Abstract
The zebrafish model has been demonstrated as an ideal vertebrate model system for a diverse range of biological studies. Along with conventional approaches, monitoring and analysis of zebrafish electrocardiogram (ECG) have been utilized for cardio-physiological screening and elucidation. ECG monitoring has been carried out with fish treated with anesthetic drugs, rendering the short period of time in recording the signals (<5 min). In this work, a prolonged sedation system for continuous ECG monitoring of multiple zebrafish was proposed and developed. We built a circulation system to provide prolonged mild anesthesia which allows more consistent and intrinsic ECG measurement. The use of prolonged anesthesia helped reduce the concentration of the anesthetic drug (MS222 or Tricaine) from 200 mg/L to 100 mg/L and even lower; thus, maintaining the integrity of intrinsic ECG. Moreover, heartrate variation during recording was investigated, showing minute changes (±3.2 beats per minute - BPM). The development of this prolonged ECG monitoring system would open the possibility of long-term monitoring for studies such as drug screening and forward genetic screening.
Keywords: Zebrafish, ECG, Prolonged Anesthesia, Cardiac Disease, Drug Screening
I. Introduction
Zebrafish is a crucial model for studies of cardiac development, regeneration, and physiological screening. The similarity of the electrocardiogram (ECG) waveform characteristics (P waves, QRS complexes and T waves) of adult zebrafish and humans’ has been remarkably evidenced in previous studies [1, 2]. These further indicate zebrafish as an alternative model for pharmaceutical toxicity screening, such as via assessment of drug-induced QT prolongation.
ECG acquisition and analysis have been proven to be a desired method to access heart functions in human cardiac disease. However, this technique has remained largely inaccessible to many laboratories in studies of zebrafish cardiology despite the popularity of this animal model. There are several factors that should be considered before conducting experiments with this model. While common factors come from confusion regarding the electrode placement and low signal-to-noise ratio (SNR), anesthetic drug concentrations and measuring procedures can also contribute to an incomplete understanding of ECG patterns and the cardiac etiology in this model. ECG is the only measuring tool for abnormal phenotype rhythms in live zebrafish in prolonged mild anesthesia (PMA) to eliminate side-effects of anesthetic drugs for the cardiac conduction system. There is an obvious need for a PMA-based ECG measurement and standardized methods to measure intrinsic ECG signals while providing ease in accessibility and operation, as well as reproducibility. In addition, existing ECG devices and systems possess long-entrenched shortcomings: 1) only a short period of time can be recorded, potentially missing significant signals during study; 2) the ECG acquisition requires zebrafish under full anesthesia, rendering the fish stressful and inadequate to provide intrinsic cardiac electrophysiological signals; and 3) the processing is done manually and tediously.
Our team and others have demonstrated the acquisition of ECG signal from both adult and embryonic zebrafish, revealing distinct P waves and QRS complexes which solidly consolidate the similarity of human and zebrafish’s homology [3, 4]. In attempt to provide favorable temporal and spatial resolution as well as signal-to-noise ratio (SNR), our group developed the microelectrode array (MEA) membranes and demonstrated the capacity in acquiring site-specific ECG signals [4, 5]. Recently, we have built acquisition and automated analysis systems to further facilitate the process [5].
As aforementioned, zebrafish ECG assessment also holds promise in various screening applications, calling for systems with prolonged recording and analysis. The combination of current tricaine methane sulfonate (MS222 or Tricaine) and other drugs, i.e. isoflurane [6, 7], possibly extends the time of anesthesia, and helps the zebrafish recover faster. However, based on our trials and observation, the lack of oxygen due to isolation from water environment affected ECG signal quality as well as introduced potential risk to the fish when experiments last more than half an hour.
In this work, we introduced a novel-yet-simple PMA system (Fig. 1, left panel) with the following features: 1) allowing real-time ECG acquisition for multiple zebrafish; 2) enabling the circulation water system to run at least 1 hour long; and 3) recovering the zebrafish treated by lower dose (i.e., 100mg/L Tricaine) after the experiment. This procedure allowed the fish maintaining their physiological nature and stress comfortability and reduce recovery time after measurement. A real-time LabVIEW graphical user interface (GUI) was also developed to simultaneously measure four zebrafish. Finally, we conducted several experiments to demonstrate the efficacy of the system.
Fig. 1.
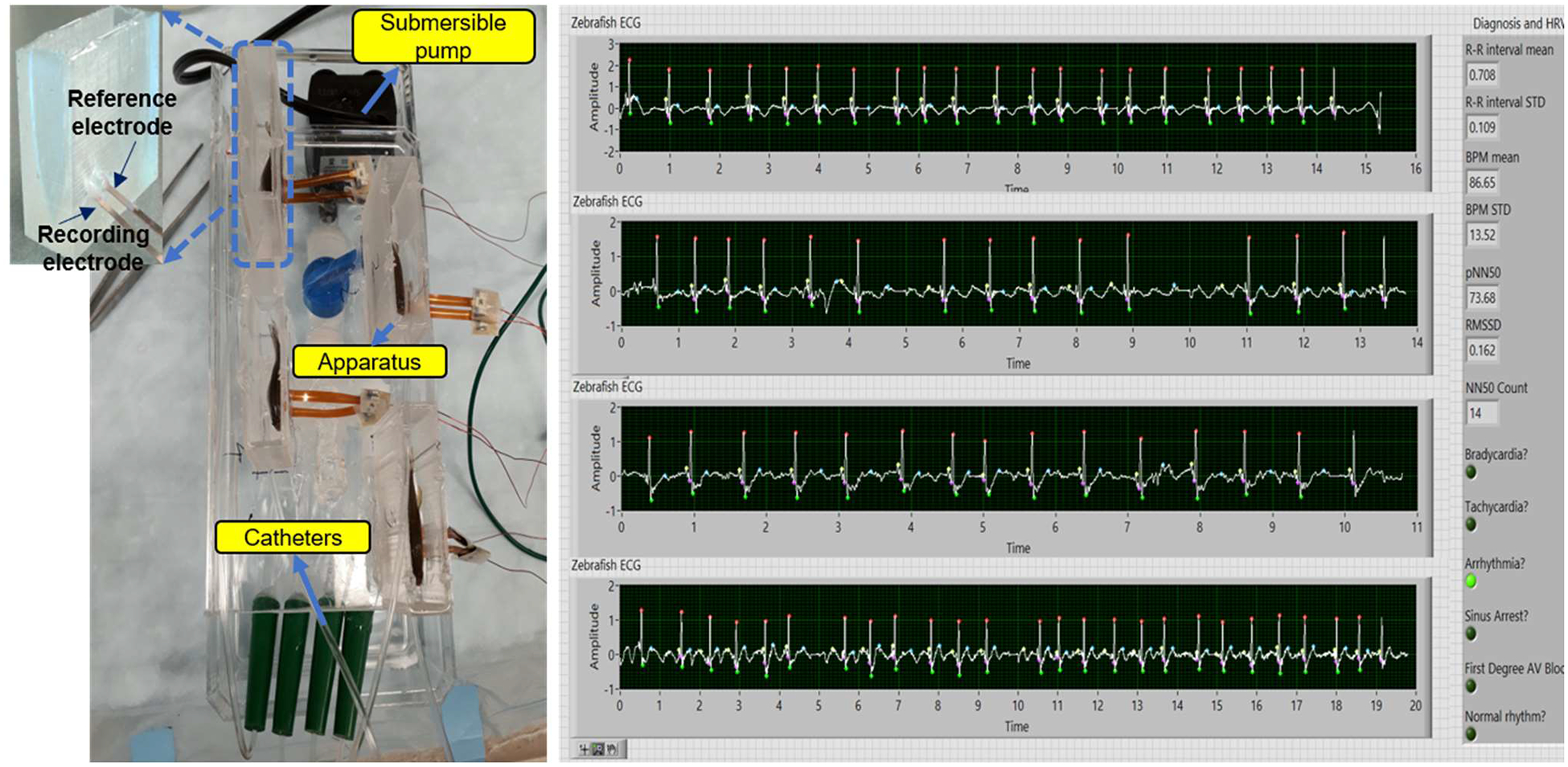
The prolonged anesthesia system for continuous zebrafish ECG monitoring (left) and the LabView GUI (right) for 4 fish simultaneously.
II. System Overview
A. Prolonged Mild Anesthesia System
The entire system consists of three unique subsystems: the housing system, recording system, and anesthetic drug delivery system. The housing system includes in-house apparatuses made of polydimethylsiloxane (PDMS) with 3D-printed molds. The apparatus was designed to fit a range of fish comfortably and secure them for recording (Fig. 1 – left panel). The detail of design could be found in our previous work [8]. The apparatus also had two electrodes made of 125-μm thick polyimide (Kapton, DuPont, Wilmington, DE) with sputtered-Au embedded at the bottom of the housing for recording. The electrodes were placed so that when the fish is loaded into the apparatus, they would be securely positioned at the chest and abdominal areas. These electrodes were then connected to differential AC amplifier that fed into a real-time LabVIEW GUI which was described in the next section.
The drug delivery system is illustrated in Fig. 1, including a submersible pump, a 4-way splitter, four catheters, a reservoir and tubing. The peristaltic pump placed inside the reservoir was to create the proper flow rate of system, which can be done by adjusting a valve connected to the pump. Here, a flow rate of 5.5–6 ml/min was chosen since it would provide zebrafish with adequate oxygen during the recording. The flow design of the system starts with the submerged pump inside the reservoir. After traveling through the pump, the solution is split 4 ways at the splitter. It goes through the catheters and into the fish directly though their mouths. Subsequently, the water exits through the gills and traveled back to the reservoir via the hole in the bottom of the apparatus.
B. Real-time Data Acquisition and Signal Processing GUI
A Labview GUI was developed to process and analyze zebrafish ECG data in real time. Here, the ECG signal collected from data acquisition (AM systems, 1700) was digitalized by NI DAQ (National Instruments, USB-6001) with 14-bit ADC at 1000 samples per second. The data stream would then be further processed to remove noise components such as baseline wander due to breathing or gill motion. A Dolph-Chebyshev low-pass filter with cutoff frequency of 40 Hz and the Wavelet Transform method were utilized to process the signal which was described comprehensively in [8]. Fig. 1 – right panel illustrates four zebrafish ECG data collected simultaneously with specific labels for the ECG features (P, QRS and T wave). Moreover, anomaly feature detection could be saved into word file for further use [5].
III. Experiments
All experiments were compliant with the Institutional Animal Care and Use Committee (IACUC) protocol (#AUP-18–115 at University of California, Irvine). Adult zebrafish targeted to the experiments were opened chest by doing surgery to form an incision and after two days they would be ready for the experiments. Owning to the use of prolonged anesthesia, the amount of Tricaine used for zebrafish could be reduced by at least 50 mg/L (25% of the original concentration) providing the ECG signal with more intrinsic features. Therefore, the first experiment was to confirm this assumption. We successively treated zebrafish with the Tricaine concentrations 100 mg/L and 150 mg/L compared with that of the original concentration (200 mg/L) reported previously [8]. Specifically, zebrafish were sedated under full anesthesia before being loaded into in-house apparatuses. Subsequently, a solution (100 mg/L) was filled in the reservoir, initiating the circulation system. The catheters were then put though zebrafish’s mouths. The GUI was turned on and data collection was run for 15 mins. The experiment was replicated exactly with 150 mg/L of Tricaine.
In the second experiment, we validated our system regarding to how long data can be collected while maintaining a stable stress level for zebrafish in the system. To conduct this experiment, we kept all setups as they were in the first one; however, we tested the system for different time periods, for 20, 40 min and 1 hour long. All data were then analyzed in terms of heartrate variation and significant changes during the measurement.
Finally, the proposed system was tested in an experiment to study methamphetamine (Meth)-induced heartrate. Methamphetamine (Meth) is a well-known stimulant that affects the cardiovascular system by inducing the release of monoamine neurotransmitters such as dopamine and norepinephrine [9]. While methamphetamine is consistently consumed due to its euphoric effect, studies showed that Meth intoxication can lead to numerous cardiovascular diseases, such as arrhythmia, hypertension, acute myocardial infarction, and cardiac death [10]. While tools are being developed to assess the effects of drugs on the zebrafish cardiovascular physiology, a simple assessment of heartrate is currently not available [11]. Therefore, this validation would provide evidence on the application of our fish system. In this experiment, a group of 4 fish was first anesthetized by Tricaine and tested for ECG before drug treatment. Then, the fish were treated with 25 μM methamphetamine (dissolved in fish water) for 5 minutes, followed by the Tricaine treatment before being reanalyzed for ECG. The two ECG results obtained before and after Meth treatment were compared to test if the system is efficient in determining parameters such as heartrate and ECG waves of fish.
IV. Results and Discussion
Fig. 2 presents the zebrafish ECG signal measured by the prolonged anesthesia system, and the recovery transition of the zebrafish from anesthesia to normal stages. Being treated with 50% of the original Tricaine concentration, fish were highly active, thereby interfering the signal (Fig. 2a) with signal-to-noise ratio (SNR) of 34.88 dB. Similarly, fish under the Tricaine concentration (150 mg/L) induced noise due to gill’s motion, making the SNR of 50.4 dB (Fig. 2b); however, noise level was not as strong as that in fish with the Tricaine concentration (100mg/L). Fig. 2c and Fig. 2d illustrate the processed ECG signals from the data collected by fish under 50% and 75% Tricaine, respectively. Here, a combination of signal processing methods was used. First, three finite impulse response (FIR) filters with 200th - order and Hanning window were utilized: 1) A high pass filter with cutoff frequency of 3 Hz for the gill’s motion reduction; 2) A low pass filter with 150 Hz cutoff frequency to obtain useful frequency range of the ECG signal; 3) 60 Hz notch filter to eliminate power line noise. Second, Wavelet transform with adaptive thresholds was implemented. After filtering, full features (P wave, QRS complex and T wave) of zebrafish ECG signal were clearly distinguishable.
Fig. 2.
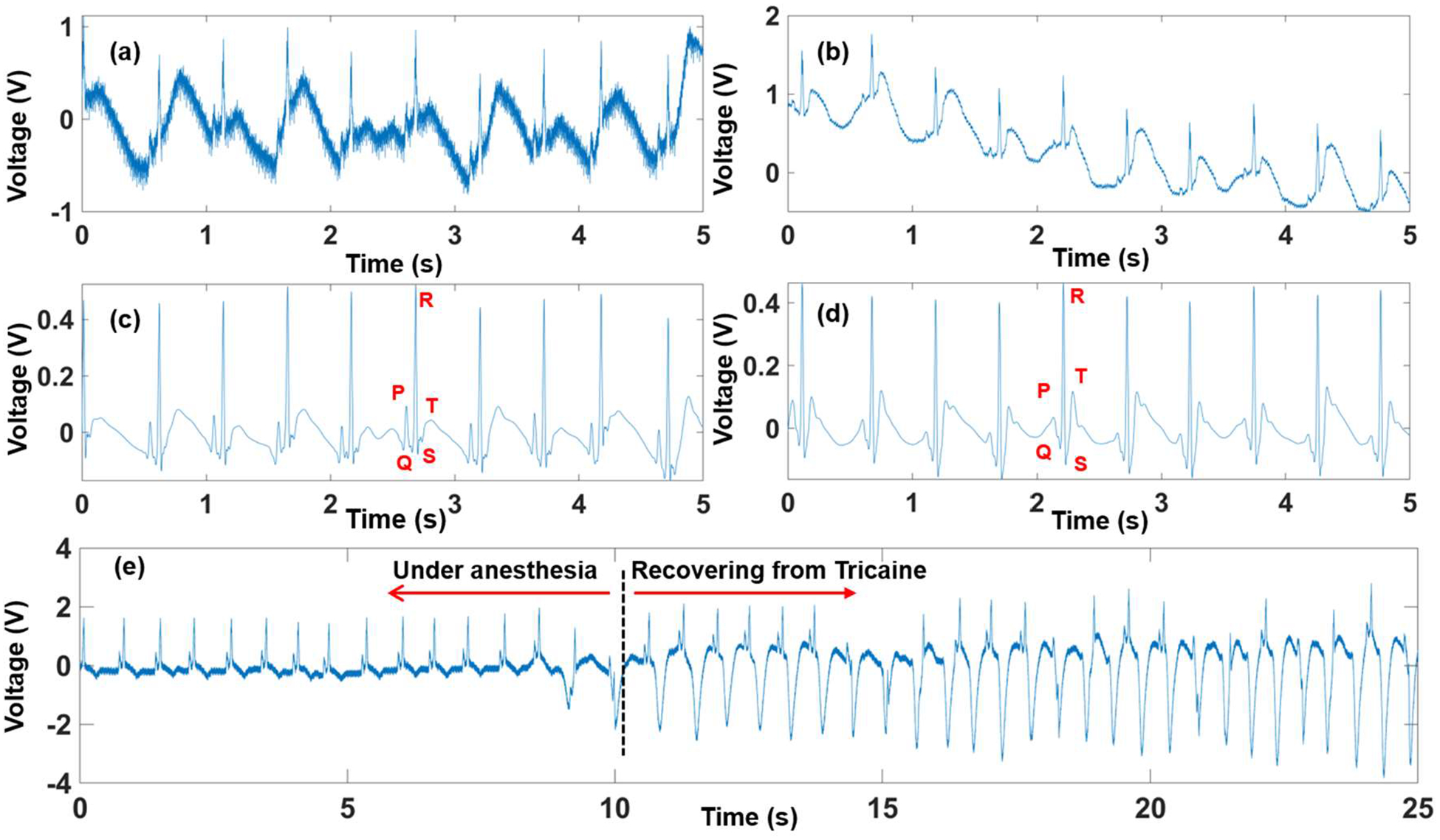
The zebrafish ECG collected with 100 mg/mL and 150 mg/mL Tricaine both raw data (2a) & (2b) and processed data (2c) & (2d). The transition stage when fish recover after being under anesthesia (2e).
Fig. 3 describes the heartrate variation in one-hour long measurement under 150 mg/L Tricaine. During the first five minutes, the heartrate was 80.3 beats per minute (BPM). It then significantly reduced to 69.8 BPM in the next five minutes, followed by slight changes in subsequent periods of time, making the heartrate average of 70.7 ± 3.2 BPM during the experiment. After one-hour long data collection, water was filled in reservoir to recover the fish. Fig. 2e depicts the moment when the fish started waking up. We can notice clearly the recovery transition with significant changes in the ECG signal. Specifically, the gills’ motion started dominating the signal as shown in the right side of the black dotted line in Fig. 2e compared with that noise level once the fish was under anesthesia. It should be noted that significantly depressed T-wave after recovering from Tricaine happened due to the gills’ motion as evidenced by its periodic repetition with a frequency of around 5 Hz. However, there were no abrupt and significant changes in the heartrate.
Fig. 3.
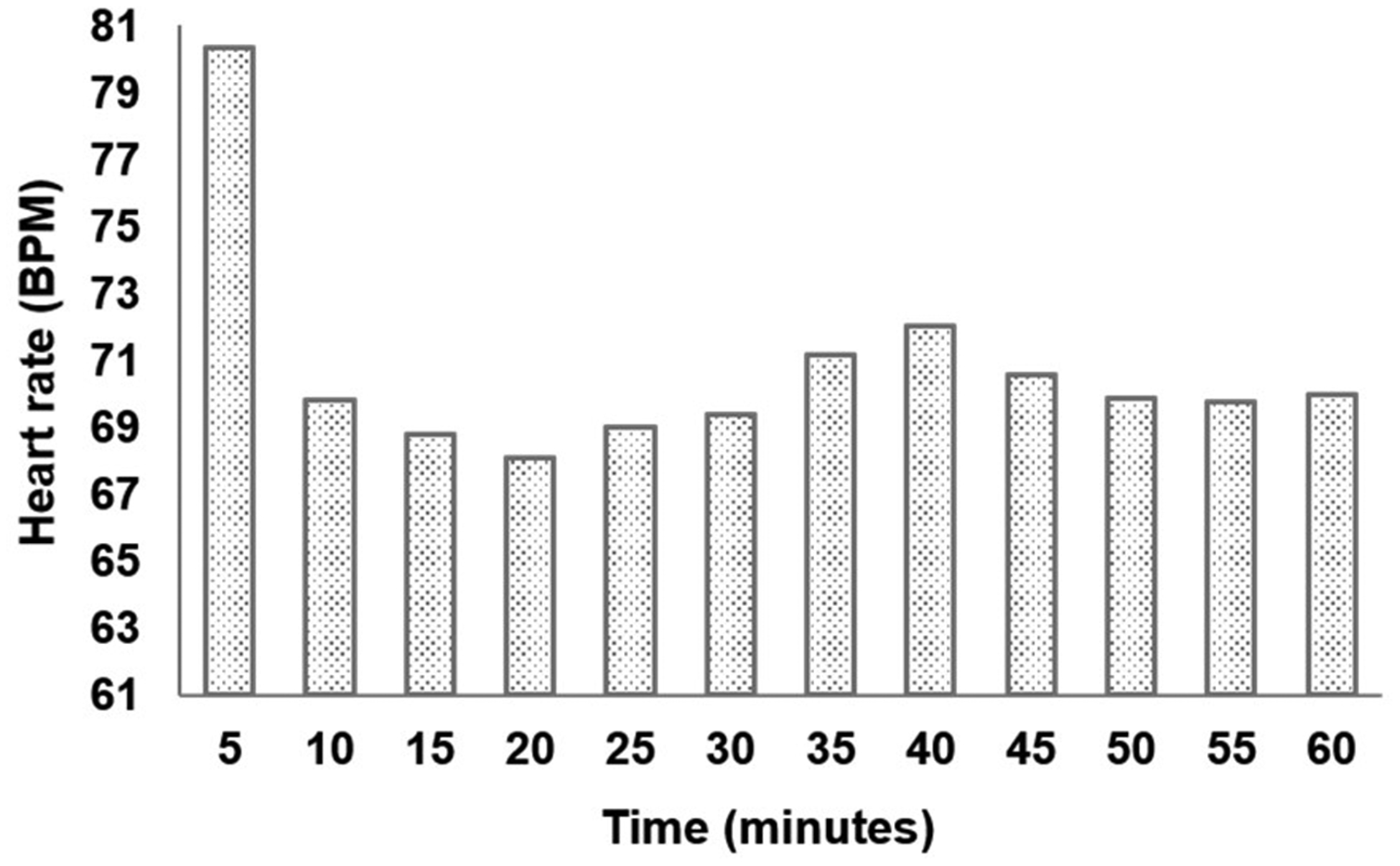
The heartrate value in every five minutes during one-hour long measurement.
Fig. 4 illustrates the heartrate from fish treated with Tricaine and Meth (Fig. 4A) by following the third experiment described in the previous section. Overall, fish with Meth showed higher heartrate values than those with Tricaine. While the average heartrate of fish 1 under Tricaine treatment is around 47.7 ± 11.78 BPM, it significantly goes up to 71.1 ± 12.01 BPM under Meth treatment. The discrepancy in the heartrate value between two groups is minimal in fish 2 and fish 4 with only a 0.5 BPM difference. It is noticeable that with the same scale, fish treated with Meth provided ECG with significantly higher amplitudes, i.e., nearly doubles the value found before Meth treatment and having higher P peaks than that treated by Tricaine (Fig. 4B), reflecting the effect of Meth to the fish.
Fig. 4.
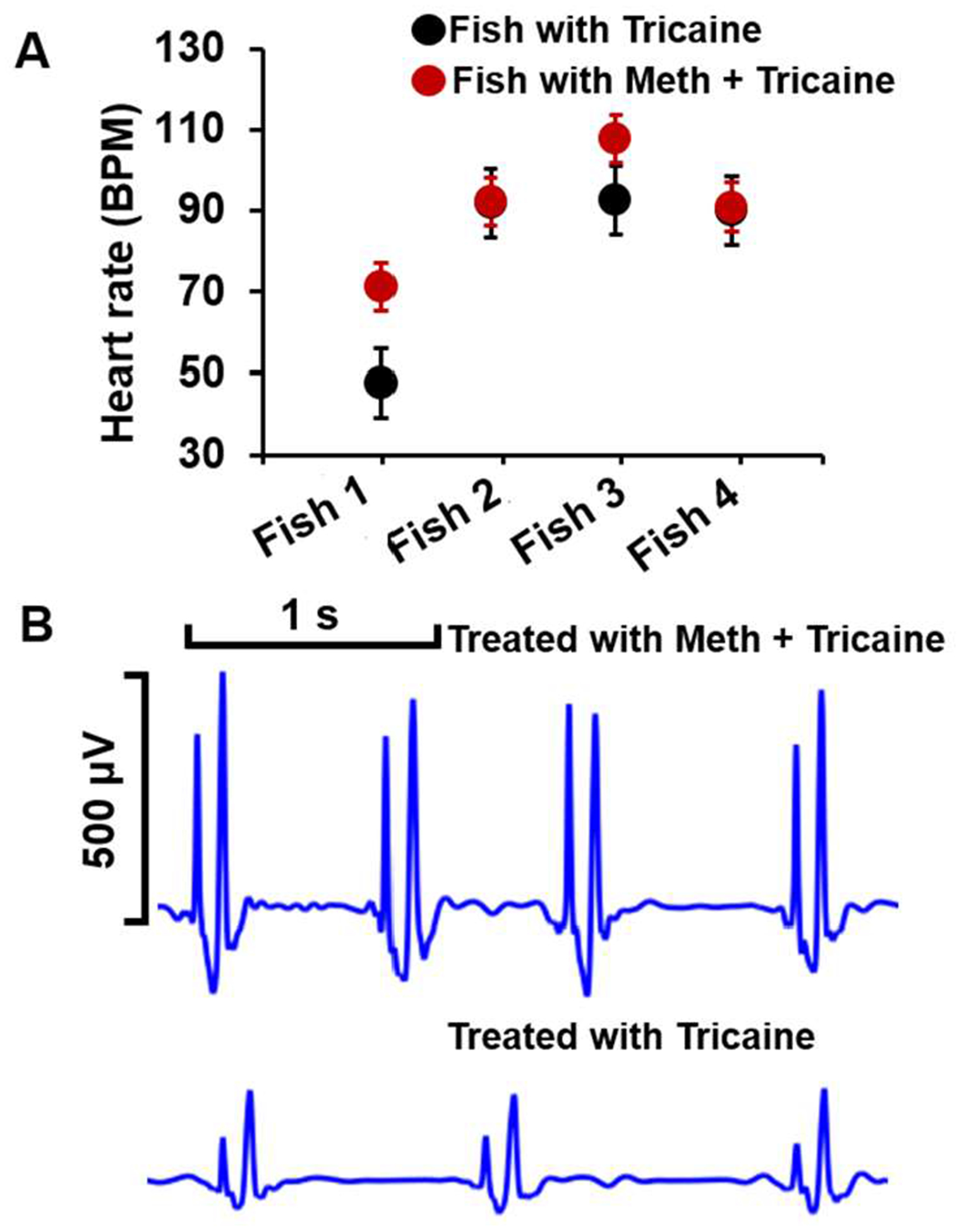
A. The heart rate of fish with tricaine and Meth treatment. B. Representative fish ECG with Tricaine and Meth treatment.
V. Conclusions
In this work, we have successfully demonstrated a prolonged mild anesthesia system for zebrafish ECG monitoring. After one-hour long measurement with small heartrate changes (±3.2 BPM), the fish could be fully recovered and ready for other experiments. Furthermore, the use of reduced Tricaine concentrations help in acquiring more-intrinsic ECG signals, indicating the usefulness of the system in a wide range of applications. The demonstration of the system using Meth treatment proved its effectiveness for drug screening as well as drug interaction studies with minimal side effects.
Acknowledgment
The authors would like to thank Yonghe Ding, Ph.D. and Jian H. Yan, M.D at Mayo Clinic for giving us insightful comments to improve the system.
Research supported by the NSF CAREER Award #1917105 and NIH R41 #OD024874.
Contributor Information
Xiaolei Xu, Department of Biochemistry and Molecular Biology, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN 55905 USA.
Isaac Clark, Biomedical Engineering, the College of Science and Engineering, University of Minnesota, MN 55455.
Michael P.H Lau, Sensoriis, Inc, Edmonds, WA, 98026..
Hung Cao, Electrical Engineering, the Samueli School of Engineering, UC Irvine, Irvine, CA 92697 USA; Sensoriis, Inc, Edmonds, WA, 98026..
References
- [1].Au - Zhao Y, Au - Yun M, Au - Nguyen SA, Au - Tran M, and Au - Nguyen TP, “In Vivo Surface Electrocardiography for Adult Zebrafish,” JoVE, p. e60011, 2019/August/01/ 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DYR, Tristani-Firouzi M, et al. , “Zebrafish model for human long QT syndrome,” Proceedings of the National Academy of Sciences, vol. 104, p. 11316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu F, Huang J, Adlerz K, Jadvar H, Hamdan MH, Chi N, et al. , “Evolving Cardiac Conduction Phenotypes in Developing Zebrafish Larvae: Implications to Drug Sensitivity,” Zebrafish, vol. 7, pp. 325–331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cao H, Yu F, Zhao Y, Zhang X, Tai J, Lee J, et al. , “Wearable multi-channel microelectrode membranes for elucidating electrophysiological phenotypes of injured myocardium,” Integrative Biology, vol. 6, pp. 789–795, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lenning M, Fortunato J, Le T, Clark I, Sherpa A, Yi S, et al. , “Real-Time Monitoring and Analysis of Zebrafish Electrocardiogram with Anomaly Detection,” Sensors, vol. 18, p. 61, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang W-C, Hsieh Y-S, Chen I-H, Wang C-H, Chang H-W, Yang C-C, et al. , “Combined Use of MS-222 (Tricaine) and Isoflurane Extends Anesthesia Time and Minimizes Cardiac Rhythm Side Effects in Adult Zebrafish,” Zebrafish, vol. 7, pp. 297–304, 2010. [DOI] [PubMed] [Google Scholar]
- [7].Lin M-H, Chou H-C, Chen Y-F, Liu W, Lee C-C, Liu LY-M, et al. , “Development of a rapid and economic in vivo electrocardiogram platform for cardiovascular drug assay and electrophysiology research in adult zebrafish,” Scientific Reports, vol. 8, p. 15986, 2018/October/30 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Le T, Lenning M, Clark I, Bhimani I, Fortunato J, Marsh P, et al. , “Acquisition, Processing and Analysis of Electrocardiogram in Awake Zebrafish,” IEEE Sensors Journal, vol. 19, pp. 4283–4289, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kish SJ, “Pharmacologic mechanisms of crystal meth,” Cmaj, vol. 178, pp. 1679–82, June 17 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kaye S, McKetin R, Duflou J, and Darke S, “Methamphetamine and cardiovascular pathology: a review of the evidence,” Addiction, vol. 102, pp. 1204–1211, 2007. [DOI] [PubMed] [Google Scholar]
- [11].Zakaria ZZ, Benslimane FM, Nasrallah GK, Shurbaji S, Younes NN, Mraiche F, et al. , “Using Zebrafish for Investigating the Molecular Mechanisms of Drug-Induced Cardiotoxicity,” BioMed Research International, vol. 2018, p. 10, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


