Abstract
Background: Functional respiratory imaging (FRI) is a computational fluid dynamics-based technique using three-dimensional models of human lungs and formulation profiles to simulate aerosol deposition.
Methods: FRI was used to evaluate lung deposition of extrafine beclomethasone dipropionate (BDP)/formoterol fumarate (FF)/glycopyrronium bromide (GB) and extrafine BDP/FF delivered through pressurized metered dose inhalers and to compare results with reference gamma scintigraphy data. FRI combined high-resolution computed tomography scans of 20 patients with moderate-to-severe chronic obstructive pulmonary disease (mean forced expiratory volume in 1 second 42% predicted) with in silico computational flow simulations, and incorporated drug delivery parameters to calculate aerosol airway deposition. Inhalation was simulated using profiles obtained from real-life measurements.
Results: Total lung deposition (proportion deposited in intrathoracic region) was similarly high for both products, with mean ± standard deviation (SD) values of 31.0% ± 5.7% and 28.1% ± 5.2% (relative to nominal dose) for BDP/FF/GB and BDP/FF, respectively. Pairwise comparison of the deposition of BDP and FF gave a mean intrathoracic BDP/FF/GB:BDP/FF deposition ratio of 1.10 (p = 0.0405). Mean intrathoracic, central and peripheral deposition ratios for BDP were 1.09 (95% confidence interval [CI]: 1.05–1.14), 0.92 (95% CI: 0.89–0.96), and 1.20 (95% CI: 1.15–1.26), respectively, and for FF were 1.11 (95% CI: 1.07–1.15), 0.94 (95% CI: 0.91–0.98), and 1.21 (95% CI: 1.15–1.27), within the bioequivalence range (0.80–1.25) for intrathoracic and central regions, and slightly exceeding the upper boundary in the peripheral region. Mean ± SD central:peripheral deposition (C:P) was 0.48 ± 0.13 for BDP/FF/GB and 0.62 ± 0.17 for BDP/FF, indicating a higher proportion of drug deposition in the small airways than in the large airways.
Conclusion: FRI demonstrated similar deposition patterns for extrafine BDP/FF/GB and BDP/FF, with both having a high lung deposition. Moreover, the deposition patterns of BDP and FF were similar in both products. Furthermore, the C:P ratios of both products indicated a high peripheral deposition, supporting small airway targeting and delivery of these two extrafine fixed combinations, with a small difference in ratios potentially due to mass median aerodynamic diameters.
Keywords: combination drug, extrafine, functional respiratory imaging, inhaled corticosteroid, lung deposition, pressurized metered-dose inhaler
Introduction
Inhaled drug lung deposition is conventionally assessed using in vivo scintigraphy.(1) However, this involves complex methodology with product radiolabeling, and consequent exposure of patients to radiation during the procedure. Various alternatives use mathematical modeling to predict drug delivery and airways deposition.(2) Functional respiratory imaging (FRI) is such a technology, combining three-dimensional (3D) lung models (obtained from standard high-resolution computed tomography [HRCT] scans) with computational fluid dynamics (CFD). In contrast to scintigraphy, FRI allows modeling of patient-specific deposition in all peripheral airways without the need for patient recruitment, and does not necessarily result in the exposure of individuals to additional radiation since data from prior studies can be reused. This technique has been validated by De Backer et al. in a crossover study evaluating regional lung deposition by FRI and single-photon emission computed tomography (SPECT) in patients with asthma, in which there was excellent agreement between calculated FRI and measured SPECT.(3) Subsequent studies have confirmed the consistency between FRI and scintigraphy (Table 1).(4–16)
Table 1.
Comparison between Functional Respiratory Imaging and Scintigraphy
| Product | FRI (% LD) | Scintigraphy (% LD) |
|---|---|---|
| BDP/FF through pMDI (solution) in COPD | 28(4) | 31–34(7,8) |
| FP/FF through pMDI (suspension) in asthma | 42(9) | 41(5) |
| BUD/FF through DPI in asthma | 23(9) | 22(10) |
| BDP through pMDI (solution) in asthma | 54* | 53(11) |
Data on file.
BDP, beclomethasone dipropionate; BUD, budesonide; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FP, fluticasone propionate; FF, formoterol fumarate; FRI, functional respiratory imaging; LD, lung deposition; pMDI, pressurized metered-dose inhaler.
An extrafine formulation of a fixed-dose dual combination of the inhaled corticosteroid (ICS) beclomethasone dipropionate (BDP) and the long-acting β2-agonist formoterol fumarate (FF) has been available since 2006 for regular treatment of asthma, and since 2014 for symptomatic treatment of severe chronic obstructive pulmonary disease (COPD) as a pressurized metered-dose inhalation (pMDI; Foster®; Chiesi Farmaceutici SpA, Parma, Italy). In 2017, a fixed-dose triple combination of BDP, FF, and the long-acting muscarinic antagonist glycopyrronium bromide (GB; Trimbow®; Chiesi Farmaceutici SpA), in a similar extrafine pMDI formulation was approved for the maintenance treatment of COPD and is in development for severe asthma. Extrafine inhaled drugs are defined as those with particles having a mass median aerodynamic diameter (MMAD) <2 μm.(17) Inhaled drugs penetrate more deeply into the smaller peripheral airways as a consequence of the particle size, with greater deposition of smaller, extrafine particles than nonextrafine.(18) This may be a more optimal drug delivery pattern for inhaled medications, which would be beneficial in asthma and COPD since the peripheral airways are an important site of inflammation in both diseases.(19,20) Consequently, in the development of new therapeutics a key attribute is the potential to effectively target the small airways; this ability should be assessed through the use of discriminating assessment techniques.(21)
In a previous study, De Backer et al. assessed lung deposition of BDP/FF (100/6 μg) in patients with severe COPD using scintigraphy.(7) The average lung deposition of BDP/FF was 33.01 ± 8.9% (relative to nominal dose). From in vitro studies, the MMAD of BDP/FF/GB and BDP/FF is similar (BDP/FF/GB 1.1 μm; BDP/FF 1.3 μm). However, even small differences in MMAD influence deposition,(22) and it was therefore important to formally evaluate BDP/FF/GB deposition. Given the good consistency between FRI and scintigraphy,(4–16) we decided to use FRI for this evaluation.
Materials and Methods
FRI methodology is based on four building blocks: (1) patient-specific 3D airway geometry modeling; (2) inhaler characteristics; (3) inhalation profile; and (4) CFD simulations to model lung deposition.
Patients and 3D airway modeling
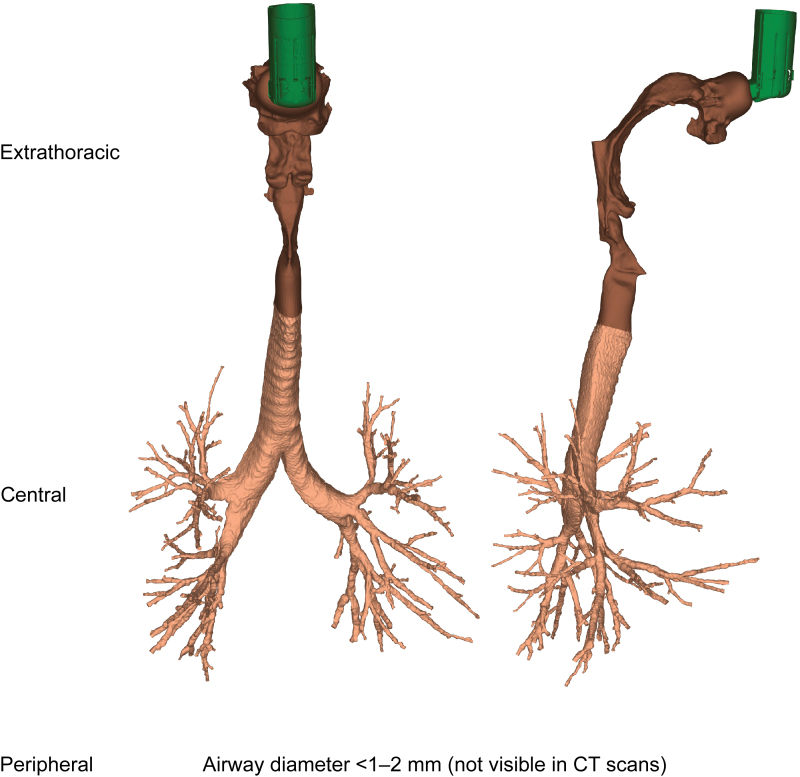
This study investigated respiratory aerosol drug delivery in 20 patients with COPD and moderate-to-severe airflow obstruction. Patient-specific volumetric, HRCT-based 3D lung models were used, providing insights on the structural and functional characteristics of the respiratory system of each patient. HRCT scans were acquired retrospectively; informed consent was obtained from each patient, with ethics approval by the Ethics Committee of Antwerp University Hospital, Belgium. For each patient a scan was taken during inspiration and during expiration. The inspiratory scan was used to segment and model patient-specific extrathoracic (upper) and intrathoracic (lower) airways until the intraluminal and alveolar airways could no longer be distinguished—that is, airways with a minimum diameter of 1–2 mm, corresponding to a HRCT scan voxel size of ∼0.5 mm3. Insights on airways further downstream in the peripheral region were retrieved through the lobar expansion induced by internal airflow distribution, by using expiratory and inspiratory scans to measure the change in lobar volume from expiration to inspiration. An example of a 3D airway model from a representative patient with COPD is shown in Figure 1, illustrating the extrathoracic region (mouth and throat), the central (large) airways, and the peripheral (small) airways of the respiratory tract. Inspiratory (total lung volume) and expiratory (functional residual capacity) scans were used to produce the lung lobe data (with lobar expansion assumed to be uniform), with scans taken at the end of inhalation/exhalation.
FIG. 1.
Patient-specific three-dimensional model of the extrathoracic and intrathoracic airways generated by HRCT scans. CT, computed tomography; HRCT, high-resolution computed tomography. Color images are available online.
Commercially available validated software packages (Mimics 15.0 and 3-Matic 7.0; Materialise nv, Leuven, Belgium) were used for all segmentation and modeling operations. HRCT scans were obtained from previous studies in which patient consent and approval from relevant Institutional Review Boards were procured; this study did not actively recruit patients.
Inhaler characteristics
To evaluate lung deposition of BDP/FF/GB and BDP/FF, the aerosol plume characteristics plume mass, cone angle, plume ejection velocity, and duration were measured for a representative BDP/FF/GB and BDP/FF pMDI (Table 2). Plume ejection velocity was measured at 10 mm (just after actuator exit) and 100 mm (simulating the throat distance).
Table 2.
Plume and Particle Characteristics for BDP/FF/GB and BDP/FF
| BDP/FF/GB | BDP/FF | ||||
|---|---|---|---|---|---|
| Mass (g) | 0.073 | 0.058 | |||
| Angle (°) | 21.24 | 15.61 | |||
| Velocity (m/s) | 21.0 (at 10 mm) | 11.4 (at 10 mm) | |||
| 6.0 (at 100 mm) | 5.1 (at 100 mm) | ||||
| Duration (ms) | 229.4 | 169.0 | |||
| API | BDP | FF | GB | BDP | FF |
| MMAD (μm) | 1.1 | 1.1 | 1.1 | 1.3 | 1.3 |
| GSD | 2.0 | 2.0 | 2.0 | 2.4 | 2.1 |
| FPF (% of DD) | 43 | 44 | 42 | 42 | 42 |
| DD (μg) | 87 | 5 | 11 | 85 | 5 |
API, active pharmaceutical ingredient; BDP, beclomethasone dipropionate; DD, delivered dose; FF, formoterol fumarate; FPF, fine particle fraction; GB, glycopyrronium bromide; GSD, geometric standard deviation; MMAD, mass median aerodynamic diameter.
Formulation characteristics were provided by Chiesi Farmaceutici SpA. Particle characteristics (MMAD, geometrical standard deviation, fine particle fraction [FPF], and delivered dose) of the compounds were measured using a Next-Generation Impactor for BDP/FF/GB and Andersen Cascade Impactor for BDP/FF (Table 2). Actual particle size measurements were used to create a particle size distribution as input for the CFD calculations.
Inhalation profile
Two types of inhalation flow profile were tested, optimal, and measured, to observe differences in regional deposition during ideal and real-life device use, respectively. All patients were coupled to the same optimal flow profile, specifically a slow and deep inhalation with a duration of 4–5 seconds, to achieve the ideal flow rate of ∼30 L/min.(23) Measured flow profiles came from the inspiratory volume and inspiratory time of 20 patients with COPD, matched by disease severity to those from whom we obtained the 3D lung models (the profiles for an example patient are shown in Supplementary Fig. S1).(24) These were used to generate a realistic and representative inhalation profile for each COPD patient scan used in the study. The mean flow of the measured flow profiles was 29.35 L/min (range 16.15–68.83 L/min).
CFD simulations
The 3D respiratory tract model was divided into two regions: extrathoracic (from mouth to extrathoracic airways) and intrathoracic (from top of the sternum to airways further downstream). This is similar to the global regions defined in scintigraphy, where the intrathoracic region is determined by the lung borders on an 81mKrypton ventilation scan and the extrathoracic region accounts for the upper parts of the respiratory tract, that is, the oropharynx and trachea. However, in scintigraphy, the extrathoracic region also includes the esophagus and stomach. Furthermore, for the 3D model, the intrathoracic region is subdivided into the central airways, with a diameter above 1–2 mm visible on a HRCT scan, and the peripheral airways that are not visible in the HRCT scan (Fig. 1).
Triangulated surface meshes created in 3-Matic (Materialise nv, Leuven, Belgium) were converted to tetrahedral 3D volume meshes using TGrid 14.0 (Ansys Inc., Canonsburg, PA). Subsequently, CFD simulations were performed on the 3D models by taking into account the boundary conditions: the inhalation profile is applied at the inhaler inlet to account for flow turbulence generated by the device; the percentage of flow exiting the model toward a lobe is equal to the relative lobar expansion obtained from patient-specific inspiratory and expiratory lobar 3D models; particles not deposited in the extrathoracic or central airways are considered to be deposited in the peripheral airways; no-slip conditions are chosen for the airway walls, that is, particles are trapped when hitting the wall. All inhaled particles are assumed to deposit, and so exhalation of particles cannot be modeled. The mathematical model and appropriate boundary conditions have been validated and previously published.(3)
Statistical analyses
Analyses were conducted using R version 3.2.5 or higher (R Foundation for Statistical Computing, Vienna, Austria). To analyze intrathoracic BDP/FF deposition, a linear model was used. Deposition values (as percentage of nominal dose) were logarithmically transformed before analysis. The model included fixed effects for product (BDP/FF/GB and BDP/FF) and flow (optimal and measured flow). The Satterthwaite approximation for degrees of freedom was used. The mean deposition ratio (standard error) of BDP/FF/GB over BDP/FF for the intrathoracic BDP/FF deposition was obtained by pairwise comparison (significance level, p < 0.05) of the estimated marginal mean of intrathoracic BDP/FF deposition for each product at optimal and measured flow, respectively.
To investigate statistical equivalence between the deposition patterns of BDP/FF/GB and BDP/FF for each of the common active pharmaceutical ingredients (APIs), that is, BDP and FF, a two one-sided t-test (significance level, p < 0.05) was used to assess paired differences in deposition patterns per lung region. The equivalence plot presents the mean ratio, with 95% confidence interval (CI), of BDP/FF/GB over BDP/FF for the deposition of each common API per lung region. According to the Food and Drug Administration guidance for Industry on Statistical Approaches to Establishing Bioequivalence, the calculated confidence interval of the ratio Test over Reference should fall within the bioequivalence boundaries [0.8, 1.25].(25)
Descriptive results of the deposition in the global (i.e., extrathoracic and intrathoracic) and lobar regions are given as mean ± standard deviation (SD). The central-to-peripheral deposition (C:P) ratio defines the distribution of intrathoracic deposition over the larger and smaller airways by dividing deposition in the central region by deposition in the peripheral region. C:P ratios were calculated for each individual API, with these ratios averaged over the two or three APIs to give an overall value for each product.
Results
Patients
Imaging data for 20 patients with moderate-to-severe COPD were selected from the FluidDA database (15 males, 5 females; mean ± SD age, 64.0 ± 7.68 years; height, 168.9 ± 8.40 cm; postbronchodilator forced expiratory volume in 1 second, 42.3% ± 14.8% predicted).
Extrathoracic deposition
At the optimal flow mean ± SD deposition in the extrathoracic region was 55.3% ± 4.8% for BDP/FF/GB and 56.7% ± 5.6% (nominal dose) for BDP/FF. At the measured flow, extrathoracic deposition was 55.1% ± 5.9% for BDP/FF/GB and 55.9% ± 5.1% (nominal dose) for BDP/FF (Supplementary Tables S1 and S2; Supplementary Fig. S2).
Intrathoracic deposition
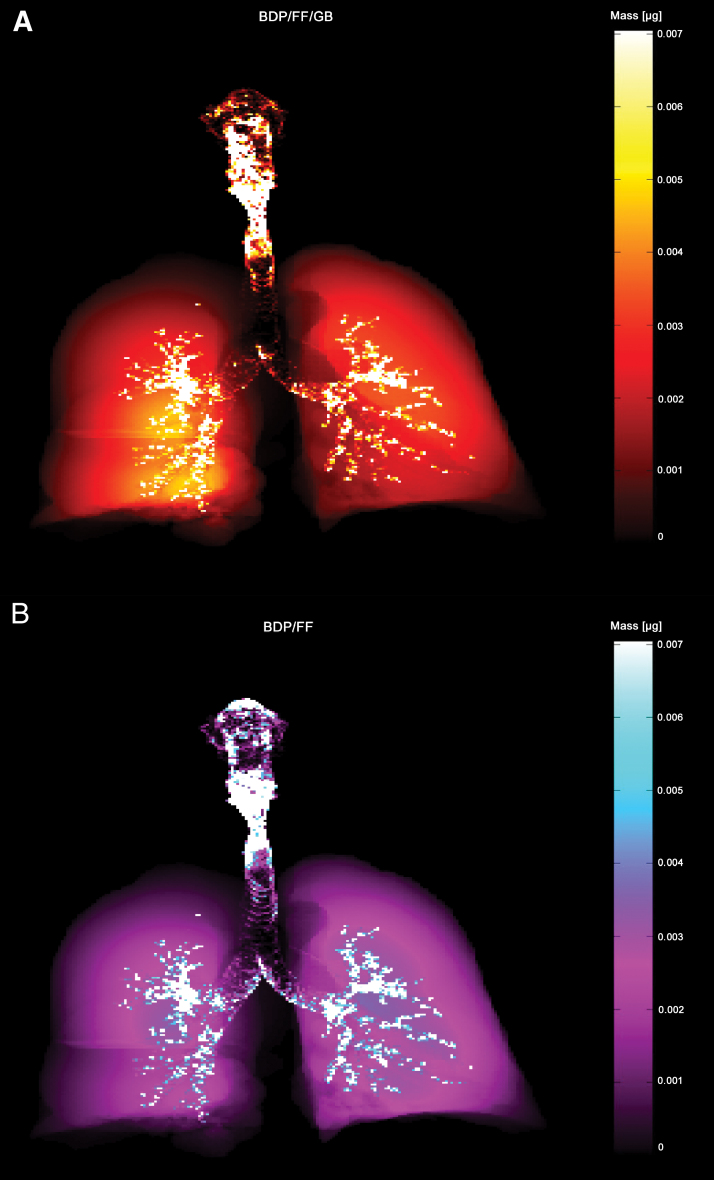
At the optimal flow, total lung deposition (defined as the proportion of the formulation that reached the intrathoracic region) was similarly high for both products, with mean ± SD values of 30.9% ± 4.5% for BDP/FF/GB and 27.3% ± 5.6% (nominal dose) for BDP/FF. Results were similar at the measured flow, with mean ± SD values of 31.0% ± 5.7% for BDP/FF/GB and 28.1% ± 5.2% (nominal dose) for BDP/FF (Supplementary Tables S1 and S2; Supplementary Fig. S2). The deposition in the lungs and airways of an example patient is depicted in Figure 2.
FIG. 2.
Representative modeled deposition of (A) BDP/FF/GB and (B) BDP/FF from one patient. Scintigraphy-like two-dimensional visualization in which lighter areas correspond to higher regional deposition concentrations. Both BDP/FF/GB and BDP/FF have high deposition throughout the extrathoracic and peripheral regions. BDP, beclomethasone dipropionate; FF, formoterol fumarate; GB, glycopyrronium bromide. Color images are available online.
C:P ratio
At the optimal flow, the mean ± SD C:P ratio for BDP/FF/GB was 0.52 ± 0.14 and for BDP/FF was 0.65 ± 0.17. At the measured flow, the ratios were 0.48 ± 0.13 for BDP/FF/GB and 0.62 ± 0.17 for BDP/FF, indicating that higher drug deposition occurred in the small airways relative to the large airways. At the two flow profiles, BDP/FF/GB and BDP/FF had consistent central and peripheral patterns. The central and peripheral patterns of BDP/FF/GB and BDP/FF at optimal and measured flow are given in Supplementary Tables S1 and S2. Descriptive results of the deposition patterns at the measured flow are given in Supplementary Figure S2.
Lobar deposition
The deposition (as a percentage of nominal dose) in each lung lobe was calculated at both the optimal and the measured flow for BDP/FF/GB and BDP/FF (Supplementary Tables S3 and S4). BDP/FF/GB and BDP/FF showed similar lobar deposition patterns, equally distributed over the left and right lung halves. Descriptive results of the lobar deposition patterns at the measured inhalation flow are given in Supplementary Figure S3.
Equivalence between BDP/FF/GB and BDP/FF
For BDP and FF, as delivered by both products, the mean intrathoracic deposition (as percentage of nominal dose) ratio of BDP/FF/GB over BDP/FF was calculated at the optimal and the measured flow. The mean deposition ratio was 1.14 at the optimal flow and 1.10 at the measured flow. This indicates that at both flow profiles the intrathoracic deposition of the BDP and FF components were comparable for the BDP/FF/GB and BDP/FF pMDI products (Table 3).
Table 3.
Mean Deposition Ratios by Pairwise Comparison of the Estimated Marginal Mean of the Intrathoracic Deposition of Extrafine BDP/FF for the Optimal and Measured Inhalation Flow Profile
| Inhalation flow profile | BDP/FF/GB vs. BDP/FF ratio (SE) | p |
|---|---|---|
| Optimal | 1.14 (0.052) | 0.0049 |
| Measured | 1.10 (0.051) | 0.0405 |
Significance of the statistical test is indicated by p < 0.05.
BDP, beclomethasone dipropionate; FF, formoterol fumarate; GB, glycopyrronium bromide; SE, standard error.
Statistical equivalence between BDP/FF/GB and BDP/FF for the deposition of BDP and FF was also calculated for the intrathoracic, central, and peripheral regions (Table 4; Supplementary Fig. S4). All mean deposition ratios and 95% CIs in the intrathoracic and central region for BDP and FF were within the range of 0.80 and 1.25. In the peripheral region the CIs slightly exceeded the upper limit of the upper bioequivalence boundary. When compared with BDP/FF, the BDP and FF particles from BDP/FF/GB had smaller MMADs but higher FPFs, with resultant higher deposition deeper in the lungs.
Table 4.
Statistical Equivalence for the Deposition of the Common Compounds BDP and FF Per Lung Region for the Optimal and Measured Inhalation Flow Profile
| Inhalation flow profile | API | Mean deposition ratio (95% CI) |
||
|---|---|---|---|---|
| Intrathoracic | Central | Peripheral | ||
| Optimal | BDP | 1.13 (1.08–1.19) | 0.97 (0.92–1.02) | 1.24 (1.18–1.30) |
| FF | 1.15 (1.10–1.20) | 0.99 (0.94–1.05) | 1.25 (1.18–1.31) | |
| Measured | BDP | 1.09 (1.05–1.14) | 0.92 (0.89–0.96) | 1.20 (1.15–1.26) |
| FF | 1.11 (1.07–1.15) | 0.94 (0.91–0.98) | 1.21 (1.15–1.27) | |
API, active pharmaceutical ingredient; BDP, beclomethasone dipropionate; CI, confidence interval; FF, formoterol fumarate.
Discussion
The current study is the first to evaluate the proportion of inhaled drug reaching the small airways (i.e., those with diameter <1 to 2 mm) for extrafine formulations of BDP/FF/GB and BDP/FF pMDIs. The findings yielded precise region-specific insights about inhaled particle deposition, providing a useful tool for the optimization of drug design (given it is an easy and quick method of evaluating regional lung deposition), and of therapeutic targeting and comparative performance. This study showed that a large fraction of inhaled extrafine BDP/FF/GB and BDP/FF were deposited in the lungs, specifically 31.0% and 28.1%, respectively. The C:P ratios in this study were 0.48 for BDP/FF/GB and 0.62 for BDP/FF, indicating that within the intrathoracic region approximately twice the amount of drug may reach the smaller peripheral airways than deposits in the larger central airways. The small difference in C:P ratios between BDP/FF/GB and BDP/FF could potentially be due to differences in MMAD; we have previously shown that C:P ratio decreases with decreasing MMAD (i.e., relative peripheral deposition increases).(22)
Deposition into the lung was calculated using FRI, an in silico technology that applies CFD calculations in combination with patient-specific airway and lung geometries to provide regional deposition metrics of aerosols and powders. FRI is able to assess the influence of various drug delivery parameters, such as inhalation flow profile, particle characteristics, device and disease population, on the aerosol deposition by investigating different delivery scenarios in a controlled environment. The patient-specific nature and core characteristics of FRI makes it an alternative to scintigraphy, providing similar results in a more time- and cost-effective manner without the need for active patient recruitment. In this study, the extrathoracic and intrathoracic deposition values for BDP/FF were very close to the deposition values found in the BDP/FF scintigraphy study by De Backer et al.(7): the extrathoracic deposition was 55.9% of the nominal dose with FRI versus 55.0% with scintigraphy, whereas intrathoracic deposition was 28.1% of the nominal dose with FRI versus 33.1% with scintigraphy. Although FRI results are often consistent with scintigraphy (regardless of the molecules or devices; Table 1), a key difference between the techniques is how lung images are processed. Scintigraphy is a two-dimensional technique, in which small airways to the front and back of the central airways are included in the central compartment. In contrast, the 3D processing of FRI allows all peripheral airways to be assessed, regardless of location.
The C:P ratio obtained with FRI cannot be compared with the C:P ratio obtained from scintigraphy, due to differences in the definitions of the central and peripheral regions. In the scintigraphy study of De Backer et al. deposition was determined on a bidimensional planar image of a Krypton ventilation scan, in which rectangular regions of interest were drawn to define the central and peripheral regions.(7) As a consequence, the central and peripheral region in scintigraphy comprises both intermediate/small airways and alveoli. In contrast, one of the major scientific advantages of the use of 3D models in FRI is that this enables aerosol deposition in the large and small airways to be distinguished and separately quantified. However, mucociliary clearance is not taken into account since it does not affect particle deposition. In contrast, redistribution of the particles following initial deposition could be impacted by clearance mechanisms, which may then affect the efficacy and safety of a compound.
A few limitations of this study are worth noting. First, the measured inhalation flow profile applied in the simulations was generated by the inhalation time and inhalation volume measurements in 20 representative patients from a previous study, rather than the 20 patients who provided the computed tomography (CT) scans for the 3D airway geometries. On an individual patient level, a more accurate deposition pattern might have been obtained if each CT scan was coupled with the inhalation flow profile from the same patient. Second, inhaled particles that were not trapped in the extrathoracic or central airways were assumed to be deposited in the smaller peripheral airways, rather than being exhaled.(26,27) This assumption is supported by the results from the in vivo extrafine BDP/FF scintigraphy study by De Backer et al.,(7) which showed that the exhaled fraction of the extrafine particles was on average only 3.4% of the nominal dose in patients with COPD.(28)
The deposition values calculated in this study are consistent with those in available literature on aerosol deposition, which showed similar lung deposition of extrafine drugs (MMAD <2 μm). Clinically it has been acknowledged that small airways (<2 mm diameter) are a major site of inflammation both in asthma and COPD,(19,20) and small airways disease is present at all stage of the diseases.(29–31) Therefore, it is important that ICS-containing inhaled drugs are able to reach the small airways, where their anti-inflammatory activity is most needed.
In conclusion, the results reported in this study further support FRI as a validated method to assess lung aerosol deposition, without the setup of a clinical trial and the modification of the drug for radiolabeling as required in scintigraphy. This FRI deposition study provided evidence of the lung deposition of extrafine BDP/FF/GB and confirmed the lung deposition of extrafine BDP/FF previously measured with scintigraphy.(7) Moreover, similar deposition patterns, with high lung deposition, were found for BDP/FF/GB and BDP/FF. Furthermore, the C:P ratios of both products indicated a high peripheral deposition, supporting the small airway targeting and delivery of these two extrafine ICS-containing fixed combinations.
Supplementary Material
Acknowledgment
Editorial support (in the form of editing the content for English grammar and journal style) was provided by David Young of Young Medical Communications and Consulting Ltd. This support was funded by Chiesi Farmaceutici SpA.
Author Disclosure Statement
O.S.U. has received industry to academic funding from Boehringer Ingelheim, Chiesi, Edmond Pharma, GlaxoSmithKline, and Mundipharma International, and has received consultancy or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Edmond Pharma, GlaxoSmithKline, NAPP, Novartis, Mundipharma International, Pearl Therapeutics, Roche, Sandoz, Takeda, Trudell Medical, UCB, and Vectura. B.M. and I.K. are employees of FluidDA nv; FluidDA nv received funding from Chiesi Farmaceutici SpA for conducting this work. R.D.M., D.C., and G.G. are employees of Chiesi. N.S. has received financial support for research and consulting from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, Sanofi.
Funding Information
This study was funded by Chiesi Farmaceutici SpA.
Supplementary Material
Reviewed by:
Gordon Prisk
Akira Isuda
References
- 1. Newman S, Bennett WD, Biddiscombe M, Devadason SG, Dolovich MB, Fleming J, Haeussermann S, Kietzig C, Kuehl PJ, Laube BL, Sommerer K, Taylor G, Usmani OS, and Zeman KL: Standardization of techniques for using planar (2D) imaging for aerosol deposition assessment of orally inhaled products. J Aerosol Med Pulm Drug Deliv. 2012;25(Suppl. 1):S10–S28 [DOI] [PubMed] [Google Scholar]
- 2. de Matas M, Shao Q, Biddiscombe MF, Meah S, Chrystyn H, and Usmani OS: Predicting the clinical effect of a short acting bronchodilator in individual patients using artificial neural networks. Eur J Pharm Sci. 2010;41:707–715 [DOI] [PubMed] [Google Scholar]
- 3. De Backer JW, Vos WG, Vinchurkar SC, Claes R, Drollmann A, Wulfrank D, Parizel PM, Germonpré P, and De Backer W: Validation of computational fluid dynamics in CT-based airway models with SPECT/CT. Radiology 2010;257:854–862 [DOI] [PubMed] [Google Scholar]
- 4. Usmani O, Vos W, Mignot B, Georges G, Scuri M, Valente I, De Maria R, and Scichilone N: Lung deposition of extrafine inhaled corticosteroid (ICS)-containing fixed combinations drug in COPD patients using functional respiratory imaging (FRI). Eur Respir J. 2018;52(Suppl. 62):PA1015 [Google Scholar]
- 5. Kappeler D, Sommerer K, Kietzig C, Huber B, Woodward J, Lomax M, and Dalvi P: Lung deposition of fluticasone propionate/formoterol administered via a breath-triggered inhaler. Eur Respir J. 2017;50(Suppl. 62):PA522. [DOI] [PubMed] [Google Scholar]
- 6. Müllinger B, Brand P, Fischer A, Häußermann S, Scheuch G, Seitz J, Sommerer K, Stegemann J, Meyer T, and Wachall B: Intra-pulmonal deposition of two different tobramycin formulations. J Cyst Fibros. 2005;4(Suppl. 1):S53 (abstract 198). [Google Scholar]
- 7. De Backer W, Devolder A, Poli G, Acerbi D, Monno R, Herpich C, Sommerer K, Meyer T, and Mariotti F: Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD Patients. J Aerosol Med Pulm Drug Deliv. 2010;23:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Maria R, Zagnoni I, Bodria A, Bonelli S, Alberi MD, Lewis DA, Johnson R, and O'Shea H: Foster®: A high-efficiency combination metered dose inhaler with consistent particle size distribution at alternative flow rates. Comb Prod Ther. 2014;4:1–5 [Google Scholar]
- 9. Iwanaga T, Kozuka T, Nakanishi J, Yamada K, Nishiyama O, Sano H, Murakami T, and Tohda Y: Aerosol deposition of inhaled corticosteroids/long-acting β2-agonists in the peripheral airways of patients with asthma using functional respiratory imaging, a novel imaging technology. Pulm Ther. 2017;3:219–231 [Google Scholar]
- 10. Hirst PH, Bacon RE, Pitcairn GR, Silvasti M, and Newman SP: A comparison of the lung deposition of budesonide from Easyhaler, Turbuhaler and pMDI plus spacer in asthmatic patients. Respir Med. 2001;95:720–727 [DOI] [PubMed] [Google Scholar]
- 11. Leach CL, Kuehl PJ, Chand R, and McDonald JD: Respiratory tract deposition of HFA-beclomethasone and HFA-fluticasone in asthmatic patients. J Aerosol Med Pulm Drug Deliv. 2016;29:127–133 [DOI] [PubMed] [Google Scholar]
- 12. Nikander K, Prince I, Coughlin S, Warren S, and Taylor G: Mode of breathing-tidal or slow and deep-through the I-neb adaptive aerosol delivery (AAD) system affects lung deposition of (99m)Tc-DTPA. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl. 1):S37–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hull D, Black A, and Vos W: Use of computational fluid dynamics (CFD) to model aerosol deposition in the lungs of patients with cystic fibrosis. J Cyst Fibros. 2018;17(Suppl. 3):S26 [Google Scholar]
- 14. Lenney W, Edenborough F, Kho P, and Kovarik JM: Lung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebuliser in healthy and cystic fibrosis subjects. J Cyst Fibros. 2011;10:9–14 [DOI] [PubMed] [Google Scholar]
- 15. Fischer A, Stegemann J, Scheuch G, and Siekmeier R: Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur J Med Res. 2009;14(Suppl. 4):71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munro S, Main M, and Vos W: Matching delivery device to a patient's condition: Use of lung deposition modelling to optimise delivery in idiopathic pulmonary fibrosis. In: Drug Delivery to the Lungs Annual Congress. 2017 [Google Scholar]
- 17. Hillyer EV, Price DB, Chrystyn H, Martin RJ, Israel E, van Aalderen WMC, Papi A, Usmani OS, and Roche N: Harmonizing the nomenclature for therapeutic aerosol particle size: A proposal. J Aerosol Med Pulm Drug Deliv. 2018;31:111–113 [DOI] [PubMed] [Google Scholar]
- 18. Usmani OS, Biddiscombe MF, and Barnes PJ: Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–1504 [DOI] [PubMed] [Google Scholar]
- 19. Bonini M, and Usmani OS: The role of the small airways in the pathophysiology of asthma and chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2015;9:281–293 [DOI] [PubMed] [Google Scholar]
- 20. Lavorini F, Pedersen S, Usmani OS, and on behalf of the Aerosol Drug Management Improvement Team (ADMIT): Dilemmas, confusion, and misconceptions related to small airways directed therapy. Chest 2017;151:1345–1355 [DOI] [PubMed] [Google Scholar]
- 21. McNulty W, and Usmani OS: Techniques of assessing small airways dysfunction. Eur Clin Respir. J. 2014;1:25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Holsbeke C, De Backer J, Vos W, and Marshall J: Use of functional respiratory imaging to characterize the effect of inhalation profile and particle size on lung deposition of inhaled corticosteroid/long-acting β2-agonists delivered via a pressurized metered-dose inhaler. Ther Adv Respir Dis. 2018;12:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laube BL, Janssens HM, de Jongh FHC, Devadason SG, Dhand R, Diot P, Everard ML, Horvath I, Navalesi P, Voshaar T, and Chrystyn H: What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–1331 [DOI] [PubMed] [Google Scholar]
- 24. De Backer W, De Backer J, Vos W, Verlinden I, Van Holsbeke C, Clukers J, Hajian B, Siddiqui S, Jenkins M, Reisner C, and Martin UJ: A randomized study using functional respiratory imaging to characterize bronchodilator effects of glycopyrrolate/formoterol fumarate delivered by a metered dose inhaler using co-suspension delivery technology in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2673–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Food and Drug Administration: Statistical approaches to establishing bioequivalence. 2001. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-approaches-establishing-bioequivalence. Accessed January10, 2020
- 26. Verbanck S, Kalsi HS, Biddiscombe MF, Agnihotri V, Belkassem B, Lacor C, and Usmani OS: Inspiratory and expiratory aerosol deposition in the upper airway. Inhal Toxicol. 2011;23:104–111 [DOI] [PubMed] [Google Scholar]
- 27. Verbanck S, Biddiscombe MF, and Usmani OS: Inhaled aerosol dose distribution between proximal bronchi and lung periphery. Eur J Pharm Biopharm. 2020;152:18–22 [DOI] [PubMed] [Google Scholar]
- 28. Jabbal S, Poli G, and Lipworth B: Does size really matter?: Relationship of particle size to lung deposition and exhaled fraction. J Allergy Clin Immunol. 2017;139:2013–2014 [DOI] [PubMed] [Google Scholar]
- 29. Crisafulli E, Pisi R, Aiello M, Vigna M, Tzani P, Torres A, Bertorelli G, and Chetta A: Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration 2017;93:32–41 [DOI] [PubMed] [Google Scholar]
- 30. Koo H-K, Vasilescu DM, Booth S, Hsieh A, Katsamenis OL, Fishbane N, Elliott WM, Kirby M, Lackie P, Sinclair I, Warner JA, Cooper JD, Coxson HO, Paré PD, Hogg JC, and Hackett T-L: Small airways disease in mild and moderate chronic obstructive pulmonary disease: A cross-sectional study. Lancet Respir Med. 2018;6:591–602 [DOI] [PubMed] [Google Scholar]
- 31. Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, Papi A, Van der Molen T, Rabe KF, Siddiqui S, Singh D, Nicolini G, Kraft M, ATLANTIS study group E, Cukier A, Stelmach R, Olivenstein R, Zhang Q, Badorrek P, Gessner C, Scichilone N, Chetta A, Paggiaro P, Milleri S, D'Amato M, Spanevello A, Foschino MP, Boersma WG, Broeders M, Vroegop JS, Moral VP, Djukanovic R, Usmani O, Schilz R, Martin R, and Hanania N: Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7:402–416 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.