LETTER
The direct use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) on positive blood culture (PBC) bottles may accelerate the identification of bloodstream infection (BSI)-causing organisms (1), particularly anaerobic bacteria for which BSI-associated mortality rates are high (2). Until now (3, 4), performing MALDI-TOF MS-based identification on direct colonies (i.e., on bacteria isolated from PBC subcultures) has allowed circumvention of the need for time-consuming biochemical/enzymatic tests, often performed under controlled anaerobic conditions, on which conventional identification methods rely. Despite being accurate, such an identification approach depends so far on the time required for growth of anaerobic bacteria. Thus, performing MALDI-TOF MS directly on PBC bottles is faster and, therefore, desirable to expedite the management of patients with anaerobic bacteremia (2). Furthermore, direct MALDI-TOF MS is a nonbiased approach that permits identification of a wide array of bacterial organisms and, importantly, is not target dependent like most of the currently available rapid molecular diagnostics (1). To date, few studies have explored the identification yield from direct MALDI-TOF MS testing for anaerobic PBC bottles in routine clinical microbiology laboratory practice (5, 6).
Here, we report the results of direct MALDI-TOF MS testing performed on positive BacT/Alert FN Plus (bioMérieux, Marcy l’Étoile, France) resin-containing anaerobic blood culture bottles (hereafter referred to as PBCs). Bottles were consecutively processed in the bioMérieux BacT/Alert 3D or Virtuo automated blood culture systems over 5 years of routine activity (January 2016 to December 2020) in the clinical microbiology laboratory of a large tertiary care hospital in Rome, Italy. We compared these results with those of colony MALDI-TOF MS testing, which was performed on bacterial BSI isolates obtained by subculturing PBC samples on solid medium as previously described (7). Briefly, purification of bacterial cells from PBC samples (8 ml of which was centrifuged to remove erythrocytes) was followed by protein extraction and loading onto a Microflex LT mass spectrometer (Bruker Daltonics, Bremen, Germany) target plate (7). Bacterial spectra were analyzed using the Bruker Daltonics MALDI BioTyper 3.1 or, later, 4.1 software versions, and results were interpreted as high-confidence or low-confidence correct identification or as no identification based on (log)score values of ≥1.9, <1.9 to ≥1.7, and <1.7, respectively (8). For isolates with a (log)score value of ∼1.7 (i.e., 1.699 to 1.750), identification was confirmed by means of PCR sequencing-based molecular analyses (9). Of 20,191 routinely processed PBCs, 1,016 (5.0%) yielded anaerobic bacteria, and 406, accounting for 3.6% of 11,154 PBCs corresponding to first BSI patient episodes, were included in this study. Using the colony MALDI-TOF MS testing as the reference method, we calculated the percentage of correct identification results with direct MALDI-TOF MS testing overall or by genera for monomicrobial or polymicrobial cultures, respectively. Fisher’s exact test was used to assess differences for each comparison. The MALDI BioTyper log(score) values between the two testing methods were compared by genera using the Wilcoxon matched-pairs signed rank test for comparison of means. Statistical significance was set at a P value of <0.05.
Of 406 studied PBCs, 340 (83.7%) were monomicrobial and 66 (16.3%) were polymicrobial, accounting for 425 anaerobic species in total (see Table S1 in the supplemental material). As shown in Table 1, compared to colony MALDI-TOF MS testing, the correct (species-level) identification rate of anaerobic bacteria with direct MALDI-TOF MS testing was 52.7% (224/425), of which 60.3% (205/340) was from monomicrobial PBCs and 22.3% (19/85) was from polymicrobial PBCs (P < 0.0001). The remaining 47.3% (201/425) of PBCs did not give identification. When only monomicrobial cultures were considered, the identification rate ranged from 97.1% (133/137) for Bacteroides/Parabacteroides species to 9.8% (7/71) for Cutibacterium species (P < 0.0001). When only polymicrobial cultures were considered, the highest (40.0%) identification rate was found for Veillonella species (2/5), while there was no identification (0.0%) for Prevotella (0/2; P = 0.47), Fusobacterium (0/1; P = 0.66), or Capnocytophaga gingivalis (0/1; P = 0.66) among Gram-negative species or for Eggerthella (0/4; P = 0.27) among Gram-positive species. Among the Gram-negative genera that gave identification by direct or colony testing methods (Table 1), mean log(score) values were 2.023 and 2.126 for Bacteroides/Parabacteroides (P < 0.0001), 1.945 and 2.192 for Prevotella (P = 0.02), 1.898 and 2.002 for Fusobacterium (P = 0.07), and 1.948 and 2.149 for Veillonella (P = 0.07), respectively. Among the Gram-positive genera that gave identification by direct or colony testing methods (Table 1), mean log(score) values were 1.839 and 2.029 for Cutibacterium (P = 0.02), 1.986 and 2.257 for Clostridium (P < 0.0001), 1.836 and 2.024 for non-spore-forming rods other than Cutibacterium (P = 0.03), and 1.931 and 2.128 for Parvimonas and other cocci (P < 0.001), respectively.
TABLE 1.
Comparison of colony and direct MALDI-TOF MS testing results for 406 anaerobic PBCs included in the study
| Anaerobic isolates | No. of isolates identified/total isolates (%) by log(score) levela |
|||||
|---|---|---|---|---|---|---|
| Monomicrobial cultures (n = 340) |
Polymicrobial cultures (n = 66) |
|||||
| ≥1.7 | ≥1.9 | <1.9 to ≥1.7 | ≥1.7 | ≥1.9 | <1.9 to ≥1.7 | |
| Gram-negative genera | 146/170 (85.9) | 140/170 (82.4) | 6/170 (3.5) | 10/35 (28.5) | 6/35 (17.1) | 4/35 (11.4) |
| Bacteroides/Parabacteroides | 133/137 (97.1) | 129/137 (94.2) | 4/137 (2.9) | 8/26 (30.8) | 4/26 (15.4) | 4/26 (15.4) |
| Prevotella | 7/11 (63.6) | 6/11 (54.5) | 1/11 (9.1) | 0/2 (0.0) | 0/2 (0.0) | 0/2 (0.0) |
| Fusobacterium | 4/9 (44.4) | 3/9 (33.3) | 1/9 (11.1) | 0/1 (0.0) | 0/1 (0.0) | 0/1 (0.0) |
| Veillonella | 2/7 (28.6) | 2/7 (28.6) | 0/7 (0.0) | 2/5 (40.0) | 2/5 (40.0) | 0/5 (0.0) |
| Othersb | 0/6 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/1 (0.0) | 0/1 (0.0) | 0/1 (0.0) |
| Gram-positive genera | 59/170 (34.7) | 47/170 (27.6) | 12/170 (7.1) | 9/50 (18.0) | 6/50 (12.0) | 3/50 (6.0) |
| Cutibacterium | 7/71 (9.8) | 4/71 (5.6) | 3/71 (4.2) | 0/0 (NAc) | 0/0 (NA) | 0/0 (NA) |
| Clostridium | 33/40 (82.5) | 31/40 (77.5) | 2/40 (5.0) | 6/35 (17.1) | 4/35 (11.4) | 2/35 (5.7) |
| Non-spore-forming rods other than Cutibacteriumd | 5/30 (16.6) | 1/30 (3.3) | 4/30 (13.3) | 1/8 (12.5) | 1/8 (12.5) | 0/8 (0.0) |
| Parvimonas and other coccie | 14/29 (48.2) | 11/29 (37.9) | 3/29 (10.3) | 2/7 (28.6) | 1/7 (14.3) | 1/7 (14.3) |
| Total isolates | 205/340 (60.3) | 187/340 (55.0) | 18/340 (5.3) | 19/85 (22.3) | 12/85 (14.1) | 7/85 (8.2) |
With direct MALDI-TOF MS testing, none of positive blood cultures with log(score) values of ≥1.7 yielded a misidentification result, whereas all of positive blood cultures with log(score) values of <1.7 yielded no identification. Fifteen isolates had log(score) values of 1.699 to 1.750. Using PCR sequencing analysis, isolates were confirmed to be Alloscardovia omnicolens (n = 1), Aggregatibacter actinomycetemcomitans (n = 1), Atopobium parvulum (n = 2), Capnocytophaga gingivalis (n = 1), Clostridium hathewayi (n = 1), Dialister pneumosintes (n = 1), Leptotrichia trevisani (n = 2), Prevotella nanceiensis (n = 1), Ruminococcus gnavus (n = 2), Staphylococcus saccharolyticus (n = 2), or Tissierella praeacuta (n = 1).
Others include A. actinomycetemcomitans (n = 1), Butyricimonas virosa (n = 1), D. pneumosintes (n = 1), L. trevisani (n = 2), T. praeacuta (n = 1) in monomicrobial cultures and C. gingivalis (n = 1) in polymicrobial cultures.
NA, not applicable.
Includes isolates of Eggerthella (n = 19 [15 in monomicrobial cultures and 4 in polymicrobial cultures]) and Bifidobacterium (n = 11 [9 in monomicrobial cultures and 2 in polymicrobial cultures]), among others (n = 8).
Includes isolates of Parvimonas (n = 26 [22 in monomicrobial cultures and 4 in polymicrobial cultures]) and Peptoniphilus (n = 4 [2 in monomicrobial cultures and 2 in polymicrobial cultures]), among others (n = 6).
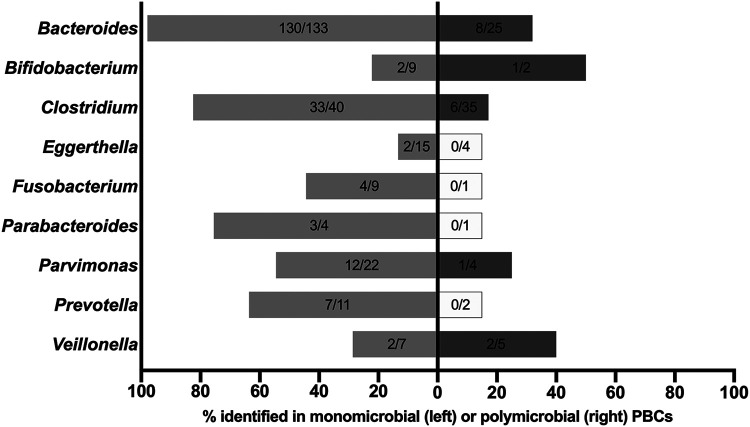
Figure 1 shows direct MALDI-TOF MS testing results for the nine most identified genera in both monomicrobial (n = 250) and polymicrobial (n = 79) cultures. Regarding monomicrobial cultures, 3 (2.3%) of 133 PBCs for Bacteroides species (1 B. fragilis, 1 B. ovatus, and 1 B. uniformis) and 7 (17.5%) of 40 PBCs for Clostridium species (1 C. aldenense, 1 C. clostridioforme, 3 C. perfringens, 1 C. sporogenes, and 1 C. subterminale) did not yield identification. Conversely, 10 (45.5%) of 22 PBCs for Parvimonas micra and 13 (86.7%) of 15 PBCs for Eggerthella lenta did not yield identification. Of note, direct identification for E. lenta was 40-fold less likely than for Bacteroides species. Regarding polymicrobial cultures, 17 (68.0%) of 25 PBCs for Bacteroides species and 29 (82.9%) of 35 PBCs for Clostridium species did not yield identification with direct MALDI-TOF MS testing.
FIG 1.
Direct MALDI-TOF MS identification results for 329 anaerobic bacteria according to nine most frequently identified genera in both monomicrobial (n = 250) and polymicrobial (n = 79) cultures. Not shown are results for Cutibacterium bacteria (n = 71), which were isolated only from monomicrobial cultures. Gray bars indicate percentages of bacterial identification (no. identified/no. tested) ranging from 13.3% to 97.1% in monomicrobial (light gray) and polymicrobial (dark gray) cultures, whereas white bars indicate no identification for the tested bacteria in polymicrobial cultures.
In spite of low occurrence, i.e., about 0.5 to 12% of all PBCs, anaerobic bacteremia may be a life-threatening infection, thus requiring rapid administration of antimicrobial agents with anaerobic activity to decrease the mortality among patients with such an infection (10). Although the etiological spectrum may be very broad (11, 12), Bacteroides species were the main clinically relevant organisms identified in anaerobic bacteremia cases (10). Not surprisingly, rapid diagnostic platforms commercially developed to test PBCs, such as the BioFire FilmArray blood culture identification panel (13), have included B. fragilis among molecular targets associated with BSI. Consistent with previous studies (1, 5), we showed that direct MALDI-TOF MS testing of PBCs for anaerobic bacteria not only is accurate but also has a high rate of success for many of the most common bacterial organisms isolated from anaerobic blood cultures. This was particularly true for monomicrobial BSIs or for common causative pathogens such as B. fragilis. At a glance (Table 1), these findings might not be so attractive if one considers that direct MALDI-TOF MS testing allowed species-level identification for about 60% of monomicrobial PBCs in our study. However, the likelihood of rapidly distinguishing BSIs due to Bacteroides species from BSIs due to non-Bacteroides Gram-negative species (e.g., Prevotella species) counterbalances the likelihood of failure when applying direct MALDI-TOF MS testing to anaerobic PBCs. We acknowledge that the mortality rate for Bacteroides bacteremia (up to 50%) is considerably higher than that for Clostridium bacteremia, which is often transient, or for bacteremia due to Cutibacterum acnes (formerly Propionibacterium acnes), which is part of the skin microbiota (2). We also acknowledge that performing a Gram stain prior to processing anaerobic PBCs is an essential step in primarily aerobic bacterial BSI-driven MALDI-TOF MS workflows (1). By including this step, it is possible to not only distinguish Gram-positive organisms from Gram-negative organisms (which is likely to succeed with MALDI-TOF MS identification) but also to mitigate the risk of testing polymicrobial cultures (which is likely to not succeed with MALDI-TOF MS identification) (1). This would allow saving precious work by the BSI-dedicated laboratory personnel as well as avoiding the costs associated with unsuccessful MALDI-TOF MS identification. In an ideal BSI workflow, laboratories should consider both MALDI-TOF MS and molecular identification for direct testing of PBCs (7), which would allow optimization of both diagnostic performance and patient care (1).
While colony MALDI-TOF MS testing is indispensable, we conclude that incorporating direct MALDI-TOF MS testing into laboratory workflows may enhance etiological diagnosis and, meanwhile, facilitate the timely initiation of effective therapy for anaerobic bacteremia.
ACKNOWLEDGMENT
We thank Franziska Lohmeyer for English revision of the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Maurizio Sanguinetti, Email: maurizio.sanguinetti@unicatt.it.
Nathan A. Ledeboer, Medical College of Wisconsin
REFERENCES
- 1.Faron ML, Buchan BW, Ledeboer NA. 2017. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for use with positive blood cultures: methodology, performance, and optimization. J Clin Microbiol 55:3328–3338. doi: 10.1128/JCM.00868-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook I. 2010. The role of anaerobic bacteria in bacteremia. Anaerobe 16:183–189. doi: 10.1016/j.anaerobe.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Nagy E, Becker S, Kostrzewa M, Barta N, Urbán E. 2012. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. J Med Microbiol 61:1393–1400. doi: 10.1099/jmm.0.043927-0. [DOI] [PubMed] [Google Scholar]
- 4.Barreau M, Pagnier I, La Scola B. 2013. Improving the identification of anaerobes in the clinical microbiology laboratory through MALDI-TOF mass spectrometry. Anaerobe 22:123–125. doi: 10.1016/j.anaerobe.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Almuhayawi M, Altun O, Abdulmajeed AD, Ullberg M, Özenci V. 2015. The performance of the four anaerobic blood culture bottles BacT/ALERT-FN, -FN Plus, BACTEC-Plus and -Lytic in detection of anaerobic bacteria and identification by direct MALDI-TOF MS. PLoS One 10:e0142398. doi: 10.1371/journal.pone.0142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeverica S, Nagy E, Mueller-Premru M, Papst L. 2018. Sample preparation method influences direct identification of anaerobic bacteria from positive blood culture bottles using MALDI-TOF MS. Anaerobe 54:231–235. doi: 10.1016/j.anaerobe.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Fiori B, D'Inzeo T, Giaquinto A, Menchinelli G, Liotti FM, de Maio F, De Angelis G, Quaranta G, Nagel D, Tumbarello M, Posteraro B, Sanguinetti M, Spanu T. 2016. Optimized use of the MALDI BioTyper system and the FilmArray BCID panel for direct identification of microbial pathogens from positive blood cultures. J Clin Microbiol 54:576–584. doi: 10.1128/JCM.02590-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect DIS 49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 9.La Scola B, Fournier PE, Raoult D. 2011. Burden of emerging anaerobes in the MALDI-TOF and 16S rRNA gene sequencing era. Anaerobe 17:106–112. doi: 10.1016/j.anaerobe.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Ny P, Ozaki A, Pallares J, Nieberg P, Wong-Beringer A. 2019. Antimicrobial stewardship opportunities in patients with bacteremia not identified by BioFire FilmArray. J Clin Microbiol 57:e01941-18. doi: 10.1128/JCM.01941-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller-Premru M, Jeverica S, Papst L, Nagy E. 2017. Performance of two blood culture systems to detect anaerobic bacteria. Is there any difference? Anaerobe 45:59–64. doi: 10.1016/j.anaerobe.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Gajdács M, Ábrók M, Lázár A, Terhes G, Urbán E. 2020. Anaerobic blood culture positivity at a University Hospital in Hungary: a 5-year comparative retrospective study. Anaerobe 63:102200. doi: 10.1016/j.anaerobe.2020.102200. [DOI] [PubMed] [Google Scholar]
- 13.Cortazzo V, D’Inzeo T, Giordano L, Menchinelli G, Liotti FM, Fiori B, De Maio F, Luzzaro F, Sanguinetti M, Posteraro B, Spanu T. 2021. Comparing BioFire FilmArray BCID2 and BCID panels for direct detection of bacterial pathogens and antimicrobial resistance genes from positive blood cultures. J Clin Microbiol 59:e03163-20. doi: 10.1128/JCM.03163-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download JCM.00521-21-s0001.pdf, PDF file, 244 KB (243.7KB, pdf)