Abstract
Traumatic brain injury (TBI) is a significant public health burden, and the development of advanced countermeasures to mitigate and prevent these injuries during automotive, sports, and military impact events requires an understanding of the intracranial mechanisms related to TBI. In this study, the efficacy of tissue-level injury metrics for predicting TBI was evaluated using finite element reconstructions from a comprehensive, multi-species TBI database. The database consisted of human volunteer tests, laboratory-reconstructed head impacts from sports, in vivo non-human primate (NHP) tests, and in vivo pig tests. Eight tissue-level metrics related to brain tissue strain, axonal strain, and strain-rate were evaluated using survival analysis for predicting mild and severe TBI risk. The correlation between TBI risk and most of the assessed metrics were statistically significant, but when injury data was analyzed by species, the best metric was often inconclusive and limited by the small datasets. When the human and animal datasets were combined, the injury analysis was able to delineate maximum axonal strain as the best predictor of injury for all species and TBI severities, with maximum principal strain as a suitable alternative metric. The current study is the first to provide evidence to support the assumption that brain strain response between human, pig, and NHP result in similar injury outcomes through a multi-species analysis. This assumption is the biomechanical foundation for translating animal brain injury findings to humans. The findings in the study provide fundamental guidelines for developing injury criteria that would contribute towards the innovation of more effective safety countermeasures.
Keywords: axonal strain, concussion, diffuse axonal injury, finite element model, strain-rate
Introduction
The Centers for Disease Control and Prevention estimates that traumatic brain injury (TBI) accounts for approximately 2.5 million emergency department visits, 282,000 hospitalizations, and 56,000 deaths annually in the United States.1 This substantial public health and economic burden warrants continued TBI prevention efforts and countermeasure innovation. To facilitate the development of effective countermeasures and safety testing standards, TBI risk assessments have been proposed based on human head kinematics.2–5 Many assessments were developed using computational brain finite element models (FEM) to predict the tissue-level response during applied head kinematics, with the underlying assumption that tissue deformation is a predictor of brain injury. Based on the correlation between the mechanical stimuli and axonal stretch using in vitro/in vivo models,6–10 many recent FEM studies have suggested using axonal strain11–13 or a combination of strain and strain-rate14–16 as a tissue-level injury predictor. The correlation between these tissue-level metrics and TBI pathology has been demonstrated using pig experimental TBI data,17,18 but further work in this area is needed.
Data with known head kinematics and injury outcomes are necessary for evaluating tissue-level injury metrics. This type of injury data typically comes from field measurements, reconstruction of impacts, and animal tests. Field data directly collected from sensors worn by athletes19,20 or reconstructed from the field impacts13,21,22 is generally considered mild TBI, which impeded the evaluation of injury metrics for more severe TBI. On the other hand, non-rodent in vivo TBI models23–26 provides accessibility to pathophysiological mechanisms and can be tested for a broader range of injury severities at well-control and characterized loading conditions. However, applying these results to humans requires a scaling technique,27–29 which also heavily depends on the accuracy of tissue metrics as an injury predictor. Existing scaling methods have translated animal data to humans by determining equivalent biomechanical impact conditions that result in similar tissue-level responses for different species,23,30 which the fundamental assumption that comparable tissue responses result in comparable clinical outcomes. To our knowledge, the validity of this “tissue-level equivalence” assumption has never been tested.
The objective of this study was to evaluate the efficacy of tissue-level brain injury metrics for predicting TBI outcome using a compiled injury database of human, non-human primate (NHP), and pig TBI data. Tissue-level response associated with the TBI data were predicted using species-specific simulations with advanced brain FEMs of the human, pig, macaque, and baboon constructed with similar modeling techniques. Injury metrics measured at similar injury severities were compared between different groups of species to examine the fundamental tissue-level equivalence assumption underlying interspecies integration of injury data. The findings from these analyses would provide guidance for developing kinematics-based injury criteria and give insight into the TBI mechanisms.
Methods
Injury database
This work focused on simulating head impact conditions associated with either no injury or diffuse TBI and excluded those injuries that are focal in nature (e.g., skull fracture, contusion). Applying this criterion, an injury dataset (n = 223) consisting of sub-injurious volunteer tests (n = 50), laboratory reconstruction data of professional football impacts (n = 53), non-human primate (NHP) tests (n = 78), and pig TBI experiments (n = 42) was established. The degrees of injury severity in the dataset were classified for this study as “no injury,” “mild TBI (mTBI),” and “severe TBI (sTBI).” A concussion was considered mTBI, while sTBI cases included diffuse axonal injury (DAI) and some intracerebral hemorrhage cases, which are believed to have the same shearing mechanism that underlies DAI.31,32 The diagnosis of injury for individual data was adopted from the corresponding data sources, as briefly introduced below:
-
1.
An existing database of 31 National Football League (NFL) head impact reconstruction cases that included 6 degrees-of-freedom (DOF) head kinematic data from 58 players was originally published by Pellman and colleagues21 and re-evaluated by Sanchez and colleagues.22 Of this data, 53 head kinematics were considered valid reconstructed head impacts and 20 of these cases were from players diagnosed with concussion and labeled as mTBI.
-
2.
An existing database of human volunteer tests from the Naval Biodynamics Laboratory (NBDL) that included 6 DOF head kinematics data involving 22 subjects from 335 sled tests (frontal, lateral, and oblique) up to 16 Gs peak sled acceleration was collected.33,34 Only 50 cases of the most severe runs were used in the current study, and all cases were non-injurious.
-
3.
An injury database consisting of multiple NHP datasets performed by different groups from the 1960s to the 1980s was collected. The first dataset came from the University of Pennsylvania using controlled, non-impact rotational accelerations to the head of more than 100 specimens of different NHP species.2,35 From this dataset, 56 acceleration traces (from axial and coronal loading conditions) were collected and corrected,36 and pathology results from these cases revealed that all 56 tests produced sTBI (DAI). The second dataset came from the Japan Automobile Research Institute using three apparatuses and a variety of loading conditions were utilized to deliver a total of 193 head impacts to the frontal, lateral or occipital part of the head of 89 specimens (mostly macaques).37 Most of the subjects were repeatedly impacted several times before autopsy, which resulted in the ambiguities of the injury diagnosis. For that reason, only five macaque tests which did not have injury from single or repeated lateral impact conditions were used for the current study. The last dataset came from the University of Michigan using a pneumatic impacting device to deliver padded and rigid impacts on the heads of two species of macaque.38,39 From this dataset, there were 17 macaque tests from lateral and occipital impact conditions that resulted in injury outcomes that were consistent with diffuse TBI and categorized for this study as having no injury (n = 4), mTBI (n = 8), or sTBI (n = 5).
-
4.
A dataset consisting of 26 four-week-old and 16 two-month-old pig TBI tests conducted using a rapid non-impact head rotation device25 was collected. All the subjects received a single head rotation in the axial (n = 19) or sagittal plane (n = 23). The tests produced clinical outcomes from no injury (n = 13) to mTBI (n = 29), as identified in post-mortem neuropathological examination.16,18
The resultant head peak rotational kinematics sustained by the subjects in the compiled injury database covers a broad range of acceleration and velocity magnitudes for both the human and animal (Fig. 1).
FIG. 1.
Distribution of peak resultant angular velocities and angular accelerations in the injury database containing no injury, mild TBI (mTBI), and severe TBI (sTBI) data. (Left) human data; (right) Animal data. Color image is available online.
Harmonized species-specific finite element simulations
Four species of brain FEMs were used in this study (Fig. 2), including human,40 pig,16 rhesus macaque,29 and baboon.29 All FEMs were harmonized in their modeling approach and formulation of the visco-hyperelastic constitutive model used for brain tissue.41 The parameters used to define the material response for each model were established independently using the best estimates for each species and were not adjusted for the purposes of this study. The human brain material models were developed using human tissue data42 and validated with brain deformation data from in situ experimental studies.43–46 The NHP models were modeled using the same material coefficients as the human model given the mechanical similarity between human and NHP brain material properties.47,48 The pig brain material properties were developed using pig brain tissue data49 and validated with brain deformations measured in ex vivo hemi-section experiments.14,30 All brain models were constructed using the embedded axon technique.40 All the FE simulations were performed using LS-DYNA (v971 R9.2.0, double precision; LSTC).
FIG. 2.
Brain finite element models used in this study. They were modeled with similar anatomical details and the same hyper-viscoelastic constitutive model for brain tissue. Color image is available online.
The head impacts were simulated by applying the experimentally measured 6 DOF head kinematics of each species directly to the rigid dura of the corresponding species FEM at an origin located at the head center of gravity, or at the experimental center of rotation, depending on the source of the data. The NHP and pig simulations were performed using FEMs scaled in geometry to match with the individual specimen's brain mass documented in the experiments. Individual-specific information for the size of the brain was not available for the human simulations, and the model represented the anatomy of a 50th percentile male adult.
Tissue-level injury metrics
A range of tissue-level injury metrics widely used in the literature to quantify tissue-level brain responses were considered (Table 1). For each simulated case, strain, strain-rate (based on the first-order discrete derivative of strain), and the product of strain and strain-rate was calculated for each element of the brain at every instance in time. The strain measurement was different depending on the element type: maximum principal strain (MPS) was used for the solid elements of the whole brain (including cerebellum, cerebrum, corpus callosum, and brain stem), and tensile strain (maximum axonal strain, [MAS]) was used for the embedded axon tract elements. At the end of the simulation, each element was assigned the values of the maximum strain (MPS and MAS), strain-rate (MPSR and MASR), and strain × strain-rate (MPS × SR and MAS × SR) encountered during the simulation. Injury metrics for the whole brain were calculated from these element-wise results, using the 95th percentile values to avoid any potential complications that may occur with localized numerical instabilities.50 Finally, the cumulative strain damage measure (CSDM), which represents the fraction of the brain that exceeds a specified MPS threshold, was calculated for each case using two commonly used thresholds (15% and 25%). All strains reported in this study were using the true strain measurement.
Table 1.
Tissue-Level Injury Metrics Assessed in This Study
 |
The suffix 95 is used to denote the 95th percentile value was used, or the specified MPS threshold for CSDM.
The current state-of-the-art in FEM analysis for TBI risk assessment uses strain-based deformation metrics for injury prediction.51 However, an additional analysis was performed using metrics based on the 95th percentiles of maximum von Mises stress and maximum axonal stress generated from the simulated impact cases in this study. All stresses reported in this ancillary analysis were using truss stress measurements, and the results of the analysis are provided in Supplementary Figures 1–5.
Statistical analysis
Correlation between injury metrics (Table 1) and injury outcomes was evaluated using a two-fold process:
Intraspecies analysis: The evaluation was performed separately in the group of human, NHP, and pig data. This analysis was limited to combinations of species and injury type where there was sufficient data to generate injury risk functions (IRFs). The human and pig data was used to evaluate the ability of the metrics to distinguish between no injury and mTBI, while the NHP data was used to evaluate their ability to discriminate sTBI and not sTBI (includes no injury and mTBI).
Interspecies analysis: The evaluation was then performed in the compiled database of human, NHP, and pig data, assuming the tissue-level metrics are equivalent across the TBI simulations of different species. Tissue-level injury metrics measured at similar injury severity (mTBI) were compared between different groups of species to verify the assumption.
Statistical models correlating an injury metric to the risk of injury were developed using parametric survival analysis with a Weibull distribution.52 A survival analysis approach was chosen because the injury metrics generated by the simulations are considered doubly censored, and a Weibull IRF (Equation 1) was selected because it natively achieves a zero-risk response for zero load.
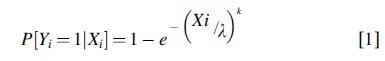
where Xi and Yi represents the injury metric and injury outcome (1 for injury, 0 for no injury) for the ith case, and the parameters l > 0 and k > 0, referred to as the scale and shape parameters, are estimated from the X, Y data using Maximum Likelihood Estimation.
Akaike information criterion (AIC)53 was used to estimate the quality of each model/metric. A lower AIC value indicates a better fit to the data. The weight of evidence in favor of the good models was evaluated using Akaike weights.

where K is the number of investigated models/metrics. With the sum of Akaike weights of all models in the candidate set being 1, a model's Akaike weight is analogous to the probability that the given model is the best approximating model.54 We identified the IRFs that constitute a 95% confidence set by summing the Akaike weights from largest to smallest until the sum is just 0.95.55
All survival models were assessed using the Hosmer-Lemeshow goodness of fit test56 with 10 bins determined by decile. A p value below alpha = 0.05 indicated that the null hypothesis is rejected, and that the IRF was not a good fit. The area under the receiver operating characteristic curve (AUC) was used to assess the discrimination capacity of the model/metric to separate individuals with different injury outcomes. A model with an AUC above 0.9 was considered to have outstanding discrimination.56 All the statistical analyses mentioned above were conducted using the R-Studio (version 1.1.456; R Studio, Inc.) and Matlab (version R2019b; Mathworks).
Results
Intraspecies analysis
The survival analysis related continuous injury metrics and binary injury results for human, pig, and NHP databases via IRFs, respectively (Table 2, and Supplementary Fig. S1 and S2). Most models and metrics had excellent to outstanding discrimination according to the AUC values, and the IRFs of all the metrics were regarded as a good fit, except for mTBI risk in humans using CSDM25, and sTBI risk in NHP using MASR95. With the human and pig IRFs, metrics that included strain-rate (MPSR95, MPS × SR95, MASR, and MAS × SR95) were the best predictors of injury outcome, and within the 95% confidence set. MPS95 was also within the 95% confidence set for the human mTBI risk model. This contrasts the NHP IRFs, which showed strain-rate metrics as the worst predictors of injury outcome, and strain-based metrics (MPS95, MAS95, CSDM15, and CSDM25) were within the 95% confidence set. This observation may be attributed to the differences in TBI severity between these injury databases: Human and pig databases included head kinematics that produced no axonal injury and mTBI data, while the majority of the NHP data were sTBI.
Table 2.
Statistical Test Results for Survival Analysis Models using Different Metrics and Different Databases
 |
mTBI, mild traumatic brain injury; sTBI, severe traumatic brain injury; AIC, Akaike information criterion; AUC, area under the receiver operating characteristic curve; MPS, maximum principal strain CSDM, cumulative strain damage; MAS, maximum axonal strain; MPSR, rate of the maximum principal strain; SR, strain-rate. Color table is available online.
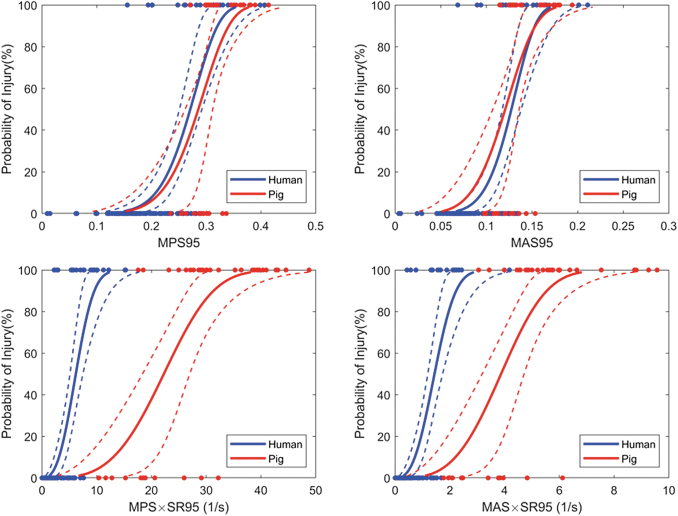
Although the mTBI risk models suggest strain-rate based metrics were better predictors of injury, we observed that risk models derived independently from human and pig data using strain-based metrics were in excellent agreement (Fig. 3). The 50% risk of mTBI using the MPS95 and MAS95 injury metrics was 27.1% and 12.7% from the human data, and 28.6% and 12.1% from the pig data, respectively. However, when comparing the mTBI risk functions predicted using strain-rate based metrics, substantial differences between the human and pig responses were identified (Fig. 3). This discrepancy was likely due to the differences in the loading rates needed to cause injury: the human mTBI data had angular velocities up to 70 rad/s whereas the pig mTBI data had angular velocities up to 200 rad/sec. As a result, the predicted maximum strain-rates between these species did not overlap (MPSR95: 0.5 – 65 for human and 77 – 235 for pig).
FIG. 3.
Comparison of injury risk functions for mild TBI developed independently using injury metrics predicted by finite element models and human and pig injury data. The solid lines represent the risk function estimate, and the dashed lines represent the 95% percentile confidence interval. Color image is available online.
Interspecies analysis
Our goal was to combine datasets across species and determine robust, unifying injury predictors. The survival analysis was conducted between continuous metrics and binary injury results from combining the human, pig, and NHP databases, respectively (Table 3 and Supplementary Fig. S3). All models and metrics had quasi-perfect separation (AUC >0.95), and most of the IRFs were regarded as a good fit for the injury data, except when using the CSDM metrics (for both mTBI and sTBI), the MASxSR95 metric for mTBI, and the MPS95 and MASR95 metrics for sTBI. The best injury predictor that emerged for distinguishing mild and/or severe TBI risk across multiple species was the axonal strain metric MAS95. The MPSxSR95 metric was also a strong predictor for both mild and severe TBI risk, despite the disparate ranges of strain-rates among the species (MPSR95: 29 – 447 for NHP).
Table 3.
Statistical Test Results for Survival Analysis Models using Different Metrics and Combined Database
 |
mTBI, mild traumatic brain injury; sTBI, severe traumatic brain injury; AIC, Akaike information criterion; AUC, area under the receiver operating characteristic curve; MPS, maximum principal strain; CSDM, MAS, maximum axonal strain; MPSR, rate of the maximum principal strain; SR, strain-rate. Color table is available online.
The 50% risk of mTBI using the MPS95 injury metric was 27.0% (± 1.5%, with 95% confidence) and using MAS95 was 12.3% (± 0.7%, with 95% confidence). The 50% risk of sTBI using the MPS95 injury metric was 40.0% (± 2.1%, with 95% confidence) and using MAS95 was 19.6% (± 1.2%, with 95% confidence), which was higher than the corresponding risk and injury metrics from the NHP sTBI risk model (32.0% and 16.7%, respectively). The risk functions for mTBI and sTBI were separated such that a high likelihood of mTBI corresponded to a low likelihood of sTBI (Fig. 4). For instance, an MPS95 value of 33% corresponds to a 90% risk of mTBI and a 10% risk of sTBI. In general, the 90%/10% overlap in transition from mild risk to severe risk was consistent for all injury metrics except the CSDM15 and CSDM25.
FIG. 4.
Comparison of mild and severe traumatic brain injury (mTBI; sTBI) risk functions developed from a combined dataset human and animal injury response using injury metrics predicted by FE models. The solid lines represent the risk function estimate, and the dashed lines represent the 95% percentile confidence interval. Black dots are non-injury data points, blue dots are injury points for the mTBI risk function and non-injury data points for the sTBI, and red dots are injury data points for both mTBI and sTBI risk functions. Color image is available online.
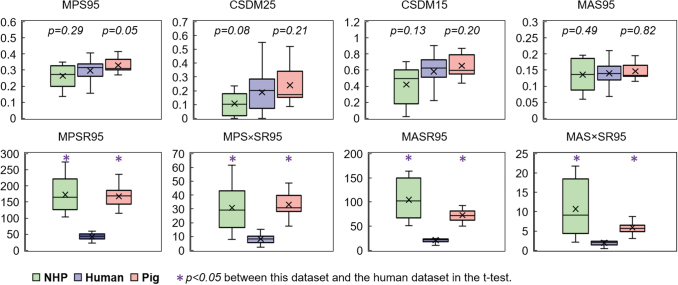
A critical assumption in integrating animal and human TBI data was that comparable strain metrics result in an equal clinical outcome. Because both the animal and human datasets had several mTBI cases, the values of the metrics from the human (n = 20), pig (n = 29), and NHP (n = 8) mTBI cases were analyzed for differences using an independent two-tailed t-test (Fig. 5). The averages of the strain-rate related metrics in the animal cases were significantly different from those of the human cases, while the strain-based metrics were more consistent across species. The significant discrepancy in the strain-rate related metrics might indicate that the tissue-level strain-rate injury thresholds are different across species and explain the poor performance of these metrics in the statistical tests for the complied database.
FIG. 5.
Distribution of metric values for mild traumatic brain injury cases in the human and animal dataset. For each box, the central band is the median, and the central “cross” marker is the mean, the box extends vertically between the 25th and 75th percentiles, the whiskers extend to the most extreme data that are not considered outliers. Color image is available online.
Discussion
This study is the first to evaluate tissue-level injury metrics with FE models using injury data from multiple sources and multiple species to assess the correlation between injury metrics and injury outcomes. The findings of this study partially confirm the hypothesis that deformation is the biomechanical mechanism of brain injury, as most of the deformation-related tissue-level metrics show good fit for the data and a capability of distinguishing between injury severities (AUC >0.9). While the independent analysis of different datasets leads to different optimal injury metrics, the combined multi-species, multi-severity analysis revealed that maximum axonal strain (MAS95) was the best metric for distinguishing mild and/or severe TBI. An alternative strain-based metric, maximum principal strain (MPS95), was also a strong predictor of injury across species, particularly for mild TBI.
Potential bias in the database
While we scrutinized the data in the existing literature to assemble a comprehensive, multi-species, multi-severity database of diffuse-type TBI, there are always challenges in combining data from different sources, studies, and eras. The non-injurious human volunteer tests from the NBDL were conducted when mTBI was defined by a loss of consciousness. While a few cases in this dataset reported transient headaches not considered clinically significant at the time,34 it cannot be completely certain that these cases would be considered non-injurious using the current state-of-knowledge in diagnostics. The diagnoses for the injured cases in the professional football dataset were more consistent with the contemporary definition of sports mTBI.21 However, the fidelity of the laboratory reconstruction process, and the biofidelity of the dummy, provide some level of uncertainty into the kinematics derived in this dataset.22 The severity of the brain injury in the pig and the NHP tests were identified by pathology and autopsy, only revealing more severe structural abnormalities, rather than subtle, transient functional alterations. For the NHP tests, the invasive methods adopted to measure head kinematics also brought up ambiguities in injury diagnosis. Future studies to acquire and diagnose TBI injury data in a consistent manner from both human and animal subjects would improve the findings made in this work.
Tissue-level injury metrics
The current study found strong evidence in favor of using axonal strain in predicting injury for all species and severities assessed. It has been suggested that tract-oriented strain14 or axonal strain11,13 is a better predictor of injury in other studies. Although we recommend using MAS95 at the predictor of injury based on the statistical analysis of this study, it was often only marginally better than MPS95 (the most significant differences were observed in the pig database). Almost all existing FE brain models can calculate the brains MPS95 from an impact, where only some models contain tractography information at the level of detailed established using the embedded axon method.40 Considering that most modern kinematics-based TBI metrics were based on correlations with the principal strain responses,3–5 MPS95 should still be a popular choice of tissue-level injury metric that balances the simplicity and performance. We also observed that the commonly used CSDM metrics were poor predictors of injury for most species and severities and should not be considered in future injury analysis.
From a biomechanical perspective, neuronal tissue is a viscoelastic material,57 and it is reasonable to hypothesize that neuronal injury response is dependent on both the magnitude and rate of applied strain. Therefore, strain-rate and the product of the strain and strain-rate were proposed as injury metrics in the literature.14 The current analysis indicates these metrics might correlate well with injury in relatively homogeneous cases where the range of the strain-rates is small. However, across species these metrics varied by orders of magnitude for brains that sustained an injury of similar severity (Fig. 5). Thus, we cannot recommend a species-independent strain-rate metric for predicting TBI risk. However, these metrics may be appropriate for intraspecies analysis.
The interspecies analysis also confirmed an important tissue-level equivalence assumption: equal tissue-level deformation would cause TBI in both animal and human, which was never formally tested while being heavily relied on for interspecies scaling. This study is the first to investigate this assumption in a combined multi-species analysis. We acknowledge these findings were based on datasets with small sample size, and limitations exist within potentially inconsistent diagnostic methods across different species. Additionally, this assumption was only verified for macaque, baboon, and pig; whether it applies to other animal species (e.g., rodents, ferrets) requires further investigation. The cellular composition of the brain has substantial variability across mammalian species. With an average of 86 billion neurons and 85 billion non-neuronal cells, the human brain has the same overall 1:1 non-neuronal/neuronal ratio as other generic primates, while the pig brain has an overall 2:1 nonneuronal/neuronal ratio.58 Apart from the consideration of cellular composition across species, other neuroanatomical differences between animals and humans (e.g., the ratio of gray and white matter, the proportions of the cerebrum in the brain) would likely influence the tissue-level equivalence as only global measures of the injury metrics were considered.
The current state-of-the-art in FEM analysis for TBI risk assessment uses strain-based deformation metrics for injury prediction.51 Strain-based tissue-level assessments are the most relevant for examining neurotrauma response across multiple species or in vitro models because they are measurements that are independent of neuronal density. Stress-based metrics have been correlated to injury in previous investigations using human models and data but were generally less predictive than strain-based metrics.13,59 In this study, we have demonstrated in an ancillary analysis that stress-based metrics were not consistent across species and did not satisfy the tissue-level equivalence assumption. This result strengthens the field's current focus on strain-based tissue-level deformation metrics as the predictor of injury.
Harmonization of different models
This work relies on the application of harmonized computational models to evaluate the capability of tissue-level metrics for predicting injury. It should be re-emphasized that the harmonization of these models only refers to the numerical methods used in developing these biomechanical models, and not a harmonization of material properties. In this study, all models were developed and validated independently, and were used in this study without deviating from their originally published material properties. This is an efficient technique for combining and comparing interspecies injury data to overcome the main caveat of scaling methods.28,29
Other limitations
Unfortunately, the human and pig FE model used in this study were only partially validated, and data for validating NHP brain FE models were unavailable. Improving the fidelity of the FE models is an ongoing process that progresses with new advances in in vivo and in situ experimental studies on brain biomechanics.45,60 Further, tissue-level metrics used in this study were based on global measures of brain deformation. Increasing the specificity of the analysis by introducing region-specific strain metrics61,62 may eventually improve correlation with injury outcome, but would also introduce new challenges for interpreting those tissue-level metrics across species.
Conclusion
This work leveraged advanced computational models to evaluate several tissue-level injury metrics as predictors of TBI using human and animal injury data. The correlation between TBI risk and injury metrics were statistically significant, but when injury data was analyzed by species, the choices of optimal tissue-level metrics was inconclusive and limited by the small datasets. When the human and animal datasets were combined, the injury analysis was able to delineate maximum axonal strain as the best predictor of injury for all species and TBI severities, with maximum principal strain as a suitable alternative metric. Although similar tissue thresholds were found for human and pig in previous studies,11,13,16,17 the current study is the first to provide evidence to support the assumption that strain metrics between human, pig, and NHP result in similar injury outcomes. This assumption is the biomechanical foundation for translating animal brain injury findings to humans. Inclusion of the strain-rate effect on tissue-level injury metric marginally improved the goodness-of-fit and prediction performance for the human and pig data separately, but these metrics were poorly correlated in the combined multi-species dataset. This study increases our understanding of the tissue-level mechanisms related TBI and will enable the development of more effective injury mitigation strategies to help reduce the incidence, consequences, and societal burden of TBI.
Supplementary Material
Acknowledgments
The research presented in this paper was made possible in part by a grant from Football Research, Inc. (FRI), and NIH R01NS097549. The views expressed are solely those of the authors and do not represent those of FRI, NIH, or any of its affiliates or funding sources.
Funding Information
The research presented in this paper was made possible in part by a grant from Football Research, Inc. (FRI), and NIH R01NS097549.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Centers for Disease Control and Prevention. (2015). Report to congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pdf/tbi_report_to_congress_epi_and_rehab-a.pdf (Last accessed February1, 2021) [DOI] [PubMed]
- 2. Margulies, S.S., and Thibault, L.E. (1992). A proposed tolerance criterion for diffuse axonal injury in man. J. Biomech. 25, 917–923 [DOI] [PubMed] [Google Scholar]
- 3. Takhounts, E.G., Craig, M.J., Moorhouse, K., McFadden, J., and Hasija, V. (2013). Development of brain injury criteria (BrIC). Stapp Car Crash J. 57, 243–266 [DOI] [PubMed] [Google Scholar]
- 4. Gabler, L.F., Crandall, J.R., and Panzer, M.B. (2018). Development of a metric for predicting brain strain responses using head kinematics. Ann. Biomed. Eng. 46, 972–985 [DOI] [PubMed] [Google Scholar]
- 5. Gabler, L.F., Crandall, J.R., and Panzer, M.B. (2019). Development of a second-order system for rapid estimation of maximum brain strain. Ann. Biomed. Eng. 47, 1971–1981 [DOI] [PubMed] [Google Scholar]
- 6. Bain, A.C., and Meaney, D.F. (2000). Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng., 122, 615–622 [DOI] [PubMed] [Google Scholar]
- 7. Cater, H.L., Sundstrom, L.E., and Morrison III, B. (2006). Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J. Biomech. 39, 2810–2818 [DOI] [PubMed] [Google Scholar]
- 8. Elkin, B.S. and Morrison III, B. (2007). Region-specific tolerance criteria for the living brain. Stapp Car Crash J. 51. [DOI] [PubMed] [Google Scholar]
- 9. Nakadate, H., Kurtoglu, E., Furukawa, H., Oikawa, S., Aomura, S., Kakuta, A., and Matsui, Y. (2017). Strain-rate dependency of axonal tolerance for uniaxial stretching. Stapp Car Crash J. 61, 53. [DOI] [PubMed] [Google Scholar]
- 10. Chierto, E., Simon, A., Castoldi, F., Meffre, D., Cristinziano, G., Sapone, F., Alex Carrete, A., Borderie, D., Etienne, F., Rannou, F., Morrison 3rd,, B., Massaad, C., and Morrison, B. (2019). Mechanical stretch of high magnitude provokes axonal injury, elongation of paranodal junctions, and signaling alterations in oligodendrocytes. Mol. Neurobiol. 56, 4231–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giordano, C., and Kleiven, S. (2014). Evaluation of axonal strain as a predictor for mild traumatic brain injuries using finite element modeling. Stapp Car Crash J. 58, 29. [DOI] [PubMed] [Google Scholar]
- 12. Ji, S., Zhao, W., Ford, J.C., Beckwith, J.G., Bolander, R.P., Greenwald, R.M., Flashman, L.A., Paulsen, K.D., and McAllister, T.W. (2015). Group-wise evaluation and comparison of white matter fiber strain and maximum principal strain in sports-related concussion. J. Neurotrauma, 32, 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahoo, D., Deck, C., and Willinger, R. (2016). Brain injury tolerance limit based on computation of axonal strain. Accid. Anal. Prev. 92, 53–70 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan, S., Eucker, S.A., Gabrieli, D., Bradfield, C., Coats, B., Maltese, M.R., Lee, J., Smith, C., and Margulies, S.S. (2015). White matter tract-oriented deformation predicts traumatic axonal brain injury and reveals rotational direction-specific vulnerabilities. Biomech. Model. Mechanobiol. 14, 877–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghajari, M., Hellyer, P.J., and Sharp, D.J. (2017). Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain, 140(2), 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hajiaghamemar, M., Wu, T., Panzer, M.B., and Margulies, S.S. (2020). Embedded axonal fiber tracts improve finite element model predictions of traumatic brain injury. Biomech. Model. Mechanobiol. 19, 1109–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hajiaghamemar, M. and Margulies, S. (2020). Multi-scale white matter tract embedded brain finite element mode predicts the location of traumatic diffuse axonal injury. Journal of Neurotrauma 38, 144–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hajiaghamemar, M., Seidi, M., and Margulies, S.S. (2020). Head rotational kinematics, tissue deformations, and their relationships to the acute traumatic axonal injury. J. Biomechan. Eng. 142, 0310061-03100613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rowson, S., Duma, S.M., Beckwith, J.G., Chu, J.J., Greenwald, R.M., Crisco, J.J., Gunnar Brolinson, P, Duhaime, A.C., McAllister, T.W., and Maerlender, A.C. (2012). Rotational head kinematics in football impacts: an injury risk function for concussion. Ann. Biomed. Eng. 40, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rowson, S. and Duma, S.M. (2013). Brain injury prediction: assessing the combined probability of concussion using linear and rotational head acceleration. Ann. Biomed. Eng. 41, 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pellman, E.J., Viano, D.C., Tucker, A.M., Casson, I.R., and Waeckerle, J.F. (2003). Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery 53, 799–814 [DOI] [PubMed] [Google Scholar]
- 22. Sanchez, E.J., Gabler, L.F., Good, A.B., Funk, J.R., Crandall, J.R., and Panzer, M.B. (2019). A reanalysis of football impact reconstructions for head kinematics and finite element modeling. Clin. Biomech. 64, 82–89 [DOI] [PubMed] [Google Scholar]
- 23. Ommaya, A.K. and Hirsch, A.E. (1971). Tolerances for cerebral concussion from head impact and whiplash in primates. J. Biomech. 4, 13–21 [DOI] [PubMed] [Google Scholar]
- 24. Gennarelli, T.A., Thibault, L.E., Adams, J.H., Graham, D.I., Thompson, C.J., and Marcincin, R.P. (1982). Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 12, 564–574 [DOI] [PubMed] [Google Scholar]
- 25. Margulies, S.S., Kilbaugh, T., Sullivan, S., Smith, C., Propert, K., Byro, M., Saliga, K., Costine, B.A., and Duhaime, A.C. (2015). Establishing a clinically relevant large animal model platform for TBI therapy development: using Cyclosporin a as a case study. Brain Pathol. 25, 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cullen, D.K., Harris, J.P., Browne, K.D., Wolf, J.A., Duda, J.E., Meaney, D.F., Margulies, S.S., and Smith, D.H. (2016). A porcine model of traumatic brain injury via head rotational acceleration Methods Mol. Biol. 1462, 289–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margulies, S.S. and Thibault, L.E. (1989). An analytical model of traumatic diffuse brain injury. J. Biomech. Eng. 111, 241. [DOI] [PubMed] [Google Scholar]
- 28. Panzer, M.B., Wood, G.W., and Bass, C.R. (2014). Scaling in neurotrauma: how do we apply animal experiments to people? Exp. Neurol. 261, 120–126 [DOI] [PubMed] [Google Scholar]
- 29. Wu, T., Antona-Makoshi, J., Alshareef, A., Giudice, J.S., and Panzer, M.B. (2020). Investigation of cross-species scaling methods for traumatic brain injury using finite element analysis. J. Neurotrauma 37, 410–422 [DOI] [PubMed] [Google Scholar]
- 30. Ibrahim, N.G., Ralston, J., Smith, C., and Margulies, S.S. (2010). Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma 27, 1021–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mata-Mbemba, D., Mugikura, S., Nakagawa, A., Murata, T., Ishii, K., Kushimoto, S., Tominaga, T., Takahashi, S., and Takase, K. (2018). Traumatic midline subarachnoid hemorrhage on initial computed tomography as a marker of severe diffuse axonal injury. J. Neurosurg. 129, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 32. Mata-Mbemba, D., Mugikura, S., Nakagawa, A., Murata, T., Kato, Y., Tatewaki, Y., Li, L., Takase, K., Ishii, K., Kushimoto, S., Tominaga, T., and Tominaga, T. (2015). Intraventricular hemorrhage on initial computed tomography as marker of diffuse axonal injury after traumatic brain injury. J. Neurotrauma 32, 359–365 [DOI] [PubMed] [Google Scholar]
- 33. Ewing, C.L. and Thomas, D.J. (1972). Human head and neck response to impact acceleration, NAMRL monograph, 21. Naval Aerospace Medical Research Laboratory, Naval Aerospace Medical Institute, Naval Aerospace and Regional Medical Center: Pensacola, FL [Google Scholar]
- 34. Sanchez, E.J., Gabler, L.F., McGhee, J.S., Olszko, A.V., Chancey, V.C., Crandall, J.R., and Panzer, M.B. (2017). Evaluation of head and brain injury risk functions using sub-injurious human volunteer data. J. Neurotrauma 34, 2410–2424 [DOI] [PubMed] [Google Scholar]
- 35. Gennarelli, T.A., Thibault, L.E., Tomei, G., Wiser, R., Graham, D., and Adams, J. (1987). Directional dependence of axonal brain injury due to centroidal and non-centroidal acceleration. SAE Technical Paper 872197 [Google Scholar]
- 36. Mendis, K. (1992). Finite element modeling of the brain to establish diffuse axonal injury criteria [PhD dissertation], The Ohio State University [Google Scholar]
- 37. Kikuchi, A. (1982). Human head tolerance to lateral impact deduced from experimental head injuries using primates. SAE Technical Paper 801303 [Google Scholar]
- 38. Stalnaker, R., Roberts, V., and McElhaney, J.H. (1973). Side impact tolerance to blunt trauma. SAE Technical Paper 730979, [Google Scholar]
- 39. Nusholtz, G.S., Kaiker, P.S., and Lehman, R.J. (1986). Critical limitations on significant ractors in head injury research. SAE Technical Paper 861890 [Google Scholar]
- 40. Wu, T., Alshareef, A., Giudice, J.S., and Panzer, M.B. (2019). Explicit modeling of white matter axonal fiber tracts in a finite element brain model. Ann. Biomed. Eng. 47, 1908–1922 [DOI] [PubMed] [Google Scholar]
- 41. Gasser, T.C., Ogden, R.W., and Holzapfel, G.A. (2006). Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface, 3, 15–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin, X., Zhu, F., Mao, H., Shen, M., and Yang, K.H. (2013). A comprehensive experimental study on material properties of human brain tissue. Journal of biomechanics, 46, 2795–2801 [DOI] [PubMed] [Google Scholar]
- 43. Hardy, W.N., Foster, C.D., Mason, M.J., Yang, K.H., King, A.I., and Tashman, S. (2001). Investigation of head injury mechanisms using neutral density technology and high-speed biplanar X-ray. Stapp Car Crash J. 45, 2001-P-375. [DOI] [PubMed] [Google Scholar]
- 44. Hardy, W.N., Mason, M.J., Foster, C.D., Shah, C.S., Kopacz, J.M., Yang, K.H., King, A.I., Bishop, J., Bey, M., Anderst, W., and Tashman, S. (2007). A study of the response of the human cadaver head to impact. Stapp car Crash J. 51, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alshareef, A., Giudice, J.S., Forman, J., Salzar, R.S., and Panzer, M.B. (2018). A novel method for quantifying human in situ whole brain deformation under rotational loading using sonomicrometry. J. Neurotrauma, 35, 780–789 [DOI] [PubMed] [Google Scholar]
- 46. Alshareef, A., Giudice, J.S., Forman, J., Shedd, D.F., Reynier, K.A., Wu, T., Sochor, S., Sochor, M.R., Salzar, R.S., and Panzer, M.B. (2020). Biomechanics of the human brain during dynamic rotation of the head. J. Neurotrauma 37, 1546–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Estes, M.S. and McElhaney, J.H. (1970). Response of brain tissue to compressive loading, in: Mechanical Engineering. American Society of Mechanical Engineering: New York, NY, p. 58 [Google Scholar]
- 48. McElhaney, J.H., Melvin, J.W., Roberts, V.L., and Portnoy, H.D. (1973). Dynamic characteristics of the tissues of the head, in Perspectives in Biomedical Engineering. Palgrave Macmillan, London, U.K., pps. 215–222 [Google Scholar]
- 49. Rashid, B., Destrade, M., and Gilchrist, M.D. (2014). Mechanical characterization of brain tissue in tension at dynamic strain rates. J. Mech. Behav. Biomed. Mater. 33, 43–54 [DOI] [PubMed] [Google Scholar]
- 50. Panzer, M.B., Myers, B.S., Capehart, B.P., and Bass, C.R. (2012). Development of a finite element model for blast brain injury and the effects of CSF cavitation. Ann. Biomed. Eng. 40, 1530–1544 [DOI] [PubMed] [Google Scholar]
- 51. Horstemeyer, M.F., Panzer, M.B., and Prabhu, R.K. (2019). State-of-the-art modeling and simulation of the brain's response to mechanical loads. Ann. Biomed. Eng., 47, 1829. [DOI] [PubMed] [Google Scholar]
- 52. McMurry, T.L. and Poplin, G.S. (2015). Statistical considerations in the development of injury risk functions. Traffic Inj. Prev. 16, 618–626 [DOI] [PubMed] [Google Scholar]
- 53. Akaike, H. (1974). A new look at the statistical model identification. IEEE transactions on automatic control, 19, 716–723 [Google Scholar]
- 54. Symonds, M.R. and Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21 [Google Scholar]
- 55. Posada, D. and Buckley, T.R. (2004). Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic biology, 53, 793–808 [DOI] [PubMed] [Google Scholar]
- 56. Hosmer Jr, D.W., Lemeshow, S., and Sturdivant, R.X. (2013). Applied Logistic Regression. John Wiley and Sons: Hoboken, NJ [Google Scholar]
- 57. Chatelin, S., Constantinesco, A., and Willinger, R. (2010). Fifty years of brain tissue mechanical testing: from in vitro to in vivo investigations. Biorheology 47, 255–276 [DOI] [PubMed] [Google Scholar]
- 58. Herculano-Houzel, S. and Dos Santos, S.E. (2018). You do not mess with the glia. Neuroglia, 1, 193–219 [Google Scholar]
- 59. Kleiven, S. (2007). Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash J. 51, 2007-P-401. [DOI] [PubMed] [Google Scholar]
- 60. Chan, D.D., Knutsen, A.K., Lu, Y.C., Yang, S.H., Magrath, E., Wang, W.T., Bayly, P.V., Butman, J.A., and Pham, D.L. (2018). Statistical characterization of human brain deformation during mild angular acceleration measured in vivo by tagged magnetic resonance imaging. J. Biomech. Eng. 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elkin, B.S., Gabler, L.F., Panzer, M.B., and Siegmund, G.P. (2019). Brain tissue strains vary with head impact location: a possible explanation for increased concussion risk in struck versus striking football players. Clin. Biomech. 64, 49–57 [DOI] [PubMed] [Google Scholar]
- 62. Wu, S., Zhao, W., Ghazi, K., and Ji, S. (2019). Convolutional neural network for efficient estimation of regional brain strains. Sci. Rep. 9, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.