Abstract
Background and Aims
Etrasimod is an oral, selective, sphingosine 1-phosphate receptor modulator. In a phase 2, randomised, double-blind, placebo-controlled trial in adults with moderately-to-severely active ulcerative colitis [OASIS], etrasimod 2 mg provided significant benefit versus placebo and was generally well tolerated. This open-label extension [OLE] evaluated safety and efficacy of etrasimod for up to 52 weeks.
Methods
In OASIS, 156 patients received etrasimod 1 mg, etrasimod 2 mg, or placebo, once daily for 12 weeks. After completing OASIS, patients could enrol in the OLE and receive etrasimod 2 mg for an additional 34–40 weeks.
Results
In all, 118 patients enrolled in the OLE; 112 patients received etrasimod 2 mg at any point and were evaluated for safety and efficacy. A total of 92 [82%] patients who received etrasimod 2 mg in the OLE completed the study. Treatment-emergent adverse events occurred in 60% [67/112] of patients receiving etrasimod 2 mg at any time, most commonly worsening ulcerative colitis and anaemia; 94% of adverse events were mild/moderate. At end of treatment, 64% of patients met the criteria for clinical response, 33% for clinical remission, and 43% for endoscopic improvement. Week 12 clinical response, clinical remission, or endoscopic improvement was maintained to end of treatment in 85%, 60%, or 69% of patients, respectively. Steroid-free clinical remission occurred in 22% of overall patients.
Conclusions
In this long-term extension study, etrasimod 2 mg demonstrated a favourable safety profile. Most patients with clinical response, clinical remission, or endoscopic improvement at Week 12 maintained that status to end of treatment.
Keywords: Ulcerative colitis, etrasimod, long-term extension study
1. Introduction
Ulcerative colitis [UC] is a chronic, disabling, immune-mediated disorder of the large intestine.1,2 Goals of UC treatment include achieving long-term, sustained, and durable steroid-free clinical and endoscopic remission, and preventing the need for colectomy.1–4
Anti–tumour necrosis factor alpha [TNFα] agents [eg, infliximab, adalimumab, golimumab], as well as vedolizumab, tofacitinib, and ustekinumab, are used for treatment of moderately-to-severely active UC.2–4 However, current treatment options have relatively low remission rates and/or loss of response over time and may be associated with side effects.5–8 In addition, despite the advent of novel treatments, 10–15% of patients still require a colectomy.5,9–12 Thus, a significant unmet need remains for novel therapies to treat this disorder.
Sphingosine 1-phosphate [S1P] receptor modulation has been investigated as a potential treatment pathway for a number of immune-mediated conditions and has been widely used in multiple sclerosis over the past decade. The interaction of S1P with S1P receptors 1 [S1P1] through 5 [S1P5] modulates a wide range of biological functions, including lymphocyte trafficking and endothelial barrier integrity.13,14 The S1P receptor modulators fingolimod, siponimod, and ozanimod have been approved by regulators for treatment of multiple sclerosis,15–20 and ozanimod has also been studied for treatment of moderate-to-severe UC for up to 32 weeks.21
Etrasimod is an oral, selective S1P1, S1P4, and S1P5 receptor modulator in development for treatment of immune- and inflammatory-mediated diseases.22 Etrasimod 2 mg provided significant benefit compared with placebo and was generally well tolerated in the OASIS study [NCT02447302], a 12-week phase 2 trial in adult patients with moderately-to-severely active UC and previous inadequate response, loss of response, or intolerance to conventional or biologic therapy.23 In addition to its safety and efficacy in UC, etrasimod has been shown to rapidly decrease mean lymphocyte counts in healthy volunteers24,25 and in patients with UC23; after drug discontinuation, lymphocyte levels recovered to within 5% of baseline in 7 days.24,25
This open-label extension [OLE] study [NCT02536404] evaluated, for up to an additional 34 to 40 weeks [46 to 52 weeks total], the safety and efficacy of once-daily etrasimod 2 mg in achieving and maintaining clinical response and/or remission in patients who completed OASIS.
2. Materials and Methods
The OLE study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and was approved by the institutional review board at each centre. All patients provided written informed consent. No study data will be made available to others.
2.1. Study design and treatment
OASIS was a randomised, double-blind [DB], placebo-controlled, parallel-group, multicentre study in 156 patients. Details of the OASIS study design have been previously reported, including description of the modified Mayo Clinic score [mMCS; range, 0–9; composed of endoscopic, rectal bleeding, and stool frequency subscores, each with range 0–3] and the full inclusion and exclusion criteria.23 Briefly, patients enrolled in the DB study were 18–80 years old with UC and had an mMCS of 4–9, a centrally read endoscopic subscore of ≥ 2, and a rectal bleeding subscore of ≥ 1. During the OASIS study, patients received DB treatment with once daily etrasimod 1 mg, etrasimod 2 mg, or placebo for 12 weeks.
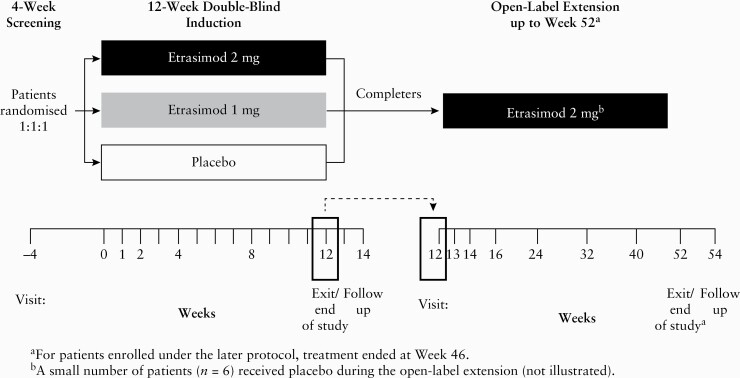
Patients who completed the DB study were eligible to enrol in the OLE and receive open-label, once daily etrasimod 2 mg, irrespective of their treatment assignment or response in the DB study, for up to an additional 34 to 40 weeks [46 to 52 weeks total across the DB and OLE phases; Figure 1].
Figure 1.
Study design.
Patients were enrolled under either early or late protocol amendments [see Supplementary Table 1, available as Supplementary data at ECCO-JCC online, for details]. Under the early protocol amendment, a small subgroup of patients received placebo instead of open-label etrasimod or received placebo followed by open-label etrasimod 2 mg.
The end of treatment [EOT] time point occurred at Week 46 [or at Week 52 for patients enrolled under the early protocol amendment], or earlier for patients who terminated before the scheduled end of therapy. At EOT, patients were assessed for efficacy using the Mayo Clinic Score [MCS] and flexible proctosigmoidoscopy that was centrally read. Compliance was assessed based on investigator records of study medication dispensed and used by each patient.
Corticosteroids, which had been permitted at stable dosage in the DB study, were allowed to be tapered as tolerated during the OLE study.
2.2. Outcome measures
The primary objective of the study was the long-term safety and tolerability of etrasimod. Safety was assessed by treatment-emergent adverse events [TEAEs] and treatment-emergent serious adverse events [AEs] up to 30 days following discontinuation of study drug. TEAEs were defined as any AE that occurred after the first dose in the OLE, including any AEs that started in the DB study and were ongoing, worsened, or ended during the OLE. Post hoc analyses were performed to summarise TEAEs excluding worsening UC and the TEAEs occurring in the subgroup of patients who did not have worsening UC during the OLE. Lymphocyte counts were measured during the DB and OLE studies at least every 4 weeks [except at Week 40] up to Week 44 and at EOT. Change from DB baseline in lymphocyte count was evaluated in the evaluable cohort using ‘as observed’ analyses, as described below.
Key efficacy endpoints included the proportion of patients with clinical response, clinical remission, or endoscopic improvement at EOT, or sustained from Week 12 to EOT. Endpoint definitions were: clinical remission as Mayo Clinic endoscopic score ≤ 1 [with absence of friability], rectal bleeding score ≤ 1, and stool frequency score ≤ 1 with ≥ 1-point decrease from DB baseline at EOT; clinical response as clinical remission or decrease relative to DB baseline in the mMCS of ≥ 2 points and ≥ 30% decrease, with either a decrease from DB baseline in rectal bleeding of ≥ 1 or rectal bleeding score of ≤ 1 at EOT; and endoscopic improvement as Mayo Clinic endoscopic subscore ≤ 1 at EOT. For a particular outcome, sustained response was defined as meeting the criteria of response at both Week 12 and EOT. Steroid-free clinical remission was defined as clinical remission at EOT among patients who either did not use oral corticosteroids at any point in the OLE or were corticosteroid-free for at least 12 weeks before EOT.
2.3. Study populations and statistical analyses
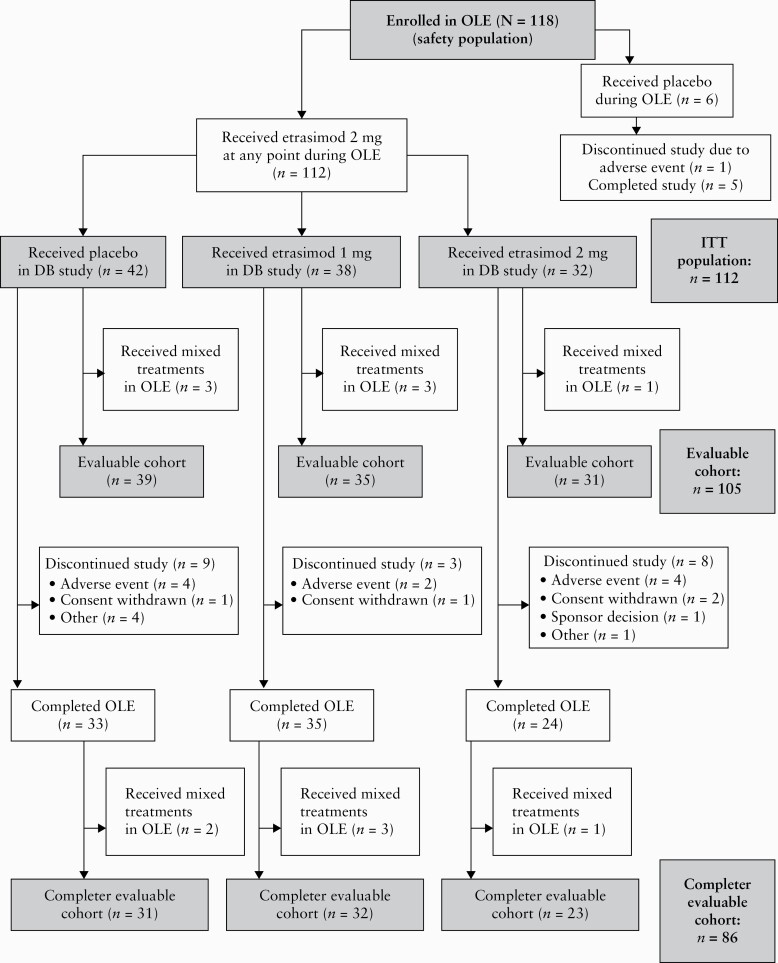
The patient populations for analysis are shown in Figure 2. The safety population included all patients enrolled in the OLE. The intention-to-treat [ITT] population was used for the key efficacy analyses and included all patients who received etrasimod 2 mg during the OLE. Due to early and late protocol amendments [see Supplementary Table 1], some patients were eligible to receive placebo in the OLE before switching to etrasimod 2 mg. The evaluable cohort included those patients whose only treatment assignment during the OLE was to etrasimod 2 mg; patients who switched treatments during the OLE period for any reason were excluded from the evaluable cohort. The completer evaluable cohort included patients who completed the OLE and whose only treatment assignment during the OLE period was to etrasimod 2 mg. The etrasimod 2 mg treat-through group comprised patients who received etrasimod 2 mg during both the DB period and the OLE.
Figure 2.
Patient disposition. DB, double-blind; ITT, intention-to-treat; OLE, open-label extension.
All statistical analyses were descriptive. No formal comparisons were performed between DB treatment groups. Descriptive statistics of the key efficacy endpoints included the proportion [%] and its 90% exact confidence interval of patients who met the criteria for each key endpoint by treatment group in the DB study. Efficacy for the key endpoints was evaluated according to the ITT principle. Non-responder imputation [NRI] analysis was used for all proportion-based endpoints. Data missing for any reason, including study discontinuation, were imputed as non-response. Evaluation of outcomes not based on proportions used ‘as observed’ analyses of the full evaluable cohort and/or completer evaluable cohort. In the ‘as observed’ analyses, no missing data were imputed or carried forward from earlier time points; therefore, the number of patients varied for each endpoint. To account for the treatment complexity due to the various protocol amendments, ‘as observed’ analyses were also conducted for proportion-based outcomes and are reported in the Supplementary Materials, available as Supplementary data at ECCO-JCC online.
3. Results
3.1. Patient disposition and characteristics
The OLE study was conducted from 25 January 2016 to 1 November 2018, at 51 study sites in 16 countries. A total of 118 patients [safety population; 84% of the 141 patients who completed the DB induction study] entered the OLE. A total of 43 patients were enrolled under the early protocol amendment and could receive treatment for up to 52 weeks, and 75 patients were enrolled under later amendments and could receive treatment for up to 46 weeks.
Of the 118 patients who entered the OLE, 112 patients received etrasimod 2 mg at any point and formed the ITT population; six patients received placebo [Figure 2].
Among the 112 patients in the ITT population, 105 patients [the evaluable cohort] received etrasimod 2 mg throughout the OLE, and seven patients received placebo initially, followed by etrasimod 2 mg. The completer evaluable cohort included 86 patients and comprised patients who completed the OLE and whose only treatment assignment during the OLE was to etrasimod 2 mg. A total of 32 patients in the ITT population received etrasimod 2 mg during both the DB study and the OLE and formed the etrasimod 2 mg treat-through group.
Of patients in the ITT population, 82% [92/112] completed the study; 75% [24/32] of patients in the etrasimod 2 mg treat-through group completed the study.
The median [range] study drug exposure of the ITT population during the OLE was 34 [0.7–44] weeks in addition to the 12 weeks of treatment during the DB study. Of the 112 patients in the ITT population, 22 [19.6%] were treated for at least 52 weeks. Mean treatment compliance of patients in the ITT population was 98%. In the ITT population, mean (standard deviation [SD]) age was 44 [13] years, 39% were female, and 95% were White [Table 1].
Table 1.
Baseline characteristics [safety population].
| Treatment in OLE: | Etrasimod 2 mg | Placebo | |||
|---|---|---|---|---|---|
| Treatment in DB study: | Placebo [n = 42] |
Etrasimod 1 mg [n = 38] |
Etrasimod 2 mg [n = 32] |
Overall [n = 112] |
Total [n = 6] |
| Age, mean [SD], y | 46.2 [15.1] | 44.6 [12.2] | 39.2 [11.0] | 43.7 [13.3] | 50.2 [13.9] |
| Female, n [%] | 15 [35.7] | 16 [42.1] | 13 [40.6] | 44 [39.3] | 3 [50.0] |
| Race, n [%]a | |||||
| White | 39 [92.9] | 35 [92.1] | 32 [100] | 106 [94.6] | 6 [100] |
| Weight, mean [SD], kg | 75.9 [15.9] | 73.1 [12.6] | 70.9 [17.3] | 73.5 [15.3] | 84.7 [22.2] |
| BMI, mean [SD], kg/m2 | 25.8 [4.8] | 24.8 [3.5] | 24.0 [5.2] | 24.9 [4.5] | 28.6 [6.3] |
| Baseline total MCS, mean [SD] | 6.6 [2.6] | 5.8 [3.1] | 4.9 [3.4] | 5.8 [3.1] | 5.8 [1.9] |
| Baseline mMCS, mean [SD] | 5.0 [2.1] | 4.3 [2.5] | 3.6 [2.5] | 4.4 [2.4] | 4.7 [1.4] |
| Duration of UC, median, yb | 5.8 | 4.7 | 4.4 | 4.9 | 6.7 |
| Disease extent, n [%]b,c | |||||
| Proctosigmoiditis | 27 [64.3] | 27 [71.1] | 21 [65.6] | 75 [67.0] | 1 [16.7] |
| Pancolitis | 20 [47.6] | 11 [28.9] | 6 [18.8] | 37 [33.0] | 3 [50.0] |
| Baseline faecal calprotectin, mean [SD], µg/g | 2276 [3055] | 2267 [4448] | 988 [1592] | 1896 [3293] | 1475 [938] |
| Baseline C-reactive protein, mean [SD], nmol/L | 10.0 [18.6] | 9.1 [13.1] | 8.6 [13.6] | 9.3 [15.4] | 3.4 [3.4] |
| Previous and concomitant treatments for UC | |||||
| Current oral corticosteroids at DB baseline, n [%]b | 14 [33.3] | 11 [28.9]d | 13 [40.6] | 38 [33.9]d | 3 [50.0] |
| Previous anti-TNFα agents, n [%]b | 15 [35.7] | 8 [21.1] | 9 [28.1] | 32 [28.6] | 4 [66.7] |
| Previous immunosuppressants, n [%]b | 24 [57.1] | 12 [31.6] | 16 [50.0] | 52 [46.4] | 4 [66.7] |
| Previous anti-integrin agents, n [%]b | 9 [21.4] | 3 [7.9] | 3 [9.4] | 15 [13.4] | 2 [33.3] |
| Previous or current oral 5-aminosalicylates at DB baseline, n [%]b | 41 [97.6] | 37 [97.4] | 29 [90.6] | 107 [95.5] | 5 [83.3] |
Unless noted, demographic and baseline characteristics are presented as at DB Week 12 [OLE Day −1]. The overall group includes patients who received any treatment [placebo, etrasimod 1 mg, or etrasimod 2 mg] during the DB period.
BMI, body mass index; DB, double-blind; MCS, Mayo Clinic score; mMCS, modified Mayo Clinic score; OLE, open-label extension; SD, standard deviation; TNFα, tumour necrosis factor alpha; UC, ulcerative colitis; y, year.
aPatients with multiple races were counted once in each race category.
bCollected at DB baseline.
cFor history of proctosigmoiditis and pancolitis, the responses are not mutually exclusive. Some patients reported a history of both proctosigmoiditis and pancolitis: for patients receiving etrasimod 2 mg in the OLE [by DB treatment group], placebo, n = 8; etrasimod 1 mg, n = 7; etrasimod 2 mg, n = 3; for total patients receiving placebo in the OLE, n = 0.
dOne patient in the group who received etrasimod 1 mg during the DB study received oral corticosteroid treatment for a condition other than UC and is not included in the number of patients with current oral corticosteroid treatment for UC.
3.2. Safety
The occurrence of TEAEs in the safety population is summarised in Table 2 with additional detail provided in Supplementary Table 2, available as Supplementary data at ECCO-JCC online. Of patients treated with any etrasimod 2 mg in the OLE, 60% [67/112] experienced ≥ 1 TEAE [Table 2]. When patients with worsening UC [n = 21] were excluded from the analysis, 51% [46/91] of patients treated with any etrasimod 2 mg in the OLE experienced ≥ 1 TEAE [Supplementary Table 3, available as Supplementary data at ECCO-JCC online]. Among patients receiving any etrasimod 2 mg in the OLE, most TEAEs (238/252 [94.4%]) were of mild or moderate severity. The most commonly reported TEAEs in patients receiving any etrasimod 2 mg in the OLE, occurring in ≥ 10% of patients, were worsening UC (21/112 [19%] patients) and anaemia (12/112 [11%] patients]).
Table 2.
Summary of treatment-emergent adverse events [safety population].a
| Treatment in OLE: | Etrasimod 2 mg | Placebo | |||
|---|---|---|---|---|---|
| Treatment in DB study: | Placebo [n = 42] | Etrasimod 1 mg [n = 38] | Etrasimod 2 mg [n = 32] | Overall [n = 112] | Total [n = 6]b |
| Patients with ≥ 1 TEAE, n [%] | 25 [59.5] | 25 [65.8] | 17 [53.1] | 67 [59.8] | 5 [83.3] |
| Number of TEAEs | 111 | 85 | 56 | 252 | 22 |
| Number of TEAEs, excluding TEAE of worsening UCc | 105 | 77 | 47 | 229 | 21 |
| Patients with TEAEs leading to death, n | 0 | 0 | 0 | 0 | 0 |
| Patients with TEAEs leading to study discontinuation, n [%] | 4 [9.5] | 2 [5.3] | 4 [12.5] | 10 [8.9] | 1 [16.7] |
| Ulcerative colitis—worseningc | 2 [4.8] | 2 [5.3] | 4 [12.5] | 8 [7.1] | 1 [16.7] |
| Atrial fibrillation | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Headache | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Patients with serious TEAEs, n [%] [no. of events]d | 4 [9.5] [11] | 0 | 3 [9.4] | 7 [6.3] [14] | 0 |
| Gastrointestinal disorders | 2 [4.8] [5] | 0 | 1 [3.1] | 3 [2.7] [6] | 0 |
| Ulcerative colitis—worseninge | 2 [4.8] | 0 | 1 [3.1] | 3 [2.7] | 0 |
| Pancreatitis | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Large intestine perforation | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Blood and lymphatic system disorders | 0 | 0 | 2 [6.3] | 2 [1.8] | 0 |
| Iron-deficiency anaemia | 0 | 0 | 2 [6.3] | 2 [1.8] | 0 |
| Infections and infestations | 1 [2.4] [2] | 0 | 0 | 1 [0.9] [2] | 0 |
| Gastroenteritis | 1 [2.4] [2] | 0 | 0 | 1 [0.9] [2] | 0 |
| Renal and urinary disorders | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Cystitis, haemorrhagic | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Nervous system disorders | 2 [4.8] | 0 | 0 | 2 [1.8] | 0 |
| Fine motor skill dysfunction | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Transient ischaemic attack | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Cardiac disorders | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Atrial fibrillation | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
| Severity [all TEAEs], n [%] [no. of events]d,f | |||||
| Grade 1—mild | 18 [42.9] [44] | 17 [44.7] [46] | 10 [31.3] [19] | 45 [40.2] [109] | 4 [66.7] [9] |
| Grade 2—moderate | 20 [47.6] [59] | 23 [60.5] [38] | 12 [37.5] [32] | 55 [49.1] [129] | 5 [83.3] [13] |
| Grade 3—severe | 5 [11.9] [8] | 1 [2.6] | 5 [15.6] | 11 [9.8] [14] | 0 |
| Grade 4—life-threatening | 0 | 0 | 0 | 0 | 0 |
| Grade 5—death related to TEAE | 0 | 0 | 0 | 0 | 0 |
| Severity [treatment-related TEAEs], n [%] [no. of events]d,f,g | |||||
| Grade 1—mild | 6 [14.3] [9] | 3 [7.9] | 1 [3.1] [2] | 10 [8.9] [14] | 0 |
| Grade 2—moderate | 8 [19.0] [12] | 1 [2.6] | 2 [6.3] | 11 [9.8] [15] | 1 [16.7] |
| Grade 3—severe | 0 | 0 | 1 [3.1] | 1 [0.9] | 0 |
| Grade 4—life-threatening | 0 | 0 | 0 | 0 | 0 |
| Grade 5—death related to TEAE | 0 | 0 | 0 | 0 | 0 |
| TEAE relation to study drug, n [%] [no. of events]d,f,g | |||||
| Not related | 24 [57.1] [90] | 25 [65.8] [81] | 17 [53.1] [51] | 66 [58.9] [222] | 5 [83.3] [21] |
| Related | 9 [21.4] [21] | 3 [7.9] [4] | 4 [12.5] [5] | 16 [14.3] [30] | 1 [16.7] |
| Treatment-related TEAEs of special interest, n [%]f,g | |||||
| Atrioventricular block first degree [grade 1 severity] | 1 [2.4] | 0 | 0 | 1 [0.9] | 0 |
The overall group includes patients who received any treatment [placebo, etrasimod 1 mg, or etrasimod 2 mg] during the DB period.
AE, adverse event; DB, double-blind; OLE, open-label extension; TEAE, treatment-emergent AE; UC, ulcerative colitis.
aTEAEs were defined as any AE that occurred after the first dose of study medication in the OLE, including any AEs that started in the DB study and were ongoing, worsened, or ended in the OLE. Events were coded using the Medical Dictionary for Regulatory Activities, version 20.1.
bOf the six patients who received placebo in the OLE, in the DB study two received placebo, one received etrasimod 1 mg, and three received etrasimod 2 mg.
cIncludes ‘colitis ulcerative’ and ‘colitis’.
dAt each level of patient summarisation, a patient was counted once if the patient reported one or more events. Unless otherwise indicated, the number of events = the number of patients.
eIncludes ‘colitis ulcerative’, ‘colitis’, and ‘proctitis ulcerative’.
fSeverity of TEAEs was assessed by investigator and graded according to the US National Cancer Institute Common Terminology Criteria for Adverse Events [Version 4.03] definitions. At each level of patient summarisation, a patient was counted once for the most severe event.
gRelatedness was determined by investigator judgement.
Fourteen serious TEAEs were reported in seven patients treated with any etrasimod 2 mg in the OLE [Table 2], of which only one was considered treatment-related as determined by the investigator [worsening UC]. Excluding patients with worsening UC, three patients reported a total of three serious TEAEs [Supplementary Table 3]. There were no treatment-related serious infections, and no patient had an infection of severity grade ≥ 3. There were two cases of herpes zoster, neither leading to study discontinuation: one was considered unrelated to treatment in a patient receiving etrasimod 2 mg in the OLE, and one was considered treatment-related in a patient receiving placebo in the OLE. No patient died during the study. Ten of 112 [9%] patients in the etrasimod 2 mg safety population discontinued study drug due to a TEAE [eight patients with worsening UC and one patient each with atrial fibrillation and headache].
The impact on heart rate and atrioventricular [AV] conduction was minimal. Among patients who first received etrasimod 2 mg in the OLE after receiving placebo in the DB study, change from the OLE baseline heart rate was greatest at 3 h after the initial dose, when a mean [SD] reduction of 8.8 [10.0] beats/min was recorded. One patient experienced an AE of heart rate lowering [grade 1 severity], with a nadir of 48 beats/min, which did not lead to change in dose or study discontinuation. Among patients who received etrasimod 1 mg in the DB study and an increased etrasimod dose of 2 mg in the OLE, the largest mean change from baseline heart rate on Day 1 was −3.6 beats/min, occurring at 2 hours post-dose. No patient discontinued due to low heart rate.
Three patients who received etrasimod 2 mg experienced first-degree AV block, two of whom received placebo in the DB study and one of whom received etrasimod 2 mg in the DB study. In two cases, the AV block was considered clinically insignificant and was not reported as an AE; in one case it was reported as a mild AE [grade 1 severity]. No patient discontinued due to AV block. One patient who experienced AV block [not considered clinically significant or reported as an AE by the investigator] also experienced an event of atrial fibrillation [grade 3 severity] leading to study discontinuation that was considered not related to study drug. Additional details are included in Supplementary Table 4, available as Supplementary data at ECCO-JCC online.
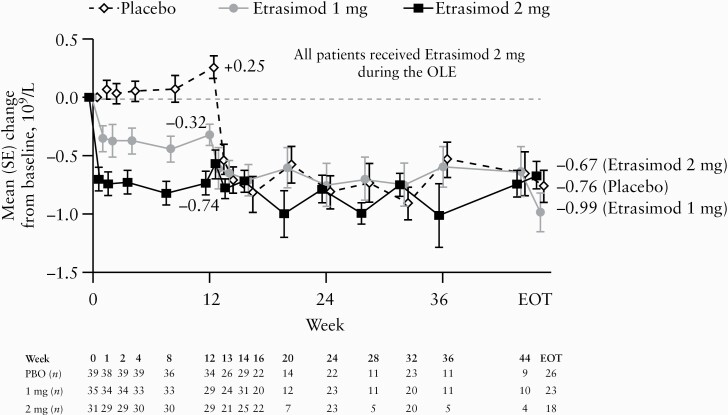
Mean lymphocyte reduction from the beginning of the DB study to EOT was 0.81 GI/L [43% relative reduction] in the overall evaluable cohort. In the evaluable etrasimod 2 mg treat-through group, mean lymphocyte reduction from the beginning of the DB study was 0.74 GI/L [43% relative reduction] at Week 12 and 0.67 GI/L [38% relative reduction] at EOT [Figure 3]. Significant reductions in mean lymphocyte count occurred within 1 or 2 weeks of initiating etrasimod 2 mg in the OLE and plateaued to EOT in the evaluable cohort groups that had received placebo or etrasimod 1 mg, respectively, in the DB period. One of 92 [1.1%] patients in the evaluable cohort developed lymphocyte count below 0.5 GI/L during the OLE. No patients discontinued due to low lymphocyte count.
Figure 3.
Change from DB baseline in lymphocyte count by treatment in the DB study [evaluable cohort]. All patients received etrasimod 2 mg during the OLE. During the DB study, 1 mg and 2 mg groups were treated with etrasimod 1 mg and 2 mg, respectively. The evaluable cohort included patients who received any etrasimod 2 mg during the OLE and had the same treatment assignment throughout the OLE. In this ‘as observed’ analysis, only patients with non-missing assessments were included, and no missing data were imputed. DB, double-blind; EOT, end of treatment; OLE, open-label extension; PBO, placebo; SE, standard error.
3.3. Efficacy
At EOT 64% [72/112] of patients had a clinical response, 33% [37/112] were in clinical remission, and 43% [48/112] had endoscopic improvement [Table 3]. Clinical response and clinical remission rates were numerically lower in patients with previous exposure to biologics [anti-integrin or anti-TNFα agents] [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. Results of the ‘as observed’ analyses are provided in Supplementary Table 5, available as Supplementary data at ECCO-JCC online.
Table 3.
Key efficacy endpoints by treatment in the DB study [ITT population].
| Placebo [n = 42] |
Etrasimod 1 mg [n = 38] |
Etrasimod 2 mg [n = 32] | Overall [n = 112] | Placebo [n = 42] | Etrasimod 1 mg [n = 38] | Etrasimod 2 mg [n = 32] | Overall [n = 112] | |
|---|---|---|---|---|---|---|---|---|
| Efficacy outcome | Week 12 | End of treatment | ||||||
| Patients with clinical response | ||||||||
| n [%] 90% CI | 13 [31.0] 19.4, 44.6 | 16 [42.1] 28.5, 56.7 | 17 [53.1] 37.3, 68.5 | 46 [41.1] 33.2, 49.3 | 25 [59.5] 45.7, 72.3 | 28 [73.7] 59.5, 85.0 | 19 [59.4] 43.3, 74.0 | 72 [64.3] 56.2, 71.8 |
| Patients with clinical remission | ||||||||
| n [%] 90% CI | 4 [9.5] 3.3, 20.5 | 6 [15.8] 7.1, 28.8 | 15 [46.9] 31.5, 62.7 | 25 [22.3] 16.0, 29.8 | 12 [28.6] 17.4, 42.1 | 14 [36.8] 23.8, 51.5 | 11 [34.4] 20.6, 50.4 | 37 [33.0] 25.7, 41.1 |
| Patients with endoscopic improvement | ||||||||
| n [%] 90% CI | 7 [16.7] 8.1, 29.0 | 8 [21.1] 10.9, 34.8 | 16 [50.0] 34.4, 65.6 | 31 [27.7] 20.8, 35.5 | 15 [35.7] 23.5, 49.5 | 20 [52.6] 38.2, 66.7 | 13 [40.6] 26.0, 56.7 | 48 [42.9] 34.9, 51.1 |
All patients received etrasimod 2 mg during the OLE. Groups are based on treatment during the DB period. The overall group includes patients who received any treatment [placebo, etrasimod 1 mg, or etrasimod 2 mg] during the DB period. Week 12 was the end of the DB period. The ITT population included all patients who received any etrasimod 2 mg during the OLE. In the NRI analysis, data missing due to any reason, including study discontinuation, were imputed as non-response. 90% CI are exact CI for the % of patients with the given endpoint.
CI, confidence interval; DB, double-blind; n, number of patients; NRI, non-responder imputation; OLE, open-label extension; ITT, intention-to-treat.
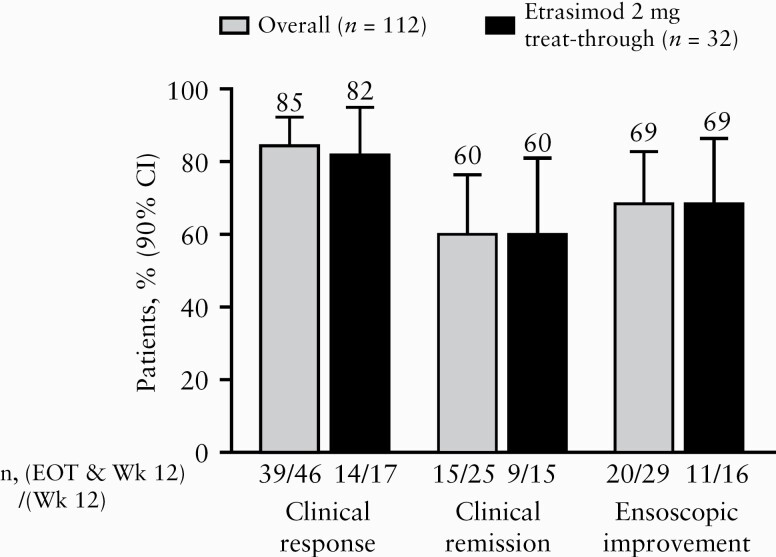
Among patients with clinical response, clinical remission, or endoscopic improvement at Week 12 of the DB study, treatment effects were maintained at EOT in the majority of patients [Figure 4]. Of patients with each respective response at Week 12, 85% [39/46] maintained clinical response to EOT, 60% [15/25] experienced sustained clinical remission, and 69% [20/29] maintained endoscopic improvement. Among patients with clinical response at Week 12, 54% [25/46] were in clinical remission at EOT. Results of the ‘as observed’ analyses are provided in Supplementary Figure 2, available as Supplementary data at ECCO-JCC online.
Figure 4.
Proportion of patients with sustained response from Week 12 to EOT [ITT population]. All patients received etrasimod 2 mg during the OLE. The overall group includes patients who received any treatment [placebo, etrasimod 1 mg, or etrasimod 2 mg] during the DB study. The etrasimod 2 mg treat-through group received etrasimod 2 mg during both the DB study and OLE. In these NRI analyses, data missing for any reason were imputed as non-response. CI, confidence interval; DB, double-blind; EOT, end of treatment; ITT, intention-to-treat; n, number of patients; NRI, non-responder imputation; OLE, open-label extension; Wk, week.
Treatment with etrasimod 2 mg in the OLE resulted in mean [SD] improvement in mMCS from DB baseline of 2.1 [2.2] points at Week 12 which continued to EOT, with mean [SD] improvement in mMCS from DB baseline at EOT of 3.4 [2.3] points for the evaluable cohort ‘as observed’ analysis overall group [Supplementary Table 6, available as Supplementary data at ECCO-JCC online]. At EOT, the evaluable groups that had received placebo or etrasimod 1 mg in the DB study had reached comparable mean [SD] improvements in mMCS to the etrasimod 2 mg group (3.4 [2.4], 3.4 [2.1], and 3.4 [2.6] points, respectively).
A majority of patients [65%; 73/112] were not using oral corticosteroids at the start of the OLE. At EOT, 67% [75/112] of patients either had not used oral corticosteroids at any time during the OLE [n = 73] or had been steroid-free for at least 12 weeks [n = 2]. Of these patients, 33% [25/75] had steroid-free clinical remission at EOT [Table 4]. Overall, 22% [25/112] of patients in the study had steroid-free clinical remission at EOT. Results of the evaluable cohort ‘as observed’ analyses are provided in Supplementary Table 7, available as Supplementary data at ECCO-JCC online.
Table 4.
Clinical remission at EOT in patients who were steroid-free at EOT by DB treatment [ITT population].
| Placebo [n = 42] |
Etrasimod 1 mg [n = 38] | Etrasimod 2 mg [n = 32] | Overall [n = 112] | |
|---|---|---|---|---|
| Patients with steroid-free clinical remission at EOT | ||||
| N n [%] 90% CI | 29 6 [20.7] 9.4, 36.8 | 25 9 [36.0] 20.2, 54.4 | 21 10 [47.6] 28.6, 67.2 | 75 25 [33.3] 24.3, 43.3 |
All patients received etrasimod 2 mg during the OLE. Groups are based on treatment during the DB period. The overall group includes patients who received any treatment [placebo, etrasimod 1 mg, or etrasimod 2 mg] during the DB period. Week 12 was the end of the DB period. Patients were considered to have steroid-free clinical remission at EOT if they either did not use oral corticosteroids at any point during the OLE or were corticosteroid-free for at least 12 weeks before EOT. The ITT population included all patients who received any etrasimod 2 mg during the OLE. In the NRI analysis, data missing due to any reason, including study discontinuation, were imputed as non-response.
CI, confidence interval; DB, double-blind; EOT, end of treatment; ITT, intention-to-treat; N, number of patients who either did not use oral corticosteroids at any point during the OLE or were corticosteroid-free for at least 12 weeks before EOT; n, number of patients with observation; NRI, non-responder imputation; OLE, open-label extension.
4. Discussion
In this long-term, OLE study of once daily etrasimod 2 mg for treatment for up to a total of 52 weeks of adults with moderately-to-severely active UC, etrasimod was well tolerated. Most [94%] TEAEs experienced by patients who received any etrasimod 2 mg during the study were mild to moderate in severity, and the most common TEAE was worsening UC. One patient who received etrasimod 2 mg in the OLE after receiving placebo in the DB study experienced a TEAE of heart rate lowering, but this did not lead to study discontinuation. The overall results of the OASIS study and the OLE indicate there is no need for dose titration at the beginning of treatment. There were no treatment-related serious infections, and no new safety signals were observed.
Of note, although the DB study allowed the enrolment of patients up to 80 years of age,23 only a small number of patients [n = 5] in the etrasimod 2 mg ITT population were older than 65 years of age. Additional studies will be needed to evaluate long-term etrasimod treatment in patient populations that more closely reflect these patients.
In the OLE, roughly two-thirds of patients across DB treatment groups met the criteria for clinical response at EOT. About one-third of patients experienced clinical remission at EOT, and almost half had endoscopic improvement. At EOT, the subgroup of patients with previous exposure to biologics had a numerically smaller proportion of patients with clinical remission compared with those who did not have previous exposure to biologics. Notably however, in OASIS although patients with previous exposure to biologics had a lower rate of clinical remission at Week 12 compared with patients without previous exposure, etrasimod 2 mg still provided benefit versus placebo in patients with previous biologic exposure. Overall these results suggest that etrasimod may be an effective therapeutic option for a broad range of patients, regardless of their earlier treatment. In the treatment groups who switched from placebo or etrasimod 1 mg in the DB study to etrasimod 2 mg in the OLE, there was a substantial increase in the number of patients with clinical response, clinical remission, or endoscopic improvement during the OLE period and substantial improvement in mean mMCS.
Importantly, most patients maintained the benefits that occurred in the DB period through to EOT. Clinical response observed at Week 12 was sustained to EOT in the vast majority of patients. Clinical remission was sustained from Week 12 to EOT in more than half of patients and, notably, endoscopic improvement was sustained from Week 12 to EOT in more than two-thirds of patients.
Steroid-free clinical remission occurred in one-third of patients at EOT; however, the lack of standardisation for corticosteroid tapering and withdrawal limits the ability to draw conclusions about the potential for steroid-free remission with etrasimod.
Limitations of this OLE study include a relatively small sample size, open-label administration of study drug during the extension phase, the lack of a comparison [placebo] group during the OLE, and the lack of standardised corticosteroid withdrawal. Several patients discontinued treatment because of worsening UC, which may have led to more positive results in the completer evaluable cohort compared with the ITT population. Histological analyses, if they had been included, might have enhanced the analysis of prognostic factors and response.
In this long-term OLE, etrasimod was well tolerated for up to 52 weeks. Dose escalation [from placebo or etrasimod 1 mg to etrasimod 2 mg] was not associated with significant AEs. Etrasimod also demonstrated benefit for maintenance of response. Other advanced therapeutics used to treat UC also have reported maintenance of response in UC, including biologics [anti-TNFα, anti-α4β7 integrin, and anti-interleukin 12 and 23 agents] and a Janus kinase inhibitor,7 but there continues to be an unmet need for therapies with different mechanisms of action, greater durability of response, and improved safety profiles. Etrasimod, an oral small molecule, has the potential to add to the range of treatment options for UC.
In conclusion, clinical response, clinical remission, or endoscopic improvement observed with etrasimod 2 mg at Week 12 in a randomised, controlled, DB study was sustained [or improved] for up to 52 weeks in most patients participating in the OLE. Etrasimod 2 mg demonstrated a favourable safety profile and tolerability with a large majority of patients remaining on therapy over approximately 1 year of treatment. Most TEAEs were of mild to moderate severity, and no new safety signals were observed. This long-term extension study provides support for a long-term safety profile and sustained clinical effects of etrasimod in treating patients with moderately-to-severely active UC.
The study protocol and data collected for the study, including individual patient data, will not be made available to others.
Supplementary Material
Acknowledgements
Lisa Baker, PhD, and Elizabeth Strickland, PhD, inScience Communications, Springer Healthcare [Philadelphia, PA, USA], provided medical writing support funded by Arena Pharmaceuticals.
Funding
This work was supported by Arena Pharmaceuticals [San Diego, CA, USA], which funded medical writing support provided by in Science Communications, Springer Healthcare.
Conflict of Interest
SV has received grants from AbbVie, J&J, Pfizer, and Takeda; and has received consulting and/or speaking fees from AbbVie, Arena Pharmaceuticals, Avaxia, Boehringer Ingelheim, Celgene, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, Hospira, Janssen, Mundipharma, MSD, Pfizer, ProDigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Takeda, Theravance Biopharma, and Tillots Pharma AG. JP has received financial support for research from AbbVie, Merck Sharp & Dohme, and Pfizer; lecture fees from AbbVie, Janssen, Merck Sharp & Dohme, Pfizer, Shire, and Takeda; and consultancy honoraria from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Janssen, Merck Sharp & Dohme, Nestlé, Oppilan Pharma, Pfizer, Progenity, Roche, Shire, Takeda, Theravance Biopharma, and TiGenix. MC has received consulting and/or speaking fees from AbbVie, Arena Pharmaceuticals, Celgene, Janssen, Medtronic, Pfizer, Prometheus, Takeda, and UCB. LP-B has received personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion Healthcare, Takeda, Boehringer Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestlé, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, Bristol Myers Squibb, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera, Theravance Biopharma; grants from AbbVie, Merck Sharpe & Dohme, and Takeda; and stock options from Clinical Trials Mobile Application. BES has received consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Capella Bioscience, Celgene, Celltrion Healthcare, Ferring, Gilead, Hoffmann-La Roche, Ironwood Pharmaceuticals, Janssen, Lilly, Otsuka, Pfizer, Palatin Technologies, Prometheus Laboratories, Rheos Medicines, Salix Pharmaceuticals, Shire, Takeda, Target Pharma Solutions, and Theravance Biopharma; and has received grant/research support for his institution from Celgene and Theravance Biopharma. WJS received consulting fees and medical writing support from Arena Pharmaceuticals relevant to the submitted work; research grants from Atlantic Healthcare, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, Celgene/Receptos, Pfizer, Prometheus Laboratories [now Prometheus Biosciences]; consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion Healthcare, Conatus, Cosmo Pharmaceuticals NV, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Reistone Biopharma, Ritter Pharmaceuticals, Robarts Clinical Trials [owned by Health Academic Research Trust, HART], Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, TiGenix, Tillotts Pharma AG, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse received stock or stock options from Escalier Biosciences, Oppilan Pharmaceuticals, Opthotech, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Ventyx Biosciences, and Vimalan Biosciences; received consulting fees from Opthotech; and was an employee of Escalier Biosciences, Oppilan Biosciences, Opthotech, Prometheus Biosciences, Ventyx Biosciences, and Vimalan Biosciences. SUN, JZ, KL, and CHC are employed by Arena Pharmaceuticals. PK was employed by Arena Pharmaceuticals at the time this study was conducted. He is currently employed by Metacrine.
Author Contributions
All authors made a substantial contribution to study design, data acquisition, analysis, and/or data interpretation and provided intellectual contributions to manuscript development. JZ provided statistical support for the concept and design of the study and data acquisition. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Conference presentation: some of the results reported in this article were presented at United European Gastroenterology Week [UEG Week], October 19–23, 2019, Barcelona, Spain, and at the American College of Gastroenterology Annual Scientific Meeting [ACG], October 25–30, 2019, San Antonio, TX, USA.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 3. Harbord M, Eliakim R, Bettenworth D; European Crohn’s and Colitis Organisation . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- 4. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee . AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018;16:343–56.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George J, Singh S, Dulai PS, et al. Corticosteroid-free remission vs overall remission in clinical trials of moderate-severe ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 2020;26:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh S, Allegretti JR, Siddique SM, Terdiman JP. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1465–96.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47: 162–75. [DOI] [PubMed] [Google Scholar]
- 9. Burisch J, Katsanos KH, Christodoulou DK, et al. ; Epi-IBD Group. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort—an Epi-IBD study. J Crohns Colitis 2019;13:198–208. [DOI] [PubMed] [Google Scholar]
- 10. Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol 2012;107:1228–35. [DOI] [PubMed] [Google Scholar]
- 11. Solberg IC, Lygren I, Jahnsen J, et al. ; IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort [IBSEN Study]. Scand J Gastroenterol 2009;44:431–40. [DOI] [PubMed] [Google Scholar]
- 12. Reinisch W, Reinink AR, Higgins PD. Factors associated with poor outcomes in adults with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:635–42. [DOI] [PubMed] [Google Scholar]
- 13. Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 2017;16:495–503. [DOI] [PubMed] [Google Scholar]
- 14. Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res 2014;55:1596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayzent [prescribing information ]. East Hanover, NJ: Novartis Pharmaceuticals; 2021. [Google Scholar]
- 16. Mayzent [summary of product characteristics]. Dublin: Novartis Europharm; 2020. [Google Scholar]
- 17. Gilenya [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals; 2019. [Google Scholar]
- 18. Gilenya [summary of product characteristics]. Dublin: Novartis Europharm; 2020. [Google Scholar]
- 19. Zeposia [prescribing information]. Summit, NJ: Celgene, 2020. [Google Scholar]
- 20. Zeposia [summary of product characteristics]. Utrect: Celgene Europe; 2020. [Google Scholar]
- 21. Sandborn WJ, Feagan BG, Wolf DC, et al. ; TOUCHSTONE Study Group. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med 2016;374:1754–62. [DOI] [PubMed] [Google Scholar]
- 22. Al-Shamma H, Lehmann-Bruinsma K, Carroll C, et al. The selective sphingosine 1-phosphate receptor modulator etrasimod regulates lymphocyte trafficking and alleviates experimental colitis. J Pharmacol Exp Ther 2019;369:311–7. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Peyrin-Biroulet L, Zhang J, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology 2020;158:550–61. [DOI] [PubMed] [Google Scholar]
- 24. Schreiber S, Morgan M, Christopher R, et al. Etrasimod [APD334], a potent, selective, oral S1P receptor modulator with preclinical autoimmune disease-modifying activity exhibits favorable PK/PD properties in healthy volunteers. In: Advances in Inflammatory Bowel Diseases [AIBD]; December 8–10, 2016; Orlando, FL.
- 25. Peyrin-Biroulet L, Adams J, Turner S, Trokan L, Panes J. Safety and immune modulatory properties of etrasimod [APD334], a next-generation oral, selective sphingosine 1-phosphate receptor [S1PR] modulator, in healthy volunteers. In: 13th Congress of the European Crohn’s and Colitis Organisation [ECCO]; February 14–17, 2018; Vienna, Austria.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.