Abstract
Background
Periodontal disease continues to be prevalent globally, but little clinical research has been undertaken to evaluate the long-term benefits of a daily oral hygiene regimen on progression of gingivitis/early periodontitis. The objective of this study was to evaluate the effects of an oral hygiene regimen (OHR) on the periodontal health of adults in good general health with established gingivitis and early periodontitis over 24 months.
Methods
A randomized controlled trial was conducted in adults with established gingivitis, with isolated sites of probing pocket depth >4 mm. Study participants were randomized to the OHR (bioavailable stannous fluoride dentifrice, oscillating-rotating electric toothbrush, cetylpyridinium chloride rinse, and floss; P&G) or usual care products (sodium fluoride dentifrice and manual toothbrush; P&G) groups. At baseline and every 6 months, gingivitis and periodontal measures were assessed and a prophylaxis was conducted. The primary outcome was Gingival Bleeding Index–Bleeding Sites (GBI–BS). Analyses used ANCOVA at 5% significance levels.
Results
A total of 107 individuals were enrolled; 87 completed the study. Mean GBI–BS, Modified Gingival Index, and Probing Pocket Depth (PPD) scores were significantly lower at each visit for the OHR versus usual care group by 28% to 39%, 12% to 18%, and 6% to 13%, respectively (p≤ 0.0009). The magnitude of reduction in median number of ≥2 mm PPD loss events for OHR versus the usual care group at 24 months was 74%.
Conclusion
Long-term use of the OHR produced significant periodontal health improvements versus the usual care products.
Keywords: cetylpyridinium chloride, dental floss, gingivitis, oral hygiene, periodontal diseases, stannous fluoride
Abstract
Contexte
La maladie parodontale continue d’être prévalente sur le plan mondial, mais peu de recherches cliniques ont été effectuées pour évaluer les avantages à long terme d’un régime d’hygiène buccodentaire sur la progression de la gingivite ou de la parodontite précoce. L’objectif de cette étude était d’évaluer les effets d’un régime d’hygiène buccodentaire (RHB) sur la santé parodontale des adultes en bonne santé générale qui présentent une gingivite établie et une parodontite précoce au cours de 24 mois.
Méthodologie
Un essai contrôlé randomisé a été effectué chez des adultes présentant une gingivite établie et des sites isolés de profondeurs de poches au sondage >4 mm. Les participants de l’étude ont été confiés à un groupe de RHB aléatoire (pâte dentifrice au fluorure stanneux biodisponible, une brosse à dents électrique rotative et oscillante, un rince-bouche au chlorure de cétylpyridinium et la soie dentaire; P & G) ou à un groupe de produits de soins habituels (dentifrice au fluorure de sodium et une brosse à dents manuelle; P & G). La gingivite et les mesures parodontales ont été évaluées au début de l’intervention et tous les 6 mois et une prophylaxie avait été effectuée. Le résultat primaire était l’Indice de saignement gingival–les sites de saignements (ISG–SS). L’analyse de covariance a été utilisée à des seuils de signification de 5 %.
Résultats
Un total de 107 personnes ont été inscrites : 87 ont terminé l’étude. Les cotes moyennes de l’ISG–SS, de l’indice gingival modifié et des cotes de profondeurs des poches au sondage (PPS) étaient significativement plus faibles à chaque visite du groupe de RHB par rapport au groupe de soins habituels, de 28 % à 39 %, 12 % à 18 % et 6 % à 13 %, respectivement (p≤ 0,0009). L’ampleur de la réduction en nombre médian d’événements de perte de PPS ≥2 mm du groupe de RHB par rapport au groupe de soins habituels était de 74 % à 24 mois.
Conclusion
L’utilisation à long terme du RHB a produit des améliorations significatives de la santé parodontale par rapport aux produits de soins habituels.
PRACTICAL IMPLICATIONS OF THIS RESEARCH.
An oral hygiene home care regimen including an electric toothbrush, stannous fluoride dentifrice, CPC rinse, and dental floss improved gingival health and slowed the rate of disease progression compared to a regimen involving a manual toothbrush and sodium fluoride dentifrice only.
At-home use of the regimen can provide long-term periodontal health benefits.
INTRODUCTION
Surveys find the earliest form of periodontal disease, gingivitis, in the majority of populations worldwide.1-3 The more severe form of periodontal disease, periodontitis, affects approximately 10% of surveyed populations.2,3 Gingivitis and periodontitis result from an inflammatory response in the periodontal tissues due to localized toxic effects of dental plaque microbial biofilms.4-6 Host response competence also plays an important role in the progression from gingivitis to periodontitis.7 The cost and effort to repair (or compensate for) damaged periodontium (e.g., periodontal therapy, dental implants, prosthodontics) support the importance of disease prevention, principally consistent, effective oral hygiene directed towards thorough dental plaque removal. While professional dental scaling and root planing provide this benefit on an infrequent basis, daily thorough personal oral hygiene is considered to be the most effective approach to maintaining a healthy periodontium.4,8-12 Though most populations carry out some form of daily oral hygiene, the underlying epidemiological statistics and specific studies show most individuals find it challenging to maintain periodontal health through their own efforts.13-15
Numerous clinically proven products have been developed to assist clients in improving gingival health, including manual toothbrushes with angled bristles,16 oscillating–rotating electric toothbrushes,17,18 irrigators,19 polytetrafluoroethylene dental floss,20 and a number of antimicrobial dentifrices and mouthrinses.21 Several studies have begun to evaluate the effect of these oral hygiene measures on more advanced periodontal disease progression.22-25 Recent studies have been initiated to explore the benefits of combination oral hygiene for controlling plaque and gingivitis at home.26-28
The purpose of this study was to examine the clinical benefits of combined mechanical (oscillating–rotating electric toothbrush + polytetrafluoroethylene dental floss) and chemotherapeutic (bioavailable stannous fluoride toothpaste and cetylpyridinium chloride mouthrinse) technologies on the prevention of gingivitis and progression of probing pocket depth (PPD) over a 2-year period in generally healthy adults with established gingivitis and isolated sites with PPD >4 mm.
METHODS
Study design and population
This was a randomized, controlled, examiner-blind, 2-treatment parallel group study approved by an Institutional Review Board (Ref# 0482-13-HMO, Hadassah Medical Organization Helsinki Committee, Jerusalem, Israel) and registered in the ISRCTN database (ISRCTN66780304). The study was conducted over a 24-month period at the Kibbutz Na’an in Israel. One hundred and ten physically healthy adult volunteers with established gingivitis and isolated sites of PPD >4 mm but no PPD >6 mm were qualified to be enrolled in the study. All participants provided written, informed consent. The target population consisted of individuals between 18 and 65 years of age with at least 16 natural teeth, a minimum of 20 bleeding sites, and 9 sites for possible plaque sampling, including 3 healthy sites (PPD ≤ 3 mm, no bleeding), 3 gingivitis sites (PPD ≤ 3 mm, bleeding), and 3 periodontal sites (PPD 4 mm to 6 mm, bleeding). Individuals with moderate to severe periodontal disease, undergoing active treatment for periodontitis, and/or any diseases or conditions that could be expected to interfere with safe completion of the study were excluded. Plaque sampling was conducted for purposes of future research. Participants were stratified and randomly assigned equally to either a regimen group (antimicrobial paste, rinse, floss, and electric toothbrush) or a usual care group (standard anti-cavity toothpaste and regular manual toothbrush,). Participants were requested to carry out home oral hygiene with the assigned products twice daily for the duration of the study according to the written and verbal usage instructions given to them during product distribution. At the same time points, participants received oral soft tissue exams and had gingival inflammation, bleeding, and periodontal evaluations. Both groups received supragingival dental prophylaxes every 6 months at study visits, consistent with local norms and standards. Products were resupplied approximately every 6 months post-baseline. Study participants were contacted periodically to check compliance with product use.
Investigational product(s) and instructions
The regimen group was provided an oscillating–rotating electric toothbrush with brush head comprising angled bristles (Oral-B Professional Care 5000 with Smart Guide and CrossAction [EB50] brush head); dentifrice with 1100 ppm F bioavailable stannous fluoride and 350 ppm F sodium fluoride (Oral-B Pro-Expert All Around Protection toothpaste); 0.07% cetylpyridinium chloride rinse (Crest Pro-Health Multi-Protection rinse); and floss (Oral-B Essential). Participants were instructed to brush with the assigned electric toothbrush and the marketed dentifrice for 2 minutes twice daily (morning and evening) following the manufacturer’s usage instructions and to floss the whole mouth once daily for the duration of the study. They were instructed to glide the floss between teeth to the gumline and to curve the floss to contact as much of the tooth as possible and to rinse with 20 mL of the mouth rinse for 30 seconds after each brushing. Participants used only the assigned products in place of normal oral hygiene products for the duration of the study.
The usual care group was provided a dentifrice with 1450 ppm F sodium fluoride (Oral-B 123) and a soft regular manual toothbrush (Oral-B Indicator 35). They were instructed to brush with the assigned products twice daily (morning and evening) in their customary manner, using only the assigned products in place of normal oral hygiene products during the study (continued use of floss was allowed if that was part of their usual care). All products in both groups were manufactured by The Procter and Gamble Co., Cincinnati, Ohio, USA.
Study visits
Study participants refrained from performing any oral hygiene procedures the morning prior to scheduled evaluation visits and from using medicated lozenges, breath mints, eating, drinking (except for water), smoking, and chewing gum for 4 hours prior to the visit. A comprehensive oral examination was conducted, and demographic information and study inclusion and exclusion criteria were obtained. Participants then received Modified Gingival Index (MGI), Gingival Bleeding Index (GBI), CAL, and PPD assessments in that order by the same experienced examiner (AZ). They were instructed to continue using regular home oral hygiene products until the baseline visit, approximately 2 weeks after screening.
At baseline, continuance criteria were verified and a comprehensive oral examination was conducted. Participants were randomized to either the regimen or usual care group by site staff using a computer-generated sequence produced by the study sponsor based on screening mean PPD, mean GBI, age, gender, and tobacco use. They received assigned products from site staff to use twice daily in an area separated from the examination area to ensure blinding of the examiner to the identity of the test products. Participants in both groups also received supervised oral hygiene and product usage instructions (verbal and written) and used the assigned products in front of a mirror supervised by site staff, which was considered one of the participant’s 2 daily brushings. Approximately 1 week later, and periodically throughout the study, site staff not blinded to the products reconnected with all individuals via phone to ensure proper product usage and compliance. Within 1 month from baseline visit, all participants received a dental prophylaxis.
At months 6, 12, 18, and 24 participants returned to the site and continuance criteria were verified. Participants returned used brush heads (regimen group), manual brushes (usual care group), paste, rinse, and floss. Site staff monitored compliance based on the product returns, and if low product consumption was suspected study participants were reinstructed on product usage (at months 6, 12, and 18). At each visit, participants used their assigned treatment products in front of a mirror supervised by site staff. A comprehensive oral examination was then conducted followed by an MGI assessment by the experienced examiner. Participants then received GBI, CAL/GR, and PPD examinations in that order by the experienced examiner.
Following that, participants received a supplemental kit box containing resupply of assigned products in an area separated from the examination area to ensure blinding of the examiner to the identity of the test products. Within 1 month from month 6, 12, 18, and 24 visits, participants received a dental prophylaxis.
The same experienced examiner conducted clinical assessments for each participant: MGI,29 GBI,30 CAL/GR, and PPD. The examiner was blinded to the treatment group assigned to each subject. Clinical information was recorded for all scorable teeth present, excluding 3rd molars, teeth with crowns or large restorations (i.e., covering 50% or more of the tooth surface), bridges, orthodontic appliances or implants. At screening, direct measurements of MGI, GBI, PPD, and CAL were made and GR was calculated as follows GR = CAL – PPD. After completion of screening measurements, it was decided, based on practical considerations, to replace direct measurements of CAL at subsequent visits with direct measurements of GR and to calculate CAL as follows: CAL = GR + PPD (where CEJ/recession = positive values and overgrowth = negative values). The impact of this change in measurement methodology for CAL and GR between baseline and ensuing visits resulted in confounding of CAL and GR measurements with respect to magnitude and ≥2 mm loss events. Oral soft tissue assessment was conducted by a licensed dental professional. Abnormal findings were recorded and categorized by location; hard tissue findings were categorized as “other.” An adverse event was recorded if a new abnormal finding was noted after product distribution or if any previously noted abnormal finding increased in severity during the treatment period. All self-reported adverse events were recorded. Whole body adverse events were collected only if potentially related to product use.
Statistical methods
Statistical power calculations were conducted and a sample size of 94 individuals (47 per group) would yield at least 85% power to detect a statistically significant difference between treatment groups estimating a mean difference of 9.0 bleeding sites with a standard deviation of 14.4. In addition, there would be at least 85% power to estimate a mean difference of 0.125 for MGI with a standard deviation of 0.200. These power calculations utilized a 2-sided 5% significance level. Up to 110 participants (55 per group) were enrolled in the study to account for the possibility of up to 15% subject dropout.
Participants were stratified based on screening mean GBI (equal to/above or below 0.49), mean PPD (equal to/above or below 2.23 mm), mean age (equal to/above or below 45 years of age), gender (male or female), and tobacco use (yes or no). Within strata, participants were randomized to 1 of the 2 study groups using a balance and assignment procedure on site. Participants from the same household were assigned to the same study group.
The primary variable was number of bleeding sites (GBI-BS) as measured from GBI. Summary statistics (e.g., means, standard deviations, frequencies) of the demographic characteristics as well as each efficacy endpoint were calculated for each study group and visit. For each efficacy variable and visit, the means for the study groups were compared using the analysis of covariance method with the screening values of the respective endpoint as the covariate. For each efficacy variable and visit, mean comparisons to screening for each study group were investigated using paired difference t-tests. Additionally, the average number of persistent bleeding sites (e.g., sites bleeding at consecutive visits) were summarized by visit for each study group. The location of the persistent bleeding sites at each visit was estimated by determining highest site frequency within each group. The average number of sites with pocket depth progression of 2 mm or greater from the screening visit was also summarized by visit and study group. The location of the sites with pocket depth incidence 2 mm or greater at each visit were estimated by determining the highest site frequency within each study group. To analyse the number of ≥2 mm PPD loss events, a nonparametric ANOVA was carried out at each visit to determine between groups differences.
Statistical tests were 2-sided using a 5% significance level.Pvalue adjustments were not carried out for multiple testing, and missing data were not imputed since only 2% of post-baseline data was missing.
RESULTS
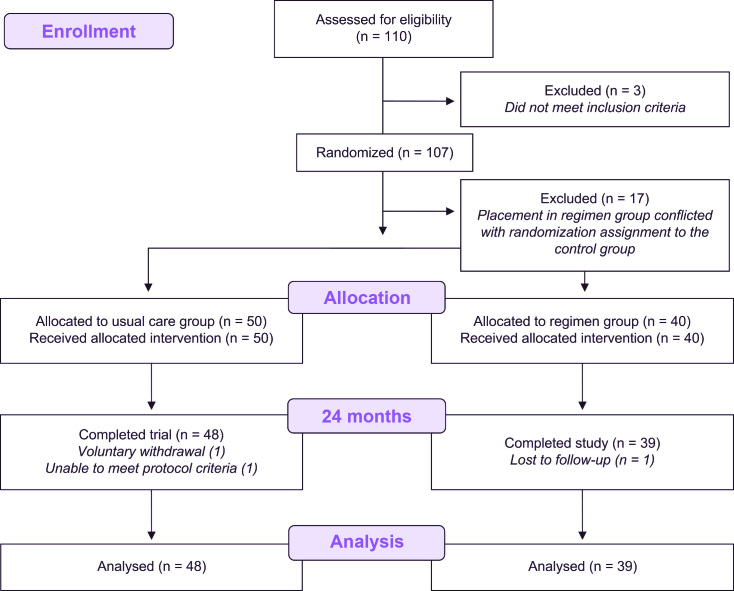
One hundred and ten (110) individuals were screened; 107 signed informed consents for study participation and were enrolled. Seventeen subjects were dropped at baseline because they were inappropriately placed in the regimen group in conflict with their randomization assignment to the control group, leaving 90 who were assigned to treatment and received products. Three enrollees did not complete the study—2 were lost to follow-up and 1 dropped out due to pregnancy—leaving 87 participants completing the 24-month clinical evaluations (Figure 1). Table 1 shows baseline demographics and clinical measures. Individuals who entered into the study ranged in age from 28 years to 64 years, with an average of 46.7 years. Fifty percent (50%) of participants were female and 14% were tobacco users.
Self-reported oral hygiene practices at baseline indicated that 98% of all study participants had regular dental cleanings or checkups; 84% reported having biannual visits. More than 80% reported using a manual toothbrush and toothpaste at least twice daily. The majority (≥69%) reported using mouthwash, dental floss, and an electric rechargeable toothbrush no more than once a week.
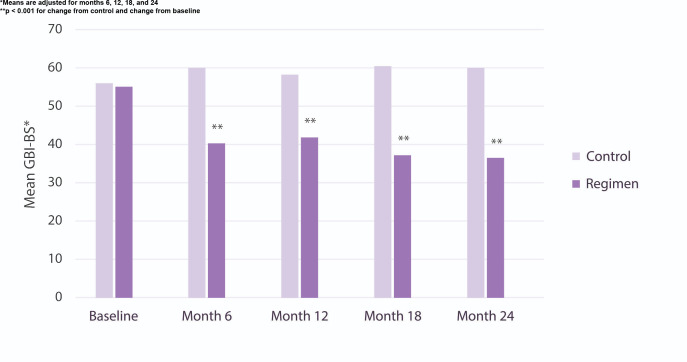
At baseline, the mean number of gingival bleeding sites (GBI-BS) was 55–56 (screening), with a mean MGI score of 1.68–1.69 (Table 1). Table 2 highlights gingivitis measures (GBI-BS, MGI) at months 6, 12, 18 and 24. The usual care group showed little change in either GBI-BS or MGI throughout the 24-month study period. The regimen group in contrast showed statistically significant (p< 0.001) and consistent reductions in number of GBI-BS and MGI throughout the study at months 6, 12, 18, and 24. With respect to GBI-BS, the regimen group was associated with statistically significant (p< 0.001) reductions of 33%, 28%, 38%, and 39% at 6, 12, 18, and 24 months versus the usual care group. With respect to MGI, the regimen group was associated with statistically significant (p< 0.001) reductions of 14%, 12%, 17%, and 18% at 6, 12, 18, and 24 months versus the usual care group. Importantly, the difference between regimen and usual care groups in the management of gingival bleeding increased over time (Figure 2).
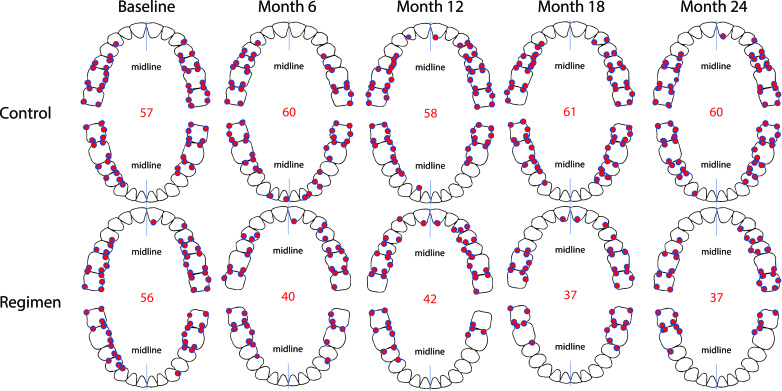
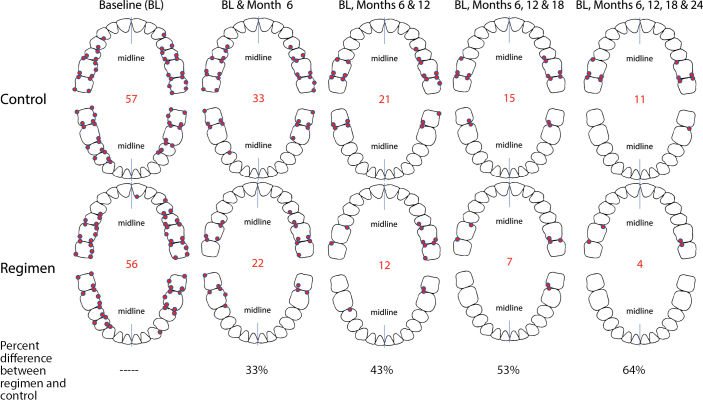
The GBI-BS data were analysed with respect to which sites manifested bleeding most frequently by both timepoint and treatment group. These frequency data were plotted on whole mouth tooth diagrams to visualize where bleeding sites most frequently occurred through rank ordering (Figure 3). These data support that bleeding sites occur with the highest frequency in the posterior and interproximal dentition. Similar analysis examining persistent GBI-BS, defined as those sites that bled at baseline and each ensuing visit, was performed. These frequency data for persistent GBI-BS were plotted on whole mouth tooth diagrams to visualize where persistent bleeding sites most frequently occurred through rank ordering (Figure 4). With respect to persistent GBI-BS, the regimen group was associated with statistically significant (p< 0.001) reductions of 33%, 43%, 53%, and 64% at 6, 12, 18, and 24 months versus the usual care group.
Table 3 shows results for PPD. PPD loss events over the 2-year treatment period were statistically significantly (p< 0.001) lower in individuals in the regimen group compared to those in the usual care group. The PPD adjusted means for the regimen group were lower by 12%, 6%, 11%, and 13% at 6, 12, 18, and 24 months, respectively.
Table 1.
Baseline demographics summary and clinical parameters
Demographic/statistic or category |
Control (n = 53) |
Regimen (n = 54) |
Overall (N = 107) |
pvalue |
Age (years) | ||||
Mean (SD) |
47.6 (10.29) |
45.9 (10.79) |
46.7 (10.53) |
0.4075a |
Min–Max |
28–64 |
29–63 |
28–64 |
|
Sex | ||||
Femaleb, n (%) |
26 (49) |
27 (50) |
53 (50) |
1.0000c |
Maleb, n (%) |
27 (51) |
27 (50) |
54 (50) |
|
Tobacco | ||||
Nob, n (%) |
46 (87) |
46 (85) |
92 (86) |
1.0000c |
Yesb, n (%) |
7 (13) |
8 (15) |
15 (14) |
|
Mean GBI-BS |
56.11 (14.921) |
55.36 (13.723) |
55.73 (14.266) |
0.784 |
Mean MGI score (SD) |
1.69 (0.154) |
1.68 (0.129) |
1.68 (0.141) |
0.723 |
Mean PPD mm (SD) |
2.25 (0.186) |
2.25 (0.206) |
2.25 (0.195) |
0.980 |
Mean CAL mm (SD) |
2.62 (0.228) |
2.60 (0.241) |
2.61 (0.234) |
0.783 |
Mean GR mm (SD) |
0.36 (0.174) |
0.35 (0.139) |
0.36 (0.157) |
0.741 |
a2-sided ANOVApvalue for the treatment comparison
bThe number (percent) of participants in each category
c2-sided Fisher’s exact testpvalue for the treatment comparison
Figure 1.
Study flow diagram
Table 2.
Gingivitis clinical results (GBI-BS, MGI) per visit
Treatment |
n |
Adjusted mean GBI-BS (SE) |
% change versus control |
2-sidedpvaluea |
Adjusted mean MGI Score (SE) |
% change versus control |
2-sidedpvaluea |
Month 6 |
|
|
|
|
|||
Control |
50 |
60.11 (2.564) |
|
|
1.80 (0.021) |
|
|
Regimen |
40 |
40.36 (1.887) |
32.9 |
<0.0001 |
1.54 (0.025) |
14.1 |
<0.0001 |
Month 12 |
|
|
|
|
|||
Control |
49 |
58.37 (2.689) |
|
|
1.55 (0.024) |
|
|
Regimen |
39 |
42.18 (2.127) |
27.7 |
<0.0001 |
1.37 (0.025) |
11.8 |
<0.0001 |
Month 18 |
|
|
|
|
|||
Control |
48 |
60.63 (2.593) |
|
|
1.57 (0.022) |
|
|
Regimen |
39 |
37.48 (2.144) |
38.2 |
<0.0001 |
1.31 (0.020) |
16.9 |
<0.0001 |
Month 24 |
|
|
|
|
|||
Control |
48 |
60.01 (2.144) |
|
|
1.65 (0.019) |
|
|
Regimen |
39 |
36.63 (2.064) |
39.0 |
<0.0001 |
1.35 (0.018) |
18.4 |
<0.0001 |
aANCOVA with respective screening value as the covariate
Figure 2.
Mean GBI-BS per visit
Figure 3.
Location of most frequent bleeding sites by visit
Figure 4.
Location of most frequent persistent bleeding sites by visit
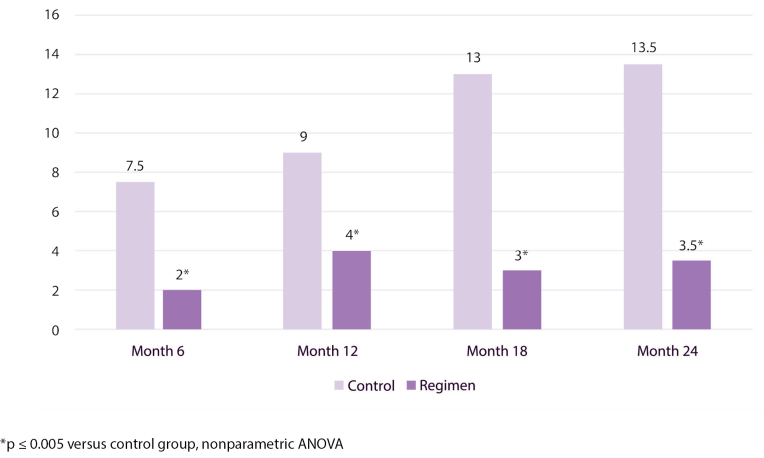
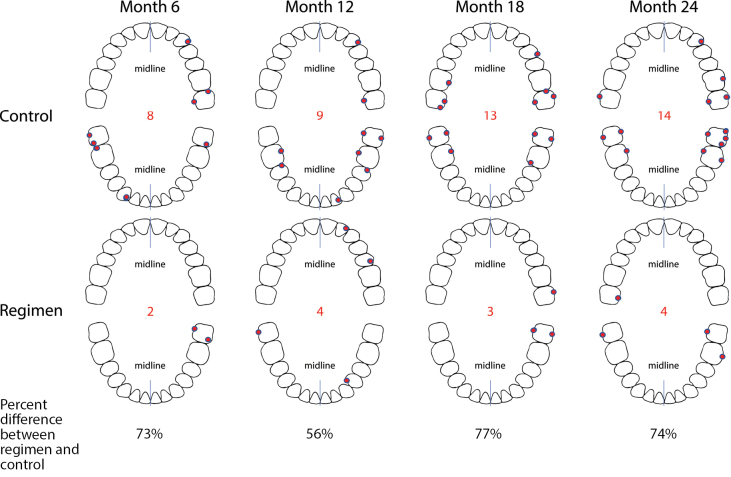
Figure 5 shows median number of sites with ≥2 mm PPD loss events from baseline per subject analyses. PPD loss events during the study period were lower in participants in the regimen group than in those in the usual care group. For median number of ≥2 mm PPD loss events, results were statistically significantly (p≤ 0.005) lower for the regimen group with fewer median number of events by 73%, 56%, 77%, and 74% at 6, 12, 18, and 24 months, respectively, versus the usual care group. Figure 6 shows the location of the most frequent ≥2 mm PPD loss events by tooth and site over the course of the study.
There was 1 non-serious adverse event (mild burning mouth) reported in the regimen group at month 6 which resolved. All components of the test regimen as well as the usual care products were well tolerated.
DISCUSSION
Clinical data support efficacy for the individual therapeutic interventions of oscillating–rotating electric toothbrushes, bioavailable stannous fluoride dentifrice, cetylpyridinium chloride mouthrinse, and dental floss for reducing plaque and gingivitis.16,17,20,21 However, a limited number of studies have examined these individual therapies for periodontal disease progression extending up to 2 years.23 Likewise, combinations of these therapies have been examined in shorter-term studies showing high levels of efficacy.26-28
This study combined 4 clinically proven technologies for their gingivitis effects and progression of periodontal indices over a longer period (2 years) in adults with established gingivitis and isolated sites of incipient periodontitis. Results demonstrated significant and consistent efficacy of the regimen in reducing number of GBI-BS and gingival inflammation (MGI) over 2 years. The periodontal measure of PPD progressed consistently throughout the study. In the regimen group, PPD increased over the 24 months. However, the increase in this periodontal parameter was statistically significantly lower versus the usual care group. The magnitude of reduction in GBI-BS for the regimen versus usual care group was 39% at 24 months. The magnitude of reduction in median PPD for the regimen versus usual care group at 24 months was 13%. The magnitude of reduction in ≥ 2 mm PPD loss events for the regimen versus usual care group at 24 months was 74%. These represent clinically important reductions in gingival inflammation and periodontal measures.
Clearly, the regimen slowed the rate of periodontal disease progression in the population. The decreased progression of PPD in the regimen group might be expected from the reductions in GBI-BS observed during the study for the regimen group versus the usual care regimen. Lang and others31-34 have systematically studied the longitudinal progression of periodontal disease in populations and assessed the role of localized gingivitis on disease progression towards tooth loss. In these studies, sites with persistent gingival bleeding on probing exhibited significantly elevated rates of CAL as compared to sites with infrequent or no gingival bleeding. These studies went on to determine that approximately one-third of sites with consistent gingival bleeding during adulthood are at risk of future tooth loss due to periodontal disease. PPD and CAL are highly correlated, as CAL is calculated by adding PPD and GR. The results of this study support the benefits that reducing gingival bleeding has on reducing the rates of PPD progression.
With respect to study limitations, the change in measurement methodology for CAL (direct) and GR (imputed) at screening/baseline to CAL (imputed) and GR (direct) at 6, 12, 18, and 24 months introduces a potential confounding effect on change from baseline analyses in terms of absolute magnitude of change, as well as the ability to measure CAL events. Importantly, the GBI-BS, MGI, and PPD measures were not affected by this change in methodology. Due to the nature of the study in which the test variable was the regimen itself rather than the individual products within the regimen, another limitation is that it is impossible to determine the relative contribution of each product within the regimen with respect to the observed clinical benefits. Nevertheless, the study results provide compelling evidence of the importance of oral hygiene measures in improving the gingival health of clients with at-home care. Furthermore, these gingival health improvements have the long-term benefit of slowing the progression of periodontal disease (PPD).
Table 3.
Periodontal clinical results (PPD) per visit
Treatment |
n |
Adjusted mean PPD mm (SE) |
% change versus control |
2-sidedpvaluea |
Month 6 | ||||
Control |
50 |
2.65 (0.047) |
||
Regimen |
40 |
2.33 (0.041) |
12.3 |
<0.0001 |
Month 12 | ||||
Control |
49 |
2.75 (0.030) |
||
Regimen |
39 |
2.57 (0.041) |
6.3 |
0.0009 |
Month 18 | ||||
Control |
48 |
2.86 (0.031) |
||
Regimen |
39 |
2.54 (0.033) |
11.4 |
<0.0001 |
Month 24 | ||||
Control |
48 |
2.91 (0.035) |
||
Regimen |
39 |
2.54 (0.026) |
12.6 |
<0.0001 |
aANCOVA with respective screening value as the covariate
Figure 5.
Median number of ≥2 mm PPD loss events
Figure 6.
Location of most frequent ≥2 mm PPD loss events from baseline by visits
CONCLUSIONS
In summary, a regimen of oral care hygiene aids including an oscillating–rotating electric toothbrush, bioavailable stannous fluoride dentifrice, cetylpyridinium chloride mouthrinse, and dental floss was found to be effective in reducing GBI-BS, MGI, and PPD compared to a usual care routine including a manual toothbrush and fluoride toothpaste over a period of 2 years. The regimen was well tolerated by study participants.
CONFLICTS OF INTEREST AND SOURCE OF FUNDING
This study was sponsored by The Procter & Gamble Company. HT, MLB, JMG, RWG, and ARB are employees of The Procter & Gamble Company. AZ and SM have been investigators for work sponsored by The Procter & Gamble Company.
Acknowledgments
The authors thank Ms Phyllis Hoke for her role in study execution and Dr. Donald J White for preparing the manuscript.
Footnotes
CDHA Research Agenda category: risk assessment and management
References
- Burt BResearch, Science and Therapy Committee of the American Academy of Periodontology Position paper: Epidemiology of periodontal diseases J Periodontol 2005;76:1406–1419 [DOI] [PubMed] [Google Scholar]
- Dye BAGlobal periodontal disease epidemiology Periodontol 2000 2012;58(1):10–25 [DOI] [PubMed] [Google Scholar]
- Eke PI, Dy BA, Wei L, Thornton-Evans GO, Genco RJPrevalence of periodontitis in adults in the United States: 2009 and 2010 J Dent Res 2012;91(10):914–920 [DOI] [PubMed] [Google Scholar]
- Löe H, Theilade E, Jensen SBExperimental gingivitis in man J Periodontol 1965;36:177–87 [DOI] [PubMed] [Google Scholar]
- Mombelli AW.The role of dental plaque in the initiation and progression of periodontal diseases. In: Lang NP, Attstrom R, Löe H. Proceedings of the European Workshop on Mechanical Plaque Control.Berlin:Quintessenz Verlag;1998. pp. 85–97. [Google Scholar]
- Theilade E, Wright WH, Jensen SB, Löe HExperimental gingivitis in man II. A longitudinal clinical and bacteriological investigation J Periodontal Res 1966;1:1–13 [DOI] [PubMed] [Google Scholar]
- Nair S, Faizuddin M, Dharmapalan JRole of autoimmune responses in periodontal disease Autoimmune Dis 2014; 596824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S, Blanco J, Buchalla W, Carvalho JC, Dietrich T, Dörfer C, et al. Prevention and control of dental caries and periodontal diseases at individual and population level: Consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases J Clin Periodontol 2017;44(Suppl 18):S85–S93. [DOI] [PubMed] [Google Scholar]
- Lamont T, Worthington HV, Clarkson JE, Beirne PVRoutine scale and polish for periodontal health in adults Cochrane Database Syst Rev 2018;27;12:CD004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang NP, Bartold PMPeriodontal health J Clin Periodontol 2018;45(Suppl 20):S9–S16 [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Eickolz P, Loos BGPrinciples in prevention of periodontal diseases: Consensus report of group I of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases J Clin Periodontol 2015;42(Suppl 16):S5-S11 [DOI] [PubMed] [Google Scholar]
- Van der Weijden FA, Slot DEEfficacy of homecare regimens for mechanical plaque removal in managing gingivitis a meta review J Clin Periodontol 2015;42(Suppl 16):S77–S91 [DOI] [PubMed] [Google Scholar]
- Kotsakis GA, Lian Q, Ioannou AL, Michalowicz BS, John MT, Chu HA network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation J Periodontol 2018;89(5):558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden IMMotivating patients Prim Dent J 2014;3(3):30–33 [DOI] [PubMed] [Google Scholar]
- Schou LBehavioral aspects of dental plaque control measures: An oral health promotion perspective. In: Lang NP, Attstrom R, Löe H. Proceedings of the European Workshop on Mechanical Plaque Control.Berlin:Quintessenz Verlag; 1998. pp. 287–99. [Google Scholar]
- Cugini M, Warren PRThe Oral-B CrossAction manual toothbrush: a 5-year literature review J Can Dent Assoc 2006;72(4):323–333 [PubMed] [Google Scholar]
- Walters PA, Cugini M, Biesbrock AR, Warren PRA novel oscillating-rotating power toothbrush with SmartGuide: Designed for enhanced performance and compliance J Contemp Dent Pract 2007;8(4):1–9 [PubMed] [Google Scholar]
- Yacoob M, Worthington HV, Deacon SA, Deery C, Walmsley AD, Robinson PG, et al. Powered versus manual toothbrushing for oral health Cochrane Database Syst Rev 2014; (6): CD002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascella JA, Fernández P, Gilbert RD, Cugini MA randomized, clinical evaluation of the safety and efficacy of a novel oral irrigator Am J Dent 2000;13(2):55–58 [PubMed] [Google Scholar]
- Biesbrock AR, Corby PMA, Bartizek R, Corby AL, Coelho M, Costa S, et al. Assessment of treatment responses to dental flossing in twins J Periodontol 2006;77(8):1386–1391 [DOI] [PubMed] [Google Scholar]
- He T, Qu L, Chang J, Wang JGingivitis models—Relevant approaches to assess oral hygiene products J Clin Dent 2018;29(2):45–51 [PubMed] [Google Scholar]
- Matthews DCPowered toothbrush plus triclosan only as effective as manual brush and fluoride toothpaste for periodontal maintenance patients Evid Based Dent 2008;9(3):74–75 [DOI] [PubMed] [Google Scholar]
- Papas A, He T, Martuscelli G, Singh M, Bartizek RD, Biesbrock ARComparative efficacy of stabilized stannous fluoride/sodium hexametaphosphate dentifrice and sodium fluoride/triclosan/copolymer dentifrice for the prevention of periodontitis in xerostomic patients: a 2-year randomized clinical trial J Periodontol 2007;78(8):1505–1514 [DOI] [PubMed] [Google Scholar]
- Pera C, Ueda P, Casarin R, et al. Double-masked randomized clinical trial evaluating the effect of a triclosan/copolymer dentifrice on periodontal healing after one-stage full-mouth debridement J Periodontol 2012;83(7):909–916 [DOI] [PubMed] [Google Scholar]
- Seymour GJ, Palmer JE, Leishman SJ, Do HL, Westerman B, Carle AD, et al. Influence of a triclosan toothpaste on periodontopathicbacteria and periodontitis progression in cardiovascular patients:a randomized controlled trial J Periodontol Res 2017;52(1):61–73 [DOI] [PubMed] [Google Scholar]
- Biesbrock AR, Bartizek RD, Gerlach RW, Terézhalmy GTOral hygiene regimens, plaque control, and gingival health: a two-month clinical trial with antimicrobial agents J Clin Dent 2007;18(4):101–105 [PubMed] [Google Scholar]
- Feng X, He T, Cao M, He Y, Ji NA randomized clinical trial to assess anti-plaque effects of an oral hygiene regimen with a stannous-containing sodium fluoride dentifrice, advanced manual toothbrush, and CPC rinse Am J Dent 2016;29(2):120–124 [PubMed] [Google Scholar]
- Zini A, Timm H, Barker ML, Gerlach RWA randomized controlled clinical trial to evaluate the efficacy of an oscillating-rotating electric toothbrush, a stannous fluoride dentifrice, and floss on gingivitis J Clin Dent 2018;29(2):64–68 [PubMed] [Google Scholar]
- Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker LA modified gingival index for use in clinical trials Clin Prev Dent 1965;8:3–6 [PubMed] [Google Scholar]
- Saxton CA, van der Ouderaa FJThe effect of a dentifrice containing zinc citrate and triclosan on developing gingivitis J Periodontol Res 1989;4:75–80 [DOI] [PubMed] [Google Scholar]
- Lang NP, Schätzle MA, Löe HGingivitis as a risk factor in periodontal disease J Clin Periodontol 2009;36(Suppl 10):3–8 [DOI] [PubMed] [Google Scholar]
- Ramseier CA, Anerud A, Dula M, Lulic M, Cullinan MP, Seymour GJ, et al. Natural history of periodontitis: Disease progression and tooth loss over 40 years J Clin Periodontol 2017;44(12):1182–1191 [DOI] [PubMed] [Google Scholar]
- Schätzle M, Löe H, Bürgin W, Anerud A, Boysen H, Lang NPClinical course of chronic periodontitis I Role of gingivitis J Clin Periodontol 2003;30(10):887–901 [DOI] [PubMed] [Google Scholar]
- Schätzle M, Löe H, Lang NP, Bürgin W, Anerud A, Boysen HThe clinical course of chronic periodontitis J Clin Periodontol 2004;31(12):1122–1127 [DOI] [PubMed] [Google Scholar]