABC (61). AdeABC is the most clinically relevant RND efflux pump because it is shown to be overexpressed in the largest number of clinical isolates compared to the other RND efflux pumps, and is the major contributor to the MDR phenotype (11, 62, 63). The adeABC operon is present in around 80% of A. baumannii isolates (11), although, interestingly, the adeABC operon can often be found without the adeC gene (64–66). It has been observed that even in strains missing adeC, upregulation of the adeAB operon results in reduced susceptibility to antibiotics, thus suggesting that AdeAB in these instances uses an alternate outer membrane protein (OMP), likely AdeK, of the constitutive efflux pump, AdeIJK (65, 66). This would not be out of the ordinary for RND efflux pumps, as this is commonly seen in Pseudomonas aeruginosa, e.g., MexAB and MexXY share the use of OMP OprM (67).
AdeABC is able to efflux aminoglycosides, trimethoprim, chloramphenicol, fluoroquinolones, tetracyclines, and ethidium bromide (Table 1) (61). There is some suggestion it is able to also use tigecycline as its substrate (68), but overexpression of adeABC does not necessarily result in decreased susceptibility to tigecycline, therefore the ability of AdeABC to efflux tigecycline remains an area of study (63). More recently, dicationic compounds, specifically pentamide, have been shown to be substrates of AdeAB (69).
In addition to the efflux of antibiotics, overexpression of adeABC has also been shown to contribute to a decrease in biofilm formation, a decrease in plasmid transfer, and natural transformation (70). These changes may be linked to the changes in bacterial membrane composition, such as a reduction in Csu-pili, which are seen when AdeABC is overproduced (70). Deletion of adeABC in A. baumannii AB5075 and A. baumannii AYE also results in the downregulation of genes involved in iron acquisition, competence, and motility (65), suggesting the AdeAB(C) pump may be involved in various physiological functions in A. baumannii.
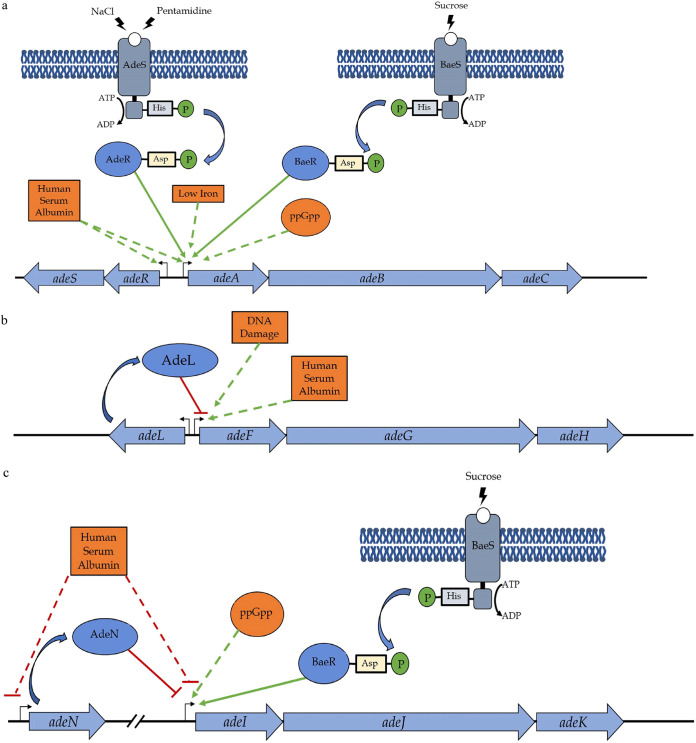
AdeRS, a two-component system, has been shown to positively regulate AdeABC expression (71). TCSs comprise a histidine kinase (HK) and a response regulator (RR) (72). The HK is generally incorporated into a membrane and senses an environmental signal (72). The HK (in this case AdeS) auto-phosphorylates and subsequently transfers that phosphoryl group to its cognate RR (AdeR in this case) to pass on the signal (72). The RR can then activate or repress the transcription of its target genes. AdeRS activates the transcription of adeABC (71, 72) (Fig. 3A). One of the environmental stimuli that AdeS senses is saline stress, as cells are more susceptible to saline stress in the absence of adeRS (73). Pentamidine has shown to lead to increased adeAB expression through the AdeRS TCS and, therefore, leads to subsequent increased resistance to dicationic compounds (69). The regulon of AdeR has been shown to affect not only adeABC expression but also genes involved in biofilm formation, motility, and virulence (74), which has been phenotypically verified as well (73). Another TCS involved in the regulation of AdeABC is BaeSR (68) (Fig. 3A). This TCS appears to be stimulated by osmotic stress (68). Deletions of this TCS lead to decreased expression of adeAB and increased susceptibility to tigecycline (68), suggesting that BaeSR is also an activator of the adeAB operon. It remains to be seen, however, whether there is any cross talk between the AdeRS and BaeSR systems. A ppGpp-deficient strain in which the ppGpp synthetase was deleted showed reduced expression of adeB, suggesting ppGpp may also play a regulatory role for the adeABC operon (52) (Fig. 3A). Human serum albumin and low iron conditions present in medium has been shown to lead to increased expression of adeABC and adeRS (75, 76).
FIG 3.
Regulation of AdeABC, AdeFGH, and AdeIJK efflux pumps in A. baumannii. Expression of the AdeABC pump (a) is activated by two two-component systems, AdeRS and BaeRS, low iron conditions, presence of human serum albumin, and presence of ppGpp. AdeFGH (b) expression is repressed by AdeL, a LysR-family protein, encoded upstream of the operon. Human serum albumin and DNA damage are known to increase adeFGH expression. AdeIJK (c) regulation involves the repression by TetR-type regulator AdeN and presence of human serum albumin, and activation by TCS BaeSR and ppGpp. Green solid arrows indicate direct activation of expression. Green dashed arrows indicate an unknown mechanism of activation of expression. Red solid lines indicate direct repression of expression. Red dashed lines indicate an unknown mechanism of inhibition of expression.
Clinical isolates have been shown to have mutations in the AdeRS TCS that will lead to even higher expressions of adeABC (63). One of the most common mutations seen are those that inactivate the phosphatase activity of AdeS, leading to AdeS being unable to dephosphorylate AdeR and thus continuous overexpression of adeABC (63). Specifically, the most common mutations seen to date in AdeS are mutations within the histidine kinase, adenylyl cyclase, methyl-accepting protein, and phosphatase (HAMP) linker domain (63). In AdeR, mutations to the phosphorylation site and effector binding pockets are more common but appear to mainly affect the stability of the protein (63).
AdeDE.
The AdeDE pump, found in Acinetobacter pittii, lacks the OMP-encoding gene in its operon (77). This efflux pump is not well studied but its presence has been linked to a reduction in susceptibility to meropenem, tetracycline, amikacin, ciprofloxacin, rifampin, ceftazidime, chloramphenicol, and erythromycin (Table 1) (77).
Mechanism(s) that regulate the expression of the AdeDE pump are not yet known.
AdeFGH.
AdeFGH was discovered via transcriptomic microarray analysis of spontaneous chloramphenicol mutants of A. baumannii BM4652 (78). The adeFGH operon is highly conserved but transcriptomic data suggest it is expressed at low levels in laboratory conditions (64). The overexpression of adeFGH confers reduced susceptibility to chloramphenicol, trimethoprim, tetracycline-tigecycline, clindamycin, and fluoroquinolones (Table 1) (78). AdeFGH has also been shown to efflux dyes, such as safranin O, acridine orange, and ethidium bromide (Table 1) (78).
Deletion of adeFGH has been shown to increase the expression of adeAB but the inverse is not true, suggesting that cross regulation occurs via an unknown mechanism in the one direction but not the other (65, 66). Overexpression of AdeFGH was found to be more common in Canadian clinical isolates compared to overexpression of AdeIJK or even AdeABC (79). This is especially interesting considering the fact that overexpression of AdeFGH was shown to have the greatest detrimental effect on cellular fitness compared to strains overexpressing either of the other two RND efflux pumps (80). Investigation into the links between a quorum-sensing gene (abaI), efflux pump genes, and biofilm formation showed the greatest increases in biofilm formation when abaI and adeG were overexpressed (81).
The expression of adeFGH is regulated by a LysR-type transcriptional repressor, AdeL, encoded immediately upstream of the operon (78) (Fig. 3B). Mutations in AdeL have been seen in clinical isolates, leading to multidrug-resistance phenotypes due to AdeFGH overexpression (63). LysR-type regulators contain N-terminal DNA-binding domains that follow the common helix-turn-helix motif (82). The C termini of LysR-type regulators contain co-inducer-binding domains that overlap the substrate-binding region of the regulator (82) and it is in this domain that mutations are most often found in AdeL, leading to the constitutive expression of the gene targets of AdeL (70).
The presence of human serum albumin increases the expression of adeFGH (75) (Fig. 3B). Overexpression of adeFGH is also seen in response to DNA damage and may be due to ddrR, a DNA-damage-inducible gene first identified in Acinetobacter baylyi but that appears to be conserved across Acinetobacter spp. (83, 84).
AdeIJK.
AdeIJK is the only RND efflux pump that has been found in all strains of A. baumannii to date, suggesting its physiological role within the cell to be of importance to the bacterial life cycle (85). AdeIJK is constitutively expressed and is believed to be one of the major contributors to the intrinsic resistance seen in A. baumannii (70). This efflux pump appears to prefer amphiphilic compounds, as suggested by its experimentally determined substrate profile, which is β-lactams, chloramphenicol, tetracycline, erythromycin, lincosamides, fluoroquinolones, fusidic acid, novobiocin, rifampin, trimethoprim, safranin, pyronine, and sodium dodecyl sulfate (Table 1) (85). It was also shown that AdeABC and AdeIJK together contribute to tetracycline, minocycline, and tigecycline resistance in a more-than-additive manner (85).
Homologues of the AdeIJK proteins are seen across Acinetobacter spp., with the members of the Acinetobacter calcoaceticus/Acinetobacter baumannii complex (A. calcoaceticus, Acinetobacter pittii, A. baumannii, and A. nosocomialis) displaying a high degree (>90%) of amino acid residue identities (86). This strong conservation suggests an important physiological role for AdeIJK in Acinetobacter spp. (86). Membrane composition alterations due to the overexpression of AdeIJK have been noted as well, suggesting that the presence of AdeIJK is critical for membrane composition stability (66, 70). Recent work has shown that AdeIJK is vital for lipid homeostasis maintenance, specifically as a lipid export mechanism (87).
Overexpression of the adeIJK operon is rarely seen in clinical isolates (70, 86). It has been suggested due to several in vivo studies that overexpression of adeIJK may be detrimental to the bacterial cell (85). When overexpression of adeIJK does occur in lab strains, it usually falls into a narrow range that is still relatively close to wild-type levels of expression, ranging from 1- to 2-fold increases in expression compared to adeABC and adeFGH, which can easily reach the mid to high double digits (63). Deletion of adeIJK leads to a broad transcriptomic response, including changes in pathways of lipid biosynthesis and turnover, as well as upregulation of motility genes, though this did not translate to an increase in motility (65).
Unlike other RND pump-encoding operons, where the regulator is encoded immediately upstream, the adeIJK operon does not have any regulator-encoding gene in close vicinity. Its expression is repressed by a TetR-type transcriptional regulator, AdeN, transcribed nearly a quarter of the genome away from the adeIJK operon (86) (Fig. 3C). AdeN has since been shown to be a global regulator, not only regulating adeIJK expression but also the expression of many other factors involved in complex processes such as virulence and biofilm formation (13). The ability of AdeN to control a variety of processes comes as no surprise, since TetR-type transcriptional regulators have been linked to controlling a bacterium’s response to changing environments (88, 89). The N terminus of the regulator, with its conserved helix-turn-helix DNA binding motif (89), is not a mutational hot spot in AdeN; instead, mutations leading to AdeN C-terminal truncations have been more commonly observed when AdeIJK is overexpressed (63). The TCS BaeSR has been shown to positively regulate adeIJK expression in response to sucrose stress (47, 48) (Fig. 3C). The involvement of BaeSR in the expression of AdeIJK, as well as AdeABC (as discussed above), suggests there may potentially be some cross talk between the expression of these two pumps. The absence of ppGpp leads to a reduction in adeIJK transcripts, suggesting that the levels of ppGpp can affect the transcription of adeIJK, though the manner in which this regulation is achieved is unknown (52) (Fig. 3C). The presence of human serum albumin has been shown to lead to reduced expression of adeIJK and, somewhat surprisingly, also of adeN (75) (Fig. 3C). It remains to be seen how downregulation of the repressor AdeN is accompanied by the downregulation of AdeIJK. The rarity in seeing adeIJK overexpression in Acinetobacter spp. suggests tight control of adeIJK expression, even in the absence of functional AdeN, further suggesting the existence of as- yet-unknown additional regulatory mechanisms (86).
AdeXYZ.
Acinetobacter baylyi and Acinetobacter GDG3 contain AdeXYZ (90). It is worth noting that AdeIJK and AdeXYZ share a nucleotide identity of 93% and an amino acid identity of 99%, suggesting that they may in fact be the same pump (91). The substrates of this efflux pump have not been elucidated due to the inability to generate a deletion or disruption of the pump (90). Nothing is known about the regulation or conditions leading to increases in AdeXYZ expression.
AbeD.
Identified in the clinical isolate A. baumannii AYE, AbeD is an orphan RND transporter (without the usual accompanying PAP or OMP genes) that was found to have an effect on oxidative stress tolerance (92). It was shown that deletion of abeD lead to increased susceptibility to benzalkonium chloride, ceftriaxone, gentamicin, tobramycin, and rifampin (92). However, it is still unknown which PAP and OMP this RND transporter partners with to form a functional complex.
Studies show that SoxR of the SoxSR two-component system, which regulates oxidative stress response, is a direct regulator of abeD expression. (92). This correlation is not all that surprising, since abeD was shown to be overexpressed under oxidative stress conditions.
ArpAB.
ArpAB was identified as a functional efflux pump in A. baumannii AB5075, with ArpA being a PAP and ArpB an RND transporter (93). Deletion of arpB resulted in increased susceptibility to tobramycin and amikacin, suggesting this is an aminoglycoside pump (93). However, it remains to be seen if the ArpAB pump is able to efflux other antibiotics. Intriguingly, the ArpAB pump was found to play a role in the opaque/translucent colony phase variation in A. baumannii, as disruption of arpA by a transposon insertion showed a 55-fold reduction in opaque-to-translucent switching (93, 94). Since opaque/translucent colony phase variation affects the virulence of most A. baumannii strains, the ArpAB pump may contribute to the virulence of A. baumannii in addition to its antibiotic susceptibility.
ArpR, a TetR-type regulator divergently transcribed in front of the arpAB operon, acts as a repressor of arpAB expression (93).
AcrAB.
A. nosocomialis contains an RND efflux pump named AcrAB which is homologous to ArpAB in A. baumannii (95). The absence of AcrAB leads to increased susceptibility to acriflavine and tobramycin, which are most likely substrates of AcrAB (96). Interestingly, the absence of AcrAB has also been shown to increase susceptibility to colistin, though the mechanism of this change in susceptibility will still need to be deduced (96).
AcrR is a repressor of acrAB expression and is divergently transcribed upstream of the pump operon (95). Though no changes in N-acyl-homoserine lactone secretion are observed in the presence or absence of AcrAB, the quorum-sensing regulator AnoR appears to activate acrAB expression (96). The AnoIR quorum-sensing system in turn is repressed by the same regulator as AcrAB, AcrR (96). This interplay between quorum sensing and efflux has not been shown with any of the pumps in Acinetobacter spp. before but has been seen in other genera, such as Pseudomonas spp. (97).
CzcABCD.
The CzcABCD RND efflux pump is a five-gene operon consisting of a gene coding for a hypothetical protein and czcB, czcA, and czcD coding for the PAP, RND, and OMP, respectively (23). The other gene, czcC, has no significant homology to any genes or proteins in E. coli or P. aeruginosa (23). This efflux pump has shown to play a role in heavy metal tolerance, specifically copper (23).
The CopRS/CuxRS TCS has been shown to play a role in regulating czcABCD expression in response to the presence of copper (23).
In conclusion, efflux pumps and transporters have been shown to play a critical role in multidrug resistance. Intrinsic and acquired resistance to antibiotics, antiseptics, and detergents can be linked to the expression of efflux pumps and transporters. In A. baumannii this has certainly been shown to be the case. But much work remains to be done, especially in elucidating the biological functions of these efflux pumps and transporters beyond antimicrobial efflux. Most of the research in the area of efflux pumps and transporters in Acinetobacter spp. focuses on the RND family of efflux pumps, primarily because of their ability to efflux clinically relevant antibiotics and because they are commonly found to be overexpressed in clinical isolates. While AdeABC, AdeFGH, and AdeIJK efflux pumps are the most studied pumps, other RND pumps, as well as those belonging to families other than RND, are being increasingly reported, mostly from lab-generated mutants. Therefore, the clinical relevance of pumps other than AdeABC, AdeFGH, and AdeIJK remains to be established. Regulation mechanisms of RND efflux pumps in other organisms such as P. aeruginosa have been shown to be multifaceted and complex. In Acinetobacter spp., this regulation of RND efflux pumps is still poorly understood and requires further investigation. Characterization of regulatory pathways that control the expression of RND pumps in A. baumannii cannot only shed light on their natural function(s), but regulatory mechanism(s) can also potentially be exploited and novel antimicrobials that prevent the expression of RND efflux pumps could be designed. Additionally, further study of the structure and function of these efflux pumps will lead to greater understanding and therefore the ability to design efflux pump inhibitors (98). Previous attempts to inhibit efflux have been successful within the lab environment but have not made it past preclinical testing (99). A promising candidate for efflux inhibition is the phytochemical resveratrol, which can be found in the skin of grapes (100). This compound has been shown to resensitize A. baumannii to antimicrobials such as chlorhexidine (101). Continued research into efflux pump inhibition is a vital component of discovering new methods of increasing susceptibility to antibiotics in use today.
ACKNOWLEDGMENTS
A.K.’s laboratory is funded by operating grants from the Natural Science and Engineering Council of Canada and the Canadian Institutes of Health Research. V.K. is supported by a Graduate Enhancement of Tri-Agency Stipends (GETS) from the Faculty of Graduate Studies, University of Manitoba.
REFERENCES
- 1.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 [Google Scholar]
- 2.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance—treatment options. Semin Respir Crit Care Med 36:85–98. 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochman S, Phillips M. 2020. Acinetobacter species, p 2718–2724. In Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 9th edition. Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 5.Rock C, Harris AD, Johnson JK, Bischoff WE, Thom KA. 2015. Infrequent air contamination with Acinetobacter baumannii of air surrounding known colonized or infected patients. Infect Control Hosp Epidemiol 36:830–832. 10.1017/ice.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz-Price LS, Fajardo-Aquino Y, Arheart KL, Cleary T, DePascale D, Pizano L, Namias N, Rivera JI, O’Hara JA, Doi Y. 2013. Aerosolization of Acinetobacter baumannii in a trauma ICU. Crit Care Med 41:1915–1918. 10.1097/CCM.0b013e31828a39c0. [DOI] [PubMed] [Google Scholar]
- 7.Morgan DJ, Liang SY, Smith CL, Johnson JK, Harris AD, Furuno JP, Thom KA, Snyder GM, Day HR, Perencevich EN. 2010. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol 31:716–721. 10.1086/653201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang S-R, Jung F, Tang H-J, Chen C-H, Chen C-C, Chou H-Y, Chuang Y-C. 2018. Desiccation and ethanol resistances of multidrug resistant Acinetobacter baumannii embedded in biofilm: the favorable antiseptic efficacy of combination chlorhexidine gluconate and ethanol. J Microbiol Immunol Infect 51:770–777. 10.1016/j.jmii.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Tipton KA, Chin C-Y, Farokhyfar M, Weiss DS, Rather PN. 2018. Role of capsule in resistance to disinfectants, host antimicrobials, and desiccation in Acinetobacter baumannii. Antimicrob Agents Chemother 62:e01188-18. 10.1128/AAC.01188-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo Z, Chapartegui-González I, Lázaro-Díez M, Ramos-Vivas J. 2018. Acinetobacter pittii maintains its ability to form biofilms on inanimate surfaces after long-term desiccation. J Hosp Infect 98:74–82. 10.1016/j.jhin.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Coyne S, Courvalin P, Périchon B. 2011. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother 55:947–953. 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisinger E, Huo W, Hernandez-Bird J, Isberg RR. 2019. Acinetobacter baumannii: envelope determinants that control drug resistance, virulence, and surface variability. Annu Rev Microbiol 73:481–506. 10.1146/annurev-micro-020518-115714. [DOI] [PubMed] [Google Scholar]
- 13.Ruth H. 2017. Antibiotic-independent adaptive effects of antibiotic resistance mutations. Trends Genet 33:521–528. 10.1016/j.tig.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Wolloscheck D, Krishnamoorthy G, Nguyen J, Zgurskaya HI. 2018. Kinetic control of quorum sensing in Pseudomonas aeruginosa by multidrug efflux pumps. ACS Infect Dis 4:185–195. 10.1021/acsinfecdis.7b00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bou G, Martinez-Beltran J. 2000. Cloning, nucleotide sequencing, and analysis of the gene encoding an AmpC-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 44:428–432. 10.1128/aac.44.2.428-432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers RA, Swanson HC, Taracila MA, Florek NW, Romagnoli C, Caselli E, Prati F, Bonomo RA, Wallar BJ. 2014. Biochemical and structural analysis of inhibitors targeting the ADC-7 cephalosporinase of Acinetobacter baumannii. ACS Biochem 53:7670–7679. 10.1021/bi500887n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon JH, Hong M-K, Lee JH, Lee JJ, Park KS, Karim AM, Jo JY, Kim JH, Ko KS, Kang L-W, Lee SH. 2014. Structure of ADC-68, a novel carbapenem-hydrolyzing class C extended-spectrum β-lactamase isolated from Acinetobacter baumannii. Acta Crystallogr D Biol Crystallogr 70:2924–2936. 10.1107/S1399004714019543. [DOI] [PubMed] [Google Scholar]
- 18.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, Macdonald IJ, Martin KM, Russo T, Campagnari AA, Hujer AM, Bonomo RA, Gill SR. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol 190:8053–8064. 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neonakis IK, Spandidos DA, Petinaki E. 2011. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents 37:102–109. 10.1016/j.ijantimicag.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Varela M, Corral J, Aranda J, Barbé J. 2019. Roles of efflux pumps from different superfamilies in the surface-associated motility and virulence of Acinetobacter baumannii ATCC 17978. Antimicrob Agents Chemother 63:e02190-18. 10.1128/AAC.02190-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJ, Luisi BF. 2018. Multidrug efflux pumps: structure, function and regulation. Nat Rev Microbiol 16:523–539. 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 23.Williams CL, Neu HM, Gilbreath JJ, Michel SLJ, Zurawski DV, Merrell DS. 2016. Copper resistance of the emerging pathogen Acinetobacter baumannii. Appl Environ Microbiol 82:6174–6188. 10.1128/AEM.01813-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law CJ, Maloney PC, Wang D-N. 2008. Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol 62:289–305. 10.1146/annurev.micro.61.080706.093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca I, Marti S, Espinal P, Martínez P, Gibert I, Vila J. 2009. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 53:4013–4014. 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajamohan G, Srinivasan VB, Gebreyes WA. 2010. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J Antimicrob Chemother 65:1919–1925. 10.1093/jac/dkq195. [DOI] [PubMed] [Google Scholar]
- 27.Hassan KA, Cain AK, Huang T, Liu Q, Elbourne LDH, Boinett CJ, Brzoska AJ, Li L, Ostrowski M, Nhu NTK, Nhu TDH, Baker S, Parkhill J, Paulsen IT. 2016. Fluorescence-based flow sorting in parallel with transposon insertion site sequencing identifies multidrug efflux systems in Acinetobacter baumannii. mBio 7:e01200-16. 10.1128/mBio.01200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A, Sharma R, Bhattacharyya T, Bhando T, Pathania R. 2017. Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a major facilitator superfamily (MFS) transporter—AbaF. J Antimicrob Chemother 72:68–74. 10.1093/jac/dkw382. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Varela M, Corral J, Aranda J, Barbé J. 2018. Functional characterization of AbaQ, a novel efflux pump mediating quinolone resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 62:e00906-18. 10.1128/AAC.00906-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foong WE, Wilhelm J, Tam H-K, Pos KM. 2020. Tigecycline efflux in Acinetobacter baumannii is mediated by TetA in synergy with RND-type efflux transporters. J Antimicrob Chemother 75:1135–1139. 10.1093/jac/dkaa015. [DOI] [PubMed] [Google Scholar]
- 31.Fournier P-E, Vallenet D, Rie Barbe V, Phane Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie J-M. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulsen IT, Skurray RA, Tam R, Saier MH, Turner RJ, Weiner JH, Goldberg EB, Grinius LL. 1996. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol 19:1167–1175. 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 33.Bay DC, Rommens KL, Turner RJ. 2008. Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta 1778:1814–1838. 10.1016/j.bbamem.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan BV, Rajamohan G, Gebreyes WA. 2009. Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob Agents Chemother 53:5312–5316. 10.1128/AAC.00748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajamohan G, Srinivasan VB, Gebreyes WA. 2010. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J Antimicrob Chemother 65:228–232. 10.1093/jac/dkp427. [DOI] [PubMed] [Google Scholar]
- 36.Abuzaid A, Hamouda A, Amyes SGB. 2012. Klebsiella pneumoniae susceptibility to biocides and its association with cepA, qacDE and qacE efflux pump genes and antibiotic resistance. J Hosp Infect 81:87–91. 10.1016/j.jhin.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Yu T, Liu L, Li Y, Zhang K, Wang H, Shi L. 2017. Examination of quaternary ammonium compound resistance in Proteus mirabilis isolated from cooked meat products in China. Front Microbiol 8:2417. 10.3389/fmicb.2017.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen IT, Littlejohn TG, Radstrom P, Sundstrom L, Skold O, Swedberg G, Skurray RA. 1993. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother 37:761–768. 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomaa F, Helal Z, Khan M. 2017. High prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 genes and their association with decreased susceptibility to antibiotics and common hospital biocides in clinical isolates of Acinetobacter baumannii. Microorganisms 5:18. 10.3390/microorganisms5020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu WJ, Fu L, Huang M, Zhang JP, Wu Y, Zhou YS, Zeng J, Wang GX. 2017. Frequency of antiseptic resistance genes and reduced susceptibility to biocides in carbapenem-resistant Acinetobacter baumannii. J Med Microbiol 66:13–17. 10.1099/jmm.0.000403. [DOI] [PubMed] [Google Scholar]
- 41.Lin F, Xu Y, Chang Y, Liu C, Jia X, Ling B. 2017. Molecular characterization of reduced susceptibility to biocides in clinical isolates of Acinetobacter baumannii. Front Microbiol 8:1836. 10.3389/fmicb.2017.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson AL, Chen J. 2004. ATP-binding cassette transporters in bacteria. Annu Rev Biochem 73:241–268. 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 43.Davidson AL, Dassa E, Orelle C, Chen J. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72:317–364. 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greene NP, Kaplan E, Crow A, Koronakis V. 2018. Antibiotic resistance mediated by the MacB ABC transporter family: a structural and functional perspective. Front Microbiol 9:950. 10.3389/fmicb.2018.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crow A, Greene NP, Kaplan E, Koronakis V. 2017. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proc Natl Acad Sci U S A 114:12572–12577. 10.1073/pnas.1712153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaswamy VK, Vargiu AV, Malloci G, Dreier J, Ruggerone P. 2017. Molecular rationale behind the differential substrate specificity of bacterial RND multi-drug transporters. Sci Rep 7:8075. 10.1038/s41598-017-08747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, Nation RL, Li J, Harper M, Adler B, Boyce JD. 2012. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-β-1,6-N-acetylglucosamine. Antimicrob Agents Chemother 56:59–69. 10.1128/AAC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin M-F, Lin Y-Y, Lan C-Y. 2015. The role of the two-component system BaeSR in disposing chemicals through regulating transporter systems in Acinetobacter baumannii. PLoS One 10:e0132843. 10.1371/journal.pone.0132843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown MH, Paulsen IT, Skurray RA. 1999. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol 31:394–395. 10.1046/j.1365-2958.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 50.Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. 2006. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci 27:587–593. 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Su X-Z, Chen J, Mizushima T, Kuroda T, Tsuchiya T. 2005. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. AAC 49:4362–4364. 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung H-W, Kim K, Islam MM, Lee JC, Shin M. 2020. Role of ppGpp-regulated efflux genes in Acinetobacter baumannii. J Antimicrob Chemother 75:1130–1134. 10.1093/jac/dkaa014. [DOI] [PubMed] [Google Scholar]
- 53.Hassan KA, Jackson SM, Penesyan A, Patching SG, Tetu SG, Eijkelkamp BA, Brown MH, Henderson PJF, Paulsen IT. 2013. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc Natl Acad Sci U S A 110:20254–20259. 10.1073/pnas.1317052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hassan KA, Liu Q, Henderson PJF, Paulsen IT. 2015. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio 6:e01982-14. 10.1128/mBio.01982-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassan KA, Naidu V, Edgerton JR, Mettrick KA, Liu Q, Fahmy L, Li L, Jackson SM, Ahmad I, Sharples D, Henderson PJF, Paulsen IT. 2019. Short-chain diamines are the physiological substrates of PACE family efflux pumps. Proc Natl Acad Sci U S A 116:18015–18020. 10.1073/pnas.1901591116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Hassan KA, Ashwood HE, Gamage HKAH, Li L, Mabbutt BC, Paulsen IT. 2018. Regulation of the aceI multidrug efflux pump gene in Acinetobacter baumannii. J Antimicrob Chemother 73:1492–1500. 10.1093/jac/dky034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolla JR, Howes AC, Fiorentino F, Robinson CV. 2020. Assembly and regulation of the chlorhexidine-specific efflux pump AceI. Proc Natl Acad Sci U S A 117:17011–17018. 10.1073/pnas.2003271117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan KA, Liu Q, Elbourne LDH, Ahmad I, Sharples D, Naidu V, Chan CL, Li L, Harborne SPD, Pokhrel A, Postis VLG, Goldman A, Henderson PJF, Paulsen IT. 2018. Pacing across the membrane: the novel PACE family of efflux pumps is widespread in Gram-negative pathogens. Res Microbiol 169:450–454. 10.1016/j.resmic.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putman M, Van Veen HW, Konings WN. 2000. Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev 64:672–693. 10.1128/MMBR.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piddock LV. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol 4:629–636. 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 61.Magnet S, Courvalin P, Lambert T. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob Agents Chemother 45:3375–3380. 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu L-J, Pan Y, Gao C-Y, Hou P-F. 2020. Distribution of carbapenemases and efflux pump in carbapenem-resistance Acinetobacter baumannii. Ann Clin Lab Sci 50:241–246. [PubMed] [Google Scholar]
- 63.Yoon EJ, Courvalin P, Grillot-Courvalin C. 2013. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob Agents Chemother 57:2989–2995. 10.1128/AAC.02556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roe C, Williamson CHD, Vazquez AJ, Kyger K, Valentine M, Bowers JR, Phillips PD, Harrison V, Driebe E, Engelthaler DM, Sahl JW. 2020. Bacterial genome wide association studies (bGWAS) and transcriptomics identifies cryptic antimicrobial resistance mechanisms in Acinetobacter baumannii. Front Public Health 8:451. 10.3389/fpubh.2020.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leus IV, Adamiak J, Trinh AN, Smith RD, Smith L, Richardson S, Ernst RK, Zgurskaya HI. 2020. Inactivation of AdeABC and AdeIJK efflux pumps elicits specific nonoverlapping transcriptional and phenotypic responses in Acinetobacter baumannii. Mol Microbiol 114:1049–1065. 10.1111/mmi.14594. [DOI] [PubMed] [Google Scholar]
- 66.Leus CV, Weeks IV, Bonifay JW, Smith V, Richardson L, Zgurskaya SI. 2018. Substrate specificities and efflux efficiencies of RND efflux pumps of Acinetobacter baumannii. J Bacteriol 200:e00049-18. 10.1128/JB.00049-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poole K. 2013. Pseudomonas aeruginosa efflux pumps, p 175–206. In Microbial efflux pumps. Yu E, Zhang Q, Brown MH (ed). Caister Academic Press, Norfolk, UK. [Google Scholar]
- 68.Lin M-F, Lin Y-Y, Yeh H-W, Lan C-Y. 2014. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol 14:119. 10.1186/1471-2180-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams FG, Stroeher UH, Hassan KA, Marri S, Brown MH. 2018. Resistance to pentamidine is mediated by AdeAB, regulated by AdeRS, and influenced by growth conditions in Acinetobacter baumannii ATCC 17978. PLoS One 13:e0197412. 10.1371/journal.pone.0197412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon E-J, Nait Chabane Y, Goussard S, Snesrud E, Courvalin P, Dé E, Grillot-Courvalin C. 2015. Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. mBio 6:e00309-15. 10.1128/mBio.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother 48:3298–3304. 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiwari S, Jamal SB, Hassan SS, Carvalho PVSD, Almeida S, Barh D, Ghosh P, Silva A, Castro TLP, Azevedo V. 2017. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: an overview. Front Microbiol 8:1878. 10.3389/fmicb.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Silva MP, Kumar A. 2018. Effect of sodium chloride on surface-associated motility of Acinetobacter baumannii and the role of AdeRS two-component system. J Membr Biol 251:5–13. 10.1007/s00232-017-9985-7. [DOI] [PubMed] [Google Scholar]
- 74.Richmond GE, Evans LP, Anderson MJ, Wand ME, Bonney LC, Ivens A, Chua KL, Webber MA, Mark SJ, Peterson ML, Piddock LJV. 2016. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. mBio 7:e00430-16. 10.1128/mBio.00430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quinn B, Rodman N, Jara E, Fernandez JS, Martinez J, Traglia GM, Montaña S, Cantera V, Place K, Bonomo RA, Iriarte A, Ramírez MS. 2018. Human serum albumin alters specific genes that can play a role in survival and persistence in Acinetobacter baumannii. Sci Rep 8:14741. 10.1038/s41598-018-33072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Modarresi F, Azizi O, Shakibaie MR, Motamedifar M, Valibeigi B, Mansouri S. 2015. Effect of iron on expression of efflux pump adeABC and quorum sensing lux I, lux R genes in clinical isolates of Acinetobacter baumannii. APMIS 123:959–968. 10.1111/apm.12455. [DOI] [PubMed] [Google Scholar]
- 77.Chau S-L, Chu Y-W, Houang ETS. 2004. Novel resistance-nodulation-cell division efflux system AdeDE in Acinetobacter genomic DNA group 3. Antimicrob Agents Chemother 48:4054–4055. 10.1128/AAC.48.10.4054-4055.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coyne S, Rosenfeld N, Lambert T, Courvalin P, Perichon B. 2010. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 54:4389–4393. 10.1128/AAC.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernando D, Zhanel G, Kumar A. 2013. Antibiotic resistance and expression of resistance-nodulation-division pump and outer membrane porin-encoding genes in Acinetobacter species isolated from canadian hospitals. Can J Infect Dis Med Microbiol 24:17–21. 10.1155/2013/696043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoon E-J, Balloy V, Fiette L, Chignard M, Courvalin P, Grillot-Courvalin C. 2016. Contribution of the Ade resistance-nodulation-cell division-type efflux pumps to fitness and pathogenesis of Acinetobacter baumannii. mBio 7:e00697-16. 10.1128/mBio.00697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He X, Lu F, Yuan F, Jiang D, Zhao P, Zhu J, Cheng H, Cao J, Lu G. 2015. Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob Agents Chemother 59:4817–4825. 10.1128/AAC.00877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maddocks SE, Oyston PC, Sarah Maddocks CE. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology (Reading) 154:3609–3623. 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 83.Hare JM, Bradley JA, Lin CL, Elam TJ. 2012. Diverse responses to UV light exposure in Acinetobacter include the capacity for DNA damage-induced mutagenesis in the opportunistic pathogens Acinetobacter baumannii and Acinetobacter ursingii. Microbiology (Reading) 158:601–611. 10.1099/mic.0.054668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peterson MA, Grice AN, Hare JM. 2020. A corepressor participates in LexA-independent regulation of error-prone polymerases in Acinetobacter. Microbiology (Reading) 166:212–226. 10.1099/mic.0.000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Damier-Piolle L, Magnet S, Brémont S, Lambert T, Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother 52:557–562. 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenfeld N, Bouchier C, Courvalin P, Périchon B. 2012. Expression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulator. Antimicrob Agents Chemother 56:2504–2510. 10.1128/AAC.06422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang J-H, Hassan KA, Begg SL, Rupasinghe TWT, Naidu V, Pederick VG, Khorvash M, Whittall JJ, Paton JC, Paulsen IT, McDevitt CA, Peleg AY, Eijkelkamp BA. 2019. Identification of novel Acinetobacter baumannii host fatty acid stress adaptation strategies. mBio 10:e02056-18. 10.1128/mBio.02056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saranathan R, Pagal S, Sawant AR, Tomar A, Madhangi M, Sah S, Satti A, Arunkumar KP, Prashanth K. 2017. Disruption of tetR type regulator adeN by mobile genetic element confers elevated virulence in Acinetobacter baumannii. Virulence 8:1316–1334. 10.1080/21505594.2017.1322240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. 2005. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev 69:326–356. 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu YW, Chau SL, Houang ETS. 2006. Presence of active efflux systems AdeABC, AdeDE and AdeXYZ in different Acinetobacter genomic DNA groups. J Med Microbiol 55:477–478. 10.1099/jmm.0.46433-0. [DOI] [PubMed] [Google Scholar]
- 91.Roca I, Espinal P, Martí S, Vila J. 2011. First identification and characterization of an AdeABC-like efflux pump in Acinetobacter genomospecies 13TU. Antimicrob Agents Chemother 55:1285–1286. 10.1128/AAC.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Srinivasan VB, Venkataramaiah M, Mondal A, Rajamohan G. 2015. Functional characterization of AbeD, an RND-type membrane transporter in antimicrobial resistance in Acinetobacter baumannii. PLoS One 10:e0141314. 10.1371/journal.pone.0141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tipton KA, Farokhyfar M, Rather PN. 2017. Multiple roles for a novel RND-type efflux system in Acinetobacter baumannii AB5075. MicrobiologyOpen 6:e00418. 10.1002/mbo3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tipton KA, Dimitrova D, Rather PN. 2015. Phase-variable control of multiple phenotypes in Acinetobacter baumannii strain AB5075. J Bacteriol 197:2593–2599. 10.1128/JB.00188-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Subhadra B, Kim J, Kim DH, Woo K, Oh MH, Choi CH. 2018. Local repressor AcrR regulates AcrAB efflux pump required for biofilm formation and virulence in Acinetobacter nosocomialis. Front Cell Infect Microbiol 8:270. 10.3389/fcimb.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Subhadra B, Surendran S, Lim BR, Yim JS, Kim DH, Woo K, Kim H-J, Oh MH, Choi CH. 2020. Regulation of the AcrAB efflux system by the quorum-sensing regulator AnoR in Acinetobacter nosocomialis. J Microbiol 58:507–518. 10.1007/s12275-020-0185-2. [DOI] [PubMed] [Google Scholar]
- 97.Dik DA, Fisher JF, Mobashery S. 2018. Cell-wall recycling of the Gram-negative bacteria and the nexus to antibiotic resistance. Chem Rev 118:5952–5984. 10.1021/acs.chemrev.8b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakashima R, Sakurai K, Yamasaki S, Hayashi K, Nagata C, Hoshino K, Onodera Y, Nishino K, Yamaguchi A. 2013. Structural basis for the inhibition of bacterial multidrug exporters. Nature 500:102–106. 10.1038/nature12300. [DOI] [PubMed] [Google Scholar]
- 99.Opperman TJ, Nguyen ST. 2015. Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol 6:421. 10.3389/fmicb.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang D, Lim Y-H. 2019. Resveratrol controls Escherichia coli growth by inhibiting the AcrAB-TolC efflux pump. FEMS Microbiol Lett 366:fnz030. 10.1093/femsle/fnz030. [DOI] [PubMed] [Google Scholar]
- 101.Singkham-In U, Higgins PG, Wannigama DL, Hongsing P, Chatsuwan T. 2020. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS One 15:e0243082. 10.1371/journal.pone.0243082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elbourne LDH, Tetu SG, Hassan KA, Paulsen IT. 2017. TransportDB 2.0: a database for exploring membrane transporters in sequenced genomes from all domains of life. Nucleic Acids Res 45:D320–D324. 10.1093/nar/gkw1068. [DOI] [PMC free article] [PubMed] [Google Scholar]