Abstract
Background and Aims
The long-term course of ulcerative colitis [UC] is difficult to predict. Mortality, colectomy, cancer, and hospitalisation represent hard outcomes of disease. Moreover, knowledge on the risk of relapses and need for potent medication add important information about living with UC. We aimed to evaluate the course and prognosis of UC during the first 20 years after diagnosis, and to identify early prognostic risk factors.
Methods
From 1990 to 1994, a population-based inception cohort of patients with inflammatory bowel disease was enrolled in South-Eastern Norway. A systematic follow-up [FU] was conducted at 1,5, 10, and 20 years after diagnosis. Clinical outcomes were recorded continuously, and possible relationships between early disease characteristics and outcomes were analysed using multiple regression analysis.
Results
Among 519 UC patients, 119 died, 60 were lost to FU, and 340 were included in the FU cohort. The 20-year cumulative risk of colectomy was 13.0% (95% confidence interval [CI] [11.4‐14.6]). Extensive colitis at diagnosis was independently associated with an increased risk of colectomy compared with proctitis (hazard ratio [HR] = 2].8, 95% CI [1.3–6.1]). In contrast, mucosal healing at 1-year FU was independently associated with reduced risk of colectomy [HR = 0.4, 95% CI [0.2–0.8]), and inversely associated with subsequent risk of relapse [adjusted HR = 0.5, 95% CI [0.3–0.7]).
Conclusions
The overall risk of colectomy in our cohort was lower than expected from previous studies, although considerable for patients with extensive colitis at diagnosis. Early mucosal healing was associated with better disease outcomes 20 years after diagnosis.
Keywords: Ulcerative colitis, population-based inception cohort, clinical outcome
1. Introduction
Ulcerative colitis is an idiopathic chronic inflammatory disease involving the mucosal lining of the colon to various extents.1 With respect to clinical activity, the disease is heterogeneous, ranging from mild flares to severe therapy-resistant colitis associated with significant morbidity and increased risk of colectomy.2 Thus, population-based cohort studies including all newly diagnosed patients are necessary to assess outcomes and prognostic risk factors of the disease. Since UC mainly develops in early adulthood and lasts throughout life, longitudinal studies with several decades of follow-up are needed to evaluate hard endpoints.
The Inflammatory Bowel disease in South-East Norway [IBSEN] study consecutively recruited all newly diagnosed patients with inflammatory bowel disease [IBD] in a well-defined geographical area between 1990 and 1994.3 A comprehensive and standardised follow-up of this inception cohort has been performed. Results regarding different outcomes of the disease during the first 10 years after diagnosis have been reported previously.4–7
The primary aim of the present study was to determine the outcome of UC with respect to relapse, colectomy, and mortality during 20 years of FU from diagnosis. Secondarily, we aimed to identify possible risk factors of disease outcomes based on information obtained at early disease presentation.
2. Material and Methods
The regional committees for medical research ethics approved the study, and permission was obtained from the Norwegian Data Inspectorate.
2.1. Study population
From January 1, 1990, to December 31, 1993, the IBSEN cohort was established. Altogether, 843 newly diagnosed patients with inflammatory bowel disease [IBD], or possible IBD, were included from four counties in South-Eastern Norway [Oslo, Østfold, Telemark, and Aust-Agder]. Detailed information regarding the design and inclusion criteria for the study has been described previously.3
Prescheduled FU visits at the hospitals were carried out at 1, 5, 10, and 20 years [±1 year] after inclusion, and information in between these visits was captured retrospectively. The same group of gastroenterologists reviewed the diagnosis and disease course.4–6,8 Each visit included a structured interview, clinical examination, laboratory tests, and colonoscopy with biopsies. Additionally, magnetic resonance cholangiography [MRC] was performed at the 20-year FU.9
For a few patients who were unable to attend the hospital, a telephone interview was conducted and supplemented by information from the hospital records. In rare cases in which the patient could not be reached by telephone, the information was based on hospital records alone, provided that these were up to date. Medical and surgical treatments during the study were administered according to established clinical practice.
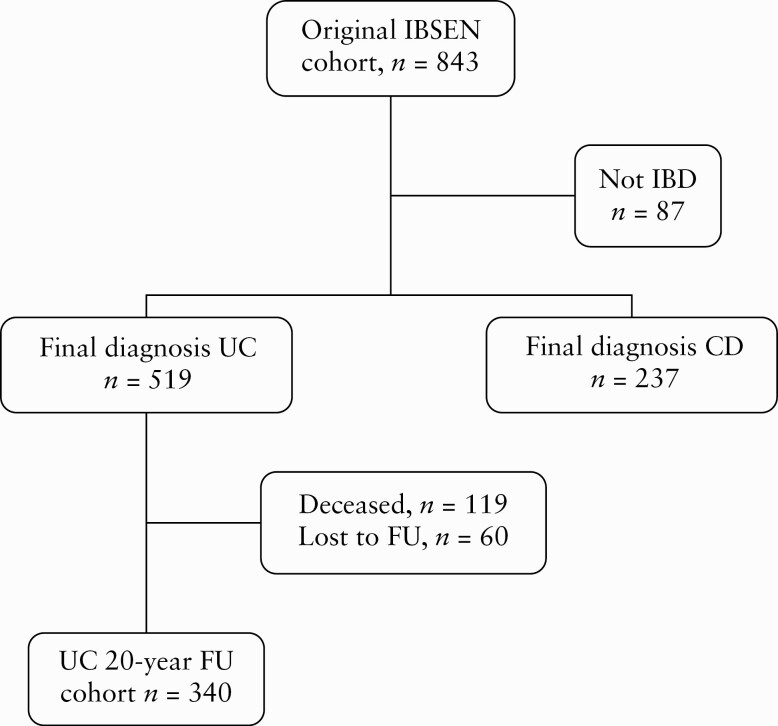
Of the patients included in the IBSEN cohort, a total of 519 patients had a final diagnosis of UC. A flowchart of the study population is shown in Figure 1.
Figure 1.
Flowchart of the IBSEN cohort from inception to the end of the 20-year follow-up [FU] period.
2.2. Classification and definitions
Patients were diagnosed according to the Lennard‐Jones criteria and initially classified as suffering from UC, Crohn’s disease [CD], indeterminate colitis, or possible IBD.10,11 At each FU visit, the patients were systematically re-evaluated, and the diagnosis was changed when appropriate. After 5 years, indeterminate colitis was reclassified as UC, CD, or non-IBD, as previously described.12 The extent of colitis during FU was determined endoscopically, and after 20 years, the patients were classified in accordance with the Montreal Classification.13 The overall mortality at 20 years FU for our UC cohort compared with geography-, age-, and sex-matched controls are published elsewhere.14 In the present study, further analysis on mortality risk based on initial disease behaviour was assessed. Data on deaths, including causes of death, were cross-matched with the Norwegian Cause of Death Registry from Statistics Norway. The International Classification of Diseases [ICD]-10 was used to classify causes of death.
Disease relapse was defined as any worsening of UC symptoms resulting in the need for more intensive medical therapy or colectomy, and was registered at the scheduled FU visits and complemented with hospital records.
Colectomies were recorded consecutively, and in cases lost to FU, the hospital records were reviewed. Patients who had undergone colectomy were excluded from further analysis of the disease course, as they no longer could be categorised as having ulcerative colitis.
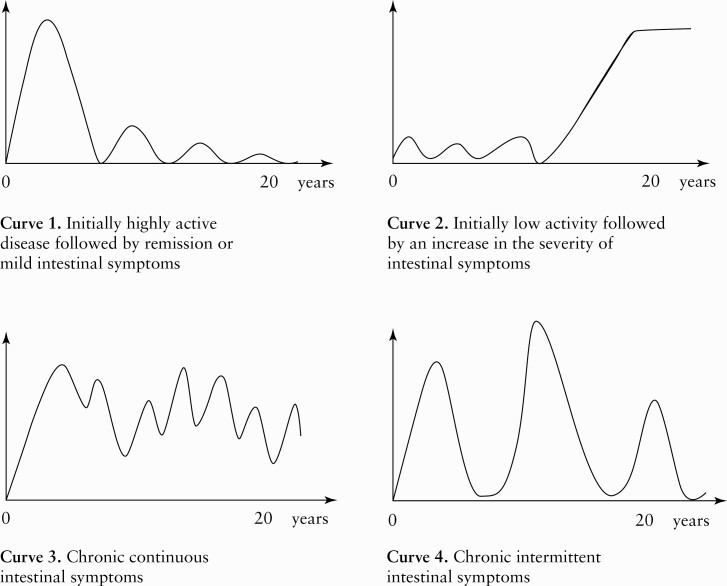
The clinical course of the disease from diagnosis onwards was illustrated by four predefined curves. Each curve reflected a different pattern in terms of bowel symptom severity from the patient´s perspective [Figure 2]. The endoscopies were evaluated according to criteria that were previously described by Froslie et al.7 Mucosal healing was defined as mucosa that had a normal appearance, slight erythema, or granularity, comparable to Mayo endoscopic subscore 0–1.15 The entire colonic mucosa was inspected, and patients who had incomplete colonoscopies were excluded from the analysis. Hospitalisation was defined as any UC-related hospital admission. Current smokers were defined as those who smoked one or more cigarette per day at diagnosis.
Figure 2.
Four predefined curves depicting different courses of ulcerative colitis [UC] from diagnosis to the end of the 20-year follow-up [FU] period. Patients’ self-assessment of disease course.
2.3. Statistical analyses
Continuous variables were described with median and range, and categorical variables with counts and percentages. Crude comparisons between pairs of categorical data were performed using the chi square test or Fisher’s exact test when appropriate. Crude comparisons between groups regarding continuous variables were performed using non-parametric methods.
The crude cumulative incidences of colectomy and relapse were estimated for the whole cohort and for selected subgroups using the Kaplan‐Meier method. Cox proportional hazards models were fitted to identify possible risk factors for mortality, relapse, and colectomy. The following covariates were included in the models: gender, age [</≥40 years], disease extent, use of systemic steroids, erythrocyte sedimentation rate/C-reactive protein [ESR/CRP] [</≥30 mm/h and 30 mg/L, respectively], and smoking status at diagnosis, in addition to mucosal healing at 1-year FU.
The patients who underwent colectomy the first year were omitted from the analysis including mucosal healing at 1 year.
Possible excess mortality due to UC in our cohort was computed using relative survival [RS] analysis Ederer II, where the matched individual is considered at risk until the corresponding UC patient dies or is censored.16 Data on Norwegian background population were retrieved from the national life tables provided by the Cancer Registry of Norway from 2020. Relative survival is defined as the ratio of observed survival estimated for the patients to the expected survival rate derived from life tables. Thus it is adjusted for the background mortality expected for persons of the same sex, age, and calendar year of investigation. The closer the relative survival is to one, the less are the patients effected by possible access mortality. The estimates of relative, observed, and expected survival were calculated using the strs Stata command.
Binary logistic regression analysis with a forward selection method was applied to determine the odds of hospitalisation, the use of systemic steroids, and patients-reported course of disease in the 20-year FU cohort.
All tests were two-sided. To correct for multiple testing, p-values ≤ 0.01 were considered statistically significant. All analyses were performed using SPSS version 24 or STATA version 16.
3. Results
Altogether, 119 [23%] of the 519 UC patients died during the 20-year period after diagnosis. Among the remaining 400 patients, 340 [85%] completed the 20-year FU. A total of 288 patients were examined at the hospital, clinical data were based on telephone interviews and hospital records for 26 patients, and on hospital records alone for a further 26 patients. Among the 60 patients defined as lost to FU, 35 were unwilling to participate, 10 were unable due to comorbidities, five had moved out of the area, and 10 were untraceable. Selected clinical characteristics at diagnosis for the FU cohort, those who were lost to follow-up, and those who died during the study period are shown in Table 1. There were no statistically significant differences between the groups, except that those who had died were older, had a lower incidence of proctitis, and a higher ESR/CRP at diagnosis.
Table 1.
Selected clinical characteristics of the UC cohort at early presentation used in the statistical analysis.
| Variables | 20-year FU cohort N [%] | Lost to FU N [%] | Deceased N [%] | Total cohort N [%] |
|---|---|---|---|---|
| Male | 166 [62] | 33 [12] | 68 [26] | 267 [100] |
| Female | 174 [69] | 27 [11] | 51 [20] | 252 [100] |
| Age at diagnosis | ||||
| Median [range] | 32.9 [5.4–69.6] | 32.9 [3.8–68.2] | 68.3 [13.8–88.2]* | 37.5 [3.8–88.2] |
| <40 years | 238 [70] | 41 [68] | 10 [8] | 289 [56] |
| ≥40 years | 102 [30] | 19 [32] | 109 [92] | 230 [44] |
| Extent at diagnosis | ||||
| E1: proctitis | 125 [37] | 24 [40] | 22 [19]** | 171 [33] |
| E2: left-sided colitis | 112 [33] | 14 [23] | 56 [47] | 182 [35] |
| E3: extensive colitis | 103 [30] | 22 [37] | 41 [34] | 166 [32] |
| Biomarkers at diagnosis | ||||
| ESR [mm/h] or CRP [mg/L] | ||||
| <30 | 221 [65] | 38 [63] | 65 [54] | 324 [62] |
| ≥30 | 114 [34] | 19 [32] | 52 [44]*** | 185 [36] |
| Missing | 5 [1] | 3 [5] | 2 [2] | 10 [2] |
| Systemic steroids at diagnosis | ||||
| No | 241 [71] | 39 [65] | 77 [65] | 357 [69] |
| Yes | 99 [29] | 20 [33] | 42 [35] | 161 [31] |
| Missing | - | 1 [2] | - | 1 |
| Smoking status at diagnosis | ||||
| Non-smoker | 301 [88] | 46 [77] | 101 [85] | 448 [86] |
| Current smoker | 38 [12] | 14 [23] | 17 [14] | 69 [13] |
| Missing | 1 | - | 1 | 2 |
| Mucosal healing at 1 year | ||||
| No | 150 [44] | 15 [25] | 48 [40] | 213 [41] |
| Yes | 136 [40] | 21 [35] | 42 [35] | 199 [38] |
| Missing | 54 [16] | 24 [40] | 29 [25] | 107 [21] |
| Total | 340 | 60 | 119 | 519 |
Patients who died were older [*p <0.001], were more likely to have left-sided colitis [**p <0.001], and had higher ESR/CRP [***p <0.001] than patients in the 20-year FU cohort.
UC, ulcerative colitis; FU, follow-up; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
3.1. Mortality
Eight of the 119 deaths were probably related to IBD. Five patients died of colorectal cancer—a female aged 36 and four patients above 80 years of age. Two patients with concomitant primary sclerosing cholangitis [PSC] died of cholangiocarcinoma at 65 and 75 years of age, and one patient aged 48 died of intestinal perforation 9 months after restorative surgery with ileal pouch-anal anastomosis [IPAA]. Two more deaths could possibly be classified as IBD-related: a male patient aged 55 died of anal cancer, and a female patient aged 45 with chronic severe colitis, on prednisolone treatment, committed suicide.
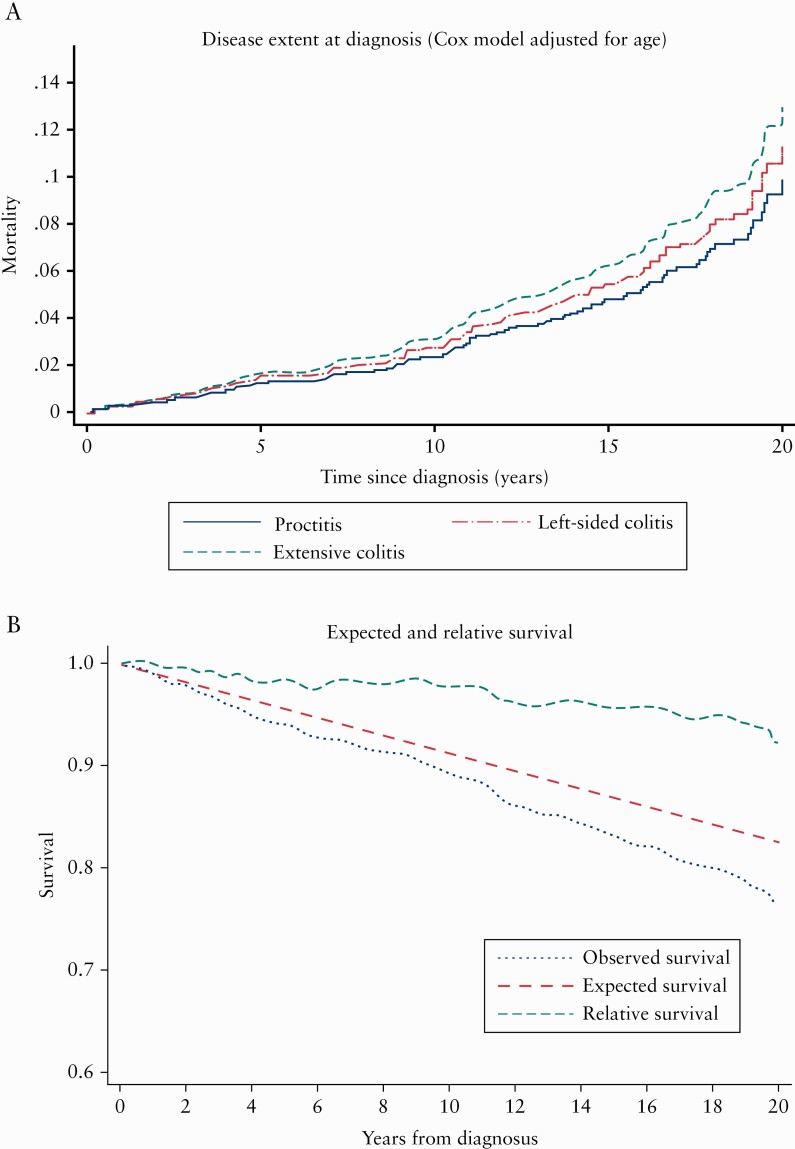
The observed survival rates for our UC patients were comparable to the expected survival rate in matched individuals [same age, sex, and calendar year of investigation] from the general Norwegian population. The relative survival rate at 20 years was 92.4%, 95% confidence interval [CI] [87.2‐96.9%].
The Cox proportional hazards models revealed that only older age at diagnosis was independently associated with increased risk of mortality (hazard ratio [HR] = 1.1, 95% CI [1.09–1.12], p <0.005). In the univariate analysis, patients with left-sided colitis at diagnosis had more than twice the risk of mortality [HR = 2.3, 95% CI [1.4–3.8], p = 0.002] than those with proctitis. However, these patients were older at diagnosis, so when adjusted for age this association was no longer statistically significant, as shown in Figure 3. Neither gender, ESR/CRP levels, use of systemic steroids, nor smoking status at diagnosis was independently associated with increased mortality. Moreover in the exploratory analyses, use of systemic mesalazine had no significant impact on the mortality risk.
Figure 3.
a. Mortality [%] in UC according to disease extent during the 20-year FU period. b. Observed survival rate for the UC cohort [n = 519] during 20 years of follow-up compared with expected survival rate derived from life tables, and their ratio: the relative survival rate. UC, ulcerative colitis; FU, follow-up.
3.2. Relapse
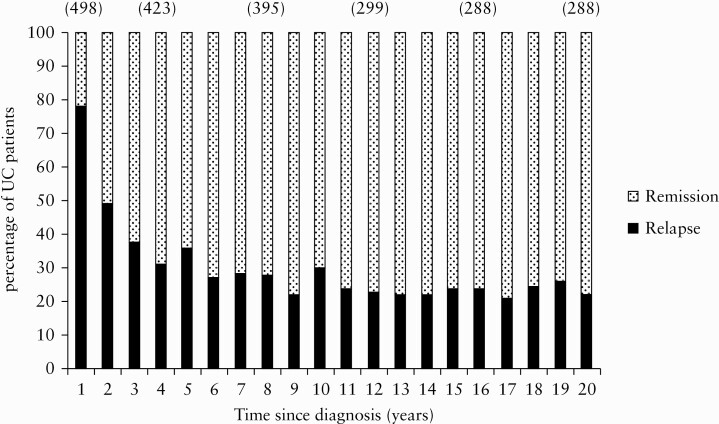
Figure 4 depicts the year-by-year proportion of patients with relapse versus remission during the 20-year period. During the last 10 years of FU, about one-fifth of the patients had one or more relapses per year.
Figure 4.
Proportion of UC [ulcerative colitis] patients [%] in the total cohort [n = 519] in relapse or remission per year from diagnosis onwards. The patients were followed up until the date of their last visit in the study, their death, or colectomy. The number of patients is shown in parentheses. The median follow-up period was 18.6 years [range: 0–22 years].
Among those patients who had a relapse in the first year, 52.7% (95% CI [47.7–57.9]) experienced a relapse the following year, 73.0% (95% CI [68.4–77.5]) within 5 years, 80% (95% CI [75.4–84.0]) within 10 years, and 85% (95% CI [81.0–88.5]) within 20 years.
In the Cox proportional hazards models, comparing the patients who had a relapse in the first or second year after diagnosis with those who had no relapse, only mucosal healing at 1-year FU was independently associated with the risk of further relapse. Patients with mucosal healing at 1-year FU had approximately half the risk of further relapse compared with those with mucosal inflammation at 1 year (adjusted HR = 0.5, 95% CI [0.3–0.7], p = 0.001).
3.3. Colectomy
The cumulative colectomy rate for the total cohort [n = 519] was 13.0% (95% CI [11.4–14.6]) 20 years after diagnosis.
Total or subtotal colectomy was performed in 54 patients and large bowel resection in four patients. Among those who underwent total or subtotal colectomy, 33 patients [61%] received an IPAA, 17 patients [32%] an ileostomy, and four patients [7%] an ileorectal anastomosis [IRA]. All four patients with initial IRA were males, and had undergone colectomy due to colorectal neoplasia; two patients were older than 60 years at diagnosis and the other two had concomitant PSC and underwent later proctectomy with IPAA as cancer prophylaxis, after 3 and 9 years, respectively. None of the patients developed rectal cancer. However, one of the older patients, at age 81 years, developed a visible dysplasic lesion in the rectum, 7 years after IRA.
Almost half [n = 25] of the colectomies were performed during the first 2 years after diagnosis. The majority of patients [n = 49] had surgery due to moderate-to-severe active disease that was refractory to medical therapy, and nine patients were operated on due to colorectal neoplasia.
The multivariate Cox proportional hazard model revealed that patients with extensive colitis at diagnosis had an almost three times higher risk of colectomy than patients with proctitis (HR = 2.8, 95% CI [1.3–6.1]), whereas patients with mucosal healing had about 60% lower risk of colectomy than those with mucosal inflammation at 1 year of follow-up (HR = 0.4, 95% CI: [0.2–0.8]). Also age ≥40 years at diagnosis was inversely associated with the risk of colectomy [Table 2].
Table 2.
Risk of colectomy in UC analysed by Cox regression. No. = number of patients included in the analysis.
| Variables | No. | HR | CI | p-value | HR | CI | p-value |
|---|---|---|---|---|---|---|---|
| Female [ref.] | 252 | 1.0 | |||||
| Male | 267 | 0.9 | 0.6–1.6 | 0.9 | Ni | ||
| Age at diagnosis | |||||||
| <40 years [ref.] | 289 | 1.0 | |||||
| ≥40 years | 230 | 0.5 | 0.3–0.9 | 0.018 | 0.5 | 0.2–0.9 | 0.036 |
| Extent at diagnosis | |||||||
| E1 [ref.] | 171 | 1.0 | |||||
| E2 | 182 | 1.3 | 0.6–2.9 | 0.49 | Excl. | ||
| E3 | 166 | 3.4 | 1.7–6.7 | 0.001 | 2.8 | 1.3–6.1 | 0.011 |
| Biomarkers at diagnosis | |||||||
| ESR [mm/h] or CRP [mg/L] | |||||||
| <30 [ref.] | 324 | 1.0 | |||||
| ≥30 | 185 | 0.9 | 0.5–1.5 | 0.63 | Ni | ||
| Systemic steroids at diagnosis | |||||||
| No [ref.] | 357 | 1.0 | |||||
| Yes | 161 | 1.2 | 0.7–2.0 | 0.60 | Ni | ||
| Smoking status at diagnosis | |||||||
| Non-smoker [ref.] | 448 | 1.0 | |||||
| Current smoker | 69 | 0.5 | 0.2–1.4 | 0.18 | Excl. | ||
| Mucosal healing at 1 year | |||||||
| No [ref.] | 213 | 1.0 | 1.0 | ||||
| Yes | 199 | 0.4 | 0.2–0.8 | 0.007 | 0.4 | 0.2–0.8 | 0.007 |
HR, hazard ratio; CI, 95% confidence interval; Ni, the variable was not entered in the multiple model; Excl, the variable was entered in the multiple models, but was excluded because of later non-significance; [ref.], reference category; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; UC ulcerative colitis.
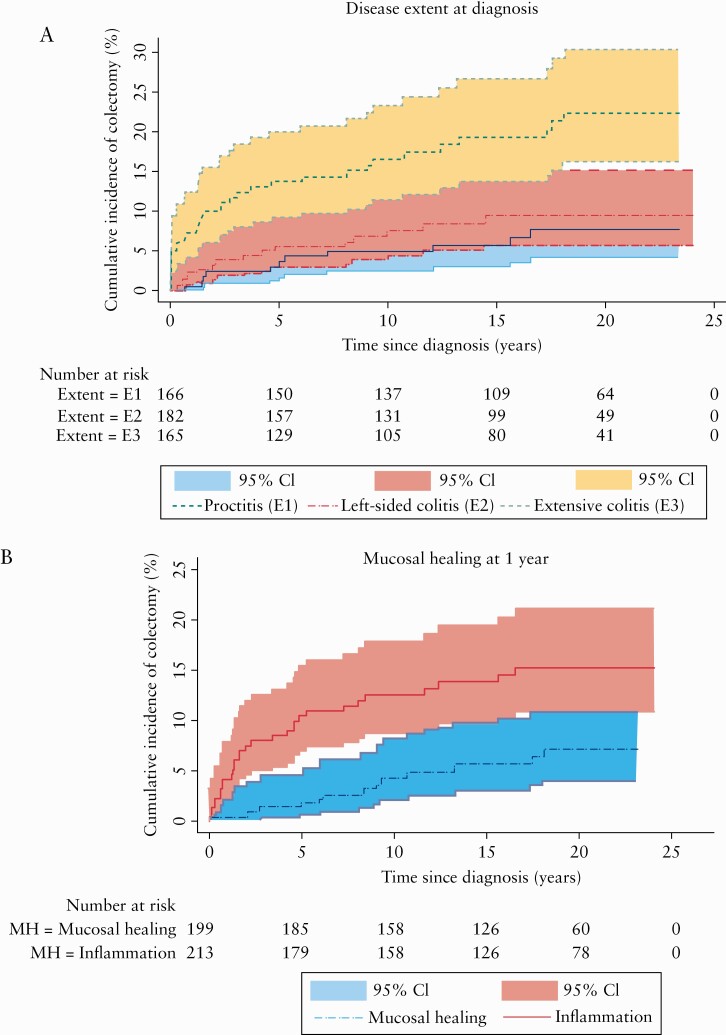
Kaplan‐Meier plots of the cumulative colectomy rates according to disease extent at diagnosis and mucosal healing at 1-year FU are depicted in Figure 5. Correspondingly, the 20-year cumulative colectomy rates were 22.4% (95% CI: [18.8–26.0]) in extensive colitis, 8.5% (95% CI: [6.1–10.9]) in left-sided colitis, and 7.6% (95% CI: [5.4–9.8]) in proctitis. For patients with mucosal healing at 1-year FU, the 20-year cumulative colectomy rate was significantly lower than for those with inflammation at 1 year, with rates of 7.2% (95% CI: [5.2–9.2]) and 15.3% (95% CI: [11.4–14.6]), respectively.
Figure 5.
a. Cumulative rate [%] of colectomy during the 20-year FU period according to the extent of UC at diagnosis. b. Cumulative rate [%] of colectomy during the first 20 years after the diagnosis of UC in patients with mucosal healing vs. mucosal inflammation at 1-year FU. UC, ulcerative colitis; FU, follow-up.
3.4. Medical treatment
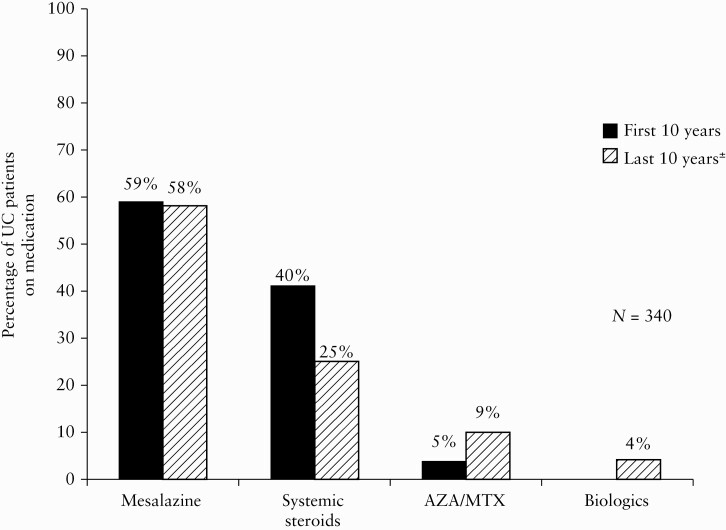
Medical therapy during the first and second decade of FU is shown in Figure 6. Almost 60% of the UC patients were receiving oral treatment with mesalazine throughout the period. The use of systemic steroids decreased during follow-up, and the use of immunosuppressives doubled during the second decade. Only 11 patients received biologics.
Figure 6.
Cumulative drug consumption after initial treatment for ulcerative colitis [UC] during the first 10 years [black bars] versus the last 10 years [patterned bars]. ±Data missing in 17 cases.
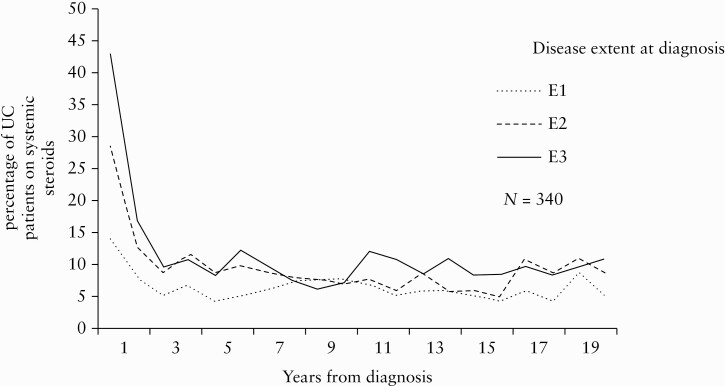
The proportion of patients on systemic steroids year-by-year according to disease extent at diagnosis is depicted in Figure 7; 34 [11%] patients had three or more courses of steroids during the last 10 years of FU. We found no significant differences in use of steroids with regard to gender, smoking status, age, disease extent, ESR/CRP levels, need for systemic steroids at diagnosis, or mucosal healing at 1-year FU.
Figure 7.
Proportions of UC patients [%] using systemic steroids in the 20-year FU cohort according to disease extent at diagnosis. E1: proctitis, E2: left-sided colitis, E3: extensive colitis. UC, ulcerative colitis; FU, follow-up.
3.5. Hospitalisation
Among the patients in the 20-year FU cohort who did not undergo colectomy [n = 308], 32 [10.4%] had been hospitalised due to worsening of UC symptoms during the last 10 years of FU. The majority of these patients [n = 18] had only one hospitalisation, but some had repeated hospital stays, including one patient who had 20 hospital admissions during the last decade. Only age below 40 years at diagnosis was significantly associated with increased odds of hospital admission (odds ratio [OR] = 3.7, 95% CI [1.3–11.1]) adjusting for disease extent and ESR/CRP levels at diagnosis.
3.6. Course of disease
Patients’ reports on the four predefined disease curves [Figure 1] showed that more than two-thirds [69%] of patients assessed their disease course to be mild or in remission after initial high activity [Curve 1]. Only 4% reported the inverse course, with initially low activity and a worsening of symptoms over time [Curve 2]. Chronic continuous symptoms [Curve 3] were reported by 8%, and 19% reported having chronic intermittent symptoms [Curve 4]. Results were missing for 21 patients [6%], and 32 patients were excluded from this analysis since they had undergone a colectomy. There were no statistically significant differences in patients’ perception of their disease activity with regard to gender, smoking status, age, disease extent, ESR/CRP levels, need for systemic steroids at diagnosis, or mucosal healing at 1-year FU.
4. Discussion
In this population-based, long-term FU study, the majority of the UC patients had a mild disease course. This is reflected by the low colectomy rate overall and few UC-related hospitalisations. Additionally, there was infrequent use of immunosuppressives and biologics. Moreover, the patients’ self-assessment of disease indicates that a large proportion of the patients experienced a decline in symptoms over time.
After 20 years, the main indication for colectomy remained moderate-to-severe disease with continuous symptoms and resistant to medical therapy. Only a few patients had surgery due to neoplasia. Although the overall risk of colectomy for UC remained low 20 years after diagnosis, this risk was not insignificant in the subgroup of patients diagnosed with extensive colitis.
In the present study, no increased risk of mortality in any of the Montreal Classification subgroups was found. The study from Olmstead County revealed a trend towards increased mortality among patients with extensive colitis compared with those with proctitis. However, this result is probably influenced by the available treatment options, which were limited at the time when the study was conducted.17 In two more recent European studies with a shorter follow-up time, including one that involved a prospective, Europe-wide, population-based cohort, the disease extent had no significant impact on mortality.18,19 Since the risk of developing cancer in IBD seems to increase with time, future studies with an even longer follow-up time are needed to demonstrate whether there is a relationship between the extent of disease and mortality rate.
The anti-inflammatory effects of mesalazine are well documented, but its direct chemopreventive effects have been harder to demonstrate, especially in population based studies.20–22 However, a recent meta-analysis showed a protective effect of mesalazine on colorectal cancer [CRC] development even in population-based studies.23 The effect was dose-dependent. In contrast, sulphasalazine did not exhibit significant chemopreventive effect.23,24 The inhibition of folic acid absorption may partly explain a reduced chemopreventive effect of this drug.25,26 In our cohort, most patients were started on sulphasalazine, the available agent in Norway in 1990, and switched to one of the newer 5-aminosalicylic acid preparations when these became available. Moreover, the low number of CRC in our cohort did not justify further statistical analysis.
The cumulative colectomy rate in our cohort was comparable to that found in the Manitoba region in Canada, in which the cumulative colectomy rate was 14.2% 20 years after diagnosis.27 In contrast, in the Olmstead County cohort from Minnesota, the cumulative incidence of colectomy was found to be as high as 25.4% 20 years after diagnosis.28 This discrepancy is apparently due to the difference in available medical therapy, as the inclusion period in the Olmstead County study began in the 1940s, when neither prednisolone nor 5-aminosalicylates were in use, whereas the Manitoba cohort was included in approximately the same time frame as the cohort in our study. However, the Copenhagen cohort study is the only study comparable to ours, as it is the only prospective IBD cohort study with 20 years of follow-up. In that study, the colectomy rate was 30% after 20 years,29 but the authors included patients from 1962, which was almost 30 years before our inclusion period. Although cortisone and salazopyrine/5-aminosalicylates were available, it is likely that the optimal use of these were not yet established. For the IBSEN cohort, azathioprine was also available from the latter part of the first decade and anti-tumour necrosis factor [TNF] alpha inhibitors from the latter half of the second decade. However, as shown, only a small percentage of our UC patients used these therapies.
Although it seems distant from patients’ concern about their symptoms and general well-being, mucosal healing has been shown to be an important and objective treatment target in UC.30–34 Clinical activity indices are mainly based on symptom scores, which are likely to be influenced by psychological factors and coexisting irritable bowel symptoms33 and have been demonstrated to be rather poorly related to inflammation.35 Moreover, since UC is primarily a mucosal disease, mucosal healing represents an obvious treatment target.
Several studies have confirmed that mucosal healing is a predictor of a favourable disease course, with sustained steroid-free remission,31,34,36 decreased hospitalisations,30 and a better quality of life.37 In line with these findings, we demonstrated that the risk of subsequent relapse was increased 2-fold for UC patients with ongoing inflammation compared with that for patients who achieved mucosal healing at 1 year. Furthermore, several studies demonstrate that mucosal healing in UC exerts an influence on hard endpoints, such as reducing both the risk of colorectal cancer38–40 and the risk of colectomy early in the disease course.30,41–43 Our study confirms that early mucosal healing is associated with a more favourable outcome with a lower risk of colectomy, even in the long term. Recent studies have implied that histological normalisation may be an even better predictor of outcome than mucosal healing. However, histological analysis entails a considerable delay and is resource-demanding, and histological indices are yet not fully validated.44
Conflicting results have been demonstrated related to age as a prognostic factor. Younger age at diagnosis of UC has in several studies been associated with a more aggressive disease course.45–48 Comparably, in our study, age below 40 years at diagnosis was associated with an increased risk of UC-related hospitalisation and colectomy. However, this trend is not confirmed in all studies.49,50 Moreover, the results are difficult to compare due to differences in study design. Furthermore, results from tertiary centres may be biased due to selected patients.
Our results may be considered outdated in the present scenario with biologics and more widespread use of immunosuppressives. However, one could argue that they still have relevance since this study largely demonstrates ‘the natural course’ of ulcerative colitis just before the introduction of biologic treatment in IBD. The majority of our patients underwent colectomy during the first years after diagnosis, when anti-TNF were not yet available. Hence, our data might be used as comparison for population-based study cohorts in the biologic era. The consequences of the long-term use of biologics are still unclear.51 Furthermore, it is not yet known whether biologics reduce the risk of colectomy in the long term or just postpones it.52
The majority of our patients, regardless of disease extent, experienced a decline in disease activity over time. Our findings may suggest that many UC patients do well without biologics, and also highlight the need for prognostic factors to better select those patients who require such therapy in the early course of disease. On the other hand, the close follow-up in this study setting may have contributed to the overall favourable disease course.
The strengths of our study include the prospective recruitment and long-term FU. In addition, the patients were unselected. The disease definition, diagnostic tools, and available therapy were uniform. The inclusion period was short and within the same geographical area, thereby minimising differences in environmental factors.
The limitations of our study include the potential risk of bias due to missing data and patients lost to FU. However, most patients defined as lost to FU were in fact traceable and living in the study area; hence the likelihood that they had undergone colectomy elsewhere is small. Moreover, there were no statistically significant differences in disease characteristics between the patients included in the study and those lost to FU. Clinical information between the scheduled visits was recorded retrospectively and complemented with hospital records, and was thus potentially subject to inaccuracy and recall bias. Furthermore, data from the Norwegian prescription database were only obtainable after 2004. Hence, confirmed information on drug use was not available for the first 10–15 years of the study. An accurate estimation of the area under the curve for drug consumption was therefore not justified.
With regard to subgroup analysis, we were unable to perform a more advanced analysis because the statistical power would be too low. Moreover a recent flare, presence of irritable bowel symptoms, acquired disease coping, and the influence of the attending physician may have influenced the patients’ self-assessment of symptoms. However, it is likely that some of these factors may counterbalance each other.
In conclusion, our population-based, longitudinal study indicates that after the first flare[s], the majority of newly diagnosed UC patients will have a mild course of disease rarely needing hospitalisation. The mortality in our cohort was not increased, and few patients underwent colectomy compared with other studies. In spite of the low colectomy rate, the incidence of neoplasia remained low. Furthermore, our results demonstrate that early mucosal healing has an important impact on surgery even 20 years after diagnosis, and thereby emphasises its importance as a treatment target in UC.
Acknowledgments
We thank the remaining members of the IBSEN study Group, in particular Elisabeth Finnes, Ragnhild Husom, and Marte Lie Hoivik at department of Gastroenterology, Oslo University Hospital, Ulleval, and Tomm Bernklev, Vestfold Hospital Trust and Institute of Clinical Medicine, University of Oslo.
Funding
This work did not receive any financial support.
Conflict of Interest
There was no conflict of interest.
Author Contributions
ILM, ICS, JJ were involved in the concept of the study, data acquisition, interpreting the data, drafting and revising the manuscript. BM and MV were involved in the concept of the study and revised the document. O.Hovde, GHH, EG, NS were involved in data acquisition. O.Hoie was involved in data acquisition and revised the document. MC was involved in the statistical analysis and revised the document. All authors approved the final document.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. [DOI] [PubMed] [Google Scholar]
- 3. Moum B, Vatn MH, Ekbom A, et al. . Incidence of inflammatory bowel disease in southeastern Norway: evaluation of methods after 1 year of registration. Southeastern Norway IBD Study Group of Gastroenterologists. Digestion 1995;56:377–81. [DOI] [PubMed] [Google Scholar]
- 4. Henriksen M, Jahnsen J, Lygren I, et al. ; IBSEN Study Group. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study [the IBSEN study]. Inflamm Bowel Dis 2006;12:543–50. [DOI] [PubMed] [Google Scholar]
- 5. Moum B, Ekbom A, Vatn MH, et al. . Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn’s disease. Results of a large, prospective population-based study in southeastern Norway, 1990-93. Scand J Gastroenterol 1997;32:1005–12. [DOI] [PubMed] [Google Scholar]
- 6. Solberg IC, Lygren I, Jahnsen J, et al. ; IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort [IBSEN Study]. Scand J Gastroenterol 2009;44:431–40. [DOI] [PubMed] [Google Scholar]
- 7. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group . Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 8. Moum B, Ekbom A, Vatn MH, et al. . Inflammatory bowel disease: re-evaluation of the diagnosis in a prospective population based study in south eastern Norway. Gut 1997;40:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lunder AK, Hov JR, Borthne A, et al. . Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology 2016;151:660–9.e4. [DOI] [PubMed] [Google Scholar]
- 10. Moum B, Vatn MH, Ekbom A, et al. . Incidence of ulcerative colitis and indeterminate colitis in four counties of southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway [IBSEN] Study Group of Gastroenterologists. Scand J Gastroenterol 1996;31:362–6. [DOI] [PubMed] [Google Scholar]
- 11. Moum B, Vatn MH, Ekbom A, et al. . Incidence of Crohn’s disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway [IBSEN] Study Group of Gastroenterologists. Scand J Gastroenterol 1996;31:355–61. [DOI] [PubMed] [Google Scholar]
- 12. Henriksen M, Jahnsen J, Lygren I, et al. ; Ibsen Study Group. Change of diagnosis during the first five years after onset of inflammatory bowel disease: results of a prospective follow-up study [the IBSEN Study]. Scand J Gastroenterol 2006;41:1037–43. [DOI] [PubMed] [Google Scholar]
- 13. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A–36A. [DOI] [PubMed] [Google Scholar]
- 14. Hovde Ø, Småstuen MC, Høivik ML, et al. . Mortality and causes of death in ulcerative colitis: results from 20 years of follow-up in the IBSEN Study. Inflamm Bowel Dis 2016;22:141–5. [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Regula J, Feagan BG, et al. . Delayed-release oral mesalamine 4.8 g/day [800-mg tablet] is effective for patients with moderately active ulcerative colitis. Gastroenterology 2009;137:1934–43.e1–3. [DOI] [PubMed] [Google Scholar]
- 16. Jansen L, Hakulinen T, Brenner H. Standard errors of non-standardised and age-standardised relative survival of cancer patients. Br J Cancer 2012;106:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jess T, Loftus EV Jr, Harmsen WS, et al. . Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut 2006;55:1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrokhyar F, Swarbrick ET, Grace RH, Hellier MD, Gent AE, Irvine EJ. Low mortality in ulcerative colitis and Crohn’s disease in three regional centers in England. Am J Gastroenterol 2001;96:501–7. [DOI] [PubMed] [Google Scholar]
- 19. Höie O, Wolters F, Riis L, et al. ; European Collaborative Study Group of Inflammatory Bowel Disease [EC-IBD]. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol 2007;102:1692–701. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen GC, Gulamhusein A, Bernstein CN. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: a meta-analysis of non-referral populations. Am J Gastroenterol 2012;107:1298–304; quiz 1297, 1305. [DOI] [PubMed] [Google Scholar]
- 21. Qiu X, Ma J, Wang K, Zhang H. Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: a systematic review with meta-analysis. Oncotarget 2017;8:1031–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mak JWY, So J, Tang W, et al. . Cancer risk and chemoprevention in Chinese inflammatory bowel disease patients: a population-based cohort study. Scand J Gastroenterol 2020;55:279–86. [DOI] [PubMed] [Google Scholar]
- 23. Bonovas S, Fiorino G, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017;45:1179–92. [DOI] [PubMed] [Google Scholar]
- 24. OʼConnor A, Packey CD, Akbari M, Moss AC. Mesalamine, but not sulfasalazine, reduces the risk of colorectal neoplasia in patients with inflammatory bowel disease: an agent-specific systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:2562–9. [DOI] [PubMed] [Google Scholar]
- 25. Burr NE, Hull MA, Subramanian V. Folic acid supplementation may reduce colorectal cancer risk in patients with inflammatory bowel disease: a systematic review and meta-analysis. J Clin Gastroenterol 2017;51:247–53. [DOI] [PubMed] [Google Scholar]
- 26. Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets 2019;19:1100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samuel S, Ingle SB, Dhillon S, et al. . Cumulative incidence and risk factors for hospitalisation and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis 2013;19:1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jess T, Loftus EV Jr, Velayos FS, et al. . Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis 2006;12:669–76. [DOI] [PubMed] [Google Scholar]
- 29. Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology 1994;107:3–11. [DOI] [PubMed] [Google Scholar]
- 30. Ardizzone S, Cassinotti A, Duca P, et al. . Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol 2011;9:483–9.e3. [DOI] [PubMed] [Google Scholar]
- 31. Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. . Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing [Mayo 0 vs 1]: a longitudinal cohort study. J Crohns Colitis 2016;10:13–9. [DOI] [PubMed] [Google Scholar]
- 32. Colombel JF, Keir ME, Scherl A, et al. . Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2017;66:2063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012;107:1474–82. [DOI] [PubMed] [Google Scholar]
- 34. Kim JH, Cheon JH, Park Y, et al. . Effect of mucosal healing [Mayo 0] on clinical relapse in patients with ulcerative colitis in clinical remission. Scand J Gastroenterol 2016;51:1069–74. [DOI] [PubMed] [Google Scholar]
- 35. Gracie DJ, Williams CJ, Sood R, et al. . Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol 2016;111:541–51. [DOI] [PubMed] [Google Scholar]
- 36. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1245–55.e8. [DOI] [PubMed] [Google Scholar]
- 37. Feagan BG, Reinisch W, Rutgeerts P, et al. . The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol 2007;102:794–802. [DOI] [PubMed] [Google Scholar]
- 38. Gupta RB, Harpaz N, Itzkowitz S, et al. . Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 2007;133:1099–105; quiz 1340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin DT, Huo D, Kinnucan JA, et al. . Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol 2013;11:1601–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutter MD, Saunders BP, Wilkinson KH, et al. . Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut 2004;53:1813–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colombel JF, Rutgeerts P, Reinisch W, et al. . Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 42. Ferrante M, Vermeire S, Fidder H, et al. . Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008;2:219–25. [DOI] [PubMed] [Google Scholar]
- 43. Sandborn WJ, Rutgeerts P, Feagan BG, et al. . Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009;137:1250–60; quiz 520. [DOI] [PubMed] [Google Scholar]
- 44. Marchal Bressenot A, Riddell RH, Boulagnon-Rombi C, et al. . Review article: the histological assessment of disease activity in ulcerative colitis. Aliment Pharmacol Ther 2015;42:957–67. [DOI] [PubMed] [Google Scholar]
- 45. Kalkan IH, Dağli U, Oztaş E, Tunç B, Ulker A. Comparison of demographic and clinical characteristics of patients with early vs. adult vs. late onset ulcerative colitis. Eur J Intern Med 2013;24:273–7. [DOI] [PubMed] [Google Scholar]
- 46. Charpentier C, Salleron J, Savoye G, et al. . Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–32. [DOI] [PubMed] [Google Scholar]
- 47. Ha CY, Newberry RD, Stone CD, Ciorba MA. Patients with late-adult-onset ulcerative colitis have better outcomes than those with early onset disease. Clin Gastroenterol Hepatol 2010;8:682–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dias CC, Rodrigues PP, Coelho R, et al. ; on behalf of GEDII. Development and validation of risk matrices for Crohn’s disease outcomes in patients who underwent early therapeutic interventions. J Crohns Colitis 2017;11:445–53. [DOI] [PubMed] [Google Scholar]
- 49. Tremaine WJ, Timmons LJ, Loftus EV Jr, et al. . Age at onset of inflammatory bowel disease and the risk of surgery for non-neoplastic bowel disease. Aliment Pharmacol Ther 2007;25:1435–41. [DOI] [PubMed] [Google Scholar]
- 50. Lee JH, Cheon JH, Moon CM, et al. . Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older? Digestion 2010;81:237–43. [DOI] [PubMed] [Google Scholar]
- 51. Bonovas S, Fiorino G, Allocca M, et al. . Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385–97.e10. [DOI] [PubMed] [Google Scholar]
- 52. Murthy SK, Begum J, Benchimol EI, et al. . Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population-based interrupted time series study. Gut 2020;69:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]