This article reports a phenotypic and genetic relationship between two water use-related traits operating at leaf level and canopy level in a C4 model crop species.
Keywords: Canopy temperature, drought, optical tomography, quantitative trait loci, Setaria, stomata
Abstract
Mechanistic modeling indicates that stomatal conductance could be reduced to improve water use efficiency (WUE) in C4 crops. Genetic variation in stomatal density and canopy temperature was evaluated in the model C4 genus, Setaria. Recombinant inbred lines (RILs) derived from a Setaria italica×Setaria viridis cross were grown with ample or limiting water supply under field conditions in Illinois. An optical profilometer was used to rapidly assess stomatal patterning, and canopy temperature was measured using infrared imaging. Stomatal density and canopy temperature were positively correlated but both were negatively correlated with total above-ground biomass. These trait relationships suggest a likely interaction between stomatal density and the other drivers of water use such as stomatal size and aperture. Multiple quantitative trait loci (QTL) were identified for stomatal density and canopy temperature, including co-located QTL on chromosomes 5 and 9. The direction of the additive effect of these QTL on chromosome 5 and 9 was in accordance with the positive phenotypic relationship between these two traits. This, along with prior experiments, suggests a common genetic architecture between stomatal patterning and WUE in controlled environments with canopy transpiration and productivity in the field, while highlighting the potential of Setaria as a model to understand the physiology and genetics of WUE in C4 species.
Introduction
Drought stress is the primary limiting factor to crop production worldwide (Boyer, 1982). This is underpinned by the unavoidable loss of water vapor from leaves, via stomata, to the atmosphere in order for CO2 to move in the reverse direction and be assimilated through photosynthesis. In the coming decades, crops are likely to experience increasingly erratic rainfall patterns, with more frequent and intense droughts, due to climate change (Stocker et al., 2013). Irrigation of crops already accounts for ~70% of freshwater use, limiting the sustainability of any increase in irrigation to address drought limitations (Hamdy et al., 2003). Consequently, there is great interest in understanding and improving crop water use efficiency (WUE; Leakey et al., 2019) as well as crop drought resistance (Cattivelli et al., 2008).
Substantial advances have been made in understanding WUE and drought resistance at the genetic, molecular, biochemical, and physiological levels in the model species, Arabidopsis thaliana (Zhang et al., 2004; Valliyodan and Nguyen, 2006; Nakashima et al., 2012). Unfortunately, efforts to translate this knowledge into improved performance of crop plants in the production environment have not resulted in success as frequently as hoped (e.g. Nelson et al., 2007; Nemali et al., 2015). Physiological, agronomic, and breeding studies directly in crops have also resulted in improved drought avoidance and drought tolerance (e.g. Condon et al., 2004; Sinclair et al., 2017), but there are challenges associated with trying to apply modern systems biology and bioengineering tools to crops that are relatively large in stature and have generation times of several months. Consequently, Setaria viridis (L.) has been proposed as a model C4 grass that has characteristics that make it tractable for systems and synthetic biology while also being closely related to key C4 crops, so that discoveries are more likely to translate to production crops (Brutnell et al., 2010; Li and Brutnell, 2011). This study aimed to assess natural genetic variation in Setaria for two key traits related to WUE and drought response: stomatal density and canopy temperature (as a proxy for the rate of whole-plant water use).
Setaria italica and S. viridis are model C4 grasses belonging to the panicoideae subfamily, which also includes maize, sorghum, sugarcane, miscanthus, and switchgrass (Brutnell et al., 2010; Li and Brutnell, 2011). Foxtail millet (S. italica) is also a food crop in China and India (Devos et al., 1998). The availability of sequence data for its relatively small diploid (2n=18) genome, short life cycle, small stature, high seed production, and amenability for transformation make Setaria a good model species for genetic engineering (Brutnell et al., 2010; Bennetzen et al., 2012). In addition, Setaria is adapted to arid conditions and is a potential source of genes conferring WUE and drought resistance.
Whole-plant WUE is the ratio of plant biomass accumulated to the amount of water used over the growing season (Condon et al., 2004; Morison et al., 2007; Blum, 2009; Tardieu, 2013). WUE at the leaf level is a complex trait controlled by factors including photosynthetic metabolism, stomatal characteristics, mesophyll conductance, and hydraulics (Farquhar et al., 1989; Condon et al., 2002; Hetherington and Woodward, 2003). At the whole-plant scale, it is modified by canopy architecture, and root structure and function (Martre et al., 2001; White and Snow, 2012).
Stomata regulate the exchange of water and carbon dioxide (CO2) between the internal leaf airspace and the atmosphere (Hetherington and Woodward, 2003; Bertolino et al., 2019). Stomatal conductance (gs), which is the inverse of the resistance to CO2 uptake and water loss, is controlled by a combination of stomatal density, patterning across the leaf surface, maximum pore size, and operating aperture (Faralli et al., 2019; Nunes et al., 2020). Of these traits, stomatal density is most simple to measure (Dow and Bergmann, 2014). Consequently, genetic variation in stomatal density has been explored in a range of species, including the identification of quantitative trait loci (QTL) in rice (Laza et al., 2010), wheat (Schoppach et al., 2016; Shahinnia et al., 2016), barley (Liu et al., 2017), Arabidopsis (Dittberner et al., 2018; Delgado et al., 2019), brassica (Hall et al., 2005), poplar (Dillen et al., 2008), and oak (Gailing et al., 2008). However, there is a notable knowledge gap regarding genetic variation in stomatal density within C4 species. While many genes involved in the regulation of stomatal development are known in Arabidopsis, investigation of whether their orthologs retain the same function in grasses and other phylogenetic groups that include the major crops is still relatively nascent (e.g. Raissig et al., 2017; Lu et al., 2019; Mohammed et al., 2019). This is in part because standard protocols for measuring stomatal density are still laborious and time consuming, which slows the application of quantitative, forward, and reverse genetics approaches to identifying candidate genes and confirmation of their function. Therefore, improved methods for acquiring and analyzing images of stomatal guard cell complexes and other cell types in the epidermis are an area of active research (Haus et al., 2015; Dittberner et al., 2018; Fetter et al., 2019; Li et al., 2019). In addition, alternative approaches to rapidly screen stomatal conductance or rates of transpiration at the leaf and canopy scales (including temperature as a proxy) have also been developed and used to reveal genetic variation in traits related to drought stress and WUE (Liu et al., 2011; Bennett et al., 2012; Awika et al., 2017; Prado et al., 2018; Deery et al., 2019; Vialet-Chabrand and Lawson, 2019). However, the links between genetic variation in stomatal density and measures of water use, which would be expected in theory, are rarely tested and, when tested, the results are inconsistent (e.g. Fischer et al., 1998; Ohsumi et al., 2007; Kholová et al., 2010; Schoppach et al., 2016).
To address these questions, we used a field study of a biparental mapping population developed from an interspecific cross between S. viridis (A10) and S. italica (B100).
The study was designed with the aim of (i) applying rapid, image-based methods for phenotyping stomatal density and canopy water use; (ii) identifying variation in stomatal patterning, canopy temperature, and productivity; (iii) assessing trait relationships between stomatal density, canopy temperature, and biomass production; and (iv) identifying QTL for these traits in Setaria, grown in the field under wet and dry treatments.
Materials and methods
Plant material
This study used a population of 120 F7 recombinant inbred lines (RILs), which were generated by an interspecific cross between domesticated S. italica accession B100 and a wild-type S. viridis accession A10 (Devos et al., 1998; Wang et al., 1998).
Greenhouse experiment
Variation in stomatal density among the RILs was assessed in a greenhouse study at the University of Illinois, Urbana-Champaign in 2015. Plants were grown in pots (10×10×8.75 cm) filled with potting mixture (Metro-Mix 360 plus, Sun Gro Horticulture). Three seeds were sown directly into the pot. After germination, plants were thinned to one plant per pot. Growth conditions were 30/24 °C during the day/night and plants received supplemental photosynthetically active radiation from high-pressure sodium and metal halide lamps during the day (350 µmol m−2 s−1 on a 16 h day/8 h night cycle). Throughout the growing period, water was added to pot capacity along with fertilizer (EXCEL-CAL-MAG 15-5-5) 2–3 times a week.
The youngest fully expanded leaf was excised from the plant 17–22 days after sowing (DAS), covered in a wet paper towel, sealed in airtight bags, and stored at 4 °C. Within 48 h, a sample was excised with a razor blade from midway along the leaf to provide a cross-section from one leaf margin to the midrib (~20–30 mm length, 3–20 mm wide). This sample was attached to a glass microscope slide using double-sided adhesive tape, and the abaxial surface was immediately imaged using an μsurf explorer optical topometer (Nanofocus, Oberhausen, Germany; Haus et al., 2015). Two fields of view in a transect from the midrib to the edge of a single leaf were imaged using a ×20 magnification objective lens with 0.6 numerical aperture. The instrument generates a grayscale image in the proprietary *.nms format with dimensions of 0.8×0.8 mm in the x- and y-axes by stacking all the focused pixels across planes of the z-axis. The images were then exported into TIF files (Supplementary Fig. S1) and the stomatal number was manually counted using the cell counter tool in ImageJ software (http://rsbweb.nih.gov/ij/). Stomatal density was calculated by normalizing the number of stomata with the area of the field of view (0.64 mm2). Data from each of the four fields of view were treated as subsamples and averaged to estimate mean stomatal density for each replicate plant of a given RIL (Supplementary Table S1).
Field experiment
The field experiment to assess variation in canopy temperature and total above-ground biomass was conducted at the SoyFACE field site, University of Illinois, Urbana-Champaign in 2015, in the manner described by Feldman et al. (2017). The average air temperature over the growing season was 21.5 °C with a relative humidity of 82% (Supplementary Fig. S2). In brief, plants were germinated in plug trays in the greenhouse and then, at 9 DAS, seedlings were hand transplanted (15 July 2015) into plots at the field site. Twelve retractable awnings (Gray et al., 2016) were placed over the plots to block all water from any rainfall event in both wet and dry treatments (Supplementary Fig. S3). Drip irrigation was supplied once a week in order to maintain greater soil moisture in the wet treatment.
Each genotype subplot in the experiment measured 25×20 cm and contained 30 plants with a grid spacing of 5 cm between the plants. There was a 25 cm space for the aisle between two columns of plots and 10 cm spacing between the rows of plots. Each awning contained 66 subplots including six check plots of the B100 accession. The volumetric water content in the center of each awning was measured every 15 min throughout the growing season using soil moisture probes (CS650; Campbell Scientific) at 5 cm and 25 cm depths.
Canopy temperature of all field plots under both wet and dry treatments was measured 30 and 32 DAS once canopy closure had occurred in all plots (Supplementary Table S2). A telescopic boom lift was used to collect images from a height of 9.1 m above the ground using a hand-held infrared camera (FLIR T400, FLIR Systems, Boston, MA, USA). On each date, one infrared and one RGB image was acquired for each awning, which consisted of 66 plots (Fig. 1). The time of the measurements was between 11.00 h and 15.00 h. Infrared imaging was performed only during clear and sunny weather conditions. Data from the 36 pixels at the center of each genotype subplot were used to estimate the canopy temperature (FLIR Tools, FLIR Systems). This ensured that temperature data were only sampled from pixels completely covered by plant canopy and not containing data from soil in the nearby aisles between plots. The data from the two dates were not structured in a way that would justify treating them as a repeated measure and therefore they were considered as two separate traits (CT-T1 and CT-T2).
Fig. 1.
Aerial infrared and RGB images of Setaria subplots under awnings in wet and dry treatments. Infrared image of wet awning (A) and dry awning (B). RGB image of wet awning (C) and dry awning (D). The square boxes are the measured area of each subplot canopy.
Three plants from the center of each plot were destructively harvested 30 d after panicle emergence to estimate the shoot biomass (Supplementary Table S3). The plants were cut at the base, and the leaf, stem, and the panicles were separated and dried at 65 °C. The dried weights of leaf, stem, and panicle were summed to obtain the total shoot biomass. Culm height and tiller height were measured on the same plants from the base of the plant to the ligule of the youngest fully expanded leaf (Supplementary Table S4). Panicle emergence was measured as the number of days after sowing at which the panicle head was seen past the collar of the culm flag leaf in at least half of the individuals in a genotype-specific subplot (Supplementary Table S4).
Data analysis
The greenhouse experiment was conducted with four replicates of each RIL arranged in a randomized complete block design with 120 genotypes as described in the equation below, where Yij is the individual observation of the trait of interest, μ is the overall mean, Genotypei is the effect of the ith genotype, Blockj is the effect of the jth block, and ε ij is the error term.
The field experiment was conducted as a randomized complete block design in a split plot arrangement with three blocks, two treatment conditions, 12 awnings nested within treatments and blocks, and 120 genotypes as described below:
where Yijkl is the individual observation of the trait of interest, μ is the overall mean, Blocki is the effect of the ith block, Treatmentj is the effect of the jth treatment, ε ij is the first error term, Awningk(ij) is the kth awning nested within Blocki and Treatmentj, Genotypel is the lth genotype, Genotype×Treatmentlj is the interaction between Genotypel and Treatmentj, and ε ijkl is the second error term.
The broad sense heritability was computed using the variance components from the mixed model using the formula below.
The variance components from the mixed model were extracted using the lme4 package in R (Bates et al., 2015). Best linear unbiased predictors (BLUPs) were calculated for each trait of interest using the experimental designs discussed earlier where genotypes and blocks were considered as random effects and treatment and awning as fixed effects. Phenotypic correlations were computed using the ggplot2 package (Wickham, 2016) in R software to determine the strength and directionality of the relationship between all the traits collected in this study.
The QTL mapping was performed on the BLUP values for stomatal density and canopy temperature under different treatments and sampling dates using ~1400 single nucleotide polymorphism (SNP) markers. Mapping was performed using a custom biparental linkage mapping program (Feldman et al., 2017) based upon the functionality encoded within the R/qtl (Broman et al., 2003) and funqtl (Kwak et al., 2014) packages in R. All codes used can be found at https://github.com/maxjfeldman/foxy_qtl_pipeline. A two-step procedure was performed (Feldman et al., 2017). First, a single QTL model genome scan was performed using Haley–Knott regression to identify QTL with a logarithm of odds (LOD) score higher than the significant threshold obtained through 1000 permutations at alpha 0.05. Second, a stepwise forward/backward selection procedure was performed to identify an additive, multiple QTL model based upon maximization of the penalized LOD score. The two-step procedure was conducted on all the traits and time points. QTL that lie within a 20 cM window were considered to be co-located (Feldman et al., 2017).
Results
Soil moisture profile
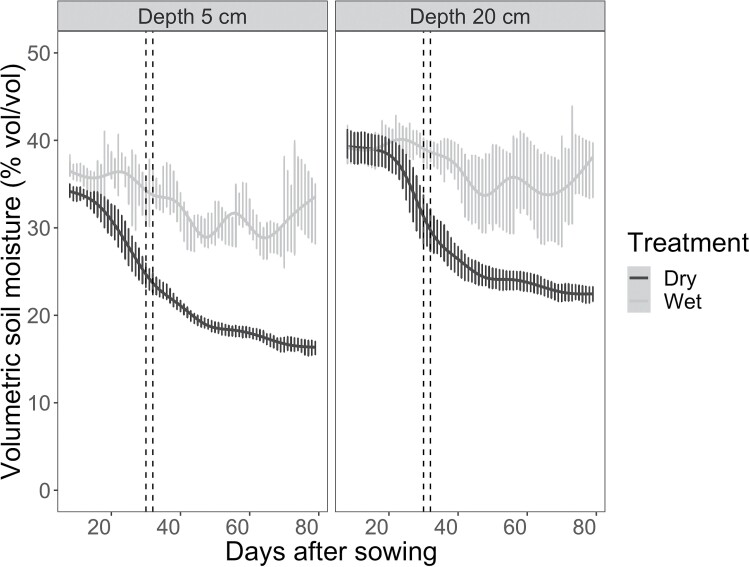
Soil moisture content was equivalent in the wet and dry treatments at the beginning of the experiment (Fig. 2). As time progressed, plants in the wet treatment continued to have adequate water supply (30–40% v/v) throughout the growing period. In contrast, plants in the dry treatment experienced progressively drier soil conditions as the water they transpired was not replaced by rainfall or irrigation. The soil moisture was reduced in the dry treatment compared with the wet treatment at 5 cm and 25 cm depth by 20 DAS, resulting in a statistically significant interaction between treatment and time (P<0.001) as well as significant overall effects of drought treatment (P<0.001), depth (P<0.001), and time (P<0.001). Midday canopy temperature data were collected after this date, 30 and 32 DAS, when plants in the dry treatment were experiencing rapidly decreasing availability of soil moisture. This indicates that while plants in the dry treatment were subjected to limited water supply, they were still physiologically active; that is, drought stress was moderate.
Fig. 2.
Soil volumetric water content (% v/v) at depths of 5 cm and 25 cm over the growing season in plots of Setaria supplied with either regular irrigation to maintain adequate water supply (wet treatment; light gray) or receiving no irrigation (dry treatment; dark gray). Rainfall was blocked from entering plots of both treatments using retractable rainout shelters. Data points and error bars shown the mean and SE of three replicates per treatment. The dashed vertical lines indicate the dates when canopy temperature was measured.
Genotypic variation in stomatal density and canopy temperature
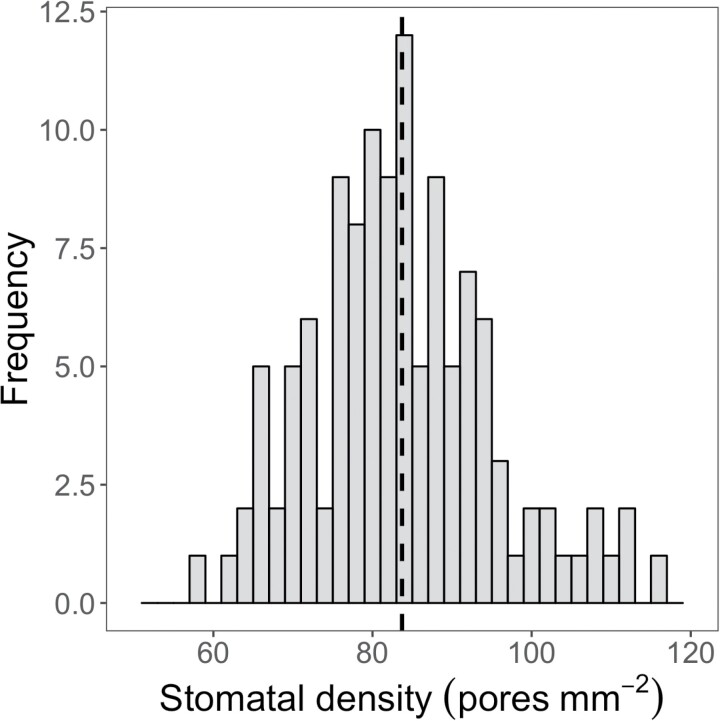
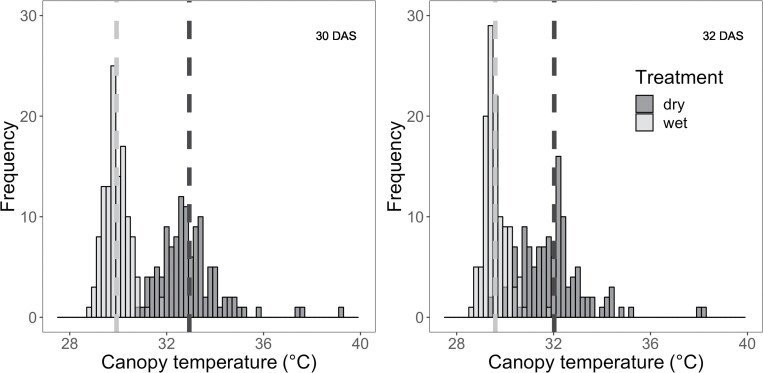
Among the 120 RILs, stomatal density on the abaxial surface of the youngest fully expanded leaf ranged between 58 and 115 stomata mm–2, with a mean of 84 stomata mm–2 (Fig. 3; Supplementary Fig. S4). The broad sense heritability of stomatal density was 0.58. Among the 120 RILs, the mean canopy temperature at midday ranged from 28.8 °C to 31.9 °C at 30 DAS and from 28.6°C to 31.9 °C at 32 DAS in the wet treatment, and from 30.9°C to 39.2 °C at 30 DAS and from 29.3°C to 38.1 °C at 32 DAS in the dry treatment. The mean midday canopy temperature across the RIL population was greater in the dry treatment than in the wet treatment at both 30 DAS (32.9 °C versus 29.9 °C; P<0.001) and 32 DAS (32.0 °C versus 29.6 °C; P<0.001; Fig. 4), with the treatment effect being slightly greater at 30 DAS (3.0 °C) than at 32 DAS (2.4 °C). Midday canopy temperature was positively correlated between the two measurement dates for both wet (ρ=0.78, P<0.001) and dry (ρ=0.66, P<0.001) conditions, which gives confidence in the phenotyping method (Supplementary Fig. S5). The broad sense heritability of canopy temperature was 0.54 and 0.40 at 30 and 32 DAS, respectively.
Fig. 3.
Frequency distribution of stomatal density (pores mm−2) of 120 recombinant inbred lines derived from a cross of S. italica and S. viridis, and the B100 parental line. Data are genotype means derived from two fields of view per leaf from each of four replicate plants. The dotted vertical lines represent the population mean value.
Fig. 4.
Frequency distribution of canopy temperature (°C) of 120 RILs in wet (light gray) and dry (dark gray) treatments at 30 and 32 days after sowing (DAS). Data are means derived from all pixels in the interior of three replicate plots per genotype. The dashed vertical lines represent the treatment mean value for each treatment.
Phenotypic relationships among canopy temperature, stomatal density, and total biomass
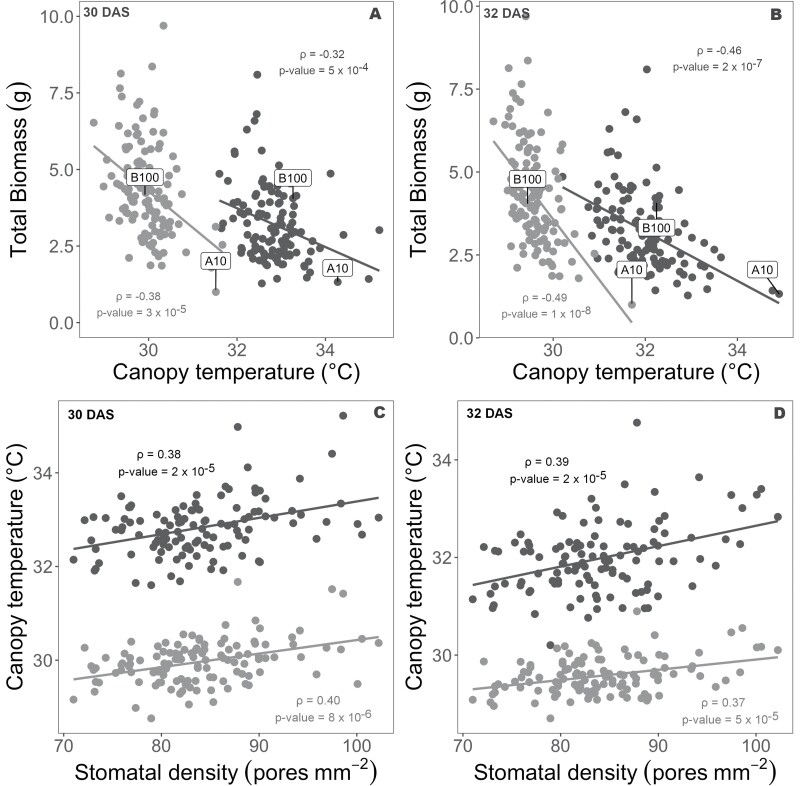
Midday canopy temperature was negatively correlated with total above-ground biomass under both wet and dry treatments at both 30 DAS (wet: ρ= –0.38, P<0.001; dry: ρ= –0.32, P<0.001; Fig. 5A) and 32 DAS (wet: ρ= –0.49, P<0.001; dry: ρ= –0.46, P<0.001; Fig. 5B). The average increase in total above-ground biomass production associated with a decrease in midday canopy temperature of 1 °C was greater in the wet treatment than in the dry treatment on both measurement dates (Table 1). The amount of variation in total above-ground biomass production explained by variation in midday canopy temperature was slightly greater in the wet treatment than in the dry treatment on both sampling dates (Table 1). The parental line A10 was one of the genotypes with the lowest biomass and highest canopy temperature under both treatments and days of measurement, while the parental line B100 had trait values that were close to the mean of the population.
Fig. 5.
(A and B) Scatterplot of total biomass (g per plant) in relation to canopy temperature (°C) for Setaria RILs and the parent lines (A10 and B100) under wet (gray circles) and dry conditions (black circles) at 30 and 32 days after sowing (DAS). (C and D) Scatterplot of canopy temperature (°C) in relation to stomatal density (pores mm−2) for Setaria RILs and the parent lines (A10 and B100) under wet (gray) and dry (black) conditions at 30 and 32 DAS. Data are best linear unbiased predicted (BLUP) values for each genotype. Lines of best fit are shown along with the Pearson’s correlation coefficient (ρ) and associated P-value.
Table 1.
Regression parameters for total above-ground biomass (g per plant) in relation to canopy temperature (°C) and stomatal density (pores per mm2) of Setaria genotypes grown under wet and dry treatments
| Intercept (b) | Slope (a) | R 2 | P-value | |||
|---|---|---|---|---|---|---|
| Biomass=Intercept (b)+a (Canopy temperature) | ||||||
| Canopy temperature | 30 DAS | Wet | 40.00 | –1.19 | 0.13 | <0.001 |
| Dry | 24.02 | –0.63 | 0.09 | <0.001 | ||
| 32 DAS | Wet | 58.21 | –1.82 | 0.24 | <0.001 | |
| Dry | 27.01 | –0.74 | 0.20 | <0.001 | ||
| Biomass=Intercept (b)+a (Stomatal density) | ||||||
| Stomatal density | Wet | 8.94 | –0.05 | 0.05 | 0.012 | |
| Dry | 8.31 | –0.06 | 0.10 | <0.001 |
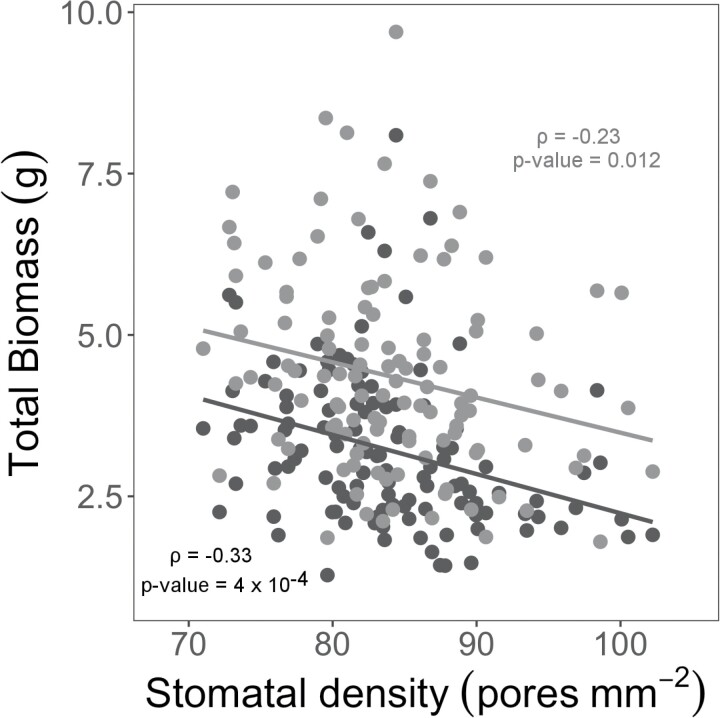
Stomatal density was positively correlated with midday canopy temperature under both wet and dry treatments at both 30 DAS (wet: ρ=0.40, P<0.001; dry: ρ=0.38, P<0.001; Fig. 5C) and 32 DAS (wet: ρ=0.37, P<0.001; dry: ρ=0.39, P≤0.001; Fig. 5D). Correspondingly, stomatal density was negatively correlated with total above-ground biomass under both dry (ρ= –0.33, P≤0.001) and wet (ρ= –0.23, P=0.012) conditions (Fig. 6). The correlation between stomatal density and total biomass was stronger under the dry treatment than under the wet treatment. Stomatal density was not significantly correlated with panicle emergence date, tiller height, or culm height in either wet or dry treatments (Supplementary Figs S6, S7).
Fig. 6.
Scatterplot of total biomass (g per plant) relative to stomatal density (pores mm−2) for Setaria RILs and the parent lines (A10 and B100) under wet (gray) and dry (black) conditions. Data are best linear unbiased predicted (BLUP) values for each genotype. Lines of best fit are shown along with the Pearson’s correlation coefficient (ρ) and associated P-value.
QTL mapping results
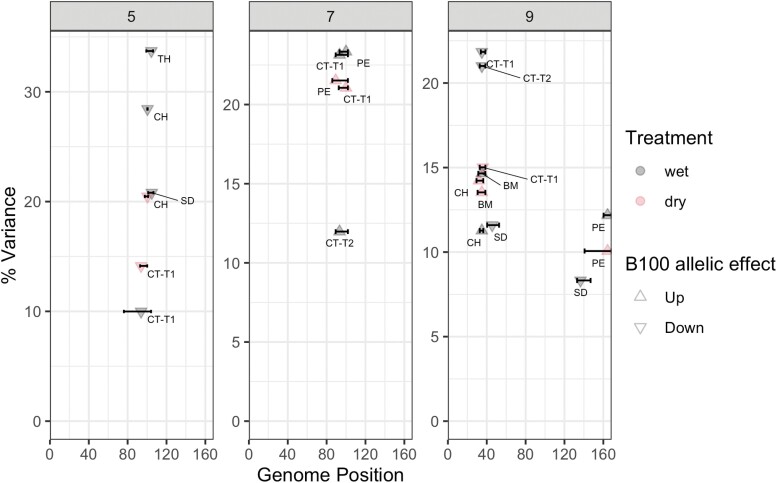
QTL analysis identified a total of 32 QTL across seven traits, including three significant loci for stomatal density and eight significant loci for canopy temperature (Table 2; Fig. 7). The proportion of phenotypic variation associated with these QTL ranged between 8% and 23% for both stomatal density and canopy temperature.. On chromosome 9 at ~40 cM, a QTL for stomatal density co-located with QTL for canopy temperature, biomass production, and culm height under both wet and dry treatments (Fig. 7). The effect of the B100 allele was negative for stomatal density and canopy temperature while being positive for biomass and culm height (Fig. 7). On chromosome 5, a QTL for stomatal density co-located with QTL for canopy temperature, culm height, and tiller height. The effect of the B100 allele at this location was negative for all traits (Fig. 7). The QTL for the date of panicle emergence overlapped with the QTL for canopy temperature on chromosome 7, with consistent allelic effects across all trait and treatment combinations (Fig. 7).
Table 2.
Putative quantitative trait loci (QTL) for stomatal density (SD), canopy temperature at 30 DAS (CT-T1) and 32 DAS (CT-T2), biomass (BM), culm height (CH), panicle emergence (PE), and tiller height (TH) traits in the 120 F7 recombinant inbred line population derived from a cross of S. italica and S. viridis, and the B100 parental line
| Trait | Treatment | Peak marker | Chr | Pos (cM)a | LOD at peakb | Variance (%)c | Additive effect | Left CI (cM)d | Right CI (cM) |
|---|---|---|---|---|---|---|---|---|---|
| BM | Dry | S2_37761700 | 2 | 69.7 | 5.0 | 12.6 | –1.8 | 69.2 | 70.0 |
| Dry | S2_37820883 | 2 | 70.0 | 6.9 | 17.9 | 2.1 | 69.7 | 71.1 | |
| Dry | S9_6724364 | 9 | 34.9 | 5.4 | 13.5 | 0.4 | 30.6 | 38.6 | |
| Wet | S1_31298551 | 1 | 66.9 | 2.5 | 5.4 | 0.2 | 61.1 | 83.0 | |
| Wet | S2_37761700 | 2 | 69.7 | 4.8 | 10.8 | –1.7 | 69.2 | 70.0 | |
| Wet | S2_37820883 | 2 | 70.0 | 6.9 | 16.2 | 2.1 | 69.7 | 71.1 | |
| Wet | S9_6724364 | 9 | 34.9 | 6.3 | 14.7 | 0.4 | 31.3 | 38.6 | |
| CH | Dry | S5_41999990 | 5 | 100.4 | 7.5 | 20.5 | –50.6 | 97.7 | 101.1 |
| Dry | S9_5686516 | 9 | 32.0 | 5.4 | 14.2 | 41.8 | 29.4 | 36.4 | |
| Wet | S1_35287681 | 1 | 80.1 | 7.1 | 8.0 | 36.5 | 78.3 | 83.5 | |
| Wet | S2_26339986 | 2 | 43.7 | 4.2 | 4.4 | 37.1 | 39.9 | 44.9 | |
| Wet | S2_37820883 | 2 | 70.0 | 3.4 | 3.6 | 28.5 | 59.6 | 75.4 | |
| Wet | S3_2542615 | 3 | 16.5 | 6.6 | 7.3 | 35.2 | 11.4 | 20.7 | |
| Wet | S5_41999990 | 5 | 100.4 | 19.4 | 28.4 | –76.5 | 100.2 | 100.7 | |
| Wet | S9_6724364 | 9 | 34.9 | 9.5 | 11.3 | 45.8 | 32.8 | 36.4 | |
| CT-T1 | Dry | S5_39309008 | 5 | 93.8 | 5.7 | 14.1 | –0.2 | 92.8 | 100.2 |
| Dry | S7_32133319 | 7 | 99.9 | 8.0 | 21.1 | 0.4 | 92.5 | 101.9 | |
| Dry | S9_7218054 | 9 | 35.9 | 6.0 | 15.0 | –0.2 | 32.8 | 38.6 | |
| Wet | S5_39309008 | 5 | 93.8 | 4.4 | 10.0 | –0.2 | 76.2 | 104.1 | |
| Wet | S7_31494503 | 7 | 93.3 | 9.2 | 23.1 | 0.3 | 89.3 | 101.9 | |
| Wet | S9_6724364 | 9 | 34.9 | 8.8 | 21.8 | –0.2 | 33.9 | 38.6 | |
| CT-T2 | Wet | S7_31494503 | 7 | 93.3 | 3.8 | 12.0 | 0.2 | 89.3 | 101.9 |
| Wet | S9_6724364 | 9 | 34.9 | 6.4 | 21.0 | –0.2 | 32.8 | 38.6 | |
| PE | Dry | S7_31178325 | 7 | 89.3 | 6.7 | 21.5 | 1.8 | 85.8 | 101.9 |
| Dry | S9_54618932 | 9 | 164.4 | 3.3 | 10.1 | 1.1 | 140.7 | 168.5 | |
| Wet | S2_43563669 | 2 | 90.6 | 3.8 | 9.5 | 1.1 | 69.7 | 95.7 | |
| Wet | S7_32133319 | 7 | 99.9 | 8.5 | 23.3 | 2.2 | 93.3 | 101.9 | |
| Wet | S9_54618932 | 9 | 164.4 | 4.8 | 12.2 | 1.2 | 160.2 | 168.5 | |
| SD | Wet | S5_42996052 | 5 | 104.8 | 8.3 | 20.8 | –3.8 | 101.1 | 106.6 |
| Wet | S9_10073675 | 9 | 45.6 | 5.0 | 11.6 | –2.3 | 40.4 | 52.7 | |
| Wet | S9_50690449 | 9 | 136.5 | 3.7 | 8.3 | –2.0 | 133.0 | 146.9 | |
| TH | Wet | S5_42757204 | 5 | 104.4 | 9.8 | 33.7 | –62.8 | 99.2 | 106.1 |
a Position of the peak marker in centiMorgans (cM).
b Logarithm of odds (LOD) of the peak marker.
c Percentage of phenotypic variance explained by the QTL.
d Left confidence interval of the QTL.
Fig. 7.
QTL identified for stomatal density (SD) and canopy temperature at 30 DAS (CT-T1) and 32 DAS (CT-T2), total biomass (BM), panicle emergence (PE), culm height (CH), and tiller height (TH) under wet (gray) and dry (pink) treatments in the Setaria RIL population. Each panel corresponds to a chromosome. The arrows indicate the direction of the B100 allelic effect.
Discussion
This study successfully characterized phenotypic and genetic variation in stomatal density, rates of canopy water use, and productivity in Setaria, which can be used as a foundation for future studies to apply systems biology approaches to advance understanding of WUE and drought resistance in C4 species. Significant trait correlations were detected among stomatal density, canopy temperature, and total above-ground biomass in both the wet and dry treatments.
The stomatal densities of RILs in this population (58–115 mm−2; Fig. 3) were slightly greater than previously reported for faba bean (30–75 mm−2, Khazaei et al., 2014) and wheat (36–92 mm−2,Schoppach et al., 2016; 43–92 mm−2, Shahinnia et al., 2016), but generally lower than for Arabidopsis (90–210 mm−2Dittberner et al., 2018) and rice (273–697 mm−2, Laza et al., 2010; 200–400 mm−2, Kulya et al., 2018). While the magnitude of variation in stomatal density among the RIL population was sufficient to allow for QTL mapping and analysis of trait correlations, the parents of the population were not selected on the basis of this trait. Thus, the resulting magnitude of variation across the population was relatively modest. It would be valuable to investigate how much more variation for stomatal density may be found among genotypes within either S. italica or S. viridis, as well as the genus as a whole. The present study provided a proof of concept for the use of optical tomography to image the leaf epidermis. As proposed by Haus et al. (2015), optical tomography does not require sample preparation steps and can also be used on frozen leaf samples. This was significantly less laborious and more convenient than standard methods of taking leaf imprints of fresh leaves with dental gum and nail varnish (Rowland-Bamford et al., 1990).
The magnitude of variation in canopy temperature across the Setaria RIL population was similar to that observed for sorghum (Awika et al., 2017) and wheat (Mason et al., 2013) RIL populations. Variations in canopy temperature among the RIL population were similar on 30 DAS (wet 3.1 °C, dry 8.3 °C; Fig. 4) and 32 DAS (wet 3.3 °C, dry 8.8 °C; Fig. 4), and canopy temperature was correlated across the two dates sampled for both the wet (r=0.78) and dry treatments (r=0.66) (Supplementary Fig. S5). This might be considered surprising given the highly dynamic nature of canopy temperature in response to wind gusts, diurnal variation in solar radiation, and daily or seasonal variation in climate. However, the reproducibility of the data across dates is consistent with the comprehensive analysis by Deery et al. (2019), which analyzed 98 independent time points of canopy temperature data collected for a wheat population over 14 dates in 2 years. These authorts concluded that canopy temperature could be reliably screened from one or two sampling points if data were collected under clear sky conditions in the afternoon, as was done in the current study. The present study also highlighted Setaria as a highly tractable model for field trials because its small stature allows non-destructive, remote-sensing approaches to phenotyping, such as thermal imaging, to be performed on hundreds of replicated plots using hand-held cameras and a boom lift. This is significantly simpler in terms of data acquisition and data analysis than using drones or vehicles to gather data across field trials of crops with larger stature that require field trials covering larger areas (Deery et al., 2016; Sagan et al., 2019).
Canopy temperature was negatively correlated with the total above-ground biomass of the Setaria RILs under both wet and dry conditions (Fig. 5A, B). This is consistent with RILs that had higher temperatures due to less evaporative cooling being able to assimilate less CO2, and therefore producing less biomass, which was expected based on theory and previous studies (Fischer et al., 1998; Jones, 2004). In addition, canopy temperature was significantly greater in the dry treatment compared with the wet treatment (Fig. 5A, B), which was consistent with stomatal closure reducing water use and evaporative cooling when there is limited water availability (Turner et al., 2001). The relationship between canopy temperature and biomass was stronger in the wet treatment than in the dry treatment on both measurement dates (Fig. 5A, B). This was reflected in canopy temperature explaining a greater proportion of variation in biomass (i.e. greater correlation coefficient) and a greater loss of biomass production per unit increase in canopy temperature under wet than under dry conditions (Fig. 5A, B). This pattern of response is also consistent with prior observations (Bennett et al., 2012; Mason et al., 2013), but does not appear to have been the subject of much discussion. While it may seem initially counterintuitive that the relationship between the rate of water use and productivity would be weaker when water is limiting, it is consistent with genotypes that have inherently high rates of transpiration (i.e. cooler canopies) having greater reductions in productivity in response to drought stress than genotypes with inherently low rates of transpiration (i.e. warmer canopies). We suggest that this differential response may be conserved. Also, it adds weight to the argument that genetic variation in WUE is best screened under well-watered conditions (Leakey et al., 2019).
The positive correlation of stomatal density with the canopy temperature under drought stress suggests that the relationship between these two traits is complicated (Fig. 5C,D), since—if all else is equal—greater stomatal density would be expected to increase transpiration and lead to canopy cooling (Dow and Bergmann, 2014). Consistent with that theory, previous studies have reported that stomatal density is positively correlated with WUE (Xu and Zhou, 2008). However stomatal conductance is influenced by multiple factors, including stomatal density, maximum size, and operating aperture (Dow and Bergmann, 2014; Faralli et al., 2019). In addition, there are multiple examples across diverse species where the expected positive correlation between stomatal density and stomatal conductance was not observed (Jones, 1977; Liao et al., 2005; Ohsumi et al., 2007). So, it is plausible that greater stomatal density within this population of Setaria RILs was associated with a developmental or functional shift that led to smaller stomatal apertures and lower rates of transpiration. As a result, within this population, lower stomatal density was also associated with greater biomass production. However, it should be noted that this relationship may be a function of the forced recombination across many parental alleles that is found in a RIL population. Breaking up gene linkage that can result from selection has been proposed to be a powerful approach to understand the biophysical basis for phenotypic relationships (Des Marais et al., 2013). The observed positive correlation may reflect the developmental trade-off where stomatal size and stomatal density are widely found to be negatively correlated due to a limited amount of space on the epidermis (Shahinnia et al., 2016; Faralli et al., 2019), but this needs to be confirmed experimentally. In contrast, stomatal density was either not correlated or was weakly, positively correlated with yield in wheat grown under both well-watered and drought treatments (Khazaie et al., 2011; Schoppach et al., 2016; Shahinnia et al., 2016; Faralli et al., 2019). So, the balance of trade-offs between stomatal density and aperture may be different among different biparental mapping populations, if not more generally in Setaria versus wheat. It would be valuable to compare if the same phenotypic relationship is observed across other biparental populations within these species as well as across natural accessions of these crops. New machine learning-enabled phenotyping methods for measuring stomatal size (e.g. Xie et al., 2020, Preprint) will aid this effort, because manual estimation of stomatal size currently takes ~30 times longer than manually measuring stomatal density, making it infeasible to assess in many experiments.
This study identified three unique QTL each for stomatal density and canopy temperature (Fig. 7). All three of the canopy temperature QTL were robust in terms of being observed in both the wet and dry treatments. In addition, the canopy temperature QTL on chromosomes 5 and 9 co-localized with QTL for stomatal density (Fig. 7). Genetic fine mapping would be required to discount the possibility that there are two loci in linkage at those locations, as <10% of the lines are discordant between the identified SNPs in each case. However, the observed pattern could be the result of pleiotropy, where a single locus regulates both traits. Additionally, this would be concordant with the consistent direction of the allelic effects and the positive correlation between stomatal density and temperature, as well as the theoretical expectation that stomatal patterning on the epidermis influences transpiration rates. It is notable that the allelic effects of the QTL identified for biomass production and culm height at ~40 cM on chromosome 9 are also consistent with the phenotypic correlations among the traits (Table 2). This opens up the possibility of pleiotropic effects at that locus across multiple measures of plant carbon and water relations which are logically linked to stomatal function.
Flowering time genes can have pleiotropic effects on stomatal apertures and stomatal conductance in Arabidopsis (Ando et al., 2013; Kimura et al., 2015; Auge et al., 2019), but data were not reported in those studies on stomatal patterning. Flowering time in wheat also impacts WUE in a complex manner that is environmentally dependent (Condon et al., 2004). Overlapping QTL for the date of panicle emergence and either stomatal density (chromosome 9) or canopy temperature (chromosome 7) opens up the possibility that similar processes occur in Setaria. However, the underlying basis of these interactions is not easily interpreted from the current data.
The ability to detect the same QTL in a greenhouse screen of stomatal density as for canopy temperature in the field suggests that rapid controlled-environment screening might be a tractable way to accelerate progress in understanding and manipulating epidermal patterning and WUE in Setaria. Such an approach would avoid the challenges associated with the lower heritability for stomatal density that can be observed under stress in some species (De Kort et al., 2020). At the same time, stomatal density varies in response to many environmental conditions (Casson et al., 2010), and genotype×environment interactions are still poorly understood in C4 species. So, further investigation of stomatal traits alongside plant water use and productivity in the field is needed. The small stature and rapid life cycle of Setaria make it particularly amenable for addressing these various next research steps. In that context, it is useful to know that the proportion of phenotypic variation explained by the stomatal density QTL in Setaria was similar to those of faba bean (Khazaei et al., 2014), rice (Laza et al., 2010), and wheat (Shahinnia et al., 2016; Wang et al., 2016).
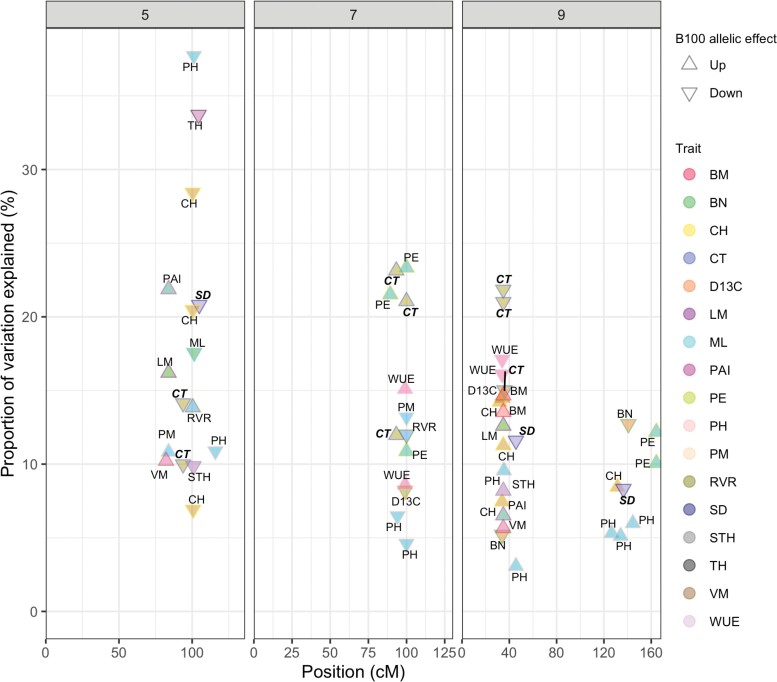
Previous studies have identified many QTL for different morphological and physiological traits using the same RIL population in Setaria in both controlled-environment and field experiments (Mauro-Herrera and Doust, 2016; Feldman et al., 2017, 2018; Banan et al., 2018; Ellsworth et al., 2020). These include measurements of traits with direct relevance to this study such as WUE of biomass production (i.e. biomass production relative to water use, as assessed by image analysis and metered irrigation on a high-throughput phenotyping platform linked to a controlled-environment chamber). Meta-analysis of all the studies (Fig. 8) reveals that QTL for stomatal density and canopy temperature overlap with QTL for WUE, δ 13C (Ellsworth et al., 2020), plant height, panicle emergence, and various measures of above-ground productivity Also, the effect of the B100 allele at each locus on canopy temperature was logically consistent with lower water use being associated with greater WUE, as measured gravimetrically on an indoor high-throughput phenotyping facility. This adds further evidence for the notion that controlled-environment and field studies of Setaria can be used in conjunction with one another when studying these traits. It is noteworthy that on chromosome 7 and at ~40 cM on chromosome 9, the percentage of the phenotypic variance explained by these QTL for stomatal density and canopy temperature, along with WUE, was generally greater than, or equal to, that for the other traits assessed to date. One explanation for this would be that these loci directly regulate traits related to stomatal function and then indirectly influence the other traits via effects on crop water use. There is no reason to think the experimental design used here results in any greater statistical power to detect genotype–phenotype associations than the other studies. However, additional experimentation where all traits are measured simultaneously is needed to test this notion definitively.
Fig. 8.
QTL on chromosomes 5, 7, and 9 identified across multiple studies of S. italica×S. viridis RIL populations (Mauro-Herrera and Doust, 2016; Feldman et al., 2017, 2018; Banan et al., 2018; Ellsworth et al., 2020). The arrows indicate the direction of the B100 allelic effect. The QTL for stomatal density and canopy temperature identified in this study are denoted in bold and italics. BM, biomass, BN, branch number; CH, culm height; CT, canopy temperature, D13C, ∆ 13C; LM, leaf mass; ML, mesocotyl length; PAI, plant area index; PE, panicle emergence; PH, plant height; PM, panicle mass; RVR, reproductive to vegetative mass ratio; SD, stomatal density; STH, secondary tiller height; VM, vegetative mass, WUE, water use efficiency.
In conclusion, this study identified genetic loci in Setaria that are associated with variation in stomatal density as well as other traits important to WUE, productivity, and drought resistance. This suggests that Setaria is an experimentally tractable model system that would be highly suitable for more in-depth investigation of the mechanisms underpinning stomatal development and their influence on WUE in C4 species. An additional benefit to identifying QTL and genes in Setaria is that it is also an agronomic crop, so the findings could have direct relevance to crop improvement programs as well as potentially translating into benefits for close relatives including maize, sorghum, and sugarcane.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Representative images from optical tomography of abaxial leaf surfaces of Setaria viridis (A10) and Setaria italica.
Fig. S2. Daily average values of air temperature and relative humidity at the SoyFACE experimental field site
Fig. S3. Field experiment layout for canopy temperature and biomass measurements.
Fig. S4. Stomatal density of 120 recombinant inbred lines derived from a cross of S. italica and S. viridis, and the B100 parental line.
Fig. S5. Scatterplot of midday canopy temperature for Setaria RILs and B100 on 30 DAS versus 32 DAS under wet and dry treatments.
Fig. S6. Phenotypic trait correlations of stomatal density versus canopy temperature at 30 DAS (CT-T1) and 32 DAS (CT-T2), total biomass, panicle emergence, culm height, and tiller height under wet treatment conditions in this study.
Fig. S7. Phenotypic trait correlations of stomatal density versus canopy temperature at 30 DAS (CT-T1) and 32 DAS (CT-T2), total biomass, panicle emergence, culm height, and tiller height under dry treatment conditions in this study.
Table S1. Stomatal density per field of view of setaria abaxial leaf surface.
Table S2. Plot mean values for canopy temperature.
Table S3. Plot mean values for above-ground biomass.
Table S4. Plot mean values for tiller height, culm height and panicle emergence date.
Acknowledgements
Funded by the U.S. Department of Energy under Prime Agreement nos DE-SC0008769 and DE-SC0018277. We thank Dr Timothy Wertin for helping with the stomatal density sample collection, and other undergraduates and summer interns for their help with field management. We also thank many project partners from the Danforth Plant Science Center, Carnegie Institute, Washington State University, and University of Minnesota that helped with transplanting seedlings.
Author contributions
ADBL and IB: conceptualization; ADB, PTP, DB, and REP: supervision; PTP: collection and processing of the thermal images; DX: collection of the stomatal images; PTP, DB, REP, and LF: management of the experiment and collection of biomass data; PTP, MF, IB, and ADBL: analysis and interpretation of the data; PTP and ADBL: writing; MF, IB, DB, REP, and LF: reviewing and commenting on the article.
Data availability
Raw data are available as supplementary tables, and all images are available via the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.crjdfn33z (Prakash et al., 2021).
References
- Ando E, Ohnishi M, Wang Y, Matsushita T, Watanabe A, Hayashi Y, Fujii M, Ma JF, Inoue S, Kinoshita T. 2013. TWIN SISTER OF FT, GIGANTEA, and CONSTANS have a positive but indirect effect on blue light-induced stomatal opening in Arabidopsis. Plant Physiology 162, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge GA, Penfield S, Donohue K. 2019. Pleiotropy in developmental regulation by flowering-pathway genes: is it an evolutionary constraint? New Phytologist 224, 55–70. [DOI] [PubMed] [Google Scholar]
- Awika H, Hays D, Mullet J, Rooney W, Weers B. 2017. QTL mapping and loci dissection for leaf epicuticular wax load and canopy temperature depression and their association with QTL for staygreen in Sorghum bicolor under stress. Euphytica 213, 207. [Google Scholar]
- Banan D, Paul RE, Feldman MJ, Holmes MW, Schlake H, Baxter I, Jiang H, Leakey ADB. 2018. High-fidelity detection of crop biomass quantitative trait loci from low-cost imaging in the field. Plant Direct 2, e00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48. [Google Scholar]
- Bennett D, Reynolds M, Mullan D, Izanloo A, Kuchel H, Langridge P, Schnurbusch T. 2012. Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential environments. Theoretical and Applied Genetics 125, 1473–1485. [DOI] [PubMed] [Google Scholar]
- Bennetzen JL, Schmutz J, Wang H, et al. 2012. Reference genome sequence of the model plant Setaria. Nature Biotechnology 30, 555–561. [DOI] [PubMed] [Google Scholar]
- Bertolino LT, Caine RS, Gray JE. 2019. Impact of stomatal density and morphology on water-use efficiency in a changing world. Frontiers in Plant Science 10, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. 2009. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research 112, 119–123. [Google Scholar]
- Boyer JS. 1982. Plant productivity and environment. Science 218, 443–448. [DOI] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen Ś, Churchill GA. 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19, 889–890. [DOI] [PubMed] [Google Scholar]
- Brutnell TP, Wang L, Swartwood K, Goldschmidt A, Jackson D, Zhu XG, Kellogg E, Van Eck J. 2010. Setaria viridis: a model for C4 photosynthesis. The Plant Cell 22, 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson SA, Hetherington AM. 2010. Environmental regulation of stomatal development. Current Opinion in Plant Biology 13, 90–95. [DOI] [PubMed] [Google Scholar]
- Cattivelli L, Rizza F, Badeck F-W, Mazzucotelli E, Mastrangelo AM, Francia E, Marè C, Tondelli A, Stanca AM. 2008. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Research 105, 1–14. [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2002. Improving intrinsic water-use efficiency and crop yield. Crop Science 42, 122–131. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460. [DOI] [PubMed] [Google Scholar]
- Deery DM, Rebetzke GJ, Jimenez-Berni JA, Bovill WD, James RA, Condon AG, Furbank RT, Chapman SC, Fischer RA. 2019. Evaluation of the phenotypic repeatability of canopy temperature in wheat using continuous-terrestrial and airborne measurements. Frontiers in Plant Science 10, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deery DM, Rebetzke GJ, Jimenez-Berni JA, James RA, Condon AG, Bovill WD, Hutchinson P, Scarrow J, Davy R, Furbank RT. 2016. Methodology for high-throughput field phenotyping of canopy temperature using airborne thermography. Frontiers in Plant Science 7, 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kort H, Panis B, Helsen K, Douzet R, Janssens SB, Honnay O. 2020. Pre-adaptation to climate change through topography-driven phenotypic plasticity. Journal of Ecology 108, 1465–1474. [Google Scholar]
- Delgado D, Sánchez-Bermejo E, de Marcos A, Martín-Jimenez C, Fenoll C, Alonso-Blanco C, Mena M. 2019. A genetic dissection of natural variation for stomatal abundance traits in Arabidopsis. Frontiers in Plant Science 10, 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DL, Hernandez KM, Juenger TE. 2013. Genotype-by-environment interaction and plasticity: exploring genomic responses of plants to the abiotic environment. Annual Review of Ecology, Evolution, and Systematics 44, 5–29. [Google Scholar]
- Devos KM, Wang Z, Beales J, Sasaki T, Gale M. 1998. Comparative genetic maps of foxtail millet (Setaria italica) and rice (Oryza sativa). Theoretical and Applied Genetics 96, 63–68. [Google Scholar]
- Dillen SY, Marron N, Koch B, Ceulemans R. 2008. Genetic variation of stomatal traits and carbon isotope discrimination in two hybrid poplar families (Populus deltoides ‘S9-2’ × P. nigra ‘Ghoy’ and P. deltoides ‘S9-2’ × P. trichocarpa ‘V24’). Annals of Botany 102, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittberner H, Korte A, Mettler-Altmann T, Weber APM, Monroe G, de Meaux J. 2018. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Molecular Ecology 27, 4052–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GJ, Bergmann DC. 2014. Patterning and processes: how stomatal development defines physiological potential. Current Opinion in Plant Biology 21, 67–74. [DOI] [PubMed] [Google Scholar]
- Ellsworth PZ, Feldman MJ, Baxter I, Cousins AB. 2020. A genetic link between leaf carbon isotope composition and whole-plant water use efficiency in the C4 grass Setaria. The Plant Journal 102, 1234–1248. [DOI] [PubMed] [Google Scholar]
- Faralli M, Matthews J, Lawson T. 2019. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Current Opinion in Plant Biology 49, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G, Hubick K, Condon A, Richards R. 1989. Carbon isotope fractionation and plant water-use efficiency. In: Rundel PW, Ehleringer JR, Nagy KA, eds. Stable isotopes in ecological research. New York: Springer, 21–40. [Google Scholar]
- Feldman MJ, Ellsworth PZ, Fahlgren N, Gehan MA, Cousins AB, Baxter I. 2018. Components of water use efficiency have unique genetic signatures in the model C4 grass Setaria. Plant Physiology 178, 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MJ, Paul RE, Banan D, et al. 2017. Time dependent genetic analysis links field and controlled environment phenotypes in the model C4 grass Setaria. PLoS Genetics 13, e1006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter KC, Eberhardt S, Barclay RS, Wing S, Keller SR. 2019. StomataCounter: a neural network for automatic stomata identification and counting. New Phytologist 223, 1671–1681. [DOI] [PubMed] [Google Scholar]
- Fischer R, Rees D, Sayre K, Lu ZM, Condon A, Saavedra AL. 1998. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475. [Google Scholar]
- Gailing O, Langenfeld-Heyser R, Polle A, Finkeldey R. 2008. Quantitative trait loci affecting stomatal density and growth in a Quercus robur progeny: implications for the adaptation to changing environments. Global Change Biology 14, 1934–1946. [Google Scholar]
- Gray SB, Dermody O, Klein SP, et al. 2016. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nature Plants 2, 16132. [DOI] [PubMed] [Google Scholar]
- Hall N, Griffiths H, Corlett J, Jones H, Lynn J, King G. 2005. Relationships between water-use traits and photosynthesis in Brassica oleracea resolved by quantitative genetic analysis. Plant Breeding 124, 557–564. [Google Scholar]
- Hamdy A, Ragab R, Scarascia-Mugnozza E. 2003. Coping with water scarcity: water saving and increasing water productivity. Irrigation and Drainage 52, 3–20. [Google Scholar]
- Haus MJ, Kelsch RD, Jacobs TW. 2015. Application of optical topometry to analysis of the plant epidermis. Plant Physiology 169, 946–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Jones HG. 1977. Transpiration in barley lines with differing stomatal frequencies. Journal of Experimental Botany 28, 162–168. [Google Scholar]
- Jones HG. 2004. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Advances in Botanical Research 41, 107–163. [Google Scholar]
- Khazaie H, Mohammady S, Monneveux P, Stoddard F. 2011. The determination of direct and indirect effects of carbon isotope discrimination (Δ), stomatal characteristics and water use efficiency on grain yield in wheat using sequential path analysis. Australian Journal of Crop Science 5, 466. [Google Scholar]
- Khazaei H, O’Sullivan DM, Sillanpää MJ, Stoddard FL. 2014. Use of synteny to identify candidate genes underlying QTL controlling stomatal traits in faba bean (Vicia faba L.). Theoretical and Applied Genetics 127, 2371–2385. [DOI] [PubMed] [Google Scholar]
- Kholová J, Hash CT, Kakkera A, Kocová M, Vadez V. 2010. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.]. Journal of Experimental Botany 61, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Aoki S, Ando E, et al. 2015. A flowering integrator, SOC1, affects stomatal opening in Arabidopsis thaliana. Plant & Cell Physiology 56, 640–649. [DOI] [PubMed] [Google Scholar]
- Kulya C, Siangliwb JL, Toojindab T, Lontoma W, Pattanagula W, Sriyota N, Sanitchon J, Theerakulpisuta P. 2018. Variation in leaf anatomical characteristics in chromosomal segment substitution lines of KDML105 carrying drought tolerant QTL segments. ScienceAsia 44, 197–211. [Google Scholar]
- Kwak IY, Moore CR, Spalding EP, Broman KW. 2014. A simple regression-based method to map quantitative trait loci underlying function-valued phenotypes. Genetics 197, 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laza MRC, Kondo M, Ideta O, Barlaan E, Imbe T. 2010. Quantitative trait loci for stomatal density and size in lowland rice. Euphytica 172, 149–158. [Google Scholar]
- Leakey ADB, Ferguson JN, Pignon CP, Wu A, Jin Z, Hammer GL, Lobell DB. 2019. Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annual Review of Plant Biology 70, 781–808. [DOI] [PubMed] [Google Scholar]
- Li K, Huang J, Song W, Wang J, Lv S, Wang X. 2019. Automatic segmentation and measurement methods of living stomata of plants based on the CV model. Plant Methods 15, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Brutnell TP. 2011. Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. Journal of Experimental Botany 62, 3031–3037. [DOI] [PubMed] [Google Scholar]
- Liao JX, Chang J, Wang GX. 2005. Stomatal density and gas exchange in six wheat cultivars. Cereal Research Communications 33, 719–726. [Google Scholar]
- Liu X, Fan Y, Mak M, et al. 2017. QTL for stomatal and photosynthetic traits related to salinity tolerance in barley. BMC Genomics 18, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Subhash C, Yan J, Song C, Zhao J, Li J. 2011. Maize leaf temperature responses to drought: thermal imaging and quantitative trait loci (QTL) mapping. Environmental and Experimental Botany 71, 158–165. [Google Scholar]
- Lu J, He J, Zhou X, Zhong J, Li J, Liang YK. 2019. Homologous genes of epidermal patterning factor regulate stomatal development in rice. Journal of Plant Physiology 234–235, 18–27. [DOI] [PubMed] [Google Scholar]
- Martre P, Cochard H, Durand JL. 2001. Hydraulic architecture and water flow in growing grass tillers (Festuca arundinacea Schreb.). Plant, Cell & Environment 24, 65– 76. [Google Scholar]
- Mason RE, Hays DB, Mondal S, Ibrahim AM, Basnet BR. 2013. QTL for yield, yield components and canopy temperature depression in wheat under late sown field conditions. Euphytica 194, 243–259. [Google Scholar]
- Mauro-Herrera M, Doust AN. 2016. Development and genetic control of plant architecture and biomass in the panicoid grass, Setaria. PLoS One 11, e0151346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed U, Caine RS, Atkinson JA, Harrison EL, Wells D, Chater CC, Gray JE, Swarup R, Murchie EH. 2019. Author Correction: rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Scientific Reports 9, 14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JI, Baker NR, Mullineaux PM, Davies WJ. 2007. Improving water use in crop production. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 639–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. 2012. NAC transcription factors in plant abiotic stress responses. Biochimica et Biophysica Acta 1819, 97–103. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, et al. 2007. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proceedings of the National Academy of Sciences, USA 104, 16450–16455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemali KS, Bonin C, Dohleman FG, et al. 2015. Physiological responses related to increased grain yield under drought in the first biotechnology-derived drought-tolerant maize. Plant, Cell & Environment 38, 1866–1880. [DOI] [PubMed] [Google Scholar]
- Nunes TDG, Zhang D, Raissig MT. 2020. Form, development and function of grass stomata. The Plant Journal 101, 780–799. [DOI] [PubMed] [Google Scholar]
- Ohsumi A, Kanemura T, Homma K, Horie T, Shiraiwa T. 2007. Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.). Plant Production Science 10, 322–328. [Google Scholar]
- Prado SA, Cabrera-Bosquet L, Grau A, Coupel-Ledru A, Millet EJ, Welcker C, Tardieu F. 2018. Phenomics allows identification of genomic regions affecting maize stomatal conductance with conditional effects of water deficit and evaporative demand. Plant, Cell & Environment 41, 314–326. [DOI] [PubMed] [Google Scholar]
- Prakash PT, Banan D, Paul RE, Feldman MJ, Xie D, Freyfogle L, Baxter I, Leakey ADB. . 2021. Data from: Correlation and co-localization of QTL for stomatal density, canopy temperature, and productivity with and without drought stress in Setaria. Journal of Experimental Botany 72, XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raissig MT, Matos JL, Anleu Gil MX, et al. 2017. Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 355, 1215–1218. [DOI] [PubMed] [Google Scholar]
- Rowland-Bamford AJ, Nordenbrock C, Baker JT, Bowes G, Allen LH Jr. 1990. Changes in stomatal density in rice grown under various CO2 regimes with natural solar irradiance. Environmental and Experimental Botany 30, 175–180. [Google Scholar]
- Sagan V, Maimaitijiang M, Sidike P, Eblimit K, Peterson KT, Hartling S, Esposito F, Khanal K, Newcomb M, Pauli D. 2019. UAV-based high resolution thermal imaging for vegetation monitoring, and plant phenotyping using ICI 8640 P, FLIR Vue Pro R 640, and thermomap cameras. Remote Sensing 11, 330. [Google Scholar]
- Schoppach R, Taylor JD, Majerus E, Claverie E, Baumann U, Suchecki R, Fleury D, Sadok W. 2016. High resolution mapping of traits related to whole-plant transpiration under increasing evaporative demand in wheat. Journal of Experimental Botany 67, 2847–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinnia F, Le Roy J, Laborde B, Sznajder B, Kalambettu P, Mahjourimajd S, Tilbrook J, Fleury D. 2016. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biology 16, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Devi J, Shekoofa A, Choudhary S, Sadok W, Vadez V, Riar M, Rufty T. 2017. Limited-transpiration response to high vapor pressure deficit in crop species. Plant Science 260, 109–118. [DOI] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM. 2013. Climate change 2013: the physical science basis. Cambridge: Cambridge University Press. [Google Scholar]
- Tardieu F. 2013. Plant response to environmental conditions: assessing potential production, water demand, and negative effects of water deficit. Frontiers in Physiology 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NC, Wright GC, Siddique K. 2001. Adaptation of grain legumes (pulses) to water-limited environments. Advances in Agronomy 71, 193–231. [Google Scholar]
- Valliyodan B, Nguyen HT. 2006. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Current Opinion in Plant Biology 9, 189–195. [DOI] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Lawson T. 2019. Dynamic leaf energy balance: deriving stomatal conductance from thermal imaging in a dynamic environment. Journal of Experimental Botany 70, 2839–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Devos K, Liu C, Wang R, Gale M. 1998. Construction of RFLP-based maps of foxtail millet, Setaria italica (L.) P. Beauv. Theoretical and Applied Genetics 96, 31–36. [Google Scholar]
- Wang SG, Jia SS, Sun DZ, Hua F, Chang XP, Jing RL. 2016. Mapping QTL for stomatal density and size under drought stress in wheat (Triticum aestivum L.). Journal of Integrative Agriculture 15, 1955–1967. [Google Scholar]
- White T, Snow V. 2012. A modelling analysis to identify plant traits for enhanced water-use efficiency of pasture. Crop and Pasture Science 63, 63–76. [Google Scholar]
- Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Xie X, Mayfield-Jones D, Erice G, Choi M, Leakey ADB. 2020. Optical topometry and machine learning to rapidly phenotype stomatal patterning traits for QTL mapping in maize. BioRxiv doi: 10.1101/2020.10.09.333880v1 [Preprint]. [DOI] [PMC free article] [PubMed]
- Xu Z, Zhou G. 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany 59, 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Creelman RA, Zhu JK. 2004. From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiology 135, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available as supplementary tables, and all images are available via the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.crjdfn33z (Prakash et al., 2021).