Abstract
Importance:
Patients with cancer are at risk for unplanned hospitalizations during treatment which can increase the cost of care.
Objectives:
To determine demographic and clinical factors associated with healthcare utilization and costs among clinical trial participants.
Design, Setting and Patients:
We conducted a retrospective analysis among breast cancer patients over the age of 65 treated on SWOG clinical trials from 1999–2011 with trial data linked to Medicare claims.
Main Outcomes and Measures:
The outcomes were healthcare utilization (emergency room visits (ER), hospitalizations) and costs from Medicare Claims. Demographic, clinical and prognostic factors were captured from clinical trial records. We identified cardiovascular comorbidities/risk factors (CVD-RFs) of diabetes, hypertension, hypercholesterolemia, and coronary artery disease (CAD) from Medicare claims. Multivariable logistic and linear regression were used to assess the association between CVD-RFs and outcomes.
Results:
Among the 708 patients included in the analysis, 160 (22.6%) experienced 234 separate hospitalizations, and 193 (27.3%) experienced 311 separate ER visits. Black race was associated with an increase in hospitalizations (OR[95% CI], 2.52 [1.10–5.79], p=.03), but not emergency room visits compared to white race. Diabetes, hypertension, hypercholesterolemia, and CAD were all independently associated with increased risk of both hospitalizations and ER visit. Hypertension had the strongest association, with more than a threefold risk of hospitalization for those with hypertension compared to those without (OR[95% CI], 3.16[1.85–5.40], p<0.001). For those with ≥3 RFs, the risk of hospitalization was nearly 3 times greater compared to 0 or 1 CVD-RFs (OR[95% CI], 2.74 [1.71–4.38], p<0.001). Similar results were seen for ER visits. In the first 12 months after trial registration, patients with diabetes ($38,324 vs $30,923, 23.9% increase, p=0.05), hypercholesterolemia ($34,168 vs $30,661, 11.4% increase, p=0.02), and CAD ($37,781 vs $31,698, 19.2% increase, p=0.04) had statistically significantly higher total healthcare costs. Additionally, those with 2 significant CVD-RFs ($35,353 vs. $28,899, 22.3% increase, p=.005) had statistically significantly higher total healthcare costs.
Conclusions:
Among participants treated on clinical trials, black race and presence of multiple cardiovascular comorbidities was associated with a substantial increase in ER visits, hospitalizations and healthcare costs. Efforts to reduce unplanned hospitalizations should focus on this high-risk group.
INTRODUCTION
Breast cancer (BC) is a serious disease associated with substantial medical and economic burden. High medical costs place a considerable burden on patients and society. Most studies have shown the largest drivers of costs in the 12 months after diagnosis are related to differences in chemotherapy costs1, however less is known about factors associated with other drivers of healthcare cost, such as emergency room (ER) visits and hospitalizations. Reduction of unplanned acute care is a major priority for clinical practice change in oncology and many new models of care, such as the oncology care model, focus on reducing unplanned acute care.2 Much research effort has focused on end of life care, however little work has looked at patients earlier in their disease.
Elderly patients with comorbid conditions may be at a particularly high risk for unplanned acute care during therapy. Observational studies have reported that the number and severity of toxicities during treatment are associated with increased age, history of comorbid conditions and race.3 In a cross sectional survey of women with early stage BC, only 9% had an unplanned office visit and only 5% had an ER visit or hospitalization for a treatment related toxicity,3 However, among low-income BC patients treated in North Carolina, 19% had at least 1 in-patient admission and 25% had at least one ER visit for an AE in the 15 months following diagnosis4. Comorbidity was one of the strongest factors associated with unplanned admissions and ER visits.4 These previous studies could not account for dose or other treatment selection criteria.
We have previously shown that comorbidities, and specifically the presence of CVD-RFs, are associated with worse overall and progression-free survival5 among women with BC treated on clinical trials. In this study, we assessed the relationship between CVD-RFs and healthcare utilization, specifically ER visits and hospitalizations. Patients on clinical trials are often followed closely, and one may expect lower rates of unplanned healthcare utilization as a result. We also examined the impact of CVD-RFs on cost of care during the first 12 months of treatment among patients age 66 or older enrolled in a series of NCI-sponsored adjuvant and advanced BC clinical treatment trials.
METHODS
We obtained data from the SWOG Cancer Research Network, a member of the National Cancer Institute’s (NCI’s) Clinical Trials Network. We examined the SWOG database to identify patients registered to phase II/III BC trials between 1999 and 2011, the period during which Medicare claims data were available for linkage to clinical records. We identified five eligible trials: S0012, S0221, S0226, S0307, and S0500 (Supplemental Table 1).6–9 Clinical records from these studies were linked to Medicare claims data according to social security number, sex, and date of birth. To be included in the current analysis, patients were required to be aged at least 66 years at baseline and to have at least 12 months’ Medicare Parts A & B coverage immediately prior to registration and 12 months after registration (including the active study period), and simultaneously have had no HMO coverage. Seventeen participants took part in more than one included trial; the trial with the earlier registration date was retained.
Demographic variables including age (66–69 (reference group) vs. 70–74 vs. 75+ years), sex, self-reported race and ethnicity, and baseline body mass index (BMI) were obtained from questionnaires administered at the time of enrollment. Planned use of a treatment with potentially high impact on utilization outcomes was defined as assignment to anthracycline-based treatment (trials S0012 or S0221; Supplemental Table 1). Potential differences in prognostic risk for recurrence/death across the panel of different studies were accounted for using a prognostic risk score, defined as the sum of the number of adverse risk factors from among the prognostic variables used for randomization stratification in each study (Supplemental Table 3). Comorbid conditions were identified using ICD9 codes from Medicare claims data and included diabetes (with and without chronic complications), hypertension, hypercholesterolemia, and coronary artery disease or ischemic heart disease (CAD). Baseline obesity was defined as BMI≥30 using trial records; post-baseline obesity status was determined using ICD9 codes. Cardiac events (heart failure, CAD) were identified by ICD9, HCPCS, and Surgery codes (Supplemental Table 2). Conditions and events were identified as any hospital claim, or ≥2 physician or outpatient claims at least 30 days apart.
OUTCOMES:
Hospital Stays and Emergency Room Visits
Hospital stays were defined using the MedPAR file, by specifying NCH claims codes 60–64, 71, or 82, or, if NCH claims code was missing, by specifying Skilled Nursing Facility indicator was not missing and was not “N” (“N” indicates SNF). Hospital stays with an admission date occurring within one year after registration were included. All hospital stays occurring in this time frame were included, regardless of their attribution, with potentially multiple observations per person. Hospital stays with different MedPAR ID were considered unique, even if dates of stay between two hospitalizations were overlapping.
ER visits were identified using two data sources: (1) Outpatient revenue center data, using Revenue Center codes 0450–0459 and 0981. This file was merged with the outpatient base claims file, using Claim ID, to identify accompanying ICD9 diagnosis codes; and (2) the MedPAR file, when the ER Charge Amount field was non-missing and non-zero. As with hospital stays, all ER visits occurring within one year after registration were included, regardless of their attribution, with potentially multiple observations per person.
Healthcare Utilization Costs
To analyze healthcare costs as an outcome, claims cost data were compiled from MedPAR, Home Health Agency, outpatient, carrier, hospice, and DME databases. Overall costs were examined within the first 12 months after registration. Claims were coded, using ICD9 codes, for the above risk factors and events (Supplemental Table 2), and CVD- and cardiac event-specific costs were compiled separately as a secondary outcome using the cost associated code for each event. Costs were inflated to constant 2017 US dollars based on the Personal Consumption Expenditure (PCE) price index for health care services.10
STATISTICAL METHODS:
To assess the potential for bias introduced by exclusion criteria, baseline participant characteristics were compared between those included in this analysis, and those aged 66+ from the same studies that were not included, using t-tests for continuous variables and chi-square tests for categorical variables.
Logistic regression was used to examine healthcare utilization outcomes. Analyses were conducted separately for both utilization endpoints, hospital stay (>=1 vs. 0) or ER visit (>=1 vs. 0). Each of the five CVD-RFs, as well as baseline demographic and clinical factors, were considered as the primary independent variable of interest in separate analyses. Both univariate and multivariable analyses were performed. Multivariable regression analyses included covariates for age (continuous), race (black vs non-black), and baseline prognostic risk. All models, both multivariable and unadjusted, were stratified by study and treatment arm.
In secondary analyses, to more broadly adjust for the impact of treatment, we stratified by anthracycline vs. non-anthracycline-based planned treatment and treatment arm, rather than study and arm. We also examined the relationship between each of the five CVD risk factors and each of the baseline demographic and clinical factors with utilization outcomes in the subset of patients receiving adjuvant therapy (S0012, S0221, S0307) and advanced therapy (S0226, S0500). Interaction p-values were calculated to test whether the relationship between each predictor variable and utilization outcomes differed between adjuvant and advanced patient groups.
Mean values of health utilization costs are presented, separately by baseline cardiovascular risk factors. P-values were calculated using linear regression, adjusted for age, race, baseline prognostic risk score, and SWOG study. Alpha=.05 was considered statistically significant.
RESULTS
Participant characteristics are shown in Table 1. Of the 1,460 eligible subjects, 48% (n=708) were linked to their Medicare claims. Linked patients were less likely to be black, more likely to be registered prior to 2004, and more likely to have a lower prognostic risk score, than those patients that were not linked. Among the patients included in the analysis, the median age was 70 years, 6.1% were minority, 2.0% were Hispanic, and 72.3% had low prognostic risk.
Table 1:
Patient characteristics
| Linked patients (included in analysis) | Patients not linked (not included in analysis) | p-value | |||
|---|---|---|---|---|---|
| Characteristic | (N=708) | (N=752) | |||
| Age (median, range) | 70 | 66–91 | 70 | 66–92 | 0.99 |
| Race | 0.01 | ||||
| Asian/Pacific Islander | 12 | 1.7% | 16 | 2.1% | |
| Black | 30 | 4.2% | 53 | 7.0% | |
| Native | 1 | 0.1% | 9 | 1.2% | |
| Unknown | 6 | 0.8% | 10 | 1.3% | |
| White | 659 | 93.1% | 664 | 88.3% | |
| Ethnicity | 0.08 | ||||
| Not Hispanic | 694 | 98.0% | 726 | 96.5% | |
| Hispanic | 14 | 2.0% | 26 | 3.5% | |
| Time of initial registration | 0.008 | ||||
| Before 20041 | 13 | 1.8% | 3 | 0.4% | |
| 2004 or later | 695 | 98.2% | 749 | 99.6% | |
| Baseline prognostic risk score2 | 0.0009 | ||||
| Low | 512 | 72.3% | 494 | 65.7% | |
| High | 179 | 25.3% | 250 | 33.2% | |
| Missing | 17 | 2.4% | 8 | 1.1% | |
| Study Type | 0.08 | ||||
| Adjuvant (S0012, S0221, S0307) | 502 | 70.9% | 501 | 66.6% | |
| Advanced (S0226, S0500) | 206 | 29.1% | 251 | 33.4% | |
| Baseline Cardiovascular Conditions | |||||
| Diabetes with or without complications | 172 | 24% | |||
| Hypertension | 519 | 73% | |||
| Hypercholesterolemia | 414 | 58% | |||
| CAD | 125 | 18% | |||
| Obesity3 | 227 | 32% | |||
| Number of baseline cardiovascular conditions | |||||
| None | 93 | 13% | |||
| One | 163 | 23% | |||
| Two | 186 | 26% | |||
| Three | 159 | 22% | |||
| Four | 90 | 13% | |||
| Five | 17 | 2% | |||
| Number of Hospital stays | |||||
| None | 548 | 77% | |||
| One | 111 | 16% | |||
| Two | 35 | 5% | |||
| Three or more | 14 | 2% | |||
| Number of ER visits | |||||
| None | 515 | 73% | |||
| One | 133 | 19% | |||
| Two | 35 | 5% | |||
| Three or more | 25 | 3% | |||
Median of the range.
Baseline prognostic risk score is defined as the sum of the number of adverse risk factors from among the prognostic variables used for randomization stratification in each study. See Supplemental Table 3 for details.
Obesity is defined using both Medicare claims and clinical records, and is defined as BMI ≥30 kg/m2.
Healthcare Utilization Outcomes
Out of 708 participants, 160 experienced hospital stays, with 49 experiencing more than one stay, representing 234 separate hospital stays. Of these hospital stays, 124 were coded with one of the CVD RFs, and 13 were coded with a cardiac event code. One hundred ninety-three participants experienced ER visits, with 60 experiencing more than one visit, representing 311 separate ER visits. Of these, 112 were coded with a CVD risk factor, and 6 were coded with a cardiac event code.
Association of Baseline Clinical, Treatment, and Demographic Factors and Utilization
In a multivariable analysis, black race was associated with an increased risk of hospitalizations (2.7% vs 9.4%, OR 2.52 (1.10–5.79), p=0.03). CAD was associated with black race, but no other baseline CVD-RFs. When adjusted for CAD, the association between black race and hospitalizations remained statistically significant (OR 2.34 (1.00–5.44), p=0.05). No association was found between black race and ER visits. Planned use of an anthracycline-based regimen was associated with notably higher risk of both hospital stays (OR=3.17, 95%CI: 1.95–5.15, p<0.001) and ER visits (OR=1.98, 95% CI: 1.24–3.17, p=.004). No other demographic or clinical characteristics were associated with either ER visits or hospital stays (Table 2).
Table 2:
Baseline Demographic, Treatment, and Clinical Factors and Risk of Hospital Stay and Emergency Room Visit
| Hospital Stay or ER Visit | Adjusted1 | Unadjusted2 | ||||
|---|---|---|---|---|---|---|
| No, N (%) | Yes, N (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Hospital Stays | ||||||
| Age | ||||||
| 66–69 | 233 (43%) | 75 (47%) | Reference | Reference | ||
| 70–74 | 176 (32%) | 46 (29%) | 0.81 (0.52–1.25) | 0.35 | 0.85 (0.56–1.29) | 0.44 |
| 75+ | 139 (25%) | 39 (24%) | 0.89 (0.56–1.40) | .60 | 0.88 (0.57–1.38) | 0.59 |
| Black race | 15 (2.7%) | 15 (9.4%) | 2.52 (1.10–5.79) | 0.03 | 2.98 (1.34–6.61) | 0.007 |
| Hispanic | 12 (2.2%) | 2 (1.3%) | 0.39 (0.08–1.99) | 0.26 | 0.43 (0.09–2.04) | 0.29 |
| High baseline prognostic risk score | 130 (24.2%) | 49 (32.0%) | 1.04 (0.67–1.62) | 0.85 | 1.05 (0.68–1.62) | 0.83 |
| Advanced disease studies | 157 (28.6%) | 49 (30.6%) | 1.09 (0.71–1.66) | 0.70 | 1.11 (0.75–1.65) | 0.61 |
| HER2 positive vs negative/equivocal/unknown | 62 (11.9%) | 24 (15.7%) | 1.06 (0.60–1.89) | 0.83 | 1.21 (0.70–2.08) | 0.50 |
| ER positive vs negative/unknown | 472 (86.3%) | 127 (79.9%) | 1.12 (0.64–1.98) | 0.68 | 0.96 (0.57–1.63) | 0.89 |
| Planned anthracycline-based therapy | 64 (11.7%) | 51 (31.9%) | 3.17 (1.95–5.15) | <.001 | 3.59 (2.27–5.65) | <.001 |
| Emergency Room Visits | ||||||
| Age | ||||||
| 65–69 | 223 (43%) | 85 (44%) | Reference | Reference | ||
| 70–74 | 164 (32%) | 58 (30%) | 0.96 (0.64–1.44) | 0.85 | 0.96 (0.65–1.42) | 0.84 |
| 75+ | 128 (25%) | 50 (26%) | 1.42 (0.64–2.01) | 0.89 | 1.04 (0.68–1.57) | 0.87 |
| Black race | 20 (3.9%) | 10 (5.2%) | 1.06 (0.45–2.49) | 0.90 | 0.96 (0.41–2.22) | 0.92 |
| Hispanic | 11 (2.1%) | 3 (1.6%) | 0.73 (0.20–2.69) | 0.63 | 0.71 (0.19–2.62) | 0.61 |
| High baseline prognostic risk score | 121 (24.1%) | 58 (30.9%) | 1.16 (0.77–1.73) | 0.48 | 1.16 (0.77–1.73) | 0.47 |
| Advanced disease studies | 146 (28.3%) | 60 (31.1%) | 1.06 (0.71–1.57) | 0.78 | 1.16 (0.80–1.68) | 0.44 |
| HER2 positive vs negative/equivocal/unknown | 62 (12.5%) | 24 (13.3%) | 0.91 (0.53–1.56) | 0.73 | 0.96 (0.57–1.63) | 0.89 |
| ER positive vs negative/unknown | 435 (84.6%) | 164 (85.4%) | 1.46 (0.85–2.49) | 0.17 | 1.45 (0.86–2.44) | 0.16 |
| Planned anthracycline-based therapy | 70 (13.6%) | 45 (23.3%) | 1.98 (1.24–3.17) | 0.004 | 1.85 (1.18–2.90) | 0.007 |
Odds ratios, 95% CI, and p-values are calculated using logistic regression and are adjusted for age (continuous), race (black vs non-black), and baseline prognostic risk (low vs hi), and stratified by SWOG study and SWOG treatment arm
Odds ratios, 95% CI, and p-values are stratified by SWOG study and SWOG treatment arm
Association of CVD-RFs and Hospital Stays
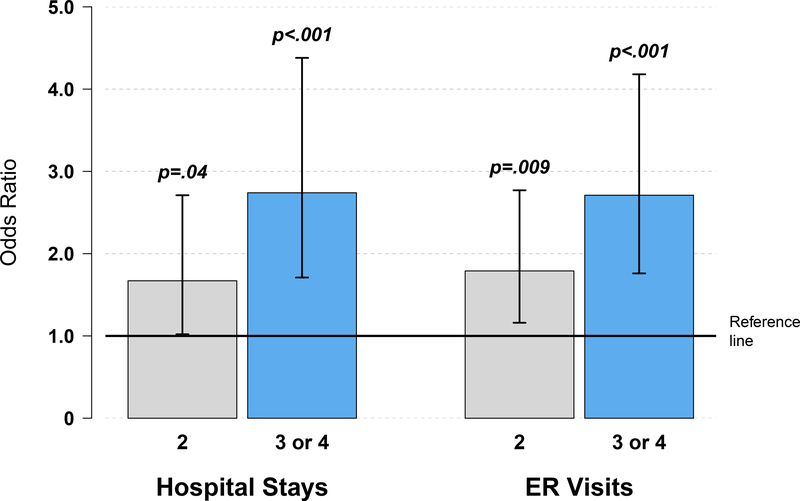
Diabetes, hypertension, hypercholesterolemia, and coronary artery disease were all associated with increased risk of hospital stays (Table 3). Hypertension had the strongest association, with a more than threefold risk of hospital stays for those with hypertension compared to those without (OR [95% CI], 3.16 [1.85–5.40], p<0.001). Additionally, the risk of a hospital stay increased with the number of baseline CVD-RFs (Figure 1). For those with >2 CVD-RFs listed above, the risk of hospital stay was nearly 3 times the risk for those none or one risk factors (OR [95% CI], 2.74 [1.71–4.38], p<0.001). Estimates of the association of the number of CVD-RFs and hospital stays were similar when stratifying by potential impact of planned treatment (anthracycline vs. non-anthracycline-based) rather than study (data not shown).
Table 3:
Baseline Cardiovascular Risk Factors and Risk of Hospital Stay and Emergency Room Visit
| Hospital Stay or ER Visit | Adjusted1 | Unadjusted2 | ||||
|---|---|---|---|---|---|---|
| CVD Risk Factor | No, N (%) | Yes, N (%) | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Hospital Stays | ||||||
| Diabetes with or without complications | 117 (21.4%) | 55 (34.4%) | 1.77 (1.17–2.66) | 0.007 | 1.81 (1.21–2.71) | 0.004 |
| Hypertension | 381 (69.5%) | 138 (86.3%) | 3.16 (1.85–5.40) | <0.001 | 2.96 (1.78–4.92) | <0.001 |
| Hypercholesterolemia | 312 (56.9%) | 102 (63.8%) | 1.58 (1.05–2.38) | 0.03 | 1.47 (1.00–2.17) | 0.05 |
| CAD | 86 (15.7%) | 39 (24.4%) | 1.83 (1.16–2.89) | 0.01 | 1.70 (1.08–2.66) | 0.02 |
| Obesity | 165 (30.1%) | 62 (38.8%) | 1.31 (0.85–2.04) | 0.22 | 1.37 (0.90–2.11) | 0.15 |
| Number of risk factors3 | ||||||
| None or One | 251 (45.8%) | 49 (30.6%) | Ref | Ref | ||
| Two | 168 (30.7%) | 47 (29.4%) | 1.67 (1.02–2.71) | 0.04 | 1.60 (1.01–2.56) | 0.05 |
| Three or Four | 129 (23.5%) | 64 (40.0%) | 2.74 (1.71–4.38) | <0.001 | 2.54 (1.62–4.00) | <0.001 |
| Risk Factors, 2 or more3 | 297 (54.2%) | 111 (69.4%) | 2.15 (1.42–3.25) | <0.001 | 2.03 (1.37–3.01) | <0.001 |
| Per additional risk factor | 1.46 (1.23–1.73) | <0.001 | 1.42 (1.21–1.67) | <0.001 | ||
| Emergency Room Visits | ||||||
| Diabetes with or without complications | 105 (20.4%) | 67 (34.7%) | 1.99 (1.37–2.91) | <0.001 | 2.00 (1.38–2.91) | <0.001 |
| Hypertension | 352 (68.3%) | 167 (86.5%) | 3.35 (2.06–5.45) | <0.001 | 3.09 (1.95–4.90) | <0.001 |
| Hypercholesterolemia | 288 (55.9%) | 126 (65.3%) | 1.69 (1.17–2.45) | 0.005 | 1.57 (1.10–2.24) | 0.01 |
| CAD | 77 (15.0%) | 48 (24.9%) | 1.85 (1.21–2.83) | 0.005 | 1.85 (1.22–2.81) | 0.004 |
| Obesity | 158 (30.7%) | 69 (35.8%) | 1.24 (0.83–1.84) | 0.30 | 1.27 (0.86–1.87) | 0.24 |
| Number of risk factors3 | ||||||
| None or One | 240 (46.6%) | 60 (31.1%) | Ref | Ref | ||
| Two | 156 (30.3%) | 59 (30.6%) | 1.79 (1.16–2.77) | 0.009 | 1.64 (1.08–2.50) | 0.02 |
| Three or Four | 119 (23.1%) | 74 (38.3%) | 2.71 (1.76–4.18) | <0.001 | 2.53 (1.67–3.83) | <0.001 |
| Risk Factors, 2 or more3 | 275 (53.4%) | 133 (68.9%) | 2.20 (1.51–3.20) | <0.001 | 2.03 (1.42–2.92) | <0.001 |
| Per additional risk factor | 1.54 (1.31–1.80) | <0.001 | 1.49 (1.28–1.74) | <0.001 | ||
Odds ratios, 95% CI, and p-values are calculated using logistic regression and are adjusted for age (continuous), race (black vs non-black), and baseline prognostic risk (low vs hi), and stratified by SWOG study and SWOG treatment arm
Odds ratios, 95% CI, and p-values are stratified by SWOG study and SWOG treatment arm
Number of risk factors includes diabetes with or without complications, hypertension, hypercholesterolemia, and CAD
Figure 1:
Association of number of cardiovascular disease risk factors and hospital stays and emergency room (ER) visits.
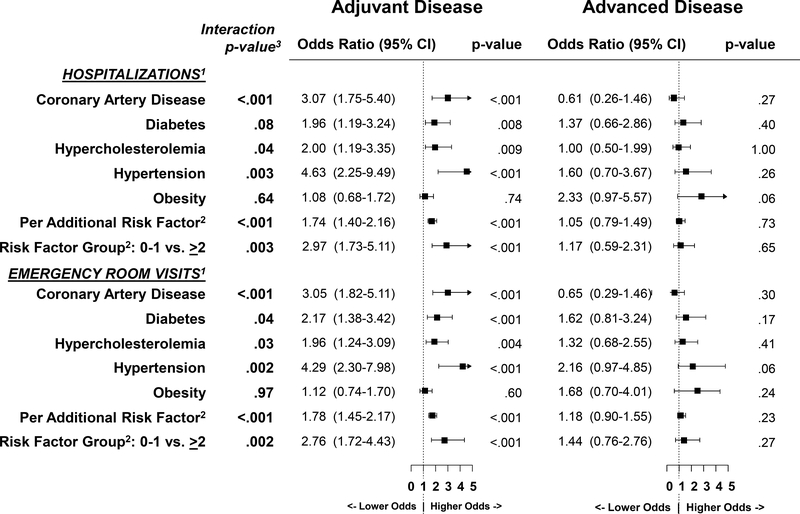
As indicated in Figure 2, the risk of hospitalization was statistically significantly greater for patients with non-metastatic disease compared to advanced disease with respect to coronary artery disease, hypercholesterolemia, hypertension, and for multiple risk factors. In fact, among advanced disease patients, there was no statistically significantly different risk of hospitalization for any CVD risk condition.
Figure 2:
Forest plot of the association of cardiovascular disease risk factors and utilization by adjuvant versus advanced disease. The boxes show the odds ratio and the horizontal lines show the 95% confidence intervals. The vertical lines shows the line of equal odds.
Footnotes:
1 – Odds ratios, 95% CI, and p-values are calculated using logistic regression and are adjusted for age (continuous), race (black vs non-black), and baseline prognostic risk (low vs hi), and stratified by SWOG study and SWOG treatment arm
2 – Odds ratios, 95% CI, and p-values are stratified by SWOG study and SWOG treatment arm
3 – Number of risk factors includes diabetes with or without complications, hypertension, hypercholesterolemia, and CAD
4 – p-value tests interaction between study type (adjuvant vs advanced) and CVD risk factor
Association of CVD-RFs and Emergency Room Visits
All baseline CVD-RFs except obesity were associated with ER visits, and hypertension again had the strongest association, with a more than threefold risk of ER visits for those with hypertension versus those without (OR [95% CI], 3.35 [2.06–5.45], p<0.001) (Table 3). The risk of ER visits increased as the number of concurrent risk factors increased (Figure 1). Those with >2 CVD-RFs had nearly 3 times the risk of ER visits compared to those with none or one of the significant risk factors (OR [95% CI], 2.71 [1.76–4.18], p<0.001). Estimates of the association of the number of CVD-RFs and ER visits were similar when stratifying by potential impact of planned treatment (anthracycline vs. non-anthracycline-based) rather than study (data not shown).
The risk of emergency room visits was statistically significantly greater for patients with adjuvant disease compared to advanced disease with respect to all CVD RFs except obesity (Figure 2). Within the subset of advanced disease patients, there was no statistically significantly different risk of ER visit for any individual CVD risk condition.
Association of CVD-RFs and Healthcare Utilization Costs
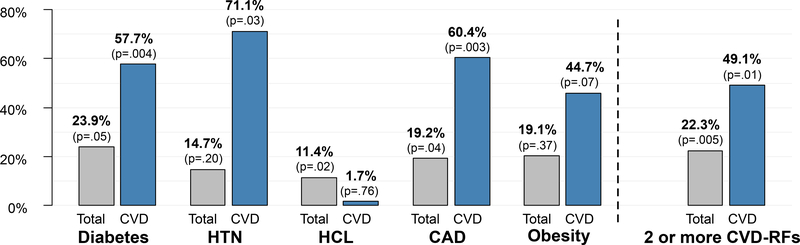
In the first 12 months after trial registration, patients with diabetes ($38,325 vs $30,923, 23.9% increase, p=0.05), hypercholesterolemia ($34,168 vs $30,661, 11.4% increase, p=0.02), CAD ($37,781 vs $31,698, 19.2% increase, p=0.04), and 2 or more CVD-RFs ($35,353 vs. $28,899, 22.3% increase, p=.005) had statistically significantly higher total healthcare costs than those without (Figure 3; Supplemental Table 4). Total healthcare costs related to CVD-RFs were higher for patients with diabetes ($7,691 vs. $4,876, 57.7% increase, p=.004), hypertension ($6,056 vs. $3,540, 71.1% increase, p=.03), CAD ($8,115 vs. $5,059, 60.4% increase, p=.003), and 2 or more CVD-RFs ($6,340 vs. $4,251, 49.1% increase, p=.01).
Figure 3:
Percent increase in total and cardiovascular-disease related costs by individual cardiovascular disease risk factor and number of risk factors
Both payer (i.e. Medicare) and patient-related total costs trended higher for those with vs. without individual CVD-RFs. Mean total costs for both the payer (24.8% increase, p=.005) and the patient (22.7% increase, p=.006) were significantly greater for those with 2 or more CVD-RFs.
DISCUSSION
In this population of elderly patients who met the strict enrollment criteria allowing participation in a therapeutic clinical trial, 64% had 2 or more CVD-RFs at baseline. Black race and the presence of CVD-RFs were associated with an increased risk of hospitalizations and ER visits; in fact, patients with 2 or more CVD-RFs had nearly a 3-fold increased risk. The findings are primarily limited to patients enrolled in trials that included non-metastatic patients. Also, of concern, having 2 or more CVD-RF was associated with a 24.8% increase in cost to the payer and a 22.7% increase in out-of-pocket costs to the patient.
Most clinical trials report toxicities, but few report rates of hospitalizations or ER visits during and following completion of treatment, so we could not compare our results to other clinical trials. However, there are publications evaluating rates of hospitalizations and ER visits among patients treated in the community who receive and do not receive chemotherapy. Two population-based studies reported significantly higher rates of ER visits and/or hospitalizations among patients treated with chemotherapy. The first used data from Marketscan data and found higher rates of ER visits (21% vs 15%) and higher rates of hospitalizations (52% vs. 33%) among those who received chemotherapy.11 A study conducted in Ontario compared BC patients in the community who received adjuvant chemotherapy to a non-cancer cohort matched on age, comorbidity and geographic location. ER visits and hospitalizations were found among 43% in the chemotherapy group compared to 9.4% in the control group. In a multivariable analysis, comorbidity, type of chemotherapy, and geographic region were associated with increased odds of hospitalizations and ER visits.12 Compared to patients with a Charlson comorbidity score of 0, patients with a comorbidity score of 2 had a 2-fold increased risk of hospitalizations and ER visits.12 In addition to hospitalizations and ER visits, elderly women undergoing chemotherapy are at increased risk for functional adverse events, which can also be associated with morbidity and increased costs. A SEER-Medicare analysis of early stage BC reported an association of chemotherapy with increased number of claims for skilled nursing facilities and durable medical equipment.13 None of these studies evaluated the association between cardiovascular risk factors and healthcare utilization among those who were selected to receive chemotherapy. Prior observational studies included patients with variable performance status. Our study reports on patients healthy enough to enroll in clinical trials, and yet the risks still remain high.
While the number of black women in our sample was relatively small (4%), we found that in an adjusted analysis, black race was associated with more than a 2-fold increased risk of hospitalizations. These results persisted despite controlling for CVD-RF’s. This is of particular concern as minorities have a higher comorbidity burden, and a higher risk for cardiovascular disease. Furthermore, minorities are often an economically disadvantaged group. This is troubling given the increased financial toxicity (out of pocket costs) associated with an unplanned hospitalization in this vulnerable population.
We have previously shown that the presence of each additional CVD-RF was associated with an increased risk of subsequent cardiac event in patients with BC enrolled in clinical trials.5 We have also shown that women with CVD-RFs who are adherent to their oral medications to control chronic conditions are more likely to become non-adherent to these medications following diagnosis.14 Of concern, non-adherence to CVD medications increases the risk of CVD events following treatment.15 The hospitalizations observed in this study were mostly attributable to CVD events, and therefore it is possible that more intensive management may prevent these complications.
The rising cost of cancer care is a major public health issue, and with newer, more targeted therapies, the costs are likely to increase. Decreasing unplanned hospitalizations provides an opportunity to decrease costs. Several studies have evaluated ways to decrease unplanned hospitalizations. In a randomized trial evaluating remote electronic patient-reported symptom monitoring vs. usual care in advanced cancer patients, the patients randomized to the intervention had fewer ER visits, fewer hospitalizations, and improved overall survival.16 The results suggest that increased communication and intensive symptom management can reduce healthcare utilization and cost.
There are several strengths to our study. Participants were prospectively enrolled and baseline data was collected on all patients. For each study, subjects were required to adhere to uniform protocol-specific therapy. A major limitation of prior studies is an inability to assess the influence of chemotherapy treatment or pre-planned dose-reductions. All of the patients in this cohort were treated uniformly. Uniform access to protocol therapy also limits the confounding influence of access to cancer care.
We also acknowledge several limitations. Patients were required to be enrolled in Medicare to be included in this study, so all analyzed patients were over age 66; given that elderly patients are often underrepresented in clinical trials, selection bias may limit the generalizability of the results. We used ICD9 codes to identify patients with CVD-RFs but there are circumstances in which patients’ conditions may not be properly codified and thus subject to misclassification bias. We were also unable to collect other CVD-RFs such as smoking and family history. All five SWOG studies mandated a Zubrod score of 0 to 2, specifying that patients needed to be at least ambulatory and capable of self-care, as part of the inclusion criteria, so patients with severe complications of their comorbidities and who may have been at higher cardiovascular event risk may not have been captured, also potentially limiting the generalizability of our results. The potential influence of on-study adverse events – especially cardiac-related events – on patterns of utilization outcomes fell outside the scope of our analysis, but future research is planned. In order to treat high-impact treatments as a predictor variable, we used planned – rather than actual – use of anthracycline-based treatments as a predictor variable; thus this analysis make not reflect actual use of assigned treatment. Because these are trial patients, the observed associations may not be generalizable to the non-trial treatment population. Finally, the reason for the hospitalization or ER visit were not available, so it is not possible to know how many could have been avoided.
In conclusion, our study demonstrates that the presence of CVD-RFs as well as ER visits and hospitalizations are frequent among elderly BC patients treated on clinical trials. The risk of both ER visits and hospitalizations is higher among black patients and patients with CVD risk factors. Because increased unplanned hospitalizations are associated with increased costs to both providers and patients, this represents a significant burden. Prospective studies should evaluate if aggressive management of cardiovascular risk factors and aggressive symptom management reduce unplanned hospitalizations and ER visits.
Supplementary Material
Acknowledgments
Dr. Hershman is the recipient of a NCORP Research Base grant 1UG1CA189974-01. Dr. Unger is a recipient of a grant from the HOPE Foundation. Dr. Hershman is the recipient of grants from the Conquer Cancer Foundation, the Breast Cancer Research Foundation, and the Susan G. Komen Research Foundation.
Contributor Information
Dawn L. Hershman, Columbia University Medical Center, New York, NY
Cathee Till, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Research Center, Seattle, WA.
Jason D. Wright, Columbia University Medical Center, New York, NY
Melissa Accordino, Columbia University Medical Center, New York, NY.
Riha Vaidya, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Research Center, Seattle, WA.
William E. Barlow, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Research Center, Seattle, WA
Scott Ramsey, Fred Hutchinson Cancer Research Center, Seattle, WA.
Joseph M. Unger, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Research Center, Seattle, WA
REFERENCES
- 1.Blumen H, Fitch K, Polkus V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am Health Drug Benefits. 2016;9(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 2.Handley NR, Schuchter LM, Bekelman JE. Best Practices for Reducing Unplanned Acute Care for Patients With Cancer. J Oncol Pract. 2018;14(5):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friese CR, Harrison JM, Janz NK, et al. Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer. 2017;123(11):1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal RK, Wheeler SB, Kohler RE, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231–238. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Till C, Shen S, et al. Association of Cardiovascular Risk Factors With Cardiac Events and Survival Outcomes Among Patients With Breast Cancer Enrolled in SWOG Clinical Trials. J Clin Oncol. 2018:JCO2017774414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis GK, Barlow WE, Gralow JR, et al. Phase III comparison of standard doxorubicin and cyclophosphamide versus weekly doxorubicin and daily oral cyclophosphamide plus granulocyte colony-stimulating factor as neoadjuvant therapy for inflammatory and locally advanced breast cancer: SWOG 0012. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(8):1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budd GT, Barlow WE, Moore HC, et al. SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. The New England journal of medicine. 2012;367(5):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32(31):3483–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Bureau of Economic Analysis, Personal consumption expenditures: Services: Health care (chain-type price index) [DHLCRG3A086NBEA], retrieved from FRED, Federal Reserve Bank of St. Louis; https://fred.stlouisfed.org/series/DHLCRG3A086NBEA, January 16, 2019.. [Google Scholar]
- 11.Hassett MJ, O’Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. Journal of the National Cancer Institute. 2006;98(16):1108–1117. [DOI] [PubMed] [Google Scholar]
- 12.Enright K, Grunfeld E, Yun L, et al. Population-based assessment of emergency room visits and hospitalizations among women receiving adjuvant chemotherapy for early breast cancer. J Oncol Pract. 2015;11(2):126–132. [DOI] [PubMed] [Google Scholar]
- 13.Mariano C, Lund JL, Peacock Hinton S, Htoo P, Muss H, Reeder-Hayes KE. Evaluating the association between adjuvant chemotherapy and function-related adverse events among older patients with early stage breast cancer. J Geriatr Oncol. 2017;8(4):242–248. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Neugut AI, Wright JD, Accordino M, Hershman DL. Nonadherence to Oral Medications for Chronic Conditions in Breast Cancer Survivors. J Oncol Pract. 2016;12(8):e800–809. [DOI] [PubMed] [Google Scholar]
- 15.Hershman D, Accordino M, Shen S, et al. Association Between Non-Adherence to Cardiovascular Medications Following Breast Cancer Diagnosis and Cardiovascular Events. Paper presented at: San Antonio Breast Confeence2018; San Antonio, TX. [Google Scholar]
- 16.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.