Abstract
Background:
In pulmonary hypertension (PH) subgroups, elevated pulmonary vascular resistance (PVR) ≥3.0 WU is associated with poor prognosis. However, the spectrum of PVR risk in PH is not known. To address this area of uncertainty, we aimed to analyze the relationship between PVR and adverse clinical outcomes in PH.
Methods:
We analyzed retrospectively all patients undergoing right heart catheterization (RHC) in the Veterans Affairs healthcare system (2008–2016; 1,153 [570–1,971] days median follow-up). Cox proportional hazards models were used to assess the association between PVR and outcomes, and the mortality hazard was validated in a RHC cohort from Vanderbilt University Medical Center (1998–2014; 1,752 [1,281–2,999] days median follow-up).
Findings:
The primary cohort (N=40,082; male, N=38,751 [96.7%]; 66.5 [61.1–73.5] yr) included patients with a history of heart failure (N=23,201 [57.9%]) and chronic obstructive pulmonary disease (N=13,348 [33.3%]). We focused on patients at risk for PH based on mean pulmonary artery pressure (mPAP) ≥19 mmHg (N=32,725 of 40,082 [81.6%]). When modeled as a continuous variable, the all-cause mortality hazard for PVR was increased at ~2.2 WU compared to PVR=1.0 WU. Among patients with ≥19 mmHg and pulmonary artery wedge pressure ≤15 mmHg, the adjusted hazard ratios for mortality and heart failure hospitalization were 1.71 (95% CI: 1.59–1.84, P<0.0001) and 1.27 (95% CI: 1.13–1.43, P=0.0001), respectively, when comparing PVR ≥2.2 WU to <2.2 WU. The validation cohort (N=3,699, male, N=1,860 [50.3%] male, 60.4 [49.5–69.2] yr) included N=2,870 patients [77.6%] with mPAP ≥19 mmHg (male, N=1,418 [49.4%]). The adjusted mortality hazard ratio PVR ≥2.2 WU for patients with PAWP ≤15 mmHg in the mPAP ≥19 mmHg group (N=1,221 of 2,870 [42.5%]) was 1.81 (95% CI: 1.33–2.47, P=0.0002).
Interpretation:
These data widen the PVR risk continuum associated with mortality and heart failure hospitalization to ~2.2 WU among patients referred for RHC with elevated pulmonary artery pressure. Testing the generalizability of these findings in at-risk populations with fewer cardiopulmonary comorbidities is warranted.
Introduction
Elevated mean pulmonary artery pressure (mPAP) is the principal hemodynamic finding in pulmonary hypertension (PH).1 The prior definition of PH used mPAP ≥25 mmHg; however, historical data from healthy volunteers established mPAP ~19–20 mmHg as the upper limit of normal,2 with values <25 mmHg corresponding to adverse prognosis in large right heart catheterization (RHC) referral populations3,4 and among smaller well-phenotyped PH cohorts.5 This led recently to a PH hemodynamic definition that is contemporary, evidence-based, and inclusive of mPAP >20 mmHg.6
Importantly, elevated mPAP may be observed under certain physiological or immediately reversible conditions, and in-and-of-itself is not pathognomonic for pulmonary vascular disease.7 Pulmonary vascular resistance (PVR) ≥3.0 Wood units (WU) is used to prognosticate and guide clinical decision-making in pulmonary arterial hypertension (PAH), primary obstructive lung disease, and orthotopic heart transplantation candidates, among other selected PH subgroups.8–10 However, the upper limit of normal PVR may reach ~2.1 WU in some normal adult populations,11,12 and post-hoc analyses from single center studies in patients with connective tissue disease imply that PVR <3.0 WU is relevant clinically.13 Nonetheless, sufficiently powered studies focusing on the association between PVR and clinical risk in PH are lacking.
The Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA-CART) Program is a national quality and safety program for invasive cardiac procedures within the VA Healthcare System. As such, the Program collects hemodynamic data from every patient that undergoes a RHC in any of the 81 cardiac catheterization laboratories within this integrated healthcare system. Further, the CART Analytic Center is able to associate clinical and hemodynamic data to clinical outcomes, creating one of the largest cohorts containing longitudinal information on patients with PH.2,14–16 We leveraged the size of this database to analyze the association of PVR and mortality as well as heart failure-hospitalization in patients with elevated mPAP, defined here as ≥19 mmHg based on prior outcome data in this population.3 We validated our findings in a sex-balanced RHC cohort at Vanderbilt University Medical Center (VUMC).4 Overall, findings from this study aim to clarify the clinical importance of PVR when considering the recently revised mPAP criterion for PH diagnosis.6
Methods
Additional information on the methods is available in the Supplementary Material.
Primary Cohort Data Sources
The details of the CART program and assembly of the RHC registry have been reported in detail previously.2,17 Briefly, the CART program uses a software application embedded in the VA electronic health record for documentation of all cardiac catheterization procedures. Key patient characteristics and procedural data were collected and tracked for longitudinal outcomes. Regularly scheduled quality checks of the CART data are performed to ensure completeness and accuracy.
Primary Cohort Study Population
We evaluated all veterans with procedural data recorded in CART who underwent RHC as an outpatient or inpatient in the VA system between October 1, 2008 and September 30, 2016. In patients undergoing multiple RHCs, the first RHC was considered the index procedure and was the only one included in the analysis. A minimum of one year of follow-up data for outcomes was available for all subjects included in the cohort. Patients were included in the analyses if data from a complete RHC were available, defined as a recorded value for mPAP, pulmonary artery wedge pressure (PAWP), height, weight, cardiac output (CO), heart rate, systolic and diastolic PA pressure and PVR. We used the standard equation to calculate PVR as follows: (mPAP-PAWP)/CO expressed in WU. For subjects with CO measured by both assumed Fick and thermodilution methods, values acquired by thermodilution were used.14 The body surface area (BSA) was calculated by using the Du Bois formula (BSA = weight [kg].425 × height [cm].725), as reported previously.3 Using these inclusion criteria, we identified N=40,082 patient records from a total possible N=51,639 records (77.7%). Hemoglobin data within 3 months of the RHC were used in analyses. The Colorado Multiple Institutional Review Board approved this study.
Primary Cohort Outcomes
A complete list of the covariates analyzed in this study is provided in Supplementary Material. The primary outcome measure was time to all-cause mortality assessed via the VA vital status file. The file has 98.3% sensitivity and 97.6% exact agreement with the National Death Index.3 The secondary outcome measures were time post index-procedure to the composite endpoint of all-cause mortality or hospitalization due to heart failure, and the time post index-procedure to hospitalization due to heart failure.
Validation Cohort Study Population
The methods for acquiring and analyzing clinical, RHC, and echocardiographic data in the validation cohort have been published previously.4 Briefly, we queried the Synthetic Derivative database, a de-identified mirror of the Vanderbilt electronic health record, for all RHC reports between 1998–2014. The Synthetic Derivative operates under a waiver of consent because all data are de-identified. A unique algorithm using pattern matching and natural language processing was used to extract structured hemodynamic data from digitized RHC reports, as published previously.17 Non-physiologic values suggestive of data entry error (e.g. arterial saturation >100%) were deleted. Both inpatients and outpatients were included in this study, but subjects with missing mPAP, PAWP, or CO were excluded. When available, CO calculated using the thermodilution method was used. Using this approach, we identified N=4,343 patients for analysis from a total possible N=5,797 patients (75.0%). Among N=4,343 patients, there were N=3,699 (85.2%) patients with at least one year of follow up.
Comorbidity, echocardiography, and laboratory data were restricted to 6 months before or after RHC. Comorbidities were defined using either previously validated algorithms based on a combination of ICD codes and keywords or using laboratory data, as previously described.4,18 Quantitative and semi-quantitative echocardiographic data were extracted as described previously.4 The Synthetic Derivative is linked to the National Death Index, which was used to determine vital status. In concert with analyses involving the primary cohort, the follow-up time was calculated from the date of index RHC, and outcome data included only patients with at least 1 yr follow-up. If death from any cause was reported to the National Death Index, the reported date of death was recorded. Otherwise, patients were considered alive and were censored on the last search date of June 1, 2016.
Statistical Analyses
Differences in baseline clinical and hemodynamic characteristics between groups were analyzed using the Wilcoxon or Kruskal-Wallis test for continuous variables, or a chi-squared test for categorical variables.
The association between PVR and the outcomes of interest (time to event for mortality, heart failure hospitalization, or the composite event of either mortality or heart failure hospitalization) were modeled using a Cox proportional hazards models with a random intercept for RHC site (i.e., a frailty model), adjusting for baseline characteristics listed in the Supplementary Material section. Inpatient status was treated as a stratification variable due to a modest proportional hazard violation for this covariate. The predictor of primary interest (PVR) was log transformed and modeled using a natural spline with five degrees of freedom. The hazard ratio for different values of PVR was calculated relative to a reference value of PVR = 1.0 (chosen based on reports suggesting this level is within normal). The PVR was also modeled dichotomously instead of continuously (≥2.2 WU vs. <2.2 WU), and as a three level categorical variable (with cut puts at 2.2 WU and 3.0 WU). For the outcome of mortality and the composite event of mortality or heart failure hospitalization, time to event (i.e. t = 0) started at the time of procedure. For the event of heart failure hospitalization, time to event started at the date of discharge, and the cohort only included subjects who survived to discharge.
Models were fit in the following sub-groups: i) the entire cohort (which included the full range of mPAP values), ii) restricting to mPAP ≥19 mmHg, iii) restricting to mPAP ≥19 mmHg and PAWP ≤15 mmHg, iv) restricting to mPAP ≥19 mmHg and PAWP >15 mmHg, and v) restricting to mPAP ≥19 and PAWP ≤12 mmHg. When models were fit in a subgroup of the original cohort the covariate that was the basis of the exclusion was not included in the model (e.g., mPAP was included as a covariate when fitting for the entire cohort, but excluded as a covariate when fitting the subgroup with mPAP ≥19 mmHg).
Kaplan-Meier event-free curves were plotted stratified by PVR category (i.e., ≥2.2 WU vs. <2.2 WU) with death, heart failure hospitalization, or the composite event of either heart failure hospitalization or death as events. Unadjusted group comparisons for the Kaplan-Meier curves were made using a log-rank test. Similar analyses were conducted on the validation cohort. In addition, we estimated the overall mortality hazard ratio in the primary cohort adjusting only for the validation covariates. This provided a fairer comparison between the cohorts with which to assess validity since the available adjustment variables differed between them.19 The Pearson correlation coefficient is reported for the linear regression analysis comparing mPAP and PVR. Data preparation and analyses were conducted using SAS version 9.4 (SAS Institute, Cary North Carolina) and R version 3.6.1. P<0.05 was considered statistically significant.
Role of the funding source
The funding sources had no role in the study design; collection, analysis, or interpretation of the data; or, writing the report. Access to the raw data at VA was available to B.A.M., E.H., S.W.W., and G.C., and at VUMC to B.A.M., S.H., E.L.B. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Clinical and cardiopulmonary hemodynamic characteristics for the primary cohort.
The primary cohort (N=40,082) was largely male (N=38,751 [96.7%]) with a median age of 66.5 [61.1–73.5] yr and frequent history of cardiopulmonary comorbidities (Table 1). Prior data modeling mPAP as a continuous variable showed that clinical risk increases beginning ~19 mmHg.3 Here, mildly elevated mPAP (19–24 mmHg) and mPAP ≥19 mmHg were identified in 9,084 [22.7%] and 32,725 [81.6%] of 40,082 patients, respectively (Supplementary Figure 1). The correlation between mPAP and logPVR (r=0.56, P<0.0001), overall distribution of PVR, and prevalence of different PVR increments by mPAP group are provided in Supplementary Figure 2 and Supplementary Table 1. As expected in this population, increasing mPAP was associated with a right-shift in the distribution of PAWP (Supplementary Figure 3). There was an inverse relationship between CO profile and PVR for the entire cohort that was similar to our findings in patients with PAWP ≤15 mmHg (Supplementary Tables 2 and 3).
Table 1.
Patient demographic, clinical, and hemodynamic characteristics for the primary cohort.
| Primary Cohort (N=40,082) | |
|---|---|
| Age | 66.5 [61.1–73.5] |
| Male | 38,751 (96.7) |
| BMI (kg/m2) | 29.6 [25.8–34.4] |
| Inpatient RHC | 16,950 (42.3) |
| Race | |
| White | 31,994 (79.8) |
| Black | 7.261 (18.1) |
| Other | 827 (2.1) |
| Clinical Comorbidities | |
| Systemic hypertension | 35,429 (88.4) |
| Prior MI | 11,222 (28.0) |
| Congestive heart failure | 23,201 (57.9) |
| Atrial arrythmia | 11,335 (28.3) |
| Peripheral arterial disease | 8,214 (20.5) |
| Diabetes mellitus | 19,104 (47.7) |
| Prior CABG | 7,625 (19.0) |
| Prior PCI | 8,095 (20.2) |
| Prior valvular disease | 16,739 (41.8) |
| Prior stroke or TIA | 3,413 (8.5) |
| Pulmonary embolism | 1,490 (3.7) |
| Tobacco use | 24,706 (61.6) |
| COPD | 13,348 (33.3) |
| Interstitial lung disease | 252 (0.6) |
| Obstructive sleep apnea | 5,303 (13.2) |
| Portal hypertension | 298 (0.7) |
| Chronic kidney disease | 12,436 (31.0) |
| Connect tissue disease | 1,303 (3.3) |
| Renal replacement therapy | 1,870 (4.7) |
| Cancer | 6,711 (16.7) |
| Psychiatric disease | 1,152 (2.9) |
| Cardiopulmonary hemodynamics | |
| mPAP (mmHg) | 27 [20–35] |
| PASP (mmHg) | 40 [32–53] |
| PADP (mmHg) | 18 [12–25] |
| PAWP (mmHg) | 16 [11–23] |
| CO (Td), % (N) | 24,567 (61.3) |
| CO (eFick), % (N) | 34,773 (86.8) |
| CO (Td) (L/min) | 5.1 (4.2–6.2) |
| CO (eFick) (L/min) | 5.1 (4.2–6.1) |
| Sbp (mmHg) | 131 [121–140] |
| Dbp (mmHg) | 74 [67–80] |
Data for patients undergoing right heart catheterization from 2008–2016 were accessed from the Veterans Affairs (VA) Clinical Assessment and Tracking Program and from administrative data sources and served as the primary cohort. BMI, body mass index; MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous transluminal coronary intervention; TIA, transient ischemia attack; COPD, chronic obstructive pulmonary disease; mPAP, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; PAWP, pulmonary artery wedge pressure; CO, cardiac output; Td, thermodilution; eFick, estimated Fick; Sbp, systolic blood pressure; Dbp, diastolic blood pressure. Data are presented as N (%) for categorical variables and median [IQR] for continuous variables.
Association between PVR and outcome in patients with elevated pulmonary artery pressure.
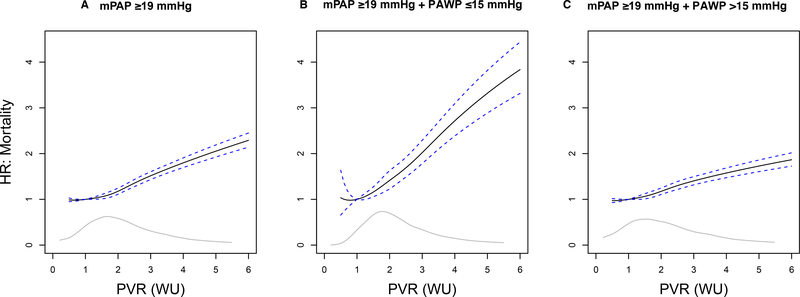
When PVR was modeled continuously in the entire CART cohort (N=40,082), the all-cause mortality hazard ratio adjusted for clinical variables and mPAP increased progressively over a wide range beginning at ~2.0 WU (Supplementary Figure 4). In healthy adults of a similar age range as our study cohort, the upper limit of normal PVR reaches ~2.1 WU.12 In this study, a PVR of 2.2 WU was the minimum level above 2.1 WU at which a clinically significant mortality hazard ratio was observed (hazard ratio [HR]=1.09; 95% confidence interval [CI]: 1.03–1.15, P=0.0034).
We focused subsequent analyses on patients with mPAP ≥19 mmHg (N=32,725). In this group, the all-cause adjusted mortality hazard increased progressively over a wide range that included PVR of 2.2 WU (HR=1.25; 95% CI: 1.18–1.32, P<0.0001) (Figure 1A). However, the CO that has been shown to be independently associated with adverse outcome14 was present only when PVR was >4.0 WU (Supplementary Table 2). More specifically, the median CO for the PVR ranges of 2.2 − 3.0 WU and ≥3.0 WU were 4.87 [4.11–5.73] L/min and 4.13 [3.4–4.97] L/min, respectively.
Figure 1. The adjusted hazard ratio for all-cause mortality stratified by pulmonary vascular resistance (PVR) in patients with elevated pulmonary artery pressure.
From the primary cohort, the hazard ratio (95% confidence interval) for all-cause mortality is plotted for PVR 1–6 WU relative to a reference value of 1.0 WU in patients with mean pulmonary artery pressure ≥19 mmHg (A). This population was then restricted to PAWP ≤15 mmHg (B) and, alternatively, to PAWP >15 mmHg (C). WU, Wood unit. The grey line inset is the kernel density estimate, representing the relative density of patients across PVR levels.
There were N=15,780 of 32,725 (48.2%) patients with PVR ≥2.2 WU (Table 2), of which 2,147 patients (13.6%) had an mPAP of 19–24 mmHg. The slope of the relationship between PVR and outcome differed after stratifying patients by high or low PAWP, defined here as ≤15 mmHg (N=12,073 [36.9%] ) vs. >15 mmHg (N=20,652 [63.1%]) (Figure 1B,C and Supplementary Table 4). For example, the adjusted mortality hazard ratio at PVR=2.2 WU was 1.52 (95% CI: 1.33–1.74, P<0.0001) and 1.23 (95% CI: 1.15–1.30, P<0.0001) for low PAWP and high PAWP patients, respectively, but at PVR=4.0 WU this hazard ratio was 2.72 (95% CI: 2.39–3.09, P<0.0001) and 1.58 (95% CI: 1.47–1.69, P<0.0001) for these subgroups.
Table 2.
Patient demographic, clinical, and hemodynamic characteristics for the primary and validation cohorts stratified by pulmonary vascular resistance.
| Characteristic | Primary Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| mPAP≥19 mmHg | mPAP≥19 mmHg | |||||
| PVR<2.2 WU (N=16,945) | PVR ≥2.2 WU (N=15,780) | P Value | PVR<2.2 WU (N=1,047) | PVR ≥2.2 WU (N=1,589) | P Value | |
| Age | 66.2 [61.0–72.6] | 67.1 [61.6–74.9] | <0.0001 | 61.3 [51.7–69.1] | 60.1 [49.0–69.6] | 0.07 |
| Male | 16,464 (97.2) | 15,181 (96.2) | <0.0001 | 623 (59.5) | 677 (42.6) | <0.0001 |
| BMI (kg/m2) | 31.2 [27.1–36.1] | 28.9 [25.2–33.7] | <0.0001 | 30.7 [26.5–36.2] | 28.6 [24.4–33.4] | <0.0001 |
| Race | <0.0001 | 0.0006 | ||||
| White | 13,892 (82.0) | 11,918 (75.5) | 888 (84.8) | 1,269 (79.9) | ||
| Black | 2,737 (16.2) | 3,526 (22.3) | 114 (10.9) | 258 (16.2) | ||
| Other | 316 (1.9) | 336 (2.1) | 45 (4.3) | 62 (3.9) | ||
| Clinical Comorbidities | ||||||
| Systemic hypertension | 15,189 (89.6) | 14,163 (89.8) | 0.73 | 891 (85.1) | 1,258 (79.2) | 0.0001 |
| Coronary heart disease | 9,493 (56.0) | 9,002 (58.3) | <0.0001 | 76.9 (80.5) | 1,074 (67.6) | <0.0001 |
| Congestive heart failure | 9,427 (55.6) | 11,233 (71.2) | <0.0001 | 490 (46.8) | 899 (56.6) | <0.0001 |
| Atrial arrythmia | 4,756 (28.1) | 5,496 (34.8) | <0.0001 | 327 (31.2) | 473 (29.8) | 0.42 |
| Diabetes mellitus | 8,652 (51.1) | 7,881 (49.9) | 0.0436 | 421 (40.2) | 606 (38.1) | 0.29 |
| COPD | 5,166 (30.5) | 6,560 (41.6) | <0.0001 | 131 (12.5) | 221 (13.9) | 0.30 |
| Interstitial lung disease | 80 (0.5) | 133 (0.8) | <0.0001 | 33 (3.2) | 122 (7.7) | <0.0001 |
| Obstructive sleep apnea | 2,583 (15.2) | 2,125 (13.5) | <0.0001 | 149 (14.2) | 168 (10.6) | 0.0047 |
| Chronic kidney disease | 5,295 (31.2) | 5,651 (35.8) | <0.0001 | 81 (7.9) | 108 (10.8) | 0.340 |
| Connect tissue disease | 496 (2.9) | 526 (3.3) | 0.0348 | 19 (1.8) | 93 (5.9) | <0.0001 |
| Cardiopulmonary hemodynamics | ||||||
| mPAP (mmHg) | 26 [22–32] | 35 [28–43] | <0.0001 | 26 [21–31] | 37 [29–46] | <0.0001 |
| PASP (mmHg) | 39 [34–47] | 53 [42–65] | <0.0001 | 37 [32–45] | 56 [43–73] | <0.0001 |
| PADP (mmHg) | 18 [14–22] | 23 [18–30] | <0.0001 | 17 [14–21] | 24 [18–31] | <0.0001 |
| PAWP (mmHg) | 18 [14–24] | 18 [12–25] | <0.0001 | 17 [13–22] | 16 [10–22] | <0.0001 |
| TPG (mmHg) | 8 [6–10] | 15 [12–20] | <0.0001 | 9 [7–11] | 18 [13–28] | <0.0001 |
| CO (Td) (L/min) | 5.7 [4.8–6.9] | 4.5 [3.7–5.4] | <0.0001 | 5.7 [4.7–6.9] | 4.4 [3.7–5.3] | <0.0001 |
| CO (eFick) (L/min) | 5.6 [4.7–6.6] | 4.5 [3.7–5.4] | <0.0001 | 6.2 [5.1–7.4] | 4.7 [3.9–5.8] | <0.0001 |
| PVR (WU) | 1.5 [1.1–1.8] | 3.3 [2.6–4.5] | <0.0001 | 1.5 [1.1–1.9] | 3.9 [2.8–6.2] | <0.001 |
| Sbp (mmHg) | 131 [122–141] | 130 [119–140] | <0.0001 | 124 [109–143] | 125 [110–143] | 0.99 |
| Dbp (mmHg) | 73 [67–80] | 73 [67–80] | 0.60 | 70 [61–78] | 72 [63–82] | <0.0001 |
| Hgb (g/dL) | 13.1 [11.6–14.4] | 13.1 [11.5–14.5] | 0.07 | 13.0 [11.4–14.2] | 12.9 [11.5–14.3] | 0.25 |
Data for patients with mean pulmonary arterial pressure (mPAP) ≥19 mmHg stratified by PVR from the primary cohort (VA-CART) and validation cohort (Vanderbilt University Medical Center) (VUMC) (1998–2014) are presented. BMI, body mass index; CAD, coronary artery disease; TIA, transient ischemia attack; COPD, chronic obstructive pulmonary disease; mPAP, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; PAWP, pulmonary artery wedge pressure; TPG, transpulmonary gradient; CO, cardiac output; Td, thermodilution; eFick, estimated Fick; Sbp, systolic blood pressure; Dbp, diastolic blood pressure; WU, Wood unit; Hgb, hemoglobin. For the VA-CART database, the entry coronary heart disease includes composite of either myocardial infarction, prior coronary artery bypass graft surgery, or a diagnosis of atherosclerotic coronary artery disease. For the VUMC database, coronary heart disease includes a diagnosis of atherosclerotic coronary artery disease. Categorical data are expressed as N (%), and continuous data are expressed as median [IQR]. The number of missing values for VA-CART: hemodynamic variables: Sbp (N=826), Dbp (N=826), CO by eFick (N=4,281), CO by Td (N=12,925). The PVR was unavailable for N=234 VUMC patients who were excluded from this table.
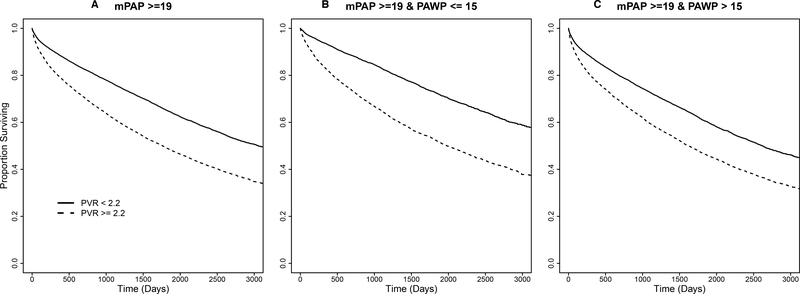
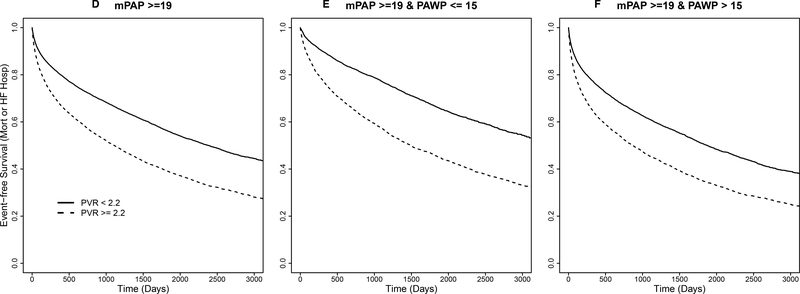
We next determined the event-free Kaplan-Meier curves for mortality (Figure 2A–C), the composite endpoint of mortality and heart failure hospitalization (Figure 2D–F), and heart failure hospitalization alone (Supplementary Figure 5) for mPAP ≥19 mmHg patients by dichotomous PVR ≥2.2 vs. <2.2 WU, subgrouped further according to low vs. high PAWP level. Overall, patients were followed for a median of 1,153 [570–1,971] days. The estimated mortality rates from the Kaplan-Meier analysis were 20.5% and 11.3% (1-year) and 43.5% and 28.5% (5-year) for patients with PVR ≥2.2 WU vs. <2.2 WU, respectively. The estimated heart failure hospitalization-free survival rates for the same groups were 32.1% and 19.5% at 1-year, and 53.5% and 37.1% at 5-years, respectively. The estimated rates for heart failure hospitalization for patients with PVR ≥2.2 WU vs <2.2 WU were 15.6% and 10.1% at 1-year, and 22.6% and 16.1% at 5-years, respectively. All the outcomes were less favorable with PVR ≥2.2 WU vs. <2.2 WU when the patients were divided further into high and low PAWP subgroups (Supplementary Table 5).
Figure 2. Time to event plot for unadjusted mortality and heart failure hospitalization-free survival stratified by pulmonary vascular resistance (PVR) for patients with elevated pulmonary artery pressure in the primary cohort.
From the primary cohort, a Kaplan-Meier analysis was performed to determine the probability of all-cause mortality according to PVR ≥2.2 WU vs. <2.2 WU in patients with mean pulmonary artery pressure ≥19 mmHg (X2=886.5, P<0.0001) (A). This population was then stratified by PAWP ≤15 mmHg (X2=539.1, P<0.0001) (B) and PAWP >15 mmHg (X2=430.5, P<0.0001) (C). A similar analysis performed for the composite of all-cause mortality and heart failure-hospitalization according to PVR ≥2.2 WU vs. <2.2 WU in patients with mean pulmonary artery pressure ≥19 mmHg (X2=1019.5, P<0.0001) (D). This population was then stratified by PAWP ≤15 mmHg (X2=578.1, P<0.0001) (E) and PAWP >15 mmHg (X2=559.3, P<0.0001) (F). Results from a log-rank test comparing strata are provided for each analysis. Censoring begins at and beyond 1 year following the index right heart catheterization, represented here as day 0. Number of patients at risk are provided at days 0, 500, 1000, 1500, 2000 post-right heart catheterization.
Elevated PVR ≥2.2 WU was associated with increased hazard for all-cause mortality after adjusting for clinical variables compared to patients with PVR <2.2 WU (HR=1.47, 95% CI: 1.42–1.53, P<0.0001) (Table 3). The association between PVR and mortality was maintained when restricting the analysis to patients with mildly elevated mPAP of 19–24 mmHg (Supplementary Tables 6). The magnitude of this association in patients with mPAP ≥19 mmHg was greater among patients with low PAWP (HR=1.71, 95% CI: 1.59–1.84, P<0.0001), but was maintained in high PAWP patients (HR=1.36, 95% CI: 1.29–1.42, P<0.0001). The hemodynamic data for these subgroups is provided in Supplementary Table 7. When using a more stringent PAWP threshold of ≤12 mmHg to define pre-capillary PH,20 patients with PVR ≥2.2 WU had a 72% (HR=1.72, 95% CI: 1.55–1.90, P<0.0001) increase in mortality hazard compared to patients with PVR <2.2 WU.
Table 3.
Hazard ratio for outcomes among patients with elevated mean pulmonary artery pressure (mPAP) stratified by pulmonary vascular resistance (PVR), and further subgrouped by low and high pulmonary artery wedge pressure (PAWP).
| Primary Cohort | Validation Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality: PVR ≥2.2 vs. <2.2 WU | Heart Failure Hospitalization: PVR ≥2.2 vs. <2.2 WU | All-Cause Mortality: PVR ≥2.2 vs. <2.2 WU | ||||||||
| Adjusted HR (95% CI) | Chi Square | P-Value | Adjusted HR (95% CI) | Chi Square | P-Value | Adjusted HR (95% CI) | Chi Square | P-Value | ||
| mPAP ≥19 mmHg | All PAWP | 1.47 (1.42 – 1.53) | 379.6 | <0.0001 | 1.17 (1.11 – 1.24) | 32.7 | <0.0001 | 1.58 (1.31 – 1.91) | 22.63 | <0.0001 |
| PAWP >15 mmHg | 1.36 (1.29 – 1.42) | 163.7 | <0.0001 | 1.15 (1.08 – 1.23) | 19.7 | <0.0001 | 1.44 (1.12 – 1.83) | 8.43 | 0.0037 | |
| PAWP ≤15 mmHg | 1.71 (1.59 – 1.84) | 214.0 | <0.0001 | 1.27 (1.13 – 1.43) | 16.2 | 0.0001 | 1.81 (1.33 – 2.47) | 13.97 | 0.0002 | |
Data for patients with mean pulmonary arterial pressure (mPAP) ≥19 mmHg stratified by PVR and then further subgrouped by low vs. high PAWP are presented for the primary cohort (VA-CART) and validation cohort (Vanderbilt University Medical Center) (VUMC) (1998–2014). Adjustment model included the following clinical variables: categorical age, sex, race, categorical body mass index, and history of systemic hypertension, congestive heart failure, left heart failure, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease (COPD), liver cirrhosis, chronic kidney disease that included the patient receiving renal replacement therapy, portal hypertension, connective tissue disease, atrial arrhythmia, interstitial lung disease, pulmonary embolism, valvular disease, tobacco use, psychiatric disease, stroke, obstructive sleep apnea, and inpatient hospital status at the time of right heart catheterization. For the validation cohort, the following variables from the adjustment used in the primary cohort analysis were available and included: age, sex, race, diabetes mellitus, body mass index, coronary artery disease, valvular heart disease, COPD, atrial arrhythmia, interstitial lung disease, connective tissue disease, systemic hypertension, chronic kidney disease, obstructive sleep apnea, congestive heart failure. For both the primary and validation cohorts, the model for analyses of patients without restricting PAWP also included the co-variate of PAWP itself. Data are presented as hazard ratio (95% confidence interval) relative to the referent group of PVR<2.2 Wood units (WU).
Irrespective of PAWP, patients with mPAP ≥19 mmHg and PVR ≥2.2 WU had a 17% (HR=1.17, 95% CI: 1.11–1.24, P<0.0001) and 37% (HR=1.37, 95% CI: 1.32–1.42, P<0.0001) increase in the adjusted hazard of heart failure hospitalization and adjusted hazard of composite endpoint of mortality and heart failure hospitalization, respectively, compared to PVR <2.2 WU. In the low PAWP and high PAWP subgroups, the increase in adjusted hazard of composite endpoint of mortality and heart failure hospitalization risk with a PVR ≥2.2 WU was 62% (HR=1.62, 95% CI: 1.52–1.73, P<0.0001) and 27% (HR=1.27, 95% CI: 1.22–1.32, P<0.0001), respectively, compared to PVR <2.2 WU.
Validating the relationship between PVR and outcome in patients with elevated pulmonary artery pressure.
Compared to the primary cohort, patients with mPAP ≥19 mmHg in the VUMC cohort (N=2,870 of 3,699 [77.6%]) had a similar median age (60.8 [50.0–69.5] yr) and BMI (29.5 [25.3–34.8]), but were sex-balanced (male, N=1,418 [49.4%]). The clinical and cardiopulmonary hemodynamic profile of this cohort is provided in Supplementary Figure 1 and Supplementary Table 8. Among patients with mPAP ≥19 mmHg, a PVR ≥2.2 WU was identified in N=1,589 (55.4%), which included N=181 (11.4%) with mPAP of 19–24 mmHg. Patients with mPAP ≥19 mmHg and PVR ≥2.2 WU had significantly smaller left ventricular end-diastolic and left atrial dimensions without differences in left ventricular ejection fraction compared to PVR <2.2 WU patients (Supplementary Table 9).
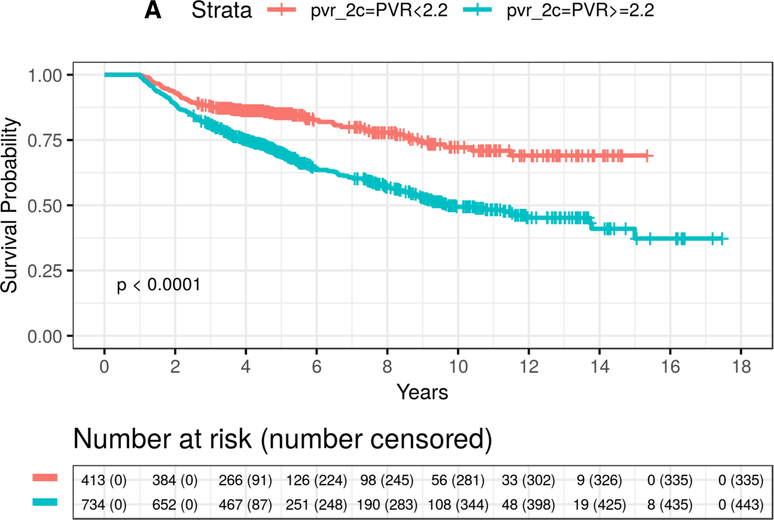
The estimated 5-year mortality rates from the Kaplan-Meier analysis were 29% and 19% for mPAP ≥19 mmHg patients with PVR ≥2.2 WU vs. <2.2 WU, respectively. Similar to findings from the primary cohort, among patients with mPAP ≥19 mmHg and PVR ≥2.2 WU, mortality was both elevated in the low PAWP subgroup and the high PAWP subgroup compared to PVR <2.2 WU (Figure 3 and Table 2). Overall, there was a 58% increase in the adjusted mortality hazard in patients with high vs. low PVR (HR=1.58, 95% CI: 1.31–1.91, P<0.0001). This increase was similar to results seen in the primary cohort: (i) a 39% increase from the comparably adjusted Cox model (HR=1.39, 95% CI: 1.34–1.44, P<0.0001), and (ii) a 47% increase estimated in the fully adjusted Cox model (HR=1.47, 95% CI: 1.42–1.53, P<0.0001) (Table 3).
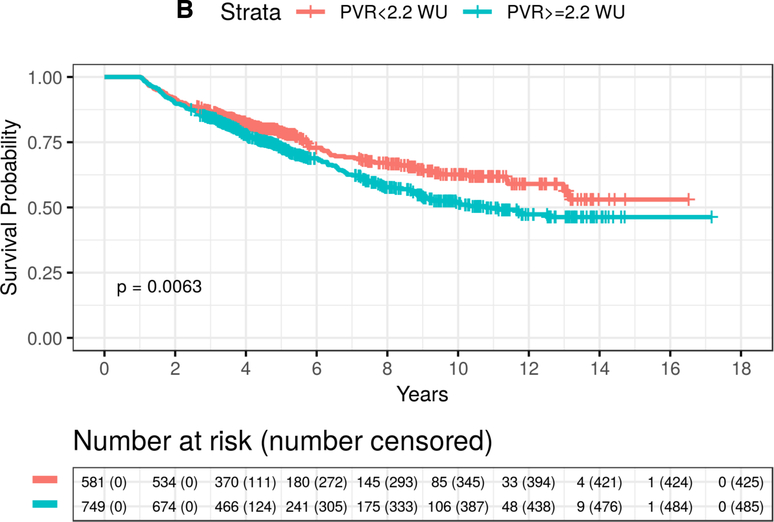
Figure 3. Time to event plot for unadjusted mortality stratified by pulmonary vascular resistance (PVR) for patients with elevated pulmonary artery pressure in the validation cohort.
From the validation cohort, Kaplan-Meier analysis was performed to determine the probability of all-cause mortality according to PVR ≥2.2 WU vs. <2.2 WU in patients with mean pulmonary artery pressure ≥19 mmHg stratified by (A) PAWP ≤15 mmHg (X2=35.2, P<0.0001) and (B) PAWP >15 mmHg (X2=7.5, P=0.0063). Number of patients at risk are provided at 2 year increments post-right heart catheterization.
In the low PAWP subgroup, the mortality rates at 5-years were 30% and 15% (log-rank P<0.0001) for PVR ≥2.2 WU compared with PVR <2.2 WU, respectively. In the low PAWP subgroup, an 81% (HR=1.81, 95% CI: 1.33–2.47, P=0.0002) increase in the adjusted hazard for mortality for PVR ≥2.2 WU compared with PVR <2.2 WU was also observed (Table 3 and Supplementary Table 10). When using PAWP ≤12 mmHg, patients with PVR ≥2.2 WU had a 65% (HR=1.65, 95% CI: 1.03–2.64, P=0.0359) increase in mortality hazard compared to patients with PVR <2.2 WU. Between the VA and VUMC cohorts, widening the PVR criterion that informs clinical risk in PH to ≥2.2 WU captured 55.9% more patients than the current PVR standard6 of ≥3.0 WU.
Discussion
To our knowledge, these data provide the first evidence-based information on the continuum of clinical risk related to PVR in patients with elevated pulmonary artery pressure. Specifically, our findings show that PVR ≥2.2 WU is associated with a sizeable increase in adjusted mortality risk, which was consistent across two RHC referral cohorts and particularly evident in the absence of frank post-capillary PH. Results from this study, therefore, establish a critical framework toward optimizing the specificity of PH cardiopulmonary hemodynamic criteria used clinically.
The current study is consistent with prior reports focusing on selected PH subgroups suggesting that PVR ≥3.0 WU is associated with poor prognosis,8–10 but adds substantially to this field by exposing the range of PVR that is an independent predictor of major clinical events, including mortality and heart failure hospitalization. We identified a lower limit of ~2.2 WU that is likely abnormal and clinically meaningful. This observation converges with extrapolated data from prior reports on normative values in healthy control patients of a similar age to our study group showing that the upper limit of normal PVR is ~2.1 WU.12 Thus, one major finding of this work is showing that opportunity exists to recalibrate the combination of variables used to diagnose PH in patients with cardiopulmonary disease.
Recently, the mPAP threshold used to define PH was decreased to >20 mmHg from ≥25 mmHg.6,21,22 This in turn, prioritized the need for greater knowledge on PVR values that modulate prognosis in populations that include patients with mPAP ~20–24 mmHg. Between the two cohorts in this study, 49% of patients had mPAP ≥19 mmHg and PVR ≥2.2 WU, among which 13% had mPAP 19–24 mmHg. In turn, a majority of patients with mPAP ranging from 19 to 24 mmHg had a PVR <2.2 WU. These results reinforce the utility of adding PVR to mPAP for optimizing the specificity of hemodynamic criteria classifying patients with elevated PAP from presumed pulmonary vascular disease. Additionally, the prevalence of patients with mildly elevated mPAP and a high-risk PVR level in this study was substantially greater than what has been suggested in the absence of empiric data.23 This lends support to an emerging focus in the field recognizing that patients with mild PH, which in this study was associated with an average 52% mortality risk increase after stratifying by PVR ≥2.2 WU, are often overlooked and require greater attention at point of care.24–26
Stratifying patients by PAWP had a major effect on outcome estimates in our study, illustrating the limitation of using the same PVR level to define clinical risk between pre- and post-capillary PH. Relative to all-comers with mPAP ≥19 mmHg and PVR ≥2.2 WU, adverse outcome was exaggerated among patients with pre-capillary PH at the time of RHC compared to post-capillary PH. Elucidating the pathophysiological mechanism by which to explain this difference is not possible from the current dataset; however, these data are consistent with outcomes in patients with isolated post-capillary PH from vascular congestion due to left heart disease.27 In these populations, stabilizing intravascular volume or treating the primary defect prior to pathogenic changes in vascular remodeling28 (and, therefore, prior to increased PVR) is often sufficient for reversing PH. By contrast, PH with elevated PVR may occur in patients with obstructive lung disease, which were well-represented in this study population; chronic left heart disease treated late in the disease course; or, other bona fide causes of pre-capillary PH.1 In these scenarios, irreversible vascular remodeling is a key driver of elevated mPAP. Our findings suggest that this population is particularly vulnerable, and that greater emphasis on PVR in risk stratification is needed for pre-capillary PH phenotypes encountered commonly, in addition to rarer but traditional subtypes such as PAH.
There are several implications of our findings to clinical medicine. First, clarifying the lower limit of PVR that is prognostic when including patients with mildly elevated mPAP favors detection of early PH. We show here that using a PVR threshold of ≥3.0 WU in patients with mPAP ~20–25 mmHg excludes a sizeable group of vulnerable patients with elevated mPAP. Although such an approach may offer higher diagnostic specificity, this also seems to emphasize detection of severe (late) PH. An abnormal CO was generally not observed in this study for patients with a PVR range of 2.2−3.0 WU. This illustrates that sizeable clinical risk exists in the absence of detrimental changes in central cardiac hemodynamics, and emphasizes the need to develop strategies that focus on early PH detection and diagnosis.
Secondly, although data from this study should not be used to justify pulmonary vasodilatory pharmacotherapy use in patients with mPAP ≥19 and PVR ≥2.2 WU (or non-PAH patients with PVR ≥3.0 WU), our findings suggest early engagement of multidisciplinary care plans without PAH pharmacotherapy could be considered for this novel subgroup. This includes close monitoring, functional (e.g., exercise) assessments, and behavior modifications focusing on tobacco cessation and prescription physical exercise, which are already recommended as first-line therapy for at-risk patients.1 Third, by determining the minimal level for mPAP and PVR that associate with adverse outcome, these data may offer an evidence-based entry point into preventive medicine for PH. It may be the case that shifting clinical trial design focus to patients near or below the hemodynamic framework identified by data in this study as high-risk exposes unexpected opportunities to delay disease progression.3,25 Accomplishing dedicated studies pursuing this line of research, however, will require developing effective strategies to recruit at-risk patients early in the disease process. This is particularly challenging owing to non-specific symptoms that are common in PH and delay diagnosis,1 although this barrier is addressable.
The primary cohort mainly included males, and both study cohorts were from the same country and excluded a sizeable number of patients with incomplete records. Together, these factors may limit the generalizability of the results. Further, despite robust adjustments, outcomes here may have been confounded due to comorbid conditions. However, it is notable that an association between mild PH and pathogenic changes to structural and functional features of the right heart has been reported recently,29–30 providing a potential pathophysiological basis for adverse outcomes that was not contingent solely on comorbid disease. We used normative data and a clinically meaningful change in risk to select the PVR threshold of 2.2 WU, but a different method could have been used to determine an alternative PVR level for which mortality and morbidity risk increases. Therefore, PVR threshold levels other than 2.2 WU could have been used in this study. For example, data on CO measured by direct Fick, which is the gold standard method, were not available. We used CO measurements acquired by thermodilution, which in the study cohorts predicts mortality more strongly than estimated Fick.13 Thus, the CO measurement method could have affected our results.
These data do not provide information on temporal trends in cardiopulmonary hemodynamics; therefore, patients in this study cannot be regarded as an early PH subgroup. Practice-specific approaches to measuring hemodynamics may have affected our results,14 although the robust size of the databases would seem to offset potential confounding effects of methodological variability during RHC. Pulmonary arterial compliance, right ventricular-pulmonary artery coupling, or greater focus on oscillatory components of afterload may provide important insight into prognosis in mild PH that is not determined by PVR.31 These and other alternative measurements were not studied in the current project, but should be considered in future similar projects.
In summary, these data expand the range of PVR that is associated with hard clinical events, including mortality, in patients at-risk for PH referred for RHC. We identified a PVR threshold of ~2.2 WU as clinically meaningful in patients with elevated pulmonary artery pressure using an unbiased analysis, which was consistent across two large at-risk populations. In particular, PVR ≥2.2 WU emerged as a central determinant of outcome estimates in patients with a pre-capillary hemodynamic phenotype. Further analyses are needed to generalize these data to PH populations with less cardiopulmonary disease that are nonetheless susceptible to abnormal vascular remodeling. Overall, these findings support reconsidering the combination of hemodynamic variables used to identify patients with PH.
Supplementary Material
Research in context.
Evidence before this study:
We searched Medline using PubMed for all full text publications from inception to April 10, 2020, using the medical subject heading (MeSH) terms “pulmonary vascular resistance” and “pulmonary hypertension” and “mortality”, as well as the term “catheterization” in any search field. We included studies in humans aged 19 years old or greater of the following types: clinical study, dataset, journal article, multicenter study, and observational study. Reviews, biographies, case reports, clinical trials, and non-academic article types or publications not in English were excluded. This search returned 38 publications; of these, two were determined to be case reports and 35 included highly selected clinical populations, such as patients with congenital heart disease, prior pneumothoroax, or post-orthotopic heart transplant status. All of these reports included <320 patients and none included patients with mildly elevated pulmonary artery pressure using the recently revised hemodynamic definition of pulmonary hypertension (PH). The remaining publication was a single-center study of 4,343 patients, and PVR emerged from a multivariate analysis as a risk factor for mortality in patients that included (but was not exclusive of) mild pulmonary hypertension. In that study, the continuum of clinical risk related to PVR was not reported. Overall, knowledge on the clinical importance of PVR in PH prior to the current work emphasized historical consensus opinion and data from small studies in selected populations analyzing outcome differences using preset definitions.
Added value of this study:
To our knowledge, this study provides the first evidence-based assessment on the continuum of clinical risk related to PVR in PH, which is derived from a sufficiently-powered national right heart catheterization database and validated in a second large patient cohort. This study also uses mortality and heart failure hospitalization, which are disease-relevant endpoints, to determine the association between PVR and outcomes using the recently revised pulmonary artery pressure criterion for determining PH risk clinically.
Implications of all the available evidence:
Data from this study demonstrate that in PH, risk for adverse outcome related to PVR emerges at ~2.2 WU, which is a level well below what is associated with the disease state in clinical practice currently. We identified patients with precapillary PH at the time of right heart catheterization as particularly vulnerable. Overall, these results suggest that reconsidering the hemodynamic parameters that define PH in patients with cardiopulmonary disease is warranted, and identify a need for developing early detection strategies to capture this large and vulnerable population.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Rocky Mountain Regional VA Medical Center, Aurora, CO, USA. The authors would like to acknowledge Mary E. Plomondon, M.S.P.H., Ph.D. for her assistance in completing this project.
Funding
Bradley A. Maron: NIH grants: R56HL131787, R01HL139613-01, R21HL145420; National Scleroderma Foundation, Cardiovascular Medical Research Education Foundation, and McKenzie Family Charitable Trust (provided salary support to conduct research); Evan L. Brittain.: NIH R01 HL146588, R01 HL146588-01S1; B.M.W.: NIH 5T32HL007633-32 (provided salary support to conduct research); G.A.A.: NIH 5KL2TR002542-02; G.C.: Department of Veterans Affairs MERIT award I01CX001892, NIH/NHLBI R01HL128661 and R01HL148727 (provided salary support to conduct research). The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH funded Shared Instrumentation Grant S10RR025141; and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. No funding source had any role in the writing of the manuscript or the decision to submit it for publication. No co-author was paid to write this manuscript by a pharmaceutical company or other agency. The corresponding author had access to all the data in the study and final responsibility for the decision to submit this manuscript for publication.
Conflicts of Interest
Dr. Maron reports being a consultant for Actelion, and co-inventor on the following patents or patent application that are related to pulmonary hypertension (U.S. Patent #9,605,047; PCT/US2015/029672; Provisional ID: #62475955; Provisional ID: #24624; Provisional ID: #24622). Dr. Brittain reports serving on the advisory board for Bayer related to topics outside the scope of the current work. Dr. Waldo receives investigator initiated research grants to the Denver Research Institute from Abiomed, Cardiovascular Systems Incorporated, Janssen Pharmaceuticals, the National Institutes of Health and Veterans Affairs Health Services Research & Development for projects outside the scope of the current work. Dr. Wertheim reports grants from National Institutes of Health, grants from Pulmonary Vascular Research Institute, and grants from Brigham and Women’s Hospital/Hearst Foundation during the conduct of the study. Dr. Tedford reports no direct conflicts pertinent to the development of this manuscript. Other general conflicts include consulting relationships with Medtronic, Arena Pharmaceuticals and United Therapeutics. Dr. Tedford reports support from Actelion, Medtronic, Abiomed, United Therapeutics and Merck, and personal fees from United Therapeutics, and Arena Pharmaceuticals, which are for efforts outside the scope for the current work. Dr. Olschewski reports personal fees and other from Bayer, grants and personal fees from Boehringer, personal fees from Chiesi, personal fees from GlaxoSmithKline, grants and personal fees from Inventiva, personal fees from Johnson&Johnson, personal fees from Menarini, personal fees from Novartis, personal fees from Pfizer, personal fees from Merck Sharp & Dohme, and personal fees from Actelion outside the scope of the current work. Dr. Galiè reports grants and personal fees from Actelion, grants and personal fees from Janssen, personal fees from Pfizer, personal fees from MSD, and grants and personal fees from Bayer, which are outside the scope of the current. Dr. Kovacs reports personal fees and non-financial support from Actelion, Bayer, GlaxoSmithKline, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, Chiesi, Vitalaire, Ferrer, and AOP outside the scope of the current work. Dr. Humbert reports grants and personal fees from Acceleron, grants and personal fees from Actelion, grants and personal fees from Bayer, personal fees from Merck, outside the scope of the current work. Dr. Choudhary reports funding from Novartis for research outside the scope of the current work.
References
- 1.Maron BA, Galiè N. Diagnosis, treatment, and clinical management of pulmonary arterial hypertension in the contemporary era: a review. JAMA Cardiol 2016; 1(9):1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34; 888–94. [DOI] [PubMed] [Google Scholar]
- 3.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting and Tracking program. Circulation 2016; 133:1240–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assad TR, Maron BA, Robbins IM, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol 2017;2:1361–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs G1, Maier R, Aberer E, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med 2009;180:881–6. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53. pii: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy SB, Bhatia ML, Mathur VS, et al. Hemodynamic effects of chronic severe anemia. Circulation 1963;28:346–56. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915–57. [DOI] [PubMed] [Google Scholar]
- 9.Tedford RJ, Beaty CA, Mathai SC, et al. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant 2014; 33:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med 1972;286:912–18. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62:D42–50. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systemic review. Eur Respir J 2012;39:319–28. [DOI] [PubMed] [Google Scholar]
- 13.Xanthoui P, Jordan S, Milde N, et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis 2019; 2020;79:370–378. [DOI] [PubMed] [Google Scholar]
- 14.Opotowsky AR, Hess E, Maron BA, et al. Thermodilution vs Estimated Fick Cardiac Output Measurement in Clinical Practice: An Analysis of Mortality From the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) Program and Vanderbilt University. JAMA Cardiol 2017;2:1090–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventetuolo CE, Hess E, Austin ED, et al. Sex-based differences in veterans with pulmonary hypertension: Results from the veterans affairs-clinical assessment reporting and tracking database. PLoS One 2017; 12:e0187734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leary PJ, Hess E, Barón AE, et al. H2 Receptor Antagonist Use and Mortality in Pulmonary Hypertension: Insight from the VA-CART Program. Am J Respir Crit Care Med 2018; 197:1638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program) Am J Cardiol 2014;114:1750–57. [DOI] [PubMed] [Google Scholar]
- 18.Assad TR, Hemnes AR, Larkin EK, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford I, Norrie J, Ahmadi S. Model inconsistency, illustrated by the Cox proportional hazards model. Stat Med 1995;14:735–746. [DOI] [PubMed] [Google Scholar]
- 20.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987;107:216–23. [DOI] [PubMed] [Google Scholar]
- 21.Maron BA, Brittain EL, Choudhary G, Gladwin MT. Redefining pulmonary hypertension. Lancet Respir Med 2018; 6:168–170. [DOI] [PubMed] [Google Scholar]
- 22.Kolte D, Lakshmanan S, Jankowich MD, Brittain EL, Maron BA, Choudhary G. Mild Pulmonary Hypertension Is Associated With Increased Mortality: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2018; 7:e009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs JSR, Torbicki A. Proposed new pulmonary hypertension definition: is 4 mm(Hg) worth re-writing medical textbooks? Eur Respir J 2019; 53 pii: 1900197. [DOI] [PubMed] [Google Scholar]
- 24.Humbert M, Gerry Coughlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev 2012; 21:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huston JH, Frantz RP, Brittain EL. Early intervention: should we conduct therapeutic trials for mild pulmonary hypertension before onset of symptoms? Pulm Circ 2019; 9:2045894019845615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52:3792–800. [DOI] [PubMed] [Google Scholar]
- 27.Obakata M, Reddy YNV, Shah SJ, et al. Effects of interatrial shunt on pulmonary vascular function in heart failure with preserved ejection fraction. J Am Coll Cardiol 2019; 74: Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Rich S, Rabinovitch S. Diagnosis and treatment of secondary (non-category 1) pulmonary hypertension. Circulation 2008; 118:2190–9. [DOI] [PubMed] [Google Scholar]
- 29.Strange G, Stewart S, Celermajer DS, et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol 2019; 73:2660–72. [DOI] [PubMed] [Google Scholar]
- 30.Huston JH, Maron BA, French J, et al. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA Cardiol 2019. September 18; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013; 6:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.