Abstract
Background: Neurofibromatosis type 1 (NF1) has no current effective treatments beyond surgery. Topical photodynamic therapy (PDT) has the potential to provide a less invasive treatment modality.
Objective: Based on murine data, we hypothesized PDT could be used for the treatment of cutaneous neurofibromas (cNF).
Methods and results: We conducted a phase I trial to examine absorption and conversion of topical aminolevulinic acid (ALA) in cNF and determine safety in a dose escalation study. ALA or control vehicle was applied to neurofibromas through microneedle-assisted delivery (n = 4) and excised specimens were examined 24 h later for protoporphyrin IX fluorescence. Fluorescence was detected in the tumors at 304 ± 94 U/μm2, while adjacent paralesional normal skin and vehicle-treated tumors showed no fluorescence (p < 0.0001). Subsequently, neurofibromas (n = 27) were treated with ALA and irradiated with 633 nm red light 18 h later, at escalating dosages of 50 and 100 mJ/cm2. Maximum tolerable dose was established at 100 mJ/cm2. Light microscopy study of tumors biopsied 48 h after PDT (ALA n = 14 and vehicle n = 4) showed mixed inflammatory infiltrate in the ALA, but not in the vehicle-treated tumors or perilesional normal skin. TUNEL evaluation showed 42.5 ± 19.9 apoptotic cells per visual field for ALA-treated and 1.1 ± 1.4 for vehicle-treated tumors (p = 0.002).
Conclusions: In the first reported clinical trial of PDT for NF1, PDT targeted neurofibromas specifically, and may offer a normal tissue-sparing treatment modality in the future.
This study is registered at Clintrials.gov (NCT01682811).
Keywords: photodynamic therapy (PDT), neurofibromatosis, NF1, ALA, aminolevulinic acid
Introduction
Background and significance
Neurofibromatosis type 1 (NF1) is among the most common neurological autosomal dominant diseases worldwide.1,2 Although there is great variability in clinical findings, cutaneous features represent localized overgrowth of a neural crest-derived tissue, in the form of benign cutaneous and plexiform neurofibromas, the most prevalent NF1 tumors. cNF involve terminal nerves, are rich in extracellular matrix, and are sparsely populated by Schwann cells, mesenchymal cells, and mast cells.
Despite their benign nature, these neurofibromas cause immeasurable stress to the parents of an affected child, the pre-adolescent affected child who is at risk for developing body dysmorphic disorder, and the adult who may face social stigmatization due to the disfiguring lesions and has to endure physical discomfort.3 These small tumors usually begin to develop during adolescence and adulthood, but rarely can also develop in early childhood.4
Study rationale
Most NF1 tumors are slow growing and refractory to conventional anticancer therapies aimed at rapidly proliferating cells. No medical therapies have been shown to benefit cutaneous lesions in patients with NF1. Several drug trials have been initiated, looking for medications that slow or halt the growth of neurofibromas.5 Current management for cutaneous neurofibromas (cNF) is entirely procedural.6 There is a desperate need for alternative therapies to eradicate progressive and infiltrative cNF tumors. Photodynamic therapy (PDT) is an established cancer therapy that involves the selective uptake of a photosensitizer (PS) by tumor cells followed by irradiation of the tumor with light to activate the PS, resulting in selective tumor destruction. Therefore, PDT may provide a cellular scalpel that selectively targets cNF tumors without sacrificing surrounding normal tissue. However, PDT has not yet been tested clinically as a therapy for NF1. PDT has an established record in dermatological applications,7 and has been approved for use in actinic keratosis, basal cell carcinoma, and Bowen's disease.8
Innovation
The Muir group (University of Florida) established cell culture and animal models of NF1 that have been characterized and utilized extensively to examine the cellular and molecular biology of NF1 tumorigenicity.9–11 In collaboration with our group at the Medical College of Wisconsin, inceptive in vitro and in vivo studies were conducted to determine the sensitivity of human NF1 tumor cells to PDT. Results showed that in vitro PDT resulted in complete ablation of various NF1 tumor cell cultures using Photofrin, talaporfin sodium, or aminolevulinic acid (ALA) PS. Dosimetry indicated that normal Schwann cells were less susceptible to PDT, requiring significantly greater doses of PS to achieve cell death. PDT using systemic PS applied to subcutaneous NF1 tumor xenografts in mice resulted in extensive tumor deterioration marked by cell lysis, nuclear condensation, rare mitotic figures, and prevalent vascular disruption and hemorrhage. TUNEL-positive cells were pervasive throughout the tumors, indicating extensive PDT-induced cell death.12
ALA is a prodrug that needs conversion to become a PS.13 ALA is a mitochondrial metabolite that is the first step in heme synthesis. Photosensitization following application of ALA occurs through the metabolic conversion of ALA to protoporphyrin IX (PpIX), which subsequently accumulates in metabolically active cells. When exposed to appropriate light energy, the accumulated PpIX produces a cytotoxic photodynamic reaction.14 ALA can be activated with blue light in the 410 nm range for superficial lesions, or longer wavelength red light (630 nm range) for deeper penetration.
ALA absorption and conversion in the tumor to PpIX are critical to the effectiveness of PDT. Therefore, we first studied whether topically applied ALA was specifically converted to PpIX in cNF. Subsequently, a phase I light-dose escalation study was conducted to determine safety and adverse effects of PDT treatment of cNF.
Materials and Methods
A prospective, controlled, nonrandomized phase I clinical trial was conducted between March 2012 and July 2016. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by the Institutional Review Board of The Medical College of Wisconsin, Milwaukee, WI. All study subjects gave their written, informed consent.
Part 1 of this study examined the absorption and conversion of ALA in cNF. Our primary hypothesis was that ALA accumulates and converts to PpIX in tumor tissue specifically compared to surrounding normal tissue. A secondary hypothesis was that tumors incubated with ALA would show greater fluorescence than untreated tumors and tumors incubated with vehicle only. In Part 2 of the study, we used dose escalation of the red light incubation to study safety and efficacy of the treatment.
Inclusion criteria included adult patients 17 years of age or older with an NF1 diagnosis as classified by the National Institutes of Health (NIH) Consensus Development Conference,15 presence of cNF scheduled to undergo tumor excision as part of standard care (for Part 1), localization on trunk or limbs, growth confirmation, and absence of any other malignancy. Exclusion criteria included life expectancy less than 1 year, pregnancy, inability to consent, photosensitivity to wavelengths used to activate PS, diagnosis of porphyria, allergy to ALA or any of the topical solution vehicle components, previous chemotherapy within 6 weeks of proposed PDT, and other concurrent tumor therapy. This study included adult patients from Froedtert Hospital in Milwaukee, Wisconsin. NF1 patients were recruited through referral by their physician. Statistical analysis was performed by Student's t test. The study drug is not labeled for the use under discussion.
Part 1—PS uptake
Subject population and treatment
Four adult subjects (two males and two females) participated in Part 1. Age ranged from 39 to 67 years. Three tumor groups for each subject consisted of untreated negative controls, tumors treated with drug vehicle, and tumors treated with ALA (Levulan Kerastick Topical Solution, Sun Pharma, Wilmington, MA). Treated tumors were incubated with ALA or vehicle for 3 h (two subjects) or overnight (two subjects), and then excised and subjected to fluorescence microscopy studies to measure mean fluorescence, indicating PpIX conversion in tumors. Importantly, ALA delivery for the subjects undergoing overnight incubation was assisted using microneedling (Dermapen, Salt Lake City, Utah) of the tumors. The tumors were degreased with alcohol, painted with ALA or vehicle, and microneedled using 1.5–2 mm depth setting until pinpoint bleeding was observed. During and immediately after microneedling, ALA or vehicle was reapplied. ALA delivery through microneedling is not a U.S. Food and Drug Administration (FDA)-approved procedure, and therefore constitutes an off-label use.
Outcome measures
The primary outcome measure was the ratio of mean fluorescence intensity in tumors compared to adjacent normal tissue, as determined by confocal microscopy.16 PpIX signals were detected with excitation at 405 nm and emission with a 600 nm long pass filter.
Part 2—adult clinical trial
Subject population and treatment
Nine adult subjects participated in Part 2, four males and five females. Age ranged from 39 to 69 years, with a median of 58. For each subject, neurofibroma lesions were selected for treatment with ALA (3–6 lesions) and vehicle (3–6 lesions) delivered with microneedling. Following an overnight (16–18 h) incubation time, all treated neurofibromas were illuminated with red light at 633 nm and 105 mW/cm2 (Omnilux Revive LED illuminator, Photo Therapeutics, Montgomeryville, PA). The dose escalation study included doses of 50, 100, or 200 J/cm2. Each subject received one single treatment.
Dose escalation and follow-up
This study follows a standard 3 + 3 phase I trial design.17 Briefly, subjects in such a study are treated starting at dose level 1 (in this study, 50 J/cm2.) Dose escalation is performed only after a minimum of three subjects have been evaluated at any current dose level. If dose-limiting toxicity (DLT) occurs in one of the three initial subjects at any dose level, three more subjects should be evaluated at that dose level. If zero of these additional three subjects at this level experience DLT, then the dose will be escalated (only one DLT in six subjects). If DLT occurs in one of the additional three subjects, the mean tolerable dose (MTD) had been exceeded (two DLTs in six subjects) and three more subjects had to be treated at the next lower dose. If DLT was noted in two of the initial three subjects at a given dose level, the MTD had been exceeded and three more subjects had to be treated at the next lower dose level. The MTD is defined as that dose level immediately below the dose level at which two subjects of a cohort (three or six subjects) experience DLT. DLT for this study was defined as pain during irradiation requiring cessation of the light treatment, or any serious cutaneous adverse events, such as erosions or blistering or crusting of the skin.
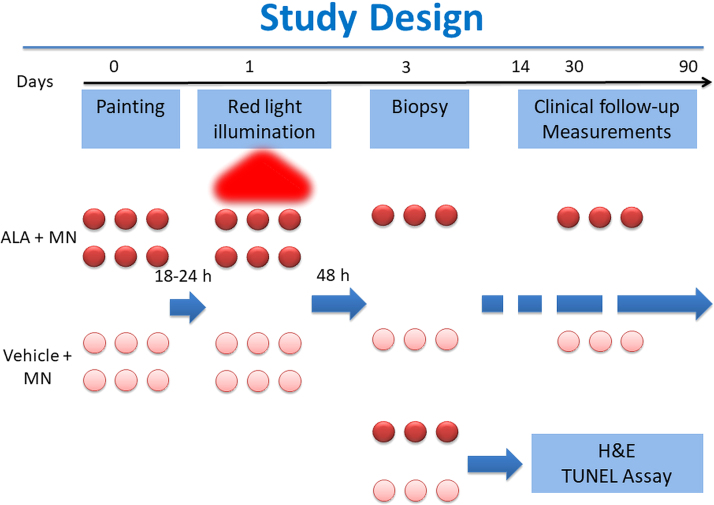
Subjects were seen 48 h (day 2) and 2–4 weeks (days 14–28) after light treatment, for evaluation of cutaneous adverse events. On day 2, tumors were biopsied using a punch technique and subjected to light microscopy examination, TUNEL assay (DeadEnd Colorimetric TUNEL System, Promega, Madison, WI), and DAPI (4′,6-diamidino-2-phenylindole) staining (ab228549; Abcam, Cambridge, MA). Additional follow-up visits for measurement of lesions were made at periods of 3 months up to 1 year. See Fig. 1 for a schematic of the study design.
FIG. 1.
Part 2 clinical study design. MN, microneedling.
Outcome measures
The primary outcome was MTD, as measured in the dose escalation using the DLT as defined above. A secondary outcome was measured efficacy defined by change in lesion size. The area of the lesion was estimated at baseline and during follow-up visits by measuring the long and short axis of the lesion. Average growth rates were calculated and comparisons made between ALA-treated and placebo lesions within the same subject. The null hypothesis was that there would be no difference in the growth rates of ALA-treated lesions when paired with same-subject placebo lesions. The alternative hypothesis was there would be a difference in the ALA and placebo lesion growth rates.
Results
Part 1—PS uptake and conversion
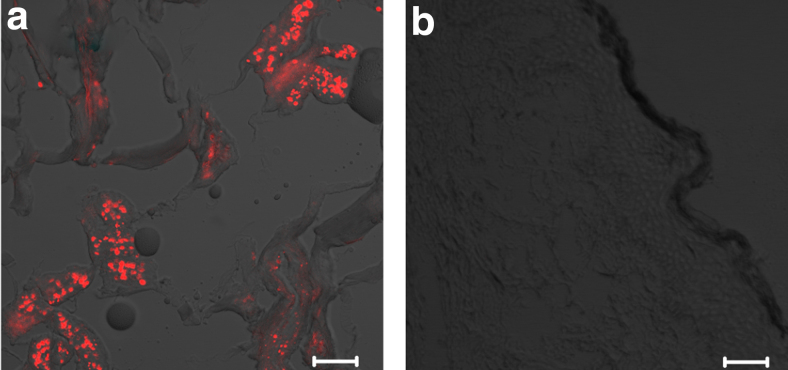
Excised NF tumor specimens from two subjects whose lesions were treated with topical ALA and incubated for 3 h showed no fluorescence in the apparent tumor zones. There was a faint stain in the dermal regions that bleached quickly (not shown). We concluded that ALA applied topically was not absorbed in adequate quantity or converted to PpIX within tumors (by endogenous enzymes), and that modifications were necessary. Interestingly, microneedle-assisted ALA delivery to NF tumors and overnight incubation resulted in detection of significant fluorescence within the tumor cells compared to the adjacent normal tissue, indicating increased uptake of the drug and specific conversion of ALA to PpIX in the tumor, but still not in the normal tissue (Fig. 2a). The average fluorescence value for PpIX-positive tumor areas was 304 ± 94 densitometry U/μm2 (n = 10). By using a one-sample t-test comparing the absolute value to an alternate expected value of zero, the results are significant with p < 0.0001. Control and vehicle-only tumors showed no visible fluorescence (Fig. 2b).
FIG. 2.
Confocal microscope fluorescence imaging. Scale bar = 50 μm. (a) Tumor tissue treated with microneedling and ALA. (b) Tumor tissue treated with microneedling and vehicle only. ALA, aminolevulinic acid.
Part 2—Phase I adult clinical trial
Clinical evaluation
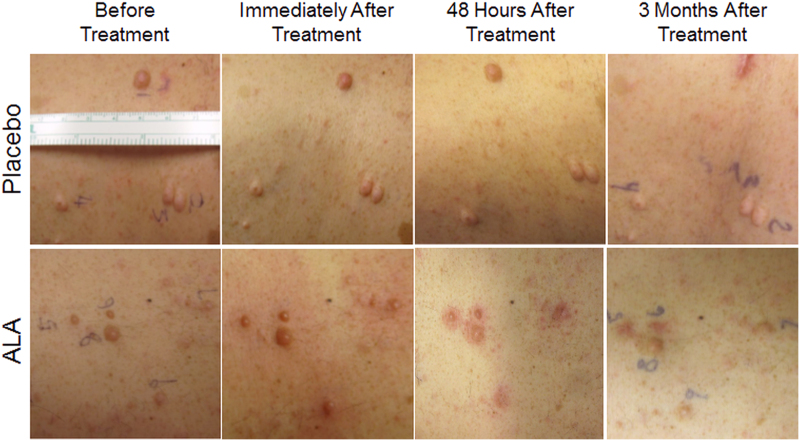
Red light at a radiant flux of 105 mW/cm2 and a dose of 50 or 100 J/cm2 resulted in marked erythema and edema around ALA-treated (n = 24), but not placebo (n = 20) lesions, which lasted for at least 48 h (Fig. 3). There was no blister formation, erosion, or crusting observed.
FIG. 3.
NF1 lesions before and after 633 nm red light treatment. Top row placebo (vehicle only) lesions; bottom row ALA-treated lesions. NF1, neurofibromatosis type 1.
Dose escalation
One subject in the initial cohort (50 J/cm2) experienced pain requiring cessation of the light treatment. Due to this DLT, three more subjects were treated at this dose level, successfully, as well as the next dose level of 100 J/cm2. No other serious cutaneous adverse event was experienced. However, red light treatment induced marked pain and discomfort in all ALA-treated lesions. While subjects could generally tolerate this level of pain, the increasing time of treatment became difficult to bear. Due to this marked pain, it was decided that the MTD would be set at 100 J/cm2, and the highest dose level foregone, even though only one subject experienced DLT. In the opinion of the clinical dermatologist, higher dose levels would likely not be tolerable, and it would be unethical to escalate the light dose further.
Light microscopy
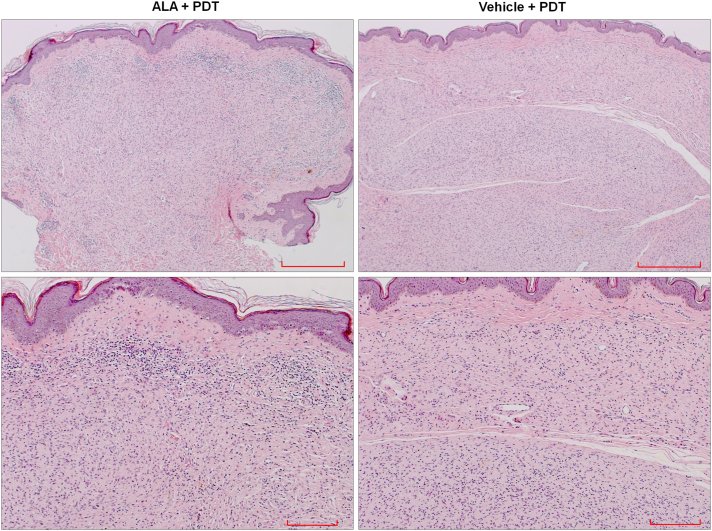
Light microscopy examination using standard hematoxylin and eosin staining showed a mixed infiltrate consisting of lymphocytes, neutrophils, and rare eosinophils in ALA-treated, but not vehicle-treated tumors or normal adjacent tissue, indicating an inflammatory response in ALA-PDT-treated neurofibromas (Fig. 4).
FIG. 4.
Light microscopic examination of NF1 lesion tissue samples using H&E (hematoxylin and eosin) staining. Top row scale bar = 500 μm, bottom row scale bar = 200 μm.
TUNEL assay
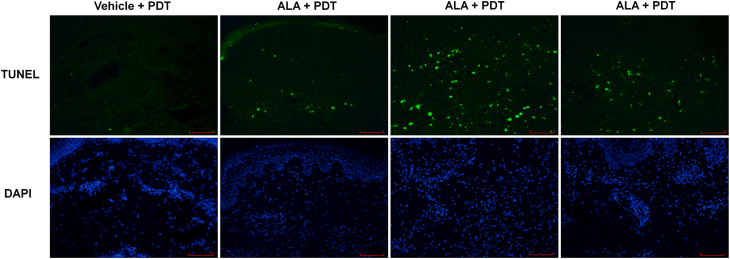
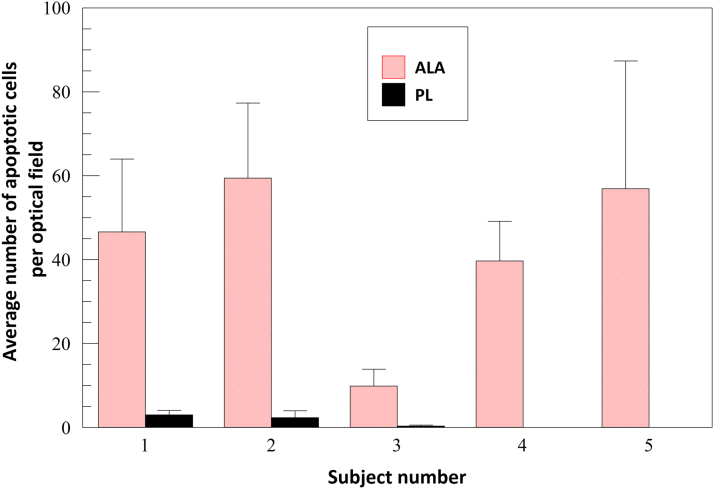
Representative neurofibromas (three ALA treated and one placebo treated) from five different subjects were evaluated for apoptotic cell death by TUNEL assay. Figure 5, from one typical subject, shows more apoptotic cells in the ALA-treated neurofibroma samples compared to the vehicle-treated samples. Figure 6 shows individual results for all evaluated tumors for each of five subjects. The average number of apoptotic cells per visual field among all TUNEL-evaluated tumor samples was 42.5 ± 19.9 for ALA-treated tumors and 1.1 ± 1.4 for vehicle-treated tumors (p = 0.002).
FIG. 5.
Fluorescent microscope imaging of NF1 lesion tissue samples 100X (vertical sections). Top row TUNEL staining; bottom row DAPI staining. Scale bar = 100 μm. DAPI, 4′,6-diamidino-2-phenylindole.
FIG. 6.
Quantification of apoptotic cells as determined by TUNEL assay. Individual results; ALA- and placebo-treated lesions. Error bars mean + standard deviation.
Tumor size
Treated tumors in five subjects were followed for measurement of size and clinical changes. Tumor sizes as measured at 2, 4, and 12 weeks after treatment did not show a significant change. A nonsignificant trend for reduction in average lesion growth rates was observed in ALA-treated tumors (−0.13 ± 0.22 mm2/day post-treatment) compared to the vehicle-treated tumors within the same subjects.
Discussion
Part 1—PS uptake and conversion
A fundamental question arising when considering the clinical use of PDT for treatment of cNF tumors is the issue of PS penetration. To be effective, ALA must penetrate through the epidermis into the tumor located in the dermis. Penetration of ALA using the Levulan Kerastick delivery stick applied to the surface of the skin in a full-thickness porcine model showed PpIX fluorescence within all layers of the epidermis, but not in the dermis.16 Normal dermal cells do not appear capable of converting the ALA into PpIX, as penetration of the ALA into the dermis does occur.18 Pre-cancerous cells and tumor cells convert ALA to PpIX. In addition, basal cell carcinomas coated with topical ALA were shown, after excision, to have detectable fluorescence in tumor cells even in the dermis.19 A lack of selectivity, however, in conversion of ALA in basal cell carcinoma tumors has been reported, with detection of fluorescence in tumors attributed to disruption of the stratum corneum by the tumor.20 In hamster models, ALA-treated amelanotic melanoma showed fluorescence intensities significantly exceeding surrounding tissues.21 Nevertheless, cNF are located in the dermis underneath a normal epidermis; hence. transepidermal delivery using microneedling is beneficial to assist ALA penetration onto tumors. Once ALA absorption in the neurofibroma is achieved, human NF1 cells, of various tumor origins, convert ALA to PpIX, and undergo PDT-mediated cell death.12
In our study, we found that tumor-specific fluorescence, resulting from ALA-PpIX conversion by the tumors, was seen in excised NF1 lesions, but not in the surrounding normal tissue. Although this does not necessarily indicate that normal dermal cells do not convert ALA to PpIX, it does show a preferential conversion by tumor areas sufficient for PDT purposes. Enhancement of topical drug delivery has recently gained attention, including concepts such as microneedling,22 skin pre-heating,23 and a wide range of other physical methods.24 The results seen in Part 1 of our study were deemed sufficient to move on to the Part 2 clinical trial phase.
Part 2—Phase I adult clinical trial
Clinical evaluation
ALA-PDT-treated NF1 lesions showed significant clinical responses compared to the vehicle-treated lesions, which demonstrated that the response was not due to the microneedling procedure or the red light treatment alone. Edema and redness subsided by day 2 after PDT, and post-inflammatory hyperpigmentation lasted up to 3 months. These effects have been reported elsewhere and are not unexpected.24
Dose escalation
An MTD of 100 J/cm2 was established in this study for PDT of cNF. The definition of DLT, pain requiring cessation of treatment, is admittedly subjective, and dependent on subject response. It is possible that a higher or lower MTD could have been established under different conditions. Nevertheless, this MTD is consistent with light treatment doses used in other dermatological ALA-PDT procedures. For actinic keratosis, dosages range from 2 to 10 J/cm2 using blue (417 nm)25,26 and 36–150 J/cm2 for red (630–635 nm) light.25,27 In basal cell carcinoma and related diseases, dosages range from 10 J/cm2 with blue light28 to 120–150 J/cm2 with red.25,29 Squamous cell carcinoma was treated at 10 J/cm2 with blue,30 and acne vulgaris at 70 J/cm2 with red light21 and 150 J/cm2 using wide-band light (550–700 nm).25 It should be noted that red light requires significantly higher doses to activate ALA-derived PpIX than blue light, a fact easily understood by considering that the Soret peak of PpIX is ∼20 times higher than the red absorption peak.31 While red light does require higher fluence (and, generally, irradiance), it has the advantage of deeper penetration (due to longer wavelengths) and is considered to be a better modality for deeper tumors.14
ALA-treated tumors
Light microscopy examination demonstrated a mixed inflammatory infiltrate and apoptotic cells, and TUNEL assays confirmed significant apoptotic cell death in ALA-treated, but not vehicle-treated NF1 lesions, while adjacent skin and vehicle-treated tumors remained normal. These results clearly demonstrate the ALA-PDT has NF1 tumor-specific effects.
Tumor size
While tumor size measurements showed a trend of tumor reduction in ALA-PDT-treated lesions, these preliminary results do not show any significant change in lesion growth rates. We do not have long-term histology on the ALA-treated tumors, so cannot say whether they consist of residual fibrous tissue or regrowth of tumor cells. Mature NF1 lesions consist mostly of metabolically inactive fibrous tissue that most likely is not responsive to PDT treatment; thus, significant tumor size reduction may not be possible, even in the event of complete death of active tumor cells. Alternatively, a better approach would entail using PDT to slow or arrest the growth of early tumor tissue, particularly in new lesions in young NF1 subjects.
Future direction
A phase II clinical effectiveness trial has begun (NCT02728388), with the primary end-point of time to progression defined as a 50% increase in lesion size. Lesion area will be measured using FotoFinder digital photography, and lesion volume using VevoMD high-frequency ultrasound. Natural history data relating to lesion size and growth rates will be collected.
Conclusions
To our knowledge, we are the first to have demonstrated that ALA topical solution, assisted by microneedle delivery, is specifically absorbed and converted to PpIX by cNF cells in vivo. We have also established, in the first reported phase I clinical trial for PDT of NF1, an MTD of 100 J/cm2 using 633 nm red light.
We report a uniquely selective treatment for cNF. Our phase I study results clearly demonstrate the ALA-PDT has NF1 tumor-specific effects. This is unique in treating neoplasia, because of the tremendous apoptosis produced in tumor cells, while completely sparing normal dermis.
These results have formed the basis for our ongoing phase II clinical trial, and show the potential to prevent the growth of neurofibromas early, rather than trying to eliminate cNF after neurofibroma tumor cells are replaced by fibrous tissue.
Acknowledgments
We would like to thank Scott Sandy, Mallory Smith, and Anna Houlihan for assistance with initial draft preparation, and Ashley Shock and Nathan Duncan for histology services.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Bleser Endowed Chair in Neurology, the Baumann Research Endowment, SUN Pharma, Children's Tumor Foundation, and Ben's Fund (all support to H.W.). Levulan Kerastick and placebo were provided by SUN Pharma.
References
- 1. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol 2009;61:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams VC, Lucas J, Babcock MA, Gutman D, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics 2009;123:124–133 [DOI] [PubMed] [Google Scholar]
- 3. Dunning-Davies BM, Parker APJ. Annual review of children with neurofibromatosis type 1. Arch Dis Child Educ Pract Ed 2016;102:102–111 [DOI] [PubMed] [Google Scholar]
- 4. Duong TA, Bastuji-Garin S, Valeyrie-Allanore L, Sbidian E, Ferkal S, Wolkenstein P. Evolving pattern with age of cutaneous signs in neurofibromatosis type 1: a cross-sectional study of 728 patients. Dermatology 2011;222:269–273 [DOI] [PubMed] [Google Scholar]
- 5. Verma SK, Riccardi VM, Plotkin SR, et al. Considerations for development of therapies for cutaneous neurofibroma. Neurology 2018;91(Suppl 1):S21–S30 [DOI] [PubMed] [Google Scholar]
- 6. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am 2019;103:1035–1054 [DOI] [PubMed] [Google Scholar]
- 7. Ceburkov O, Gollnick H. Photodynamic therapy in dermatology. Eur J Dermatol 2000;10:568–576 [PubMed] [Google Scholar]
- 8. Babilas P, Schreml S, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed 2010;26:118–132 [DOI] [PubMed] [Google Scholar]
- 9. Muir D, Neubauer D, Lim IT, Yachnis AT, Wallace MR. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am J Pathol 2001;158:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perrin GQ, Fishbein L, Li H, et al. An orthotopic xenograft model of intraneural NF1 MPNST suggests a potential association between steroid hormones and tumor cell proliferation. Lab Invest 2007;87:1092–1102 [DOI] [PubMed] [Google Scholar]
- 11. Wu M, Wallace MR, Muir D. Tumorigenic properties of neurofibromin-deficient Schwann cells in culture and as syngrafts in Nf1 knockout mice. J Neurosci Res 2005;82:357–367 [DOI] [PubMed] [Google Scholar]
- 12. Muir D, Perrin G, Neubauer D, Whelan H.. Photodynamic therapy development using NF1 tumor xenografts. Presented at Children's Tumor Foundation Conference, Bonita Springs, Florida, June 2008: p. 58 [Google Scholar]
- 13. Wachowska M, Muchowicz A, Firczuk M, et al. Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer. Molecules 2011;16:4140–4164 [Google Scholar]
- 14. Quirk BJ, Brandal G, Donlon S, et al. Photodynamic therapy (PDT) for malignant brain tumors-where do we stand? Photodiagnosis Photodyn Ther 2015;12:530–544 [DOI] [PubMed] [Google Scholar]
- 15. Stumpf DA, Alksne JF, Annegers JF, et al. Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol 1988;45:575–578 [PubMed] [Google Scholar]
- 16. Maisch T, Worlicek C, Babilas P, Landthaler M, Szeimies R. A HCl/alcohol formulation increased 5-aminolevulinic acid skin distribution using an ex vivo full thickness porcine skin model. Exp Dermatol 2008;17:813–820 [DOI] [PubMed] [Google Scholar]
- 17. Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009;101:708–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen JL, Berlin AL. Challenge: treatment of diffuse actinic keratoses on the scalp. Dermatologist 2008;16:1–2 [Google Scholar]
- 19. Szeimies RM, Sassy T, Landthaler M. Penetration potency of topical applied delta-aminolevulinic acid for photodynamic therapy of basal cell carcinoma. Photochem Photobiol 1994;59:73–76 [DOI] [PubMed] [Google Scholar]
- 20. Martin A, Tope WD, Grevelink JM, et al. Lack of selectivity of protoporphyrin IX fluorescence for basal cell carcinoma after topical application of 5-aminolevulinic acid: implications for photodynamic treatment. Arch Dermatol Res 1995;287:665–674 [DOI] [PubMed] [Google Scholar]
- 21. Pahernik S, Langer S, Botzlar A, Dellian M, Goetz AE. Tissue distribution and penetration of 5-ALA induced fluorescence in an amelanotic melanoma after topical application. Anticancer Res 2001;21:59–63 [PubMed] [Google Scholar]
- 22. Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma. Plast Surg Int 2011;2011:15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barolet D, Boucher A. Radiant near infrared light emitting Diode exposure as skin preparation to enhance photodynamic therapy inflammatory type acne treatment outcome. Lasers Surg Med 2010;42:171–178 [DOI] [PubMed] [Google Scholar]
- 24. Champeau M, Vignoud S, Mortier L, Mordon S. Photodynamic therapy for skin cancer: how to enhance drug penetration? J Photochem Photobiol B 2019;197:111544. [DOI] [PubMed] [Google Scholar]
- 25. Gupta AK, Ryder JE. Photodynamic therapy and topical aminolevulinic acid: an overview. Am J Clin Dermatol 2003;4:699–708 [DOI] [PubMed] [Google Scholar]
- 26. Ormrod D, Jarvis B. Topical aminolevulinic acid HCl photodynamic therapy. Am J Clin Dermatol 2000;1:133–139 [DOI] [PubMed] [Google Scholar]
- 27. de Oliveira ER, Inada NM, Blanco KC, Bagnato VS, Salvio AG. Cancerization field treatment using topical photodynamic therapy: a comparison between two aminolevulinate derivatives. Photodiagnosis Photodyn Ther 2020;30:101603. [DOI] [PubMed] [Google Scholar]
- 28. Gilchrest BA, Brightman LA, Thiele JJ, Wasserman DI. Photodynamic therapy for patients with Basal cell nevus syndrome. Dermatol Surg 2009;35:1576–1581 [DOI] [PubMed] [Google Scholar]
- 29. Oseroff AR, Shieh S, Frawley NP, et al. Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy. Arch Dermatol 2005;141:60–67 [DOI] [PubMed] [Google Scholar]
- 30. Willey A1, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg 2010;36:652–658 [DOI] [PubMed] [Google Scholar]
- 31. Rollakanti KR, Kanick SC, Davis SC, Pogue BW, Maytin EV. Techniques for fluorescence detection of protoporphyrin IX in skin cancers associated with photodynamic therapy. Photonics Lasers Med 2013;2:287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]