ABSTRACT
Circulating tumor cells (CTCs) are considered to be related to the prognosis of cancer patients. CTC is a powerful indicator for recurrence or metastasis. The relationship, however, between the expression of programmed cell death receptor ligand 1 (PD-L1) on CTCs in peripheral blood and the prognosis, is still controversial. Here, we conducted a meta-analysis to evaluate its prognostic value. A total of 20 articles were screened from PubMed, Embase, Cochrane, China National Knowledge Internet (CNKI) and WanFang Database, and the Hazard Ratio (HR) along with 95% confidence intervals (CIs) of each article were combined to study the relationship between PD-L1 expression on CTCs and prognosis. The expression of PD-L1 on CTCs in the peripheral blood of cancer patients is associated with poor prognosis. The pooled HRs for overall survival (OS) in cancer patients were 1.85 (95% CI, 1.29–2.66, P = .001). The pooled HRs for progression-free survival (PFS) in cancer patients were 1.50 (95% CI, 1.12–2.01; P = .007). This is the first meta-analysis to clarify the expression of PD-L1 on CTCs at baseline affects the prognosis of cancer patients. Patients with CTCs expressing PD-L1 had a shorter survival time than patients with CTCs not expressing PD-L1.
KEYWORDS: CTCs, cancer, PD-L1, prognosis
Introduction
Circulating tumor cells were first described in 1869. CTCs are cells that are shed into the blood from the primary tumor and metastatic deposits.1 With the latest development of reproducible detection technology, CTCs have been studied as diagnostic, prognostic and/or predictive biomarkers for various types of cancer.2 CTCs are negatively correlated with the prognosis of tumor patients. Patients with CTCs detected in the peripheral blood have a worse prognosis and shorter survival time.3,4 The existence of CTCs may be the cause of tumor recurrence and metastasis.4,5 A study by Tamminga et al. showed that about one-third of patients with advanced non-small cell lung cancer could be detected with CTC, and this was related to the poor prognosis of patients receiving immune checkpoint therapy.6 For patients with non-small cell lung cancer treated with tyrosine kinase inhibitors (TKI) or chemotherapy, the response rate of patients with CTC detected in peripheral blood to treatment was lower than that of patients without CTC detected.7
It is reported in the literature that the expression of PD-L1 is considered to be positively correlated with the efficacy of immune checkpoint inhibitor therapy,8 but the relationship between PD-L1 on CTCs and the prognosis is still inconclusive.
Compared with tissue biopsy, circulating tumor cells have the following advantages: (1) easy to collect, (2) serial evaluation, (3) interrogation of the entire tumor burden instead of just a limited part of the tumor. Recent progress has been made in the phenotype and genotyping of CTC, which should provide insights into the predictive effect of CTC on treatment sensitivity or resistance. In addition, changes in CTC phenotypic markers during treatment can be used as a tool for drug efficacy monitoring. Therefore, CTCs collection can be considered a “liquid biopsy” that can provide prognostic and predictive clinical information and further understanding of tumor heterogeneity.9
Whether the expression of PD-L1 on CTCs can be used as a prognostic indicator has been explored,10–12 but according to the current literature’ results, there is no consensus. Boffa et al. found that PD-L1 expressed on circulating tumor cells in peripheral blood was associated with worse survival of lung cancer.13 In the study by Khattak et al., patients with PDL1+
CTCs had longer PFS compared with patients with PD-L1− CTCs.14
Therefore, it is necessary to conduct a meta-analysis to study whether PD-L1 in the CTCs is related to the prognosis of cancer patients based on the current research status.
Materials and methods
Inclusion criteria
1. literatures with search terms in the title or abstract. 2. literatures restricted to human studies written in English or Chinese. 3. studies included the effect of PD-L1 on CTCs in the blood on the prognosis of cancer patients. 4. efficacy results expressed as PFS or OS had to be provided.
Exclusion criteria
1. reviews, case reports, notes, chapters, editorials and letters. 2. literatures were not relevant to this study. 3. literatures with insufficient data or no information available. 4. research on animal experiments.
Literature review
Titles and abstracts were screening by two investigators independently according to the inclusion criteria. After removing the duplicate literatures, the final selected literatures had been screened according to the exclusion criteria. If any disputes were encountered, the two reviewers negotiated to resolve or consulted with the third investigators, and the quality of the final enrolled literatures was evaluated after reading the full text.
Data extraction
The information was extracted from the full text includes: author, publication time and journal, region, sample size, age, tumor types, expression level of PD-L1 in CTCs or tissue specimens, cutoff value, CTCs detection methods, treatment methods, PFS, OS, HR for OS and PFS, etc. The information was extracted by two reviewers independently and when encountering controversial issues, the two reviewers consulted with a third investigator.
Quality assessment
The literatures were evaluated according to the Newcastle–Ottawa scale (NOS), which was used to assess the quality of cohort studies and case–control studies. The included literatures were evaluated by two reviewers independently, and the disagreement was resolved through discussion or consultation with a third investigator. The highest score is 9 points, and studies with a score of six or more are considered to be high-quality.15
Statistical analysis
Stata 16.0 statistical software was used for meta-analysis. Hazard ratios for OS and PFS and 95% confidence intervals were pooled to measure the time to event relationship (between the expression of PD-L1 in CTCs and prognosis of cancer patients). HRs were derived from the multivariate analysis first, followed by univariate analysis, or calculated from Kaplan–Meier survival curves using the methods previously proposed by Tierney and colleagues.16 Heterogeneity was evaluated by Q test. P > .10 was considered to have no heterogeneity or slight heterogeneity, while P < .10 implied significant heterogeneity.17 Besides, heterogeneity was assessed by the I2 statistics. I2 values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.18 When obvious heterogeneity was observed, the random effect model was used; otherwise, the fixed-effect model was used. In addition, to find the source of significant heterogeneity, sensitivity analysis and subgroup analysis were performed. Publication bias was assessed by visual inspection of the funnel plot first and further by the Egger test19 and the Begg test.20
Subgroup analysis
Subgroup analysis in this meta-analysis included the characteristics of the patients, such as area, tumor type, gender, age, methods for detecting CTCs, PD-L1 antibody, data type and treatment methods.
Results
Literature search
A total of 546 documents from five databases were obtained. The five databases are PubMed, Embase, Cochrane, CNKI, and WanFang Database. Among them, 270 documents were excluded because they overlapped in various databases. 183 articles were removed due to lack of full text. 86 articles were reviewed for full-text evaluation. 66 full texts were excluded for the following reasons. 1. They were reviews, case reports, notes, chapters, editorials, and letters. 2. They were not relevant to this study. 3. There was insufficient data or no information available. 4. They were researchers on animal experiments. Finally, 20 articles12–14,21–37 were recruited for qualitative synthesis and meta-analysis (Figure 1). The remaining documents were unanimously regarded as high-quality documents by researchers.
Figure 1.
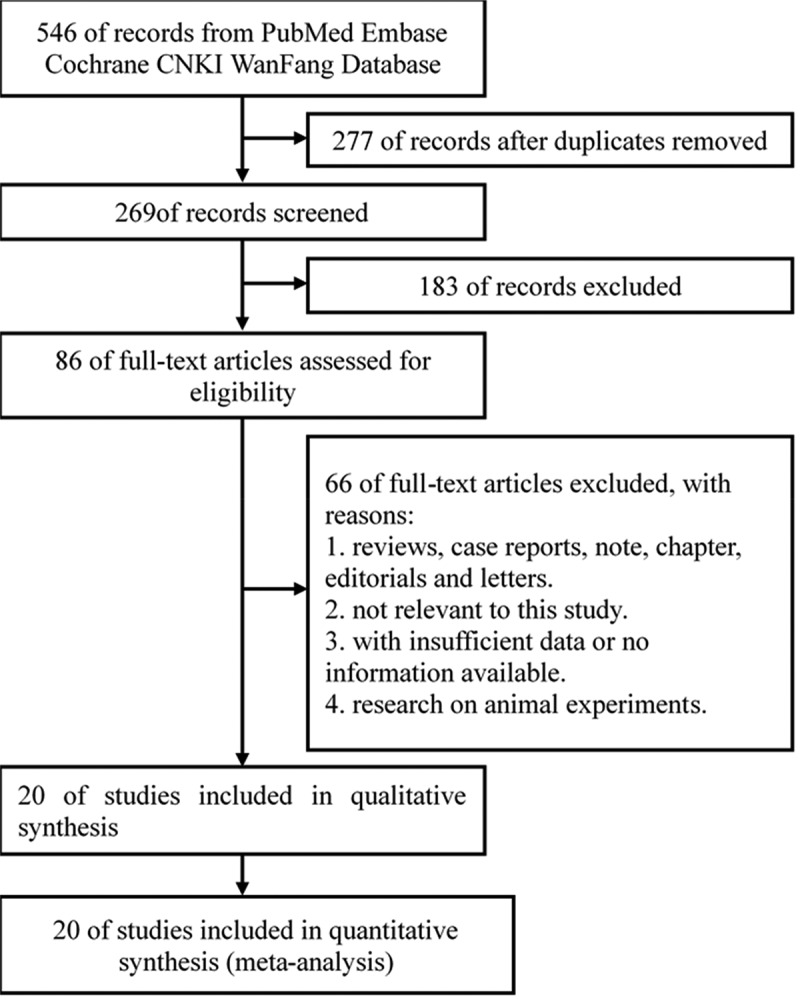
Flow Diagram of the Study Selection Process. CNKI, China National Knowledge Internet
Study characteristics
The study included 20 studies with a total of 1,344 patients from five countries: the United States, Australia, Greece, France and China. The characteristics of 20 studies are summarized in Table 1. The patients were between 21 and 91 y old. These studies included a variety of tumor types such as non-small cell lung cancer (NSCLC), squamous cell carcinoma of head and neck (HNSCC), prostate cancer, melanoma, colon cancer, gastrointestinal tumors and breast cancer. CTCs in the peripheral blood of 617 out of 1344 patients expressed PD-L1.
Table 1.
Characteristics of studies included in the meta-analysis
| Author | Year | Journal | Region | N total | Age mean (range) |
Tumor type | PD-1+ CTC |
Cutoff PD-L1+ CTC/mL |
CTC detection Method | PD-L1 antibody | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anantharaman | 2016 | BMC Cancer | America | 25 | 67(43–89) | Bladder cancer | 7 | 1 | EPIC Sciences Platform | CST | ||||||||||
| Boffa | 2017 | Cancer Epidemiol Biomarkers Prev |
America | 112 | 67.5(59–76.5) | NSCLC | 26 | 1.1 | EPIC Sciences Platform | CST | ||||||||||
| Cheng | 2020 | Cancer Management and Research |
China | 66 | 62(48–79) | NSCLC | 22 | 1% | HE pathological staining | Abcam | ||||||||||
| Dhar | 2018 | ScientIfic Reports | America | 22 | 69.4(51–91) | NSCLC | 7 | 2 | Vortex HT chip | ProSci Inc | ||||||||||
| Dong | 2019 | Front Oncol | China | 114 | 60.9 | NSCLC | 56 | - | CanPatrolTM | RNA-ISH | ||||||||||
| Guibert | 2018 | Lung Cancer | France | 96 | 60 (30–81) | NSCLC | 74 | 1% 5% 10% | ISET platform | CST | ||||||||||
| Ilie′ | 2018 | Annals of Oncology | France | 106 | 65 (41–86) | NSCLC | 71 | 1 | ISET platform | Ventana | ||||||||||
| Kallergi | 2018 | Therapeutic Advances In Medical Oncology |
Greece | 30 | - | NSCLC | 9 | 3 | ISET platform | Biolegend Novus Biologicals |
||||||||||
| Khattak | 2020 | The Oncologist | Australia | 40 | 71 | Melanoma | 14 | - | Flow Cytometric Staining | R&D System | ||||||||||
| Kulasinghe | 2018 | Cancer Medicine | Australia | 56 | 60 (21–82) | HNSCC NSCLC |
17 | - | ClearCell FX system | Abcam | ||||||||||
| Liu | 2020 | Molecular Oncology | China | 70 | 63 | Gastric cancer | 50 | 8 | Flow Cytometric Staining | CST | ||||||||||
| Papadaki | 2020 | Cancers | Greece | 198 | 60(29–84) | Breast cancer | 60 | 1 | - | CST | ||||||||||
| Satelli | 2016 | ScientIfic Reports | America | 92 | - | Colon cancer prostate cancer |
64 | 50% | Flow Cytometric Staining | Flow cytometry | ||||||||||
| Tada | 2020 | Oral Oncology | America | 44 | 66 | HNSCC | 11 | - | CellSieve™ microfilter | RT-qPCR | ||||||||||
| Yue | 2018 | Oncoimmunology | China | 35 | - | Gastrointestinal cancer |
26 | 2 20% | Pep@MNPs isolated system | KN802 | ||||||||||
| Adams | 2017 | Clinical Cancer Research | America | 41 | Lung cancer | 17 | 2 | CellSieve™ microfilter | R&D systems | |||||||||||
| Manjunath | 2019 | Cancers | America | 30 | 65(50–79) | NSCLC | 30 | 3 | CellSieve™ microfilter | CST | ||||||||||
| Strati | 2017 | Annals of Oncology | Greece | 113 | 65 | HNSCC | 24 | - | RosetteSep System | CellSearchTM analysis |
||||||||||
| Wang | 2019 | ScientIfic Reports | America | 38 | 67(57–89) | NSCLC | 25 | 5% | GO chip Immunofluorescence staining |
BioLegend | ||||||||||
| Zhang | 2020 | Cancer Letters | China | 16 | - | NSCLC | 7 | - | SE-iFISH | IF(-) | ||||||||||
Aberrations: NSLCC: Non-small cell lung carcinoma, HNSCC: Head and neck squamous cell carcinoma, PD-1: Programmed cell death-1, QA: Quality Assessment.
In five articles, percentages were used to define the threshold cutoff point for CTCs expressing PD-L1. Other articles used the number of CTCs expressing PD-L1 as the cutoff point. Eight articles received immune checkpoint inhibitor therapy. These documents applied CTC detection platforms based on different detection mechanisms. The researchers stained CTCs with PD-L1 antibody to identify how many cells in the peripheral blood expressed PD-L1, even though the antibodies selected were different (Table 1).
OS of cancer patients with PD-L1 expression on CTCs in the peripheral blood
The pooled HRs for OS in cancer patients were 1.85 (95% CI, 1.29–2.66, P = .001; heterogeneity: I2 = 43.1%, P = .055; Figure 2a). Although no significant heterogeneity observed among the selected studies, the P value is close to 0.05 (0.055), we conducted a sensitivity test and found that omitting any single study did not influence the result of OS. Hence, subgroup analyses were proposed. Among patients whose cutoff≤1 group, pooled HRs of OS were 1.82 (95% CI, 1.22–2.74, P = .004; heterogeneity: I2 = 0.0%, P = .543). Among patients under 65 y of age, the combined HRs of OS were 1.74 (95% CI, 1.18–2.56; heterogeneity: I2 = 33.7%, P = .071). When the included studies were analyzed by subgroups based on region, there were no heterogeneity in the studies from the United States (2.39,95% CI, 1.62–3.52; heterogeneity: I2 = 0.0%, P = .652), China (3.03,95% CI, 1.61–5.72; heterogeneity: I2 = 0.0%, P = .440), and France (1.23,95% CI, 0.68–2.21; heterogeneity: I2 = 0.0%, P = .625), and the heterogeneity came from the researches in Greece (1.43,95% CI, 0.40–5.05; heterogeneity: I2 = 69.9%, P = .068). According to the pooled HRs for OS in cancer patients, the expression of PD-L1 on CTCs in the peripheral blood of cancer patients was associated with poor prognosis.
Figure 2.
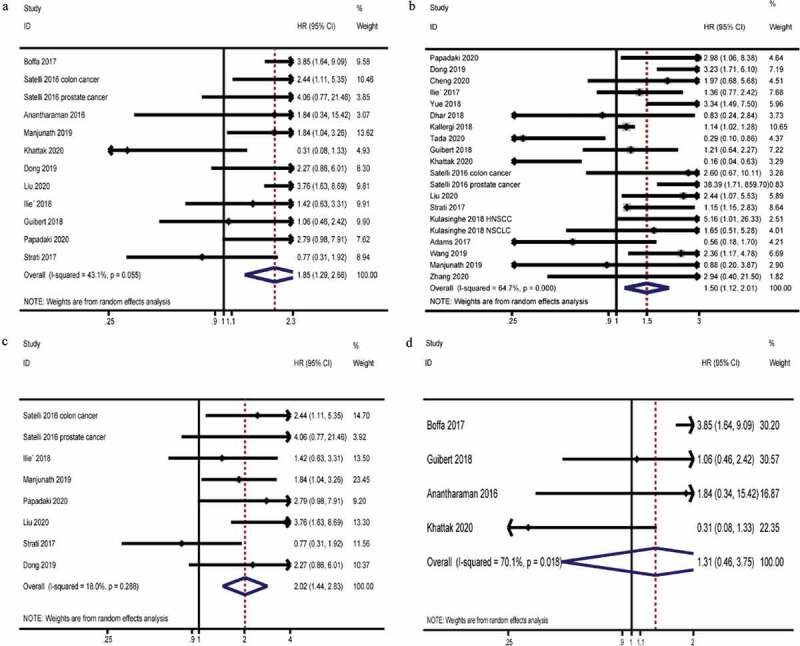
Prognosis of cancer patients with PD-L1 expression on CTCs in the peripheral blood. A, Pooled HRs and 95% CI for OS. B, Pooled HRs, and 95% CI for PFS. C, Pooled HRs, and 95% CI for OS in the subgroup that did not use ICI therapy. D, Pooled HRs and 95% CI for OS in the subgroup that uses ICI therapy
PFS of cancer patients with PD-L1 expression on CTCs in the peripheral blood
The pooled HRs for PFS in cancer patients were 1.50 (95% CI, 1.12–2.01; P = .007; heterogeneity: I2 = 64.7%, P = .000; Figure 2b). Because of significant heterogeneity observed, we also performed a similar sensitivity analysis. The sensitivity analysis result showed that omitting any single study did not influence the result of PFS. We then conducted subgroup analyses of the included studies. In the cutoff≤1 subgroup, the pooled HRs for PFS in cancer patients were 1.85 (95% CI, 1.26–2.72; P = .002; heterogeneity: I2 = 24.2%, P = .244). Differences had also been observed in age groups. In the under 65-y-old group, the pooled HRs for PFS in cancer patients were 1.82 (95% CI, 1.35–2.45; P = .000; heterogeneity: I2 = 15.3%, P = .303). When analyzing the subgroups by region, no heterogeneity was found in the China (2.85, 95% CI, 1.93–4.20; P = .000; heterogeneity: I2 = 0.0%, P = .924) and France (1.29, 95% CI, 0.84–1.97; P = .239; heterogeneity: I2 = 0.0%, P = .303) subgroups.
Whether the expression of PD-L1 on CTCs can be a prognostic indicator for immune checkpoint inhibitor therapy.
In the subgroup analysis, among studies that did not use immune checkpoint inhibitor (ICI) therapy, the pooled HRs for OS were 2.02 (95% CI, 1.44–2.83; P = .000; heterogeneity: I2 = 18.0%, P = .288; Figure 2c, Table 2). This suggested that without the use of immune checkpoint inhibitors, the expression of PD-L1 on CTCs indicated a worse prognosis. However, in the group treated with immune checkpoint inhibitors, the expression of PD-L1 on CTCs in the peripheral blood was not found to be related to the patient’s prognosis. The pooled HRs for OS were 1.31 (95% CI, 0.46–3.75; P = .618; heterogeneity: I2 = 70.1%, P = .018; Figure 2d, Table 2).
Table 2.
Subgroup analysis of the pooled HRs for OS and PFS in cancer patients with PD-L1 expressed in the CTCs
| |
OS |
PFS |
||||
|---|---|---|---|---|---|---|
| Number of studies Heterogeneity I2%, p | Pooled HRs (95% CI) | Interaction (p) | Number of studies Heterogeneity I2%, p | Pooled HRs (95% CI) | Interaction (p) | |
| Total | 43.1(0.055) | 1.96(1.34–2.88) | 0.001 | 64.7(0.000) | 1.50 (1.12–2.01) | 0.007 |
| Cutoff | ||||||
| >1 | 32.5(0.227) | 2.74(1.63–4.60) | 0.000 | 57.8(0.037) | 1.37(0.83–2.24) | 0.217 |
| ≤1 | 0.0(0.543) | 1.82(1.22–2.74) | 0.004 | 24.2(0.244) | 1.85(1.26–2.72) | 0.002 |
| unknown | 64.3(0.061) | 0.9(0.32–2.54) | 0.835 | 78.3(0.000) | 1.19(0.48–2.98) | 0.710 |
| Median Age | ||||||
| >65 | 77.2(0.012) | 1.37(0.27–7.06) | 0.707 | 82.9(0.001) | 0.59(0.16–2.12) | 0,417 |
| ≤65 | 33.7(0.171) | 1.74(1.18–2.56) | 0.005 | 15.3(0.303) | 1.82(1.35–2.45) | 0.000 |
| unknown | 0.0(0.587) | 2.67(1.31–5.45) | 0.007 | 67.3(0.009) | 1.85(0.90–3.80) | 0.093 |
| Area | ||||||
| America | 0.0(0.652) | 2.39(1.62–3.52) | 0.000 | 67.4(0.005) | 1.17(0.51–2.66) | 0.707 |
| Australia | - | - | - | 82.7(0.003) | 1.08(0.16–7.24) | 0.938 |
| China | 0.0(0.440) | 3.03(1.61–5.72) | 0.001 | 0.0(0.924) | 2.85(1.93–4.20) | 0.000 |
| France | 0.0(0.625) | 1.23(0.68–2.21) | 0.494 | 0.0(0.788) | 1.29(0.84–1.97) | 0.239 |
| Greece | 69.9(0.068) | 1.43(0.40–5.05) | 0.579 | 38.8(0.195) | 1.32(0.84–2.08) | 0.231 |
| Tumor type | ||||||
| Gastrointestinal cancer | 0.00(0.459) | 2.99(1.68–5.30) | 0.000 | 0.0(0.859) | 2.86(1.61–5.09) | 0.000 |
| Lung cancer | 39.1(0.145) | 1.64(1.07–2.51) | 0.022 | 53(0.010) | 1.36(1.00–1.84) | 0.047 |
| Treatment method | ||||||
| ICI | 70.1(0.018) | 1.31(0.46–3.75) | 0.618 | 66.9(0.000) | 1.41(0.67–2.96) | 0.370 |
| No ICI | 18.0(0.288) | 2.02(1.44–2.83) | 0.000 | 65.7(0.000) | 1.55(1.08–2.23) | 0.018 |
| Data types | ||||||
| Multivariate | 73.4(0.010) | 1.18(0.46–3.02) | 0.728 | 75.4(0.017) | 0.74(0.26–2.11) | 0.575 |
| Others | 0.0(0.572) | 2.15(1.57–2.94) | 0.000 | 64.5(0.000) | 1.71(1.21–2.42) | 0.002 |
Sensitivity analyses
Sensitivity analysis results showed that the heterogeneity of HRs for OS was mainly derived from two studies conducted by Khattak and colleagues and Strati and colleagues. Khattak and colleagues conducted a melanoma study. Because patients in this cohort were treated with the immune checkpoint inhibitor Pembrolizumab. The application of immune checkpoint inhibitors made the prognosis of patients with PD-L1 positive CTCs better than that of patients with PD-L1 negative CTCs.
Strati and colleagues conducted a prospective cohort study of head and neck squamous cell carcinoma. The sample size of this study was relatively large, with a total of 113 patients and 24 patients with PD-L1 positive on CTCs. The expression of PD-L1 on CTCs was detected by Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) method. This method represented the transcription level of PD-L1 gene to a certain extent, but might not completely represent the expression level of PD-L1 protein.
After removing these two studies, the pooled HRs for OS were 2.18 (95% CI, 1.65–2.88; P = .000; heterogeneity: I2 = 0.0%, P = .489; Figure 3e).
Figure 3.
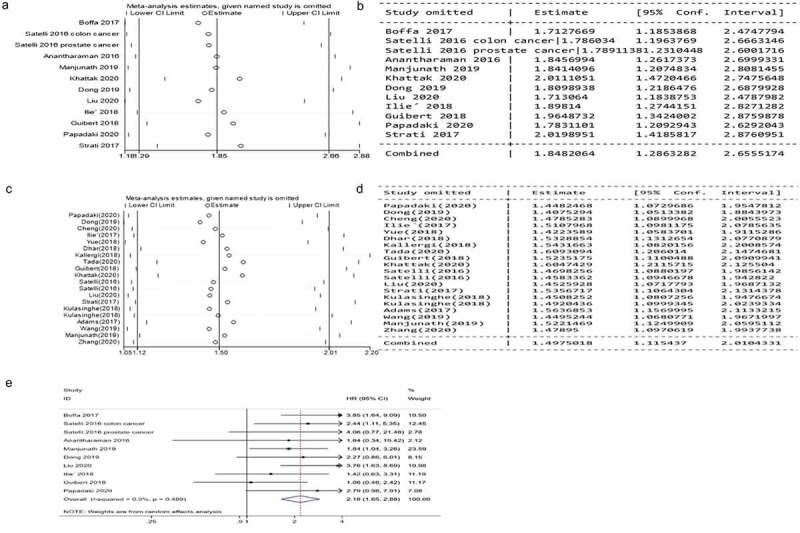
Sensitivity analyses of the pooled HRs for OS and PFS of cancer patients with PD-L1 expression on CTCs in the peripheral blood. A, B, Sensitivity analyses of the pooled HRs for OS. C, D, Sensitivity analyses of the pooled HRs for PFS. E, Pooled HRs and 95% CI for OS after removing two studies
Excluding the literature one by one in the sensitivity analysis did not affect the results and heterogeneity of PFS, and then we conducted a subgroup analysis.
Subgroup analyses
Because the pooled HRs for PFS in cancer patients had obvious heterogeneity and the pooled HRs for OS in cancer patients had moderate heterogeneity, we conducted a subgroup analysis of the selected literatures to explore the source of heterogeneity. We had set up a total of eight subgroups, namely the CTCs detection platform, the threshold of CTCs expressing PD-L1, the type of PD-L1 antibody, the research area, the median age of the enrolled patients, the data analysis method, the treatment method and tumor type.
Taken the expression of PD-L1 on 1 CTC as the dividing point, in cutoff≤1 group, the pooled HRs of OS were 1.82 (95% CI, 1.22–2.74, P = .004; heterogeneity: I2 = 0.0%, P = .543; Table 2). The pooled HRs for PFS were 1.85 (95% CI, 1.26–2.72; P = .002; heterogeneity: I2 = 24.2%, P = .244; Table 2).
There was heterogeneity between research in different regions. The pooled HRs for OS in the American subgroup were 2.39 (95% CI, 1.62–3.52; heterogeneity: I2 = 0.0%, P = .652; Table 2), the pooled HRs for OS in the China subgroup were 3.03 (95% CI, 1.61–5.72; heterogeneity: I2 = 0.0%, P = .440; Table 2), and the pooled HRs for OS in the France subgroup were 1.23 (95% CI, 0.68–2.21; heterogeneity: I2 = 0.0%, P = .625; Table 2). The pooled HRs for PFS in the China subgroup were 2.85 (95% CI, 1.93–4.20; P = .000; heterogeneity: I2 = 0.0%, P = .924; Table 2) and the pooled HRs for PFS in the France (1.29, 95% CI, 0.84–1.97; P = .239; heterogeneity: I2 = 0.0%, P = .303; Table 2).
Among patients under 65 y of age, the combined HRs for OS was 174 (95% CI, 1.18–2.56; heterogeneity: I2 = 33.7%, P = .071; Table 2). The pooled HRs for PFS in cancer patients were 1.82 (95% CI, 1.35–2.45; P = .000; heterogeneity: I2 = 15.3%, P = .303; Table 2). This indicated that the expression of PD-L1 on CTCs in younger patients had a poor prognosis.
When we performed subgroup analyses on tumor types, the pooled HRs for OS in gastrointestinal cancer were 2.99 (95% CI, 1.68–5.30; P = .000; heterogeneity: I2 = 0.0%, P = .459; Table 2). The pooled HRs for OS in Lung cancer were 1.64 (95% CI, 1.07–2.51; P = .000; heterogeneity: I2 = 39.1%, P = .145; Table 2). And the pooled HRs for PFS in gastrointestinal cancer were 2.86 (95% CI, 1.61–5.09; P = .000; heterogeneity: I2 = 0.0%, P = .859; Table 2). This represented that the conclusions in respiratory system tumors and digestive system tumors were consistent.
The pooled HRs for OS in multivariate analyses group were 1.18 (95% CI, 0.46–3.02; P = .728; heterogeneity: I2 = 73.4%, P = .010; Table 2). The pooled HRs for OS in the other group were 2.15 (95% CI, 1.57–2.94; P = .000; heterogeneity: I2 = 0.0%, P = .572; Table 2).
Detection platform for CTCs in peripheral blood had no effect on OS. The reason was that the number of studies using the same platform was too small. When analyzing PFS, there were differences between different platforms. The pooled HRs of ISET platform subgroup were 1.15 (95% CI, 1.03–1.28; P = .015; heterogeneity: I2 = 0.0%, P = .554; Figure S1A). The pooled HRs of CellSieve™ microfilter were 0.47 (95% CI, 0.24–0.84; P = .034; heterogeneity: I2 = 0.0%, P = .461; Figure S1B). The pooled HRs of ClearCell FX system were 2.51 (95% CI, 0.85–7.41; P = .095; heterogeneity: I2 = 19.9%, P = .264; Figure S1C). The pooled HRs of Flow Cytometric Staining were 1.81 (95% CI, 0.35–9.50; P = .481; heterogeneity: I2 = 81.8%, P = .001; Figure S1D).
The pooled HRs for OS in CST subgroup were 2.27 (95% CI, 1.49–3.45; P = .000; heterogeneity: I2 = 26.4%, P = .236; Figure S1E). The pooled HRs for PFS in CST subgroup were 1.71 (95% CI, 1.03–2.81; P = .036; heterogeneity: I2 = 19.6%, P = .292; Figure S1F). The pooled HRs for PFS in Abcam subgroup were 1.65 (95% CI, 1.08–2.57; P = .028; heterogeneity: I2 = 0.0%, P = .489; Figure S1G).
Publication bias
Egger and Begg tests were performed to evaluate publication bias and funnel plot symmetry was examined. Publication bias was not observed based on the visual distribution of funnel plot and P values in Egger and Begg tests (Figure S2).
Comparing results from random effect model with those from fixed effect model
As shown in Supplementary Table S1 and S2, in the absence of heterogeneity (I2 = 0.00%), the HRs and 95% CIs obtained by the random effects model and the fixed effects model were consistent. Additionally, HRs and 95% CIs from the analyses with heterogeneity (I2 > 0.00%) were slightly changed from random effect model to fixed effect model but it had no impact on prognostic analyses.
Discussion
There had been many studies on the relationship between the expression of PD-L1 on CTCs and the prognosis of cancer patients.26,35,38 Articles showed that patients with PD-L1 expression had a worse prognosis.38,39 However, there were also studies that have found that patients with PD-L1 expression can benefit from immune checkpoint inhibitor therapy.40 For a unified conclusion that has not been reached, a meta-analysis could be done to guide clinical treatment. However, no one has done a meta-analysis in this direction. Our study is the first study, which can bring important evidence for whether the expression of PD-L1 on CTCs can be used as a prognostic assessment marker.
We found that the PD-L1 expression of CTCs in the peripheral blood of patients at baseline was related to the poor prognosis of patients. Patients with 1 or more CTCs expressing PD-L1 had shorter OS and PFS. PD-L1 on tumor cells can bind to PD-1 expressed on T cells, leading to immune escape of tumors, which may be the advantage of tumor metastasis and a feature of high malignancy.
Among patients treated with immune checkpoint inhibitors, studies showed that PD-L1 expression responds better to ICI treatment and had a longer survival benefit.41,42 However, less than 30% of patients with PD-L1 expression could benefit from immune checkpoint inhibitor therapy.43 The expression of PD-L1 on CTCs in peripheral blood could not yet be used as a basis for patients to benefit from immune checkpoint inhibitor therapy. A large number of prospective randomized controlled clinical trial studies are needed.
Some limitations existed in our meta-analysis. All the comprehensive studies were selected from Chinese and English databases, so articles in other languages or unpublished articles were overlooked. Some data were obtained from univariate analyses or calculated from Kaplan–Meier survival curves,16 which might be in slight disparity with the fact. The results of some subgroup analyses might not be representative enough, because the number of studies in some subgroups was too small, such as the detection platform of CTCs and the use of PD-L1 antibodies.
Immune checkpoint inhibitor therapy currently lacks effective prognostic indicators. At present, immunohistochemical detection of PD-L1 expression level in tissues was commonly used.44 At the same time, there were studies on the relationship between microsatellite instability,45 tumor mutation burden,46the density of tumor-infiltrating lymphocyte (TIL),47 gut microbiota,48 circulating biomarkers,49 and patient previous history,50 and driving gene mutations.51–53 However, there was no perfect index, and due to the existence of tumor heterogeneity, there were certain limitations in the extraction of tissues. If a marker can be found in the peripheral blood to judge the prognosis, it will be of great significance to the patient. It will not only be able to quickly and non-invasively detect whether it is effective for immune checkpoint inhibitors and can also reduce the risk of tumor metastasis, bleeding, and spread caused by a puncture.
Supplementary Material
Funding Statement
This work is supported by the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (Grant No: 2017-I2M-1-005) and the National Key R&D Program of China (Grant No. 2017YFC1308700, No. 2017YFC1308702).
Systematic review registration
PROSPERO CRD42020188069.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Pantel K, Speicher MR.. The biology of circulating tumor cells. Oncogene. 2016;35:1216–9. [DOI] [PubMed] [Google Scholar]
- 2.Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, Bidard FC. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22(3):421–430. doi: 10.1007/s10147-017-1105-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Li Y, Xu J, Zhang A, Wang X, Tang R, Zhang X, Yin H, Liu M, Wang DD, et al. Quantified postsurgical small cell size CTCs and EpCAM(+) circulating tumor stem cells with cytogenetic abnormalities in hepatocellular carcinoma patients determine cancer relapse. Cancer Lett. 2018;412:99–107. doi: 10.1016/j.canlet.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, Entenberg D, Zhang Y, Gerratana L, Huang S, Patel DB, et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2019;9(1):96–113. doi: 10.1158/2159-8290.CD-18-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okajima W, Komatsu S, Ichikawa D, Miyamae M, Ohashi T, Imamura T, Kiuchi J, Nishibeppu K, Arita T, Konishi H, et al. Liquid biopsy in patients with hepatocellular carcinoma: circulating tumor cells and cell-free nucleic acids. World J Gastroenterology. 2017;23(31):5650–5668. doi: 10.3748/wjg.v23.i31.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamminga M, De Wit S, Hiltermann TJN, Timens W, Schuuring E, Terstappen L, Groen HJM. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunotherapy Cancer. 2019;7(1):173. doi: 10.1186/s40425-019-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamminga M, De Wit S, Schuuring E, Timens W, Terstappen L, Hiltermann TJN, Groen HJM. Circulating tumor cells in lung cancer are prognostic and predictive for worse tumor response in both targeted- and chemotherapy. Translational Lung Cancer Res. 2019;8(6):854–861. doi: 10.21037/tlcr.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Eng J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paoletti C, Hayes DF. Circulating Tumor Cells. Adv Exp Med Biol. 2016;882:235–258. [DOI] [PubMed] [Google Scholar]
- 10.Raimondi L, Raimondi FM, Di Benedetto L, Cimino G. PD-L1 expression on circulating tumour cells may be predictive of response to regorafenib in patients diagnosed with chemorefractory metastatic colorectal cancer. Int J Mol Sci. 2020;21:18. doi: 10.3390/ijms21186907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Kim TH, Fouladdel S, Zhang Z, Soni P, Qin A, Zhao L, Azizi E, Lawrence TS, Ramnath N, et al. PD-L1 expression in circulating tumor cells increases during radio(chemo)therapy and indicates poor prognosis in non-small cell lung cancer. Sci Rep. 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadaki MA, Koutsopoulos AV, Tsoulfas PG, Lagoudaki E, Aggouraki D, Monastirioti A, Koutoulaki C, Apostolopoulou CA, Merodoulaki AC, Papadaki C, et al. Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers. 2020;12(2):376. doi: 10.3390/cancers12020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boffa DJ, Graf RP, Salazar MC, Hoag J, Lu D, Krupa R, Louw J, Dugan L, Wang Y, Landers M, et al. Cellular Expression of PD-L1 in the peripheral blood of lung cancer patients is associated with worse survival. Cancer Epidemiol. 2017;26(7):1139–1145. doi: 10.1158/1055-9965.EPI-17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khattak MA, Reid A, Freeman J, Pereira M, McEvoy A, Lo J, Frank MH, Meniawy T, Didan A, Spencer I, et al. PD-L1 Expression on circulating tumor cells may be predictive of response to pembrolizumab in advanced melanoma: results from a pilot study. oncologist. 2020;25(3):e520–e7. doi: 10.1634/theoncologist.2019-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 21.Anantharaman A, Friedlander T, Lu D, Krupa R, Premasekharan G, Hough J, Edwards M, Paz R, Lindquist K, Graf R, et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer. 2016;16(1):744. doi: 10.1186/s12885-016-2758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Wang T, Lv X, Li R, Yuan L, Shen J, Li Y, Yan T, Liu B, Wang L. Detection of PD-L1 expression and its clinical significance in circulating tumor cells from patients with non-small-cell lung cancer. Cancer Manag Res. 2020;12:2069–2078. doi: 10.2147/CMAR.S245425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhar M, Wong J, Che J, Matsumoto M, Grogan T, Elashoff D, Garon EB, Goldman JW, Sollier Christen E, Di Carlo D, et al. Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci Rep. 2018;8(1):2592. doi: 10.1038/s41598-018-19245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong J, Zhu D, Tang X, Qiu X, Lu D, Li B, Lin D, Zhou Q. Detection of circulating tumor cell molecular subtype in pulmonary vein predicting prognosis of stage i-iii non-small cell lung cancer patients. Front Oncol. 2019;9:1139. doi: 10.3389/fonc.2019.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, Mourlanette J, Gouin S, Dormoy I, Favre G, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–112. doi: 10.1016/j.lungcan.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Ilié M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvée S, Selva E, Leroy S, Marquette C-H, Kowanetz M, Hedge P, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29(1):193–199. doi: 10.1093/annonc/mdx636. [DOI] [PubMed] [Google Scholar]
- 27.Kallergi G, Vetsika EK, Aggouraki D, Lagoudaki E, Koutsopoulos A, Koinis F, Katsarlinos P, Trypaki M, Messaritakis I, Stournaras C, et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758834017750121. doi: 10.1177/1758834017750121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulasinghe A, Kapeleris J, Kimberley R, Mattarollo SR, Thompson EW, Thiery JP, Kenny L, O'Byrne K, Punyadeera C. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018;7(12):5910–5919. doi: 10.1002/cam4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Wang R, Sun X, Liu Y, Wang Z, Yan J, Kong X, Liang S, Liu Q, Zhao T, et al. Prognostic significance of PD-L1 expression on cell-surface vimentin-positive circulating tumor cells in gastric cancer patients. Mol Oncol. 2020;14(4):865–881. doi: 10.1002/1878-0261.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satelli A, Batth IS, Brownlee Z, Rojas C, Meng QH, Kopetz S, Li S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci Rep. 2016;6:28910. doi: 10.1038/srep28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tada H, Takahashi H, Kuwabara-Yokobori Y, Shino M, Chikamatsu K. Molecular profiling of circulating tumor cells predicts clinical outcome in head and neck squamous cell carcinoma. Oral Oncol. 2020;102:104558. doi: 10.1016/j.oraloncology.2019.104558. [DOI] [PubMed] [Google Scholar]
- 32.Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, Li D, Wang R, Dang Y, Hu Z, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology. 2018;7(7):e1438111. doi: 10.1080/2162402X.2018.1438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams DL, Adams DK, He J, Kalhor N, Zhang M, Xu T, Gao H, Reuben JM, Qiao Y, Komaki R, et al. Sequential Tracking of PD-L1 expression and RAD50 Induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin Cancer Rese. 2017;23(19):5948–5958. doi: 10.1158/1078-0432.CCR-17-0802. [DOI] [PubMed] [Google Scholar]
- 34.Manjunath Y, Upparahalli SV, Avella DM, Deroche CB, Kimchi ET, Staveley-O’Carroll KF, Smith CJ, Li G, Kaifi JT. PD-L1 Expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer. Cancers. 2019;11(6). doi: 10.3390/cancers11060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strati A, Koutsodontis G, Papaxoinis G, Angelidis I, Zavridou M, Economopoulou P, Kotsantis I, Avgeris M, Mazel M, Perisanidis C, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol. 2017;28(8):1923–1933. doi: 10.1093/annonc/mdx206. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Kim TH, Fouladdel S, Zhang Z, Soni P, Qin A, Zhao L, Azizi E, Lawrence TS, Ramnath N, et al. PD-L1 Expression in circulating tumor cells increases during radio(chemo)therapy and indicates poor prognosis in non-small cell lung cancer. Sci Rep. 2019;9(1):566. doi: 10.1038/s41598-018-36096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Zhang X, Liu Y, Zhang T, Wang Z, Gu M, Wang DD, Li W, Lin PP. PD-L1(+) aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2020;469:355–366. doi: 10.1016/j.canlet.2019.10.041. [DOI] [PubMed] [Google Scholar]
- 38.Koh Y, Yagi S, Akamatsu H, Kanai K, Hayata A, Tokudome N, Akamatsu K, Higuchi M, Kanbara H, Nakanishi M, et al. Heterogeneous expression of programmed death receptor-ligand 1 on circulating tumor cells in patients with lung cancer. Clin Lung Cancer. 2019;20(4):270–7.e1. doi: 10.1016/j.cllc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Flaifel A, Xie W, Braun DA, Ficial M, Bakouny Z, Nassar AH, Jennings RB, Escudier B, George DJ, Motzer RJ, et al. PD-L1 expression and clinical outcomes to cabozantinib, everolimus, and sunitinib in patients with metastatic renal cell carcinoma: analysis of the randomized clinical trials METEOR and CABOSUN. Clin Cancer Rese. 2019;25(20):6080–6088. doi: 10.1158/1078-0432.CCR-19-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 41.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus Chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 42.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 43.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Rese. 2016;22(4):813–820. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 46.Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853–61.e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, Rahimi K, Shaw PA, Clarke BA, Nelson BH, et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Rese. 2017;23(4):925–934. doi: 10.1158/1078-0432.CCR-16-1433. [DOI] [PubMed] [Google Scholar]
- 48.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Man Lei Y, Jabri B, Alegre M-L, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morita R, Okishio K, Shimizu J, Saito H, Sakai H, Kim YH, Hataji O, Yomota M, Nishio M, Aoe K, et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: a multicenter retrospective observational study in Japan. Lung Cancer. 2020;140:8–18. doi: 10.1016/j.lungcan.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Fang C, Zhang C, Zhao WQ, Hu WW, Wu J, Ji M. Co-mutations of TP53 and KRAS serve as potential biomarkers for immune checkpoint blockade in squamous-cell non-small cell lung cancer: a case report. BMC Med Genomics. 2019;12(1):136. doi: 10.1186/s12920-019-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pascual M, Mena-Varas M, Robles EF, Garcia-Barchino MJ, Panizo C, Hervas-Stubbs S, Alignani D, Sagardoy A, Martinez-Ferrandis JI, Bunting KL, et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood. 2019;133(22):2401–2412. doi: 10.1182/blood.2018889931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coelho MA, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, East P, Spencer-Dene B, Nye E, Barnouin K, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity. Immunity. 2017;47(6):1083–99.e6. doi: 10.1016/j.immuni.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


