Abstract
Objectives
To determine the prevalence of renal impairment in a large cohort of youths with histologically confirmed nonalcoholic fatty liver disease (NAFLD), and to determine its association with liver disease severity.
Study design
Clinical, laboratory, and histology data were collected retrospectively in a pediatric cohort with biopsy-confirmed NAFLD at a tertiary care center between 2010 and 2017. Histological NAFLD severity was scored using validated criteria. Glomerular filtration rate (GFR) was calculated and categorized as low (<90 mL/min/1.73 m2), normal (90–136 mL/min/1.73 m2), or high (>136 mL/min/1.73 m2). Univariate and multivariate modeling were used to determine differences between the GFR groups and to control for confounders.
Results
The cohort comprised 179 patients (82% non-Hispanic; median age; 14 years; IQR, 12–16 years). One-third of the patients had abnormal renal function, including 36 (20%) with glomerular hyperfiltration and 26 (15%) with low GFR. In multivariable logistic regression, compared with normal GFR, hyperfiltration was independently associated with higher NAFLD activity score (aOR, 2.96; 95% CI, 1.49–5.87; P = .002), after adjusting for age, sex, ethnicity, obesity severity, presence of type 2 diabetes mellitus, and medications.
Conclusions
In this large cohort with histologically confirmed NAFLD, renal impairment was highly prevalent and associated with liver disease severity, independent of obesity severity. Screening patients with confirmed NAFLD for renal complication is recommended.
Nonalcoholic fatty liver disease (NAFLD) is closely linked to obesity and is considered the hepatic manifestation of the metabolic syndrome.1 Both obesity and specific components of the metabolic syndrome frequently encountered in patients with NAFLD, such as insulin resistance and hypertension, are associated with renal impairment.2–7 Thus, it is unsurprising that chronic kidney disease is highly prevalent in adults with NAFLD, affecting up to 20%-55% of patients.8 NAFLD is currently the leading adult indication for concurrent liver and kidney transplantation, further underscoring the link between these 2 conditions.9,10 Renal impairment does not simply coexist with NAFLD; in adult cohorts, it is also associated with histological liver disease severity.11 In children, despite a high prevalence of NAFLD (10% in the general US population alone), the literature on the renal function of these patients is limited and controversial.12,13 In contrast to the findings of adult cohorts with NAFLD, Manco et al previously found no association between liver histology and renal impairment in 80 children with biopsy-confirmed NAFLD.13 They also reported no links between measures of insulin resistance and renal function.
Most often, the severity of renal impairment is determined based on the glomerular filtration rate (GFR). In clinical practice, the GFR is typically calculated using the modified Schwartz equation; however, it can also be calculated using cystatin C-based estimates or even measured with nuclear medicine scans.14 The first stage of renal impairment is glomerular hyperfiltration, which is most often defined as a GFR >130 mL/min/1.73 m2.15–17 Glomerular hyperfiltration is prevalent in adults with conditions associated with NAFLD, such as obesity,18,19 prehypertension and impaired glucose tolerance,19 type 2 diabetes mellitus (T2DM),20,21 and hypertension,22–24 as well as in children with obesity-related hypertension 25 and insulin resistance.26 Glomerular hyperfiltration is hypothesized to be a precursor of intraglomerular hypertension, which ultimately leads to albuminuria. Over time, albuminuria increases, and GFR declines, leading to progressive renal dysfunction and ultimately end-stage renal disease.27 Screening high-risk patients for hyperfiltration can be beneficial, given that early intervention with medications, such as angiotensin receptor inhibitors, can prevent and/or delay progression to more severe renal disease.28–30
The objectives of this study were to investigate the prevalence and severity of renal impairment in a large cohort of children with histologically confirmed NAFLD, and to determine whether renal impairment is associated with more advanced liver disease.
Methods
This retrospective study was performed at Cincinnati Children’s Hospital Medical Center. Institutional Review Board approval and a waiver of informed consent were obtained before the initiation of data collection. Inclusion criteria were age ≤18 years, histologically confirmed NAFLD on liver biopsy (August 2010 to December 2017), and available serum creatinine level measured within 3 months of the liver biopsy. Exclusion criteria were other concurrent liver diseases (eg, autoimmune hepatitis, Wilson disease), known chronic kidney disease secondary to unrelated (non-obesity/NAFLD) causes, and steatohepatitis secondary to genetic or metabolic causes (eg, lipodystrophy, lysosomal acid lipase deficiency).
Electronic medical records were reviewed to collect demographic data (ie, age, sex, and ethnicity) and clinical characteristics (eg, anthropometrics, other diagnoses, concurrent medications) at the time of liver biopsy. Laboratory data available within 3 months of the liver biopsy (and closest to the biopsy), including serum levels of alanine aminotransferase (ALT), aspartate aminotransferase, gamma glutamyl transferase, alkaline phosphatase, hemoglobin A1c (HbA1c), albumin, prothrombin time, platelet count, and creatinine level, were also collected.
At our institution, patients typically undergo liver biopsy when more severe NAFLD is suspected, as in the case of persistently elevated ALT >50 U/L for 3–6 months and lack of body mass index (BMI) improvement, concurrent diagnosis of metabolic syndrome or obstructive sleep apnea, imaging evidence of splenomegaly and/or increased liver stiffness on magnetic resonance elastography, or when a different liver disease is also being strongly considered (eg, autoimmune hepatitis).
Definition of Estimated GFR and GFR Classification
Estimated GFR (eGFR) was calculated using the modified Schwartz equation: eGFR = 0.413 × [height (cm)/serum creatinine (mg/dL)].31 Patients were classified according to eGFR level as low GFR (<90 mL/min/1.73 m2),32 normal GFR (90–136 mL/min/1.73 m2), or high GFR (ie, glomerular hyperfiltration; >136 mL/min/1.73 m2). The cutoff for hyperfiltration used in this study was the average of most studies in the field,33,34 supported by a systematic review reporting the majority of study cutoffs values at 130–140 mL/min/1.73 m2.15
Definitions of Clinical and Histological Variables
Using BMI percentiles for age and sex based on Centers for Disease Control and Prevention growth charts, we classified the patients as overweight (BMI 85th-<95th percentile), obese class I (BMI 95th percentile to <120% of the 95th percentile), severely obese class II (BMI 120%-<140% of the 95th percentile), or severely obese class III (BMI ≥140% of the 95th percentile).35 A diagnosis of T2DM was confirmed by an oral glucose tolerance test with plasma glucose >200 mg/dL at 2 hours, hemoglobin A1C (HbA1c) value >6.4%, or confirmation of T2DM diagnosis by an endocrinologist.36 The classification developed by Kleiner et al was used to grade histological steatosis (scored as 0–3), lobular inflammation (0–3), and hepatocyte ballooning (0–2) and to stage fibrosis (0–4), and the NAFLD Activity Score (NAS) was calculated as a sum of the scores for steatosis, lobular inflammation, and ballooning degeneration.37 For the purpose of our analyses, an NAS of ≥5 was used as a cutoff to distinguish mild vs severe liver disease.38 Significant fibrosis was defined as fibrosis stage 2 or greater.
Statistical Analyses
Demographic and other characteristic variables were summarized as descriptive statistics. ORs and 95% CIs with P values were reported. The main statistical model used to study the relationships of GFR categories with other variables was the multinomial logistic model, in which 3 categories were modeled in a general logit function, with the normal GFR category as the reference category. ORs were calculated for both the low and high GFR groups separately relative to the normal group. This logistic model was used either in single variable or multiple independent variable settings. For categorical independent variables, we applied the Fisher exact test to investigate their associations with GFR categorical responses. Stepwise selection procedures were applied when multiple independent variables were considered. The potential confounders adjusted for were age, sex, ethnicity, obesity severity, presence of T2DM, and medications used at the time of liver biopsy. Statistically nonsignificant variables were eliminated from final models to maintain parsimoniousness. Statistical significance was claimed at a P value <.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
The study cohort comprised 179 children with biopsy-confirmed NAFLD. The median patient age was 14 years (range, 2–18 years); 64% were male, and 18% were Hispanic. The majority of patients had severe obesity (n = 102; 77%). The clinical, laboratory, and histological characteristics of the study cohort are presented in Table I. The median eGFR was 110 mL/min/1.73 m2 (range, 51–203 mL/min/1.73 m2; IQR, 96–129 mL/min/1.73 m2). Sixty-two patients (35%) had some degree of renal impairment (Table I); of these patients, 5 (8%) were prescribed antihypertensive medication (lisinopril in 2 and clonidine in 3).
Table I.
Demographic and baseline clinical, laboratory, and histological characteristics of the study cohort (N = 179)
| Characteristics | Values |
|---|---|
| Age, y, median (IQR) | 14 (12–16) |
| Male sex, n (%) | 115 (64) |
| Ethnicity, n (%) | |
| Hispanic | 47 (18) |
| Non-Hispanic | 147 (82) |
| BMI, kg/m2, median (IQR) | 36 (31.5–40.5) |
| Obesity classification, n (%) | |
| Overweight | 2 (1) |
| Obese, class I | 38 (21) |
| Severely obese, class II | 55 (31) |
| Severely obese, class III | 82 (46) |
| T2DM, n (%) | 20 (11) |
| Medication use at time of biopsy, n (%) | |
| Insulin | 8 (4) |
| Metformin | 41 (23) |
| Vitamin E | 6 (3) |
| Statins | 2 (1) |
| Antihypertensive drugs | 19 (11) |
| ACE inhibitors (lisinopril, enalapril) | 11 (58) |
| Amlodipine | 1 (5) |
| Clonidine | 7 (37) |
| Laboratory data, median (IQR) | |
| ALT, U/L | 85 (60–123) |
| AST, U/L | 44.5 (34–70) |
| GGT, U/L | 46 (35–74) |
| ALP, U/L | 183 (135–235) |
| HbA1c, % | 5.2 (5.0–5.5) |
| Albumin, g/dL | 4.1 (3.9–4.4) |
| Prothrombin-INR | 0.99 (0.95–1.03) |
| Platelet count, ×109/L | 312 (257–346) |
| eGFR, mL/min/1.73 m2, median (IQR) | 110 (96–129) |
| GFR status, n (%) | |
| Low GFR | 26 (15) |
| Normal GFR | 117 (65) |
| High GFR (glomerular hyperfiltration) | 36 (20) |
| Histology data | |
| Steatosis score, median (IQR) | 2 (1–3) |
| Lobular inflammation score, median (IQR) | 1 (1–2) |
| Hepatocellular ballooning score, median (IQR) | 1 (0–1) |
| NAS, median (IQR) | 4 (3–5) |
| NAS ≥5, n (%) | 70 (39) |
| Portal inflammation score, median (IQR) | 1 (0–1) |
| Fibrosis stage, median (IQR) | 1 (0–1) |
| Fibrosis stage ≥2, n (%) | 14 (8) |
ACE, angiotensin-converting enzyme; ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; INR, international normalized ratio.
The patients were subsequently divided into 3 GFR groups (high, normal, and low) and their demographic, clinical, laboratory, and histological characteristics were compared (Table II). The GFR groups differed in terms of patient age at the time of liver biopsy; however, the remaining clinical, demographic, and laboratory characteristics were not significantly different. Liver disease severity differed among the groups, as shown by a higher median NAS in patients with hyperfiltration (NAS, 5; IQR, 4–6) compared with patients with normal GFR (NAS, 4; IQR, 2–5; P = .042 compared with the hyperfiltration group) and low GFR (NAS, 3; IQR, 3–4; P = .02 compared with the hyperfiltration group). The NAS distribution by GFR category is depicted in the Figure.
Table II.
Demographic, clinical, laboratory, and histological characteristics of patients grouped by GFR status
| Variables | Glomerular hyperfiltration (N = 36) | Normal GFR (N = 117) | Low GFR (N = 26) | P value |
|---|---|---|---|---|
| Age, y, median (IQR) | 12 (11–14) | 15 (11–16) | 16 (16–17) | <.001 |
| Male sex, n (%) | 25 (69) | 72 (62) | 18 (69) | .64 |
| Hispanic ethnicity, n (%) | 8 (22) | 22 (19) | 2 (8) | .31 |
| Obesity, n (%) | .63 | |||
| Overweight | 1 (3) | 1 (1) | 0 (0) | |
| Obese class I | 8 (22) | 26 (23) | 4 (15) | |
| Severe obesity class II | 8 (22) | 39 (34) | 8 (31) | |
| Severe obesity class III | 19 (53) | 49 (42) | 14 (54) | |
| T2DM, n (%) | 7 (19) | 11 (9) | 2 (8) | .24 |
| Medications at time of biopsy, n (%) | ||||
| Insulin | 4 (11) | 4 (3) | 0 (0) | .11 |
| Metformin | 10 (28) | 25 (21) | 6 (23) | .70 |
| Vitamin E | 1 (3) | 4 (3) | 1 (4) | 1.00 |
| Statins | 0 (0) | 1 (1) | 1 (4) | .31 |
| Antihypertensive drugs | 3 (8) | 14 (12) | 2 (8) | .82 |
| ACE inhibitors | 2 (6) | 9 (8) | 0 (0) | .21 |
| Amlodipine or clonidine | 1 (2) | 5 (4) | 2 (8) | .21 |
| Laboratory data, median (IQR) | ||||
| eGFR, mL/min/1.73 m2 | 151 (140–172) | 109 (99–119) | 85 (78–86) | <.01 |
| ALT, U/L | 90 (71–141) | 85 (58–121) | 77 (56–112) | .38 |
| AST, U/L | 47.5 (34.5–100) | 45 (34–68) | 42 (37–61) | .14 |
| GGT, U/L | 50 (36–77) | 45 (32–70) | 52 (36–71) | .56 |
| ALP, U/L | 201 (136–238) | 179 (131–233) | 176 (132–249) | .82 |
| HbA1c, % | 5.2 (5.0–5.5) | 5.3 (3.0–5.6) | 5.2 (5.0–5.5) | .15 |
| Albumin, g/dL | 4.2 (3.9–4.4) | 4.1 (3.9–4.3) | 4.1 (3.8–4.4) | .80 |
| Prothrombin-INR | 1.0 (0.94–1.05) | 0.99 (0.95–1.03) | 0.99 (0.97–1.05) | .40 |
| Platelet count, ×109/L | 300 (249–324) | 315 (270–372) | 313 (234–317) | .33 |
| Histology data | ||||
| Steatosis score, median (IQR) | 3 (2–3) | 2 (1–3) | 1.5 (1–3) | .12 |
| Lobular inflammation score, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (0–1) | .28 |
| Ballooning score, median (IQR) | 1 (0–1) | 1 (0–1) | 1 (0–1) | .39 |
| NAS, median (IQR) | 5 (4–6) | 4 (2–5) | 3 (3–4) | .01 |
| NAS ≥5, n (%) | 20 (56) | 44 (38) | 6 (23) | .01 |
| Portal inflammation score, median (IQR) | 1 (1–1) | 1 (0–1) | 1 (0–1) | .24 |
| Fibrosis stage, median (IQR) | 1 (0–1) | 1 (0–2) | 0 (0–1) | .16 |
| Fibrosis ≥2, n (%) | 1 (3) | 11 (9) | 2 (8) | .11 |
Figure.
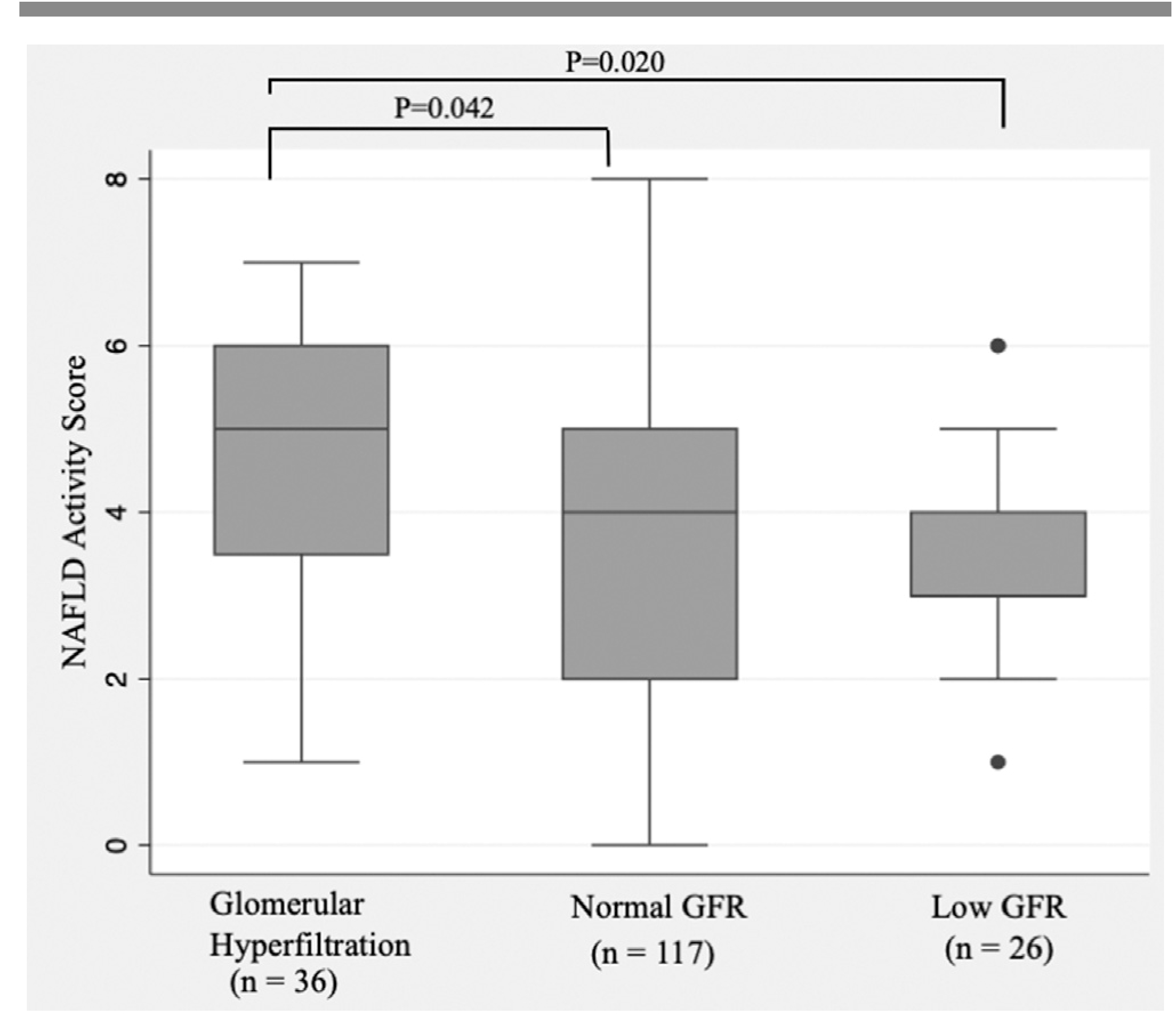
Relationship between median GFR categories and NAS in the study cohort.
In multivariable logistic regression analyses, glomerular hyperfiltration was associated with greater odds of a higher NAS (aOR, 2.96; 95% CI, 1.49–5.87; P = .002) and greater odds of NAS ≥5 (aOR, 2.58; 95% CI, 1.19–5.59; P = .002) compared with normal GFR, after adjusting for age, sex, ethnicity, obesity severity, presence of T2DM, and medications (Table III).
Table III.
Multivariable logistic regression model for predicting NAS
| Variables | Point estimates | Wald 95% CIs | P value |
|---|---|---|---|
| OR estimates of NAS* | |||
| High vs normal GFR | 2.957 | 1.489–5.870 | .002 |
| Low vs normal GFR | 0.652 | 0.307–1.384 | .266 |
| OR estimates of NAS ≥5* | |||
| High vs normal GFR | 2.582 | 1.193–5.589 | .016 |
| Low vs normal GFR | 0.383 | 0.127–1.154 | .088 |
Adjusted for age, sex, ethnicity, T2DM, obesity severity, and drug use based on model selection results.
Discussion
In a cohort of 179 children and adolescents with biopsy-confirmed NAFLD, we found that 20% had glomerular hyperfiltration and 15% had low eGFR within 3 months of their liver biopsy. Furthermore, glomerular hyperfiltration was associated with a higher NAS, independent of traditional risk factors, such as obesity severity, presence of T2DM, and antihypertensive drug use. This suggests that glomerular hyperfiltration should be taken into consideration when risk-stratifying patients with presumed NAFLD.
Our results confirm the association between renal impairment and histological severity that has been previously reported in the adult literature.11 However, our data are in contrast with the single previous pediatric report on the topic. Manco et al studied a cohort of 80 children with biopsy-confirmed NAFLD and compared their creatinine clearance and albuminuria against values of 59 age- and sex-matched normal-weight children without NAFLD.13 Although the groups differed in terms of insulin sensitivity, their renal function did not differ. In that study, renal function was not grouped using GFR cutoffs, so the association between glomerular hyperfiltration and liver disease severity was not evaluated. In another study, Pacifico et al studied 268 children with presumed NAFLD diagnosed by magnetic resonance imaging and demonstrated that obese patients with NAFLD were more likely to have decreased GFR (<90 mL/min/1.73 m2) compared with children with obesity without NAFLD and lean controls.12 Although this study underscored the association between NAFLD and renal impairment, it did not include patients with histologically confirmed NAFLD and as such could not address whether liver disease severity and renal impairment were linked. To expand this field further, future studies should include a combination of cystatin C-based calculations of GFR or technetium-99m diethylenetriaminepentacetate clearance, as well as assessment of microalbuminuria, to provide a more detailed evaluation of the renal function of patients with histologically confirmed NAFLD.
The mechanism linking renal impairment and liver disease severity is not entirely understood. It is possible that they are both the end result of the same “hit.” The renin-angiotensin system may play a key role in this process. In the liver, angiotensin II type 1 (AT1) receptor activation promotes steatosis through the stimulation of de novo lipogenesis and inhibition of fatty acid oxidation.39,40 AT1 receptor activation also promotes hepatic insulin resistance, oxidative stress, and synthesis of proinflammatory and profibrogenic cytokines, thereby potentially contributing to the entire spectrum of liver injury seen in NAFLD/nonalcoholic steatohepatitis.39,40 In the kidney, AT1 receptor activation promotes glomerular efferent arteriole vasoconstriction, glomerulosclerosis, and glomerular injury through reactive oxygen species and cytokine production.40 Notably, there is an ongoing randomized, placebo-controlled, double blind clinical trial investigating an AT1 receptor blocker, losartan, for the treatment of NAFLD in children (ClinicalTrials.gov identifier NCT03467217). It remains to be seen whether this approach can ameliorate both the liver and renal impairment of children with NAFLD/nonalcoholic steatohepatitis.
Fructose may be another common variable contributing to both liver and renal impairment in patients with NAFLD. Excess consumption of fructose is known to contribute to hepatic steatosis and fibrosis.40–43 Fructose can also elevate blood pressure and increase serum uric acid levels, both factors known to be associated with renal impairment.44 In fact, several observational studies have suggested that high fructose intake may be associated with the incidence and severity of both renal impairment and NAFLD.40 This possibility awaits further investigation.
Beyond fructose, previous studies have also linked glomerular hyperfiltration to insulin resistance in children. Lee et al demonstrated that glomerular hyperfiltration is associated with insulin resistance independent of BMI z-score in a nationally representative sample of US adolescents.26 Insulin resistance is a known driver of hepatic steatosis, the main component of the NAS. As a result, it could be postulated that insulin resistance is the common denominator for the glomerular hyperfiltration and higher NAS scores seen in these patients. It should be noted, however, that in our study revealed no differences in the prevalence of T2DM, insulin/metformin use, or HbA1c value among the GFR groups, and thus this hypothesis is not supported by our findings.
In our study, the 3 GFR groups did not differ in terms of traditional risk factors for NAFLD, specifically obesity severity and Hispanic ethnicity, or in use of medications for T2DM or hypertension. The proportion of patients with T2DM, although numerically higher in the hyperfiltration group, was also not statistically different among the groups. More importantly, serum ALT level did not differ across the 3 GFR groups, even though histologically, those with hyperfiltration were found to be 3 times more likely to have a higher NAS. Given these findings, eGFR should be calculated in all patients with NAFLD and should be taken into account when considering liver biopsy in children with presumed NAFLD. Notably, in our study, only a minority of patients with hyperfiltration (8%) were prescribed antihypertensive medications. AT1 receptor antagonists can be particularly beneficial in earlier stages of renal impairment to control hypertension and/or albuminuria, providing another reason to screen for this comorbidity.
The major strength of the present study is the large sample size of children and adolescents with biopsy-confirmed NAFLD evaluated using validated histological criteria for disease severity. In addition, the cohort was well phenotyped, including severity of obesity, dyslipidemia, insulin resistance, T2DM, and concurrent medication use. The study was limited by its retrospective design, which prevented us from performing in-depth assessments of renal function, such as cystatin C-based assessment of renal function and determination of the presence of albuminuria/microalbuminuria. To determine renal impairment, we relied on laboratory values obtained for clinical purposes, which perhaps introduced selection bias. In addition, eGFR has not been validated for use in severely obese individuals; however, it has been previously reported in other studies on the topic.33,45 Importantly, we did not include blood pressure measurements, because those obtained in our clinics are often inaccurate, as demonstrated by subsequent 24-hour blood pressure ambulatory monitoring of a subset of our patients referred for further workup. Furthermore, we chose to focus on patients who had undergone liver biopsy to assess liver disease severity. In clinical practice, we obtain liver biopsy specimens in patients with persistent significant ALT elevation (>2 times the upper limit of normal) after an initial period of lifestyle management or who have risk factors for more advanced liver disease (eg, imaging findings suggestive of more advanced fibrosis), and as such this cohort is not representative of the entire spectrum of patients with NAFLD. Moreover, in this study, the low GFR group had a lower NAS compared with the normal and high GFR groups. Although this finding seems to contradict our hypothesis that the severity of liver disease is associated with progression of renal impairment, the low GFR group had a median GFR of 85 mL/min/1.73 m2 (IQR, 78–86 mL/min/1.73 m2). This value is close to the lower limit of normal for GFR, and only 1 subject had an GFR <60 mL/min/1.73 m2. To demonstrate the association between low GFR and liver disease severity in pediatric NAFLD, further studies are needed with a larger number of patients with both NAFLD and GFR <60 mL/min/1.73 m2. Finally, in this study, renal impairment was determined using a calculated eGFR. Although calculating eGFR using the Schwartz equation is the most commonly used method in clinical practice, this approach has limitations in patients with advanced liver disease. The impaired liver function, low muscle mass, and protein malnutrition seen in patients with advanced end-stage liver disease result in reduced creatinine production and overestimation of the GFR. However, in this cohort of patients with NAFLD, no patients had evidence of end-stage liver disease (eg, elevated prothrombin time/international normalized ratio, hypoalbuminemia). Owing to the logistics and cost involved in obtaining isotopic renal scans, we recommend that prospective studies in patients with NAFLD use cystatin C-based calculations of the eGFR, along with estimates of microalbuminuria, to further investigate the renal impairment in these patients.46
In this study, no patient with hyperfiltration was prescribed AT1 receptor inhibitors. Because glomerular hyperfiltration is a precursor of chronic kidney disease, pediatricians caring for patients with NAFLD should be aware of this comorbidity. Future studies should address the impact of early intervention on the natural history of the renal impairment seen in these patients. ■
Glossary
- ALT
Alkaline aminotransferase
- AT1
Angiotensin II type 1
- BMI
Body mass index
- eGFR
Estimated glomerular filtration rate
- GFR
Glomerular filtration rate
- HbA1c
Hemoglobin A1C
- NAFLD
Nonalcoholic fatty liver disease
- NAS
Nonalcoholic fatty liver disease activity score
- T2DM
Type 2 diabetes mellitus
Footnotes
The authors declare no conflicts of interest.
References
- 1.Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr 2016;170:e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Zepeda A, Newton KP, Xanthakos SA, Behling C, Hallinan EK, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One 2014;9:e112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int 2017;91: 1224–35. [DOI] [PubMed] [Google Scholar]
- 4.Correia-Costa L, Azevedo A, Caldas Afonso A. Childhood obesity and impact on the kidney. Nephron 2019;143:8–11. [DOI] [PubMed] [Google Scholar]
- 5.Jadhakhan F, Marshall T, Gill P. A systematic review investigating the cumulative incidence of chronic kidney disease in young adults with impaired glucose tolerance. Syst Rev 2015;4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 7.Mallat SG, Al Kattar S, Tanios BY, Jurjus A. Hyperuricemia, hypertension, and chronic kidney disease: an emerging association. Curr Hypertens Rep 2016;18:74. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis 2014;64:638–52. [DOI] [PubMed] [Google Scholar]
- 9.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation 2014;98:216–21. [DOI] [PubMed] [Google Scholar]
- 10.Yu JW, Gupta G, Kang L, Bandyopadhyay D, Siddiqui MS, Bhati CS, et al. Obesity does not significantly impact outcomes following simultaneous liver kidney transplantation: review of the UNOS database - a retrospective study. Transpl Int 2019;32:206–17. [DOI] [PubMed] [Google Scholar]
- 11.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11:e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacifico L, Bonci E, Andreoli GM, Di Martino M, Gallozzi A, De Luca E, et al. The impact of nonalcoholic fatty liver disease on renal function in children with overweight/obesity. Int J Mol Sci 2016;17:E1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manco M, Ciampalini P, DeVito R, Vania A, Cappa M, Nobili V. Albuminuria and insulin resistance in children with biopsy proven nonalcoholic fatty liver disease. Pediatr Nephrol 2009;24:1211–7. [DOI] [PubMed] [Google Scholar]
- 14.Huang SH, Macnab JJ, Sontrop JM, Filler G, Gallo K, Lindsay RM, et al. Performance of the creatinine-based and the cystatin C-based glomerular filtration rate (GFR) estimating equations in a heterogenous sample of patients referred for nuclear GFR testing. Transl Res 2011;157:357–67. [DOI] [PubMed] [Google Scholar]
- 15.Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 2015;10:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774–7. [DOI] [PubMed] [Google Scholar]
- 17.Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006;70:1214–22. [DOI] [PubMed] [Google Scholar]
- 18.Melsom T, Mathisen UD, Eilertsen BA, Ingebretsen OC, Jenssen T, Njølstad I, et al. Physical exercise, fasting glucose, and renal hyperfiltration in the general population: the Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6). Clin J Am Soc Nephrol 2012;7:1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 2007;71:816–21. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139: 137–47. [DOI] [PubMed] [Google Scholar]
- 21.Nitsch D, Felber Dietrich D, von Eckardstein A, Gaspoz JM, Downs SH, Leuenberger P, et al. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant 2006;21:935–44. [DOI] [PubMed] [Google Scholar]
- 22.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 23.Schmieder RE, Messerli FH, Garavaglia G, Nunez B. Glomerular hyperfiltration indicates early target organ damage in essential hypertension. JAMA 1990;264:2775–80. [PubMed] [Google Scholar]
- 24.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 2012;27:1821–5. [DOI] [PubMed] [Google Scholar]
- 25.Kelishadi R, Qorbani M, Assadi F, Motlagh ME, Djalalinia S, Shahsavari A, et al. Glomerular hyperfiltration as predictor of cardiometabolic risk factors among children and adolescents: the Childhood and Adolescence Surveillance and Prevention of Adult-V Study. Int J Prev Med 2018;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AM, Charlton JR, Carmody JB, Gurka MJ, DeBoer MD. Metabolic risk factors in nondiabetic adolescents with glomerular hyperfiltration. Nephrol Dial Transplant 2017;32:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant 2012;27: 1708–14. [DOI] [PubMed] [Google Scholar]
- 28.Park HW, Kim Y, Kim KH, Jeong HJ, Shin MH, Rozen S, et al. Angiotensin II receptor blockade blocker pre-treatment largely prevents injury from gradual renal ablation in rats. J Renin Angiotensin Aldosterone Syst 2007;8:110–7. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Hove KY, Dollerup J, Poulsen PL, Christiansen JS, Schmitz O, et al. Losartan modifies glomerular hyperfiltration and insulin sensitivity in type 1 diabetes. Diabetes Obes Metab 2001;3:463–71. [DOI] [PubMed] [Google Scholar]
- 30.Mathis KM, Banks RO. Role of nitric oxide and angiotensin II in diabetes mellitus-induced glomerular hyperfiltration. J Am Soc Nephrol 1996;7: 105–12. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–100. [DOI] [PubMed] [Google Scholar]
- 33.van Dam M, Rijks J, Dorenbos E, Horuz F, van Dael K, Vreugdenhil A. The effect of one year lifestyle intervention on eGFR in children and adolescents with overweight, obesity and morbid obesity. Sci Rep 2019;9:4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piepsz A, Tondeur M, Ham H. Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 2006;33:1477–82. [DOI] [PubMed] [Google Scholar]
- 35.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics 2007;120(Suppl 4):S193–228. [DOI] [PubMed] [Google Scholar]
- 36.Yodoshi T, Orkin S, Arce-Clachar AC, Bramlage K, Liu C, Fei L, et al. Vitamin D deficiency: prevalence and association with liver disease severity in pediatric nonalcoholic fatty liver disease. Eur J Clin Nutr 2020;74:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 38.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53: 810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthew Morris E, Fletcher JA, Thyfault JP, Rector RS. The role of angiotensin II in nonalcoholic steatohepatitis. Mol Cell Endocrinol 2013;378:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musso G, Cassader M, Cohney S, Pinach S, Saba F, Gambino R. Emerging liver-kidney interactions in nonalcoholic fatty liver disease. Trends Mol Med 2015;21:645–62. [DOI] [PubMed] [Google Scholar]
- 41.Marcuccilli M, Chonchol M. NAFLD and chronic kidney disease. Int J Mol Sci 2016;17:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cydylo MA, Davis AT, Kavanagh K. Fatty liver promotes fibrosis in monkeys consuming high fructose. Obesity (Silver Spring) 2017;25: 290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol 2013;19:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobridge KS, Haines GL, Mori TA, Beilin LJ, Oddy WH, Sherriff J, et al. Dietary fructose in relation to blood pressure and serum uric acid in adolescent boys and girls. J Hum Hypertens 2013;27:217–24. [DOI] [PubMed] [Google Scholar]
- 45.Savino A, Pelliccia P, Giannini C, de Giorgis T, Cataldo I, Chiarelli F, et al. Implications for kidney disease in obese children and adolescents. Pediatr Nephrol 2011;26:749–58. [DOI] [PubMed] [Google Scholar]
- 46.Björk J, Nyman U, Berg U, Delanaye P, Dubourg L, Goffin K, et al. Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatr Nephrol 2019;34:1087–98. [DOI] [PubMed] [Google Scholar]


