Abstract
Background and Aims
It is unclear whether pre-pouch ileitis heralds an aggressive inflammatory pouch disease in patients with ileal pouch-anal anastomosis [IPAA]. We compared outcomes of patients with pouchitis and concomitant pre-pouch ileitis with those with pouchitis alone.
Methods
Patients undergoing IPAA surgery for inflammatory bowel disease, who subsequently developed pouchitis with concomitant pre-pouch ileitis [pre-pouch ileitis group], were matched by year of IPAA surgery and preoperative diagnosis [ulcerative colitis or inflammatory bowel disease-unclassified] with patients who developed pouchitis alone [pouchitis group]. Primary outcomes were development of Crohn’s disease [CD]-like complications [non-anastomotic strictures or perianal disease >6 months after ileostomy closure] and pouch failure. Secondary outcomes were need for surgical/endoscopic interventions and immunosuppressive therapy. Log-rank testing was used to compare outcome-free survival, and Cox regression was performed to identify predictors of outcomes.
Results
There were 66 patients in each group. CD-like complications and pouch failure developed in 36.4% and 7.6% patients in the pre-pouch ileitis group and 10.6% and 1.5% in pouchitis group, respectively. CD-like complications-free survival [log-rank p = 0.0002] and pouch failure-free survival [log-rank p = 0.046] were significantly lower in the pre-pouch ileitis group. The pre-pouch ileitis group had a higher risk of requiring surgical/endoscopic interventions [log-rank p = 0.0005] and immunosuppressive therapy [log-rank p <0.0001]. Pre-pouch ileitis was independently associated with an increased risk of CD-like complications (hazard ratio [HR] 3.8; p = 0.0007), need for surgical/endoscopic interventions [HR 4.1; p = 0.002], and immunosuppressive therapy [HR 5.0; p = 0.0002].
Conclusions
Pre-pouch ileitis is associated with a higher risk of complicated disease and pouch failure than pouchitis. It should be considered a feature of CD.
Keywords: Pre-pouch ileitis, afferent limb inflammation, Crohn’s disease
1.Introduction
Ileal pouch-anal anastomosis [IPAA] has become the standard surgical procedure in patients with ulcerative colitis [UC] or inflammatory bowel disease-unclassified [IBD-U]. Patients with IPAA are at a risk of developing inflammatory complications of the ileal pouch. Pouchitis is the most common inflammatory complication, with up to 80% of patients experiencing at least one episode of pouchitis during their lifetime.1,2 A majority of these patients have a benign course and respond well to short courses of antibiotics.3,4 However, a small proportion of patients with IPAA go on to develop de novo Crohn’s disease [CD] which is associated with poorer outcomes including pouch failure.5–7
There are no established criteria for diagnosis of de novo CD.6 Commonly used criteria include the development of fistulae or non-anastomotic intestinal strictures which are not thought to be related to the IPAA surgery, histological presence of granulomas, and the development of pre-pouch ileitis. Pre-pouch ileitis is the inflammation of the afferent limb of the ileal pouch which occurs in around 5% of patients with IPAA.8,9 Since strictures and fistulae are well-known complications of CD, the diagnosis of de novo CD is relatively straightforward when patients with an ileal pouch develop one of these complications. However, presence of pre-pouch ileitis is a more controversial criterion for a de novo CD diagnosis. Though a majority of published studies on this topic have used pre-pouch ileitis as a criterion of de novo CD diagnosis,10 some studies have shown that pre-pouch ileitis is not associated with development of CD-like complications.9,11,12 Hence, it is currently debatable whether pre-pouch ileitis lies on the pouchitis spectrum or represents a more aggressive inflammatory pouch disease phenotype, like CD.
We aimed to study the impact of pre-pouch ileitis on pouch-related outcomes by comparing outcomes of patients with pouchitis and concomitant pre-pouch ileitis with patients who have pouchitis without pre-pouch ileitis. We hypothesised that patients with pre-pouch ileitis are at a higher risk of development of CD-like complications including non-anastomotic strictures and perianal disease, compared with patients with pouchitis alone.
2.Methods
2.1.Patient population
A prospectively maintained database of patients who underwent IPAA with mucosectomy and handsewn anastomosis, performed by a single colorectal surgeon [PRF] at Cedars-Sinai Medical Center for a diagnosis of UC or IBD-U between January 1993 and December 2018, was queried. Patients having an IPAA for CD or noted to have features of CD on histopathological examination of the colectomy specimen were excluded. Patients were seen for follow-up examinations and pouchoscopy with ileal intubation every 3 months for the first year after ileostomy closure and yearly afterwards. Histopathological evaluation of the pouch was not routinely performed. Only patients followed for minimum of 6 months were included in this analysis. This study was approved by the Cedars-Sinai Institutional Review Board [Pro00050625].
2.2.Assessment of clinical characteristics
Detailed clinical profiles assessing demographic information and characteristics of the disease were prospectively generated by one investigator [PRF], using chart review and patient interview. Demographic information included patient age at inflammatory bowel disease [IBD] diagnosis, age at surgery, gender, and time from stoma closure to the diagnosis of pre-pouch ileitis or pouchitis. Disease characteristics included preoperative diagnosis [UC or IBD-U], disease extent, presence of backwash ileitis or primary sclerosing cholangitis [PSC], and family history of IBD. Clinical, endoscopic, and pathological criteria were reviewed in all patients to determine if they had UC or IBD-U, according to the Montreal Classification.13 Clinically, UC patients had no perianal disease, and endoscopic features included continuous macroscopic disease extending varying distances from the dentate line. Radiological and/or endoscopic evaluation revealed the distinct absence of either a colonic stricture or small bowel disease 3 cm proximal to the ileocaecal valve. Histological features included continuous microscopic inflammation and the absence of non-caseating granulomas. Additionally, intraoperative findings and histopathology of the resected specimen were reviewed to confirm absence of features suggestive of CD. Patients were classified as having IBD-U when they predominantly met the criteria for UC with some features suggestive but not diagnostic of CD. These features included discontinuous inflammation possibly related to medical therapy, history of a simple anal fistula or ulcer which was inactive at the time of surgery, and non-caseating crypt rupture granulomas.
Backwash ileitis was defined as neutrophilic inflammation in the lamina propria and/or epithelium of the terminal ileum extending less than 3 cm proximal to the ileocaecal valve. The diagnosis of PSC was based on magnetic resonance cholangiopancreaticography or liver biopsy. Treatment characteristics included preoperative immunomodulators and/or biologic use, indication for IPAA, and number of stages used for IPAA completion.
2.3.Diagnosis of pre-pouch ileitis and pouchitis
Given the lack of a validated definition, pre-pouch ileitis was defined by the presence of visible inflammation in the distal 10 cm of the afferent limb, with one or more of these features—friability, erosions, or ulcers. Further details of pre-pouch ileitis extent and severity were not available. All patients with endoscopic pre-pouch ileitis were confirmed to have acute ileitis with or without features of chronic inflammation histologically. Patients with pouchitis and pre-pouch ileitis [pre-pouch ileitis group] were matched 1:1 by year of IPAA surgery [+/- 2 years] and pre-perative diagnosis [UC or IBD-U] with patients who developed pouchitis without pre-pouch ileitis [pouchitis group]. Pouchitis was defined as a clinical syndrome characterised by the onset of increased stool frequency often with bloody diarrhoea, pelvic discomfort, urgency, malaise, fever, and endoscopically confirmed inflammation in the ileal pouch. Patients using non-steroidal anti-inflammatory drugs and those who were noted to have granulomas on ileal pouch biopsies were also excluded from analysis.
Patients were assigned to the groups based on their final diagnosis after ileostomy closure or the diagnosis at the time they developed a study outcome. Patients who had pouchitis but did not develop pre-pouch ileitis were assigned to the pouchitis group. Those who developed pouchitis initially but later developed pre-pouch ileitis were assigned to pre-pouch ileitis group. If a patient with pouchitis developed a primary or secondary outcome and subsequently developed pre-pouch ileitis, he/she was assigned to the pouchitis group. To minimise the confounding effect of pouchitis on the study outcomes, patients with pre-pouch ileitis who did not have concomitant pouchitis were excluded. All patients entered the study when they were first diagnosed with the inflammatory pouch disorder specific to their group, and follow-up data regarding their subsequent disease course were recorded.
2.4.Long-term outcomes
Primary outcomes were development of CD-like complications and pouch failure. CD-like complications were defined as a perianal fistulae or abscesses occurring more than 6 months after the ileostomy closure, or non-anastomotic strictures in the ileal pouch or in the distal 10 cm of the afferent limb. Pouch failure was defined as the need for permanent ileostomy with or without pouch excision. Secondary outcomes were need for surgical or endoscopic interventions >6 months after the ileostomy closure and need for immunosuppressive [immunomodulator or biologic or both] therapy. Surgical or endoscopic interventions included small bowel resection, pouch revision/resection, temporary or permanent ileostomy, perianal surgery, and endoscopic dilation of non-anastomotic strictures. Lysis of adhesion surgeries and endoscopic or surgical dilations of anastomotic strictures were excluded from this outcome. Study outcomes were compared between the pouchitis with pre-pouch ileitis and the pouchitis alone groups. A subgroup analysis was performed to evaluate the outcomes of patients with pouchitis and pre-pouch ileal ulcers [excluding those who did not have ulcers in the pre-pouch ileum] compared with those with pouchitis alone.
2.5.Statistical analysis
Continuous variables were expressed as median and interquartile range and categorical variables were expressed as percentages. Wilcoxon rank sum testing was used to compare continuous variables, and chi square and Fisher’s exact tests were used to compare categorial variables as appropriate. Follow-up was censored at the date of either first occurrence of the outcome being evaluated or at date of last follow-up for those with no event. Kaplan‐Meier survival analysis was performed for each primary and secondary outcome and log ranking test was used to compare outcomes between the two groups. Univariable and multivariable Cox proportional hazard analysis was performed to identify predictors of primary and secondary outcomes and results were expressed as hazard ratios [HRs] and 95% confidence intervals [CIs]. Only variables with a p-value of ≤0.10 on univariable analysis were included in multivariable analysis. A two-tailed p-value of 0.05 was considered statistically significant for all analyses. All analyses were performed using JMP® 14.2.0 software [SAS Institute, Cary, NC]. All authors had access to the study data and reviewed and approved the final manuscript.
3.Results
3.1.Patient demographics and clinical characteristics
A total of 635 patients underwent IPAA surgery in the study period. Pouchitis with pre-pouch ileitis developed in 66 [10%] of these patients. Four patients were noted to have pre-pouch ileitis without pouchitis and were not included in the study per the exclusion criteria [Supplementary Figure S1, available as Supplementary data at ECCO-JCC online]. A total of 66 patients with pouchitis and pre-pouch ileitis [pre-pouch ileitis group] were 1:1 matched with patients who had pouchitis alone [pouchitis group] by year of IPAA surgery and preoperative UC/IBD-U diagnosis. Of the 66 patients with pre-pouch ileitis, pre-pouch ileal ulcers were present in 51, and the other 15 had friability and/or erosions in the pre-pouch ileum without ulcers.
Table 1 shows the distribution of baseline characteristics of the two patient groups. Most patients had a pre-colectomy IBD diagnosis of UC and preoperative disease extent was most commonly pancolitis. Comorbid primary sclerosing cholangitis [PSC] was present in 8% of patients in both groups and around 15% had backwash ileitis. A majority of patients had surgery for medically refractory disease. Although pre-colectomy biologic drug use was also similar between patient groups, a higher proportion of patients with pre-pouch ileitis had exposure to immunomodulators before colectomy compared with those in the pouchitis group [p = 0.05]. There was no other significant difference in clinical characteristics between the patient groups. None of the patients in entire study cohort developed any study outcomes before the diagnosis of pouchitis or pre-pouch ileitis was made.
Table 1.
Patient group characteristics.
| Pre-pouch ileitis + pouchitis [n = 66] | Pouchitis alone [n = 66] | p-value | |
|---|---|---|---|
| Age [years] | 50 [36, 64] | 50 [33, 57] | 0.11 |
| Age of IBD onset [years] | 27 [17, 36] | 25 [17, 34] | 0.48 |
| Male sex | 41 [62.1] | 41 [62.1] | 1.00 |
| Preoperative diagnosis | |||
| UC | 41 [62.1] | 41 [62.1] | 1.00 |
| IBD-U | 25 [37.9] | 25 [37.9] | |
| Disease extent | |||
| Proctitis [Montreal E1] | 5 [7.6] | 1 [1.5] | 0.27 |
| Left-sided colitis [Montreal E2] | 9 [13.6] | 11 [16.7] | |
| Pancolitis [Montreal E3] | 52 [78.8] | 54 [81.8] | |
| Family history of IBD | 19 [28.8] | 20 [30.3] | 0.85 |
| Primary sclerosing cholangitis | 5 [7.6] | 5 [7.6] | 1.00 |
| Backwash ileitis | 10 [15.2] | 11 [16.7] | 0.82 |
| Pre-colectomy immunomodulator use | 53 [80.3] | 43 [65.2] | 0.05 |
| Pre-colectomy biologic use | 36 [54.6] | 37 [56.1] | 0.86 |
| Indication for surgery | |||
| Medically refractory disease | 55 [83.3] | 62 [93.9] | 0.06 |
| Dysplasia or cancer | 11 [16.7] | 4 [6.1] | |
| IPAA stages | |||
| Two | 43 [65.2] | 36 [54.6] | 0.21 |
| Three | 23 [34.8] | 30 [45.4] | |
| Time from ileostomy closure to pre-pouch ileitis/pouchitis diagnosis [months] | 33 [5, 67] | 14 [6, 38] | 0.004 |
All values expressed as median [interquartile range] or n [%].
IBD, inflammatory bowel disease; UC, ulcerative colitis; IBD-U, IBD-unclassified; IPAA, ileal pouch-anal anastomosis.
3.2.Primary outcomes
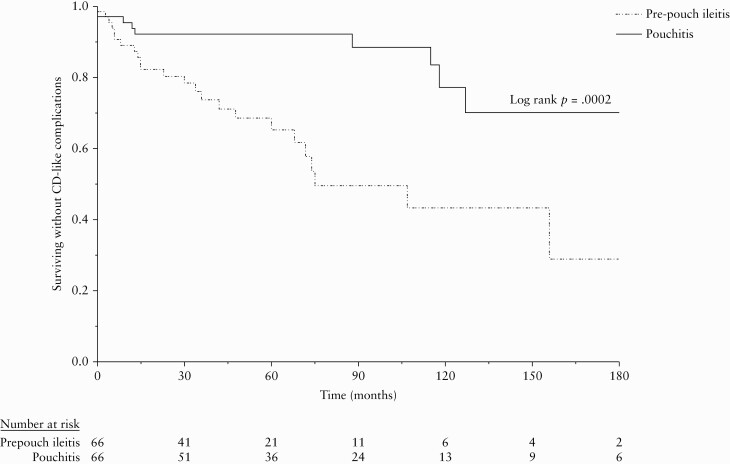
CD-like complications developed in 31 [23.5%] patients, 24 [36.4%] in the pre-pouch ileitis group and in seven [10.6%] in the pouchitis group [p = 0.0004]. CD-like complications in the pre-pouch ileitis group included development of perianal fistulae or abscesses in 14 [21.2%] and non-anastomotic strictures in 10 [15.2%] patients. All strictures were located in the distal afferent limb. In the pouchitis group, seven [10.6%] developed perianal disease and no patients developed strictures. Median time from ileostomy closure to CD-like complication was 27 (interquartile range [IQR] 7, 66) months in the pre-pouch ileitis group and 102 [IQR 13, 120] months in the pouchitis group [p = 0.09]. CD-like complications-free survival was significantly worse in the pre-pouch ileitis group compared with the pouchitis group [log rank p = 0.0002]. The rate of CD-like complications at 2, 5, and 10 years in the pre-pouch ileitis group was 19.7%, 34.8%, and 56.7% and in the pouchitis group was 7.8%, 7.8%, and 23.0%, respectively [Figure 1].
Figure 1.
Crohn’sdisease [CD]-like complication-free survival.
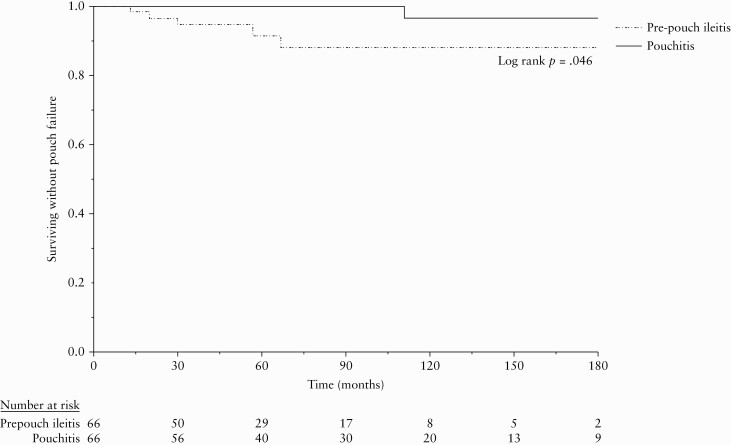
Six [5%] patients developed pouch failure in the entire cohort, five [7.7%] patients in the pre-pouch ileitis group and one [1.5%] patient in the pouchitis group [p = 0.09]. Median time from ileostomy closure to pouch failure was 30 [17, 62] months in the pre-pouch ileitis group. The only patient who developed pouch failure in the pouchitis group developed it at 111 months. Pouch failure-free survival was significantly lower in pre-pouch ileitis group with 3.5%, 8.6%, and 12.1% patients developing pouch failure at 2, 5, and 10 years compared with 0%, 0%, and 4.3% in the pouchitis group [log rank p = 0.046] [Figure 2].
Figure 2.
Pouch failure-free survival.
In the subgroup of patients who had pouchitis with pre-pouch ileal ulcers, CD-like complications developed in 20/51 [39.2%] and pouch failure developed in 4/51 [7.8%]. Patients who had pre-pouch ileal ulcers had significantly worse CD-like complication-free survival [log rank p <0.0001] and pouch failure-free survival [log rank p = 0.04] compared with those with pouchitis alone [Supplementary Figure S2, available as Supplementary data at ECCO-JCC online].
3.3.Secondary outcomes
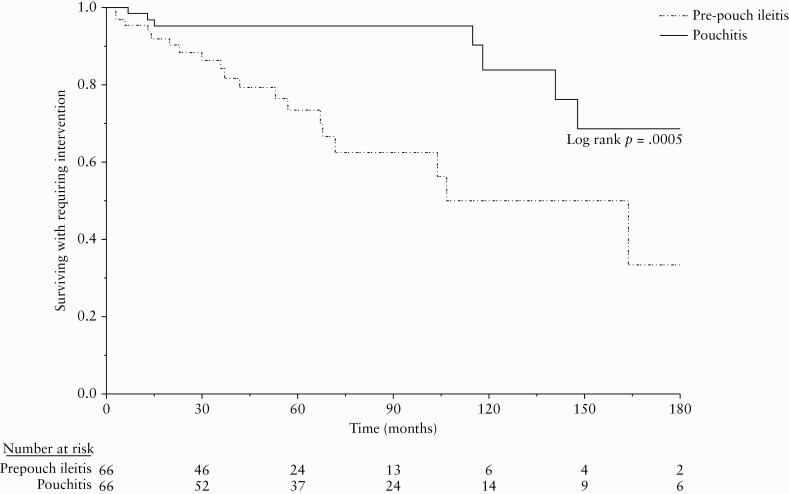
In all, 27 [20.5%] patients required surgical or endoscopic interventions, 19 [28.8%] in the pre-pouch ileitis group and eight [12.1%] in the pouchitis group [p = 0.02]. These included perianal fistula surgery [n = 9], endoscopic stricture dilation [n = 7], permanent ileostomy with or without pouch excision [n = 5], and pouch revision, small bowel resection, and diverting ileostomy [n = 1 each] in the pre-pouch ileitis group. Interventions in the pouchitis group included perianal fistula surgery [n = 7], small bowel resection, and permanent ileostomy without pouch excision [n = 1 each]. Median time from ileostomy closure to the intervention was 37 [IQR 14, 68] months in the pre-pouchitis group versus 118 [IQR 13, 148] months in the pouchitis group [p = 0.08]. Intervention-free survival [Figure 3] was significantly lower in pre-pouch ileitis group, with 11.8%, 26.6%, and 50% in the pre-pouch ileitis group and 4.8%, 4.8%, and 16.2% in the pouchitis group needing interventions at 2, 5, and 10 years, respectively [log-rank p = 0.0005].
Figure 3.
Intervention-free survival.
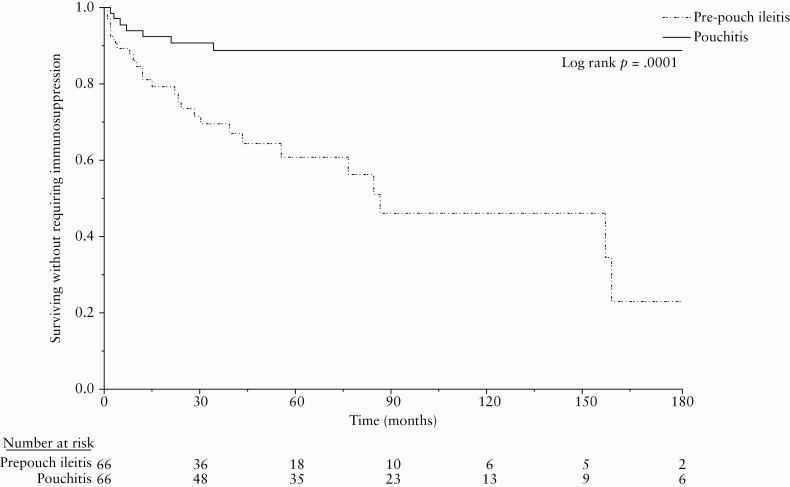
Immunosuppressive therapy was used in 33 [25%] patients in the entire cohort, 26 [39%] in the pre-pouch ileitis group and seven [10.6%] in the pouchitis group [p <0.0001]. In the pre-pouch ileitis group, 21 patients were treated with biologic therapy, two with immunomodulators, and three with combination therapy. In the pouchitis group, five patients were treated with biologic agents and two with combination therapy. Median time from ileostomy closure to immunosuppressive therapy was 19 [IQR 4, 46] months in the pre-pouch ileitis group versus 12 [IQR 3, 21] months in the pouchitis group [p = 0.39]. Immunosuppression-free survival [Figure 4] was significantly lower in the pre-pouch ileitis group, with 26.6%, 39.3%, and 54.1% patients in the pre-pouch ileitis group and 9.4%, 11.3%, and 11.3% patients in the pouchitis group starting immunosuppressive therapy at 2, 5, and 10-years, respectively [log rank p <0.0001].
Figure 4.
Immunosuppression-free survival.
In the subgroup of patients who had pouchitis with pre-pouch ileal ulcers, 15/51 [29.4%] required surgical or endoscopic interventions and 21 [41.2%] required immunosuppressive therapy. On subgroup analysis, intervention-free survival [log rank p = 0.0004] and immunosuppression-free survival [log rank p <0.0001] were significantly worse in patients with pre-pouch ileal ulcers compared with those with pouchitis alone [Supplementary Figure S3, available as Supplementary data at ECCO-JCC online].
3.4.Predictors of outcome
Table 2 shows results of Cox regression analysis performed to identify predictors of development of CD-like complications. Pre-pouch ileitis was the independently associated with a 3.8-fold£ [95% CI 1.8–8.3; p = 0.0007] increase in the hazard of developing CD-like complications. Pancolitis was associated with a decreased risk of development of CD-like complications though it was not statistically significant [HR 0.5; 95% CI 0.3–1.0; p = 0.06]. Pre-pouch ileitis was also the only variable associated with the requirement of surgical or endoscopic interventions [HR 4.1; 95% CI 1.7–9.9; p = 0.002] and immunosuppressive therapy [HR 5.0; 95% CI 2.2–11.6; p <0.0001] on multivariable analysis [Table 3]. Since the number of ileal pouch failure events in the entire cohort was small [n = 6], regression analysis for predictors of pouch failure was not performed.
Table 2.
Predictors for CD-like complications.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Age | 1.0 [0.9–1.0] | 0.49 | ||
| Male | 1.0 [0.5–2.0] | 1.00 | ||
| UC diagnosis | 1.0 [0.5–2.1] | 0.91 | ||
| Age of IBD onset | 1.0 [0.9–1.0] | 0.44 | ||
| Pancolitis | 0.5 [0.2–0.9] | 0.03 | 0.5 [0.3–1.0] | 0.06 |
| Family history of IBD | 1.2 [0.6–2.6] | 0.59 | ||
| Primary sclerosing cholangitis | 1.5 [0.5–5.0] | 0.50 | ||
| Backwash ileitis | 0.9 [0.3–2.5] | 0.81 | ||
| Pre-colectomy IMM use | 2.1 [0.8–5.5] | 0.12 | ||
| Pre-colectomy biologic | 1.1 [0.5–2.2] | 0.82 | ||
| Disease activity as IPAA indication | 1.2 [0.4–3.4] | 0.73 | ||
| Two-stage IPAA | 1.4 [0.6–3.8] | 0.43 | ||
| Pre-pouch ileitis | 3.9 [1.8–8.6] | 0.0005 | 3.8 [1.8–8.3] | 0.0007 |
IBD, inflammatory bowel disease; UC, ulcerative colitis; IPAA, ileal pouch-anal anastomosis, IMM, immunomodulator; HR, hazard ratio; CI, confidence interval; CD, Crohn’s disease.
Bold numbers represents statistically significant results.
Table 3.
Predictors of surgical/endoscopic interventions and immunosuppressive therapy.
| Need for surgical / endoscopic interventions | Need for immunosuppressive therapy | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Age | 1.0 [1.0–1.1] | 0.73 | 1.0 [1.0–1.1] | 0.86 | ||||
| Male gender | 0.9 [0.4–2.1] | 0.88 | 1.3 [0.6–2.7] | 0.50 | ||||
| UC diagnosis | 1.2 [0.6–2.7] | 0.62 | 0.9 [0.4–1.7] | 0.68 | ||||
| Age of IBD onset | 1.0 [1.0–1.1] | 0.71 | 1.0 [1.0–1.1] | 0.97 | ||||
| Pancolitis | 0.5 [0.2–1.0] | 0.07 | 0.5 [0.2–1.1] | 0.09 | 0.5 [0.2–01.0] | 0.04 | 2.0 [1.0–4.1] | 0.06 |
| Family history of IBD | 1.7 [0.8–3.7] | 0.20 | 1.4 [0.7–2.9] | 0.32 | ||||
| Primary sclerosing cholangitis | 2.1 [0.6–7.0] | 0.24 | 1.0 [0.2–4.2] | 0.99 | ||||
| Backwash ileitis | 1.0 [0.3–3.4] | 0.99 | 0.1 [0.4–2.9] | 0.81 | ||||
| Pre-colectomy IMM use | 1.2 [0.5–2.9] | 0.72 | 1.2 [0.5–2.6] | 0.71 | ||||
| Pre-colectomy biologic use | 0.7 [0.3–1.7] | 0.45 | 0.9 [0.5–1.9] | 0.81 | ||||
| Disease activity as IPAA indication | 0.9 [0.3–2.7] | 0.88 | 0.7 [0.3–1.8] | 0.44 | ||||
| Two-stage IPAA | 1.6 [0.7–3.9] | 0.28 | 1.6 [0.7–3.5] | 0.23 | ||||
| Pre-pouch ileitis | 4.3 [1.8–10.2] | 0.001 | 4.1 [1.7–9.9] | 0.002 | 5.1 [2.2–11.7] | 0.0002 | 5.0 [2.2–11.6] | 0.0002 |
IBD, inflammatory bowel disease; UC, ulcerative colitis; IPAA, ileal pouch-anal anastomosis, IMM, immunomodulator; HR, hazard ratio; CI, confidence interval.
Bold numbers represents statistically significant results.
4.Discussion
Pre-pouch ileitis is an inflammatory complication that occurs in a small proportion of patients who undergo IPAA surgery for IBD. Whereas its presence is commonly used as a criterion for diagnosis of de novo CD, its implication on the clinical course of the patients is unclear.10 Some authors have suggested that pre-pouch ileitis is a manifestation of immune-mediated pouchitis that occurs in patients with primary sclerosing cholangitis and IgG4-associated pouchitis, and is usually refractory to antibiotic therapy.14–16 Others have noted good clinical and endoscopic response of pre-pouch ileitis to antibiotic therapy and concluded that pre-pouch ileitis and pouchitis may have a shared aetiopathogenesis, with bacteria playing a significant role.17 In addition, several studies evaluating outcomes of patients with pre-pouch ileitis have found no evidence to suggest that it could be a manifestation of CD.9,11,12 In this study, we compared the outcomes of the largest reported cohort of patients with pouchitis and pre-pouch ileitis with an age and sex-matched cohort of patients with pouchitis alone. Our findings suggest that presence of pre-pouch ileitis is associated with an increased risk of development of CD-like complications in the form of non-anastomotic strictures and perianal disease, pouch failure, need for endoscopic or surgical interventions, and need for immunosuppressive therapy. Since ulcers are the most unequivocal sign of inflammation in the small bowel, we also performed a subgroup analysis comparing patients who had pouchitis and pre-pouch ileal ulcers with those who had pouchitis alone. The fact that our study results remained unchanged on this subgroup analysis highlights the strength of our findings.
Previous studies evaluating the significance of pre-pouch ileitis have shown mixed results. A 2004 study by Wolf et al. showed that patients with an ileal pouch and CD were more like to have afferent limb ulcers [45%] on pouch endoscopy compared with those with UC [14%].18 A recent Italian study of 57 patients with pre-pouch ileitis showed that almost half of these patients developed afferent limb stenosis, 32% required small bowel or pouch surgery, and 14% developed pouch failure.8 However, both these studies included patients whose diagnosis was changed from UC to CD at the time of IPAA surgery, based on histopathological examination of the colectomy specimen. These patients likely had missed or covert CD. Not surprisingly, the latter study found poorer outcomes in this specific subset of patients [n = 8], with 100% requiring surgery and 50% developing pouch failure.8 To avoid this bias, we only included patients with UC or IBD-U diagnosis in our study.
Two other studies have evaluated the outcomes of small cohorts of patients who underwent IPAA for UC or IBD-U and developed pouchitis with pre-pouch ileitis. In an uncontrolled study by Segal et al. of 31 patients with pre-pouch ileitis, only two [6.5%] patients developed CD-like complications, and seven [23%] patients went on to develop pouch failure after a median follow-up of 98 months.12 In another study comparing 33 patients with pre-pouch ileitis with those who had pouchitis, no difference was noted in development of strictures between the two groups, though a significant proportion of patients with pre-pouch ileitis required immunosuppressive therapy.9 On the contrary, we found that 36% of subjects with pre-pouch ileitis developed non-anastomotic strictures and perianal fistulae, which was significantly higher than the complication rate in patients without pre-pouch ileitis in the time-to-event analysis. These differences may be due to the larger sample size and longer follow-up in our cohort. The significantly higher need for immunosuppressive therapy and surgical or endoscopic procedure in patients with pre-pouch ileitis, compared with pouchitis alone, in our study are likely directly related to higher CD-like complications in this group. Pouch failure rate in patients with pre-pouch ileitis in our study was 7.7%, which is much lower than previously reported by Segal et al. and Rottoli et al.8,12 This could potentially be attributed to a greater use of immunosuppressive therapy in our pre-pouch ileitis cohort, with around 40% of patients being treated with an immunomodulator or biologic therapy.
We also found pre-pouch ileitis to be an independent predictor of an increased risk of developing CD-like complications, requiring surgical/endoscopic interventions, and being on immunosuppressive therapy. These findings could have significant implications on clinical management of these patients. First and foremost, they reinforce the need for afferent limb intubation in all patients undergoing a pouch endoscopy. They also suggest that the presence of pre-pouch ileitis heralds a more aggressive disease course than idiopathic pouchitis. Though the extent and/or severity of pre-pouch ileitis may affect the risk of development of complications, it is appropriate to assume that patients with pre-pouch ileitis have de novo CD and treat them as such. Biologic therapy is increasingly recognised as effective in treatment of CD of the ileal pouch.19–21 However, its effectiveness in treating pre-pouch ileitis is largely unknown except for a small observational study showing complete healing of pre-pouch ileitis in around 25% of patients within 1 year with anti-tumour necrosis factor-α therapy.22 Moreover, the efficacy of biologic therapy in preventing pouch-related complications in patients with de novo CD is unclear. It was not possible to evaluate the impact of immunosuppressive therapy on the development of CD-like complications in our cohort, because in 25/33 [75.8%] of patients who were treated with immunosuppressive therapy, this was initiated after the development of CD-like complications. We also could not study the impact of immunosuppressive therapy on pouch failure in our cohort, as the number of pouch failure events was very small. Until further research answers these questions, it may be prudent to treat patients with pre-pouch ileitis using the CD treatment principles, i.e. treat early and monitor closely for development of complications. The reason pancolitis was borderline protective against development of CD-like complications is unclear, but could be related to the genetic determinants of the extent of colitis.23 This finding also needs to be studied and validated in future studies.
There are several strengths to our study. This is the largest reported cohort of patients with pre-pouch ileitis. The availability of a well-maintained prospective registry enabled us to appropriately select patients and controls. The use of complications relevant to the natural history of inflammatory ileal pouch disorders as study outcomes makes the study results clinically relevant. Our study also has some limitations. It is a retrospective analysis of a prospectively maintained database of a single-centre experience. Given a relatively small study population, we were unable to use rigorous methods like propensity score matching of study groups to reduce the effect of confounders on the study outcomes. However, we matched controls with cases based on the year of IPAA surgery and preoperative UC/IBD-U diagnosis, which are arguably the two most important confounding factors, and performed multivariable regression analysis to allow us to evaluate the independent effect of pre-pouch ileitis on study outcomes. We were unable to use validated inflammation scores or obtain detailed information on the extent and the severity of afferent limb inflammation. Last, given the nature of the study, we were not able to evaluate the impact of pre-pouch ileitis on the patient symptoms and functional outcomes or the effect of medical therapy on pre-pouch ileitis or development of CD-like complications. Future studies should not only validate our findings but should also evaluate the importance of the extent and severity of afferent limb inflammation in determining outcomes.
In conclusion, patients with pouchitis and pre-pouch ileitis are at a higher risk of developing CD-like complications, requiring surgical or endoscopic interventional procedures and being treated with immunosuppressive therapy, compared with patients with pouchitis alone. Hence, pre-pouch ileitis should not be regarded as mere extension of pouch inflammation into the afferent limb. Given the risk of development of complications associated with pre-pouch ileitis, it should be considered a feature of de novo CD and treated proactively with close monitoring for development of complications.
The data underlying this article cannot be shared publicly according to the Cedars-Sinai Medical Center’s Institutional Review Board policy to preserve the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Supplementary Material
Funding
DPBM is supported by the National Institute of Health [P01 DK046763 and U01 DK062413], the Litwin Foundation, and the Leona M. and Harry B. Helmsley Charitable Trust grants.
Conflict of Interest
None of the authors declares any financial, professional, or personal conflict of interest relevant to this study.
Authors Contributions
GS, study concept, study design, data collection and analysis, manuscript writing and revision.RS, data collection and analysis. NB, manuscript review and final approval. EJF, manuscript review and final approval. EAV, manuscript review and final approval. KZ, manuscript review and final approval. CYH, manuscript review and final approval. SRT, manuscript review and final approval. GYM, manuscript review and final approval. DPBM, study concept, manuscript review, and final approval. PRF, study concept, study design, manuscript revision, final approval.
References
- 1. Peyrin-Biroulet L, Germain A, Patel AS, Lindsay JO. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther 2016;44:807–16. [DOI] [PubMed] [Google Scholar]
- 2. Lightner AL, Mathis KL, Dozois EJ, et al. Results at up to 30 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Inflamm Bowel Dis 2017;23:781–90. [DOI] [PubMed] [Google Scholar]
- 3. Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol 2013;11:1538–49. [DOI] [PubMed] [Google Scholar]
- 4. Quinn KP, Lightner AL, Faubion WA, Raffals LE. A comprehensive approach to pouch disorders. Inflamm Bowel Dis 2019;25:460–71. [DOI] [PubMed] [Google Scholar]
- 5. Kayal M, Plietz M, Rizvi A, et al. Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Inflamm Bowel Dis 2020;26:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lightner AL, Pemberton JH, Loftus EJ Jr. Crohn’s disease of the ileoanal pouch. Inflamm Bowel Dis 2016;22:1502–8. [DOI] [PubMed] [Google Scholar]
- 7. Zaghiyan K, Kamiński JP, Barmparas G, Fleshner P. De novo Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis and inflammatory bowel disease unclassified: long-term follow-up of a prospective inflammatory bowel disease registry. Am Surg 2016;82: 977–81. [PubMed] [Google Scholar]
- 8. Rottoli M, Vallicelli C, Bigonzi E, et al. Pre-pouch ileitis after ileal pouch-anal anastomosis: patterns of presentation and risk factors for failure of treatment. J Crohns Colitis 2018;12:273–9. [DOI] [PubMed] [Google Scholar]
- 9. Samaan MA, de Jong D, Sahami S, et al. Incidence and severity of pre-pouch ileitis: a distinct disease entity or a manifestation of refractory pouchitis? Inflamm Bowel Dis 2016;22:662–8. [DOI] [PubMed] [Google Scholar]
- 10. Barnes EL, Kochar B, Jessup HR, Herfarth HH. The incidence and definition of Crohn’s disease of the pouch: a systematic review and meta-analysis. Inflamm Bowel Dis 2019;25:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLaughlin SD, Clark SK, Bell AJ, Tekkis PP, Ciclitira PJ, Nicholls RJ. Incidence and short-term implications of pre-pouch ileitis following restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum 2009;52:879–83. [DOI] [PubMed] [Google Scholar]
- 12. Segal JP, McLaughlin SD, Faiz OD, Hart AL, Clark SK. Incidence and long-term implications of pre-pouch ileitis: an observational study. Dis Colon Rectum 2018;61:472–5. [DOI] [PubMed] [Google Scholar]
- 13. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen B, Bennett AE, Navaneethan U, et al. Primary sclerosing cholangitis is associated with endoscopic and histologic inflammation of the distal afferent limb in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis 2011;17:1890–900. [DOI] [PubMed] [Google Scholar]
- 15. Navaneethan U, Venkatesh PG, Kapoor S, Kiran RP, Remzi FH, Shen B. Elevated serum IgG4 is associated with chronic antibiotic-refractory pouchitis. J Gastrointest Surg 2011;15:1556–61. [DOI] [PubMed] [Google Scholar]
- 16. Seril DN, Yao Q, Lashner BA, Shen B. Autoimmune features are associated with chronic antibiotic-refractory pouchitis. Inflamm Bowel Dis 2015;21:110–20. [DOI] [PubMed] [Google Scholar]
- 17. McLaughlin SD, Clark SK, Bell AJ, Tekkis PP, Ciclitira PJ, Nicholls RJ. An open study of antibiotics for the treatment of pre-pouch ileitis following restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment Pharmacol Ther 2009;29:69–74. [DOI] [PubMed] [Google Scholar]
- 18. Wolf JM, Achkar JP, Lashner BA, et al. Afferent limb ulcers predict Crohn’s disease in patients with ileal pouch-anal anastomosis. Gastroenterology 2004;126:1686–91. [DOI] [PubMed] [Google Scholar]
- 19. Huguet M, Pereira B, Goutte M, et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and Crohn’s disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm Bowel Dis 2018;24: 261–8. [DOI] [PubMed] [Google Scholar]
- 20. Gregory M, Weaver KN, Hoversten P, et al. Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter US cohort. Inflamm Bowel Dis 2019;25:1569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weaver KN, Gregory M, Syal G, et al. Ustekinumab is effective for the treatment of Crohn’s disease of the pouch in a multicenter cohort. Inflamm Bowel Dis 2019;25:767–74. [DOI] [PubMed] [Google Scholar]
- 22. Segal JP, Rottoli M, Felwick RK, et al. Biological therapy for the treatment of pre-pouch ileitis: a retrospective observational study from three centers. Clin Exp Gastroenterol 2018;11: 461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu F, Liu Y, Wheaton AG, Rabarison KM, Croft JB. Trends and factors associated with hospitalization costs for inflammatory bowel disease in the United States. Appl Health Econ Health Policy 2019;17:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.