Abstract
Recent studies have shown that long noncoding RNAs (lncRNAs) are critical regulators in the central nervous system (CNS). However, their roles in the cerebellum are currently unclear. In this work, we identified the isoform 204 of lncRNA Gm2694 (designated as lncRNA-Promoting Methylation (lncRNA-PM)) is highly expressed in the cerebellum and derived from the antisense strand of the upstream region of Cerebellin-1 (Cbln1), a well-known critical cerebellar synaptic organizer. LncRNA-PM exhibits similar spatiotemporal expression pattern as Cbln1 in the postnatal mouse cerebellum and activates the transcription of Cbln1 through Pax6/Mll1-mediated H3K4me3. In mouse cerebellum, lncRNA-PM, Pax6/Mll1, and H3K4me3 are all associated with the regulatory regions of Cbln1. Knockdown of lncRNA-PM in cerebellum causes deficiencies in Cbln1 expression, cerebellar synaptic integrity, and motor function. Together, our work reveals an lncRNA-mediated transcriptional activation of Cbln1 through Pax6-Mll1-H3K4me3 and provides novel insights of the essential roles of lncRNA in the cerebellum.
The long non-coding RNA lncRNA-PM activates transcription of the cerebellar synaptic organizer Cbln1 by promoting Pax6-Mll1-mediated H3K4me3 methylation, thereby helping to maintain cerebellar synaptic integrity and motor function.
Introduction
Cerebellum plays a crucial role in motor functions including coordination, posture, and balance [1–6]. The cerebellar cortex consists of 3 sagittal-orientated zones: molecular layer, Purkinje cell (PC) layer, and granule cell (GC) layer [1–6]. A core cerebellar circuit, which mediates all of the cerebellar functions, is mainly comprised of GCs and PCs [1,5]. GCs project parallel fibers (PFs) and send excitatory signals to PCs. The growing PFs form thousands of synapses with the dendritic arborization of PCs in the molecular layer. PCs function as the sole output of the cerebellar cortex.
Cerebellin-1 (Cbln1) plays essential roles in cerebellar synaptic integrity and plasticity. It is expressed and secreted from the GCs and helps to establish and maintain the synaptic connections between PFs (axons of the GCs) and the dendrites of PCs through interaction with the presynaptic neurexin (Nrx) and the postsynaptic glutamate receptor delta2 (GluD2) [7–10]. Cbln1-null mice exhibited profound reduction in the number of synapses formed between PFs and PCs during development [7]. The observations that Cbln1 could rapidly induce the formation of functional and structurally normal PF synapses in the adult murine cerebellum indicated that Cbln1 functions in the mature neurons as well [11]. However, despite its well-known functions and therapeutic potential for cerebellar ataxic disorders, the gene expression regulation of Cbln1 is yet to be elucidated.
Long noncoding RNAs (lncRNAs) are enormously enriched in the central nervous system (CNS) [12]. Despite some reports on the role of lncRNAs in the CNS [13–15], how lncRNAs are involved in the brain function and regulation is largely unexplored. Recently, we and other independent groups have revealed dynamic spatiotemporal expression patterns of lncRNAs in mammalian brain [16–19]. Interestingly, we found that the number of cerebellar-expressing lncRNAs are relatively higher and that their profiling is quite unique compared with the other tested brain areas [16,17], raising a possibility that these cerebellum highly expressed lncRNAs may be essential regulators of cerebellar function.
In the present study, we found that our designated lncRNA-PM (Gm2694-204) is a specific isoform of Gm2694 to promote the expression of Cbln1. LncRNA-PM increases the DNA-bound fractions of Pax6 and Mll1, promotes the recruitment of Pax6, Mll1, and H3K4me3 to the upstream regulatory regions of Cbln1, and thus activates Cbln1 transcription in cultured Neuro2a cells. In the mouse cerebellum, lncRNA-PM, Pax6, and Mll1 are associated and located to the high H3K4me3 marked regions of Cbln1. Knockdown of lncRNA-PM specifically in the cerebellum causes deficiencies in Cbln1 expression, cerebellar synaptic integrity, and motor function. Moreover, we found that Gm2694 has a broader distribution in the cerebellum than Cbln1 through data mining of the reported cerebellar single-cell data and that lncRNA-PM regulates genes functioning in GABAergic synaptic transmission and morphogenesis of branching structure. Collectively, we characterize lncRNA-PM as a regulator of Cbln1 transcriptional activation and suggest lncRNA-PM as a multifunctional regulator in the cerebellum.
Results
Identification of lncRNA-PM, a splicing isoform of Gm2694, which promotes Cbln1 transcriptional activation
Our previous work showed that cerebellum is enriched for lncRNAs [17]. In order to explore the biological roles of lncRNAs in the cerebellum, we further analyzed the 57 identified cerebellum highly expressed lncRNAs (representing 63 reported transcripts) (S1 Table; [17]). Among these, lncRNA Gm2694 is derived from the antisense strand of the upstream region of Cbln1 (Fig 1A, left panel), exhibiting as a divergent lncRNA gene to Cbln1. Previous study has reported that “divergent lncRNAs” often positively regulate the transcription of their nearby coding genes and participate in similar biological processes as the neighboring genes [20]. Through reverse transcription-quantitative PCR (RT-qPCR), our results showed that lncRNA Gm2694 is relatively highly expressed in the cerebellum than olfactory bulb, hypothalamus, and hippocampus (Fig 1A, right panel). Such relatively high cerebellar expression of Gm2694 is similar to Cbln1 [7,21]. Moreover, data mining from the available developing cerebellum single-cell RNA sequencing (RNA-seq) datasets also revealed that Gm2694 and Cbln1 were coexpressed in certain subgroups of the cerebellar GCs and cerebellar nuclear neurons (S1 Fig; [21,22]). These results raised a very likely possibility that Gm2694 acts as a regulator of Cbln1 in these cells. Owing to the known essential role of Cbln1 as a synaptic organizer in the cerebellum and the unknown regulatory mechanisms of its expression, we focused our attention on the Cbln1 neighboring lncRNA, Gm2694, in this study.
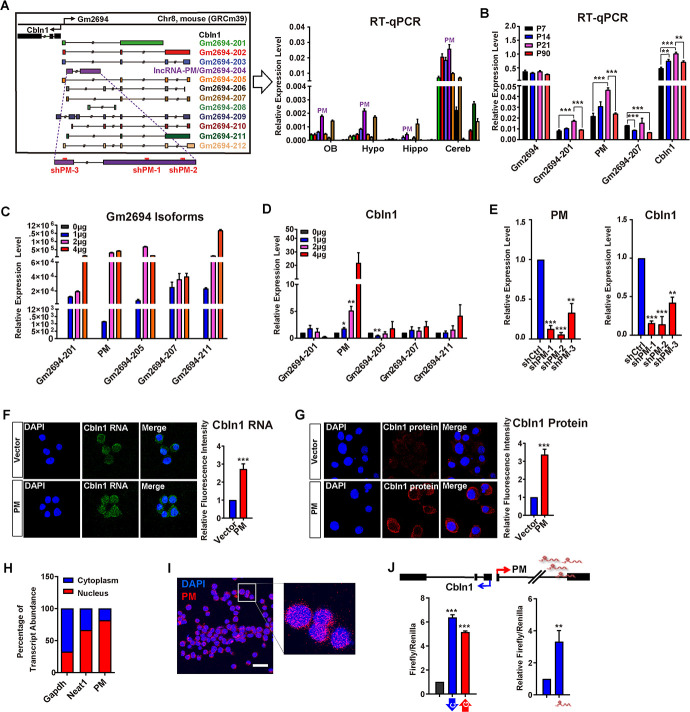
Fig 1. Identification of lncRNA-PM, a splicing isoform of Gm2694, which is required for Cbln1 activation.
(A) Left: illustration of 12 Gm2694 isoforms indicated as different colors. The shPM-1, shPM-2, and shPM-3 used in the study were indicated. Right: the relative expression levels of the 12 isoforms of Gm2694 in the indicated brain regions. Data are shown as means ± SEMs, n = 3. Gm2694-204 is designated as lncRNA-PM (PM). (B) The relative expression levels of Gm2694 (for 201, 202, 203, 205, 206, 207, 209, and 210), Gm2694-201, PM, Gm2694-205, Gm2694-207, and Cbln1 at the indicated cerebellar developmental time points were measured by RT-qPCR. Data are shown as means ± SEMs, n = 3. (C, D) The expression levels of the indicated Gm2694 isoforms (C) and Cbln1 (D) under the indicated treatments. (E) The expression levels of Cbln1 in control or PM shRNAs (shPM-1/2/3)-treated Neuro2a cells, detected by RT-qPCR. Data are shown as means ± SEMs, n = 3. (F) Left: representative images of Cbln1 mRNA in the control or PM-overexpressed Neuro2a cells, detected by FISH. Right: quantification of the left. Data are shown as means ± SEMs, n = 3. (G) Left: representative immunofluorescence images of Cbln1 protein in the control (Vector) or PM-overexpressed (PM) Neuro2a cells. Right: quantification of the left. Data are shown as means ± SEMs, n = 3. (H) Subcellular distributions of PM, Gapdh, and Neat1 by fractionating assay in Neuro2a cells. Gapdh and Neat1 RNA served as positive controls for RNAs predominantly expressed in cytoplasm and nucleus, respectively. Data are shown as means ± SEMs, n = 3. (I) Representative FISH image of PM RNA (red) in Neuro2a cells. Nuclei were stained with DAPI (blue). (J) Top: schematic illustration of the Cbln1/PM locus. Bottom left: luciferase assays indicate the activity changes of pGL3-Cbln1 (blue) and pGL3-Gm2694 (red), compared with pGL3 vector control (black). Bottom right: Luciferase assays indicate the activity changes of pGL3-Cbln1 in the presence of control or PM-expressing plasmid. Data are shown as means ± SEMs, n = 3. Scale bar, 13 um. All RT-qPCR results were normalized to Gapdh. All the data of this figure can be found in the S1 Data file. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; Cereb, Cerebellum; FISH, fluorescence in situ hybridization; Hippo, hippocampus; Hypo, hypothalamus; lncRNA-PM, lncRNA-Promoting Methylation; OB, olfactory bulb; RT-qPCR, reverse transcription-quantitative PCR.
The Ensembl database (version 101) has cataloged 12 splicing isoforms of Gm2694 (Fig 1A, left panel). Our qPCR results showed that except for isoforms 208 and 209—which were not detectable in the tested brain samples—the remaining 10 isoforms of Gm2694 were expressed at higher levels in the postnatal mouse cerebellum than in other tested brain regions (Fig 1A, right panel). When we examined cerebellum tissues at 4 key postnatal stages (P7, P14, P21, and P90) [2,4–6], we found that Gm2694-201, Gm2694-204 (PM), and Cbln1 were induced from P7 to P21, and then reduced at P90 (Fig 1B). The net expression of Gm2694-201, 202, 203, 205, 206, 207, 209, and 210, shown as Gm2694 in Fig 1B, showed to be relatively stable across the 4 tested time points. Next, we cloned 5 abundant Gm2694 isoforms (201, 204, 205, 207, and 211; Fig 1A, right panel), and tested their gain of functions on Cbln1 in cultured Neuro2a cells. Surprisingly, despite the similar cerebellum expression patterns among these tested isoforms, only the overexpression of Gm2694-204 altered Cbln1 expression and caused a significant increase of Cbln1 mRNA level (Fig 1C and 1D). Owing to the later identified role of Gm2694-204 in recruiting H3K4me3 to the regulatory regions of Cbln1, we designated Gm2694-204 as lncRNA-Promoting Methylation (PM). LncRNA-PM is then used in the following context and all the figures. In order to characterize the 5′ and 3′ of lncRNA-PM and Cbln1 transcripts, we conducted rapid amplification of cDNA ends (RACE) from mouse cerebellum total RNA and confirmed that lncRNA-PM and Cbln1 are positioned head-to-head (S2 Fig). The positive effect of lncRNA-PM on Cbln1 mRNA was further confirmed by RT-qPCR in lncRNA-PM knockdown cells (Fig 1E) and by fluorescence in situ hybridization (FISH) analysis in lncRNA-PM–overexpressed cells (Fig 1F). Consistently, the protein level of Cbln1 was dramatically increased in lncRNA-PM–overexpressed cells detected by immunofluorescence (Fig 1G). Together, these results indicated that lncRNA-PM can promote the transcriptional activation of Cbln1.
Next, both fractionation and FISH analysis detected the nuclear expression of lncRNA-PM in the cultured Neuro2a cells (Fig 1H and 1I) and the cerebellar GCs (Fig 2B). In order to test whether the effect of lncRNA-PM on Cbln1 is at the transcriptional level, we constructed a pGL3 vector containing the promoter sequence of Cbln1 (pGL3-Cbln1) and observed that overexpression of lncRNA-PM could further activate the pGL3-Cbln1 promoter activity in Neuro2a cells by luciferase assays (Fig 1J). To test the impacts of lncRNA-PM on other genes located near the Cbln1/Gm2694 locus, we designed specific qPCR primer sets for 12 nearby genes, including 4933402J07Rik, Gm24841, Zfp423, Cnep1r1, Heatr3, Tent4b, Brd7, N4bp1, Siah1a, Lonp2, Abcc12, and Phkb. With one exception (Abcc12, a relatively distal gene to the Cbln1/Gm2694 locus), none of these genes displayed significantly altered expression upon lncRNA-PM knockdown (S3 Fig). Collectively, our results suggested that lncRNA-PM promotes the expression of its divergent gene Cbln1 at the transcriptional level.
Fig 2. LncRNA-PM knockdown in mouse cerebellum results in decreased Cbln1, impaired synapse integrity, and motor behavior.
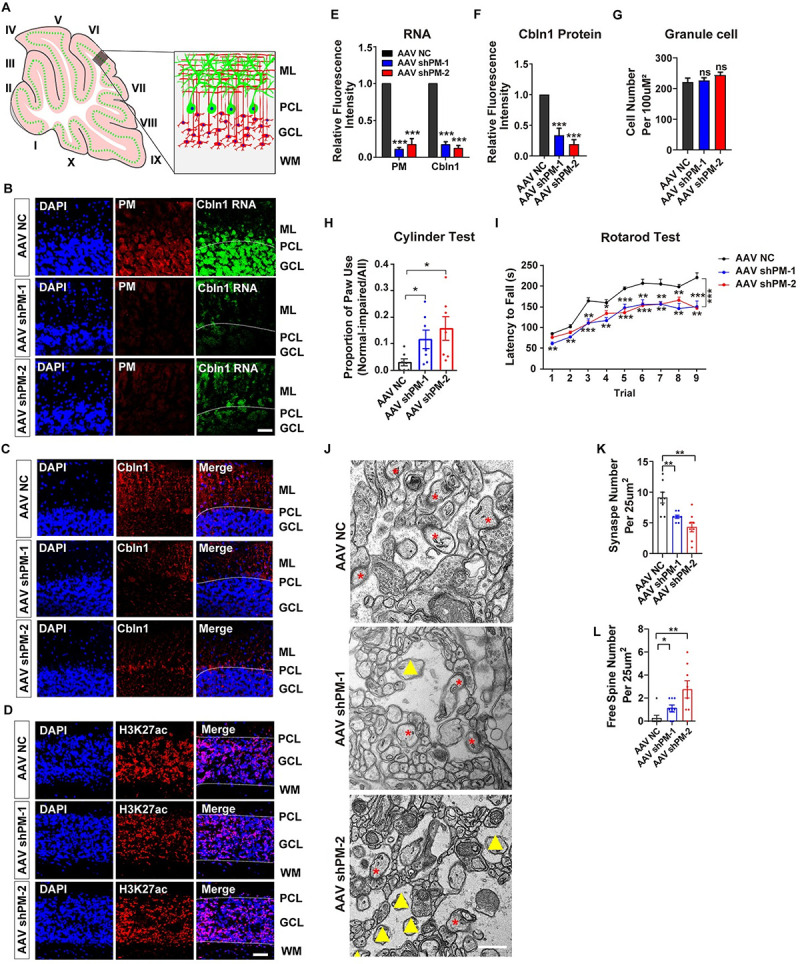
(A) Illustrated structure in the investigated cerebellar area. (B) The expression levels of PM (red) and Cbln1 (green) RNA in the cerebellar sections obtained from control (AAV NC) or lncRNA-PM (PM, red) shRNA-injected mice (AAV shPM-1 and AAV shPM-2). Scale bar, 40 um. (C and D) Immunofluorescence images of Cbln1 protein (C) and H3K27ac (D) in the cerebellar sections obtained from AAV NC and AAV shPMs mice. Scale bar, 40 um. (E) Quantification of the RNA levels of PM and Cbln1 obtained from B. Data are shown as means ± SEMs, n = 3. (F) Quantification of the protein levels of Cbln1 obtained from C. (G) Quantification of the GC numbers. (H) Evaluation of the control and shPMs-injected mice on cylinder test. Cylinder test was designed to assess limb preference on mice. It showed that compared with the control mice, PM shRNA-injected mice exhibited asymmetric high frequency in forepaw usage. The paw usage was counted and analyzed by normal-impaired/all. Data are shown as means ± SEMs, n = 7 (per group). (I) Evaluation of the control and shPMs-injected mice on rotarod test. Latency to fall provides the quantification of motor ability on the accelerating rotarod. It showed that compared with the control mice, shPMs-injected mice exhibited significant reduced duration in latency to fall. Data are shown as means ± SEMs, n = 6 (per group). (J) Representative electron microscope images for the control and shPM-injected mice. Red asterisk: intact synapse. Yellow triangle: free spine. Scale bar, 500 nm. (K) Quantification of the numbers of intact synapses obtained from J in the indicated treatments. (L) Quantification of the numbers of free spines obtained from (J) in the indicated treatments. All the data of this figure can be found in the S1 Data file. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; GCL, granule cell layer; lncRNA-PM, lncRNA-Promoting Methylation; ML, molecular layer; PCL, Purkinje cell layer; WM, white matter.
Selective knockdown of lncRNA-PM in mouse cerebellum results in synaptic terminal reduction and motor behavioral deficiencies
Next, we attempted to investigate the in vivo function of lncRNA-PM. In order to avoid the removal of DNA cis-acting elements on the Cbln1/Gm2694 locus, we chose a knockdown strategy, instead of knockout, for this particular case of lncRNA-PM. Control shRNA (negative control, NC) and 2 shRNAs to lncRNA-PM exon2 (shPM-1 and shPM-2) (indicated in Fig 1A, left panel) were generated into the pAAV-eGFP backbone and were utilized in the cerebellum injection. The virus infection resulted in wide distribution in the mice cerebellum (S4 Fig). When compared with the negative control, the expression levels of lncRNA-PM in the cerebellar GCs were sharply reduced in shPM-injected mice (Fig 2A and 2B). Consistently with the positive regulation of lncRNA-PM on Cbln1 in the Neuro2a cells shown in Fig 1, lncRNA-PM knockdown also resulted in sharply reduced expression levels of Cbln1 mRNA (Fig 2B and 2E) and protein (Fig 2C and 2F) in the shPM-injected mice. In contrast, a control antibody of H3K27ac displayed no difference between negative control and shPM mice (Fig 2D). Note that the number of GCs in the shPM-injected mice showed no significant difference with the negative control-injected mice, eliminating the possibility that the decrease of Cbln1 could have resulted from an altered number of GCs (Fig 2G).
The rotarod test and cylinder test were designed to assess motor coordination and limb preference on mice. In cylinder test, the shPM-injected mice showed significant asymmetric high frequency of forepaw usage when compared with negative control-injected mice (Fig 2H). In rotarod test, latency to fall provided the quantification of motor ability on the accelerating rotarod, and our data showed a significant decrease of latency to fall in the shPM-injected mice than negative control-injected mice (Fig 2I).
To further confirm the reported PF–PC synapse deficiencies in Cbln1-null mice [7], we used electron microscopy to examine the ultrastructure and analysis for the shPM-injected mice. We found that the number of synapses was markedly reduced in the shPM-injected mouse cerebellum, when compared to the negative control-injected mice (Fig 2J and 2K). The “naked” synapses contain postsynaptic density (PSD)-like condensations but lack of presynaptic contact. In the shPM-injected mouse cerebellum, we also observed significantly increased number of “naked” spines (Fig 2L).
Vesicular glutamate transporter 1 (VGluT1) and VGluT2 are PF and climbing fiber terminals markers, respectively [7]. It is known that Cbln1-null mice displayed impaired VGluT1 and VGluT2 expression patterns [7]. In order to examine whether lncRNA-PM influenced the general properties of VGluT1 and VGluT2, we performed immunofluorescence assay. Our data showed a significant reduction of VGluT1 (Fig 3A and 3D) and VGluT2 (Fig 3B and 3D) in shPM-injected mice. Moreover, we found that the longitudinal outgrowth of PC dendrites, detected by Calbindin, was decreased in shPM-injected mice (Fig 3C and 3D). Together, our results showed that lncRNA-PM is critical for normal synaptic terminal formation and cerebellar motor functions.
Fig 3. Reduced density of VGluT1, VGluT2, and Calbindin in the shPMs-injected mice.
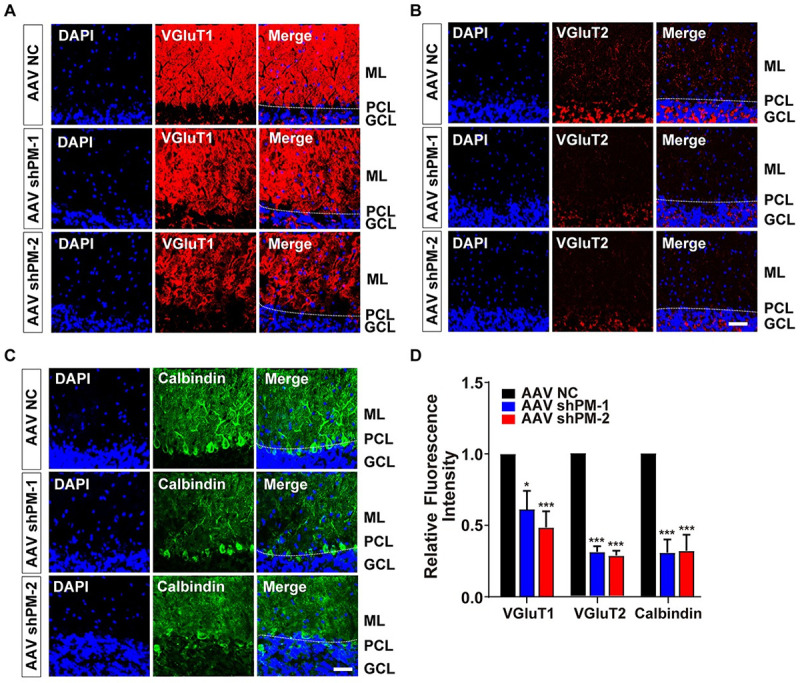
(A–C) Representative immunofluorescence images showed the density of VGluT1 (A), VGluT2 (B), or Calbindin (C) in the control and indicated shPMs-injected mice. VGluT1, VGluT2, and Calbindin are markers for PF terminals, climbing fiber terminals, and PCs, respectively. (D) Quantification of the indicated markers obtained from A–C. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. Scale bar, 40 um. *P < 0.05 and ***P < 0.001. GCL, granule cell layer; ML, molecular layer; PCL, Purkinje cell layer; PF, parallel fiber; VGluT1, vesicular glutamate transporter 1; VGluT2, vesicular glutamate transporter 2.
LncRNA-PM mediates H3K4me3 on Cbln1 via Mll1
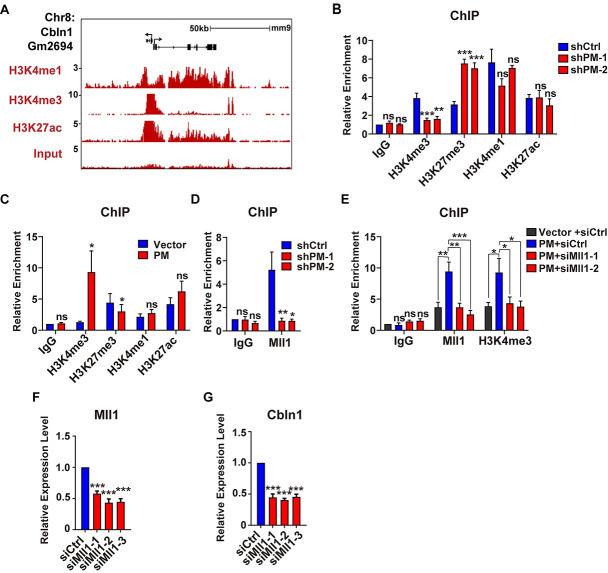
The Cbln1-Gm2694 locus showed highly enrichments of H3K4me3/H3K27ac markers, which encompass the transcription start sites and the upstream regulatory regions of Cbln1 (Fig 4A; S2 Table). In order to test the effect of lncRNA-PM on the histone markers located on the upstream regulatory regions of Cbln1, we either knocked down or overexpressed lncRNA-PM in cultured Neuro2a cells and performed chromatin immunoprecipitation (ChIP) assays. Our results showed that the H3K4me3 occupancy was consistently and dramatically increased by lncRNA-PM (Fig 4B and 4C). In contrast, the H3K27me3 level was consistently significantly decreased by lncRNA-PM (Fig 4B and 4C). No significant alteration of H3K4me1 or H3K27ac levels was detected upon modulation of lncRNA-PM (Fig 4B and 4C).
Fig 4. LncRNA-PM mediates H3K4me3 recruitment on the 5′ regulatory region of Cbln1 via Mll1.
(A) Indicated histone markers on Gm2694/Cbln1 locus in mouse cerebellum based on ENCODE data. (B) ChIP-qPCR detection of the indicated histone markers on the 5′ regulatory region of Cbln1 in the presence of control (shCtrl) or PM shRNAs (shPM-1 and shPM-2) in Neuro2a cells. Data are shown as means ± SEMs, n = 3. (C) ChIP-qPCR detection of the indicated histone markers on the 5′ regulatory region of Cbln1 in the control (Vector) or PM-overexpressed (PM) Neuro2a cells. Data are shown as means ± SEMs, n = 3. (D) ChIP-qPCR detection of Mll1 on the 5′ regulatory region of Cbln1 in the control or the indicated shPMs in Neuro2a cells. Data are shown as means ± SEMs, n = 3. (E) Changes of the Mll1 and H3K4me3 deposition on the 5′ regulatory region of Cbln1 in PM-overexpressed Neuro2a cells, with the treatments of Mll1 (siMll1-1 and siMll1-2) or control siRNAs (siCtrl). Data are shown as means ± SEMs, n = 3. (F and G) The expression levels of Mll1 (F) and Cbln1 (G) mRNAs in the control or Mll1 siRNAs-treated Neuro2a cells. All results were normalized to Gapdh. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; ChIP, chromatin immunoprecipitation; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance; qPCR, quantitative PCR.
Mll family contains 4 members (Mll1-4) and is known for their histone methyltransferase activities. Among them, Mll1 is a specific methyltransferase for H3K4me3 [23,24]. ChIP-qPCR assay showed that overexpression of lncRNA-PM in Neuro2a cells increased the recruitment of Mll1 instead of Mll2-Mll4 to the upstream regulatory regions of Cbln1 (S5 Fig). Consistently, knockdown of lncRNA-PM decreased recruitment of Mll1 to the upstream regulatory regions of Cbln1 (Fig 4D). These results suggested an involvement of Mll1 in lncRNA-PM-mediated Cbln1 activation. To test our hypothesis, we knocked down Mll1 in lncRNA-PM-overexpressed Neuro2a cells and found that the effect of lncRNA-PM-induced H3K4me3 enrichment was completely blocked by Mll1 knockdown (Fig 4E). Supporting these results, Mll1 knockdown significantly decreased the expression level of Cbln1 (Fig 4F and 4G). Taken together, our data suggested that lncRNA-PM–mediated H3K4me3 of Cbln1 is Mll1 dependent.
Pax6 is required for the lncRNA-PM-mediated Mll1-H3K4me3 enrichment
It has been reported that Mll1 forms a complex with Pax6 [24], a transcriptional factor that is highly expressed in mouse cerebellum [25] and a potential upstream regulator of Cbln1 suggested by genome-wide microarray analysis in the developing mouse cerebellum [26]. Very recently, a single-cell transcriptional study of the developing murine cerebellum also identified Pax6 as a lineage marker for the granule neuron progenitors and granule neurons [22]. Our data mining results showed that cells containing Gm2694 share strong overlap with Pax6-positive cell lineages (S1 Fig). These findings collectively indicated that lncRNA-PM may regulate Cbln1 through Pax6.
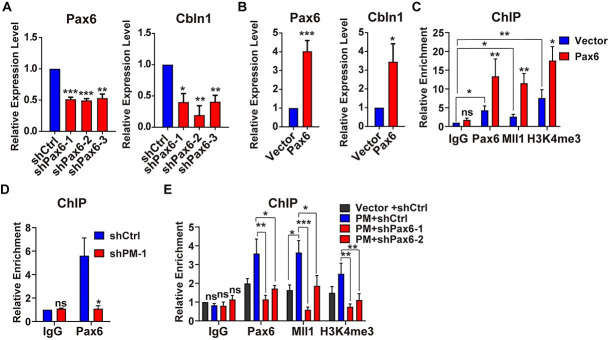
We first confirmed the positive regulation of Pax6 on Cbln1 mRNA by either knockdown or overexpression of Pax6 in Neuro2a cell (Fig 5A and 5B). ChIP assay showed that the recruitments of Mll1 and H3K4me3 were significantly increased in Pax6-overexpressed cells (Fig 5C). In order to rule out the possibility that Pax6 regulated the expression level of Mll1, we performed RT-qPCR assay and found that the expression level of Mll1 was not altered in Pax6-overexpressed cells (S6 Fig). Vice versa, Mll1 siRNAs had no effects on the expression level of Pax6 (S6 Fig). These results suggested that Pax6 activates Cbln1 through Mll1-H3K4me3 axis.
Fig 5. Pax6 is required for the lncRNA-PM-mediated Mll1-H3K4me3 recruitment.
(A) The expression levels of Pax6 and Cbln1 mRNAs in the control or Pax6 shRNAs-treated Neuro2a cells. All results were normalized to Gapdh. Data are shown as means ± SEMs, n = 3. (B) The expression levels of Pax6 and Cbln1 mRNAs in the control (Vector) or Pax6-overexpressed Neuro2a cells. All results were normalized to Gapdh. Data are shown as means ± SEMs, n = 3. (C) ChIP-qPCR detection of the indicated Pax6, Mll1, and H3K4me3 recruitment on the 5′ regulatory region of Cbln1 in the control or Pax6-overexpressed Neuro2a cells. Data are shown as means ± SEMs, n = 3. (D) ChIP-qPCR detection of Pax6 on the 5′ regulatory region of Cbln1 in the control or the indicated shPM in Neuro2a cells. Data are shown as means ± SEMs, n = 3. (E) Changes of the Pax6, Mll1, and H3K4me3 deposition on the 5′ regulatory region of Cbln1 in PM-overexpressed Neuro2a cells, with the treatments of Pax6 (shPax6-1 and shPax6-2) or control shRNAs (shCtrl). All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; ChIP-qPCR, chromatin immunoprecipitation couple with quantitative PCR; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance.
To answer the question whether Pax6 was involved in lncRNA-PM-mediated Mll1-H3K4me3 activation of Cbln1, we checked whether the deposition of Pax6 was affected by modulation of lncRNA-PM. Our results showed that knocking down of lncRNA-PM significantly decreased the occupancies of Pax6 (Fig 5D), and overexpression of lncRNA-PM increased the occupancies of Pax6 (Fig 5E). In addition, knockdown of Pax6 in the lncRNA-PM-overexpressed Neuro2a cells completely blocked the lncRNA-PM-induced recruitment of Mll1 and H3K4me3 on Cbln1 (Fig 5E). Next, we conducted fractionationing experiments upon overexpression of lncRNA-PM in the Neuro2a cells and tested the changes of Pax6 and Mll1. Our results showed that lncRNA-PM promoted the DNA-bound fractions of both Pax6 and Mll1 (S7 Fig). Together with the results showed in Fig 4, our data suggested that lncRNA-PM activates Cbln1 through recruitment of Pax6, Mll1, and H3K4me3. A possible underlying mechanism for such regulation is that lncRNA-PM helps to retain or translocate the chromatin associated fraction of Pax6 and Mll1.
In vivo association of lncRNA-PM and Pax6/Mll1 complex with Cbln1
In order to provide in vivo evidence of the above identified regulatory mechanism, we conducted a series of experiments in mouse cerebellum. Firstly, we applied the UV-RNA immunoprecipitation (UV-RIP) assay to detect binding of Mll1 and Pax6 to lncRNA-PM. We found that Mll1 and Pax6 were specifically associated with lncRNA-PM transcripts in the mouse cerebellum (Fig 6A). Serving as negative controls, unrelated lncRNA-Neat1 and another cerebellum expressed lncRNA-lhx1os showed no interaction with either Mll1 or Pax6 (Fig 6A). Secondly, to answer whether lncRNA-PM is bound to the regulatory regions of Cbln1 in cerebellum, we conducted chromatin isolation by RNA purification (ChIRP) experiments. To exclude the potential interference from other isoforms, we designed specific antisense oligonucleotides (ChIRP probes) of lncRNA-PM that did not overlap with other isoforms of Gm2694. These probes showed to be highly effective and specific in the mouse cerebellum extracts (Fig 6B). When compared to the negative control probes of lacZ, our ChIRP-qPCR analysis of lncRNA-PM revealed significant signals of lncRNA-PM to those upstream regulatory regions of Cbln1 that are highly marked by H3K4me3 revealed by ChIP-seq (Fig 6C and 6D). Our ChIRP results suggested that lncRNA-PM is accumulating at its derived genomic locus to direct local histone modification changes. Consistently with these ChIRP results, our ChIP assays also revealed the lncRNA-PM-dependent recruitments of Pax6, Mll1, and H3K4me3 at multiple regions on the upstream regulatory regions of Cbln1 (Figs 4 and 5, S8 Fig). Thirdly, in order to test whether Mll1 and Pax6 function as a complex on Cbln1 in mouse cerebellum, we conducted Re-chromatin immunoprecipitation (Re-ChIP) assay and showed that Pax6 and Mll1 were simultaneously bound to Cbln1 (Fig 6E). Taken all together, our data provided strong evidence of an association of lncRNA-PM and Pax6/Mll1 complex with the regulatory regions of Cbln1 in cerebellum (Fig 6F).
Fig 6. In vivo association of lncRNA-PM and Pax6/Mll1 complex with Cbln1.
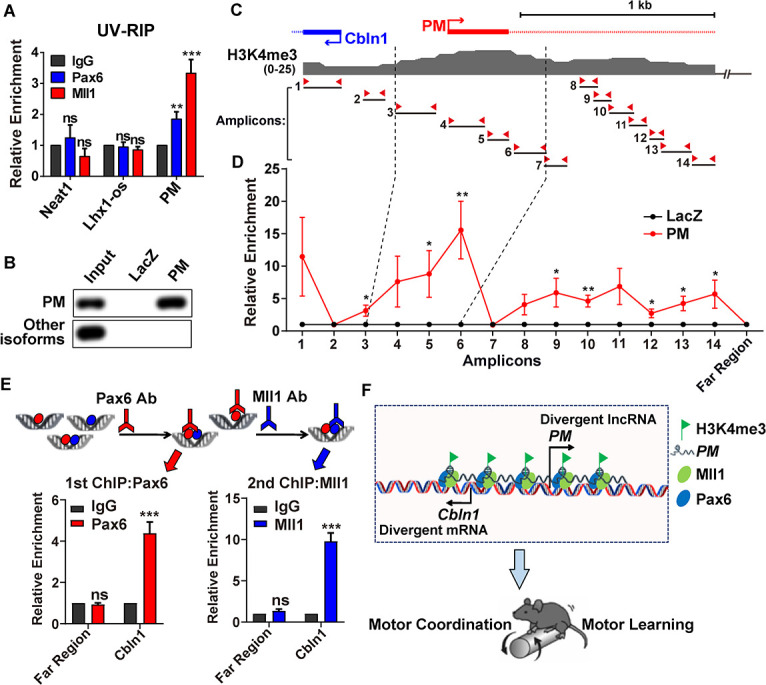
(A) UV-RIP assay showed PM:Pax6 and PM:Mll1 interactions in mouse cerebellum. Neat1 and lncRNA-lhx1os: negative controls. Data are shown as means ± SEMs, n = 3. (B) RT-qPCR detection following PM RNA pull-down using cerebellum tissue. PM: PM probes; LacZ: control probes. (C) Illustration of H3K4me3 signature on the upstream regulatory region of Cbln1 locus from +300 to −2,000 base pair based on ENCODE data in mouse cerebellum. The 14 pairs of amplicons are indicated. (D) ChIRP-qPCR showed occupancy of PM to the regions amplified by the indicated 14 amplicons and a control region that is 85 kb upstream of Cbln1 transcription start site (Far Region). Data are shown as means ± SEMs, n = 3. (E) Re-ChIP of Pax6 and Mll1. Top: illustrated flowchart of double ChIP experiment. Bottom left: recruitments of Pax6 or IgG to Cbln1 or the negative control region (Far Region) after first ChIP in mouse cerebellum. Bottom right: recruitments of Mll1 or IgG to Cbln1 or Far Region after second ChIP in mouse cerebellum. Data are shown as means ± SEMs, n = 3. (F) Model: LncRNA-PM mediates the transcriptional activation of Cbln1 through Pax6/Mll1-mediated H3K4me3 and maintains mouse motor coordination and motor learning. All the data of this figure can be found in the S1 and S2 Data files. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; ChIP, chromatin immunoprecipitation; ChIRP, chromatin isolation by RNA purification; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance; qPCR, quantitative PCR; Re-ChIP, Re-chromatin immunoprecipitation; RT-qPCR, reverse transcription-quantitative PCR; UV-RIP, UV-RNA immunoprecipitation.
LncRNA-PM regulates genes in neuronal associated pathways
Through analysis of the single-cell transcriptional study of the murine cerebellum, we found that lncRNA-PM exhibited a broader distribution than the Cbln1 (S1 Fig). In addition, the shPM-injected mice resulted in defects on PC dendrites that were not observed in the Cbln1-null mice [7] (Fig 3C and 3D). These results suggested that lncRNA-PM acts as a broader cerebellar regulator. In order to gain a full scope of the genome-wide expression control of lncRNA-PM, we performed RNA-seq upon knockdown of lncRNA-PM by shPM-1 and shPM-2 and analyzed the common downstream targets from both shRNA transfectants. LncRNA-PM knockdown resulted in statistically significant changes in the expression levels of 504 genes (|fold change|>1.5) compared to the vector control; 352 (70%) of these genes were down-regulated and 152 (30%) were up-regulated. Importantly, we found that 245 (46.8%) of lncRNA-PM-regulated genes were also targeted by Pax6, identified from Pax6 overexpression RNA-seq (S9 Fig; S3 Table). The Gene Ontology (GO) analysis showed that these comprised 245 genes were significantly enriched in neuronal associated pathways, including GABAergic synaptic transmission, morphogenesis of branching structure, neurotransmitter biosynthetic process, and regulation of transmission of nerve impulse (Fig 7A). We further validated 14 downstream genes that were commonly regulated by lncRNA-PM and Pax6 in the same direction (Fig 7B–7D).
Fig 7. LncRNA-PM regulates neuronal associated genes.
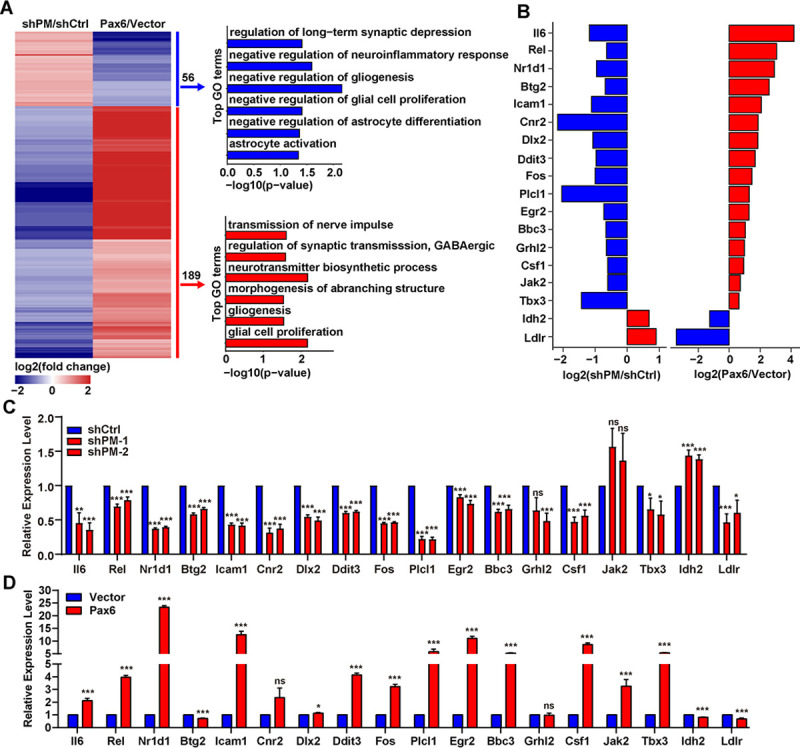
(A) Left: heatmap of dysregulated genes (|Fold change|>1.5, FDR < 0.05) in PM knockdown and Pax6 overexpression RNA-seq. Right: GO biological processes of PM and Pax6 coregulated genes. Red: positively regulated genes shared by PM and Pax6. Blue: negatively regulated genes shared by PM and Pax6. (B) Modulations of lncRNA-PM and Pax6 impact neuronal associated genes. Expression changes for selected genes are shown as the log2 expression ratio. Red: positively regulated genes shared by PM and Pax6. Blue: negatively regulated genes shared by PM and Pax6. (C and D) qPCR detection of the indicated genes upon PM knockdown (C) and Pax6 overexpression (D). All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. FDR, false discovery rate; GO, Gene Ontology; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance; qPCR, quantitative PCR; RNA-seq, RNA sequencing.
Discussion
Cbln1 plays essential roles in developing and mature cerebellum [7,11]. Despite the exceptional high expression level of Cbln1 in cerebellum, the molecular mechanism of Cbln1 gene expression control is still unexplored. Recently, Krishnan V. and colleagues found that a ubiquitin-ligase and transcriptional coregulator Ube3a down-regulated Cbln1 in several mouse brain regions and proposed a possible regulation at the transcriptional level of Cbln1 [27]. In the present work, we found that Cbln1 and its neighboring lncRNA lncRNA-PM are coexpressed in the cerebellar GCs. Our finding that lncRNA-PM activates the gene expression of Cbln1 unveils the mystery of gene activation control of this critical cerebellar synapse organizer.
Pax6 has been recently identified as a cerebellar lineage marker [22,28]. In cerebellum, Pax6 is highly expressed in GC layer of developing cerebellum and maintains in adult cerebellar GCs [29]. Loss of Pax6 causes aberrant organization of GC layer, GC survival, and neurite extension [29]. Neural behavior analysis for Pax6-null mice/rats and investigation of clinical patients with Gillespie or WAGR syndrome indicated that Pax6 is associated with cerebellar ataxia and other neurologic diseases [30–33]. Pax6 is known to interact with TrxG/MLL complex responsible for H3K4me3 [24]. In the present work, we demonstrated that the lncRNA-PM-mediated Cbln1 activation is achieved through recruitments of Pax6, Mll1, and H3K4me3. Our findings provide a novel mechanism to explain the roles of Pax6 and Mll1 (via activating Cbln1) in cerebellum.
LncRNA-PM-mediated Cbln1 activation occurs in both cultured cell system and the murine cerebellum. The lncRNA-PM knockdown mice exhibit similar phenotypes as the Cbln1-null mice in synaptic integrity and motor function. Intriguingly, the Cbln1-null mice expressed a higher density of VGluT2 and showed no overt difference in PC dendrites, while significant reductions of VGluT2 and Calbindin signals have been found in our lncRNA-PM knockdown mice. Such discrepancies may be resulted from 2 aspects: the different methodology and animal stages utilized for generating the 2 types of animal models and the broader distribution of Gm2694 than Cbln1 in the cerebellum. First, the reported Cbln1-null mice were generated by replacing the Cbln1 gene with Cbln1 promoter-driven LacZ. Thus, any impacts resulted from Cbln1 depletion would have been present throughout the course of development. However, some impacts also might be compromised during development. Distinct from those Cbln1-null mice, our study used 2-month-old adult mice for the lncRNA-PM knockdown. It is well known that synapse remodeling of diverse fiber types to their destination neurons is highly dynamic in the developing and mature cerebellum. For example, during the first 3 postnatal weeks, redundant climbing fibers (marked by VGluT2) are eliminated, culminating in a situation where the majority of PCs are innervated by a single climbing fiber [34,35]. Second, Gm2694 is evident in GABAergic progenitors, interneurons, and glutamatergic cerebellar nuclear neurons, in addition to the coexpression with Cbln1 in the cerebellar GCs.
Increasing the number of isoforms is a general strategy used by different types of cells to expand the transcriptome diversity. For instance, in the CNS, alternative splicing-generated mRNA isoforms are important in the development, migration, synaptic transmission, and plasticity of neurons [36,37]. LncRNAs have also been recently reported as targets of alternative splicing [36–42]. Although a few studies have shown that lncRNAs participate in the dynamic regulation process of alternative splicing in CNS [43,44], it is currently largely unclear about the biological significance of lncRNA isoforms in CNS. Our identification that Gm2694 regulates Cbln1 transcriptional activation through an isoform-specific manner expands the current understanding on the specificity mediated by lncRNA isoforms.
Collectively, our work provides a previously unidentified mechanism of lncRNA-PM-Pax6-Mll1-H3K4me3-mediated Cbln1 activation and expands our understandings on the biological roles of lncRNA in the cerebellum. Our observation that lncRNA-PM functions in a different way than other Gm2694 isoforms sheds light on the biological significance of lncRNA-based isoform in nervous system.
Materials and methods
Animal ethics statements
All animal experiments were approved and carried out in accordance with the Institutional Animal Care and Use Committee of the University of Science and Technology of China (permission number: USTCACUC1801023) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell culture and transfection
The HEK 293T (ATCC, USA) and Neuro2a cells (ATCC, USA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA), 1% penicillin–streptomycin (WISENT, Canada), and 1% L-glutamine. Transfections were conducted using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s instructions.
SiRNAs and shRNAs
Three siRNAs of Mll1 were designed and synthesized from Ribobio (Guangzhou, China). Three independent shRNAs specifically targeting 3 different regions of lncRNA-PM or Pax6 were designed and separately cloned into pLKO.1 vector to generate 3 lncRNA-PM or Pax6 shRNAs. Two of 3 lncRNA-PM shRNAs inserted pLKO.1 vector were separately cloned into pAAV-CAG-eGFP-U6-shRNA vector (OBIO, China) to generate 2 pAAV-CAG-eGFP-U6-shPM vectors. The primers are listed in S4 Table.
Plasmids construction
The ectopic expression constructs of 5 Gm2694 isoforms (ENSMUST00000180700.8/Gm2694-201, ENSMUST00000181898.1/Gm2694-204/lncRNA-PM, ENSMUST00000182174.7/Gm2694-205, ENSMUST00000182758.2/Gm2694-207, and ENSMUST00000211631.1/Gm2694-211) were amplified from mouse cerebellum cDNA and cloned into the pZW1-sno vector [45], respectively. The Pax6 (Gene ID: 18508) expression plasmid was constructed by cloning Pax6 ORF from mouse cerebellum cDNA and inserting it into the multiple cloning site (MCS) of the p3xflag-Myc-CMV-24 vector (Sigma, USA).
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA of cultured cell or mouse brain tissues was isolated using the TRizol Reagent (Ambion, USA), treated with RNase-free DNase I (Thermo Scientific, USA). Reverse transcription was performed using HiScript II One Step RT-PCR Kit (Vazyme, China) according to the manufacturer’s instructions. qPCR was performed using SYBR Green reagents (Vazyme, China) in Light Cycle 96 (Roche, USA). Relative gene expression was calculated using the 2−ΔΔCt method, and Gapdh served as an internal control. The primers are listed in S5 Table.
Rapid amplification of cDNA ends (RACE)
RACE was conducted using the 5′ RACE System and 3′ RACE System Kit (Invitrogen, USA) following the manufacturer’s instructions. Briefly, for the 5′ RACE, cerebellum RNA was reverse transcribed with the antisense gene specific primers (GSPs) to lncRNA-PM or Cbln1, and the first-strand cDNA product was homopolymeric tailed. Two rounds of nest PCR amplification were then performed with sense generic primer and antisense GSPs (Flexcycler, Germany). For the 3′ RACE, the cerebellum RNA was reverse transcribed using the adapter primer containing a 3′ stretch of poly(dT). Amplification was sequentially performed by nest PCRs using oligonucleotides to the non-poly(dT) portion of the adapter primer and the GSPs specific to lncRNA-PM or Cbln1. The PCR products were subjected to electrophoresis, purification, and DNA sequence analysis. The GSPs used in RACE experiments are as follows:
5′ RACE:
Reverse transcription:
Cbln1: 5′ - AAACTGTAGATGCCTT-3′;
lncRNA-PM: 5′ - CAAGTGAAGACCATAC-3′;
First-round PCR amplification:
Cbln1: 5′ - GTGCTGCGTTCTGAGTCAAA-3′;
lncRNA-PM: 5′ - TCCTTCCCTGGTCCCTTTAC-3′;
Second round of amplification:
Cbln1: 5′ - TCCAGAACAAATGTCCCCGC-3′;
lncRNA-PM: 5′ - CAGGCCATGAGAACACTGTG-3′;
3′ RACE:
First-round PCR amplification:
Cbln1: 5′ - GGTGACTGGTTTGACTATAC-3′;
lncRNA-PM: 5′ -TGAAACTACATCCACGCCCA-3′;
Second round of amplification:
Cbln1: 5′ - AGGCTGCTTGGTGTTTGTTC-3′;
lncRNA-PM: 5′ - GCGTATGGTCTTCACTTGCC-3′.
Dual luciferase reporter assay
The 5′ regulatory sequences of Gm2694 and Cbln1 were amplified using PCR from mouse cerebellum genomic DNA and sequentially ligated into pGL3-basic vector to generate pGL3-Gm2694 or pGL3-Cbln1 reporter plasmid. To measure the reporters’ activity, either pGL3-basic vector, pGL3-Gm2694, or pGL3-Cbln1 plasmid was cotransfected into HEK 293T cells together with Renilla luciferase reporter plasmid. To measure lncRNA-PM effect on the pGL3-Cbln1, either pZW1-sno vector or pZW1-sno-lncRNA-PM plasmid was cotransfected into HEK 293T cells together with pGL3-Cbln1 and Renilla luciferase reporter plasmid. Two days after transfection, firefly and Renilla luciferase activity were measured by a dual luciferase reporter assay system (GeneCopoeia, USA). The data are represented as mean ± SEM of 3 independent experiments.
Cerebellum lncRNA-PM knockdown mice
Mice were group housed in groups of 2 to 5 with a 12-h light/dark cycle (lights off at 6 PM) and free access to food and water ad libitum under specific pathogen-free conditions. Male C57BL/6J mice were purchased from Beijing SPF Biotechnology limited company and used for all experiments. For pAAV-CAG-eGFP-U6-shRNA (OBIO, China) injection, 3 AAV-based plasmids (AAV NC, AAV shPM-1, or AAV shPM-2) were respectively injected into normal 2-month mice to produce 3 groups of experimental mice. Briefly, glass micropipettes attached to 10 μl syringes were placed bilaterally into the cerebellum (A/P: −7.05 mm; M/L: ±1.2 mm; D/V: −1.6 mm), and 1 μl of virus was infused to each hemisphere at a rate of 50 nl/min using a pump (KD Scientific, USA). Mice were allowed to recover for 2 months before behavioral tests or immunofluorescence.
Cylinder test
The cylinder test was designed to assess forepaw preference on mice and conducted in a transparent cylinder. A video recorder was placed behind the explorative area to allow recording forelimb movements in all directions. The animals were not habituated to the cylinder prior to filming. The forepaw use was counted and normal-impaired/all was used a quantitative outcome parameter. Seven mice were tested in each group.
Rotarod test
We used the rotarod training system (Softmaze, China) to test the motor coordination and motor learning skill of mice. Briefly, before the first testing sessions, the mice were habituated to stay on a stationary rod for 2 min. To assess motor skill, a total of 9 trials for the rotarod test were carried out using an accelerating protocol from 0 to 60 rpm in 300 s with 30-min intertrial intervals. Three testing trials per day were performed for 3 consecutive days. After falling, the mice were immediately placed back to their home cages, and the time to fall was automatically recorded by the rotarod software. Six male mice in each group were used for the experiment. Six mice in each group were divided into 2 experiments, and 3 mice were simultaneously tested.
Brain tissue preparation
Mice were deeply anesthetized by intraperitoneal (IP) injection of pentobarbital (40 mg/kg), and then perfused with chilled PBS, followed by 4% paraformaldehyde (PFA) (Biosharp, China). Brains were removed and immersed in 4% PFA overnight, and subsequently incubated in 30% (wt/vol) sucrose in PBS at 4°C for 18 h. Sagittal brain sections with 25 μm thickness were made using a cryostat (Leica CM1950, Germany) at −20°C and conducted for desired experiments.
Immunofluorescence (IF) and FISH
Cultured Neuro2a cells growing on coverslips were fixed by 4% PFA for 10 min and subsequently incubated with 0.2% Triton X-100 in PBS. After 30 min of blocking using 1% BSA in PBST (0.05% Tween-20 in PBS), Neuro2a cells were incubated with rabbit anti-Cbln1 antibody (Novus, NBP1-17239, 1:200) in PBST overnight at 4°C. On the following day, the cells were washed with PBST and incubated with rhodamine-labeled secondary antibody (1:100) for 30 min at room temperature on a shaker. After PBST wash and stained with DAPI for 10 min, the coverslips were sealed with 75% glycerin and subjected to laser scanning confocal microscope (ZEISS710, Germany).
For brain sections, sections were incubated with 0.5% Triton X-100 in PBS for 15 min after wash (3 times of PBS, each time 5 min). After 40 min of blocking (3% albumin from bovine serum, 3% concentrated goat serum in PBS), the sections were incubated with rabbit anti-Cbln1 antibody (Abcam, ab64184, 1:50), rabbit anti-H3K27ac antibody (Abcam, ab4729, 1:100), mouse anti-Calbindin antibody (Abcam, ab82812, 1:100), rabbit anti-VGluT1 (Abcam, ab227805, 1:100), or rabbit anti-VGluT2 antibody (Abcam, ab216463, 1:100) in PBS at 4°C for 24 h. On the following day, the sections were washed in PBS and incubated with rhodamine- or Alexa Fluor 647–labeled secondary antibody (1:100) for 30 to 60 min.
FISH assays were performed using FISH Kits (Genepharma, China) according to the manufacturer’s instructions. lncRNA-PM and Cbln1 probes were designed by Biosearch Technologies website (https://www.biosearchtech.com/stellarisdesigner/). All probes were synthesized from General Biosystems, China (Probes for Cbln1: 5′-TACGATGGGCTCTGTCTCAT-3′, 5′-TTCAACATGAGGCTCACCTG-3′, 5′-GAAGGTTGAGTACTTCCAGC-3′; Probes for lncRNA-PM: 5′-TTTTGTATGCATGCTGACCG-3′, 5′-CTCAGGGAAGGTCTTCTTAA-3′, 5′-GTTTATTATTCCTTGTGGAG-3′). Laser scanning confocal microscope (ZEISS710, Germany) was used for images.
To quantify the signal intensity obtained from the above described IF and FISH images, we followed a previously reported analytic procedure with some modifications [11]. Briefly, 3 square images of 40 × 40 μm were taken within the cerebellar molecular layer. All images were obtained by exposures and gains at fixed parameters for each fluorescent channel. The images were analyzed using ImageJ to evaluate the signal intensity. After subtracting the background signals, the values of signal intensity from 3 regions were averaged to represent the data for each treatment, and the experiment was performed 3 times independently. All the comparable treatments were simultaneously performed and analyzed on the same day.
Electron microscopy
Mice were perfused with 2% PFA and 2.5% glutaraldehyde. Cerebellum were sliced sagittally and fixed with 2.5% glutaraldehyde. The cerebellum slices were postfixed with 1% osmium acid, dehydrated through graded acetone, and embedded in epoxy resin. Ultrathin sections (70 nm) were made from the epoxy resin blocks using an ultramicrotome (Leica UC7, Germany) and stained with uranium acetate and lead citrate. Micrographs were taken by electron microscope (FEI, USA) at 8,200×.
Western blot
The cells were washed in PBS and lysed with RIPA buffer (Vazyme, China) supplemented with protease inhibitor cocktail (Roche, Germany). After quantification using One DropTM 1000+ (ANTPEDIA, China), proteins were separated by SDS-PAGE under denaturing conditions and transferred to PVDF membranes (Millipore, USA). Membranes were blocked with 5% fat-free milk for 2 h at room temperature and incubated with primary antibodies for Gapdh (Proteintech, 60004-1-Ig, 1:5,000), Mll1 (CST, D668N, 1:1,000), Mll2 (CST, E6A8V, 1:1,000), Pax6 (Abcam, ab5790, 1:500), and H2b (Abcam, ab52599, 1:10,000). The membranes were then incubated with secondary antibodies conjugated with horseradish peroxidase (Proteintech, USA). Protein signals were visualized using the ECL detection reagent (Thermo Scientific) on UVP (Analytik Jena AG, Germany).
Subcellular fractionation assay
Neuro2a cells (7 × 106) were lysed in RSB-100 buffer (100 mM Tris-HCl (pH 7.4); 2.5 mM MgCl2; 100 mM NaCl; 40 μg/mL digitonin) at 4°C for 15 min. After centrifugation at 2,000g for 8 min, the supernatant was collected as the cytosolic fraction and saved for western blot. The pellets were resuspended in RSB-100T buffer (RSB-100 containing 0.5% Triton X-100) and rotated at 4°C for 15 min. After centrifugation at 2,000g for 8 min, the supernatant was collected as the nuclear fraction and saved for western blot. The pellets were resuspended in RSB-100T buffer and subjected to sonication (Scientz, China). After centrifugation at 4,000g for 15 min, the soluble DNA-bound fraction was collected and subjected for western blot with the previously obtained cytosolic and nuclear fraction.
UV-RNA immunoprecipitation (UV-RIP), chromatin immunoprecipitation (ChIP), and Re-ChIP
For the UV-RIP assay, dounced cerebellum tissues were irradiated with UV light (400 mJ/cm2). After centrifuged at 16,000 rpm at 4°C for 10 min, the cerebellum tissues were lysed for 30 min in RIPA buffer supplemented with RNase inhibitors (Beyotime, China) and protease inhibitor cocktail. After centrifuged at 16,000 rpm for 10 min at 4°C, the supernatant was precleared with 25 μl of Protein A/G Plus-Agarose beads (Santa Cruz Biotechnology, USA) at 4°C for 2 h. Then, the precleared lysate was incubated with 50 μl of Protein A/G Plus-Agarose beads that were precoated with 4 μg of IgG (Abcam, 172730), Pax6 (Abcam, ab5790), or Mll1 (Sigma, PLA0100) antibody. After 12-h incubation, the beads were washed 5 times using RIPA buffer. Finally, RNA was extracted using TRizol Reagent (Ambion, USA). The protein-bound RNA was analyzed by RT-qPCR.
The ChIP assay was performed as previously reported [46]. Briefly, dounced cerebellum tissues or cultured cells were cross-linked with 1% formaldehyde and neutralized with 125 mM glycine. After cell lysing, the lysates were sonicated. Supernatants were then incubated with 2 μg desired antibodies for IgG (Abcam, 172730), H3K4me1 (Abcam, ab8895), H3K4me3 (Abcam, ab8580), H3K27ac (Abcam, ab4729), H3K27me3 (Abcam, ab6002), Mll1 (Sigma, PLA0100), or Pax6 (Abcam, ab5790) at 4°C overnight. Immunoprecipitated complexes were collected using Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, USA). The isolated chromatin fraction was purified by EasyPure Genomic DNA Kit (TransGene Biotech, Beijing, China). For Re-ChIP experiments, complex obtained from the first ChIP were eluted by incubation for 30 min at 37°C in 25 μl 10 mM DTT. After centrifugation, the supernatant was diluted 20 times with Re-ChIP buffer (1% TritonX-100, 2 mM EDTA, 20 mM Tris-HCL, 150 mM NaCl (pH 8.1)) and subjected again to the ChIP procedure. The DNA product was analyzed by qPCR. For the data analysis, the control IgG group of each set of data was used as the normalizer for the following antibody groups to determine the binding specificity of the desired antibody. The related primers are listed in S6 Table.
Chromatin isolation by RNA purification (ChIRP)
Antisense RNA oligonucleotides (probes) for lncRNA-PM and LacZ (negative control) were designed by Biosearch Technologies website (https://www.biosearchtech.com/stellarisdesigner/) and synthesized with 3′-biotin-TEG modification (Sangon, China). Probes targeting to each tested RNA molecule were pooled and diluted to a stock concentration of 100 μM. The ChIRP assay was performed as previously reported with some modifications [47]. Briefly, 5 dounced mouse cerebellum were cross-linked with 3% formaldehyde for 30 min at room temperature on a shaker, and the reaction was quenched with 0.125 mM glycine for 5 min. Cells were collected by spinning at 2,000g for 5 min, and nuclei were collected with Covaris truChIP Chromatin shearing tissue kit (Covaris, USA) supplemented with RNase inhibitors (Beyotime, China) and protease inhibitors (Roche, USA). Nuclei were immediately sonicated by Covaris M220 (Covaris, USA) to 200 to 500 bp fragments and verified by gel electrophoresis. The sonicated samples were spun at 16,100g for 10 min at 4°C, and the supernatant was then transferred to a new tube. Before continuing to the next steps, 2 sets of 10% supernatant were removed into 2 separate tubes and saved as DNA or RNA input control, respectively. Double volume of hybridization buffer and the desired probes (1 μl of 100 μM probes per 1 ml of lysate) were added to the remaining supernatant and incubated at 37°C for 6 h in a rotator. After incubation, prewashed Dynabeads MyOne Streptavidin C1 magnetic beads (Invitrogen, USA) (100 μl of beads per 1ml of lysate) were added and incubated for 30 min at 37°C on a rotator. The Dynabeads were then captured by magnets (Invitrogen, USA) and washed with Wash Buffer for 5 min at 37°C for a total of 5 times. After the last wash, beads were resuspended in 1 ml Wash Buffer. A volume of 100 μl was taken for RNA isolation, and the rest was used for DNA isolation. RNA enrichment was confirmed by RT-qPCR. RNA-bound DNA was analyzed by qPCR. For the data analysis, the LacZ control was used as the normalizer to determine the binding specificity to each tested region. The related primers and probes are listed in S6 and S7 Tables.
RNA-seq
RNA library construction and sequencing were performed with BGISEQ-500 platform. For each experiment, 3 biological repeats were sequenced. The differential expressed genes (DEGs) were determined by using |fold change| >1.5.
Quantification and statistical analysis
Statistical analysis was carried out using Microsoft Excel software and GraphPad Prism to assess the differences between experimental groups. Statistical significance was analyzed by two-tailed Student t test and expressed as a P value. Especially, two-way ANOVA test was performed to measure significance of interaction in rotarod test. P < 0.05 were considered to be statistical significance.
The numerical data used in all figures are included in S1 Data. The raw images of blot and gel used in all figures are included in S2 Data.
Supporting information
(A) t-SNE plot displaying the distribution of the main cerebellar cell types. (B) The t-SNE distributions of Gm2694, Pax6, and Cbln1. The data used for these analyses are from [10]. Cbln1, Cerebellin-1; RNA-seq, RNA sequencing; t-SNE, t-Stochastic Neighbor Embedding.
(TIF)
(A) Electrophoresis gel images of the 5′ and 3′ RACE results of Cbln1. (B) Sequence of Cbln1. (C) Electrophoresis gel images of the 5′ and 3′ RACE results of lncRNA-PM. (D) Sequence of lncRNA-PM. All the data of this figure can be found in the S2 Data file. Cbln1, Cerebellin-1; lncRNA-PM, lncRNA-Promoting Methylation; RACE, rapid amplification of cDNA ends.
(TIF)
(A) Illustration of the Cbln1, Gm2694, and the tested 12 nearby genes. (B) Relative expression levels of the indicated 12 genes upon the indicated treatments in Neuro2a cells. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; lncRNA-PM, lncRNA-Promoting Methylation.
(TIF)
eGFP, enhanced green fluorescent protein.
(TIF)
ChIP-qPCR detection of Mll1, Mll2, Mll3, and Mll4 on the indicated upstream regulatory regions of Cbln1 in the control or PM-overexpressed Neuro2a cells. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. ***P < 0.001. Cbln1, Cerebellin-1; ChIP, chromatin immunoprecipitation; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance; qPCR, quantitative PCR.
(TIF)
The relative expression levels of Mll1 (A) and Pax6 (B) under the indicated treatments in Neuro2a cells. All results were normalized to Gapdh. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. ns, no significance.
(TIF)
(A) Representative images of the effect of lncRNA-PM on the protein levels of Pax6 and Mll1 in the whole cell lysates (Input), cytoplasm (Cyto), nucleoplasm (Nucl), and DNA-bound fractions. (B) Quantification of A. Gapdh and H2b are markers for cytoplasm and nucleus fractions, respectively. All the data of this figure can be found in the S1 and S2 Data files. Data are shown as means ± SEMs, n = 3. *P < 0.05 and **P < 0.01. lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance.
(TIF)
(A, B) Occupancies of H3K4me3 and H3K27ac (A) and Mll1 (B) on the 5′ regulatory region of Cbln1 detected by amplicon 3 (indicated in Fig 6C) in the control (Vector) or PM-overexpressed (PM) Neuro2a cells. Data are shown as means ± SEMs, n = 3. (C) Occupancies of Mll1 and Pax6 on the 5′ regulatory region of Cbln1 detected by amplicon 3 (indicated in Fig 6C) in the control or the indicated shPMs in Neuro2a cells. Data are shown as means ± SEMs, n = 3. (D) Occupancies of the Mll1 and H3K4me3 on the 5′ regulatory region of Cbln1 detected by amplicon 3 (indicated in Fig 6C) in PM-overexpressed Neuro2a cells, with the treatments of Mll1 (siMll1-1 and siMll1-2) or control siRNAs (siCtrl). All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; ChIP, chromatin immunoprecipitation; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance.
(TIF)
(A) Venn diagram of the positively regulated genes shared by PM and Pax6. (B) Venn diagram of the negatively regulated genes shared by PM and Pax6. lncRNA-PM, lncRNA-Promoting Methylation.
(TIF)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(PDF)
Acknowledgments
We thank Dr. Xiaohua Shen from Tsinghua University and Dr. Shouhong Guang from University of Science and Technology of China (USTC) for their constructive suggestions. We also thank Core Facility Center for Life Sciences, USTC for their technical support to this project.
Abbreviations
- Cbln1
Cerebellin-1
- ChIP
chromatin immunoprecipitation
- ChIRP
chromatin isolation by RNA purification
- CNS
central nervous system
- DEG
differential expressed gene
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- FISH
fluorescence in situ hybridization
- GC
granule cell
- GluD2
glutamate receptor delta2
- GO
Gene Ontology
- GSP
gene specific primer
- IP
intraperitoneal
- lncRNA
long noncoding RNA
- lncRNA-PM
lncRNA-Promoting Methylation
- MCS
multiple cloning site
- Nrx
neurexin
- PC
Purkinje cell
- PF
parallel fiber
- PFA
paraformaldehyde
- PSD
postsynaptic density
- RACE
rapid amplification of cDNA ends
- Re-ChIP
Re-chromatin immunoprecipitation
- RNA-seq
RNA sequencing
- RT-qPCR
reverse transcription-quantitative PCR
- UV-RIP
UV-RNA immunoprecipitation
- VGluT1
vesicular glutamate transporter 1
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by the National Key Research and Development Program of China (http://www.most.gov.cn; 2016YFC1000605 to XW and 2019YFA0802600 to GS) and National Natural Science Foundation of China (http://www.nsfc.gov.cn; 31671306 to XW), Anhui Mental Health Center (http://www.ahmhcentre.com; 2019LH01 to XW), and the Major/Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology (http://cpst.ustc.edu.cn; 2018CXFX006 to XW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Manto M, De Zeeuw CI. Diversity and complexity of roles of granule cells in the cerebellar cortex. Editorial. Cerebellum. 2012;11(1):1–4. Epub 2012/03/08. doi: 10.1007/s12311-012-0365-7 . [DOI] [PubMed] [Google Scholar]
- 2.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2(7):484–91. Epub 2001/07/04. doi: 10.1038/35081558 . [DOI] [PubMed] [Google Scholar]
- 3.Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, Gallo V. Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci. 2019;20(5):298–313. Epub 2019/03/30. doi: 10.1038/s41583-019-0152-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butts T, Green MJ, Wingate RJ. Development of the cerebellum: simple steps to make a ’little brain’. Development. 2014;141(21):4031–41. Epub 2014/10/23. doi: 10.1242/dev.106559 . [DOI] [PubMed] [Google Scholar]
- 5.Hibi M, Shimizu T. Development of the cerebellum and cerebellar neural circuits. Dev Neurobiol. 2012;72(3):282–301. Epub 2011/02/11. doi: 10.1002/dneu.20875 . [DOI] [PubMed] [Google Scholar]
- 6.Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(Pt 2):290–2. Epub 2006/01/26. doi: 10.1093/brain/awh729 . [DOI] [PubMed] [Google Scholar]
- 7.Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E, et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci. 2005;8(11):1534–41. Epub 2005/10/20. doi: 10.1038/nn1576 . [DOI] [PubMed] [Google Scholar]
- 8.Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328(5976):363–8. Epub 2010/04/17. doi: 10.1126/science.1185152 . [DOI] [PubMed] [Google Scholar]
- 9.Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, et al. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141(6):1068–79. Epub 2010/06/12. doi: 10.1016/j.cell.2010.04.035 . [DOI] [PubMed] [Google Scholar]
- 10.Elegheert J, Kakegawa W, Clay JE, Shanks NF, Behiels E, Matsuda K, et al. Structural basis for integration of GluD receptors within synaptic organizer complexes. Science. 2016;353(6296):295–9. Epub 2016/07/16. doi: 10.1126/science.aae0104 ; PubMed Central PMCID: PMC5291321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito-Ishida A, Miura E, Emi K, Matsuda K, Iijima T, Kondo T, et al. Cbln1 regulates rapid formation and maintenance of excitatory synapses in mature cerebellar Purkinje cells in vitro and in vivo. J Neurosci. 2008;28(23):5920–30. Epub 2008/06/06. doi: 10.1523/JNEUROSCI.1030-08.2008 ; PubMed Central PMCID: PMC6670322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89. Epub 2012/09/08. doi: 10.1101/gr.132159.111 ; PubMed Central PMCID: PMC3431493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of Long Non-coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution. Neuron. 2015;88(5):861–77. Epub 2015/12/08. doi: 10.1016/j.neuron.2015.09.045 . [DOI] [PubMed] [Google Scholar]
- 14.Aprea J, Calegari F. Long non-coding RNA s in corticogenesis: deciphering the non-coding code of the brain. EMBO J. 2015;34(23):2865–84. doi: 10.15252/embj.201592655 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry G. Integrating the roles of long and small non-coding RNA in brain function and disease. Mol Psychiatry. 2014;19(4):410–6. doi: 10.1038/mp.2013.196 . [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Wang Z, Chen D, Zhang B, Tian RR, Wu J, et al. Annotation and cluster analysis of spatiotemporal- and sex-related lncRNA expression in rhesus macaque brain. Genome Res. 2017;27(9):1608–20. Epub 2017/07/09. doi: 10.1101/gr.217463.116 ; PubMed Central PMCID: PMC5580719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Ren D, Liang X, Ke S, Zhang B, Hu B, et al. A long noncoding RNA cluster-based genomic locus maintains proper development and visual function. Nucleic Acids Res. 2019;47(12):6315–29. Epub 2019/05/28. doi: 10.1093/nar/gkz444 ; PubMed Central PMCID: PMC6614851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XQ, Wang ZL, Poon MW, Yang JH. Spatial-temporal transcriptional dynamics of long non-coding RNAs in human brain. Hum Mol Genet. 2017;26(16):3202–11. Epub 2017/06/03. doi: 10.1093/hmg/ddx203 . [DOI] [PubMed] [Google Scholar]
- 19.Goff LA, Groff AF, Sauvageau M, Trayes-Gibson Z, Sanchez-Gomez DB, Morse M, et al. Spatiotemporal expression and transcriptional perturbations by long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2015;112(22):6855–62. Epub 2015/06/03. doi: 10.1073/pnas.1411263112 ; PubMed Central PMCID: PMC4460505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo S, Lu JY, Liu L, Yin Y, Chen C, Han X, et al. Divergent lncRNAs Regulate Gene Expression and Lineage Differentiation in Pluripotent Cells. Cell Stem Cell. 2016;18(5):637–52. Epub 2016/03/22. doi: 10.1016/j.stem.2016.01.024 . [DOI] [PubMed] [Google Scholar]
- 21.Otsuka S, Konno K, Abe M, Motohashi J, Kohda K, Sakimura K, et al. Roles of Cbln1 in Non-Motor Functions of Mice. J Neurosci. 2016;36(46):11801–16. Epub 2016/11/18. doi: 10.1523/JNEUROSCI.0322-16.2016 ; PubMed Central PMCID: PMC6705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter RA, Bihannic L, Rosencrance C, Hadley JL, Tong Y, Phoenix TN, et al. A Single-Cell Transcriptional Atlas of the Developing Murine Cerebellum. Curr Biol. 2018;28(18):2910–20.e2. Epub 2018/09/18. doi: 10.1016/j.cub.2018.07.062 . [DOI] [PubMed] [Google Scholar]
- 23.Herz HM, Mohan M, Garruss AS, Liang K, Takahashi YH, Mickey K, et al. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;26(23):2604–20. Epub 2012/11/21. doi: 10.1101/gad.201327.112 ; PubMed Central PMCID: PMC3521626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Zhao Y, McGreal R, Cohen-Tayar Y, Rockowitz S, Wilczek C, et al. Pax6 associates with H3K4-specific histone methyltransferases Mll1, Mll2, and Set1a and regulates H3K4 methylation at promoters and enhancers. Epigenetics Chromatin. 2016;9(1):37. Epub 2016/09/13. doi: 10.1186/s13072-016-0087-z ; PubMed Central PMCID: PMC5018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan D, Fu Y, Paxinos G, Watson C. Spatiotemporal expression patterns of Pax6 in the brain of embryonic, newborn, and adult mice. Brain Struct Funct. 2013;218(2):353–72. Epub 2012/02/23. doi: 10.1007/s00429-012-0397-2 . [DOI] [PubMed] [Google Scholar]
- 26.Ha TJ, Swanson DJ, Kirova R, Yeung J, Choi K, Tong Y, et al. Genome-wide microarray comparison reveals downstream genes of Pax6 in the developing mouse cerebellum. Eur J Neurosci. 2012;36(7):2888–98. Epub 2012/07/24. doi: 10.1111/j.1460-9568.2012.08221.x ; PubMed Central PMCID: PMC4189819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJ, Ozkaynak E, et al. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature. 2017;543(7646):507–12. Epub 2017/03/16. doi: 10.1038/nature21678 ; PubMed Central PMCID: PMC5364052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Rockowitz S, Chauss D, Wang P, Kantorow M, Zheng D, et al. Chromatin features, RNA polymerase II and the comparative expression of lens genes encoding crystallins, transcription factors, and autophagy mediators. Mol Vis. 2015;21:955–73. Epub 2015/09/04. ; PubMed Central PMCID: PMC4551281. [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson DJ, Tong Y, Goldowitz D. Disruption of cerebellar granule cell development in the Pax6 mutant, Sey mouse. Brain Res Dev Brain Res. 2005;160(2):176–93. Epub 2005/11/18. doi: 10.1016/j.devbrainres.2005.09.005 . [DOI] [PubMed] [Google Scholar]
- 30.Umeda T, Takashima N, Nakagawa R, Maekawa M, Ikegami S, Yoshikawa T, et al. Evaluation of Pax6 mutant rat as a model for autism. PLoS ONE. 2010;5(12):e15500. Epub 2011/01/05. doi: 10.1371/journal.pone.0015500 ; PubMed Central PMCID: PMC3006426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser T, Ton CC, Mueller R, Petzl-Erler ML, Oliver C, Nevin NC, et al. Absence of PAX6 gene mutations in Gillespie syndrome (partial aniridia, cerebellar ataxia, and mental retardation). Genomics. 1994;19(1):145–8. Epub 1994/01/01. doi: 10.1006/geno.1994.1024 . [DOI] [PubMed] [Google Scholar]
- 32.Takada Y, Sakai Y, Matsushita Y, Ohkubo K, Koga Y, Akamine S, et al. Sustained endocrine profiles of a girl with WAGR syndrome. BMC Med Genet. 2017;18(1):117. Epub 2017/10/25. doi: 10.1186/s12881-017-0477-5 ; PubMed Central PMCID: PMC5654094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dollfus H, Joanny-Flinois O, Doco-Fenzy M, Veyre L, Joanny-Flinois L, Khoury M, et al. Gillespie syndrome phenotype with a t(X;11)(p22.32;p12) de novo translocation. Am J Ophthalmol. 1998;125(3):397–9. Epub 1998/03/25. doi: 10.1016/s0002-9394(99)80157-3 . [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto K, Kano M. Synapse elimination in the developing cerebellum. Cell Mol Life Sci. 2013;70(24):4667–80. Epub 2013/07/03. doi: 10.1007/s00018-013-1405-2 ; PubMed Central PMCID: PMC3830199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dusart I, Flamant F. Profound morphological and functional changes of rodent Purkinje cells between the first and the second postnatal weeks: a metamorphosis? Front Neuroanat. 2012;6:11. Epub 2012/04/20. doi: 10.3389/fnana.2012.00011 ; PubMed Central PMCID: PMC3324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raj B, Blencowe BJ. Alternative Splicing in the Mammalian Nervous System: Recent Insights into Mechanisms and Functional Roles. Neuron. 2015;87(1):14–27. Epub 2015/07/04. doi: 10.1016/j.neuron.2015.05.004 . [DOI] [PubMed] [Google Scholar]
- 37.Vuong CK, Black DL, Zheng S. The neurogenetics of alternative splicing. Nat Rev Neurosci. 2016;17(5):265–81. Epub 2016/04/21. doi: 10.1038/nrn.2016.27 ; PubMed Central PMCID: PMC4861142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, Wang J, Zheng Y, Zhang J, Chen S, Zhao F. Comprehensive identification of internal structure and alternative splicing events in circular RNAs. Nat Commun. 2016;7:12060. Epub 2016/06/29. doi: 10.1038/ncomms12060 ; PubMed Central PMCID: PMC4931246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Xue Z, Yan J, Wang J, Liu Q, Jiang H. LncRNA Riken-201 and Riken-203 modulates neural development by regulating the Sox6 through sequestering miRNAs. Cell Prolif. 2019. doi: 10.1111/cpr.12573 ; PubMed Central PMCID: PMC6536386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai J, Aoto J, Sudhof TC. Alternative Splicing of Presynaptic Neurexins Differentially Controls Postsynaptic NMDA and AMPA Receptor Responses. Neuron. 2019;102(5):993–1008 e5. Epub 2019/04/22. doi: 10.1016/j.neuron.2019.03.032 ; PubMed Central PMCID: PMC6554035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fagnani M, Barash Y, Ip JY, Misquitta C, Pan Q, Saltzman AL, et al. Functional coordination of alternative splicing in the mammalian central nervous system. Genome Biol. 2007;8(6):R108. Epub 2007/06/15. doi: 10.1186/gb-2007-8-6-r108 ; PubMed Central PMCID: PMC2394768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li YP, Duan FF, Zhao YT, Gu KL, Liao LQ, Su HB, et al. A TRIM71 binding long noncoding RNA Trincr1 represses FGF/ERK signaling in embryonic stem cells. Nat Commun. 2019;10(1):1368. Epub 2019/03/27. doi: 10.1038/s41467-019-08911-w ; PubMed Central PMCID: PMC6433952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos AD, Andersen RE, Liu SJ, Nowakowski TJ, Hong SJ, Gertz C, et al. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16(4):439–47. Epub 2015/03/25. doi: 10.1016/j.stem.2015.02.007 ; PubMed Central PMCID: PMC4388801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29. doi: 10.1038/emboj.2010.199 ; PubMed Central PMCID: PMC2944070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin QF, Hu SB, Xu YF, Yang L, Carmichael GG, Chen LL. SnoVectors for nuclear expression of RNA. Nucleic Acids Res. 2015;43(1):e5. Epub 2014/11/08. doi: 10.1093/nar/gku1050 ; PubMed Central PMCID: PMC4288147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi X, Wang X. LINC00473 mediates cyclin D1 expression through a balance between activation and repression signals in breast cancer cells. FEBS Lett. 2019;593(7):751–9. Epub 2019/03/09. doi: 10.1002/1873-3468.13353 . [DOI] [PubMed] [Google Scholar]
- 47.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44(4):667–78. Epub 2011/10/04. doi: 10.1016/j.molcel.2011.08.027 ; PubMed Central PMCID: PMC3249421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) t-SNE plot displaying the distribution of the main cerebellar cell types. (B) The t-SNE distributions of Gm2694, Pax6, and Cbln1. The data used for these analyses are from [10]. Cbln1, Cerebellin-1; RNA-seq, RNA sequencing; t-SNE, t-Stochastic Neighbor Embedding.
(TIF)
(A) Electrophoresis gel images of the 5′ and 3′ RACE results of Cbln1. (B) Sequence of Cbln1. (C) Electrophoresis gel images of the 5′ and 3′ RACE results of lncRNA-PM. (D) Sequence of lncRNA-PM. All the data of this figure can be found in the S2 Data file. Cbln1, Cerebellin-1; lncRNA-PM, lncRNA-Promoting Methylation; RACE, rapid amplification of cDNA ends.
(TIF)
(A) Illustration of the Cbln1, Gm2694, and the tested 12 nearby genes. (B) Relative expression levels of the indicated 12 genes upon the indicated treatments in Neuro2a cells. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; lncRNA-PM, lncRNA-Promoting Methylation.
(TIF)
eGFP, enhanced green fluorescent protein.
(TIF)
ChIP-qPCR detection of Mll1, Mll2, Mll3, and Mll4 on the indicated upstream regulatory regions of Cbln1 in the control or PM-overexpressed Neuro2a cells. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. ***P < 0.001. Cbln1, Cerebellin-1; ChIP, chromatin immunoprecipitation; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance; qPCR, quantitative PCR.
(TIF)
The relative expression levels of Mll1 (A) and Pax6 (B) under the indicated treatments in Neuro2a cells. All results were normalized to Gapdh. All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. ns, no significance.
(TIF)
(A) Representative images of the effect of lncRNA-PM on the protein levels of Pax6 and Mll1 in the whole cell lysates (Input), cytoplasm (Cyto), nucleoplasm (Nucl), and DNA-bound fractions. (B) Quantification of A. Gapdh and H2b are markers for cytoplasm and nucleus fractions, respectively. All the data of this figure can be found in the S1 and S2 Data files. Data are shown as means ± SEMs, n = 3. *P < 0.05 and **P < 0.01. lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance.
(TIF)
(A, B) Occupancies of H3K4me3 and H3K27ac (A) and Mll1 (B) on the 5′ regulatory region of Cbln1 detected by amplicon 3 (indicated in Fig 6C) in the control (Vector) or PM-overexpressed (PM) Neuro2a cells. Data are shown as means ± SEMs, n = 3. (C) Occupancies of Mll1 and Pax6 on the 5′ regulatory region of Cbln1 detected by amplicon 3 (indicated in Fig 6C) in the control or the indicated shPMs in Neuro2a cells. Data are shown as means ± SEMs, n = 3. (D) Occupancies of the Mll1 and H3K4me3 on the 5′ regulatory region of Cbln1 detected by amplicon 3 (indicated in Fig 6C) in PM-overexpressed Neuro2a cells, with the treatments of Mll1 (siMll1-1 and siMll1-2) or control siRNAs (siCtrl). All the data of this figure can be found in the S1 Data file. Data are shown as means ± SEMs, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001. Cbln1, Cerebellin-1; ChIP, chromatin immunoprecipitation; IgG, immunoglobulin G; lncRNA-PM, lncRNA-Promoting Methylation; ns, no significance.
(TIF)
(A) Venn diagram of the positively regulated genes shared by PM and Pax6. (B) Venn diagram of the negatively regulated genes shared by PM and Pax6. lncRNA-PM, lncRNA-Promoting Methylation.
(TIF)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.