Abstract
Background and Aims
Ontamalimab, a fully-human monoclonal antibody targeting MAdCAM-1, induced remission in patients with moderate-to-severe ulcerative colitis [UC] in the TURANDOT study. We aimed to assess long-term safety, tolerability, and efficacy of ontamalimab in TURANDOT II.
Methods
TURANDOT II was a phase 2, multicentre, open-label [OL] study in patients with moderate-to-severe UC who completed TURANDOT on placebo or ontamalimab (NCT01771809). Patients were randomised to 75 mg or 225 mg ontamalimab every 4 weeks for 72 weeks [OL1]. The dosage could be increased to 225 mg from Week 8 at the investigator’s discretion. All patients then received 75 mg every 4 weeks for 72 weeks [OL2], followed by 6-month safety follow-up. The primary objective was safety, measured by adverse events [AEs], serious AEs [SAEs], and AEs leading to withdrawal. Mucosal healing [MH; centrally read endoscopy] was assessed.
Results
Of 330 patients, 180 completed OL1; 94 escalated to 225 mg; 127 completed OL2. Overall, 36.1% experienced drug-related AEs. The most common SAE [10.0%] was worsening/ongoing UC; 5.5% of patients had serious infections, the most common being gastroenteritis [0.9%]. One death and four cancers [all unrelated to ontamalimab] occurred. No PML [progressive multifocal leukoencephalopathy]/lymphoproliferative disorders occurred. Geometric mean high-sensitivity C-reactive protein [hsCRP] and faecal calprotectin decreased across OL1 in both dose groups. The proportion of patients assigned to placebo in TURANDOT achieving MH increased from 8.8% [6/68] at baseline to 35.3% at Week 16 [24/68; non-responder imputation]. The corresponding increase in the ontamalimab group was from 23.3% [61/262] to 26.7% [70/262].
Conclusions
Ontamalimab was well tolerated up to 144 weeks in patients with moderate-to-severe UC, with good safety and efficacy.
Keywords: Ulcerative colitis, phase 2, MAdCAM-1
1. Introduction
Ulcerative colitis [UC] is an inflammatory bowel disease [IBD] characterised by episodic or chronic inflammation of the colonic mucosa.1 Inflammation results in symptoms such as rectal bleeding and diarrhoea, significantly affecting health-related quality of life, and in some patients necessitates colectomy.1,2
The aim of treatment for UC is to achieve and maintain remission, which comprises alleviating symptoms and inducing endoscopic mucosal healing.3 Biologics such as infliximab, adalimumab, and golimumab, as well as small molecules including tofacitinib, have contributed to improved outcomes for patients with moderate-to-severe UC who do not respond to first-line therapy with glucocorticoids or immunosuppressants.1,4,5 However, primary non-response and secondary loss of response to anti-tumour necrosis factor [anti-TNF] therapies occur frequently,6,7 and safety concerns surrounding several current treatments remain,8,9 indicating a need for novel therapies with alternative modes of action.
One promising novel target in UC is the interaction between mucosal addressin cell adhesion molecule-1 [MAdCAM-1] and the α4β7 integrin. MAdCAM-1 binds selectively to the α4β7 integrin, which is expressed on the surface of leukocytes [including subsets of blood T lymphocytes, B lymphocytes, natural killer cells, and eosinophils]10 but, notably, does not bind to the α4β1 integrin that interacts with the much more broadly expressed vascular cell adhesion molecule-1 [VCAM-1].11 Unlike integrins, which are expressed on circulating leukocytes, MAdCAM-1 is predominantly expressed on the endothelium of high endothelial venules in the gut and gut-associated lymphoid tissue. In contrast to VCAM-1, MAdCAM-1 is not constitutively expressed in the central nervous system.12,13 MAdCAM-1 expression is upregulated in IBD, and has been shown to play a role in gut immune surveillance and homing of α4β7 integrin-expressing leukocytes during inflammation of the intestinal mucosa.13–15
Selectively targeting the α4β7 integrin or MAdCAM-1 may reduce leukocyte translocation and thereby mucosal inflammation. In line with this theory, vedolizumab, a monoclonal antibody that selectively blocks the α4β7 integrin, is an approved treatment for active moderate-to-severe UC.16 Ontamalimab, a fully-human monoclonal antibody that binds selectively and with high affinity to MAdCAM-1,17 may also reduce intestinal mucosal inflammation; this proposal is supported by positive results from clinical trials.18–21 In a 12-week phase 2 study [TURANDOT], ontamalimab was well tolerated and superior to placebo for the induction of remission in patients with moderate-to-severe UC.21 The current open-label extension study aimed to monitor the safety, tolerability, and pharmacokinetics of ontamalimab, and to assess durability of response during long-term treatment.
2. Materials and Methods
2.1. Study design
TURANDOT II [NCT01771809] was a phase 2, multicentre, two-part, open-label extension study of the anti-MAdCAM-1 antibody ontamalimab [formerly known as SHP647 and PF-00547659] in patients with moderate-to-severe UC. TURANDOT II was an extension of the 12-week randomised controlled induction trial TURANDOT [NCT01620255].21
Patients who were eligible for enrolment in TURANDOT II had completed the blinded 12-week TURANDOT study, in which they received placebo or ontamalimab 7.5, 22.5, 75 or 225 mg subcutaneously [s.c.] every 4 weeks. Eligible patients were aged 18–66 years at the time of consent and must have discontinued immunosuppressants before enrolment in TURANDOT II [with the exception of oral glucocorticoids, as outlined in Section 2.2]. Patients were excluded from the study if they had experienced any serious adverse events [SAEs] related to ontamalimab during TURANDOT or were taking part in any other interventional studies. A full list of inclusion and exclusion criteria is given in Supplementary Table 1, available as Supplementary data at ECCO-JCC online.
The final protocol and amendments were reviewed and approved by the institutional review board[s] [IRB] and/or independent ethics committee[s] [IEC] at each participating investigational centre. Signed informed consent documents were obtained from all participants and were reviewed by the sponsor and approved by the IRB/IEC.
2.2. Intervention
This study consisted of two consecutive 72-week periods: open-label treatment period 1 [OL1; baseline to Week 72] and open-label treatment period 2 [OL2; Weeks 76–144], the latter of which was added during an amendment to the protocol. In OL1, all patients were randomised to receive ontamalimab 75 mg or 225 mg s.c. every 4 weeks [without unblinding their assigned treatment in TURANDOT]. Patients assigned to ontamalimab 75 mg who experienced clinical deterioration or an unacceptable response in the opinion of the treating physician, were permitted a one-time dose escalation to 225 mg any time between Week 8 and Week 72. Dose escalation was at the investigator’s discretion, but clinical deterioration was typically characterised by an increase in total Mayo score to >6 or a partial Mayo score of >4 with an increase in rectal bleed subscore to >2 and/or an increase in stool frequency subscore to ≥2. Patients who experienced no satisfactory improvement in clinical condition within 8 weeks of dose escalation discontinued treatment and entered the follow-up period.
In OL2, all patients received ontamalimab 75 mg s.c. every 4 weeks for an additional 72 weeks. Dose escalation to 225 mg was not permitted during OL2. Patients entered a 6-month follow-up period after the last dose in OL2 or after discontinuation, consisting of two visits, 3 months apart. All patients underwent a final on-site visit at the end of the follow-up period [Week 168].
Oral glucocorticoids were permitted under specific conditions during the study. For patients who entered TURANDOT II in remission or with a clinically significant response, oral glucocorticoids were to be tapered according to local guidelines. For all other patients, tapering was to be initiated once they had achieved remission or a clinically significant response, and glucocorticoids were to be discontinued if possible by Week 40.
Oral glucocorticoids [up to a maximum of 1 mg/kg] could be administered as rescue treatment, but the patients were to be tapered off these within 12 weeks. Similarly, budesonide up to a maximum of 9 mg could be used. A tapering regimen was suggested to be budesonide 9 mg for 8 weeks, reduced to 6 mg for 2 weeks, 3 mg for 2 weeks, and then stopped. Alternate tapering regimens could be used as long as the duration for an individual rescue treatment did not exceed 12 weeks. A maximum of two courses of rescue therapy were permitted in OL1 and two further courses in OL2, and each course should not have exceeded 12 weeks. Patients who were unable to taper off either oral glucocorticoids or rescue therapy, or who relapsed within 2 months of rescue, were withdrawn from ontamalimab treatment and entered the follow-up period.
2.3. Outcome measures and assessments
2.3.1. Primary objectives and endpoints
The primary objective of this study was to assess the long-term safety and tolerability of ontamalimab. The incidences of treatment-emergent adverse events [TEAEs] and SAEs were recorded throughout OL1, OL2, and the follow-up period.
2.3.2. Secondary objectives and endpoints
The secondary objectives of this study were to assess mucosal healing and the pharmacokinetics and immunogenicity of ontamalimab. Flexible sigmoidoscopy or colonoscopy was carried out at Week 12 of TURANDOT [baseline of TURANDOT II] and at Week 16 of TURANDOT II, to assess mucosal healing [defined as a Mayo endoscopic subscore ≤1]. For patients undergoing routine cancer surveillance, an optional endoscopy was performed between Week 40 and Week 72, allowing an additional assessment of mucosal healing. In both TURANDOT and TURANDOT II, all endoscopies were read by a central reader who remained blinded to the study protocol and received no information about patient treatment history.
To assess serum ontamalimab levels, blood samples were collected at Week 12 of TURANDOT [TURANDOT II baseline], every 4 weeks until Week 72, and at Week 156 [during the follow-up period] or at early withdrawal from treatment. Serum anti-drug antibody [ADA] and, when applicable, neutralising antibody [NAb] titres were also measured at Week 12 of TURANDOT, then at Weeks 8, 16, 24, 40, 48, 64, and 156 of TURANDOT II or at early withdrawal from treatment. ADAs were assessed by an assay with a high tolerance to both soluble MAdCAM-1 and ontamalimab.22
2.3.3. Exploratory objectives and endpoints
Exploratory objectives of this study were to assess the durability of remission and response, and to explore the pharmacodynamics of long-term ontamalimab treatment. Total Mayo score was measured at Week 12 of TURANDOT [TURANDOT II baseline] and Week 16 of TURANDOT II, to assess rates of clinical response [decrease from TURANDOT baseline of ≥3 points with ≥30% change in total Mayo score, accompanied by a ≥1-point decrease in rectal bleed subscore or an absolute rectal bleed subscore of ≤1] and clinical remission [total Mayo score ≤2 with no individual subscore >1 and a rectal bleed subscore of ≤1].
Partial Mayo score was measured every 4 weeks until Week 144 to assess rates of long-term clinical response [decrease from TURANDOT baseline of ≥2 points with ≥30% change in partial Mayo score, accompanied by a ≥1-point decrease in rectal bleed subscore or an absolute rectal bleed subscore of ≤1] and remission [absolute partial Mayo score of ≤2 points with no individual subscore >1 and a rectal bleed subscore of ≤1].
Blood and stool samples were collected before dosing at TURANDOT II baseline and every 4 weeks to Week 24, and then at Weeks 32 and 72, and assessed for concentrations of high-sensitivity C-reactive protein [hsCRP, in serum] and faecal calprotectin [FC]. Soluble MAdCAM-1 levels in serum were measured at baseline and Week 16 as a pathway-specific marker of ontamalimab action.
Further assessments during the active treatment period included physical examinations, 12-lead electrocardiograms [ECGs], neurological assessments, monitoring of vital signs, and clinical laboratory values [biochemistry, haematology, and urinalysis].
2.4. Statistical analyses
The main analyses included in this study were summarised by patients initially randomised to receive 75 mg ontamalimab and those randomised to receive 225 mg ontamalimab [an intent-to-treat approach]. Adverse events and efficacy endpoints were analysed in the safety analysis set [all patients who received at least one dose of ontamalimab]. Pharmacokinetic and pharmacodynamic endpoints were analysed in patients from the safety analysis set for whom at least one pharmacokinetic/pharmacodynamic sample was collected. Data were reported for patients overall and separately for each initial randomisation group, unless otherwise stated.
2.4.1. Safety, pharmacokinetics, and immunogenicity
The number and proportions of patients who experienced TEAEs were reported. Mean (standard deviation [SD]) serum ontamalimab levels were reported every 4 weeks until Week 24, then at Weeks 32 and 72. The numbers and proportions of patients with positive ADA status [log2 titre ≥4.64] and NAb status were summarised.
2.4.2. Efficacy
Efficacy endpoints were summarised using a non-responder imputation [NRI] approach in which missing data were imputed as if patients were non-responders, and separately using an observed-cases approach, in which only the observed data were summarised. The mean proportions (90% confidence intervals [CIs]) of patients with mucosal healing [based on Mayo endoscopic subscore] and clinical response and clinical remission [based on total Mayo score] at Week 16 were reported. For the subgroup of patients who underwent an additional endoscopy, the mean proportion [90% CI] of patients with mucosal healing pooled from Weeks 40–72 was reported in a post hoc exploratory analysis. The mean proportions [90% CIs] of patients with clinical response and remission based on partial Mayo score were reported every 4 weeks during OL1. Time to dose escalation, and response and remission rates before and 16 weeks after dose escalation [based on partial Mayo score], were analysed in the subset of patients who escalated from 75 mg to 225 mg in OL1 in a post hoc analysis.
3. Results
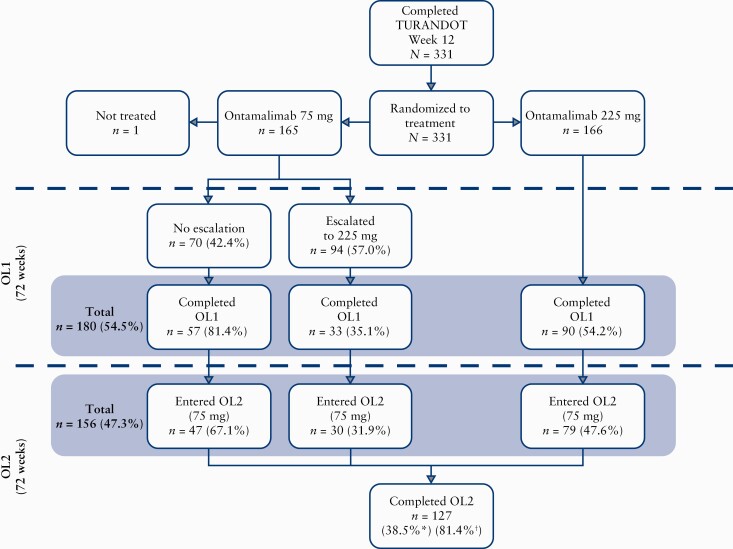
Overall, 331 patients were randomised, and 330 received ontamalimab 75 mg [n = 164] or 225 mg [n = 166] [Figure 1]. Patients initially randomised to the 75 mg and 225 mg groups were similar with respect to demographics and characteristics including age, sex, ethnicity, body mass index, and previous anti-TNF exposure [Table 1]. Within the 75 mg group, the completion rate for OL1 was 54.8% [90/164]. Of the patients who escalated to 225 mg, 35.1% [33/94] completed OL1; of the patients who did not escalate, 81.4% [57/70] completed OL1. In the group initially assigned to 225 mg, the completion rate for OL1 was 54.2% [90/166]. Overall, 81.4% [120/147] of patients who entered OL2 completed OL2. The most common reasons for study discontinuation were withdrawal of consent [23.0%; 76/330] and insufficient response [12.7%; 42/330]. Of 76 patients who withdrew consent, 41 did so during follow-up.
Figure 1.
Patient flow. Of 180 patients who completed OL1, 21 did not enter OL2 and proceeded to follow-up, owing to the timing of a protocol amendment which stipulated that OL2 be added to the study design to further evaluate the long-term safety of ontamalimab. An additional three patients completed OL1 and intended to progress to OL2; however, they proceeded directly to follow-up. OL1, open-label treatment period 1; OL2, open-label treatment period 2. *Calculated as n divided by the number of patients who received any amount of study drug [safety analysis set, n = 330]. †Calculated as n divided by the number of patients who entered OL2 [n = 156].
Table 1.
Patient demographics and baseline characteristics.
| Ontamalimab 75 mg [n = 164] | Ontamalimab 225 mg [n = 166] | Ontamalimab overall [n = 330] | |
|---|---|---|---|
| Mean [SD] age, years | 40.5 [12.75] | 41.1 [13.68] | 40.8 [13.21] |
| Sex, n [%] male | 102 [62.2] | 96 [57.8] | 198 [60.0] |
| Ethnicity, n [%] Hispanic or Latino | 4 [2.4] | 6 [3.6] | 10 [3.0] |
| Race, n [%] | |||
| White | 148 [90.2] | 143 [86.1] | 291 [88.2] |
| Black | 3 [1.8] | 2 [1.2] | 5 [1.5] |
| Asian | 11 [6.7] | 15 [9.0] | 26 [7.9] |
| Other | 2 [1.2] | 6 [3.6] | 8 [2.4] |
| Mean [SD] BMI, kg/m2 | 25.25 [5.69] | 25.24 [4.59] | 25.24 [5.16] |
| Anti-TNF naïve, n [%] | 68 [41.5] | 76 [45.8] | 144 [43.6] |
| Mean [SD] time since UC diagnosis, yearsa | 8.69 [7.04] | 7.56 [7.36] | 8.12 [7.21] |
| Mean [SD] total Mayo score | 6.0 [2.87] | 5.9 [2.84] | 6.0 [2.85] |
| Mean [SD] Mayo endoscopic subscore | 2.2 [0.07] | 2.2 [0.06] | 2.2 [0.05] |
| Mean [SD] concentration of hsCRP [mg/dL]b | n = 160 | n = 162 | n = 322 |
| 0.94 [1.266] | 0.73 [0.89] | 0.83 [1.10] | |
| Mean [SD] concentration of FC [µg/g]c | n = 142 | n = 155 | n = 329 |
| 2468.3 [3819.3] | 1924.7 [2541.8] | 2184.6 [3222.4] | |
| Receiving systemic glucocorticoids for UC, n [%] | 59 [36.0] | 71 [42.8] | 130 [39.4] |
| Smoking classification, n [%]c | |||
| Never smoked | 107 [65.2] | 102 [61.4] | 209 [63.3] |
| Smoker | 8 [4.9] | 9 [5.4] | 17 [5.2] |
| Ex-smoker | 49 [29.9] | 55 [33.1] | 104 [31.5] |
| Clinical responder at baseline, n [%]d | 79 [48.2] | 75 [45.2] | 154 [46.7] |
| Mucosal healing at baseline, n [%] | 34 [20.7] | 33 [19.9] | 67 [20.3] |
Treatment groups based on initial randomisation assignment.
BMI, body mass index; FC, faecal calprotectin; hsCRP, high-sensitivity C-reactive protein; SD, standard deviation; TNF, tumour necrosis factor; UC, ulcerative colitis.
aCalculated as: [[date of visit 1 in TURANDOT II − date of diagnosis from TURANDOT] + 1] / 365.25.
bCalculated for patients included in the pharmacodynamic analyses only.
cAt screening in TURANDOT.
dThose who achieved clinical response based on total Mayo score at Week 12 in TURANDOT.
3.1. Safety
Overall, 2339 TEAEs occurred in 293 patients [88.8%; 293/330] [Table 2]. In most patients who experienced TEAEs, these were mild or moderate in severity (mild, 73/330 patients [22.1%]; moderate, 153/330 patients [46.4%]; severe, 67/330 patients [20.3%]). TEAEs occurred in similar proportions of patients randomised to ontamalimab 75 mg (146/164 patients [89.0%]) and 225 mg (147/166 patients [88.6%]), and in a slightly higher proportion of patients who dose-escalated than in those who did not (85/94 patients [90.4%] vs 61/70 patients [87.1%]). The most frequently reported SAE was worsening of or ongoing UC (33/330 patients [10.0%]). The most frequently reported TEAE system organ class was infections and infestations; events of this type occurred in 57.6% of patients [190/330]. Serious infections occurred in 5.5% of patients [18/330] overall; the most common serious infection was gastroenteritis (3/330 patients [0.9%]). Pelvic abscess and pneumonia occurred in two patients [0.6%] each. There were no cases of tuberculosis. There were no notable changes from baseline values in laboratory results, vital signs, ECGs or results of neurological assessments [data not shown].
Table 2.
Safety characteristics of patients across OL1 [baseline to Week 72], OL2 [Weeks 76–144] and the follow-up period.
| Ontamalimab 75 mg [n = 164] | Ontamalimab 225 mg [n = 166] | Ontamalimab overall [n = 330] | |
|---|---|---|---|
| Overall TEAEs by system organ class, n [%] | |||
| Any TEAEa | 146 [89.0] | 147 [88.6] | 293 [88.8] |
| Infections and infestations | 96 [58.5] | 94 [56.6] | 190 [57.6] |
| General disorders and administration site conditions | 43 [26.2] | 58 [34.9] | 101 [30.6] |
| Skin and subcutaneous tissue disorders | 41 [25.0] | 55 [33.1] | 96 [29.1] |
| Gastrointestinal disorders | 95 [57.9] | 94 [56.6] | 189 [57.3] |
| Nervous system disorders | 33 [20.1] | 48 [28.9] | 81 [24.5] |
| Respiratory, thoracic, and mediastinal disorders | 34 [20.7] | 34 [20.5] | 68 [20.6] |
| Musculoskeletal and connective tissue disorders | 53 [32.3] | 62 [37.3] | 115 [34.8] |
| TEAEs considered related to ontamalimab | 58 [35.4] | 61 [36.7] | 119 [36.1] |
| TESAEs | 34 [20.7] | 40 [24.1] | 74 [22.4] |
| Treatment discontinuation due to TEAEsb | 12 [7.3] | 23 [13.9] | 35 [10.6] |
| Deaths | 1 [0.6] | 0 [0.0] | 1 [0.3] |
| Individual TEAEs, reported in ≥5% of patients, n [%] | |||
| Ulcerative colitisc | 55 [33.5] | 50 [30.1] | 105 [31.8] |
| Arthralgia | 27 [16.5] | 30 [18.1] | 57 [17.3] |
| Nasopharyngitis | 20 [12.2] | 28 [16.9] | 48 [14.5] |
| Upper respiratory tract infection | 23 [14.0] | 20 [12.0] | 43 [13.0] |
| Headache | 17 [10.4] | 22 [13.3] | 39 [11.8] |
| Gastroenteritis | 19 [11.6] | 14 [8.4] | 33 [10.0] |
| Cough | 20 [12.2] | 11 [6.6] | 31 [9.4] |
| Abdominal pain | 9 [5.5] | 21 [12.7] | 30 [9.1] |
| Back pain | 12 [7.3] | 18 [10.8] | 30 [9.1] |
| Nausea | 8 [4.9] | 20 [12.0] | 28 [8.5] |
| Influenza | 8 [4.9] | 15 [9.0] | 23 [7.0] |
| Pyrexia | 15 [9.1] | 7 [4.2] | 22 [6.7] |
| Rash | 8 [4.9] | 13 [7.8] | 21 [6.4] |
| Urinary tract infection | 11 [6.7] | 10 [6.0] | 21 [6.4] |
| Diarrhoea | 12 [7.3] | 7 [4.2] | 19 [5.8] |
| Pharyngitis | 2 [1.2] | 17 [10.2] | 19 [5.8] |
| Vomiting | 11 [6.7] | 8 [4.8] | 19 [5.8] |
| Bronchitis | 10 [6.1] | 8 [4.8] | 18 [5.5] |
| Influenza-like illness | 8 [4.9] | 9 [5.4] | 17 [5.2] |
| Sinusitis | 7 [4.3] | 10 [6.0] | 17 [5.2] |
Treatment groups based on initial randomisation assignment.
OL1, open-label treatment period 1; OL2, open-label treatment period 2; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
aIncludes non-treatment-related TEAEs.
bIncludes two patients in the 225 mg group who discontinued owing to TEAEs that occurred after the treatment period was completed.
cWorsening or ongoing disease activity.
The most frequently reported TEAEs were worsening of or ongoing UC (105/330 patients [31.8%]), arthralgia (57/330 patients [17.3%]), and nasopharyngitis (48/330 patients [14.5%]; Table 2). No cases of progressive multifocal leukoencephalopathy [PML] or lymphoproliferative disorders were observed throughout the study, including during the follow-up period. Four cases of malignant neoplasms were observed: three non-melanoma skin cancers (two basal cell carcinomas [0.6%] and one squamous cell carcinoma [0.3%]) and one [0.3%] malignant lung neoplasm; these were not considered treatment-related.
In total, 478 TEAEs were considered related to ontamalimab in 119/330 patients [36.1%], and 35/330 patients [10.6%] discontinued treatment owing to TEAEs [Table 2]. In OL1, general disorders and administration site conditions that were considered treatment-related [including injection-site reactions, oedema, and pyrexia] were more common in patients initially assigned to 225 mg versus 75 mg ontamalimab (24/166 patients [14.5%] vs 13/164 patients [7.9%]). No notable differences in any type of treatment-related TEAE between patients initially randomised to ontamalimab 75 mg versus 225 mg were observed in OL2 [at which point all patients were receiving 75 mg], or in the follow-up period. The proportion of patients with treatment-related TEAEs was higher in OL1 than OL2 (110/330 patients [33.3%] vs 27/156 patients [17.3%]; Supplementary Tables 2 and 3, available as Supplementary data at ECCO-JCC online).
One death occurred in OL1: a woman [26 years old, 75 mg escalated to 225 mg], started high-dose prednisone owing to increased disease activity and died of a pulmonary embolism 7 weeks later [Table 2]. This was considered by the investigator to be unrelated to treatment. There were no other cases of pulmonary embolism in this study.
The proportion of patients with ADAs was 6.3% at TURANDOT II baseline [19/301]; of these 19 patients, four had NAbs [Supplementary Table 4, available as Supplementary data at ECCO-JCC online]. Most patients who were confirmed positive at any time point had ADA concentrations in the range 4.64–9.40 log2 titres. Two patients had ADAs in the range 10.17–10.86 log2 titres between TURANDOT II baseline and Week 8. No patient had a titre that increased ≥2-fold over the course of the study. There were no reported hypersensitivity reactions that could be associated with the presence of ADAs. Whether patients had ADAs or not had no impact on serum ontamalimab concentrations in either of the dose groups [data not shown].
3.2. Efficacy
3.2.1. Mucosal healing at Week 16
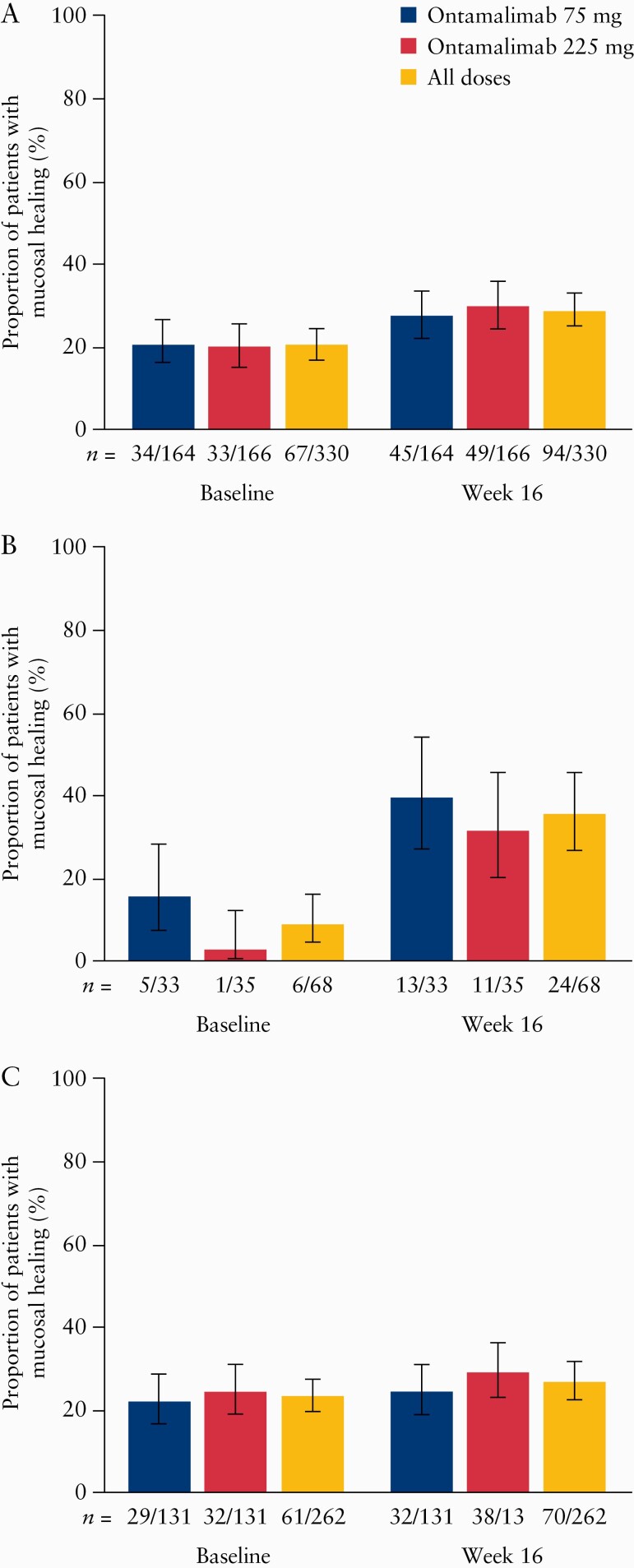
The proportion of patients with mucosal healing in this study increased from 20.3% at baseline to 28.5% overall at Week 16 when using the NRI method. The proportion increased from 20.7% [34/164] to 27.4% [45/164] in those initially assigned to ontamalimab 75 mg, and from 19.9% [33/166] to 29.5% [49/166] in patients assigned to ontamalimab 225 mg [Figure 2A].
Figure 2.
Proportion [90% CI] of patients in the safety analysis set with mucosal healing at Week 16 compared with baseline [Week 12 of TURANDOT]: [A] overall; [B] in patients who received placebo in TURANDOT; [C] in patients who received ontamalimab in TURANDOT. 90% CIs calculated using Wilson score test. Patients who were missing results for the endpoint were imputed as not meeting the endpoint. Treatment groups based on initial randomisation assignment. CI, confidence interval.
We also analysed the data by treatment received in TURANDOT. In this analysis, the proportion of patients with mucosal healing increased from 8.8% [6/68] at baseline to 35.3% [24/68] at Week 16 in those assigned to receive placebo in TURANDOT [Figure 2B]. The proportion of patients with mucosal healing in those patients assigned to receive any dose of ontamalimab in TURANDOT increased from 23.3% [61/262] at baseline to 26.7% [70/262] at Week 16 [Figure 2C]. The proportion of patients with mucosal healing in those who received ontamalimab in TURANDOT was generally maintained between baseline and Week 16 across all doses of ontamalimab. These results were similar when the observed case method was used [data not shown].
3.2.2. Long-term mucosal healing
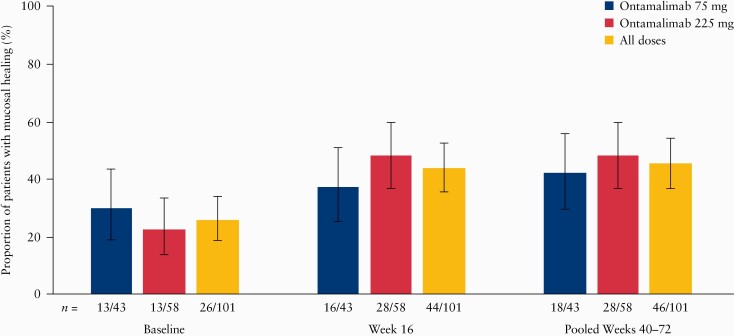
Between Weeks 40 and 72, 101 patients underwent an additional, optional, centrally read endoscopy. Baseline characteristics for this subgroup were broadly similar to those of the overall population [Supplementary Table 5, available as Supplementary data at ECCO-JCC online]. The principal difference was that a higher proportion of patients in this subgroup were clinical responders at baseline, compared with the overall population, 64.4% [65/101] versus 46.7% [154/330]. At baseline, 25.7% of these patients [26/101] had mucosal healing. The proportion of patients with mucosal healing increased to 43.6% [44/101] at Week 16 and to 45.5% [46/101] at Weeks 40–72 in this subset of patients [Figure 3]. Of the 44 patients with mucosal healing at Week 16, 75.0% [33/44] maintained mucosal healing up to Weeks 40–72.
Figure 3.
Proportion [90% CI] of patients in the long-term efficacy set* with endoscopic mucosal healing at baseline [Week 12 of TURANDOT], Week 16, and Weeks 40–72 of TURANDOT II. 90% CIs calculated using Wilson score test. Treatment groups based on initial randomisation assignment. CI, confidence interval. *The long-term efficacy set was defined as the subgroup of patients who had a second endoscopy between Week 40 and Week 72.
3.2.2. Clinical remission and response
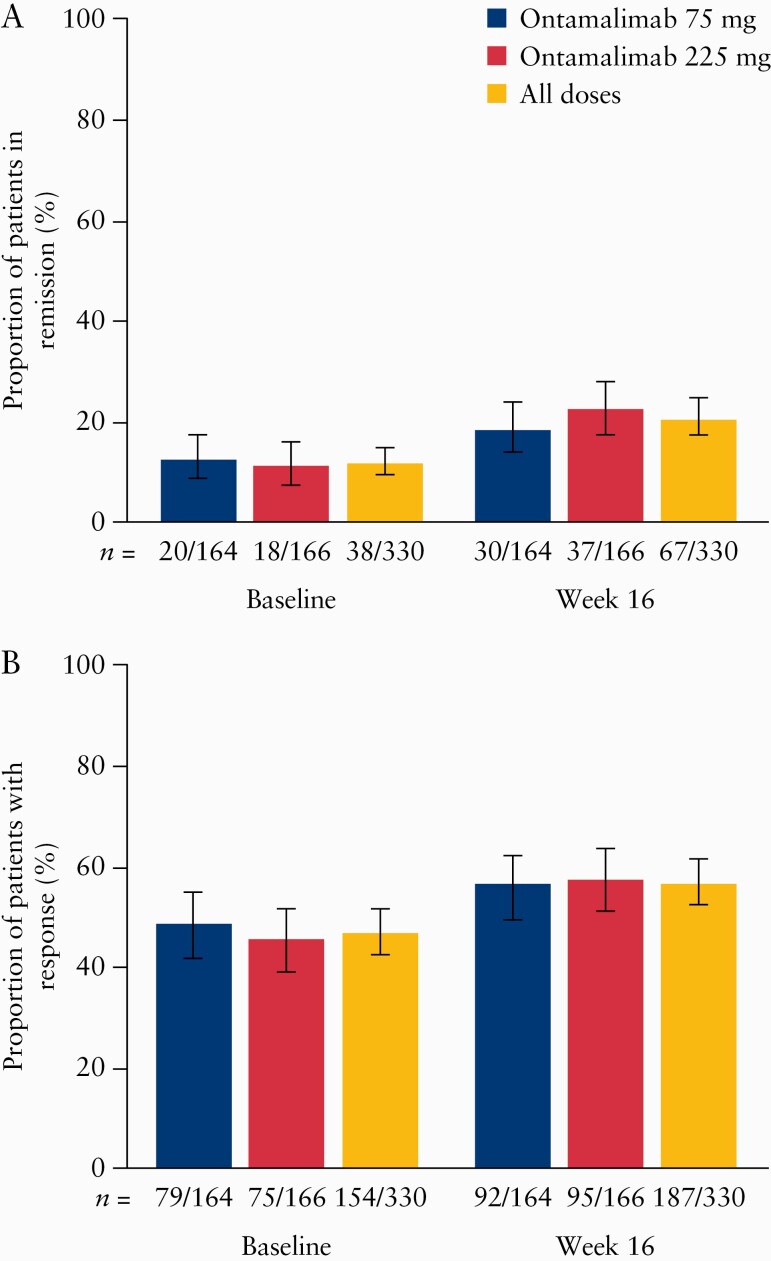
The proportion of patients with clinical remission based on total Mayo score increased from 11.5% [38/330] at baseline to 20.3% [67/330] at Week 16 [Figure 4A]. This pattern was similar in both dose groups. Of patients who were not in remission at TURANDOT II baseline, 14.0% [41/293] had achieved remission by Week 16; this proportion was 10.2% [23/226] and 27.3% [18/66] for patients who received ontamalimab and placebo in TURANDOT, respectively [Supplementary Figure 1A, available as Supplementary data at ECCO-JCC online]. Of the patients who were in clinical remission at TURANDOT II baseline, 68.4% [26/38] were still in remission at Week 16. Of 170 non-responders at baseline, one patient [0.6%] was in remission at baseline. This patient had a score of 4 at TURANDOT baseline [enrolled in error] and 2 at TURANDOT Week 12 [TURANDOT II baseline] and therefore met the remission, but not the response, criteria. The proportion of non-responders at baseline who achieved remission increased to 7.6% [13/170] by Week 16. Of 154 responders, the proportion in remission increased from 24.0% [37/154] at baseline to 35.1% [54/154] at Week 16. These results were similar in both dose groups and when using an observed case approach [data not shown].
Figure 4.
Proportion [90% CI] of patients [A] in remission and [B] with response to ontamalimab at baseline [Week 12 of TURANDOT] and Week 16, based on total Mayo score. 90% CIs calculated using Wilson score test. Patients who were missing results for the endpoint were imputed as not meeting the endpoint. Treatment groups based on initial randomisation assignment. CI, confidence interval.
The proportion of patients with clinical response based on total Mayo score increased from 46.7% [154/330] at baseline to 56.7% [187/330] at Week 16 [Figure 4B]; a similar increase was observed in both dose groups. Of 170 patients who did not have a response at the end of TURANDOT, 38.2% [n = 65] had achieved a response by Week 16 of TURANDOT II; this proportion was 34.7% [42/124] and 47.8% [22/46] for patients who received ontamalimab and placebo in TURANDOT, respectively [Supplementary Figure 1B]. Of the patients who had a response at TURANDOT II baseline, 78.6% [121/154] still had a response at Week 16. These results were similar in both dose groups, and when using an observed case approach [data not shown].
Following an increase from baseline to Week 16, the proportions of patients with clinical remission and response based on partial Mayo score decreased over time [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online]. Mean [SD] partial Mayo score improved from 3.8 [2.29] at TURANDOT II baseline to 1.0 [1.31] at Week 144 [end of OL2] in patients who remained in the study [n = 127]. The mean change from TURANDOT baseline to Week 144 was -4.7 [1.73]. This improvement was similar in both dose groups.
3.2.3. Dose escalation
In patients who escalated their dose to 225 mg [n = 94], the median time to dose escalation was 25.6 weeks; patients who dose-escalated remained on treatment for a median of 14.7 weeks, with most patients [52.1%; 49/94] having fewer than five doses after dose escalation. The response rate [based on partial Mayo score] did not change substantially between the point of dose escalation and 16 weeks after escalation (29.8% [28/94] vs 30.9% [29/94]), and remained lower than in patients who did not escalate and in patients randomised to 225 mg. Remission rates [based on partial Mayo score] appeared to increase from the point of dose escalation to 16 weeks post-escalation (8.5% [8/94] vs 25.5% [24/94]); however, remission rates remained lower than in both the group of patients who did not escalate and the group randomised to 225 mg.
3.3. Pharmacokinetics and pharmacodynamics
Overall, there was a dose-related increase in serum ontamalimab concentration during OL1 [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online]. Mean [SD] concentrations of ontamalimab increased from a baseline value of 6398.62 [8584.4] µg/L to 10 928.9 [7900.8] µg/L at Week 20 in patients receiving 75 mg, and from 8064.4 [10 629.8] µg/L to 25 583.0 [11 767.8] µg/L in the same period in those initially assigned to 225 mg. The observed concentrations in pharmacokinetic samples collected over 20 weeks suggest that the steady state was achieved around Week 12 for both doses of ontamalimab [Supplementary Figure 3], consistent with the molecule’s half-life of approximately 17 days.23
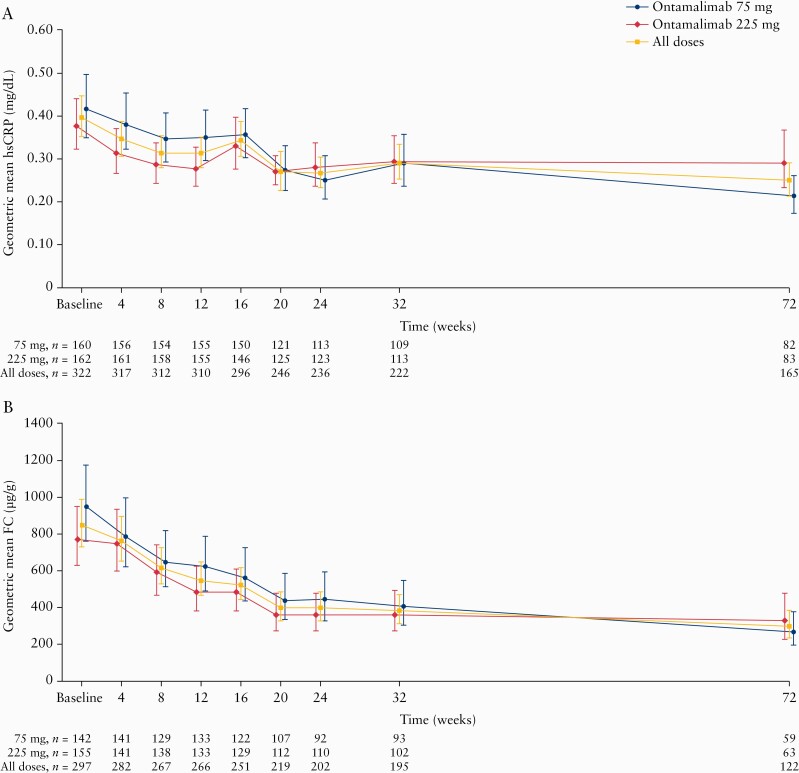
Geometric mean hsCRP and FC levels decreased across OL1 in both dose groups [Figure 5]. Geometric mean hsCRP levels decreased from 0.39 mg/dL to 0.25 mg/dL [Figure 5A], with a geometric mean percentage change from TURANDOT II baseline to Week 72 of -23.8 [90% CI -36.6 to -8.5]. There were no notable differences between dose groups. The geometric mean percentage change in hsCRP was greater in patients assigned to placebo versus ontamalimab in TURANDOT (-70.4 [-84.9 to -48.7] vs -5.9 [-22.9 to 14.8]). Geometric mean FC levels decreased from 848.37 µg/g to 300.00 µg/g in all patients [Figure 5B]. At Week 72, the geometric percentage change in FC level from TURANDOT II baseline was -41.6 [-58.0 to -18.8]. As with hsCRP, the decrease in the geometric mean percentage change in FC was larger in the group assigned to placebo versus ontamalimab in TURANDOT (-81.8 [-90.8 to -63.9] and -16.7 [-41.8 to 19.4]).
Figure 5.
Geometric mean [90% CI] concentrations of [A] hsCRP and [B] FC from baseline [Week 12 of TURANDOT] to Week 72. Treatment groups based on initial randomisation assignment. CI, confidence interval; FC, faecal calprotectin; hsCRP, high-sensitivity C-reactive protein.
There was a decrease in geometric mean soluble MAdCAM-1 from 33.5 pmol/L at TURANDOT II baseline to 6.2 pmol/L at Week 16 [-81.4% [90% CI -84.5 to -77.6]]. As with hsCRP and FC, the decrease in soluble MAdCAM-1 was larger in the group assigned to placebo versus ontamalimab in TURANDOT (-97.8 [-98.2 to -97.2] and -65.9 [-71.1 to -59.7]).
4. Discussion
This study is the first to report on the safety and efficacy of an anti-MAdCAM-1 antibody in a phase 2 trial of more than 12 weeks in patients with moderate-to-severe UC. Unlike other recent studies, this trial included patients who had received placebo during the induction trial and those who did not meet criteria for response following induction with active treatment. Based on safety data collected over a total duration of 3 years [across TURANDOT and TURANDOT II], ontamalimab was well tolerated, with a safety profile that remained stable in the long term. Moreover, the continued efficacy of ontamalimab was evident, with clinical response and remission rates persisting in the long term.
As expected based on results from TURANDOT and other ontamalimab trials,20,21 the most common TEAEs seen in this study were related to patients’ underlying disease. The overall safety profile of ontamalimab was consistent with that previously described20,21 and with that of vedolizumab.24 Furthermore, over the 144-week study period, there were no cases of lymphoproliferative disorders or PML [an opportunistic infection of the central nervous system, reported with long-term natalizumab treatment25–27], corroborating findings from TURANDOT and trials of ontamalimab in other populations.19,21 The latter is of particular significance, given that natalizumab non-selectively targets the α4 integrin subunit, blocking the interaction between the α4β1 integrin and VCAM-1. All types of serious infections were infrequent in the present study, occurring in 5.5% of patients overall. Although four patients in this trial experienced malignant neoplasms [three non-melanoma skin cancers and one lung cancer], and one patient died of pulmonary embolism, none of these five events was reported as drug-related by the investigator. Rather, increased risks of cancer and thrombosis have been associated with IBD itself and other IBD treatments such as thiopurines and glucocorticoids.28–31 Notably, although the proportion of patients with TEAEs leading to treatment withdrawal and the proportion with injection-site reactions were higher in the 225 mg group than the 75 mg group, no patient discontinued treatment owing to an injection-site reaction. The most common reason for treatment withdrawal was insufficient response, suggesting ontamalimab was generally very well tolerated.
This study assessed the immunogenicity of ontamalimab as a secondary objective. Immune response development to a therapeutic protein can affect its safety profile and can reduce treatment efficacy through increased drug clearance. Overall, the proportion of patients with ADAs was found to be low. Small decreases in the proportion of patients with ADAs over time may have resulted from patients with ADAs discontinuing the study. However, there was no evidence of increasing ADA titres over time, consistent with the absence of a clinically relevant immune response to ontamalimab.
Mucosal healing and remission rates at Week 16 in this study in patients who received placebo in TURANDOT support the efficacy of ontamalimab as induction therapy. Likewise, patients who received placebo in TURANDOT had marked reductions in the inflammatory biomarkers hsCRP and FC by the end of TURANDOT II compared with baseline, further supporting this concept. Long-term reductions in hsCRP and FC, biomarkers known to correlate with clinical response and mucosal healing in UC,32–34 were associated with both ontamalimab doses in the current study, as was a reduction in free soluble MAdCAM-1, a biomarker specific to the mode of action of this biologic.
As an exploratory objective, this study investigated the long-term efficacy of ontamalimab. Of patients entering the study who had received ontamalimab during TURANDOT and who were in clinical remission or had a response, the majority sustained that outcome after an additional 16 weeks of treatment. Patients randomised to ontamalimab 75 mg, who did not respond or lost response and underwent dose escalation to 225 mg, had lower response and remission rates than other groups. Nevertheless, there was no clear signal that dose escalation affected patient outcomes; rather, the criteria for escalation may have effectively resulted in a self-selected group of non-responders. Another hypothesis is that there is a U-shaped dose-response curve [hormesis], such that doses greater than 75 mg are associated with lower response rates. Further studies investigating dose escalation would be needed to allow a firmer conclusion on this to be drawn.
Surprisingly, differences between the 75 mg and 225 mg doses observed in TURANDOT were not confirmed in this study. This could relate to well-known differences in outcomes in open-label versus blinded studies, as was the case with the open-label study TOSCA versus the blinded study OPERA, which examined ontamalimab in patients with Crohn’s disease.18,19 Nevertheless, the clinical performance of the 225 mg dose remains uncertain, and the question of optimal dosing needs further exploration before a definitive answer can be established.
There are a few study limitations that should be noted. This trial investigated the open-label administration of two doses of ontamalimab based on results of previous studies. However, the absence of a placebo group in this trial limits quantification of the full benefit of ontamalimab over the long term. In addition, the lack of blinding may have allowed some bias. Furthermore, although dose escalation was permitted, this trial was not designed to investigate it; owing to the wide range of timings allowable for dose escalation, assessments of different doses were limited to groups based on the initial treatment randomisation. Last, the consent withdrawal rate in this study of 23% was higher than expected, but could be linked to the commercial availability of vedolizumab during this trial.
We conclude that our study substantiates and adds to results from the previous trial, TURANDOT, demonstrating a good safety and efficacy profile of ontamalimab during long-term treatment up of to 144 weeks. Continued clinical benefit in both treatment arms supports phase 3 clinical testing of ontamalimab in moderate-to-severe UC.
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results reported in this article, will be made available [within 12 months from initial request] to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Funding
This work [trial NCT01771809] was supported by Shire, a member of the Takeda group of companies, and Pfizer. These analyses were funded by Shire, a member of the Takeda group of companies. Medical writing support for the preparation of this manuscript was provided by Emma Saxon, PhD, and Katie Pillidge, PhD, of PharmaGenesis London, London, UK, and funded by Shire International GmbH, a member of the Takeda group of companies.
Conflict of Interest
WR reports personal fees from 4SC, Abbott Laboratories, AbbVie, Aesca, AM Pharma, Amgen, AOP Orphan, Aptalis, Arena Pharmaceuticals, Astellas, AstraZeneca, Avaxia, Bioclinica, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Cellerix, Celltrion, Centocor, ChemoCentryx, Covance, Danone Austria, Dr Falk Pharma GmbH, Elan, Eli Lilly, Ernst & Young, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Immundiagnostik, InDex Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, MedAhead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, Otsuka, Parexel, PDL, Peri Consulting, Pfizer, Pharmacosmos, Philip Morris Institute, PLS Education, Procter & Gamble, Prometheus, Protagonist, Provention, Robarts Clinical Trials (owned by Health Academic Research Trust [HART]), Roland Berger GmbH, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpoint Medical, Shire,a Sigmoid, Takeda, Therakos, TiGenix, UCB, Vifor, Yakult, Zealand, and Zyngenia; and grants from Abbott Laboratories, AbbVie, Aesca, Centocor, Dr Falk Pharma GmbH, Immundiagnsotik, and MSD, outside the submitted work. WJS reports personal fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, TiGenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals; research grants from AbbVie, Amgen, Atlantic Healthcare, Celgene/Receptos, Genentech, Gilead Sciences, Janssen, Lilly, Pfizer, Prometheus Laboratories, and Takeda; and other [stock or stock options] from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, and Vimalan Biosciences, outside the submitted work. SD reports personal fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor, outside the submitted work. XH reports personal fees from AbbVie, Arkopharma, Astellas, Janssen, Nutricia, Pfizer, and Takeda; and lecture fees from AbbVie, Arard, Arkopharma, Baxter, Bristol-Myers Squibb, Ferring, Janssen, MSD, Nutricia, Pfizer, Sanofi-Aventis, Takeda, and Tillotts; and other [involvement in clinical research] from AbbVie, Abivax, Alfasigma, Arena, Cellgene, Eli Lilly, Enterome, Gilead, InDex Pharmaceuticals, Janssen, Pfizer, Roche, Salix, Takeda, and Theravance, outside the submitted work. MK reports personal fees from AbbVie, Ferring, Janssen, Pfizer, Pharmabest, PRO-MED, and Takeda, outside the submitted work. DT reports personal fees from AbbVie, MSD, Pfizer, Roche, Sanofi-Aventis, and Takeda, outside the submitted work. TV reports personal fees from Hospira, Pfizer, and Takeda, outside the submitted work. MGr reports personal fees from AbbVie, Alfa Wasserman, Egis, Ferring Pharmaceuticals, Hospira, MSD, Pfizer, Takeda, and Vifor. PAH has nothing to disclose. JSK has nothing to disclose. MPS reports personal fees from AbbVie, Celgene, Ferring, Janssen, MSD, Pfizer, Shire,a and Takeda; and grants from AOP Orphan and Ferring, outside the submitted work. KJG reports personal fees from Pfizer and Shirea during the conduct of the study. MH reports other remuneration [employee] from Shirea during the conduct of the study. MGo reports other remuneration [employee and stock options] from Shirea and Pfizer during the conduct of the study. CB reports other remuneration [employee and stock options] from Shirea during the conduct of the study. CG reports other remuneration [employee] from GCE solutions, part of IQVIA, outside the submitted work and other [employee] from Cytel during the conduct of the study. FC reports other remuneration [employee] from Pfizer during the conduct of the study. SV reports personal fees from AbbVie, Arena, Celgene, Dr Falk Pharma, Eli Lilly, Ferring, Galapagos, Genentech/Roche, Gilead, Hospira, Janssen, MSD, Mundipharma, Pfizer, ProDigest, Progenity, Second Genome, Shire,a Takeda, and Tillotts; and grants from AbbVie, Janssen, MSD, Pfizer, and Takeda.
Author Contributions
All authors contributed to the manuscript and approved the final version. WR: study concept and design, acquisition and interpretation of data. WJS: acquisition and interpretation of data. SD: acquisition and interpretation of data. XH: acquisition and interpretation of data. MK: acquisition and interpretation of data. DT: acquisition and interpretation of data. TV: acquisition and interpretation of data. MGr: acquisition and interpretation of data. PAH: acquisition and interpretation of data. JSK: acquisition and interpretation of data. MPS: acquisition and interpretation of data. KJG: study concept and design, interpretation of data. MH: interpretation of data. MGo: study design, interpretation of data. CB: analysis and interpretation of data. CG: analysis and interpretation of data. FC: study concept and design, interpretation of data. SV: acquisition and interpretation of data.
Supplementary Material
Footnotes
A member of the Takeda group of companies.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paschos P, Katsoula A, Salanti G, Giouleme O, Athanasiadou E, Tsapas A. Systematic review with network meta-analysis: the impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther 2018;48:1174–85. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 4. Dubinsky MC. Reviewing treatments and outcomes in the evolving landscape of ulcerative colitis. Postgrad Med 2017;129:538–53. [DOI] [PubMed] [Google Scholar]
- 5. Paschos P, Katsoula A, Giouleme O, et al. . Tofacitinib for induction of remission in ulcerative colitis: systematic review and meta-analysis. Ann Gastroenterol 2018;31:572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopetuso LR, Gerardi V, Papa V, et al. . Can we predict the efficacy of anti-TNF-α agents? Int J Mol Sci 2017;18:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014;13:24–30. [DOI] [PubMed] [Google Scholar]
- 8. Torres J, Cravo M, Colombel JF. Anti-TNF withdrawal in inflammatory bowel disease. GE Port J Gastroenterol 2016;23:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tran V, Shammas RM, Sauk JS, Padua D. Evaluating tofacitinib citrate in the treatment of moderate-to-severe active ulcerative colitis: design, development and positioning of therapy. Clin Exp Gastroenterol 2019;12:179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med 2009;9:836–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newham P, Craig SE, Seddon GN, et al. . Alpha4 integrin binding interfaces on VCAM-1 and MAdCAM-1. Integrin binding footprints identify accessory binding sites that play a role in integrin specificity. J Biol Chem 1997;272:19429–40. [DOI] [PubMed] [Google Scholar]
- 12. Allavena R, Noy S, Andrews M, Pullen N. CNS elevation of vascular and not mucosal addressin cell adhesion molecules in patients with multiple sclerosis. Am J Pathol 2010;176:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Briskin M, Winsor-Hines D, Shyjan A, et al. . Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 14. Nakache M, Berg EL, Streeter PR, Butcher EC. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature 1989;337:179–81. [DOI] [PubMed] [Google Scholar]
- 15. Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 1988;331:41–6. [DOI] [PubMed] [Google Scholar]
- 16. Takeda Pharma A/S. Summary of product characteristics, Entyvio. European Medicines Agency, 2014. https://www.ema.europa.eu/en/documents/product-information/entyvio-epar-product-information_en.pdf. Accessed July, 2019.
- 17. Pullen N, Molloy E, Carter D, et al. . Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol 2009;157:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Haens G, Vermeire S, Vogelsang H, et al. . Effect of PF-00547659 on central nervous system immune surveillance and circulating beta7+ T cells in Crohn’s disease: report of the TOSCA study. J Crohns Colitis 2018;12:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Lee SD, Tarabar D, et al. . Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut 2018;67:1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vermeire S, Ghosh S, Panes J, et al. . The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut 2011;60:1068–75. [DOI] [PubMed] [Google Scholar]
- 21. Vermeire S, Sandborn WJ, Danese S, et al. . Anti-MAdCAM antibody [PF-00547659] for ulcerative colitis [TURANDOT]: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017;390:135–44. [DOI] [PubMed] [Google Scholar]
- 22. Wang Q, Goetsch M. An electrochemiluminescence [ECL] immunoassay for the detection of anti-drug antibodies against the anti-mucosal addressin cell adhesion molecule [MAdCAM] monoclonal antibody ontamalimab [SHP647]. In: 14th Congress of the European Crohn’s and Colitis Organisation; 2019; Copenhagen, Denmark. [Google Scholar]
- 23. Wang Y, Marier J-F, Lavigne J, Kassir N, Martin P. Population pharmacokinetics and pharmacodynamics of ontamalimab (SHP647), a fully human monoclonal antibody against mucosal addressin cell adhesion molecule-1 (MAdCAM-1), in patients with ulcerative colitis or Crohn’s disease. J Clin Pharmacol 2020;60:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colombel JF, Sands BE, Rutgeerts P, et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005;353:375–81. [DOI] [PubMed] [Google Scholar]
- 26. Van Assche G, Van Ranst M, Sciot R, et al. . Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005;353:362–8. [DOI] [PubMed] [Google Scholar]
- 27. Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol 2016;22:533–5. [DOI] [PubMed] [Google Scholar]
- 28. Axelrad JE, Lichtiger S, Yajnik V.. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22:4794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giannotta M, Tapete G, Emmi G, Silvestri E, Milla M. Thrombosis in inflammatory bowel diseases: what’s the link? Thromb J 2015;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stuijver DJF, Majoor CJ, van Zaane B, et al. . Use of oral glucocorticoids and the risk of pulmonary embolism: a population-based case-control study. Chest 2013;143:1337–42. [DOI] [PubMed] [Google Scholar]
- 31. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. ; Cesame Study Group. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011;141:1621–8.e1–5. [DOI] [PubMed] [Google Scholar]
- 32. Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology 2016;150:96–102. [DOI] [PubMed] [Google Scholar]
- 33. De Vos M, Dewit O, D’Haens G, et al. ; behalf of BIRD. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis 2012;6:557–62. [DOI] [PubMed] [Google Scholar]
- 34. Henriksen M, Jahnsen J, Lygren I, et al. ; IBSEN Study Group. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008;57:1518–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.