Abstract
The continuing emergence of SARS-CoV-2 variants calls for regular assessment to identify differences in viral replication, shedding and associated disease. In this study, African green monkeys were infected intranasally with either a contemporary D614G or the UK B.1.1.7 variant. Both variants caused mild respiratory disease with no significant differences in clinical presentation. Significantly higher levels of viral RNA and infectious virus were found in upper and lower respiratory tract samples and tissues from B.1.1.7 infected animals. Interestingly, D614G infected animals showed significantly higher levels of viral RNA and infectious virus in rectal swabs and gastrointestinal tract tissues. Our results indicate that B.1.1.7 infection in African green monkeys is associated with increased respiratory replication and shedding but no disease enhancement similar to human B.1.1.7 cases.
One-Sentence Summary:
UK B.1.1.7 infection of African green monkeys exhibits increased respiratory replication and shedding but no disease enhancement
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late 2019 as the causative agent of coronavirus disease 2019 (COVID-19). COVID-19 was declared a pandemic by the World Health Organization in March 2020 (1) and has now infected more than 170 million people with over 3.7 million deaths (2).
Enhanced sequence-based surveillance and epidemiological studies have led to the identification of multiple SARS-CoV-2 variants carrying distinct mutations that may impact transmissibility, disease severity and/or effectiveness of treatments and vaccines. The SARS-CoV-2 B.1.1.7 variant was first reported within the English county of Kent from the United Kingdom (UK) (3) and has since been classified as a ‘Variant of Concern’ (VOC) associated with increased transmissibility and potentially increased disease severity but with minimal impact on the efficacy of monoclonal antibody treatment (4). Several clinical reports supported the increase in transmissibility associated with the B.1.1.7 VOC with up to a 90% increase in transmission compared to earlier variants (5–7). However, the reported increase in mortality of the B.1.1.7 VOC (8–10) seen from earlier COVID case analysis has recently been questioned (11, 12). Apart from clinical studies, experimental infections in animals - ideally in species closely related to humans such as nonhuman primates (NHPs) - is one way to assess transmissibility and disease severity of emerging SARS-CoV-2 variants.
The rhesus macaque model of SARS-CoV-2 infection was established early in the pandemic (13–15) and has been used to test SARS-CoV-2 therapeutics and vaccines (16–19). Additional NHP species, such as cynomolgus macaques, baboons and marmosets have been investigated for their susceptibility to SARS-CoV-2 in an attempt to develop models exhibiting increased disease severity (20, 21). None of these models result in severe disease, but susceptible NHP species exhibit oral and nasal shedding and develop mild to moderate respiratory disease in the upper and lower respiratory tract. An African green monkey (AGM) model of SARS-CoV-2 infection has recently been developed, wherein animals show greater severity of disease (22, 23), with intranasal infection of AGMs resulting in significant shedding and respiratory disease (24). The AGM model may thereby represent a more natural NHP model for SARS-CoV-2 infection and disease.
In our current study, the AGM intranasal model of SARS-CoV-2 infection was used to assess differences between a contemporary SARS-CoV-2 D614G variant, which was circulating in the summer of 2020, and the B.1.1.7 VOC that emerged in the UK in late 2020. Herein, we report and discuss differences in organ tropism, replication kinetics and shedding between the two SARS-CoV-2 variants.
Infection with B.1.1.7 was not associated with a significant increase in disease severity in the AGM model.
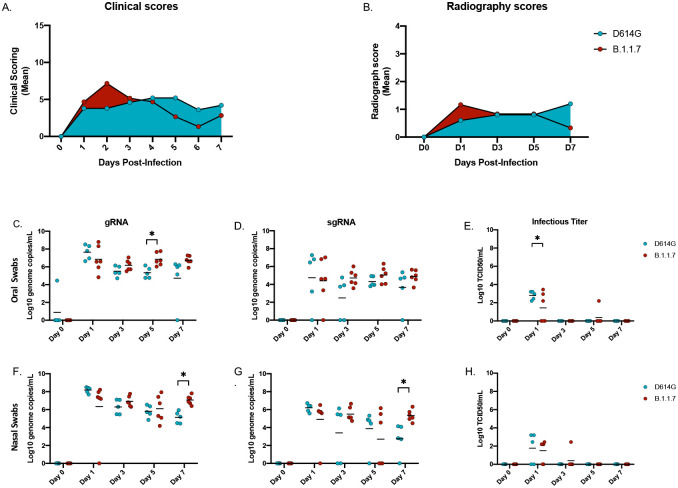
Following intranasal infection with 1×106 infectious particles of either the SARS-CoV-2 D614G (n=5) or the B.1.1.7 variant (n=6) (5×105 per naris) using a nasal atomization device, animals were monitored and scored daily for clinical signs of disease including changes in general appearance, respiration, food intake and fecal output and locomotion. Clinical signs were mild with both groups of AGMs displaying only minor changes in respiration and showing reduced appetite that negatively impacted volume of feces produced. Slight differences were observed between the two variant groups. B.1.1.7 infected animals had elevated scores early in infection peaking at 2 days post-infection (dpi), which subsequently returned toward baseline (Fig 1A). In contrast, scores for the D614G animals increased slowly peaking at 4dpi and remained stable until euthanasia (Fig 1A, Table S1). Radiographs were taken at each examination and scored for pulmonary infiltrates, progression of which were similar to the clinical scores (Fig 1B, Table S2). B.1.1.7 infected AGMs scored slightly higher earlier and peaked at 1dpi, whereas D614G infected animals scored higher later and peaked at 3dpi. Overall, the changes in clinical and radiographic scores were not significantly different between the two groups even though minor differences were noted in disease progression.
Figure 1: Clinical scoring, radiographs and oral and nasal shedding.
AGMs were infected with either the D614G or B.1.1.7 SARS-CoV-2 variant intranasally utilizing the Nasal Mucosal Atomization Device. (A) AGMs were scored daily for clinical signs of disease including changes in general appearance, respiration, food intake and feces as well as locomotion (B) Radiographs were taken on clinical exam days (0, 1, 3, 5, 7) and scored for pulmonary infiltrates. Swabs were taken on clinical exam days (0, 1, 3, 5 and 7) and used as a correlate for virus shedding. Viral RNA total genome (gRNA) and subgenome (sgRNA) copies were determined by qRT-PCR. Infectious virus was titered on VeroE6 cells. (C-E) Viral shedding in oral swabs. Statistical significance was found at day 5 in total RNA (C, p-value <0.05) and at day 1 in infectious titers (E, p-value <0.05). (F-H) Viral shedding in nasal swabs. Statistical significance was found at day 7 in gRNA (F, p-value <0.05) and at day 7 sgRNA (G, p-value <0.05). Multiple t-tests were used to compare the gRNA, sgRNA and infectious titers between groups.
B.1.1.7 replication/shedding from the upper respiratory tract was increased compared to D614G.
Oral and nasal swabs were taken at each examination to assess virus replication in the upper respiratory tract and virus shedding. SARS-CoV-2 RNA was measured with qPCR assays targeting either total viral RNA (gRNA, N assay) or subgenomic viral RNA (sgRNA, E assay) (Fig 1). Total gRNA in oral swabs was significantly higher at 5dpi in B.1.1.7 compared to D614G infected animals; this difference between variants was maintained but dropped below significance by 7dpi (Fig 1C). There were no significant differences in sgRNA levels in oral swabs at any time point, but levels consistently trended higher in the B.1.1.7 infection group starting at 3dpi (Fig 1D). Although viral RNA was detectable throughout the study, infectious virus was only isolated from oral swabs at 1dpi with significantly higher titers for the D614G infected animals (Fig 1E). Consistent with the oral swabs, higher levels of viral RNA were detected in nasal swabs collected from AGMs infected with B.1.1.7. By 7dpi these animals were all shedding significantly more viral RNA than those infected with D614G (Fig 1F, G). Infectious virus in the nasal swabs was recovered predominantly at 1dpi and there was no difference between groups (Fig 1H).
B.1.1.7 replication in the lower respiratory tract was increased compared to D614G.
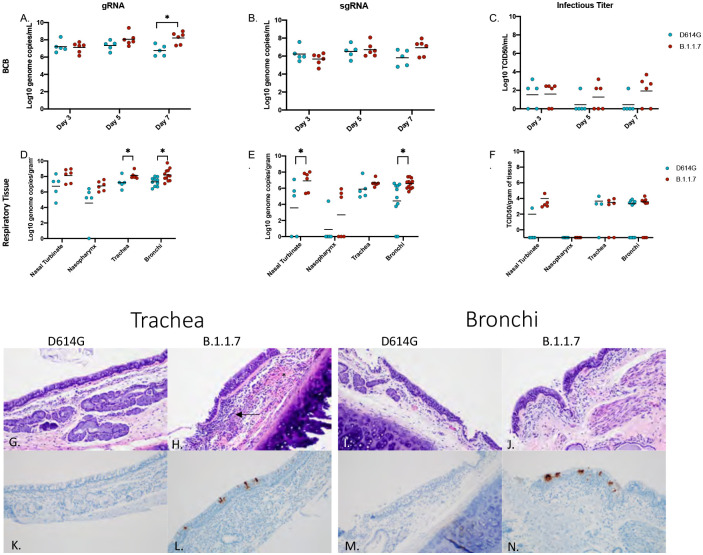
Samples to assess viral replication kinetics in the lower respiratory tract were collected with broncho cytology brushes (BCB) at 3, 5 and 7dpi and bronchoalveolar lavage (BAL) at 3 and 5dpi. Total viral RNA levels were consistent between both sampling methods (~105-107 genome copies/ml) at each day sampled (Fig 2A–C; Fig S1). The gRNA levels were consistently higher in AGMs infected with B.1.1.7 and significantly increased at the final time points of BCB (7dpi) (Fig 2A) and BAL (5dpi) sampling (Fig S1). Although not significantly different at any time point, sgRNA was higher in the B.1.1.7 AGMs at 7dpi in the BCB and in BAL at the final day of collection (5dpi) (Fig 1B; Fig S1). Higher levels of infectious virus were also isolated from animals infected with B.1.1.7, particularly in BCB samples (Fig 2C; Fig. S1).
Figure 2: Virus load in the lower respiratory tract.
AGMs were infected with either the D614G or B.1.1.7 SARS-CoV-2 variant intranasally utilizing the Nasal Mucosal Atomization Device. Bronchial cytology brush (BCB) samples were collected on days 3, 5 and 7 post-infection and samples were analyzed for gRNA, sgRNA and infectious virus. (A-C) A significant difference in gRNA collected in the BCBs was detected 7 days-post-infection (A, p-value <0.05). Animals were euthanized on day 7 post-infection and respiratory tissues were collected for analyses for gRNA, sgRNA and infectious virus (D-F). gRNA was significantly different in the trachea and bronchi (D, p-value <0.05). sgRNA differed significantly in the nasal turbinate and bronchi (E, p-values <0.05). Infectious virus did not differ significantly in any tissue (C). Multiple t-tests were used to compare tissue between groups. Pathology and immunoreactivity in the trachea and bronchi (G-N). (G, H) Normal trachea found in the D614G vs. trachea with cellular infiltrates, hemorrhage (*) and fibrin (arrow) found in the submucosa in the B.1.1.7. (K, L) immunoreactivity in the trachea of D614G vs B.1.1.7. (I, J) Normal bronchi found in the D614G vs bronchi with inflammation and cellular infiltrates found in the B.1.1.7. (M, N) Immunoreactivity in bronchi of the D614G vs B.1.1.7. (HE G, H; IHC K, L 100x; HE I, J; IHC M, N 100x).
Post-mortem tissues were collected at 7dpi for virological analysis and pathology. Respiratory tissues including nasal turbinate, nasopharynx, trachea and left and right bronchi and a section from each lung lobe were examined for viral RNA and infectious virus. Total and sgRNA was higher in all respiratory tissues in the B.1.1.7 infected animals and was significantly higher in the trachea and bronchi (Fig 2D,E). Although not statistically significant, AGMs infected with B.1.1.7 had higher levels of infectious virus in nasal turbinates, trachea and bronchi (Fig 2F). Consistent with the viral RNA and infectious titer results, most animals (5 out of 6) infected with B.1.1.7 had inflammation of the trachea, with only 1 of 5 animals infected with the D614G variant having any similar observable lesion. Similarly, lesions were found in 8 of 10 bronchi from B.1.1.7 infected AGMs compared to only 2 of 8 of D614G animals, with lesions corresponding to SARS-CoV-2 immunoreactivity by immunohistochemistry (IHC) (Fig 2G–N).
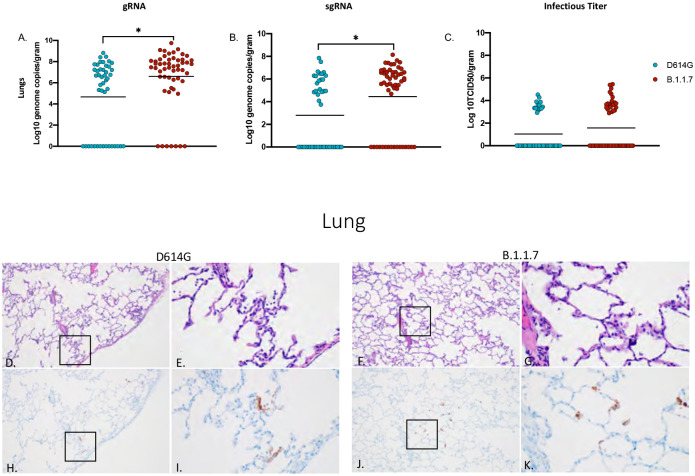
Total and sgRNA was significantly higher in the lungs of B.1.1.7- compared to D614G-infected animals (Fig 3A, B); however, elevated levels of infectious virus in B.1.1.7 animals remained just below statistical significance (Fig 3C). Lung lesions for both groups were minor but consistent with SARS-CoV-2 pneumonia and included thickening and inflammation of alveolar septa and the presence of fibrin (Fig 3D–G). SARS-CoV-2 immunoreactivity by IHC was also limited and not directly associated with foci of inflammation (Fig 3H–K).
Figure 3: Virus load, pathology and immunoreactivity in the lungs.
Animals were euthanized on day 7 post-infection and a section from each lung lobe were collected and analyzed for gRNA, sgRNA and infectious virus. Results of each assay were combined to look at the lungs in total (A-C). Significant differences were detected in lungs in both gRNA and sgRNA (D, p-value <0.05 and E. p-value <0.05) but not in infectious virus (C). Multiple t-tests were used to compare the two groups for statistical significance. Pathology and immunoreactivity in the lungs (D-K). (D, E) Minimally thickened and inflamed alveolar septa with multifocal pneumocyte immunoreactivity in the D614G and B.1.1.7 samples (HE D, F; IHC H, J 100x; HE E, G; IHC I, K 400x).
D614G replication in the gastrointestinal (GI) tract was increased compared to B.1.1.7.
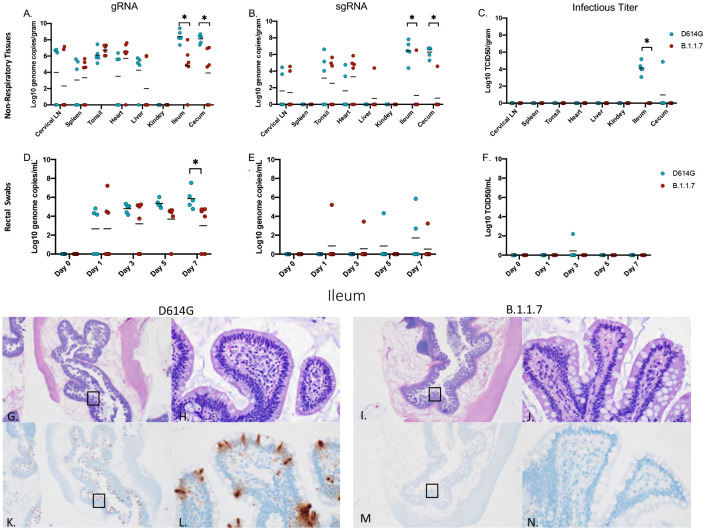
Total viral gRNA was detected in cervical lymph nodes, tonsil, heart, liver, spleen, ileum and cecum in both groups (Fig 4A). With the exception of one liver sample, sgRNA was not detected in liver, spleen nor kidneys (Fig 4B). Notably and in marked contrast to respiratory tissues, AGMs infected with D614G had significantly more total and sgRNA in the ileum and cecum than B.1.1.7 infected AGMs (Fig 4A, B). Levels of viral RNA corresponded to infectious virus with only D614G animals having detectable infectious SARS-CoV-2 in these two GI-derived tissues (Fig 4C). Similarly, gRNA in rectal swabs peaked and was significantly higher at 7dpi in D614G infected animals (Fig 4D). sgRNA was recovered intermittently across the study (Fig 4E), but infectious virus was recovered from rectal swabs of only one D614G animal (Fig 4F). Viral replication in the ileum was associated with inflammation in D614G infected AGMs and corresponded with detectable viral antigen by IHC (Fig 4G,H,K,L), while AGMs infected with the B.1.1.7 had no observable inflammation or viral antigen (Fig 4I,J,L,M). In D614G infected animals, only one AGM presented with inflammation and a small amount of associated viral antigen in the cecum. No infectious virus was isolated from any of the other non-respiratory tissue in either group and pathology was unremarkable in these tissues.
Figure 4: Viral load in gastrointestinal tract, pathology and immunoreactivity.
AGMs were euthanized 7 days post-infection and tissues were collected to determine viral load, pathology and immunoreactivity (A-C). gRNA and sgRNA was significantly different in the ileum and cecum (A, p-value <0.05 and B, p-value <0.05). Infectious virus was significantly different in the ileum (C, p-value <0.05), no other tissues were significantly different. (D-F) Viral shedding in rectal swabs. Statistical significance was found at day 7 in total RNA (D, p-value <0.05). Statistical significances was determined by multiple t-tests between the two groups. Pathology and immunoreactivity in the ileum (G-N). (G, H) Normal mucosa with multifocal mucosal immunoreactivity in the D614G challenged ileum (HE G, IHC K, 20x; HE H, IHC L, 400x). (I, J) Normal mucosa and no immunoreactivity in the B.1.1.7 challenged ileum (HE I, IHC M, 20x; HE J, IHC N, 400x).
Infection with neither variant was associated with marked changes in hematology, blood chemistry and coagulation, nor a broad systemic cytokine response.
Blood and serum samples were collected for hematology, blood chemistry, coagulation assays and cytokine analysis at every clinical examination. No differences were found in the hematology (Fig S2A–L), blood chemistry (Fig S2M–T) or coagulation assays (Fig S3) between the D614G and B.1.1.7 infected AGMs. Of the cytokines examined (Fig. S4), IL6 was the only pro-inflammatory cytokine that was significantly different between the groups, with IL6 being elevated in the D614G group at 3dpi and 5dpi compared to B.1.1.7 infected animals (Fig S4A). Levels of T cell chemo-attractants IP-10 (CXCL 10) (Fig S4B) and I-Tac (CXCL 11) (Fig S4) were also increased at 1dpi in the D614G group but were not sustained throughout the study.
The regular emergence of SARS-CoV-2 variants represents a constant public health challenge with the COVID-19 pandemic. Although epidemiological and clinical data can give insight into characteristics of a new variant, this data can be limited initially and may be biased by many factors. Animal models provide the ability to directly compare biological and clinical characteristics of multiple SARS-CoV2 variants in a study with limited variables providing invaluable data otherwise unattainable.
In the present study, we have used the AGM intranasal infection model to compare the B.1.1.7 VOC, a variant that emerged in the UK in September of 2020 and then quickly spread throughout the world (5, 7), with a contemporary D614G variant, in terms of virus replication, shedding and disease severity. A nasal atomization device was used to infect NHPs to most closely mimic natural infection in human. Following infection with either variant, animals in both groups exhibited minor differences in disease progression but overall disease signs were similar with mild respiratory disease for both B.1.1.7 and D614G (Fig. 1A,B; Table S1 & S2). Increased disease severity was initially reported for human B.1.1.7 cases (8–10), but more recent studies have contradicted these earlier claims (11, 12). The outcome of our study using a NHP surrogate model supports findings from these more recent studies indicating that B.1.1.7 VOC is not associated with increased disease severity.
Although no animals in this study developed severe disease, our analysis reveal differences between the two variants in terms of their replication within the respiratory system. Viral RNA and infectious virus in the lower respiratory tract tissues were more prevalent in the animals infected with B.1.1.7 compared to D614G, especially at later timepoints suggesting the development of a stronger respiratory component associated with the emerging VOC (Figs 2, Fig 3). Consistent with higher levels of viral replication in the upper respiratory tract, shedding of viral RNA in both the nose and oral cavity was also higher in B.1.1.7 infected animals (Fig. 1), which support reports from human infection data showing that the B.1.1.7 VOC is more transmissible than earlier variants (6, 7, 25).
The pathology associated with infections differed between the two variants. B.1.1.7 replicated at higher levels in the respiratory tract resulting in lesions that were both more numerous and severe than seen for D614G infected animals. In contrast, D614G replicated at higher levels in the GI tract and the associated pathology seen in these animals correlated with this difference in GI replication. This finding was supported by higher levels of viral RNA in rectal swabs indicating the possibility of fecal transmission (Fig. 4). This may indicate that D614G is more suited to replication in the digestive tract than other variants which is in line with clinical studies conducted in early to mid-2020 that reported GI symptoms in approximately 15–20% of COVID-19 patients (26). The difference observed in these studies may also indicate that genetic alterations in B.1.1.7 may have not merely resulted in a general increased rate of replication but may have also altered organ tropism. Clinical studies concerning B.1.1.7 have to date been mainly focused on respiratory symptoms (8–11). However, with clinical studies still underway it remains to be seen whether the observed changes in tissue tropism and replication detected here in the AGM model will correspond to a drop in reported GI symptoms in those infected with the B.1.1.7 VOC compared to infections with contemporary SARS-CoV-2. It is also possible that AGMs may be more prone to GI tract infections and an earlier SARS-CoV-2 study with AGMS using the nCoV-WA1–2020 isolate suggests this could be true. That study had a single animal that exhibited infection in the GI tract up to 10dpi but the remaining animals did not (27). Future studies should address potential differences in organ tropism associated with SARS-CoV-2 variants.
A recent study posted on a preprint server examining SARS-CoV-2 variants in the rhesus macaque model showed no difference in viral replication nor disease between the D614G and B.1.1.7 variants (28). Notably, this study used double the infectious dose via intranasal challenge using the same atomizing device, but also added an intratracheal inoculation route (28). Although AGMs and rhesus macaques share similar ACE-2/RBD binding affinities (29), we cannot rule out the different NHP species as a potential factor in the outcome of infection. The marginally higher dose and additional route of infection may have been additional factors affecting infection outcomes.
In conclusion, our results from the intranasal AGM COVID-19 surrogate model support the most recent data from B.1.1.7 in humans, providing direct empirical data for increased replication in respiratory tissue, but with no enhancement of disease. Although the lack of clear statistical significance for some of the parameters may be regarded as possible limitation of the study, the use of these multiple parameters to independently verify one another addresses this concern. One way to obtain statistical significance more uniformly is to increase NHP numbers something that is ethically sensitive and difficult given the current issues with NHP availability. NHPs remain a surrogate model for humans and results presented herein provide direct experimental evidence that support recent clinical observations, which indicate that the B.1.1.7 VOC has characteristics of increased replication in respiratory tissues with enhanced shedding from the nose and oral cavity resulting in advanced transmission. A further notable and interesting observation of differences between these two variants in terms of distinct viral tissue/organ tropism warrants further attention as such changes would have public health implications in terms of transmission and disease manifestation.
Supplementary Material
Acknowledgements:
The authors are thankful to the animal caretakers and histopathology group of the Rocky Mountain Veterinary Branch (NIAID, NIH) for their support with animal related work, to the Research Technologies Branch (NIAID, NIH) for sequencing of stock viruses, and Anita Mora (NIAID, NIH) for help with the display items. We are grateful to Emmie de Wit and Vincent Munster for their discussions and help with virus stock preparations. The B.1.1.7 variant was obtained through BEI Resources (Bassam Hallis, Sujatha Rashid), NIAID (Ranjan Mukul, Kimberly Stemple) and the NIH.
Funding:
This work was funded by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Footnotes
Competing interest: The authors have declared that no conflict of interest exists.
Data and materials availability: All data are available in the main text or the supplementary materials. Additional information can be requested through the corresponding author.
Publisher's Disclaimer: Disclaimer. The opinions, conclusions and recommendations in this report are those of the authors and do not necessarily represent the official positions of the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH). There was no conflict in interest identified for any individual involved in the study.
References
- 1.Cucinotta D., Vanelli M., WHO Declares COVID-19 a Pandemic. Acta Biomed 91, 157–160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. (2021), vol. 2021. [Google Scholar]
- 3.Wise J., Covid-19: New coronavirus variant is identified in UK. BMJ 371, m4857 (2020). [DOI] [PubMed] [Google Scholar]
- 4.CDC. (2021), vol. 2021. [Google Scholar]
- 5.Davies N. G. et al. , Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 372, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volz E. et al. , Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature, (2021). [DOI] [PubMed] [Google Scholar]
- 7.Washington N. L. et al. , Emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. Cell, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challen R. et al. , Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 372, n579 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies N. G. et al. , Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grint D. J. et al. , Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Euro Surveill 26, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham M. S. et al. , Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health 6, e335–e345 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frampton D. et al. , Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munster V. J. et al. , Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585, 268–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan C. et al. , Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Res 30, 670–677 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aid M. et al. , Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell 183, 1354–1366.e1313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J. et al. , DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369, 806–811 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doremalen N. et al. , ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586, 578–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum A. et al. , REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 370, 1110–1115 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson B. N. et al. , Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585, 273–276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh D. K. et al. , Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nature Microbiology 6, 73–86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockx B. et al. , Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368, 1012–1015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolsey C. et al. , Establishment of an African green monkey model for COVID-19 and protection against re-infection. Nat Immunol 22, 86–98 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross R. W. et al. , Intranasal exposure of African green monkeys to SARS-CoV-2 results in acute phase pneumonia with shedding and lung injury still present in the early convalescence phase. Virol J 17, 125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross R. W. et al. , Intranasal exposure of African green monkeys to SARS-CoV-2 results in acute phase pneumonia with shedding and lung injury still present in the early convalescence phase. Virology Journal 17, 125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calistri P. et al. , Infection sustained by lineage B.1.1.7 of SARS-CoV-2 is characterised by longer persistence and higher viral RNA loads in nasopharyngeal swabs. Int J Infect Dis 105, 753–755 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lui K., Wilson M. P., Low G., Abdominal imaging findings in patients with SARS-CoV-2 infection: a scoping review. Abdominal Radiology 46, 1249–1255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speranza E. et al. , Single-cell RNA sequencing reveals SARS-CoV-2 infection dynamics in lungs of African green monkeys. Sci Transl Med 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munster V. J. et al. , Subtle differences in the pathogenicity of SARS-CoV-2 variants of concern B.1.1.7 and B.1.351 in rhesus macaques. bioRxiv, 2021.2005.2007.443115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melin A. D., Janiak M. C., Marrone F. 3rd, Arora P. S., Higham J. P., Comparative ACE2 variation and primate COVID-19 risk. Commun Biol 3, 641 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.