Abstract
Water activity is an important characteristic for describing unusual waters and is a determinant of habitability for microorganisms. However, few empirical studies of water activity have been done for natural waters exhibiting an extreme chemistry. Here, we investigate water activity for acid brines from Western Australia and Chile with pH as low as 1.4, salinities as high as 32% total dissolved solids, and complex chemical compositions. These acid brines host diverse communities of extremophilic microorganisms, including archaea, bacteria, algae, and fungi, according to metagenomic analyses. For the most extreme brine, its water activity (0.714) was considerably lower than that of saturated (pure) NaCl brine. This study provides a thermodynamic insight into life within end-member natural waters that lie at, or possibly beyond, the very edge of habitable space on Earth.
Key Words: Acid brines, Extremophiles, Halophile ecology, Water activity, Habitability
1. Introduction
Acid brines are considered among the most extreme natural waters, based on their low pH, high salinities, and complex chemical compositions. However, little is known about a key thermodynamic parameter of acid brines: their water activity. For a solution or solid substance, it is water activity that defines water availability (relative humidity is used for a gas). At a given temperature and pressure, the maximum equilibrium relative humidity is designated the value 100%, whereas the maximum possible water activity, at a given temperature and pressure, is designated a value of 1. In general, the higher the concentration of solutes in a water, the lower the water activity. For example, seawater has ∼19,000 ppm (or mg/L) Cl-, ∼10,500 ppm Na+, and ∼2,700 ppm SO42-, accounting for ∼32,200 ppm, or ∼3.22% of the ∼3.5% total dissolved solids (TDS) in this natural water (Drever, 1997). Seawater has a relatively high water activity that is, nevertheless, stressful for some types of microorganisms (Brown, 1990).
Water activity is a potent determinant of growth and metabolism for microbes, and an indicator of habitability for environments that otherwise have the potential to host life. In general, the lower the water activity, the less likely the environment is to host a diverse biome (Larson and Belovsky, 2013; Stevenson et al., 2015a; Lee et al., 2018; Merlino et al., 2018). A less widely appreciated aspect of low water activity of natural waters is the likely influence on physical processes such as evaporation and fluid flow (e.g., Raup, 1970; Benison et al., 2008).
Despite the significance of specific water chemistry and geochemical processes to cellular life and the intimate interactions of ecosystems with the environment, the identification and characterization of the most-extreme waters on Earth is a relatively recent area of study. Terrestrial waters of both high salinity and low pH are among these extreme waters and are, perhaps, the least understood (Bowen and Benison, 2009; Benison and Bowen, 2015; Hallsworth, 2019a). Nevertheless, and surprising to some in the scientific community, evidence of microbial communities has been documented for a number of acid brines (Mormile et al., 2009; Escudero et al., 2013, 2018; Johnson et al., 2015, 2020; Zaikova et al., 2018).
Modern acid brines with pH as low as 1.4 and salinity as high as 32% TDS are known from ephemeral lakes in southern Western Australia (Benison et al., 2007; Bowen and Benison, 2009; Benison and Bowen, 2015). In northern Chile, some shallow lakes have pH as low as 1.3 and salinity as high as 29.7% TDS (Risacher et al., 2002; Benison, 2019a). In contrast, modern seawater has pH of approximately 8.1 and salinity of approximately 3.5% TDS (Drever, 1997). Brines are traditionally defined as waters with at least 10% TDS (Drever, 1997). Whereas there is no universally agreed pH value at which we define high-salinity, low-pH waters as acid brines, those with pH less than 5 contain no detectable carbonate or bicarbonate (Bowen and Benison, 2009). Western Australian lakes and groundwaters with pH <4 have been termed “extremely acidic” (Benison et al., 2007). At pH less than ∼3.5, relatively high concentrations of Al, Fe, and Si can exist in brines in Western Australia and Chile (Risacher et al., 2002; Bowen and Benison, 2009) and help to precipitate minerals such as alunite, jarosite, Fe-oxides, and clays (Benison et al., 2007; Story et al., 2010; Benison and Bowen, 2013; Benison, 2019a). All acid brines in Western Australia and Chile precipitate halite and gypsum as well, making these a complex form of NaCl-CaSO4 saturated brines (Risacher et al., 2002; Benison et al., 2007; Bowen and Benison, 2009; Benison, 2019a). Only extreme xerophiles or halophiles can function below 0.800, and only a very small number of microbes are able to function below 0.755 water activity (Stevenson et al., 2015a, 2015b; Lee et al., 2018). Similarly, only acidophilic microbes can thrive below pH 2 (Johnson and Quatrini, 2016). Therefore, in this study, we will consider any brines with both pH <2 and < 0.755 water activity as the most-extreme acid brines.
Empirical studies of brine water activity have traditionally focused on synthetic solutions (most commonly of NaCl-H2O mixtures) rather than compositionally complex brines such as those of extreme acid-brine lakes (e.g., Winston and Bates, 1960; Robson and Loneragan, 1970; Chirife et al., 1983; Grant, 2004; Lee et al., 2018). To understand how far life can prevail under extreme thermodynamic constraints, more work is needed to elucidate the water chemistries of acid-brine systems and how these can impact cellular function. For example, it is unclear whether microbial communities found in situ are active during the evapoconcentration phase of lakes when brine chemistry is at its most extreme. Here, we describe acid brines from lakes of Western Australia and northern Chile and reveal their measured water activities in the context of other biophysical and geochemical parameters.
2. Background
2.1. Water activity
Both equilibrium relative humidity and water activity are measures of the mole fraction of water, for the vapor phase and the liquid phase, respectively. At a given temperature and pressure, the maximum possible equilibrium relative humidity is designated the value 100% and the maximum possible water activity is designated a value of 1. All dissolved substances reduce water activity, so the more concentrated a solution of a specific salt is, the lower its water activity typically is. However, some salts/ions reduce water activity more than others, both at a given concentration, and due to their different valences and solubility limits (Winston and Bates, 1960). Generally, divalent salts such as MgCl2 depress water activity more than monovalent salts such as NaCl (Hallsworth et al., 2007). MgCl2 is, in this way, more osmotically stressful, but it is also chaotropic and becomes extremely inhibitory, even to extreme halophiles, between 2 and 3 M (Hallsworth et al., 2007; Stevenson et al., 2015a; Yakimov et al., 2015). Salts with a high solubility typically depress water activity to lower values than those that are less soluble (Winston and Bates, 1960). The water activity of a microbial substrate (or indeed any solution) of unknown composition can only be determined empirically (Brown, 1990), and such examples include the complex and undefined brines found in nature (Hallsworth et al., 2007; Yakimov et al., 2015; Rubin et al., 2017; La Cono et al., 2019). The vast majority of microbial species exhibit no metabolism or growth below 0.900 water activity, and, until recently, the metabolism/growth of extreme halophiles was thought to be limited to values of ≥0.755 water activity (Grant, 2004; Rummel et al., 2014). We now know that this water activity, which is equivalent to saturated NaCl, does not prevent life in many brine systems and, indeed, is not at all inhibitory for some extremophiles. Empirical data show that only the most extremely halotolerant archaea can grow down to water activities as low as 0.635, and extrapolated values indicate theoretical limits as low as 0.611 (Stevenson et al., 2015a). Recent studies of the fungal xerophile/halophile Aspergillus penicillioides have demonstrated differentiation and cell division at values as low as 0.585 water activity, albeit on glycerol-supplemented media, with a theoretically determined minimum of <0.565 (Stevenson et al., 2017b). This ascomycete is also known to grow at saturated NaCl (Nazareth and Gonsalves, 2014). It is not surprising, therefore, that NaCl-saturated brines can support biomass-dense and biodiverse ecosystems, which are dominated by microbial halophiles (Lee et al., 2018). What has yet to be established for acid brines is how their extreme pH and complex composition influence/determine water activities, and whether the latter lie in the known range for active life.
In studies of geochemistry, microbiology, and astrobiology, the water activity of extreme waters has generally been derived by using models and then expressed to only two decimal places. Water-activity values of these brines derived from models, however, can only be trusted if validated via empirical determinations. Yet many methods to determine water activity are also inaccurate (as discussed by Yu et al., 2009; Stevenson et al., 2015a) and do not approach the same level of sensitivity as the microbial cell (see below). An additional challenge is that many natural waters from extreme environments have complex compositions that either do not allow for complete compositional identifications and/or are difficult, if not impossible, to produce a synthetic match in the laboratory. Natural acid brines, for example, are extremely acid, extremely saline, and contain unusually high concentrations of elements and compounds typically not found in higher than trace amounts in dilute, neutral waters. There are also unknowns about the exact nature of the acids and their dissociation. Nevertheless, empirical determinations of water activity for extremely acid brines have not yet been made.
Recent studies have shown that microbial systems are sensitive to changes in water activity of <0.010, and, in some cases, may be sensitive to ≤0.001 (Stevenson et al., 2015b). It is therefore imperative to measure and express water activity to this level of accuracy. As stated by Stevenson et al. (2015b), “water activity determinations to one decimal place can lack biological meaning, and those made to two decimal places are far less [accurate] than what we would accept for biological studies [which focus on equivalent changes in] temperature or other environmental parameters.”
2.2. Acid brine lakes in Western Australia
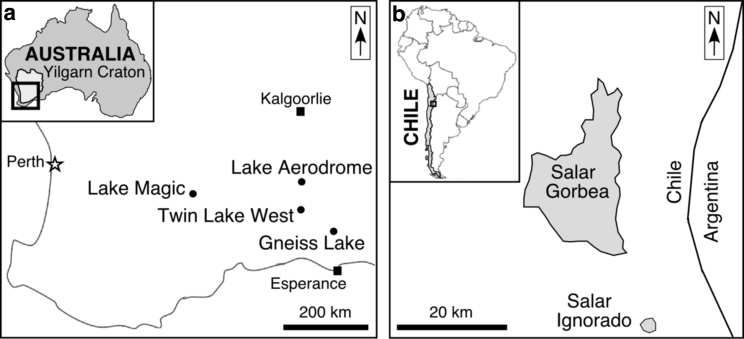
This current study investigates the water activities of lake waters from two distinctly different types of acid saline settings: lakes in Western Australia exist on an Archean craton and salars in northern Chile are hosted by volcaniclastic sediments above an active subduction zone (Figs. 1 and 2). Acid brines of Western Australia and northern Chile formed by different geologic processes, yet their geologic products (waters and minerals) are similar. These acid brines are among the most chemically extreme natural waters on Earth (Bowen and Benison, 2009; Benison and Bowen, 2015; Hallsworth, 2019a).
FIG. 1.
Locations of acid brines used in this study. (a) Four acid brine lakes on the Yilgarn Craton of southern Western Australia. (b) Two acid salars on the Altiplano of northern Chile.
FIG. 2.
Images of lakes used in this study. (a) Lake Magic near Hyden, Western Australia, on January 15, 2015. (b) Gneiss Lake, east of Grass Patch, Western Australia, on January 4, 2015. (c) Twin Lake West, near Salmon Gums, Western Australia, on January 23, 2009. (d) Lake Aerodrome, on the southern edge of Cowan Basin, near Norseman, Western Australia, on January 19, 2009. (e) Salar Gorbea, northern Chile, on March 5, 2007. (f) Salar Ignorado, northern Chile, on March 6, 2007.
Thousands of natural acid saline lakes and neutral saline lakes exist on the weathered ancient rocks of the Yilgarn Craton of southern Western Australia (Mann, 1988; McArthur et al., 1991; Benison et al., 2007). These lakes are ephemeral, undergoing periods of flooding after rainstorms and evapoconcentration during arid weather, which sometimes leads to desiccation (Benison et al., 2007). Lake-water temperatures closely conform to the local air temperatures and range from 0°C to 50°C. The regional groundwater system is acid and saline, with an average pH of ∼3.5 (Bowen and Benison, 2009). An estimated 50% of the lakes have pH less than 4; other lakes have pH in the ∼4–6 range, and rare lakes have pH ∼6–8. The lowest pH and highest salinity measured in the lakes is 1.4 pH and 34% TDS, respectively, and was measured during an evapoconcentration stage (Benison et al., 2007; Benison and Bowen, 2015). Acid lake brines and associated groundwaters are chemically complex, with unusually high concentrations of minor elements and trace elements, including metals (Bowen and Benison, 2009). Water chemistry, including pH and salinity, fluctuates through time (hours to months) and space (meters to kilometers) due to flooding-evapoconcentration-desiccation cycles and localized water-rock interaction. In turn, the saturation states of the water with respect to minerals fluctuate over the same temporal and spatial scales; halite, as well as some other minerals, dissolves during flooding and precipitates during evapoconcentration. The waters also have changing saturation states with respect to other authigenic minerals, including gypsum, jarosite, alunite, hematite, and clays. This results in natural systems with highly variable authigenic mineral suites, as well as fluctuating water composition, pH, and salinity, over relatively small spatial and temporal scales (Benison et al., 2007; Bowen and Benison, 2009; Benison and Bowen, 2013). The acid brines of Western Australia likely evolved over millions of years, involving (1) elimination by chemical weathering of any buffer minerals or compounds in a previous warm, wet climate; (2) oxidation of sulfide minerals disseminated in the host rocks to form sulfuric acid and iron oxides; and (3) the concentration of waters by evapoconcentration with aridification of climate (Benison and Bowen, 2015).
Life in acid brine lakes in Western Australia is confined to microorganisms, yet these microorganisms live in diverse communities. Mormile et al. (2009) discovered different prokaryotic communities in 11 lakes of various pH values and salinities. Genetic sequencing of one sediment sample from a 3.6 pH lake determined 529 species, some of which are novel (Johnson et al., 2015). Zaikova et al. (2018) analyzed a lake water (pH 2.3, 30% TDS), a groundwater (pH 3.4, 4% TDS), and a saline mudflat sediment (pH 1.7, 26% TDS) from Lake Magic near Hyden, Western Australia. Paradoxically for a hypersaline environment, the lake water hosted more eukaryotic microbes than bacteria, and there were very few archaea. The Lake Magic groundwater and sediment samples had more diverse communities of bacteria, with more eukaryotes than archaea. Some studies have shown that microorganisms and organic compounds from acid saline lakes in Western Australia are preserved as solid inclusions and within fluid inclusions in lake-precipitated halite and gypsum (Conner and Benison, 2013; Benison, 2019b). Work has not yet been carried out to determine the biophysical/physicochemical limits for biotic function of these acid brine ecosystems (and whether they are active during the evapoconcentration stage).
2.3. Acid brine lakes in northern Chile
Two acid salars, comprised of shallow lakes, saline and dry sandflats, and dunes, have been described in the Atacama region of the Andes Mountains at over 4000 m above sea level (Risacher et al., 2002; Benison, 2019a). Salars Gorbea and Ignorado are composed of saline minerals hosted by volcaniclastic sediments sourced from recent eruptions of the Cerro Bayo volcanic complex. At Salar Gorbea, lake waters reach a pH value as low as 1.8 and salinity as high as 29% TDS. Lake waters at Salar Ignorado are less geochemically extreme; pH ranges from 3.3 to 4.1, and salinity ranges from 0.5% to 2.0% TDS (Benison, 2019a). Lake waters at Salars Gorbea and Ignorado are most likely evapoconcentrated hydrothermal waters that migrate upward from subsurface magma bodies (Karmanocky and Benison, 2016).
Microorganisms have been documented in acid waters at Salars Gorbea and Ignorado. A diverse community of archaea and bacteria, including novel genera, has been identified at Salar Gorbea (Davis-Belmar et al., 2013; Escudero et al., 2013, 2018; Quatrini et al., 2017). In addition, prokaryotes, Dunaliella algae, and pennate diatoms have been documented as solid inclusions and within fluid inclusions in gypsum from both these Chilean salars (Benison and Karmanocky, 2014; Benison, 2019b). These two acid salars, along with four acid-brine lakes of Western Australia, are the focus of the current study.
3. Materials and Methods
3.1. Fieldwork and sampling
For this study of water activity, we sampled evapoconcentrated acid brine lake waters from Western Australia and Chile. In addition, we collected one seawater sample from Helen's Bay, County Down, Northern Ireland, to serve as a natural non-brine, non-acidic comparator.
Fieldwork was conducted at acid saline lakes and adjacent saline and dry mudflats, dunes, and desert soils in southern Western Australia during seven field campaigns between 2001 and 2015. The purpose was to provide an understanding of geological, geochemical, and biological processes, and their products at various spatial and temporal scales. For the current study, we focused on lake water samples and their corresponding field data, from four lakes sampled in Western Australia in January 2009 and January 2015: (1) one sample from Gneiss Lake, east of the town of Grass Patch; (2) two samples from Lake Magic (also known in Bowen and Benison [2009] as Wave Rock Lake) near Hyden, collected from the same site 15 days apart; (3) one sample from Lake Aerodrome, a part of the Cowan Basin near Norseman; and (4) one sample from Twin Lake West, south of Salmon Gums. These water samples were selected to include some of the lowest pH and highest salinity ever reported, in contrast to measurements made during previous field trips (e.g., Benison et al., 2007). Sampling dates and environmental conditions of lake water samples are shown in Table 1.
Table 1.
Physical and Geochemical Data and Measured Water Activity for Acid Brine Lake Waters
| Lake | Date of sampling | Water depth (cm) | Water color | pH | Salinity (% TDS) | Water temp. (°C) | Water activity |
|---|---|---|---|---|---|---|---|
| Gneiss Lake, W. Australia | 4 Jan. 2015 | 2.5 | yellow | 1.4 | 32 | 30 | 0.717 |
| Gneiss Lake, W. Australia | 4 Jan. 2015 | 2.5 | yellow | 1.4 | 32 | 34 | 0.714 |
| Lake Magic I, W. Australia | 1 Jan. 2015 | 2.5 | yellow | 2.0 | 30 | 29 | 0.742 |
| Lake Magic II, W. Australia | 15 Jan. 2015 | 5.0 | yellow | 1.6 | 30 | 31 | 0.726 |
| Lake Aerodrome, W. Australia | 19 Jan. 2009 | 1.2 | yellow | 1.8 | 29 | 24 | 0.725 |
| Twin Lake West, W. Australia | 23 Jan. 2009 | 10 | green | 2.6 | 27 | ∼30 | 0.745 |
| Salar Gorbea, site 7, Chile | 5 Mar. 2007 | 1 | clear | 2.7 | 28 | ∼30 | 0.799 |
| Salar Gorbea, site 10, Chile | 5 Mar. 2007 | 2 | pink | 1.8 | 18 | 14 | 0.807 |
| Salar Ignorado, site 3, Chile | 6 Mar. 2007 | 10 | clear | 3.8 | 5 | 15 | 0.944 |
Note that the same Gneiss Lake sample is shown twice at two different temperatures. The specific sites for Salars Gorbea and Ignorado samples are described and shown on maps in Benison (2019a). All water samples were in evapoconcentrated stage.
Fieldwork in Chile was conducted in March 2007. The remote location and high elevation discouraged repeated field trips to these salars. Three acid lake water samples from the two salars were used for comparison with the Western Australian acid brines. Salar Gorbea samples were collected from two separate sites (Gorbea site 7 and site 10), and the Salar Ignorado sample is from site 3 (Table 1). Detailed sample site locations are provided in Benison (2019a).
In the field, we used multiple Oakton Double Junction pHTestr 2 portable pH meters to measure the pH of lake waters. These pH meters are designed for use in high salinity, low pH, and/or other geochemically extreme conditions. They are equipped with automatic temperature compensation, have a range of -1.0 to 15.0 pH, and are accurate to ±0.1 pH units. pH meters were calibrated at the start of each field day with Oakton and/or Orion pH 10, 7, 4, and 1.38 buffer solutions and tested with these buffers in the middle and end of each field day; no deviation was observed. Salinity of lake waters, as represented by TDS, was measured in the field with three handheld Hauke optical salinity refractometers (model HRS-28) equipped with automatic temperature compensation. The refractometers measure refractive index of a water sample and convert it to salinity expressed in % TDS (as known for various concentrations of solutions made by dilution and concentration of seawater) and specific gravity. Because the specific gravity of the water samples used in this study exceeds the maximum measurement of 1.070 g/cm3 on the refractometer scale, the specific gravities are not reported here; we estimate that they are as high as 1.22 g/cm3. The water temperatures and air temperatures were measured with plastic-encased glass and alcohol thermometers. Lake waters were collected by filling sterile high-density polyethylene (HDPE) bottles with screw tops to the top, so there was no head space. Water samples were stored at room temperature (20–25°C), close to the temperature at which samples were collected.
3.2. Water geochemistry
Major, minor, and trace element ion concentrations in the lake water samples were measured by a variety of techniques by Activation Laboratories Ltd. of Ancaster, Ontario, Canada. Inductively coupled plasma–mass spectrometry (ICP-MS) was conducted with a Perkin-Elmer Sciex ELN 9000 ICP-MS instrument to measure 59 elements, including Na, Mg, K, Ca, Al, Si, and Fe. For inductively coupled plasma–optical emission spectrometry (ICP-OES), an Agilent Axial 730-ES instrument was used to measure concentrations of 36 elements. A Dionex DX-120 ion chromatography (IC) system analyzed for seven anions, including Cl, Br, and SO4. Methodological details are given in Bowen and Benison (2009).
3.3. Water activity measurements
Water activity was determined by measuring relative humidity above each water sample until equilibrium was reached. Most equipment manufactured to quantify water activity has levels of sensitivity which are an order of magnitude less than those of cellular systems (Stevenson et al., 2015a, 2015b) and so have limited value for studies of microbiology or habitability. For this study, we employed a Novasina Humidat IC-II, an instrument capable of accurately measuring water activity to three decimal places. It can be manually calibrated along continuous scales of water activity and temperature and has been proven accurate for quantification of water activity to the third decimal place, including for undefined and complex brines (Hallsworth and Nomura, 1999; Stevenson et al., 2015a; Yakimov et al., 2015; La Cono et al., 2019).
Calibration salts and brine samples were maintained at the temperature recorded during sampling, prior to water-activity determinations, which were also made at the same temperature. In each case, a Novasina IC-II water activity machine was first calibrated by using a saturated salt solution (i.e., NaCl, KCl, or K2SO4) with a water activity that was closest to that of the samples at the appropriate temperature. In the range of 25°C to 35°C, a saturated solution of pure NaCl (i.e., Na-Cl-H2O) has a water activity of 0.755; from 15°C to 20°C, it has a water activity of 0.760; and at 10°C, a water activity of 0.765 (Winston and Bates, 1960). Equilibration was continued until the value had stabilized at the third decimal place for a 40 min period.
A number of precautions were taken to ensure optimum accuracy, according to Stevenson et al. (2015a). For example, we used filters to prevent particulates or volatiles from interfering with the chemical electrolyte of the humidity sensor. The natural acid brines and calibration solutions were allowed to equilibrate at the required temperature for 2–3 weeks. After each reading, the sensor was allowed to de-saturate to the ambient humidity (∼50%) to ensure stability of the electrolyte and avoid the possibility of fungal growth.
To test reproducibility of results, three independent replicates of each sample were used for water activity determinations. The saturated NaCl solution was used for sensor calibration prior to and after each sensor reading to ensure consistency and stability of sensor performance after exposure to the sample. Accuracy was ±0.001 water activity in the pertinent range (0.900–0.600).
4. Results
The field geochemistry and water activity measurements for four Western Australian acid saline lakes and three pools at two Chilean acid salars are summarized in Table 1. Salinities ranged from 5% to 32% TDS, pH ranged from 1.4 to 3.8, temperatures ranged from 14°C to 34°C, and measured water activities ranged from 0.944 down to 0.714. The seawater sample had a measured water activity of 0.980 at 20.0°C.
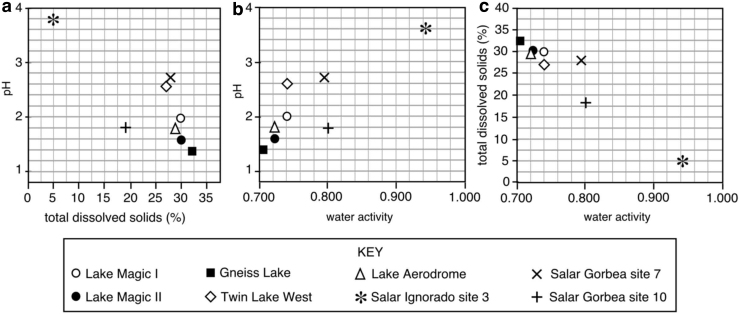
There was a general relationship between pH and salinity in these Western Australian and Chilean brines. The lower the pH in these lake waters, the higher the salinity tended to be (Fig. 3a). It is likely that water-rock interactions, which produce ions in solution, are enhanced by lower pH solutions (Benison and Bowen, 2015), thereby adding more ions to the lake waters. Likewise, there is a relationship between water activity and salinity, as well as between water activity and pH (Fig. 3b, 3c). In general, the higher the salinity, the lower the water activity, and the lower the pH, the lower the water activity. Of the water samples, that with the lowest water activity (0.714) had the highest salinity (32% TDS) and lowest pH (1.4). Three samples from three different Western Australian lakes should be considered the most-extreme brines because they had both pH less than 2 and water activity less than 0.755. Conversely, except for the seawater sample, the highest water activity (0.944) was measured for the water sample from an acid saline lake in Chile with lowest salinity (3.8% TDS) and the highest pH (3.8). However, the relationships among water activity, salinity, and pH are not linear. The two Lake Magic waters had the same salinity (30% TDS) but have different pH and water activities; the pH 1.6 Lake Magic sample had a water activity of 0.726, and the pH 2.0 Lake Magic sample has a water activity of 0.742. Salar Gorbea's site 7 water, with 28% TDS salinity and pH 2.7, had a considerably higher water activity (0.799) than Twin Lake West sample (0.745), despite a similar salinity and pH (27% TDS and pH 2.6).
FIG. 3.
Plots showing relationships among pH, salinity, and water activity. (a) pH versus salinity (expressed as percent of total dissolved solids). (b) pH versus water activity. (c) Salinity versus water activity.
Water activities for the Gneiss Lake sample were measured at two different temperatures, 30°C and 34°C, to reproduce the range of temperatures observed in this lake at the same location within an hour of sample collection. There was a slight influence of temperature on water activity; at 30°C, this 1.4 pH, 32% TDS water has a water activity of 0.717. At 4°C higher, the same acid brine sample has a measured water activity of 0.714. Although the lake waters were precipitating salt minerals at the time of collection due to evaporation in the environment, no further precipitation was observed after collection.
Compositional analyses carried out with ICP-OES, ICP-MS, and IC identified major and minor ions, as shown in Table 2. There was a variety of compositions, marked by (1) a wide range of concentrations of individual ions, (2) inconsistent ratios of major and minor ions among samples, (3) HCO3- concentrations too low to be detected, and (4) unusually high concentrations of some ions. For example, ratios of Mg:Ca ranged from 14.9:1 to 94.3:1 in Western Australian brines and 143.2:1 to 174.0:1 in Salar Gorbea brines. Ratios were much lower in Salar Ignorado brine, that is, 1.14:1. In contrast, Mg:Ca in seawater is approximately 5:1 and Al:Ca in seawater is 0.008:1. Al:Ca, however, ranges from 0.3:1 to 9.9:1 in Western Australian samples and 16.4:1 to 25.4:1 in Salar Gorbea waters. In Salar Ignorado, however, the ratio is 0.3:1. A high range and high concentration of some individual ion concentrations also characterize these acid brines. For example, we measured Br values from 1 to 938 mg/L, Si from 60 to 730 mg/L, and Fe from 0.3 to 160 mg/L (Table 2). The Al concentrations of 5660 and 3610 mg/L in the Chilean acid brines and 2870 mg/L in Gneiss Lake are among the highest dissolved Al recorded in the scientific literature. When added to the high Na and Cl in these acid brines, the unusual ratios of major elements Mg, Ca, and K, as well as high concentrations of SO4, Mg, Br, Al, Si, and Fe, can confer idiosyncratic physical properties and behaviors on these complex brines. Furthermore, natural saline waters that have negligible levels of HCO3- (below limits of detection) are scarce and are restricted to low-pH solutions (Bowen and Benison, 2009).
Table 2.
Geochemical Analyses for Acid Brine Lake Waters of This Study
| Lake | pH | % TDS | Na | Cl | Mg | SO4 | K | Ca | Br | Si | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gneiss | 1.4 | 32 | 99000 | 174000 | 28200 | 56100 | 4490 | 299 | 648 | 94 | 2870 | 160 |
| Magic I | 2.0 | 30 | 94600 | 197000 | 12000 | 17500 | 1970 | 555 | 532 | 73 | 432 | 66 |
| Magic II | 1.6 | 30 | 85500 | 205000 | 23200 | 30300 | 3240 | 388 | 938 | 62 | 751 | 116 |
| Aerodrome | 1.8 | 29 | 84300 | 219000 | 27900 | 37700 | 1490 | 348 | 592 | 68 | 346 | 38 |
| Twin L. W. | 2.6 | 27 | 116000 | 183000 | 8320 | 17700 | 1850 | 558 | 308 | 70 | 163 | 12 |
| Gorbea site 7 | 2.7 | 28 | 68800 | 12600 | 31500 | 92300 | 1310 | 220 | 60 | 60 | 3610 | 2 |
| Gorbea site 10 | 1.8 | 18 | 69000 | 190000 | 38800 | 139000 | 1590 | 223 | 100 | 730 | 5660 | 8 |
| Ignorado site 3 | 3.8 | 5 | 1700 | 776 | 622 | 7540 | 439 | 544 | 1 | 98 | 145 | 0.3 |
Major and minor cations measured by ICP-OES and ICP-MS; major and minor anions measured by IC. Elemental results are expressed in mg/L.
Whereas the measured water-activity values were more extreme than we had anticipated, the geochemical data for the samples in this study fall within the compositional ranges detected in previous, more detailed geochemical analyses of previously sampled acid brines from the same locations in Western Australia and Chile (Risacher et al., 2002; Bowen and Benison, 2009; Kipnis et al., 2020). These acid brines are considerably more enriched in major and minor ions than seawater and are more chemically complex and more spatially and temporally variable than other well-known salt-precipitating brines, such as those of Badwater Basin in Death Valley and the Dead Sea (Hunt et al., 1966; Salhotra et al., 1985; Lowenstein and Risacher, 2009).
5. Discussion
5.1. Relationships identified among salinity, pH, elemental concentrations, and water activity
This study shows that pH and ionic compositions/concentrations of solutions, in addition to total salinity and temperature, are important factors in water activity of natural brines. Previous empirical measurements of water activity in brines have most commonly focused on synthetic salt solutions with a limited number of dissolved salt compounds, most commonly NaCl or MgCl2 (e.g., Harrison et al., 2013; Stevenson et al., 2015a). The acid brines of this current study are considerably more complex, characterized by a large range of ions, including Na, Cl, SO42-, Mg, Ca, and K, but also high concentrations of Br, Al, Fe, and Si, which are absent or of negligible concentrations in most natural waters. In addition, acids such as H2SO4, HCl, and HBr make these acid brines unusual.
The relationships between salinity and water activity, or between pH and water activity, are not perfectly linear (Fig. 3). Therefore, other factors, such as elemental types and concentrations, appear to play a significant role in the water activity. Salar Gorbea site 10 water, at 18% TDS, 1.8 pH, and 14°C, had significantly higher Cl, SO4, Si, and Al than more saline, less acid, and warmer Gorbea site 7 water. All waters studied from Western Australia had water activities lower than synthetic NaCl-saturated brines, which have been measured at 0.755 in the same general temperature range (25°C to 35°C) as the Western Australian waters (Winston and Bates, 1960).
The water studied with the highest salinity and the lowest pH was from Gneiss Lake in Western Australia. This sample was measured for water activity at two different temperatures. At 30°C, the water activity was 0.717, and at 34°C, it was only lower by 0.003, with a water activity of 0.714. This study demonstrates that temperature differences, in the range of 4°C at least, may have a moderate effect on water activity in acid brines. This said, the 0.003 difference observed between samples of Gneiss Lake measured at 30°C and 34°C is considerable in relation to potential impacts on microbial systems (Stevenson et al., 2015b), assuming that active life is indeed present.
The acid brine samples from Western Australian lakes were collected during evapoconcentration stage for this study. However, there is a range of temperature, salinity, and pH at individual lakes during the days to months of a single evapoconcentration stage before a lake is either desiccated or flooded (Benison et al., 2007). Therefore, at times of greater environmental extremes, the lakes may reach even lower water activities than measured in this study. Possible water-activity values below 0.714 are likely when a lake is at a more evapoconcentrated stage, just prior to desiccation. This current study also reveals that a slight increase in temperature can lower a brine's water activity. The highest air and water temperature we have recorded at an acid saline lake in Western Australia is 50°C (Benison et al., 2007), although samples from that temperature were not included in the current study. Regardless of this, the dynamic nature of environmental conditions at these most extreme acid brine lakes suggests the potential for water-activity values lower than those measured.
5.2. Life in natural acid brines of low water activity
In earlier studies, diverse microbial communities were detected in each of the acid lakes included in this current study. Mormile et al. (2009) used denaturing gradient gel electrophoresis (DGGE) to determine DNA from waters sampled at 11 Western Australian lakes, including Gneiss Lake and Twin Lake. Phylogenetic trees for prokaryotes of four of these lakes, based on the 16S rRNA gene, were developed. Metagenomic shotgun sequencing of Gneiss Lake samples resulted in a microbial profile that included 45 genes associated with sulfur metabolism, strongly suggesting sulfur-oxidizing and sulfur-reducing archaea and bacteria (Johnson et al., 2015). Studies of Lake Magic waters have shown that there is a diverse community in which fungi and algae are more abundant and diverse than bacteria, and in which archaea are almost absent (Zaikova et al., 2018). Prokaryotes, eukaryotes, and organic compounds have been documented in fluid inclusions and as solid inclusions in halite and gypsum precipitating from Gneiss Lake, Lake Magic, Twin Lake West, and Lake Aerodrome (Benison, 2013, 2019b; Conner and Benison, 2013).
Salar Gorbea in northern Chile has been the subject of several microbial surveys. There, analyses have documented a diverse community of extremophilic prokaryotes, including Acidithiobacillus thiooxidans, as well as many novel species and genera (Demergasso et al., 2010; Davis-Belmar et al., 2013; Escudero et al., 2013, 2018; Quatrini et al., 2017). Preservation of prokaryotes, eukaryotes, and organic compounds has been observed in fluid inclusions and as solid inclusions in gypsum from Salars Gorbea and Ignorado (Benison and Karmanocky, 2014; Karmanocky and Benison, 2016; Benison, 2019b).
Acid brines host microbially diverse communities of prokaryotes and eukaryotes, many of which are novel. We cannot be certain that these microbes are metabolically active during the evaporative stage of acid-brine lakes when the brines are at their most concentrated and most acidic. However, even during flooding stage, at which time the waters are more dilute and less acidic, the combined environmental conditions of high salinity, low pH, complex water composition, fluctuating water chemistry and availability, and high solar radiation impose a selection pressure that favors polyextremophiles. A small number of microbes are known to be both halotolerant and acidophilic, most notably a species of Acidohalobacter (A. aeolianus), which can grow at 1283 mM NaCl, pH 2, 30°C, and some of which grow at pH as low as 1 (Khaleque et al., 2018, 2019). However, Acidohalobacter has not been identified in acid brine lakes thus far. Nevertheless, it is intriguing to note that it can tolerate the combination of extreme pH, reduced water activity, osmotic stress, and ionic conditions while retaining biotic function. Whereas one Acidohalobacter species (A. ferrooxidans) grows at pH 1, it has been found to do so at 1283 mM (7.5%, w/v) NaCl. Furthermore, the NaCl tolerance of 7.5% (w/v) would be insufficient for activity in the acid brines reported in the current study. The most acidophilic microbe known, Picrophilus torridus, grows at pH values as low as -0.6 (Schleper et al., 1995) but lacks halotolerance. There are many acidophiles that inhabit mine wastes and other low pH environments (Johnson and Quatrini, 2016). Some of these habitats have pH values of approximately 0, but they do not contain high concentrations of salts. Furthermore, from culture-based studies of isolated microbial strains, we have not yet identified a microbe capable of confirmed growth in saturated brines with acidity in the range pH 1–2 and water activity ≤0.755. The novel taxa identified in extreme acid brines during metagenomic surveys have yet to be studied in relation to their stress biology to determine their biophysical limits when using culture-based systems.
It should also be noted that individual salts/ions can exert multiple stresses and multiple extremes on microbial cells (Hallsworth et al., 2007, 2019b; Alves et al., 2015). The close association between microorganisms and organic compounds such as beta carotene suggests that these microorganisms are living in the lake water and not simply getting carried into the lake by wind. Spatial distribution and diversity of microbial communities in the field area in Western Australia argue against wind or floods as a primary transport agent of microbes. The observed heterogeneity of microbial communities among shallow lakes within kilometers of one another, as well as the distinctly different communities in Lake Magic lake water contrasted to sandflat sediment adjacent to Lake Magic, suggests that a majority of microorganisms are adapted to individual lakes and their specific water geochemistry (Mormile et al., 2009; Zaikova et al., 2018). Western Australian lake waters with salinity at or above 29% TDS and pH at or below 1.8 all had measured water activities less than 0.726, suggesting to us that these lakes may indeed be habitats for polyextremophiles.
Separate fractions of the Lake Magic I sample were used for this current study and for earlier metagenomic analyses (see Table 1; Zaikova et al., 2018). The work of Zaikova et al. (2018) represents the most comprehensive biological analyses done for any of the Western Australian lakes. At pH 2.0, 30% TDS, and 29°C, this shallow lake water had a water activity of 0.742. The lake water at this time and under these environmental conditions hosted a diverse community. Prokaryotes in this Lake Magic water were of low diversity and abundance, with only 0.030% of reads attributed to archaea. The alphaproteobacterial genus Methylobacterium was detected in Lake Magic waters, but the most abundant bacterium was the proteobacterial genus Salinisphaera (Zaikova et al., 2018). Eukaryotes dominated this Lake Magic water, composing 73% of reads. Most eukaryotes were fungi, the most abundant of which was the filamentous ascomycetes of the clade Leotiomyceta. Acidotolerant halophilic ascomycete genera Aspergillus and Penicillium were particularly abundant. In addition to fungi, Lake Magic I lake water sample also included several genera of green alga: Dunaliella, Volvox, Oedogonium, and Monoraphidium (Zaikova et al., 2018), but no diatoms. This Lake Magic I sample suggests that environments with a water activity beyond that of saturated NaCl might possibly support active communities of diverse microbes, and that haloarchaea are not necessarily the dominant organisms in acid brines.
Previous studies suggest a lack of biotic activity in brines of pH <3 and <0.750 water activity (Harrison et al., 2013). Such metabolic activity is, however, plausible because some halophiles are known to retain biotic activity and undergo cell division beyond the water activity of NaCl-saturated brines, and even close to or below 0.600 (Stevenson et al., 2015a, 2017a, 2017b; Lee et al., 2018). We propose that, even at low pH, active life may occur at water activity values below the relatively moderate value of 0.755 (Lee et al., 2018). The presence of microbial communities in acid brines of pH 1.8 and water activity 0.726 (see above) keeps alive the intriguing question of whether the resident acidotolerant halophiles are metabolically active at times when the waters are so concentrated.
5.3. Implications for astrobiology
The current study determined low water activity for modern, terrestrial acid brines that host diverse communities of microorganisms, including archaea, bacteria, fungi, and algae. Some ancient terrestrial acid-lake deposits were even more geochemically extreme. For example, sedimentological and geochemical studies have documented that similar lake environments as those in Western Australia, but with lower pH, existed during the Permian and Triassic Periods (∼270 to ∼240 million years ago). Bedded halite in the Permian Opeche Shale of North Dakota and South Dakota (USA) grew in shallow lakes with pH less than 0 (Benison et al., 1998). The Permian Nippewalla Group of Kansas and Oklahoma (USA) and Triassic Mercia Mudstone Group include some bedded halite that precipitated in lakes with pH less than 1 (Benison et al., 1998; Andeskie et al., 2018). These Permo-Triassic acid lake brines were also enriched in Al, Fe, and/or Si (Benison et al., 1998). Microorganisms and organic compounds are entrapped within primary fluid inclusions in these Permian and Triassic halites, strongly suggesting that life existed in these ancient extreme environments, some of which likely had water activities lower than 0.714 (Benison, 2019b).
Modern and Permo-Triassic acid brine lakes may share some geochemical similarities with Precambrian Earth environments that hosted early life. Some Archean and Paleoproterozoic sedimentary rocks are composed of high concentrations of silica and iron oxides due to depositional waters enriched in Si and Fe, like some modern acid brines. These cherts and banded iron formations host some of the first signs of life on Earth, in the form of stromatolites, microbial cellular morphologies, and carbonized matter (i.e., Konhauser and Ferris, 1996; Westall and Folk, 2003). Our study of water activities in modern acid brines may have implications for better understanding the links between geochemistry and microbial life for Precambrian Earth and perhaps on Mars.
Using a model-based approach, Tosca et al. (2008) derived a value of 0.400 water activity for ancient brines that precipitated saline minerals on the surface at Meridiani Planum, Mars, which suggests that life on Mars was highly unlikely. Whereas other studies of water availability on Mars have drawn similar conclusions for the surface and shallow subsurface (Rummel et al., 2014; Hallsworth, 2020). A study of deep-sea brines claims active life at 0.400 water activity (Steinle et al., 2018), although there is no direct biological evidence for this (Hallsworth, 2019b). It is not known how accurate modeling is for extreme acid brines; such modeling may be complicated by the complex chemical compositions, as well as low pH, high salinities, and a wide range of temperatures that would be likely on Mars. Modeling of water activity of acid brines has not yet been validated by empirical measurements. Future studies that test how well water-activity measurements compare to modeled results for acid brines are needed before potential for habitability based on water activity can be reliably predicted for Mars.
Mars likely also hosted acid brines in the past. Mineral assemblages found on Mars, including chlorides, hydrated calcium sulfates, jarosite, alunite, silica, and clay minerals, are consistent with those precipitated by modern and ancient terrestrial acid brines (e.g., Benison and LaClair, 2003; Squyres et al., 2004; Benison, 2006, 2019a; Benison and Bowen, 2006). In terrestrial acid-brine lakes, precipitation of chemical sediments halite and gypsum entraps microorganisms within primary fluid inclusions. However, the salt minerals on Mars have yet to be examined for fluid inclusions (Benison, 2019b). Studies of synthetic martian brines report water-activity values up to 0.984 (Fox-Powell et al., 2016), and studies of salt deliquescence suggest that perchlorate brines form on present-day Mars with water activities up to 0.800. We propose that the low water activities of habitable acid brines on Earth increase the likelihood that, at moderate martian temperatures, some microbes might be capable of metabolic activity on Mars.
6. Conclusions
Here, for natural, compositionally complex acid brines, we revealed their water activity that was previously unknown, and we explain why models cannot be used to accurately quantify water activity. Measured water activities of Western Australian lakes were considerably beyond those of saturated NaCl brines, from 0.745 to 0.714. Lakes in northern Chile were less extreme in terms of water activity (0.944 to 0.799), as well as their pH and salinity. In general, the lower the pH, the lower the water activity. The combination of extreme water activity, pH, and salinity in Western Australian brines, which contain phylogenetically diverse communities of microbes that are presumably acidotolerant/acidophilic and halophilic, expands our knowledge of the biophysical limits for life on Earth and beyond this planet.
Acknowledgments
Helpful discussion was provided by Thomas Koop (Bielefeld University, Germany). Fieldwork and geochemical analyses were partially funded by grants to K.C.B. from the National Science Foundation Sedimentary Geology and Paleobiology Program grant EAR-0902250 and National Aeronautics and Space Administration Exobiology Program grant 80NSSC18K1286. Water-activity measurements were funded by Biotechnology and Biological Sciences Research Council (BBSRC, United Kingdom) project BBF003471 to J.E.H. The Department of Agriculture, Environment and Rural Affairs (Northern Ireland) provided a scholarship for W.K.O. Editor Sherry Cady and the anonymous reviewers are thanked for suggested revisions.
Abbreviations Used
- IC
ion chromatography
- ICP-MS
inductively coupled plasma–mass spectrometry
- ICP-OES
inductively coupled plasma–optical emission spectrometry
- TDS
total dissolved solids
References
- Alves, F.L., Stevenson, A., Baxter, E., Gillion, J.L.M., Hejazi, F., Hayes, S., Morrison, I.E.G., Prior, B.A., McGenity, T.J., Rangel, D.E.N., et al. (2015) Concomitant osmotic and chaotropicity-stresses in Aspergillus wentii: compatible solutes determine the biotic window. Curr Genet 61:457–477 [DOI] [PubMed] [Google Scholar]
- Andeskie, A.S., Benison, K.C., Eichenlaub, L.A., and Raine, R. (2018) Acid-saline-lake systems of the Triassic Mercia Mudstone Group of County Antrim, Northern Ireland. Journal of Sedimentary Research 88:385–398 [Google Scholar]
- Benison, K.C. (2006) A martian analog in Kansas: comparing martian strata with Permian acid saline lake deposits. Geology 34:385–388 [Google Scholar]
- Benison, K.C. (2013) Acid saline fluid inclusions: examples from modern and Permian extreme lake systems. Geofluids 13:579–593 [Google Scholar]
- Benison, K.C. (2019a) The physical and chemical sedimentology of two high-altitude acid salars in Chile: sedimentary processes in an extreme environment. Journal of Sedimentary Research 89:147–167 [Google Scholar]
- Benison, K.C. (2019b) How to search for life in martian chemical sediments and their fluid and solid inclusions using petrographic and spectroscopic methods. Front Environ Sci 7, doi: 10.3389/fenvs.2019.00108 [DOI] [Google Scholar]
- Benison, K.C. and Bowen, B.B. (2006) Acid saline lake systems give clues about past environments and the search for life on Mars. Icarus 183:225–229 [Google Scholar]
- Benison, K.C. and Bowen, B.B. (2013) Extreme sulfur-cycling in acid brine lake environments of Western Australia. Chem Geol 351:154–167 [Google Scholar]
- Benison, K.C. and Bowen, B.B. (2015) The evolution of end-member continental waters: the origin of acidity in southern Western Australia. GSA Today 25:4–10 [Google Scholar]
- Benison, K.C. and Karmanocky, F.J., III. (2014) Could microorganisms be preserved in Mars gypsum? Insights from terrestrial examples. Geology 42:615–618 [Google Scholar]
- Benison, K.C. and LaClair, D.A. (2003) Modern and ancient extremely acid saline deposits: terrestrial analogs for martian environments? Astrobiology 3:609–618 [DOI] [PubMed] [Google Scholar]
- Benison, K.C., Goldstein, R.H., Wopenka, B., Burruss, R.C., and Pasteris, J.D. (1998) Extremely acid Permian lakes and groundwaters in North America. Nature 392:911–914 [Google Scholar]
- Benison, K.C., Bowen, B.B., Oboh-Ikuenobe, F.E, Jagniecki, E.A., LaClair, D.A., Story, S.L., Mormile, M.R., and Hong, B.Y. (2007) Sedimentology of acid saline lakes in southern Western Australia: newly described processes and products of an extreme environment. Journal of Sedimentary Research 77:366–388 [Google Scholar]
- Benison, K.C., LaClair, D.A., and Walker, J.W. (2008) Acid waters as physical sedimentology agents: implications for Mars. Earth Planet Sci Lett 270:330–337 [Google Scholar]
- Bowen, B.B. and Benison, K.C. (2009) Geochemical characteristics of naturally acid and alkaline saline lakes in southern Western Australia. Appl Geochem 24:268–284 [Google Scholar]
- Brown, A.D. (1990) Microbial Water Stress Physiology: Principles and Perspectives, John Wiley & Sons, Chichester, UK [Google Scholar]
- Chirife, J., Favetto, G., Fontan, C.F., and Resnik, S.L. (1983) Water activity of standard saturated salt solutions in the range of intermediate moisture foods. Lebensmittel-Wissenschaft and Technologie 16:36–38 [Google Scholar]
- Conner, A.J. and Benison, K.C. (2013) Acidophilic halophilic microorganisms in fluid inclusions in halite from Lake Magic, Western Australia. Astrobiology 9:850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Belmar, C.S., Pinto, E., Demergasso, C., and Rautenbach, G. (2013) Proteo and actinobacteria diversity at a sulfide, salt and acid-rich lake in the north of Chile. Adv Mat Res 825:37–41 [Google Scholar]
- Demergasso, C., Dorador, C., Meneses, D., Blamey, J., Cabrol, N., and Escudero, L. (2010) Prokaryotic diversity pattern in high-altitude ecosystems of the Chilean altiplano. J Geophys Res Biogeosciences 115:2156–2202 [Google Scholar]
- Drever, J.I. (1997) The Geochemistry of Natural Waters: Surface and Groundwater Environments, 3rd ed., Prentice Hall, Upper Saddle River, NJ [Google Scholar]
- Escudero, L.V., Bijman, J., Chong, G., Pueyo, J.J., and Demergasso, C. (2013) Geochemistry and microbiology in an acidic, high altitude (4,000 m) salt flat: High Andes, northern Chile. Adv Mat Res 825:28–32 [Google Scholar]
- Escudero, L.V., Oetiker, N., Gallardo, K., Tebes-Cayo, M., Gujrdo, M., Nunez, C., Davis-Belmar, C., Pueyo, J.J., Diez, G.C., and Demergasso, C. (2018) A thiotrophic microbial community in an acidic brine lake in Northern Chile. Antonie van Leeuwenhoek 111:1572–1589 [DOI] [PubMed] [Google Scholar]
- Fox-Powell, M.G., Hallsworth, J.E., Cousins, C.R., and Cockell, C.S. (2016) Ionic strength is a barrier to the habitability of Mars. Astrobiology 16:427-442 [DOI] [PubMed] [Google Scholar]
- Grant, W.D. (2004) Life at low water activity. Philos Trans R Soc Lond B Biol Sci 359:1249–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallsworth, J.E. (2019a) Microbial unknowns at the saline limits for life. Nat Ecol Evol 3:1503–1504 [DOI] [PubMed] [Google Scholar]
- Hallsworth, J.E. (2019b) Wooden owl that redefines Earth's biosphere may yet catapult a fungus into space. Environ Microbiol 21:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallsworth, J.E. (2020) Salt deliquescence can support extraterrestrial life. Nat Astron 4:739–740 [Google Scholar]
- Hallsworth, J.E. and Nomura, Y. (1999) A simple method to determine the water activity of ethanol-containing samples. Biotech Bioeng 62:242–245 [DOI] [PubMed] [Google Scholar]
- Hallsworth, J.E., Yakimov, M.M., Golyshin, P.N., Gillion, J.L.M., D'Auria, G., De Lima Alves, F., La Cono, V., Genovese, M., McKew, B.A., Hayes, S.L., et al. (2007) Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ Microbiol 9:801–813 [DOI] [PubMed] [Google Scholar]
- Harrison, J.P., Gheeraert, N., Tsigelnitskiy, D., and Cockell, C.S. (2013) The limits for life under multiple extremes. Trends Microbiol 21:204–212 [DOI] [PubMed] [Google Scholar]
- Hunt, C.B., Robinson, T.W., Bowles, W.A., and Washburn, A.L. (1966) Hydrologic Basin, Death Valley, California, Geological Survey Professional Paper 494-B, United States Government Printing Office, Washington, DC [Google Scholar]
- Johnson, D.B. and Quatrini, R. (2016) Acidophile microbiology in space and time. Curr Issues Mol Biol 39:63–76 [DOI] [PubMed] [Google Scholar]
- Johnson, S.S., Chevrette, M.G., Ehlmann, B., and Benison, K.C. (2015) Insights from the metagenome of an acid salt lake: the role of biology in an extreme depositional environment. PLoS One 10, doi: 10.1371/journal.pone.0122869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S.S., Millan, M., Graham, H., Benison, K.C., Williams, A., McAdam, A., Knudson, C., Andrejkovicova, S., and Achilles, C. (2020) Lipid biomarkers in ephemeral acid salt lake mudflat/sandflat sediments: implications for Mars. Astrobiology 20:167–178 [DOI] [PubMed] [Google Scholar]
- Karmanocky, F.J., III, and Benison, K.C. (2016) A fluid inclusion record of hydrothermal pulses in acid Salar Ignorado gypsum, northern Chile. Geofluids 16:490–506 [Google Scholar]
- Khaleque, H.N., Kaksonen, A.H., Bowall, N.J., and Watkin, E.L.J. (2018) Chloride ion tolerance and pyrite bioleaching capabilities of pure and mixed halotolerant, acidophilic iron- and sulfur-oxidizing cultures. Miner Eng 120:87–93 [Google Scholar]
- Khaleque, H.N., Gonzalez, C., Kaksonen, A.H., Bowall, N.J., Holmes, D.S., and Watkin, E.L.J. (2019) Genome-based classification of two halotolerant extreme acidophiles, Acidihalobacter prosperus V6 ( = DSM 14174 = JCM 32253) and ‘Acidihalobacter ferrooxidans’ V8 ( = DSM 14175 = JCM 32254) as two new species, Acidihalobacter aeolianus sp. nov. and Acidihalobacter ferrooxydans sp. nov., respectively. Int J Syst Evol Microbiol 69:1557–1565 [DOI] [PubMed] [Google Scholar]
- Kipnis, E.L., Bowen, B.B., Hutchings, S.J., Hynek, S.A., and Benison, K.C. (2020) Major ion geochemistry in Na-Ca-Mg-K-Cl-SO4 brines using portable X-ray fluorescence spectrometry. Chem Geol 558, doi: 10.1016/j.chemgeo.2020.119865 [DOI] [Google Scholar]
- Konhauser, K.O. and Ferris, F.G. (1996) Diversity of iron and silica precipitation by microbial mats in hydrothermal waters, Iceland: implications for Precambrian iron formations. Geology 24:323–326 [Google Scholar]
- La Cono, V., Bortoluzzi, G., Messina, E., La Spada, G., Smedile, F., Borghini, M., Stumpp, C., Schmitt-Kopplin, P., Harir, M., O'Neill, W.K., et al. (2019) The discovery of Lake Hephaestus, the youngest athalassohaline deep-sea formation on Earth. Sci Rep 9, doi: 10.1038/s41598-018-38444-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, C.A. and Belovsky, G.E. (2013) Salinity and nutrients influence species richness and evenness of phytoplankton communities in microcosm experiments from Great Salt Lake, Utah, USA. J Plankton Res 35:1154–1166 [Google Scholar]
- Lee, C.J.D., McMullan, P.E., O'Kane, C.J., Stevenson, A., Santos, I.C., Roy, C., Ghosh, W., Manicelli, R.L., Mormile, M.R., McMullan, G., et al. (2018) NaCl-saturated brines are thermodynamically moderate, rather than extreme, microbial habitats. FEMS Microbiol Rev 42:672–693 [DOI] [PubMed] [Google Scholar]
- Lowenstein, T.K. and Risacher, F. (2009) Closed basin brine evolution and the influence of Ca-Cl inflow waters: Death Valley and Bristol Dry Lake, California, Qaidam Basin, China, and Salar de Atacama, Chile. Aquat Geochem 15:71–94 [Google Scholar]
- Mann, A.W. (1988) Hydrochemistry and weathering on the Yilgarn Block, Western Australia—ferrolysis and heavy metals in continental brines. Geochim Cosmochim Acta 47:181–190 [Google Scholar]
- McArthur, J.M., Turner, J.V., Lyons, W.B., Osborn, A.O., and Thirwall, M.F. (1991) Hydrochemistry on the Yilgarn Block, Western Australia: ferrolysis and mineralization in acid brines. Geochim Cosmochim Acta 55:1273–1288 [Google Scholar]
- Merlino, G., Barozzi, A., Michoud, G., Ngugi, D.K., and Daffonchio, D. (2018) Microbial ecology of deep-sea hypersaline anoxic basins. FEMS Microbiol Ecol 94, doi: 10.1093/femsec/fiy085 [DOI] [PubMed] [Google Scholar]
- Mormile, M.R., Hong, B.-y., and Benison, K.C. (2009) Molecular analysis of the microbial communities of Mars-analog lakes in Western Australia. Astrobiology 9:919–930 [DOI] [PubMed] [Google Scholar]
- Nazareth, S. and Gonsalves, V. (2014) Aspergillus penicillioides—a true halophile existing in hypersaline and polyhaline econiches. Annal Microbiol 64:397–402 [Google Scholar]
- Quatrini, R., Escudero, L.V., Moya-Beltran, A., Galleguillos, P.A., Issotta, F., Acosta, M., Cardenas, J.P., Nunez, H., Salinas, K., Holmes, D.S., and Demergasso, C. (2017) Draft genome sequence of Acidithiobacillus thiooxidans CLST isolated from the acidic hypersaline Gorbea salt flat in northern Chile. Stan Genomic Sci 12, doi: 10.1186/s40793-017-0305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raup, O.B. (1970) Brine mixing: an additional mechanism for formation of basin evaporites. AAPG Bulletin 54:2246–2259 [Google Scholar]
- Risacher, F., Alonso, H., and Salazar, C. (2002) Hydrochemistry of two adjacent acid saline lakes in the Andes of northern Chile. Chem Geol 187:39–57 [Google Scholar]
- Robson, A.D. and Loneragan, J.F. (1970) Sensitivity of annual Medicago species to manganese toxicity as affected by calcium and pH. Aust J Agric Res 21:223–232 [Google Scholar]
- Rubin, S.S., Marin, I., Gomez, M.J., Morales, E.A., Zekker, I., San Matrin-Uriz, P., Rodriguez, N., and Amils, R. (2017) Prokaryotic diversity and community composition in the Salar de Uyuni, a large scale, chaotropic salt flat. Environ Microbiol 19:3745–3754 [DOI] [PubMed] [Google Scholar]
- Rummel, J.D., Beaty, D.W., Jones, M., Bakermans, C., Barlow, N.G., Boston, P.J., Chevrier, V.F., Clark, B., de Vera, J.-P. P., Gough, R., et al. (2014) A new analysis of Mars “Special Regions”: findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14:887–968 [DOI] [PubMed] [Google Scholar]
- Salhotra, A.M., Adams, E.E., and Harleman, D.R.F. (1985) Effect of salinity and ionic composition on evaporation: analysis of Dead Sea evaporation pans. Water Resour Res 21:1336–1344 [Google Scholar]
- Schleper, C., Puehler, G., Holz, I., Gambacorta, A., Janekovic, D., Santarius, U., Klenk, H-P., and Zillig, W. (1995) Picrophilus gen. nov. fam. nov.: a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J Bacteriol 177:7050–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squyres, S.W., Grotzinger, J.P., Arvidson, R.E., Bell, J.F., Calvin, W., Cristensen P.R., Clark B.C., Crisp, J.A., Farrand, W.H., Herkenhoff, K.E., et al. (2004) In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306:1709-1714 [DOI] [PubMed] [Google Scholar]
- Steinle, L., Knittel, K., Felber, N., Casalino, C., de Lange, G. Tessarolo, C., Stadnitskala, A., Sinninghe Damaste, J.S., Zopfi, J., Lehmann, M.F., et al. (2018) Life on the edge: active microbial communities in the Kryos MgCl-brine basin at very low water activity. ISME J 12:1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, A., Cray, J.A., Williams, J.P., Santos, R., Sahay, R., Neuenkirchen, N., McClure, C., Grant, I.R., Houghton, J.D.R., Quinn, J.P., et al. (2015a) Is there a common water-activity limit for the three domains of life? ISME J 9:1333–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, A., Burkhardt, J., Cockell, C.S., Cray, J.A., Dijksterhuis, J., Fox-Powell, M., Kee, T.P., Kminek, G., McGenity, T.J., Timmis, K.N., et al. (2015b) Multiplication of microbes below 0.690 water activity: implications for terrestrial and extraterrestrial life. Environ Microbiol 17:257–277 [DOI] [PubMed] [Google Scholar]
- Stevenson, A., Hamill, P.G., Medina, A., Kminek, G., Rummel, J.D., Dijksterhuis, J., Timson, D.J., Magan, N., Leong, E.-L.L., and Hallsworth, J.E. (2017a) Glycerol enhances fungal germination at the water-activity limit for life. Environ Microbiol 19:947–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, A., Hamill, P.G., O'Kane, C.J., Kminek, G., Rummel, J.D., Voyteck, M.A., Dijksterhuis, J., and Hallsworth, J.E. (2017b) Aspergillus penicilliodes differentiation and cell division at 0.585 water activity. Environ Microbiol 19:687–697 [DOI] [PubMed] [Google Scholar]
- Story, S. Bowen, B.B., Benison, K.C., and Schulze, D.G. (2010) Authigenic phyllosilicates in modern acid saline lake sediments and implications for Mars. J Geophys Res Planets 115, doi: 10.1029/2010JE003687 [DOI] [Google Scholar]
- Tosca, N.J., Knoll, A.H., and McLennan, S.M. (2008) Water activity and the challenge for life on early Mars. Science 320:1204–1207 [DOI] [PubMed] [Google Scholar]
- Westall, F. and Folk, R.L. (2003) Exogeneous carbonaceous microstructures in Early Archean cherts and BIFs from the Isua Greenstone Belt: implications for the search for life in ancient rocks. Precambrian Res 126:313–330 [Google Scholar]
- Winston, P.W. and Bates, D.H. (1960) Saturated solutions for the control of humidity in biological research. Ecology 41:232–237 [Google Scholar]
- Yakimov, M.M., La Cono, V., Spada, G.L., Bortoluzzi, G., Messina, E., Smedile, F., Arcadi, E., Borghini, M., Ferrer, M., Schmitt-Kopplin, P., et al. (2015) Microbial community of the deep-sea brine Lake Kryos seawater-brine interface is active below chaotrophicity limit of life as revealed by recovery of mRNA. Environ Microbiol 17:364–382 [DOI] [PubMed] [Google Scholar]
- Yu, X., Schmidt, A.R., and Schmidt, S.J. (2009) Uncertainty analysis of hygrometer-obtained water activity measurements of saturated salt slurries and food materials. Food Chem 115:214–226 [Google Scholar]
- Zaikova, E., Benison, K.C., Mormile, M.R., and Johnson, S.S. (2018) Microbial communities and their predicted metabolic functions in a desiccating acid salt lake. Extremophiles 22:367–379 [DOI] [PubMed] [Google Scholar]