Abstract
Background and Aims
Tofacitinib is an oral, small molecule JAK inhibitor for the treatment of ulcerative colitis. We report integrated analyses of infections in the Phase [P]2 and P3 OCTAVE programmes.
Methods
Three cohorts were analysed: Induction [P2/3 induction studies]; Maintenance [P3 maintenance study]; and Overall [all tofacitinib-treated patients in induction, maintenance, or ongoing, open-label, long-term extension studies; as of May 2019]. Proportions and incidence rates [IRs; unique patients with events/100 patient-years] of serious infections [SIs], herpes zoster [HZ] [non-serious and serious], and opportunistic infections [OIs] are reported [censored at time of event].
Results
In the Induction Cohort [N = 1220], no patients receiving placebo and eight [0.9%] receiving tofacitinib 10 mg twice daily [BID] developed SIs. Maintenance Cohort [N = 592] SI IRs (95% confidence interval [CI]) were 1.94 [0.23–7.00] for placebo and 1.35 [0.16–4.87] and 0.64 [0.02–3.54] for tofacitinib 5 and 10 mg BID, respectively; HZ IRs were 0.97 [0.02–5.42], 2.05 [0.42–6.00], and 6.64 [3.19–12.22], respectively. In the Overall Cohort [N = 1157; 82.9% predominantly received tofacitinib 10 mg BID], SI, HZ, and non-HZ OI IRs were 1.70 [1.24–2.27], 3.48 [2.79–4.30], and 0.15 [0.04–0.38], respectively. No SIs resulted in death.
Conclusions
During induction, SIs were more frequent with tofacitinib versus placebo. SIs were generally infrequent in the Maintenance and Overall Cohorts, with rates comparable between treatment groups. Maintenance Cohort HZ IR was numerically higher with tofacitinib 10 mg BID versus 5 mg BID. Overall Cohort HZ IRs remained stable over time. Non-HZ OIs and viral infections were rare.
Keywords: Infections, tofacitinib, ulcerative colitis
1. Introduction
Infections are an important safety concern in patients with inflammatory bowel disease [IBD], due to immunosuppression which may be associated with the disease itself1 as well as its therapies.2 Various immunosuppressant therapies for IBD, including corticosteroids, thiopurines, methotrexate, and tumour necrosis factor inhibitors [TNFi], are associated with an increased risk of serious infections and/or opportunistic infections.2–7 This risk can further increase when some therapies are used in combination.4,5,7
Tofacitinib is an oral, small molecule Janus kinase [JAK] inhibitor for the treatment of ulcerative colitis [UC]. Tofacitinib inhibits JAK-mediated signal transduction,8,9 thereby modulating immune and inflammatory responses.9,10 Risks of serious infections, herpes zoster, and opportunistic infections with tofacitinib have been reported.11–18
The safety of tofacitinib in adults with UC has been previously evaluated in an 8-week, Phase 2 induction study [NCT00787202],19 two 8-week, Phase 3 induction studies (OCTAVE Induction 1 [NCT01465763] and 2 [NCT01458951])20 and a 52-week, Phase 3 maintenance study (OCTAVE Sustain [NCT01458574]),20 and are being further evaluated in an ongoing, open-label, long-term extension [OLE] study (OCTAVE Open [NCT01470612]).21 We report integrated analyses of infection events throughout the global tofacitinib UC clinical programme, including Phase 2 and Phase 3 OCTAVE trials.
2. Materials and Methods
2.1. Studies and patients
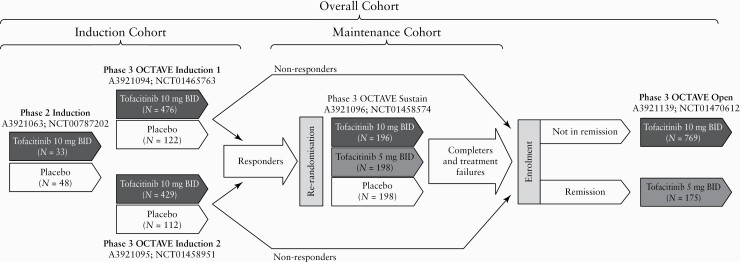
All studies used in these analyses included adult patients with moderate to severe UC, receiving placebo or tofacitinib 5 or 10 mg twice daily [BID] [Figure 1]. Details of included studies are provided in the Supplementary Data, available at ECCO-JCC online page 2 [ClinicalTrials.gov identifiers: NCT00787202; NCT01465763; NCT01458951; NCT01458574; NCT01470612]. Data from the ongoing OLE study are reported as of 27 May 2019 [database not locked].
Figure 1.
Overview of the tofacitinib UC clinical programme, showing Phase 2, Phase 3, and OLE studies, and the transfer of patients from induction studies to maintenance and/or OLE studies for treatment groups included in the analysis. Patients meeting clinical response criteria at the end of the 8-week OCTAVE Induction 1 and 2 studies were re-randomised to receive placebo or tofacitinib 5 or 10 mg BID during the 52-week maintenance study [OCTAVE Sustain]. Non-responders from the induction studies were eligible for inclusion in the OLE study, as were those completing the maintenance study. Clinical response in OCTAVE Induction 1 and 2 was defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1. Remission was defined as a total Mayo score of ≤2 with no individual subscore >1, and a rectal bleeding subscore of 0. Study A3921139 [OCTAVE Open] is ongoing. Adapted from Winthrop et al.,15 (in accordance with the CC BY-NC licence). BID, twice daily; N, total number of patients in the treatment group; OLE, open-label, long-term extension; UC, ulcerative colitis.
During OCTAVE Open, tofacitinib dose could be modified a maximum of two times after ≥8 weeks of initial treatment [from 5 to 10 mg BID for patients with flare documented by site-read endoscopy, and from 10 to 5 mg BID if specified laboratory criteria were met or if patients were in remission or partial Mayo score remission at or beyond Month 24]. In addition to assigned dose groups, patients in the Overall Cohort were analysed by predominant dose [PD], based on the average total daily dose of tofacitinib during tofacitinib treatment: PD 5 mg BID for average total daily dose <15 mg; PD 10 mg BID for average total daily dose ≥15 mg. Due to protocol design, 82.9% of patients were in the PD 10 mg BID group.
Oral corticosteroids [stable dose; ≤30 mg/day prednisolone or equivalent in the Phase 2 study and ≤25 mg/day prednisone or equivalent in Phase 3 studies] were permitted during induction studies, but with mandatory taper during OCTAVE Sustain and OCTAVE Open. Oral 5-aminosalicylates [5-ASA] were also permitted, provided the dose remained stable for ≥4 weeks preceding baseline for induction studies and remained stable during the study treatment period for induction and maintenance studies. Oral 5-ASA dose modifications were permitted during OCTAVE Open. Concomitant immunosuppressant or TNFi therapy was prohibited [immunosuppressant washout period: within 1 week of baseline in the Phase 2 study and within 2 weeks of baseline in OCTAVE Induction studies; TNFi washout period: within 8 weeks of baseline in the Phase 2 and OCTAVE Induction studies]. Further details are provided in the Supplementary Data pages 3–5 and in Supplementary Table 1, available as Supplementary data at ECCO-JCC online.
Patients entering the programme were required to have no evidence of active, latent, or inadequately treated tuberculosis [TB; see Supplementary Data page 6]. Patients with human immunodeficiency virus or hepatitis B or C virus were excluded. As per the protocols, patients with serious infections were required to discontinue from the studies.
2.2. Analysis cohorts
Safety data were analysed as three cohorts [Figure 1]:
Induction Cohort: patients who received placebo or tofacitinib 10 mg BID for up to 8 weeks in Phase 2 or 3 induction studies;
Maintenance Cohort: patients who received placebo or tofacitinib 5 or 10 mg BID for up to 52 weeks in OCTAVE Sustain;
Overall Cohort: patients who received at least one dose of tofacitinib 5 or 10 mg BID in Phase 2 or 3 induction studies, OCTAVE Sustain and OCTAVE Open, for up to 6.8 years [as of 27 May 2019, database not locked], the latter of which was open label.
2.3. Review of adverse events
Adverse events [AEs] and serious AEs [SAEs] were monitored [further details in Supplementary Data page 6]. Infection AEs of special interest included serious infections, opportunistic infections, herpes zoster, and TB. Serious infections were defined as infections that met SAE reporting criteria [see Supplementary Data page 6].
External independent adjudication committees were established to help assess specific safety events, including an Opportunistic Infections Review Committee [OIRC]. Infections reviewed by the OIRC included viral, bacterial, fungal, and parasitic infections reported as SAEs or potential opportunistic infections [see Supplementary Data page 6]. As the conduct of the Phase 2 study [NCT00787202] pre-dated establishment of the adjudication committees, this study was not included in the adjudication process. Herpes zoster events that were adjudicated as multidermatomal [defined as non-adjacent or >2 adjacent dermatomes that were not considered disseminated] or disseminated were classified as opportunistic infections. All other herpes zoster events were limited to cutaneous involvement with ≤2 adjacent dermatomes. As these studies were designed to evaluate the efficacy and safety of tofacitinib and not to specifically evaluate herpes zoster, baseline varicella zoster virus serology was not measured in the tofacitinib UC clinical programme.
2.4. Proportions and incidence rates
Infection AEs were evaluated as proportions and incidence rates [IRs], calculated as the number of unique patients with events per 100 patient-years [PY], with 95% confidence intervals [CIs; see Supplementary Data page 7]. Exposure time was censored at time of event. Events occurring during treatment and up to 28 days after the last dose were included in calculations of proportions and IRs. IRs were calculated for all opportunistic infections [including herpes zoster cases meeting the OIRC definition for herpes zoster opportunistic infections], and separately for herpes zoster opportunistic infections, non-herpes zoster opportunistic infections, and all herpes zoster [non-serious and serious; comprising all forms of herpes zoster and including cases not adjudicated as opportunistic infections].
2.5. Risk factor analysis
Cox proportional regression models were used to assess the association of demographic and clinical factors with the risk of serious infections, opportunistic infections, and herpes zoster [non-serious and serious]. Models were applied to the Overall Cohort, which included all patients exposed to tofacitinib in the UC clinical programme and excluded any time periods and events experienced while receiving placebo. Evaluated baseline factors included [but were not limited to]: age, sex, race, body weight, body mass index [BMI], previous TNFi use, previous TNFi failure, previous immunosuppressant use, oral corticosteroid use at baseline, and tofacitinib PD [full list of factors considered are provided in Supplementary Data page 8].
The modelling approach for baseline characteristics first applied univariate models to identify individual risk factors with a statistically significant relationship to each AE; factors with p <0.10 were then included in a stepwise multivariable model. The final model included all factors from the stepwise model with p <0.05.
Separate univariate models using time-varying covariates evaluated factors that could change during the study, including tofacitinib dose, oral corticosteroid dose [prednisone equivalent], and post-baseline confirmed low absolute lymphocyte count [ALC; <1 x 109/L] and low absolute neutrophil count [ANC; <1.5 x 109/L]. For post-baseline confirmed low ALC and low ANC, values observed before the AEs of interest had to meet the criteria on two consecutive visits.
2.6. Analysis by age group
To evaluate the effect of age on serious infection risk in the Overall Cohort, IRs for age groups [<30 years, ≥30 to <40 years, ≥40 to <50 years, ≥50 to <60 years, and ≥60 years, <65 years, and ≥65 years] were calculated.
2.7. Analysis over time
To evaluate changes in the incidence of infection AEs over time in the Overall Cohort, IRs for discrete 6-month intervals of tofacitinib exposure [eg. exposure for 0–6, >6 to 12, >12 to 18, >18 to 24, >24 to 30, and >30 months] were calculated.
2.8. Ethical considerations and patient consent
All studies were registered with ClinicalTrials.gov [NCT00787202; NCT01465763; NCT01458951; NCT01458574; NCT01470612], were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines, and were approved by the institutional review boards and/or independent ethics committees at each investigational centre participating in the studies or at a central institutional review board. All patients provided written informed consent.
3. Results
3.1. Patients
In total, 1220 patients were included in the Induction Cohort [placebo, N = 282; tofacitinib 10 mg BID, N = 938] and 592 patients were included in the Maintenance Cohort [placebo, N = 198; tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 196]. A total of 1157 patients received ≥1 dose of tofacitinib [as of May 2019] and were included in the Overall Cohort, with 763 patients exposed for ≥6 months, 666 patients exposed for ≥12 months, 552 patients exposed for ≥24 months, and 315 patients exposed for ≥48 months. The tofacitinib UC clinical programme encompassed 2581.3 PY of exposure to tofacitinib [median, 623 days], with up to 6.8 years of treatment. In the Overall Cohort, most patients [N = 959; 82.9%] received a PD of tofacitinib 10 mg BID.
Baseline characteristics for the Induction and Maintenance Cohorts are shown in Table 1. Characteristics associated with UC activity [eg. total Mayo score and C-reactive protein level] were notably different between the two cohorts, reflecting the entry requirements of the Maintenance Cohort [achieved clinical response with placebo or tofacitinib 10 mg BID in OCTAVE Induction 1 or 2]. Otherwise, demographics and baseline characteristics were generally similar among treatment groups in both cohorts. Exposure and baseline characteristics for the Overall Cohort [N = 1157] are shown in Supplementary Tables 2 and 3, available as Supplementary data at ECCO-JCC online.
Table 1.
Demographics and clinical characteristics of the Induction and Maintenance Cohorts.
| Induction Cohort | Maintenance Cohort | |||||
|---|---|---|---|---|---|---|
| Placebo [N = 282] | Tofacitinib 10 mg BID [N = 938] | Placebo [N = 198] | Tofacitinib 5 mg BID [N = 198] | Tofacitinib 10 mg BID [N = 196] | Tofacitinib all doses [N = 394] | |
| Mean age, years [SD] | 41.4 [14.4] | 41.3 [13.8] | 43.4 [14.0] | 41.9 [13.7] | 43 [14.4] | 42.5 [14.0] |
| Males, n [%] | 155 [55] | 557 [59.4] | 116 [58.6] | 103 [52.0] | 110 [56.1] | 213 [54.1] |
| Race, n [%] | ||||||
| White | 229 [81.2] | 756 [80.6] | 155 [78.3] | 164 [82.8] | 153 [78.1] | 317 [80.5] |
| Asian | 28 [9.9] | 114 [12.2] | 26 [13.1] | 23 [11.6] | 25 [12.8] | 48 [12.2] |
| Geographical region, n [%] | ||||||
| Asia | 26 [9.2] | 95 [10.1] | 20 [10.1] | 22 [11.1] | 21 [10.7] | 43 [10.9] |
| Eastern Europe | 90 [31.9] | 283 [30.2] | 57 [28.8] | 66 [33.3] | 63 [32.1] | 129 [32.7] |
| North America | 53 [18.8] | 187 [19.9] | 45 [22.7] | 39 [19.7] | 44 [22.4] | 83 [21.1] |
| Western Europe | 79 [28.0] | 281 [30.0] | 55 [27.8] | 47 [23.7] | 57 [29.1] | 104 [26.4] |
| Rest of world | 34 [12.1] | 92 [9.8] | 21 [10.6] | 24 [12.1] | 11 [5.6] | 35 [8.9] |
| Mean disease duration, years [SD] | 8.2 [6.8] | 8.2 [7.0] | 8.8 [7.5] | 8.3 [7.2] | 8.7 [7.0] | 8.5 [7.1] |
| Mean total Mayo score [SD] | 8.9 [1.5] | 9.0 [1.5] | 3.3 [1.8] | 3.3 [1.8] | 3.4 [1.8] | 3.4 [1.8] |
| Median CRP, mg/L [range]a | 5.3 [0.1–205.1] | 4.6 [0.1–208.4] | 1.0 [0.1–45.0] | 0.7 [0.1–33.7] | 0.9 [0.1–74.3] | 0.7 [0.1–74.3] |
| Previous TNFi treatment, n [%]b | 130 [55.6] | 488 [53.9] | 92 [46.5] | 90 [45.5] | 100 [51.0] | 190 [48.2] |
| Previous immunosuppressant treatment, n [%]b | 160 [68.4] | 683 [75.5] | 134 [67.7] | 149 [75.3] | 144 [73.5] | 293 [74.4] |
| Immunosuppressant treatment within 8 weeks preceding baseline, n [%]b | 56 [23.9] | 259 [28.6] | 44 [22.2] | 45 [22.7] | 56 [28.6] | 101 [25.6] |
| 5-ASA use at baseline, n [%]b,c | 167 [71.4] | 650 [71.8] | - | - | - | - |
| Oral corticosteroid use at baseline, n [%] | 127 [45.0] | 430 [45.8] | 100 [50.5] | 101 [51.0] | 86 [43.9] | 187 [47.5] |
| Mean oral corticosteroid daily dose at baseline—prednisone equivalent, mg/day [SD] | 16.9 [6.2] | 16.0 [6.4] | 15.9 [6.2] | 14.9 [6.2] | 14.5 [5.9] | 14.7 [6.1] |
| Extent of disease, n [%]b | ||||||
| Proctosigmoiditis | 35 [15.0] | 132 [14.6] | 21 [10.6] | 28 [14.3] | 33 [16.9] | 61 [15.6] |
| Left-sided colitis | 76 [32.6] | 307 [34.0] | 68 [34.3] | 66 [33.7] | 60 [30.8] | 126 [32.2] |
| Extensive/pancolitis | 122 [52.4] | 463 [51.3] | 108 [54.5] | 102 [52.0] | 102 [52.3] | 204 [52.2] |
| Proctitis | 0 [0.0] | 1 [0.1] | 1 [0.5] | 0 [0.0] | 0 [0.0] | 0 [0.0] |
| Baseline albumin [g/dL], mean [SD]b | 4.2 [0.4] | 4.2 [0.4] | 4.5 [0.3] | 4.5 [0.3] | 4.5 [0.3] | 4.5 [0.3] |
| Baseline ALC [x 106/L], mean [SD]b,d | 1.8 [0.8] | 1.9 [0.9] | 1.9 [0.8] | 2.0 [1.0] | 1.9 [0.8] | 2.0 [0.9] |
| Baseline ANC [x 106/L], mean [SD]b,d | 6.0 [2.7] | 5.9 [2.9] | 5.2 [2.2] | 5.1 [2.6] | 5.3 [2.6] | 5.2 [2.6] |
| Low post-baseline ALC [<1.0 x 109/L], n [%]e,f | 21 [7.6] | 61 [6.6] | 7 [3.5] | 23 [11.6] | 16 [8.2] | 39 [9.9] |
| Low post-baseline ANC [<1.5 x 109/L], n [%]e,f | 0 [0.0] | 0 [0.0] | 0 [0.0] | 0 [0.0] | 1 [0.5] | 1 [0.3] |
| Smoking status, n [%]g | ||||||
| Current smoker | 11 [3.9] | 48 [5.1] | 12 [6.1] | 7 [3.5] | 6 [3.1] | 13 [3.3] |
| Ex-smoker | 76 [27.0] | 296 [31.6] | 73 [36.9] | 49 [24.7] | 63 [32.1] | 112 [28.4] |
| Never smoked | 195 [69.1] | 593 [63.2] | 113 [57.1] | 142 [71.7] | 127 [64.8] | 269 [68.3] |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; BID, twice daily; CRP, C-reactive protein; N, total number of patients in the treatment group; n, number of patients in the specified category; SD, standard deviation; TNFi, tumour necrosis factor inhibitor; 5-ASA, 5-aminosalicylate.
aInduction Cohort: placebo, N = 278; tofacitinib 10 mg BID, N = 922. Maintenance Cohort: placebo, N = 198; tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 196.
bData from baseline of Phase 3 induction studies NCT01465763 and NCT01458951; placebo, N = 234; tofacitinib 10 mg BID, N = 905.
c5-ASA use at baseline is not available for the Maintenance and Overall Cohorts.
dInduction Cohort: placebo, N = 282; tofacitinib 10 mg BID, N = 904. Maintenance Cohort: placebo, N = 195; tofacitinib 5 mg BID, N = 197; tofacitinib 10 mg BID, N = 195; tofacitinib all doses, N = 392.
e Induction Cohort: placebo, N = 277; tofacitinib 10 mg BID, N = 928. Maintenance Cohort: placebo, N = 198; tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 195; tofacitinib all doses, N = 393.
fFor post-baseline confirmed low ALC and low ANC, values observed before the adverse events of interest had to meet the criteria on two consecutive visits.
gOne patient with missing data in Induction Cohort, tofacitinib 10 mg BID group.
3.2. Observed infection events
The proportions of patients in the Induction and Maintenance Cohorts with infection AEs are shown in Table 2. In the Overall Cohort [tofacitinib all doses], infection AEs occurred in 55.4% of patients, and 49 [4.2%] patients had their dose reduced or temporarily discontinued due to infection AEs [Table 3]. No infections resulted in death [Table 3]. Nasopharyngitis was the most frequently reported infection AE, followed by upper respiratory tract infection [Tables 2 and 3]. Pneumonia and atypical pneumonia were infrequent in the Overall Cohort (13 [1.1%] patients and 1 [0.1%] patient, respectively).
Table 2.
Treatment-emergent infections [all-causality] in the Induction and Maintenance Cohorts.
| Induction Cohort [up to 8 weeks of treatment] | ||||
|---|---|---|---|---|
| Adverse event, n [%] | Placebo [N = 282] | Tofacitinib 10 mg BID [N = 938] | ||
| Infections [all] | 43 [15.2] | 198 [21.1] | ||
| Discontinuations due to infections | 0 [0.0] | 5 [0.5] | ||
| Deaths due to infections | 0 [0.0] | 0 [0.0] | ||
| Infections occurring in ≥2% of patients | ||||
| Nasopharyngitis | 14 [5.0] | 56 [6.0] | ||
| Upper respiratory tract infection | 6 [2.1] | 26 [2.8] | ||
| Maintenance Cohort [up to 52 weeks of treatment] | ||||
| Adverse event, n [%] | Placebo [N = 198] | Tofacitinib 5 mg BID [N = 198] | Tofacitinib 10 mg BID [N = 196] | Tofacitinib all doses [N = 394] |
| Infections [all] | 48 [24.2] | 71 [35.9] | 78 [39.8] | 149 [37.8] |
| Discontinuations due to infections | 1 [0.5] | 2 [1.0] | 1 [0.5] | 3 [0.8] |
| Deaths due to infections | 0 [0.0] | 0 [0.0] | 0 [0.0] | 0 [0.0] |
| Infections occurring in ≥2% of patients in any treatment group | ||||
| Nasopharyngitis | 11 [5.6] | 19 [9.6] | 27 [13.8] | 46 [11.7] |
| Upper respiratory tract infection | 7 [3.5] | 13 [6.6] | 12 [6.1] | 25 [6.4] |
| Gastroenteritis | 5 [2.5] | 6 [3.0] | 8 [4.1] | 14 [3.6] |
| Herpes zoster [non-serious and serious] | 1 [0.5] | 2 [1.0] | 10 [5.1] | 12 [3.1] |
| Bronchitis | 3 [1.5] | 5 [2.5] | 6 [3.1] | 11 [2.8] |
| Influenza | 7 [3.5] | 4 [2.0] | 7 [3.6] | 11 [2.8] |
| Urinary tract infection | 4 [2.0] | 5 [2.5] | 6 [3.1] | 11 [2.8] |
| Oral herpes | 0 [0.0] | 4 [2.0] | 5 [2.6] | 9 [2.3] |
| Sinusitis | 2 [1.0] | 6 [3.0] | 2 [1.0] | 8 [2.0] |
| Folliculitis | 1 [0.5] | 2 [1.0] | 5 [2.6] | 7 [1.8] |
| Pharyngitis | 3 [1.5] | 6 [3.0] | 1 [0.5] | 7 [1.8] |
| Tooth abscess | 0 [0.0] | 2 [1.0] | 4 [2.0] | 6 [1.5] |
| Cystitis | 0 [0.0] | 1 [0.5] | 4 [2.0] | 5 [1.3] |
Patients are only counted once per treatment for each row.
BID, twice daily; N, total number of patients in the treatment group; n, number of patients with events.
Table 3.
Treatment-emergent infections [all-causality] in the Overall Cohort.
| Adverse event, n [%] | Tofacitinib all doses [N = 1157] | PD tofacitinib 5 mg BID [N = 198] | PD tofacitinib 10 mg BID [N = 959] |
|---|---|---|---|
| Infections [all] | 641 [55.4] | 132 [66.7] | 509 [53.1] |
| Dose reduction or temporary discontinuations due to infections | 49 [4.2] | 10 [5.1] | 39 [4.1] |
| Deaths due to infections | 0 [0.0] | 0 [0.0] | 0 [0.0] |
| Infections occurring in ≥2% of patients in any treatment group | |||
| Nasopharyngitis | 251 [21.7] | 56 [28.3] | 195 [20.3] |
| Upper respiratory tract infection | 128 [11.1] | 21 [10.6] | 107 [11.2] |
| Influenza | 99 [8.6] | 18 [9.1] | 81 [8.4] |
| Herpes zoster [non-serious and serious] | 80 [6.9] | 20 [10.1] | 60 [6.3] |
| Gastroenteritis | 76 [6.6] | 9 [4.5] | 67 [7.0] |
| Bronchitis | 58 [5.0] | 19 [9.6] | 39 [4.1] |
| Urinary tract infection | 57 [4.9] | 17 [8.6] | 40 [4.2] |
| Sinusitis | 42 [3.6] | 11 [5.6] | 31 [3.2] |
| Pharyngitis | 39 [3.4] | 12 [6.1] | 27 [2.8] |
| Folliculitis | 29 [2.5] | 6 [3.0] | 23 [2.4] |
| Oral herpes | 29 [2.5] | 4 [2.0] | 25 [2.6] |
Patients are only counted once per treatment for each row. PD groups [the average daily tofacitinib dose levels in the entire UC clinical programme] were based on the average total daily dose of tofacitinib during tofacitinib treatment: PD 5 mg BID, an average total daily dose <15 mg; PD 10 mg BID, an average total daily dose ≥15 mg.
BID, twice daily; N, total number of patients in the treatment group; n, number of patients with events; PD, predominant dose; UC, ulcerative colitis.
3.3. Serious infections
In the Induction Cohort, 0.9% of patients receiving tofacitinib 10 mg BID had serious infections, versus no patients receiving placebo [Table 4]. There was no increased risk associated with continuation of tofacitinib 10 mg BID in the Maintenance Cohort (one [0.5%] patient with a serious infection). The IRs [95% CI] for serious infection in the Overall Cohort (1.70 [1.24–2.27]) and tofacitinib 5 and 10 mg BID groups in the Maintenance Cohort (1.35 [0.16–4.87] and 0.64 [0.02–3.54], respectively) were similar to the placebo group in the Maintenance Cohort (1.94 [0.23–7.00]; Table 4).
Table 4.
Proportions and IRs of infection AEs of special interest in the Induction, Maintenance, and Overall Cohorts in the UC clinical programme
| Adverse event | Induction Cohort | Maintenance Cohort | Overall Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo [N = 282; 44.8 PY] | Tofacitinib 10 mg BID [N = 938; 156.2 PY] | Placebo [N = 198; 100.4 PY] | Tofacitinib 5 mg BID [N = 198; 146.2 PY] | Tofacitinib 10 mg BID [N = 196; 154.3 PY] | Tofacitinib all doses [N = 1157; 2581.3 PY] | |||||||
| n [%] | IR [95% CI] | n [%] | IR [95% CI] | n [%] | IR [95% CI] | n [%] | IR [95% CI] | n [%] | IR [95% CI] | n [%] | IR [95% CI] | |
| Infection [all] | 43 [15.2] | 93.23 [67.47–125.57] | 196 [20.9] | 134.25 [116.11–154.42] | 48 [24.2] | 58.2 [42.9–77.1] | 71 [35.9] | 62.5 [48.9–78.9] | 78 [39.8] | 72.8 [57.6–90.9] | 638 [55.1] | 50.37 [46.53–54.43] |
| Serious infectiona | 0 [0.0] | 0.00 [0.00–7.30] | 8 [0.9] | 4.83 [2.09–9.52] | 2 [1.0] | 1.94 [0.23–7.00] | 2 [1.0] | 1.35 [0.16–4.87] | 1 [0.5] | 0.64 [0.02–3.54] | 45 [3.9] | 1.70 [1.24–2.27] |
| Herpes zoster [non-serious and serious] | 1 [0.4] | 1.98 [0.05–11.05] | 6 [0.6] | 3.62 [1.33–7.88] | 1 [0.5] | 0.97 [0.02–5.42] | 3 [1.5] | 2.05 [0.42–6.00] | 10 [5.1] | 6.64 [3.19–12.22] | 87 [7.5] | 3.48 [2.79–4.30] |
| Opportunistic infectiona,b | 0 [0.0] | 0.00 [0.00–9.13] | 3 [0.3] | 1.89 [0.39–5.53] | 1 [0.5] | 0.97 [0.02–5.42] | 2 [1.0] | 1.36 [0.16–4.92] | 4 [2.0] | 2.60 [0.71–6.65] | 28 [2.5] | 1.07 [0.71–1.55] |
| Herpes zostera,c | 0 [0.0] | 0.0 | 2 [0.2] | 1.2 | 1 [0.5] | 0.97 [0.02–5.42] | 2 [1.0] | 1.36 [0.16–4.92] | 4 [2.0] | 2.60 [0.71–6.65] | 24 [2.1] | 0.92 [0.59–1.37] |
| Non-herpes zostera | 0 [0.0] | 0.00 [0.00–9.13] | 1 [0.1] | 0.63 [0.02–3.51] | 0 [0.0] | 0.00 [0.00–3.57] | 0 [0.0] | 0.00 [0.00–2.48] | 0 [0.0] | 0.00 [0.00–2.35] | 4 [0.4] | 0.15 [0.04–0.38] |
| Tuberculosisa | 0 [0.0] | 0.00 [0.00–9.13] | 0 [0.0] | 0.00 [0.00–2.33] | 0 [0.0] | 0.00 [0.00–3.57] | 0 [0.0] | 0.00 [0.00–2.48] | 0 [0.0] | 0.00 [0.00–2.35] | 0 [0.0] | 0.00 [0.00–0.14] |
| Death [all causes] | 0 [0.0] | 0.00 [0.00–7.30] | 1 [0.1]d | 0.60 [0.02–3.35] | 0 [0.0] | 0.00 [0.00–3.57] | 0 [0.0] | 0.00 [0.00–2.48] | 0 [0.0] | 0.00 [0.00–2.35] | 2 [0.2]e | 0.07 [0.01–0.27] |
IRs were calculated as the number of unique patients with events per 100 PY. Only events occurring within 28 days after the last dose are included in this table for calculation of proportion and IR.
AE, adverse event; BID, twice daily; CI, confidence interval; IR, incidence rate; N, total number of patients in the treatment group; n, number of patients with events; PY, patient-years; UC, ulcerative colitis.
aAdjudicated events. Adjudicated data do not include data from the Phase 2 study NCT00787202. Percentage was calculated based on the number of patients in studies in which adjudication was performed; Induction Cohort: placebo, N = 234; tofacitinib 10 mg BID, N = 905; Maintenance Cohort: placebo, N = 198; tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 196; Overall Cohort: tofacitinib all doses, N = 1124.
bExcludes tuberculosis and herpes zoster with non-adjacent or two adjacent dermatomes.
cIncludes herpes zoster that was adjudicated as multidermatomal [defined as non-adjacent or >2 adjacent dermatomes that were not considered disseminated] or disseminated herpes zoster.
dCause of death: dissecting aortic aneurysm.
eCauses of death: dissecting aortic aneurysm [n = 1] and pulmonary embolism [n = 1].
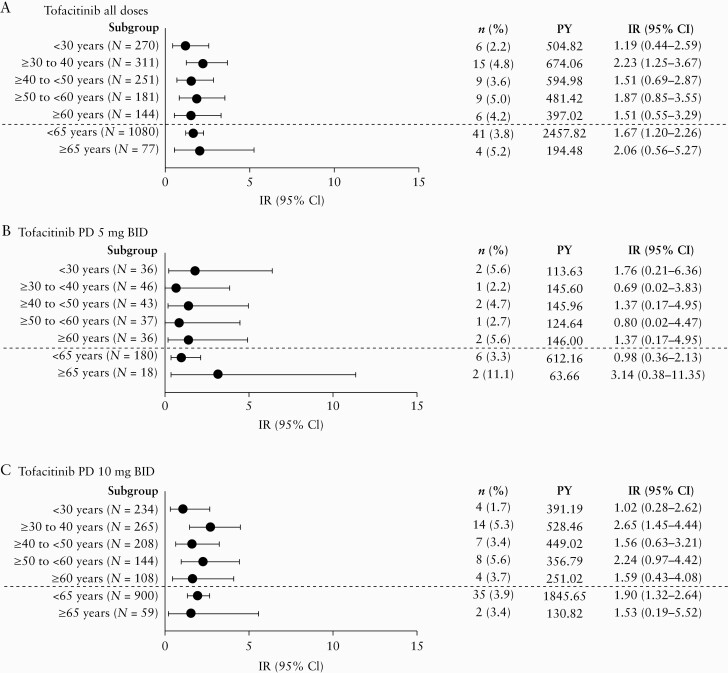
In the Overall Cohort, 49 serious infection events occurred in 46 patients [including one event of severe cellulitis reported 61 days after the last dose of tofacitinib and not included in the proportion or IR calculations]. Of these, only anal abscess [four events], appendicitis [three events], herpes zoster [five events], ophthalmic herpes zoster [two events], sinusitis [two events], and Clostridium difficile infection [two events] occurred in ≥1 patient. The median [range] time to infection for serious infections was 433 [23–1742] days. IRs of serious infection in the Overall Cohort [tofacitinib all doses] were generally similar across age groups [Figure 2], with an IR [95% CI] of 1.19 [0.44–2.59] for patients <30 years old versus 1.51 [0.55–3.29] for patients ≥60 years old. No serious infections resulted in death. Because patients with serious infections were required [per protocol] to discontinue, 38 of the 49 serious infection events [77.6%] directly resulted in discontinuation; of the remaining 11 events, one patient discontinued due to worsening UC at the same time as the serious infection, six had already discontinued [serious infection occurred after the last tofacitinib dose], and four were protocol deviations. Further details of the specific serious infections reported are provided in the Supplementary Data page 15.
Figure 2.
IRs of serious infection by age group in the Overall Cohort for [A] tofacitinib all doses, [B] tofacitinib PD 5 mg BID, and [C] tofacitinib PD 10 mg BID groups. PD groups [the average daily tofacitinib dose levels in the entire UC clinical programme] were based on the average total daily dose of tofacitinib during tofacitinib treatment: PD 5 mg BID, an average total daily dose <15 mg; PD 10 mg BID, an average total daily dose ≥15 mg. BID, twice daily; CI, confidence interval; IR, incidence rate; N, total number of patients in the treatment group; n, number of patients with events; PD, predominant dose; PY, patient-years; UC, ulcerative colitis.
When the multivariable Cox proportional hazard model was applied to the Overall Cohort [tofacitinib all doses], the only baseline risk factor that was significantly associated with serious infections was BMI (per kg/m2; hazard ratio, HR [95% CI] 1.06 [1.01–1.11]). Increasing age [10-year increments] was analysed as a variable; however, it was not identified as a statistically significant risk factor associated with serious infections. Results of the univariate analyses are shown in Supplementary Table 4, available as Supplementary data at ECCO-JCC online.
In the Overall Cohort [N = 1149 patients with available post-baseline ANC and ALC data], post-baseline confirmed low ANC and ALC occurred in 9/1149 [0.8%] and 303/1149 [26.4%] patients, respectively [Supplementary Table 3, available as Supplementary data at ECCO-JCC online]. In the analysis of time-varying post-baseline covariates, low post-baseline ANC [<1.5 x 109/L; confirmed by two consecutive observations] preceding the AE of interest was not significantly associated with serious infections (HR [95% CI] 5.54 [0.75–40.77]), but low post-baseline ALC [<1 x 109/L; confirmed by two consecutive observations] preceding the AE of interest was significantly associated with serious infections (HR [95% CI] 2.25 [1.15–4.38]). Time-varying tofacitinib dose and time-varying steroid dose were not significantly associated with serious infections.
3.4. Herpes zoster
In the Induction Cohort, herpes zoster [non-serious and serious] occurred in six [0.6%] patients receiving tofacitinib 10 mg BID, versus one [0.4%] patient receiving placebo [Table 4]. In the Maintenance Cohort, the IR [95% CI] for herpes zoster [non-serious and serious] was highest for tofacitinib 10 mg BID (6.64 [3.19–12.22]), followed by tofacitinib 5 mg BID (2.05 [0.42–6.00]) and placebo (0.97 [0.02–5.42]; Table 4). In the Overall Cohort, 92 herpes zoster [non-serious and serious] events occurred in 87 [7.5%] patients, with an IR [95% CI] of 3.48 [2.79–4.30] and a median [range] time to onset of 474 [13–1799] days.
Of the herpes zoster events, 92.1% were non-serious, 92.4% had resolved at the time of the data cut-off, and none resulted in death. Among patients with herpes zoster [non-serious and serious], eight [9.2%] patients discontinued due to herpes zoster; the remaining patients either continued tofacitinib treatment (61 [70.1%] patients) or temporarily discontinued but resumed treatment after the event (18 [20.7%] patients). Among patients with herpes zoster events, 67 [72.8%] events were limited to cutaneous involvement and ≤2 adjacent dermatomes, four [4.6%] patients had multiple or repeated herpes zoster events [three patients had two events, one patient had three events], and four [4.6%] patients had post-herpetic neuralgia.
When the multivariable Cox proportional hazard model was applied to the Overall Cohort [tofacitinib all doses], increasing age [10-year increments], previous TNFi failure, and lower body weight [per kg] were identified as significant risk factors for herpes zoster (HR [95% CI] 1.40 [1.21–1.63], 1.76 [1.13–2.74], and 1.02 [1.01–1.04], respectively). Geographical region was also associated with herpes zoster risk: patients in North America had significantly higher risk versus Europe (HR [95% CI] 2.14 [1.28–3.59]) and other regions (HR [95% CI] 1.30 [0.72–2.34]), whereas patients in Europe had significantly lower risk versus other regions (HR [95% CI] 0.61 [0.35–1.04]). No other variables analysed [see Supplementary Data page 8], including oral corticosteroid use at baseline and tofacitinib PD, were identified as significant risk factors. Results of the univariate analyses are shown in Supplementary Table 4. In the analysis of time-varying post-baseline covariates, none of the factors evaluated (tofacitinib dose, steroid dose, low post-baseline ANC [<1.5 x 109/L], and low post-baseline ALC [<1 x 109/L]) were significantly associated with herpes zoster.
3.5. Opportunistic infections and non-herpes zoster viral infections
Adjudicated opportunistic infections other than herpes zoster were infrequent in the tofacitinib UC clinical programme [Table 4]. In the Induction Cohort, three opportunistic infection events (two herpes zoster and one cytomegalovirus [CMV] colitis) occurred in three [0.3%] patients, all in the tofacitinib 10 mg BID group. In the Maintenance Cohort, IRs [95% CI] for opportunistic infection were 0.97 [0.02–5.42; one patient] for placebo, 1.36 [0.16–4.92; two patients] for tofacitinib 5 mg BID, and 2.60 [0.71–6.65; four patients] for tofacitinib 10 mg BID. All opportunistic infections in the Maintenance Cohort were herpes zoster events. The IR [95% CI] for opportunistic infection in the Overall Cohort [1.07 [0.71–1.55] was within the range observed in the Maintenance Cohort [IRs of 0.97–2.60].
In the Overall Cohort, 29 opportunistic infections occurred in 28 patients. Non-herpes zoster opportunistic infections (IR [95% CI] 0.15 [0.04–0.38]; four patients) occurred less frequently than herpes zoster opportunistic infections (IR [95% CI] 0.92 [0.59–1.37]; 24 patients). Non-herpes zoster opportunistic infections included CMV hepatitis [one], CMV colitis [one; based on a colonic biopsy showing a positive polymerase chain reaction test for CMV], and histoplasmosis [with pulmonary involvement; one] in the tofacitinib 10 mg BID group, and pulmonary mycosis [cryptococcosis; one] in the tofacitinib 5 mg BID group. No new cases of Epstein‐Barr virus [EBV] were reported; one event of lymphoma in an EBV-positive patient was reported in the tofacitinib 10 mg BID group. There was no specific clustering of viral infections or viral opportunistic infections other than herpes zoster.
No TB was reported; one event of suspected active TB was assessed to be an SAE based on the criterion of medically significant event, but OIRC review did not confirm it as TB. No progressive multifocal leukoencephalopathy [PML] was reported. Among the opportunistic infections reported, six were serious, none resulted in death, and the median [range] time to onset was 336 [22–1617] days. Six [21.4%] patients discontinued due to opportunistic infections [three due to non-herpes zoster opportunistic infections].
A total of 25 herpes zoster opportunistic infection events were reported in 24 patients [Table 4]. Most herpes zoster opportunistic infections were non-serious [22 events; 88.0%]. Eighteen herpes zoster opportunistic infection events were adjudicated as multidermatomal herpes zoster [defined as non-adjacent or >2 adjacent dermatomes], and seven as disseminated herpes zoster (defined as any of: diffuse rash [>6 dermatomes], pneumonia or other non-skin organ involvement (keratitis [one], ophthalmic herpes zoster [one], herpes zoster meningitis [encephalitis] [one]); five/seven [71.4%] had resolved at the time of data cut-off).
When the multivariable Cox proportional hazard model was applied to the Overall Cohort [tofacitinib all doses], diabetes mellitus, previous TNFi failure, lower baseline ANC [per 1 x 109/L], and lower body weight [per kg] were identified as significant risk factors for opportunistic infections (HR [95% CI] 5.78 [1.92–17.36], 2.32 [1.04–5.18], 1.26 [1.03–1.53], and 1.03 [1.00–1.05], respectively). Results of the univariate analyses are shown in Supplementary Table 4. In the analysis of time-varying post-baseline covariates, none of the factors evaluated (tofacitinib dose, steroid dose, low post-baseline ANC [<1.5 x 109/L], and low post-baseline ALC [<1 x 109/L]) were significantly associated with opportunistic infections.
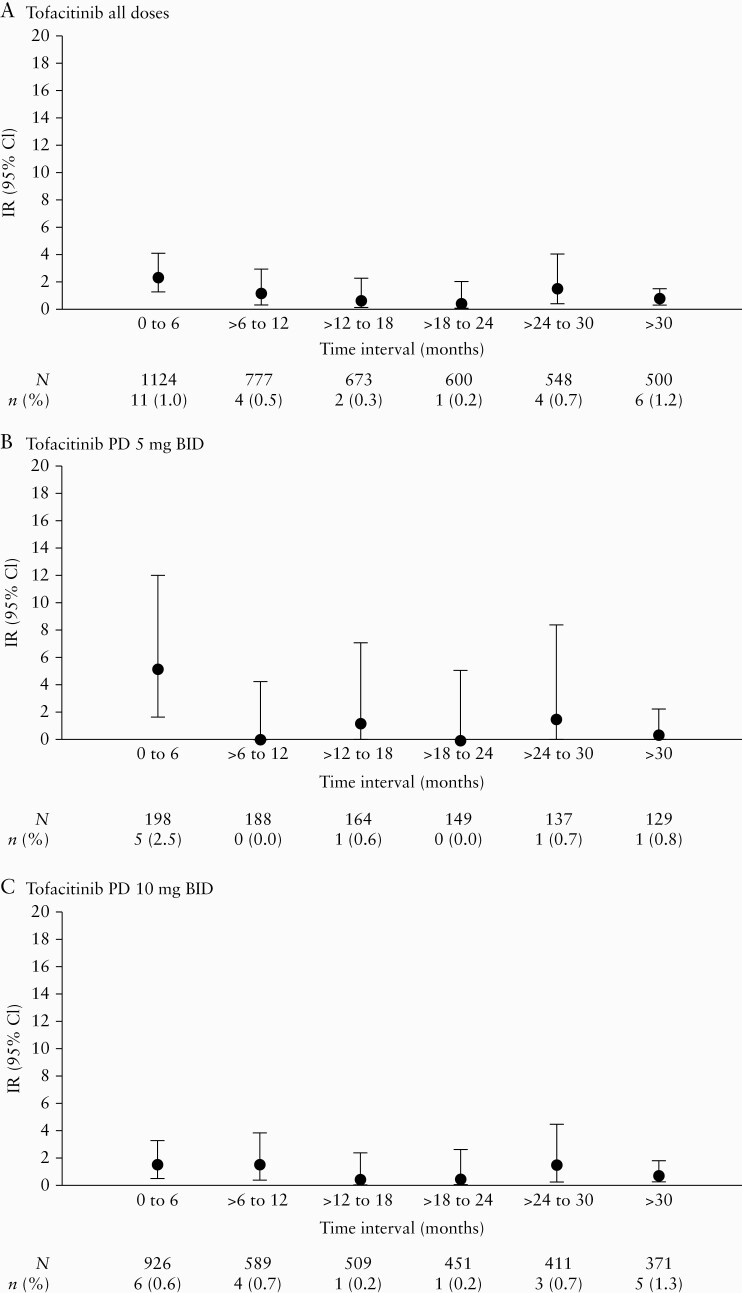
3.6. Infections incidence rates over time
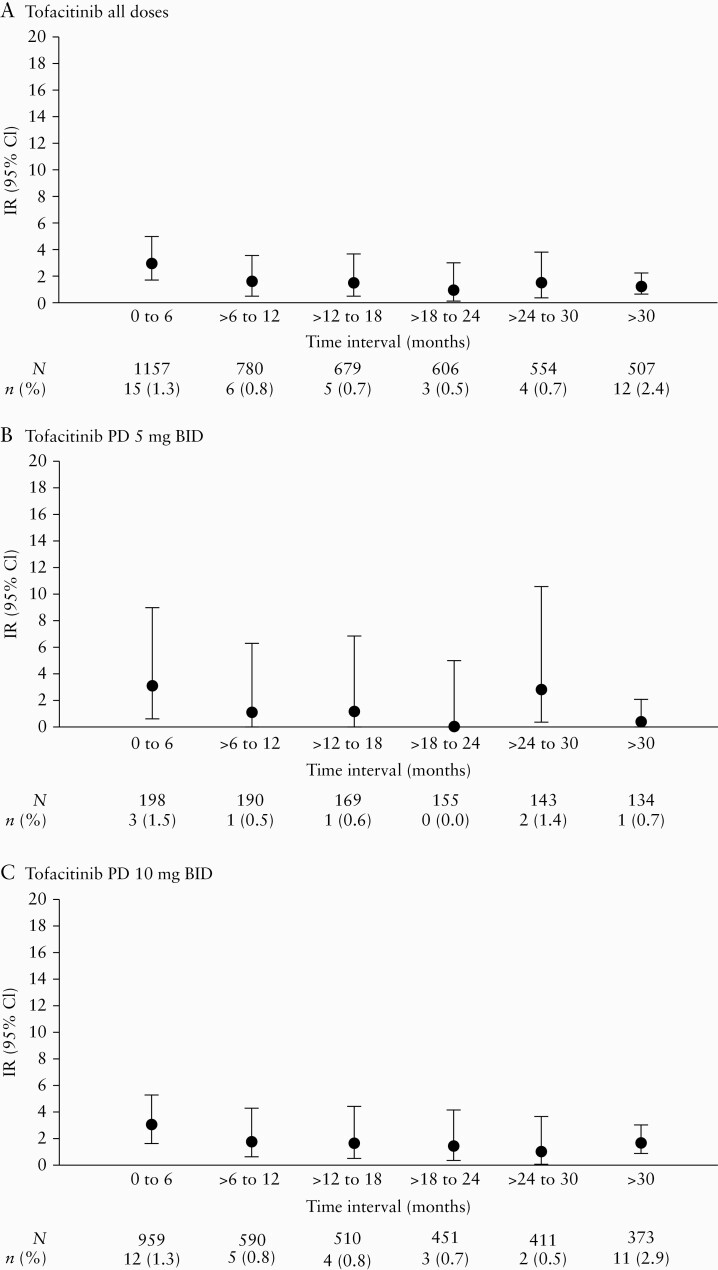
In the Overall Cohort, IRs of serious infection were generally stable when analysed in 6-month intervals [Figure 3], with the IR [95% CI] at > 30 months (1.30 [0.67–2.28]) no higher than that observed in the first 6 months (3.02 [1.69–4.98]) in the tofacitinib all doses group.
Figure 3.
IRs of serious infection by time intervals in the Overall Cohort for [A] tofacitinib all doses, [B] tofacitinib PD 5 mg BID, and [C] tofacitinib PD 10 mg BID groups. PD groups [the average daily tofacitinib dose levels in the entire UC clinical programme] were based on the average total daily dose of tofacitinib during tofacitinib treatment: PD 5 mg BID, an average total daily dose <15 mg; PD 10 mg BID, an average total daily dose ≥15 mg. BID, twice daily; CI, confidence interval; IR, incidence rate; N, total number of patients in the treatment group; n, number of patients with events; PD, predominant dose; UC, ulcerative colitis.
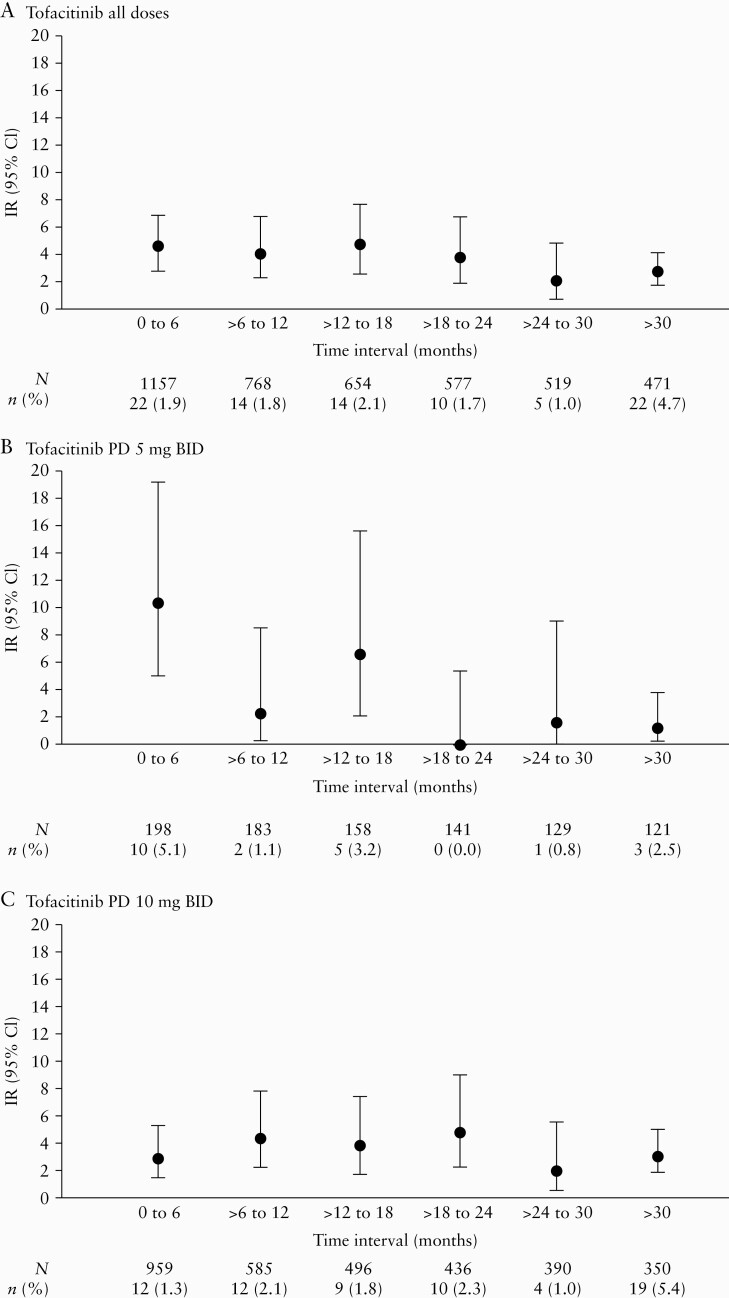
IRs of herpes zoster in the Overall Cohort remained generally stable when analysed in 6-month intervals [Figure 4], with an IR [95% CI] at >30 months of 2.66 [1.67–4.03] versus 4.46 [2.79–6.75] in the first 6 months in the tofacitinib all doses group.
Figure 4.
IRs of herpes zoster infection by time intervals in the Overall Cohort for [A] tofacitinib all doses, [B] tofacitinib PD 5 mg BID, and [C] tofacitinib PD 10 mg BID groups. PD groups [the average daily tofacitinib dose levels in the entire UC clinical programme] were based on the average total daily dose of tofacitinib during tofacitinib treatment: PD 5 mg BID, an average total daily dose <15 mg; PD 10 mg BID, an average total daily dose ≥15 mg. BID, twice daily; CI, confidence interval; IR, incidence rate; N, total number of patients in the treatment group; n, number of patients with events; PD, predominant dose; UC, ulcerative colitis.
Of the 28 patients in the Overall Cohort with opportunistic infections, 11 developed opportunistic infections in the first 6 months of tofacitinib treatment. IRs of opportunistic infection were relatively stable over time when analysed in 6-month intervals [Figure 5], with an IR [95% CI] at >30 months of 0.67 [0.24–1.45] versus 2.25 [1.12–4.03] in the first 6 months in the tofacitinib all doses group.
Figure 5.
IRs of opportunistic infection by time intervals in the Overall Cohort for [A] tofacitinib all doses, [B] tofacitinib PD 5 mg BID, and [C] tofacitinib PD 10 mg BID groups. PD groups [the average daily tofacitinib dose levels in the entire UC clinical programme] were based on the average total daily dose of tofacitinib during tofacitinib treatment: PD 5 mg BID, an average total daily dose <15 mg; PD 10 mg BID, an average total daily dose ≥15 mg. BID, twice daily; CI, confidence interval; IR, incidence rate; N, total number of patients in the treatment group; n, number of patients with events; PD, predominant dose; UC, ulcerative colitis.
4. Discussion
We evaluated the rates and risk factors for serious and opportunistic infections within the global clinical programme for tofacitinib in the treatment of UC. Whereas the incidence of serious infections was higher among those using tofacitinib in the Induction Cohort versus placebo, rates were low and similar between treatment groups in the Maintenance and Overall Cohorts. Herpes zoster incidence was numerically higher with tofacitinib 10 versus 5 mg BID in the Maintenance Cohort; however, in the Overall Cohort, rates were similar between doses. Rates of serious infections, herpes zoster, and opportunistic infections did not increase with longer tofacitinib exposure. Multivariate modelling identified higher BMI and lymphopenia as risk factors for serious infections, and risk factors for herpes zoster were older age, previous TNFi failure, lower body weight, and North American geographical region.
Although serious infections occurred more frequently with tofacitinib 10 mg BID than with placebo in the Induction Cohort, there was no apparent dose dependency in serious infection risk according to frequency or IRs with placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID in the Maintenance Cohort, and a previous analysis of the OLE study showed no difference between the tofacitinib 5 and 10 mg BID dose groups in the proportion of patients with serious infections.21 It is possible that previous treatment with other immunosuppressive therapies may have played a role in the slight increase in serious infections observed with tofacitinib treatment in the Induction Cohort. There was no increased risk of serious infections among older age groups, and IRs of serious infection in the Overall Cohort were generally stable across age groups. Analysis of IRs over time in 6-month intervals in the Overall Cohort did not suggest an increasing risk of serious infections with longer duration of tofacitinib treatment.
Obesity has been associated with a higher risk of several types of infection in the general population,22 although patients with IBD tend to have lower body mass indices versus controls without IBD.23 Lymphopenia was associated with a significant increase in serious infection risk in both the tofacitinib UC clinical programme and the tofacitinib rheumatoid arthritis [RA] programme.17 Monitoring lymphocyte and neutrophil counts is recommended with tofacitinib treatment, with dose adjustments or discontinuation recommended for patients with lymphopenia or neutropenia.24
The IR of serious infections reported here for the Maintenance Cohort was similar to those reported in UC trials of other therapies, including adalimumab at Week 52 [2.2]25 and golimumab at Week 54 [5.2].26 Furthermore, the IR of serious infections reported here for the Overall Cohort was similar to those reported in UC long-term extension trials of adalimumab [3.4],25 golimumab [3.2],26 infliximab [3.4],27 and vedolizumab [1.8],28 although these comparisons should be interpreted with caution due to differences in study design, such as whether or not concomitant use of corticosteroids or other therapies was permitted. Corticosteroid tapering was mandatory at the beginning of OCTAVE Sustain and OCTAVE Open; therefore, by design, most patients in remission would have been steroid-free. Remission was achieved at Week 52 of OCTAVE Sustain in 34.3% and 40.6% of patients receiving tofacitinib 5 or 10 mg BID, respectively.20 Although IRs from clinical trial data are not directly comparable with those from real-world data, the overall incidence of serious infections with tofacitinib reported here (IR [95% CI] 1.70 [1.24–2.27]) is lower [indicated by non-overlapping 95% CI] than that determined using data from the Truven MarketScan® health care insurance database in the USA for ‘all TNFi’ in patients with UC (IR [95% CI] 3.33 [2.73–4.02]).29 The IR of serious infection in the tofacitinib UC clinical programme (IR [95% CI] 1.70 [1.24–2.27]) was also generally consistent with that in the tofacitinib RA clinical programme (6194 patients; 19 406 PY of tofacitinib exposure; IR [95% CI] 2.7 [2.5–3.0]).17 However, it is important to recognise that there are differences between these two programmes that extend beyond the patient population and indication, such as use of corticosteroids.
Herpes zoster is a known risk for patients with IBD compared with the general population. This risk is further increased by treatment with immunosuppressive therapies, including tofacitinib, TNFi, and TNFi and thiopurine combination therapy.30 A previously published study reported an elevated risk of herpes zoster in patients with UC treated with tofacitinib, with increased rates associated with older age, Asian race, and previous TNFi failure.15 In the tofacitinib UC clinical programme, herpes zoster IR was numerically higher with tofacitinib 10 mg versus 5 mg BID in the Maintenance Cohort. However, in the Overall Cohort, IRs were similar between doses and remained stable over time. Overall herpes zoster incidence was consistent with that reported in the tofacitinib RA clinical programme (IR [95% CI] 3.9 [3.6–4.2]), where patients were older and could have been receiving background disease-modifying antirheumatic drugs or corticosteroids.17 A dose-dependent risk of herpes zoster has been reported with baricitinib in RA [IR 3.2],31 as well as with other JAK inhibitors in development, such as upadacitinib [IR 3.7–7.0].32 Similar to the experience reported for herpes zoster with tofacitinib in other indications,16–18 most herpes zoster events were restricted to one or two adjacent dermatomes. Post-herpetic neuralgia is a recognised complication of herpes zoster in immunocompromised patients33 and was reported in 4.6% of patients with herpes zoster in the tofacitinib UC clinical programme. This is consistent with proportions reported in the tofacitinib RA clinical programme [7.4%]34 and is consistent with the range reported for the general population [5–15%].35 Most patients were able to either continue tofacitinib or resume treatment after resolution. Detailed analysis of herpes zoster among patients with UC has been previously published, evaluating treatment with tofacitinib for up to 3.9 years.15 The analysis herein adds to this previous report in that IRs were examined among patients with UC with up to 6.8 years of tofacitinib treatment, demonstrating that the herpes zoster IR in the Overall Cohort in the current analysis is consistent with the herpes zoster IR in the previously published study, and therefore remained stable with longer tofacitinib exposure.
We identified several important risk factors for herpes zoster in the tofacitinib UC clinical programme, including older age, previous TNFi failure, lower body weight, and North American geographical region. Older age is associated with an increased risk of herpes zoster both in the general population2,36 and in patients with IBD,5 and the risk factors identified here are consistent with findings from the RA and psoriasis programmes, where similar risk factors were identified, with the exception of baseline corticosteroid use and Asian race, which did not reach statistical significance in our modelling.13,37 However, a previous study in tofacitinib-treated patients with UC identified Asian race to be associated with increased risk for herpes zoster.15 North American geographical region as a risk factor for herpes zoster has not previously been reported; however, it is possible that geographical differences in vaccination recommendations and uptake may contribute to herpes zoster risk. Further studies evaluating herpes zoster IR and vaccination rates in different regions could advance our understanding of this. Given that higher BMI was identified as a risk factor for serious infection, the identification of lower body weight as a risk factor for herpes zoster was interesting. Lower BMI may be an indicator of more severe UC,23 and in turn, IBD is a known risk factor for herpes zoster, independent of treatment.38 Therefore, patients with UC with lower body weight may have more severe disease and potentially be at a greater risk for herpes zoster. The association was very weak, however (HR [95% CI] 1.02 [1.01–1.04]), and unlikely to be clinically significant. Further studies are needed to understand this association. The use of immunosuppressive therapies such as corticosteroids, thiopurines, and TNFi, are well established and important risk factors for herpes zoster in this and other settings,15,39–42 and reduction or elimination of corticosteroids remains an important risk reduction tool for herpes zoster. Findings from the Phase 3 tofacitinib RA studies showed a numerically lower herpes zoster risk among patients receiving tofacitinib monotherapy, compared with those receiving tofacitinib in combination with corticosteroids and/or disease-modifying antirheumatic drugs; therefore, elimination of concomitant therapies may enable reduction of herpes zoster risk.34
Further, herpes zoster is theoretically preventable with vaccination, and guidance on the management and prevention of herpes zoster in such patients has recently been published.30 For example, one option to reduce herpes zoster risk in susceptible patients is vaccination with the newly developed non-live herpes zoster vaccine, Shingrix [zoster vaccine recombinant, adjuvanted; GlaxoSmithKline]43; however, there are currently limited published data on the safety of this adjuvanted vaccine or its effectiveness in patients with immune-mediated inflammatory diseases such as UC,44 or in patients receiving tofacitinib. The live vaccine, Zostavax (shingles [herpes zoster] vaccine live; MSD), also remains an option and appeared to be safe and adequately immunogenic in a study of patients with RA, when given 2–3 weeks before starting tofacitinib.45 Current vaccination guidelines for immunosuppressive agents recommend that the vaccine be given 4 weeks before starting biologics or JAK inhibitors46; use of live vaccines concurrently with tofacitinib is not recommended.24
Opportunistic infection risk in patients with IBD is increased by the use of corticosteroids, thiopurines, and TNFi agents individually, and particularly in combination therapy.4 Non-herpes zoster opportunistic infections were rare in the tofacitinib UC clinical programme, and longer tofacitinib exposure was not associated with increased opportunistic infection risk [based on a comparison between the Maintenance and Overall Cohorts, and analysis of IRs over time in 6-month intervals]. Neutropenia and diabetes mellitus were identified as significant risk factors for opportunistic infections in the current tofacitinib UC clinical programme. In the tofacitinib RA clinical programme, no patient with confirmed neutropenia developed a serious infection within 30 days of the lowest neutrophil count,47 whereas diabetes was identified as a significant risk factor for serious infections.48
Viral opportunistic infections, including the reactivation of latent viruses,49 have been reported in patients receiving tofacitinib for RA; these include CMV and disseminated or multidermatomal herpes zoster.14 Furthermore, patients with IBD have an increased risk of viral infections and reactivation of latent viruses compared with the general population, with some therapies further increasing that risk.41,42,50 No EBV infections occurred in the tofacitinib UC clinical programme. Two CMV cases occurred in the tofacitinib UC clinical programme, including one case reported following the protocol-specified endoscopy at Week 8 of induction therapy in a patient with previous history of CMV infection based on colonic biopsy, and one case of CMV hepatitis. There was no specific clustering of primary viral infections or viral opportunistic infections other than herpes zoster, which is the result of viral reactivation. No cases of PML were reported in this or any other tofacitinib clinical programme16–18,47 or in any post-marketing data to date.49,51 In the context of the current SARS-CoV-2 pandemic, several organisations are attempting to understand the effect of COVID-19 on the IBD population. The Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease [SECURE-IBD] database is an international registry set up to monitor outcomes of COVID-19 in patients with IBD. Current data reported on the SECURE-IBD registry suggest that JAK inhibitors are unlikely to increase the risk of COVID-19 infection or negative outcomes [intensive care unit admission, ventilation, or death]; however, patient numbers are low and further studies are required to fully understand the risk.52 Expert guidance from the International Organization for the Study of Inflammatory Bowel Diseases and American Gastroenterological Association states that discontinuation of tofacitinib is not recommended to prevent infection with SARS-CoV-2, although it is recommended in patients who develop COVID-19 and/or test positive for the virus until infection resolves or the test is negative.53,54 Per product labelling, if a serious infection develops, treatment with tofacitinib should be interrupted until the infection is controlled.24
An important limitation of these analyses is the small size of the patient population in the tofacitinib UC clinical programme. The multivariable analysis may have been limited by the low number of patients with serious infection, herpes zoster, or opportunistic infection events in the Overall Cohort, potentially impacting on the ability to draw robust conclusions. Evaluation of safety data stratified by age is limited by the sample size within certain age groups. Comparing data from patients <65 years old with patients ≥65 years old is challenging due to the small sample size of patients in the ≥65 years age group, although the ≥60 years age group was a similar size to the younger age groups. Furthermore, evaluation of tofacitinib dose dependency in the UC clinical programme is limited by several factors. The protocol requirement for patients who developed serious infections to discontinue meant that healthier patients, or those less prone to infection, remained in the study at later time points. Patients in the OLE study were not randomised to treatment, but were allocated to receive tofacitinib 5 or 10 mg BID based on their remission status in the induction or maintenance studies, leading to a higher proportion of patients receiving tofacitinib 10 mg BID. Furthermore, the use of PD in the OLE study analyses did not consider the actual dose at the time of the event.
The safety data for tofacitinib in UC presented here complement the safety data established by the tofacitinib programmes for RA,17,47 psoriatic arthritis,18,55 and psoriasis,16,56 although variation in the intrinsic infection risk in patients with these conditions may limit comparisons between programmes. Whereas IRs in the tofacitinib UC clinical programme were based on relatively few events observed in 1157 patients with a treatment duration up to 6.8 years, published analyses from the RA and psoriasis programmes have studied larger numbers of patients with longer exposure.16,17 Across all programmes, in general, tofacitinib carried risks of infections and opportunistic infections similar to those associated with biologic therapies used in those indications,17,47,56,57 with the exception of herpes zoster17,47,58,59 which is already identified as an increased risk observed with tofacitinib and other JAK inhibitors,49 in addition to TNFi and thiopurines.30,42
In summary, we have evaluated the risk of infection among patients with UC using tofacitinib in the global UC clinical programme. Our study found that although serious infections occurred in a higher proportion of patients receiving tofacitinib 10 mg BID versus placebo during induction, overall, serious infections were generally infrequent in patients treated with tofacitinib 5 or 10 mg BID, with no dose dependency or treatment duration effect. In the tofacitinib UC clinical programme, herpes zoster IR was numerically higher with tofacitinib 10 versus 5 mg BID in the Maintenance Cohort. However, over time in the Overall Cohort, IRs were similar between doses and remained stable. Non-herpes zoster opportunistic infections and viral infections occurred infrequently. Subsequent studies offering controlled comparisons between therapies for UC may further the understanding of infection risk with tofacitinib in patients with UC.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, investigators, and study teams who were involved in the tofacitinib UC programme: OCTAVE Induction 1, OCTAVE Induction 2, OCTAVE Sustain, and OCTAVE Open. Connie Chen at Pfizer Inc, New York, NY, USA, reviewed the manuscript and provided editorial support. Ronald D. Pedersen at Pfizer Inc, Collegeville, PA, USA, performed statistical analysis. Medical writing support, under the guidance of the authors, was provided by Nina Divorty, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA, in accordance with Good Publication Practice [GPP3] guidelines [Ann Intern Med 2015;163:461–4].
Data sharing statement
Upon request, and subject to certain criteria, conditions, and exceptions [see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information], Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: [1] for indications that have been approved in the USA and/or EU; or [2] in programmes that have been terminated [ie. development for all indications has been discontinued]. Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Funding
This work was supported by Pfizer Inc. The clinical trials described in this article were sponsored by Pfizer Inc. Funding for medical writing support was provided by Pfizer Inc.
Conflict of Interest
KLW has received research support or consulting fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer Inc, Roche, and UCB. EVL has received consulting fees from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion Healthcare, Eli Lilly, Genentech, Gilead, Janssen, Pfizer Inc, Takeda, and UCB; and has received research support from AbbVie, Allergan, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Pfizer Inc, Receptos, Robarts Clinical Trials, Takeda, and UCB. DCB reports institutional unrestricted grants, consulting fees, or non-personal financial support [ie. travel support or unrestricted institutional research grants] from AbbVie, Allergan, Amgen, Applied Medical, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Centocor, CSL Behring, Dr. Falk Pharma, Elan, Ferring Pharmaceuticals, Forward Pharma, Genentech, Hitachi, Janssen-Cilag, Karl Storz, Merck, Merz, MSD, Nestlé, Ocera, Ogilvy, Otsuka, PDL, Pfizer Inc, Recordati, Roche, Shield Therapeutics, Shire, Takeda, TiGenix, Tillotts, UCB, Vifor, and 4D Pharma. WR has received research support from Abbott, AbbVie, AESCA, Centocor, Dr. Falk Pharma, Immundiagnostik, and MSD; has received lecture fees from Abbott, AbbVie, AESCA, Aptalis, Celltrion, Centocor, Danone, Dr. Falk Pharma, Elan, Ferring Pharmaceuticals, Immundiagnostik, Mitsubishi Tanabe Pharma, MSD, Otsuka, PDL, Pharmacosmos, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; and has received consulting fees from Abbott, AbbVie, AESCA, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia Biologics, Bioclinica, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Cellerix, Celltrion, Centocor, ChemoCentryx, Covance, Danone, Dr. Falk Pharma, Elan, Ferring Pharmaceuticals, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Mitsubishi Tanabe Pharma, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pfizer Inc, Pharmacosmos, Procter & Gamble, Prometheus Laboratories, Robarts Clinical Trials, Schering-Plough, Second Genome, SetPoint Medical, Takeda, Therakos, TiGenix, UCB, Vifor, Zyngenia, and 4SC. CIN was an employee of Pfizer Inc at the time this research was conducted. NL, GC, RM, GSF, LS, AJT, and CS are employees and stockholders of Pfizer Inc.
Author Contributions
Planned the study/studies: NL, GC, GSF, and CS. Conducted the study/studies: NL, GC, RM, GSF, and CS. Collected or interpreted data: KLW, EVL, DCB, WR, CIN, NL, GC, RM, GSF, LS, AJT, and CS. Drafted and edited the manuscript: KLW, EVL, DCB, WR, CIN, NL, GC, RM, GSF, LS, AJT, and CS. All authors have confirmed their approval for this final draft for submission.
References
- 1. Kantsø B, Simonsen J, Hoffmann S, et al. . Inflammatory bowel disease patients are at increased risk of invasive pneumococcal disease: a nationwide Danish cohort study 1977–2013. Am J Gastroenterol 2015;110:1582–7. [DOI] [PubMed] [Google Scholar]
- 2. Rahier JF, Magro F, Abreu C, et al. . Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443–68. [DOI] [PubMed] [Google Scholar]
- 3. Bongartz T, Sutton AJ, Sweeting MJ, et al. . Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85. [DOI] [PubMed] [Google Scholar]
- 4. Toruner M, Loftus EV Jr, Harmsen WS, et al. . Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134:929–36. [DOI] [PubMed] [Google Scholar]
- 5. Khan N, Patel D, Trivedi C, et al. . Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol 2018;16:1919–27.e3. [DOI] [PubMed] [Google Scholar]
- 6. Greenberg JD, Reed G, Kremer JM, et al. . Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis 2010;69:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schneeweiss S, Korzenik J, Solomon DH, et al. . Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther 2009;30:253–64. [DOI] [PubMed] [Google Scholar]
- 8. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 2014;57:5023–38. [DOI] [PubMed] [Google Scholar]
- 9. Hodge JA, Kawabata TT, Krishnaswami S, et al. . The mechanism of action of tofacitinib ‐ an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016;34:318–28. [PubMed] [Google Scholar]
- 10. Ghoreschi K, Jesson MI, Li X, et al. . Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Vollenhoven RF, Fleischmann R, Cohen S, et al. ; ORAL Standard Investigators . Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 12. Fleischmann R, Kremer J, Cush J, et al. . Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 13. Winthrop KL, Yamanaka H, Valdez H, et al. . Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winthrop KL, Park SH, Gul A, et al. . Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winthrop KL, Melmed GY, Vermeire S, et al. . Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valenzuela F, Korman NJ, Bissonnette R, et al. . Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol 2018;179:853–62. [DOI] [PubMed] [Google Scholar]
- 17. Cohen SB, Tanaka Y, Mariette X, et al. . Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gladman D, Rigby W, Azevedo VF, et al. . Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Ghosh S, Panés J, et al. . Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Su C, Sands BE, et al. . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 21. Lichtenstein GR, Loftus EV Jr, Wei SC, et al. . Tofacitinib, an oral, small-molecule Janus kinase inhibitor, in the treatment of ulcerative colitis: analysis of an open-label, long-term extension study with up to 5.9 years of treatment. J Crohns Colitis 2020;14(Suppl 1):S100–1(DOP61). [Google Scholar]
- 22. Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37:333–40. [DOI] [PubMed] [Google Scholar]
- 23. Dong J, Chen Y, Tang Y, et al. . Body mass index is associated with inflammatory bowel disease: a systematic review and meta-analysis. PLoS One 2015;10:e0144872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration. XELJANZ ® (Tofacitinib): Highlights of Prescribing Information., 2019. http://labeling.pfizer.com/ShowLabeling.aspx?id=959Accessed April 8, 2020. [Google Scholar]
- 25. Colombel JF, Sandborn WJ, Ghosh S, et al. . Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am J Gastroenterol 2014;109:1771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibson PR, Feagan BG, Sandborn WJ, et al. . Maintenance of efficacy and continuing safety of golimumab for active ulcerative colitis: PURSUIT-SC maintenance study extension through 1 year. Clin Transl Gastroenterol 2016;7:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reinisch W, Sandborn WJ, Rutgeerts P, et al. . Long-term infliximab maintenance therapy for ulcerative colitis: the ACT-1 and -2 extension studies. Inflamm Bowel Dis 2012;18:201–11. [DOI] [PubMed] [Google Scholar]
- 28. Vermeire S, Colombel J-F, Feagan BG, et al. . Long-term safety of vedolizumab in ulcerative colitis and Crohn’s disease: final results from the GEMINI LTS study. J Crohns Colitis 2019;13(Suppl 1):S018–20(OP26). [Google Scholar]
- 29.US Food and Drug Administration. FDA Advisory Committee Meeting sNDA 203214 Supplement 018 Briefing Document. Pfizer Inc.2018. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM599514.pdfAccessed June 13, 2019. [Google Scholar]
- 30. Colombel JF. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis 2018;24:2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smolen JS, Genovese MC, Takeuchi T, et al. . Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. [DOI] [PubMed] [Google Scholar]
- 32. Cohen SB, van Vollenhoven R, Winthrop K, et al. . Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the select phase 3 clinical program. Ann Rheum Dis 2019;78(Suppl 2):357(THU0167). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Côté-Daigneault J, Peerani F, MacMahon E, et al. . Management and prevention of herpes zoster in the immunocompromised inflammatory bowel disease patient: a clinical quandary. Inflamm Bowel Dis 2016;22:2538–47. [DOI] [PubMed] [Google Scholar]
- 34. Winthrop KL, Curtis JR, Lindsey S, et al. . Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol 2017;69:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friesen KJ, Chateau D, Falk J, Alessi-Severini S, Bugden S. Cost of shingles: population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis 2017;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmader K. Herpes zoster in the elderly: issues related to geriatrics. Clin Infect Dis 1999;28:736–9. [DOI] [PubMed] [Google Scholar]
- 37. Winthrop KL, Lebwohl M, Cohen AD, et al. . Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol 2017;77:302–9. [DOI] [PubMed] [Google Scholar]
- 38. Forbes HJ, Bhaskaran K, Thomas SL, et al. . Quantification of risk factors for herpes zoster: population based case-control study. BMJ 2014;348:g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smitten AL, Choi HK, Hochberg MC, et al. . The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 2007;57:1431–8. [DOI] [PubMed] [Google Scholar]
- 40. McDonald JR, Zeringue AL, Caplan L, et al. . Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 2009;48:1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006;4:1483–90. [DOI] [PubMed] [Google Scholar]
- 42. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. US Food and Drug Administration. SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) Suspension for Intramuscular Injection, Highlights of Prescribing Information. 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdfAccessed May 04, 2020. [Google Scholar]
- 44. Satyam VR, Li P-H, Reich J, et al. . Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. Dig Dis Sci 2020;65:2986–91. [DOI] [PubMed] [Google Scholar]
- 45. Winthrop KL, Wouters AG, Choy EH, et al. . The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol 2017;69:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) . Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57:1–30. [PubMed] [Google Scholar]
- 47. Wollenhaupt J, Lee EB, Curtis JR, et al. . Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cohen S, Radominski SC, Gomez-Reino JJ, et al. . Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2924–37. [DOI] [PubMed] [Google Scholar]
- 49. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:320. [DOI] [PubMed] [Google Scholar]
- 50. Tinsley A, Navabi S, Williams ED, et al. . Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis 2019;25:369–76. [DOI] [PubMed] [Google Scholar]
- 51. Cohen S, Curtis JR, DeMasi R, et al. . Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Ther 2018;5:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brenner EJ, Ungaro RC, Colombel JF, Kappelman MD. SECURE-IBD Database Public Data Update, 2020. https://www.covidibd.orgAccessed September 02, 2020.
- 53. Rubin DT, Abreu MT, Rai V, Siegel CA; International Organization for the Study of Inflammatory Bowel Disease . Management of patients with Crohn’s disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology 2020;159:6–13.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology 2020;159:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mease P, Hall S, FitzGerald O, et al. . Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 56. Papp KA, Krueger JG, Feldman SR, et al. . Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol 2016;74:841–50. [DOI] [PubMed] [Google Scholar]
- 57. Strand V, Ahadieh S, French J, et al. . Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strangfeld A, Listing J, Herzer P, et al. . Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA 2009;301:737–44. [DOI] [PubMed] [Google Scholar]
- 59. Shalom G, Zisman D, Bitterman H, et al. . Systemic therapy for psoriasis and the risk of herpes zoster: a 500,000 person-year study. JAMA Dermatol 2015;151:533–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.