Abstract
This study describes the clinical, diagnostic, and pathological characteristics of canine nasal polyps and how they responded to medical, endoscopic, and surgical treatments. The database of a multi-center veterinary endoscopy group was searched from 2010 to 2018. All dogs with a histological diagnosis of nasal polyposis that were undergoing endoscopic investigation (N = 23), were included. Clinical signs at presentation were sneezing (91%), nasal discharge (83%), stertor (74%), and frontonasal deformation (17%). Skull radiography on 13 dogs had alterations in 77% of cases, including turbinate lysis (6/13), increased radiopacity of one (4/13) or both (6/13) nasal cavities, and lysis of the nasal vomer bone (3/13). Nasal polyposis had a characteristic endoscopic appearance. There were clinical and diagnostic similarities between this cohort of dogs and dogs with nasal neoplasia, although dogs with nasal polyps were often younger and polypoid tissue was external to the nose. Steroid therapy alone was not effective in treating polyposis in dogs; however, endoscopic debulking with a laser and forceps was more effective.
Résumé
Diagnostic et issue de la polypose nasale chez 23 chiens traités médicalement ou par débridement endoscopique. Cette étude décrit les caractéristiques cliniques, diagnostiques et pathologiques des polypes nasaux canins et comment ils ont répondu aux traitements médicaux, endoscopiques et chirurgicaux. La base de données d’un groupe multicentres d’endoscopie vétérinaire a été recherchée de 2010 à 2018. Tous les chiens avec un diagnostic histologique de polypose nasale qui faisaient l’objet d’une investigation endoscopique (N = 23), ont été inclus. Les signes cliniques à la présentation étaient des éternuements (91 %), un écoulement nasal (83 %), un stertor (74 %) et une déformation fronto-nasale (17 %). La radiographie du crâne de 13 chiens présentait des altérations dans 77 % des cas, y compris une lyse du cornet (6/13), une radio-opacité accrue d’une (4/13) ou des deux (6/13) cavités nasales et une lyse du vomer nasal (3/13). La polypose nasale avait un aspect endoscopique caractéristique. Il y avait des similitudes cliniques et diagnostiques entre cette cohorte de chiens et celle de chiens atteints de néoplasie nasale, bien que les chiens atteints de polypes nasaux étaient souvent plus jeunes et que le tissu polypoïde était externe au nez. La thérapie stéroïdienne seule n’a pas été efficace dans le traitement de la polypose chez les chiens; cependant, la réduction endoscopique avec un laser et une pince était plus efficace.
(Traduit par Dr Serge Messier)
Introduction
Canine nasal polyps are uncommon and can cause clinical signs that include nasal discharge, sneezing, and stertor (1–3). The diagnosis is suspected based on imaging studies and is confirmed with endoscopy and histological examination of nasal biopsies. The predominant treatment in humans is conservative; surgery is only recommended in cases of persistent recurrence (4,5). Ventral or dorsal rhinotomy has been reported for canine nasal polyps, but surgical complications and recurrence have also been reported (1,6). Endoscopic treatment of canine nasal polyps has not been reported.
The aims of this study were to report the clinical, diagnostic, and pathological characteristics of canine nasal polyps and their response to medical, endoscopic (i.e., debulking and laser ablation), and surgical treatments.
Materials and methods
A retrospective study was performed. A search was conducted on the database of a multi-center veterinary endoscopy group (Endovet, Rome, Italy) from May 2010 to December 2018. All dogs undergoing endoscopic investigation with a histological diagnosis of nasal polyposis were included in the study.
For each patient, we obtained the following information: breed, body weight (BW), age, sex, reproductive status, clinical and pharmacological anamnesis, clinical signs, hematological abnormalities, diagnostic imaging findings, treatment, and follow-up. With the dog under general anesthesia, ventrodorsal open-mouth projection radiographs of the skull were taken and computed tomography (CT) scans were performed with the dog in sternal recumbency, with and without administration of intravenous contrast.
Anterograde rhinoscopy and nasopharyngeal endoscopy were performed in all cases with a rigid rhinoscope (Hopkins Forward-Oblique Telescope 30° 64029 BA with diagnostic sheath 10.5 Fr 64018 VS or operating sheath 14.5 Fr with working channel 5 Fr 67065 C; Karl Storz, Tuttlingen, Germany) and flexible video bronchoscope (Video Bronchoscope EB-530S, distal diameter 4.9 mm, working channel 2 mm, length 600 mm; Fujifilm, Tokyo, Japan). For each dog, multiple biopsies of each portion of the grossly abnormal tissue were collected under direct vision with forceps inserted in the working channel of the endoscope (67161 Z Biopsy Forceps, flexible, oval, diameter 1.7 mm, length 34 cm and 60001 KL Biopsy Forceps, oval cupped jaws, diameter 1.7 mm, length 160 cm; Karl Storz) in dogs weighing <10 kg and with coaxial external biopsy forceps (69133 Biopsy and Grasping Forceps with oval cups 3 mm, sheath diameter, working length 15 cm; Karl Storz) in dogs weighing >10 kg. Biopsies were also performed in cases of nasopharyngeal extension, with forceps inserted in the working channel of the video bronchoscope in the retroversion position. The biopsied tissues were fixed in buffered formalin (Formalin 10% with Acetate Buffer; Bio-Optica, Milan, Italy) and sent to a reference laboratory to be analyzed by 1 Board-certified pathologist.
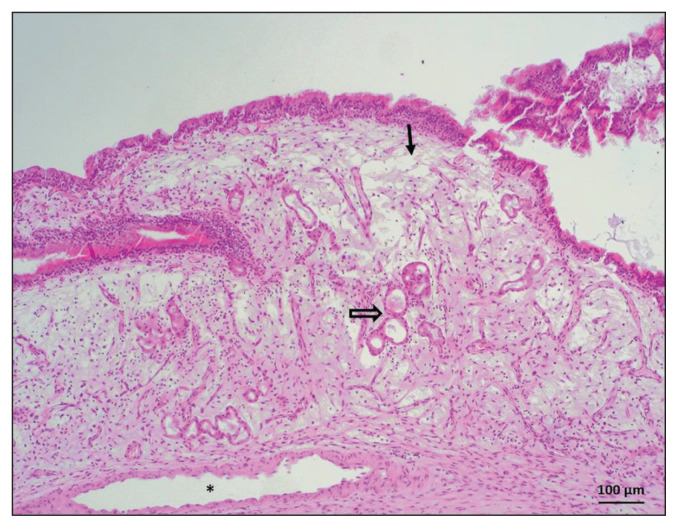
The histological pattern was considered compatible with nasal polyposis if newly formed exophytic tissue covered with respiratory epithelium was present. This tissue was either hyperplastic or exhibited squamous metaplasia with dysplasia, with underlying fibrillary myxoid to stroma. The stroma had areas of proliferation of the mesenchyme and/or tortuous and dilated blood vessels, seldom forming lacunar spaces, surrounded by highly variable numbers of serous or mucous glands and sparse inflammatory infiltrates (Figure 1).
Figure 1.
Histological appearance of canine nasal polyposis (Case 1). Exophytic mass is covered by respiratory epithelium that is either hyperplastic or exhibiting squamous metaplasia. The stroma is generally edematous and myxoid (arrow) with scant fibrils and surrounded by tortuous and ectatic nasal glands (empty arrow), which are present in highly variable quantities. In the lamina propria, mixed inflammatory infiltrates and dilation of the vascular system (*) are present. Hematoxylin and eosin, 100× magnification.
Dogs diagnosed with nasal polyps received the following treatments: medical (Group 1), endoscopic (Group 2), surgical (Group 3), or no treatment (Group 4). Medical treatment consisted of prednisolone (Prednicortone; Dechra Pharmaceuticals, Northwich, United Kingdom), 1 mg/kg BW, PO, q24h for 2 wk, then a progressively reduced dose for 3 to 6 wk, based on the clinical response. In general, this was achieved by halving the dosage every 10 d with a final administration of 0.5 mg/kg BW, PO, q48h. Small cooperative dogs were administered aerosol budesonide (Xavin; Teva Italia, Milan, Italy) 0.25 to 0.5 mg, q12h for 15 d, or fluticasone propionate (Flixotide; Aspen Bad Oldesloe, Bad Oldesloe, Germany) in a canine aerosol chamber (AeroDawg; BreathEazy, London, Ontario), 125 to 250 μg per puff, 1 or 2 times per day for 10 d then the dose was progressively reduced.
Endoscopic debulking was performed under direct vision using an alternating diode laser (400 to 600 mm diameter, continuous wave, 980 mm wavelength; Quanta System, Varese, Italy), an external grasping forceps (Karl Storz), and pressure washing. The operator used a laser inserted in the working channel of the endoscope. The procedure was carried out using an immersion in a sterile saline solution, under direct vision, and by directly contacting the fiber with the polypoid tissue using a continuous delivery (power 4 to 8 W) for 5 to 15 s. External grasping forceps were used to extract the polypoid tissue and fragments of pathological tissue were charred by the laser. In addition, after filling the oropharynx with gauze, pressure washes were performed with a sterile saline solution through a sterile 60-mL syringe (disposable syringe without needle, catheter tip; Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) inserted medially into the ventral meatus of the nose to dislocate the nasal and nasopharyngeal polypoid tissue and the fragments resulting from debulking. A residual surgical aspirator (VORTECO AS 100; Alsa, Bologna, Italy) connected to the endoscope was used to eliminate combustion fumes and washing solution residue.
All dogs recovered immediately from anesthesia and were monitored for complications for 4 to 8 h after endoscopic debulking. The dogs were then discharged with a prescription for a broad-spectrum antibiotic for 7 to 10 d and anti-inflammatory therapy with prednisolone (Dechra Pharmaceuticals), 1 mg/kg BW, PO, q24h, with the dose decreasing over the subsequent 3 to 6 wk, based on the clinical response. This protocol was also used in dogs that underwent a second endoscopic debulking.
Surgical rhinotomy was performed as described (7). New tissue was removed by traction and curettage, and hemorrhage was controlled with pressure using surgical gauze. After nasal passages were flushed, a tube was placed into the frontal sinus and secured to the skin and the periosteum. Subcutaneous tissue and skin were closed in separate layers with single interrupted sutures.
Follow-up data were obtained by telephone interview for at least 24 mo. This was done monthly for the first 6 mo and every 4 mo thereafter. Disease progress was assessed based on the presence or absence of clinical signs. For the latter, dogs were considered to be without pathology if they were capable of inducing nasal clinical signs. Clinical symptomatology similar to what was first observed at diagnosis was considered to be a possible relapse of the nasal polyposis. When possible, this was confirmed with an endoscopic examination and a repeated biopsy.
Results
Twenty-three dogs were included in the study, 11 mixed breeds and 12 purebreds. Body weights ranged from 7 to 39 kg (median = 24.4 kg) and ages ranged from 3 to 12 y (median = 9 y). Fourteen dogs were intact males, 5 were spayed females, and 4 were intact females. The duration of clinical signs ranged from 1 to 10 mo (median = 3 mo).
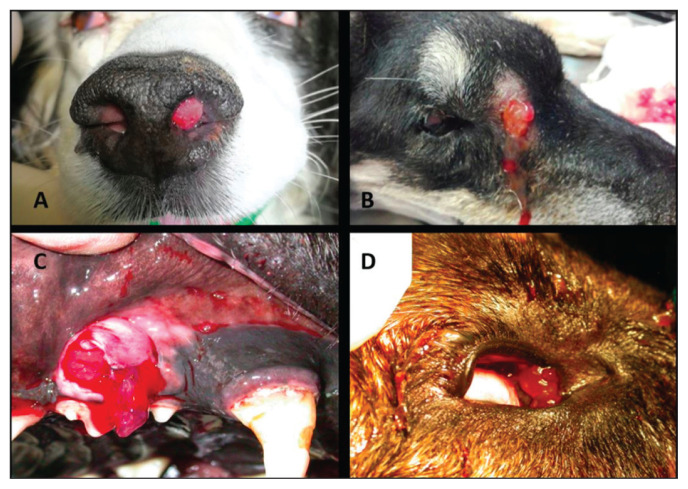
Clinical signs at presentation were sneezing (91.3%), nasal discharge [82.6% (56.5% bilateral and 26.1% unilateral)], stertor (73.9%), and frontonasal deformation (17.4%). Two dogs had clinical evidence of a fistula (1 in the zygomatic region and 1 in the frontal sinus). Translucent tissue was present in the nostril of 1 dog and at the end of the nasolacrimal duct in the medial canthus in the eye of 2 other dogs. In 1 dog, the same tissue was also present in the periodontal space of the right maxillary PM2 (Figure 2). One dog had hypoalbuminemia and 2 had neutrophil leukocytosis. None were diagnosed with an infectious disease. Cytology of the mandibular lymph nodes of the 13 dogs with mild or moderate lymphadenomegaly during their physical examination was negative for neoplastic cells in all cases and reactive in 6 patients.
Figure 2.
Clinical manifestations of canine nasal polyposis. There is evidence of polypoid tissue in the nostril (A), the zygomatic fistula (B), the periodontal space (C), and the medial canthus of the eye (D).
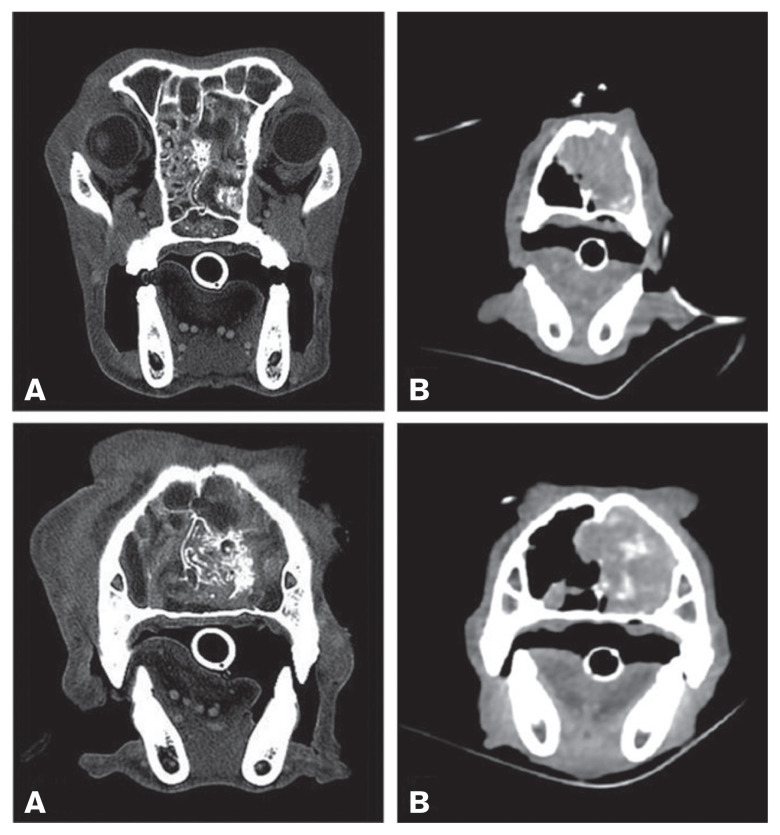
Skull radiography on 13 dogs showed alterations in 10 cases, including turbinate lysis (6/13), increased radiopacity of 1 (4/13) or both (6/13) nasal cavities, and lysis of the nasal vomer bone (3/13). Three dogs had a normal radiographic appearance. A CT scan was performed in 4 dogs. Total or partial turbinate lysis was present in all dogs and nasal septum and nasal bone lysis were present in 3. Two dogs had soft tissue with patchy contrast enhancement in 1 or both nasal cavities. Three dogs had similar tissue in the choana. One dog had frontal sinus involvement (Figure 3).
Figure 3.
Transverse CT images (A — Case 1, B — Case 15) of the bone and soft tissue window. In all images, a mass involving the nasal cavity (A and B left) is clearly visible, characterized by patchy contrast enhancement (B) and destruction of turbinates and parts of the bony nasal septum (A and B). Partial lysis of the nasal bone is also present (B). The mass extends into the choanae and the rostral aspect of the nasopharynx (A).
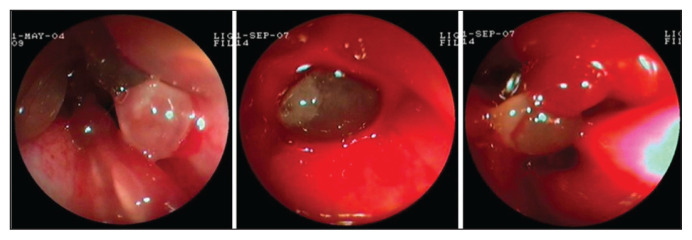
Both anterograde rhinoscopy and nasopharyngeal endoscopy revealed grossly abnormal tissue with a smooth translucent surface, pink color, and elastic consistency. This tissue occupied all the meatuses bilaterally in 13 dogs, all the meatuses unilaterally in 7 dogs, and was focally located in 3 dogs. In all subjects with focal lesions, polyposis was unilaterally located in the caudal nasal portions of the middle meatus and the ethmoturbinates. In 9 dogs, the polyposis also resulted in complete occlusion of the nasopharynx, whereas in 4 dogs, unilateral polyposis derived from the choana only partially occluded the nasopharynx. The endoscopic appearance of the nasopharyngeal polyposis was identical to that of the nasal polyposis (Figure 4).
Figure 4.
Endoscopic appearance of canine nasal polyposis. Evidence of newly formed tissue with a smooth translucent surface, pink color, and elastic consistency.
Histological examination of specimens obtained under direct endoscopic vision identified nasal polyposis in all patients. Mild epithelial dysplasia was determined in 3 patients and the type of inflammation in the lamina propria was predominantly lymphoplasma cellular in 6 cases and neutrophilic in 4 cases; there was no inflammation in the remaining cases.
Of the 23 dogs in the study, 10 were treated medically (Group 1). Six of these had follow-ups for 24 mo. Clinical signs recurred at 6 mo in 4 dogs (Cases 12, 13, 17, and 18) and at 7 mo in 1 dog (Case 14). One dog (Case 22) had no recurrence of clinical signs. In 4 dogs with recurrent signs, an endoscopic examination and at least 4 biopsies were performed with the same technique described, with histological confirmation of the presence of polyposis in all dogs. Endoscopic findings were similar to those of the initial examination in all 4 dogs. One dog (Case 18) had endoscopic debulking performed and was subsequently treated with aerosolized budesonide, with no recurrence of clinical signs at 24 mo.
The owners of 4 dogs declined the endoscopic debulking; these dogs continued to have fluctuating clinical signs throughout the study. Clinical signs were mild in 2 dogs and more severe in 2 dogs. The 5 dogs that were treated medically were alive at 24 mo. One dog (Case 18) was asymptomatic. One dog (Case 22) died of cardiac disease.
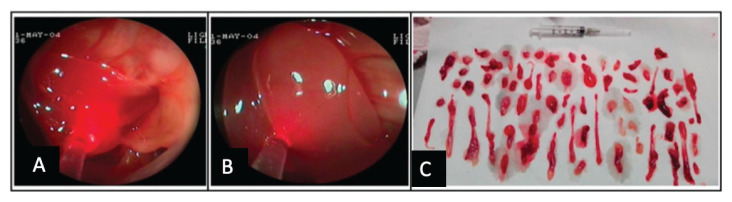
Endoscopic debulking was performed in 10 dogs (Group 2). Eight dogs had a complete follow-up. One dog had a follow-up at 6 mo. The procedure lasted 45 min on average (range: 24 to 71 min), depending on the area covered by the grossly abnormal tissue (Figure 5). None of the dogs experienced postoperative complications. Clinical signs recurred at 12 mo (2 dogs; Cases 7 and 8) and 8 mo (1 dog; Case 21). Five dogs were free of clinical signs for at least 24 mo (Cases 1, 3, 4, 19, and 20). One dog with worsening clinical signs of nasal disease was euthanized after 6 mo. One dog with focal nasal lesions (Case 20) had no recurrence for 33 mo. Two other dogs (Cases 1 and 3) had recurrence of clinical signs at 24 mo. Two dogs (Cases 4 and 19) remained asymptomatic for 24 mo.
Figure 5.
Endoscopic debulking with a diode laser of the polypoid tissue of the nasal cavity (A and B) and the removed tissue (C).
Laser debulking was repeated in 3 dogs with recurrent clinical signs (Case 21 at 8 mo and Cases 1 and 3 at 24 mo). Histopathology confirmed the recurrence of polyps in all 3 dogs. These dogs were asymptomatic for 72 mo (Case 1), 40 mo (Case 21), and 12 mo (Case 3). Two additional dogs had recurrent clinical signs 12 mo after laser debulking. One dog (Case 7) was treated with steroids and had bilateral nasal discharge and stertor at the end of the study. The second dog (Case 8) subsequently died of acute kidney injury. One dog (Case 11) had poor control of clinical signs after laser debulking and was euthanized 6 mo after initial treatment.
Rhinotomy was performed in 1 dog (Case 15). The procedure took 70 min. The dog was periodically treated with steroids after surgery and had no clinical signs 89 mo after surgery. Two dogs were not treated (Group 4). One dog (Case 16) was euthanized 15 d after diagnosis for uncontrolled epistaxis. One dog (Case 23) that had persistent clinical signs of nasal disease was euthanized after 6 mo due to chronic kidney disease. Data on treatments, timing of follow-ups, and outcomes are summarized in Table 1.
Table 1.
Results of follow-up and outcomes of cases.
| Follow-up (mo) | Time to relapse (mo) | State at the end of the study (December 2018) | |
|---|---|---|---|
| Group 1 — Medical treatment | |||
|
| |||
| Cases | |||
| 2, 5, 6, 9 | N/A | N/A | N/A |
| 12 | 26 | 6 | Symptomatic |
| 13 | 25 | 6 | Symptomatic |
| 14 | 25 | 7 | Symptomatic |
| 17 | 26 | 6 | Symptomatic |
| 18 | 32 | 6 | Asymptomatic (laser at 6 mo) |
| 22 | 28 | 24 | Died from cardiac disease |
|
| |||
| Group 2 — Endoscopic debulking | |||
|
| |||
| Cases | |||
| 1 | 96 | 24 | Asymptomatic (laser at 24 mo) |
| 3 | 36 | 24 | Asymptomatic (laser at 24 mo) |
| 4 | 24 | 24 | Euthanized for acute necrotizing pancreatitis |
| 7 | 26 | 12 | Symptomatic |
| 8 | 24 | 12 | Died from acute renal failure |
| 10 | N/A | N/A | N/A |
| 11 | 6 | 0 | Euthanized at 6 mo |
| 19 | 24 | 24 | Died from trauma |
| 20 | 33 | 33 | Asymptomatic |
| 21 | 48 | 8 | Asymptomatic (laser at 8 mo) |
|
| |||
| Group 3 — Rhinotomy | |||
|
| |||
| Case | |||
| 15 | 89 | 89 | Died from chronic renal failure |
|
| |||
| Group 4 — No treatment | |||
|
| |||
| Cases | |||
| 16 | 1 | 0 | Euthanized for uncontrollable epistaxis |
| 23 | 6 | 0 | Died from chronic renal failure |
N/A — data not available.
Discussion
This retrospective multicenter study identified 23 cases of nasal polyposis over an 8-year interval. There were many clinical and diagnostic similarities between this cohort of dogs and dogs with nasal neoplasia. However, dogs with nasal polyps were often younger than those with neoplasia; the median age of our patients was 9 y, with 52.2% of dogs <8 y and 30.4% <5 y. By contrast, dogs with nasal neoplasms are typically older, with an average age of 9 to 11 y in studies conducted over the past 15 y (8–12). Based on our data, it is necessary to consider a nasal polyposis diagnosis, especially in young dogs with nasal clinical signs.
Clinical signs of nasal polyposis are often severe and similar to those of nasal neoplasia. In our study, deformation of the frontonasal profile was present in 17.4% of cases, despite never having been reported in the literature on polyposis (1). Epistaxis, which is typical of nasal neoplasms (8,10), was not reported at first presentation in any of our cases.
Another interesting clinical observation was the presence of polypoid tissue external to the nose, which is not a feature of malignant neoplasia. We detected grossly abnormal tissue in the medial canthus of the eye (2 cases), in the periodontal space (1 case), and protruding from the nostril (1 case). To the best of the authors’ knowledge, this observation has not been reported in a malignant neoplasm. We speculate that after occupying the nasal cavity, polypoid tissue can also occupy very small free spaces, such as the nasolacrimal canal or the periodontal space. This could be confirmed with a tomographic examination to certify the integrity of the periodontal space or the nasolacrimal duct. Another possible explanation is that the epithelial tissue of the nasolacrimal duct can be affected in a manner similar to that of the nasal epithelium.
The association of polypoid tissue with fistulae in the zygomatic region is another interesting finding. This suggests that similar to what happens in the course of a malignant neoplasm, the disease can cause substantial bone erosion, allowing the polypoid tissue to extend beyond the nasal cavity through bone.
Diagnostic imaging in our study showed the same alterations previously described in both polyposis (1) and malignant neoplasms (9,13). In all cases, endoscopy also revealed the distinct characteristics of grossly abnormal tissue with a smooth translucent surface, which were pink and had an elastic consistency. In our opinion, this is an important finding, since these characteristics were present in all our cases. The macroscopic appearance of malignant neoplasms can vary substantially.
Although concurrent neoplasia was not reported in any of the cases in the present study, even on the second biopsy, the authors have observed other cases in which this occurred. In agreement with Tarrant et al (14), we believe that this concurrence in dogs with a caudal nasal neoplasm is not uncommon, especially if it is epithelial in nature. In the study by Tarrant et al (14), in 35% of cases in which histological examination of the tissue was repeated, a second biopsy revealed a malignancy. However, most of our patients had at least 1 follow-up at 24 mo, yet we did not observe a malignancy.
Tarrant et al (14) hypothesized that the tumor, due to the obstruction of the lymphatic drainage, could lead to regional edema and expansion of turbinates until polyps are formed. In the cases of polyposis that we have described, we cannot make equally valid pathophysiological hypotheses. To better understand this pathology, it would be interesting to compare the histological features of polyps that coexist with malignant neoplasms with those of idiopathic nasal polyps.
This is the first study of endoscopic debulking using a laser and forceps in combination with appropriate follow-up care in dogs with nasal polyposis. Given the retrospective nature of this study, it was not possible to conduct an adequate statistical analysis of the data. However, 7 of the 10 dogs in Group 2, which were treated with laser debulking and medical therapies, had an average recurrence time of 16.8 mo, and in 5 of these cases, the dogs were without clinical signs for at least 24 mo. We therefore believe that endoscopic treatment is more appropriate than medical therapy alone. Further prospective studies with more uniform inclusion criteria are required to confirm this hypothesis. Even when it is benign, nasal polyposis is aggressive in nature. For this reason, surgical therapy is necessary in patients with aggressive forms of the disease. This approach is also practiced in human medicine in patients with chronic rhinosinusitis with polyps, whereby the guidelines recommend the use of surgery in cases that are relapsing and not responsive to medical treatment (15–17). There is insufficient knowledge to warrant adopting the same physiopathological, diagnostic, histological, and therapeutic considerations in dogs that we do in humans, especially because in humans, the diagnosis is typically made at an early stage and the response to medical therapy is more successful (15–17).
Steroid therapy alone was not effective in treating polyposis in dogs. We hypothesize that this ineffectiveness is related to the extent of the newly formed tissue, which in many dogs, occupied much of the nasal cavity. We speculate that steroid therapy could be useful in the maintenance phase, after endoscopic treatment, in order to delay recurrence. In fact, in our study, 3 dogs in Group 2 (Cases 1, 20, and 21) that received non-standardized oral and local steroid therapies experienced recurrence after very long intervals. We cannot rule out that in cases of localized polyposis at an early stage, steroid therapy may also be effective in the long term, which is what we believe happened in Case 22. However, we cannot exclude that the small polypoid tissue would have regressed even without steroid therapy. Further studies with a greater number of dogs are needed to define when and how steroids should be used.
Two dogs in our series (Cases 16 and 11) were euthanized, 1 due to severe epistaxis and 1 due to a decline in its overall condition and worsening of nasal signs. Performing a second treatment may have led to better results and possibly identified concomitant pathologies. We were not aware of a possible clotting disorder in the dog with severe epistaxis (Case 16). In addition, both dogs may have had a malignant nasal neoplasm that was not identified through rhinoscopy. A complete inspection of the nasal cavities is fundamental, especially when there are polypoid lesions in the most rostral portions of the nose. We also believe that in the case of unclear histological results, biopsies should be repeated and a complete exploration of the nasal cavities should be performed.
The main limitation of this study lies in its retrospective nature, as the records did not provide reliable data on prognosis and response to therapy for the cases described. This prevented us from comparing the same diagnostic and therapeutic strategies among all patients. In addition, the endoscopic debulking was performed by 2 operators, which may have influenced the results obtained.
In conclusion, although canine nasal polyposis may have clinical signs similar to those of nasal neoplasia, it has unique clinical characteristics, such as the presence of newly formed tissue in the periodontal space or the medial canthus of the eye. Nasal polyposis also has a characteristic endoscopic appearance. Endoscopic debulking for the treatment of canine nasal polyposis represents a possible therapeutic option. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Holt DE, Goldschmidt MH. Nasal polyps in dogs: Five cases (2005 to 2011) J Small Anim Pract. 2011;52:660–663. doi: 10.1111/j.1748-5827.2011.01152.x. [DOI] [PubMed] [Google Scholar]
- 2.Sumner JA, Witham AI, Stent AW, Wightman PF, Mansfield CS. Emergence of nasal chondrosarcoma in a dog with nasal polyposis. Clin Case Rep. 2018;6:821–826. doi: 10.1002/ccr3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobetti RG. A retrospective study of chronic nasal disease in 75 dogs. J S Afr Vet Assoc. 2009;80:224–228. doi: 10.4102/jsava.v80i4.212. [DOI] [PubMed] [Google Scholar]
- 4.Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:1431–1440. doi: 10.1016/j.jaci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123:57–63. doi: 10.1002/lary.23671. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher DJ, Snyder JM, Messinger JS, Chiu AG, Vite CH. Ventricular pneumocephalus and septic meningoencephalitis secondary to dorsal rhinotomy and nasal polypectomy in a dog. 2006;229:240–245. doi: 10.2460/javma.229.2.240. [DOI] [PubMed] [Google Scholar]
- 7.Harvey CE. Surgery of the nasal cavity and sinus. In: Bojrab MJ, Waldron DR, Toombs JP, editors. Current Techniques in Small Animal Surgery. 2nd ed. Philadelphia, Pennsylvania: Lea & Febiger; 1983. pp. 253–257. [Google Scholar]
- 8.Avner A, Dobson JM, Sales JI, Herrtage ME. Retrospective review of 50 canine nasal tumours evaluated by low-field magnetic resonance imaging. J Small Anim Pract. 2008;49:233–239. doi: 10.1111/j.1748-5827.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Malinowsky C. Canine and feline nasal neoplasia. Clin Tech Small Anim Pract. 2006;21:89–94. doi: 10.1053/j.ctsap.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Mason SL, Maddox TW, Lillis SM, Blackwood L. Late presentation of canine nasal tumours in a UK referral hospital and treatment outcomes. J Small Anim Pract. 2013;54:347–353. doi: 10.1111/jsap.12083. [DOI] [PubMed] [Google Scholar]
- 11.Plickert HD, Tichy A, Hirt RA. Characteristics of canine nasal discharge related to intranasal diseases: A retrospective study of 105 cases. J Small Anim Pract. 2014;55:145–152. doi: 10.1111/jsap.12175. [DOI] [PubMed] [Google Scholar]
- 12.Cohn LA. Canine nasal disease: An update. Vet Clin North Am Small Anim Pract. 2020;50:359–374. doi: 10.1016/j.cvsm.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarrant JC, Holt DE, Durham AC. Co-occurrence of nasal polyps and neoplasms of the canine nasal cavity. Vet Pathol. 2019;56:885–888. doi: 10.1177/0300985819854438. [DOI] [PubMed] [Google Scholar]
- 15.Piromchai P, Kasemsiri P, Laohasiriwong S, Thanaviratananich S. Chronic rhinosinusitis and emerging treatment options. Int J Gen Med. 2013;6:453–464. doi: 10.2147/IJGM.S29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol Head Neck Surg. 2015;152:S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 17.Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: A systematic review. JAMA. 2015;314:926–939. doi: 10.1001/jama.2015.7544. [DOI] [PubMed] [Google Scholar]