Abstract
Reproduction and immunity are energy intensive, intimately linked processes in most organisms. In women, pregnancy is associated with widespread immunological adaptations that alter immunity to many diseases, whereas, immune dysfunction has emerged as a major cause for infertility in both men and women. Deciphering the molecular bases of this dynamic association is inherently challenging in mammals. This relationship has been traditionally studied in fast-living, invertebrate species, often in the context of resource allocation between life history traits. More recently, these studies have advanced our understanding of the mechanistic underpinnings of the immunity-fertility dialogue. Here, we review the molecular connections between reproduction and immunity from the perspective of human pregnancy to mechanistic discoveries in laboratory organisms. We focus particularly on recent invertebrate studies identifying conserved signaling pathways and transcription factors that regulate resource allocation and shape the balance between reproductive status and immune health.
Keywords: aging, C. elegans, fertility, immunity, pregnancy, resource allocation, transcriptional networks
INTRODUCTION
Reproduction and immunity are intimately linked processes. Historically, pregnancy in women was considered to be an extended state of immunosuppression.[1,2] This is partly due to the fact that pregnancy represents an exceptional state wherein a genetically distinct fetus is allowed to develop within the mother’s body without stimulating a deleterious immune reaction. It is now understood that rather than generalized immunosuppressionpregnancy involves a compendium of adaptations of the maternal immune system that play critical roles at every step of reproduction, from conception to birth, which also significantly alter a woman’s ability to combat disease (reviewed in [1–4]). Similarly, immune status profoundly influences reproductive health. A wide body of evidence has accrued that infections and/or immune dysfunction negatively impact fertility.[5,6] Recent estimates suggest that up to 20% of unexplained infertility in women and men may be attributable to immune dysfunction, and the field of “Immunological Infertility” is a rapidly burgeoning area in basic and clinical research.[6,7] Despite the strong immunity-fertility links evident in human pregnancy, this relationship is inherently challenging to study in people for a variety of reasons, including the fact that it is a lengthy endeavor and is also impacted by post-partum (lactation), behavioral and psychosocial aspects.
Reproduction and immunity are highly energy intensive functions. In invertebrate species, the major energetic expense during procreation is the production of eggs and the deposition of large quantities of yolk fat, proteins and organelles in them. Vertebrates, especially placental mammals, have additional complex energetic demands besides egg production as their reproduction involves remodeling of multiple organs and tissues. Nonetheless, studies have estimated that a fullterm human pregnancy requires ~88,000–89,400 kcal, in part to support the increased basal metabolic rate (BMR) necessary for fetal tissue synthesis and increased effort by the maternal organ systems (e.g., cardiovascular system). BMR is projected to increase by 4% during the first trimester and up to 24% during the third trimester during pregnancy.[8] However, precise quantification of energetic requirements is difficult as they are highly influenced by both internal (age, nutrition, individual physiology) as well as external variables (racial and population characteristics). Similarly, mounting and sustaining an immune response to an infection requires enormous energetic resources. Invertebrates rely on innate immunity to combat pathogens, whereas, vertebrates also utilize adaptive immunity. Immune activation, however, is intensely demanding across the evolutionary spectrum as all animals upregulate anti-pathogen genes and deploy multiple defense and tolerance strategies (Figure 1)[9]. Animals with more complex immune systems additionally raise their body temperature, mobilize specialized immune cells and trigger humoral responses. Increasing metabolic rate in response to infection is documented across mice, birds, and humans (reviewed in [10–12]). One estimate indicated an 8–14% increase in BMR in young adult human males infected with a respiratory virus,[10] but as with pregnancy, energy needs and metabolic changes vary among individuals due to numerous internal and external factors.
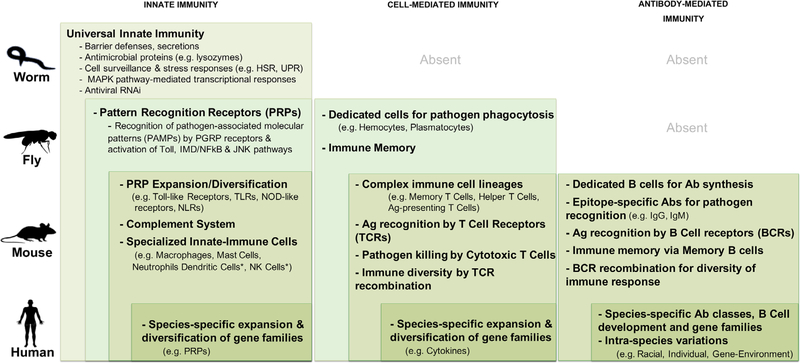
FIGURE 1.
Comparison of immune systems across invertebrate and vertebrate species. The main constituents of the immune systems of the invertebrate (worms, flies) and vertebrate (mice, humans) species discussed in this review are depicted. A universal innate immune response (left column) is conserved from worms to flies, whereas major elements of adaptive immunity (middle and right columns) are absent in invertebrates (though dedicated immune cells have been identified in flies). The major features of each arm of the immune system are recapitulated along with layers of complexity added at each evolutionary level (shown by nested rectangles of progressively darker shades of green). Ab, antibody; Ag, antigen; HSR, heat shock response; PGRP, peptidoglycan pattern recognition receptor; UPR, unfolded protein response
There is widespread evidence from model organisms and species in the wild that infections reduce fertility, whereas, increased reproduction leads to immunosuppression (reviewed in [5,13]). This research has traditionally been conducted in the context of evolutionary “life history theory” (LHT) that posits there are “tradeoffs” between competing, energy intensive, life history traits, and these are key drivers of speciation (see Box 1 for summary of LHT in the context of the immunity-fertility axis).[14,15] Fast living, invertebrate species with short lifespans and high fertility rates are exceptionally well-suited for such studies as their reproductive output is highly sensitive to endogenous or environmental perturbations such that even subtle shifts in resource allocation can be detected and measured easily.[16] For instance, the fruit fly, Drosophila melanogaster, produces ~500 progeny over a 10-day period, whereas, the nematode Caenorhabditis elegans lays ~300 progeny within 3–6 days.[17,18] Additionally, the remarkable ease of molecular and genetic manipulation in flies and worms have made it possible to begin addressing the molecular mechanisms underlying the immunity-fertility axis. Recent studies have identified signaling pathways and transcriptional regulators that mediate this dialogue. Since many of these genes and proteins are conserved, these discoveries promise to open up avenues to understand this important relationship in humans. In this article, we aim to review the state of our knowledge on the molecular connections between reproduction and immunity, from the perspective of human pregnancy to mechanistic discoveries in laboratory organisms. We first highlight the major immune adaptations of human pregnancy and clinical repercussions of this relationship to maternal-fetal health. We then summarize the research from invertebrate models on the mutual impacts of fertility and immunity. We focus on recent investigations identifying conserved signaling pathways and transcription factors involved in this dialogue, including work from our laboratory.
BOX 1: The Immunity-Fertility Relationship Explicated by the Life History Theory.
Life history theory (LHT), first proposed by MacArthur and Wilson in 1967, explains the evolutionary drivers of lifecycle diversity across species as well as individual organisms’ need to optimize survival in the face of limited energy supply and environmental challenges. LHT posits that there are “tradeoffs” between competing life history traits (e.g., growth, procreation, lifespan, immunity) where organisms will invest more in one function at the expense of another. Tradeoff strategies have been found to be remarkably prevalent across phylogenetic groups. In a recent analysis of 121 invertebrate and vertebrate species, Healy et al.,[19] suggested that tradeoff relationships may shape over 70% of life-history strategies. In this framework, reproduction and immunity, which are highly energy dependent and plastic functions, are proposed to be mutually antagonistic due to a competing reliance on limited organismal resources. Studies of life history traits in invertebrates have provided the earliest documentation of, and insights into, the complexities of this reproduction-immunity axis. This work has also shown reproduction-immunity tradeoffs can be facultative (occurring only when resources are limiting) or obligate (independent of resource availability). Moreover, despite its popularity, LHT has limitations as well as exceptions. For instance, poor correlation has been observed between the energetic demands of reproduction and consequent degree of immunity suppression, emphasizing the significance of resource-independent features. In some species (e.g., Queen Ants, Bumble Bees), mating is in fact beneficial to the mother’s immunity. The rather simplistic view of reproductive behavior proposed by LHT has been succeeded by alternative theories that do not mandate tradeoffs (e.g., Antagonistic Pleiotropy). Thus, while resource allocation is an important attribute of the immunity-fertility relationship, there are many other variables that play key roles. The molecular determinants of the immunity-fertility axis discussed in this review are liable to both match and defy the classical LHT tradeoff paradigm, and likely to explain more nuanced aspects of this relationship.
PREGNANCY WIDELY REMODELS MATERNAL IMMUNITY
In women, pregnancy is characterized by widespread shifts in the local and systemic milieu of the mother’s immune system involving a host of maternal and fetal factors.[1,3,4] The maternal–fetal interface, composed of the maternally derived decidua and the fetus-derived placenta, is the main site where local immunomodulation occurs.[20,21] Initial interactions between trophoblasts, the leading placental cells, and the decidual immune cells allow the embryo, and then the fetus, to develop in the uterus. Besides providing nutrition and oxygen to the fetus, the placenta also plays key immunomodulatory roles. For example, placenta-derived exosomes promote a local immunosuppressive environment.[22] The placenta also expresses antiviral Toll-like receptors throughout gestation and secretes a range of antimicrobial proteins and peptides that protect the fetus from direct infection.[4] The fetus additionally evades detection by not expressing antigens recognizable by the mother’s immune cells.[1,3]
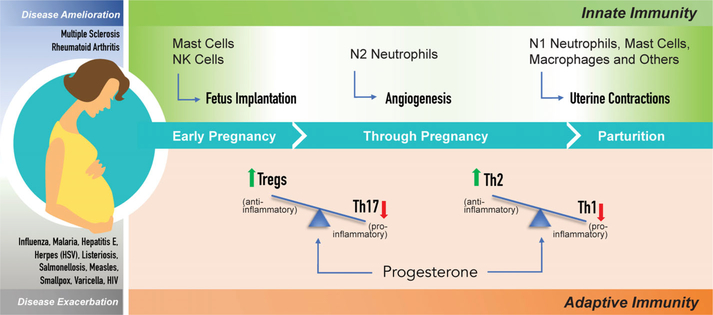
On the maternal side, both the innate- and adaptive-immune systems undergo sweeping alterations (Figure 2). Constituents of the innate-immune system are critical locally for implantation to occur, but globally innate immunity is downregulated during pregnancy, and then enhanced again at term to facilitate parturition and labor (Figure 2).[3,4] At the maternal-fetal interface, multiple populations of innate-immune cells undergo dynamic fluctuations at different stages of pregnancy to facilitate key events. For example, innate immune cells, especially natural killer (NK) and mast cells flood the uterine lining to help implant the fetus. Specialized uterine NK (uNK) cells interact with the placental cells to facilitate blood flow to the embryo. In the first trimester, a specialized population of Neutrophils, called N2 cells, becomes prominent and these are important for fetal angiogenesis. At term, inflammatory N1 Neutrophils, along with macrophages, mast cells and other innate-immune cell types, traffic to the uterus and express matrix metalloproteases that help dissolve the fetal membranes and induce uterine contractions (Figure 2) (reviewed in [1,3,4]). Similarly, the end of pregnancy is characterized by acute inflammation, particularly in the birth canal.[23] Hence, pregnancy remodels maternal innate immunity and repurposes the inflammatory response as the signal and mechanism of labor.
FIGURE 2.
Immunological adaptations of pregnancy and impacts on disease susceptibility. Pregnancy in placental mammals such as humans is characterized by unique immunological adaptations that allow the genetically distinct fetus to survive within the mother’s body. The establishment, maintenance and successful completion of healthy pregnancy relies on finely tuned alterations in the maternal innate- and adaptive- immune systems, both at the ‘local’ maternal-placental-fetal interface as well as systemically. An early rise in activity of innate-immune cells is critical for fetus implantation. It is followed by a generalized suppression during much of pregnancy and another surge at the end of term that facilitates labor and parturition. Maternal adaptive immunity also undergoes major shifts resulting in a dramatic expansion of a anti-inflammatory T cell populations, the Treg and Th2 cells, that produce anti-inflammatory cytokines and concomitant reduction in pro-inflammatory T cell types, Th17 and Th1, respectively. These widespread immune alterations impact the mother’s immune resistance, increasing the susceptibility, and/or severity, to many infections (select list in blue) while ameliorating some autoimmune diseases (select list in gray)
The maternal adaptive immune system, comprising cell-mediated immunity conferred by T Cell lymphocytes and humoral immunity provided by B Cell lymphocytes (Figure 1), also undergoes major shifts during pregnancy predominantly in T cell profiles. The key modification involves a change in the balance between two populations of maternal T cells, the T regulatory (Treg) cells and the T helper 17 (Th17) cells (Figure 2).[24,25] Treg cells expand dramatically as pregnancy progresses and are thought to be the primary means of creating tolerance at the local maternal-fetal interface via activation of the transforming growth factor β (TGFβ) signaling pathway and production of the anti-inflammatory cytokine, IL10. The Th17 subset, on the other hand, expresses potent pro-inflammatory cytokines such as IL17 and is suppressed during pregnancy.[24] The relative proportion of two other populations of T cells, the Th1 and Th2 cells (which secrete pro- and anti- inflammatory cytokines, respectively) also changes significantly such that Th2 levels are elevated and Th1 levels are reduced (Figure 2).[24,25] This is thought to protect the fetus from the maternal immune system while maintaining sufficient immune protection for the mother, although the relative significance of this adaptation for normal pregnancy has been questioned.[26] These shifts maintain humoral immunity while repressing cell-mediated immunity in the global maternal immune system. The adaptations in B cells that mediate humoral immunity are nuanced and still being discovered.[27]
Following childbirth, the post-partum period is marked by the mother’s immune system returning to a pre-pregnancy state. Activated T cells have been reported to increase 12 weeks after delivery,[28] whereas, levels of the Th1 cytokines, IFN-γ and IL-2, are restored by 3 and 4 months post-partum, respectively.[29] However, it may take up to one year for the immune system to recover from pregnancy-related changes.[30] Lactation and breast feeding – which suppresses fertility – is likely to impact this immune recovery as well,[31] but this remains poorly studied. Despite the widely-characterized benefits of breast milk to neonatal immunity,[32] to our knowledge no study has directly examined the rate or extent of maternal immune recovery among women who breast feed, either exclusively or partially versus those who do not.
THE IMMUNOSUPPRESSIVE ALTERATIONS OF PREGNANCY INCREASE DISEASE SUSCEPTIBILITY
An interesting consequence of the immune adaptations during mammalian pregnancy is the effect on immune resistance of the mother towards various disease symptoms, many of which are exacerbated while some are alleviated.[2] Immunity against a number of bacterial, parasitic and viral infections is reduced during pregnancy (Figure 2). Yet, the immune impact of pregnancy is often through infectious-disease severity rather than incidence per se. For instance, the prevalence of influenza in pregnancy is similar to that of the general population, yet influenza carries a five-fold higher risk of death in pregnant women.[33] The ultimate disease-pregnancy relationship, however, appears to depend on the branch of the immune system used to combat a given disease.[34] Defense against infections that are predominantly fought off using inflammatory responses, such as malaria, or those caused by bacterial pathogens such as Listeria and Salmonella, is reduced due to expansion of the anti-inflammatory Treg population.[35,36] By the same token, pregnancy ameliorates diseases of chronic inflammation such as Multiple Sclerosis or Rheumatoid Arthritis that are characterized by overwhelming Th17 numbers that attack healthy self-tissue.[37] Nevertheless, some autoimmune diseases such as Scleroderma and Systemic Lupus Erythematosus flare during pregnancy.[3] Inherently low-inflammation helminth infections are noted to suppress immunity and push the bias of Th2 immune responses in pregnant women even further, causing an array of immununologic effects on mother and fetus and increasing co-infection risk to other pathogens.[38] A less-specific impact of helminth infection is also a detraction of iron, lipids, and other molecular resources already in high demand by the fetus.[39] Thus, unique, pathogen-specific strategies need to be accounted for in considering the maternal impact of an infection during pregnancy. The molecular mechanisms underlying these variable susceptibilities are poorly understood but have been linked, in part, to the canonical reproductive hormones. Progesterone, the hormone whose levels rise remarkably to maintain pregnancy, appears to be the force that leads to repression of Th1-dependent immune response and increase in levels of Th2-secreted cytokines.[40]
IMMUNE ACTIVATION OFTEN IMPAIRS REPRODUCTIVE FITNESS
There is extensive evidence demonstrating that infections and/or immune dysfunction negatively impact every aspect of human reproduction from gamete production and establishment and maintenance of pregnancy to fetal and neonatal health.[6,7] Preexisting immune disorders are linked to premature ovarian failure, recurrent miscarriages and poor pregnancy outcomes.[6,41] During pregnancy, infectious diseases such as malaria or pneumonia are associated with increased incidence of fetal growth restriction, premature births and adverse pregnancy outcomes.[2] Preeclampsia, or gestational hypertension, responsible for ~15% of premature births in the US, is characterized by chronic immune activation and high pro-inflammatory cytokine levels.[42] Aberrant maternal immune activation has even been implicated in a range of neurocognitive defects in children including schizophrenia and autism, and in the effects of cocaine and opioids on the fetal respiratory system.[43] Interestingly, even during infections with TORCH pathogens, best known for their ability to cross the placenta and directly infect the fetus, pregnancy complications due to maternal immune response are noticeable.[44] In many cases, the detrimental effects of maternal infection have been ascribed to the production of inflammatory factors such as IL-6 that cause conversion of Treg cells into Th17 cells, thus, disrupting the Treg:Th17 ratio critical for healthy pregnancy.[45] However, the molecular mechanisms that control these shifts remain largely unknown.
MODEL ORGANISM STUDIES HAVE PROVIDED KEY INSIGHTS INTO IMMUNITY-FERTILITY MOLECULAR INTERACTIONS
Much of our knowledge about the immunological adaptations of pregnancy, and the links between inflammation and poor pregnancy outcomes described above, is derived from investigations in rodent models.[34,46] In particular, studies in knock-out mice have allowed for comparisons of pregnancy rates, litter sizes, and embryonic health, linking these to changes in specific immune populations. For example, a definitive role of Tregs in establishment of pregnancy was shown through a series of elegant mouse experiments in which antibody-induced depletion of Tregs was found to induce implantation defects.[47,48] Vertebrate models have been used extensively to model infections that decrease fertility, either by directly targeting reproductive tissues (e.g., Trypanosoma brucei infecting uterine and testicular cells)[49,50] or by spreading to reproductive tissues from systemic origins (e.g., ZIKA virus dissemination).[51–54] Though rodent models closely mirror human biology, studies with them are hampered by their relatively long lives and low progeny number compared to other research models (mice and rats have an average lifespan of ~2 years, whereas worms and flies live for ~3 weeks and ~6 months, respectively). The inherent complexities of innate and adaptive immune systems in vertebrates also pose challenges to mechanistic studies. Alternatively, direct assessment of the immunity-fertility crosstalk is easier in many invertebrates because these “fast-living” species have short lifespans, large brood sizes and rely on innate immune response alone (Figure 1), although the same simplicity also make relevance to mammalian biology challenging.[16] In fact, an extensive body of invertebrate literature originating from research on life history traits has documented the impact of reproductive activity on immune resistance and vice versa (reviewed in [5,15]). Drosophila studies were some of the first to reveal the direct links between matig and immune resistance in females, and in recent years, have been instrumental in revealing the genes and pathways involved in the immunity-fertility crosstalk.[5,55] Studies in C. elegans, on the impact of mating on lifespan, have supported these observations.[56,57] The emerging theme from these studies implicates signaling pathways and regulatory molecules with important roles in both reproduction and immunity as being central players in determining the outcome of the fertility-immunity dialogue (Figure 3). In the next two sections, we briefly summarize this work from the fly and worm models.
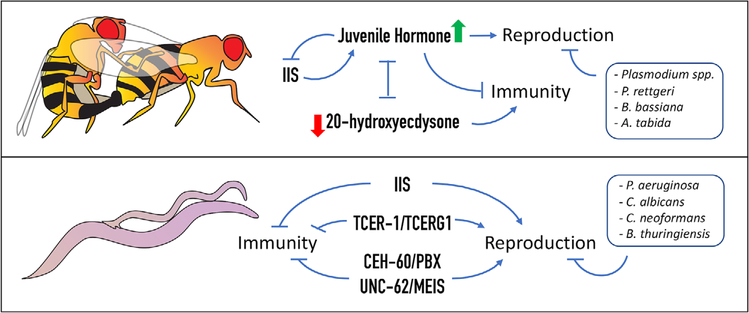
FIGURE 3.
Molecular determinants governing the fertility-immunity axis in invertebrates. There is extensive literature documenting the mutual impacts of reproductive activity and immunity in invertebrates. Studies in the fruit fly, D. melanogaster (top), and the nematode, C. elegans (bottom), have begun to reveal the underlying molecular pathways. In D. melanogaster (top panel), major endocrine signaling cascades, the juvenile hormone (JH) pathway and the 20 hydroxy ecdysterone (20E) pathway, that control growth and maturation, have antagonistic impacts on fertility and immunity. JH promotes reproduction and inhibits immunity, along with the conserved growth regulator, the insulin/IGF1 signaling (IIS) pathway, whereas, 20E acts as an immune activator. The IIS pathway also inhibits immune activity and supports reproductive health in C. elegans (bottom panel). Recent studies in worms have also identified transcription factors with roles in this relationship. TCER-1, worm homolog of human transcription elongation and splicing factor, TCERG1, promotes reproductive fitness and represses innate immunity. CEH-60 and UNC-62, worm orthologs of the TALE class of homeodomain proteins, PBX and MEIS, act in a complex to mediate fat transport into oocytes, and to repress innate-immune genes’ expression, facilitating allocation of lipids towards fertility.
IMMUNITY-FERTILITY TRADEOFFS ARE EXPLICITLY DEMONSTRATED IN INVERTEBRATE MODELS
Generally, immune activity in females in a wide variety of insect species has been reported to be associated with reduced fertility.[5] In flies, mosquitoes, crickets and beetles, not only pathogenic infection, but even exposure to bacterial cell wall components, causes reductions in ovarian protein content, egg number and overall fecundity (reviewed in [5]). In species of Anopheles mosquitoes, a targeted degradation of oocytes is observed upon infection by the malarial parasite, Plasmodium.[58] In C. elegans, we found that exposure to the opportunistic human pathogen, Pseudomonas aeruginosa, caused reduction in the number of eggs laid by the animal within 4 h, and declined ~65% by 12 h—well before the animal shows any overt signs of infection.[59] Worms exposed to the yeast Cryptococcus neoformans show substantially reduced fertility. Fertility is also reduced upon exposure to Shiga toxin-producing Escherichia coli (SHEC) strains.[60,61] In fact, the dramatic fertility suppression induced by C. neoformans has been used as a rapid screening measure to identify conserved, virulence-determining pathogen genes.[62] Thus, fertility is responsive to immune perturbations and impacts are pathogen specific.[63] Lastly, Drosophila strains selected for high resistance to bacteria show reduced fecundity even when uninfected, suggesting that constitutive elevation of immunity is also detrimental for reproduction. This is reminiscent of the poor reproductive outcomes associated with chronic inflammatory diseases such as asthma in women.[1,2]
Conversely, increased reproductive activity has been associated with reduced immune fitness in many species.[5,64] The most explicit demonstrations of this effect have been made in Drosophila where female survival after an array of bacterial infections is reduced by mating. Mated females exhibit higher pathogen loads and reduced induction of anti-bacterial peptides.[65] The molecular basis of this effect can be traced to specific proteins in the seminal fluid transferred by the male during copulation. In fact, transferring just the seminal fluid without sperm—or sperm proteins alone—is sufficient to make un-mated female flies immune-susceptible.[55] It is worth noting here that, in both flies and worms, mating also shortens female longevity per se, even in the absence of infection.[56,66] In worms, mating has been shown to cause shrinkage of the mother’s body and increased susceptibility to osmotic stress.[57] So far, no reports have tested the impact of mating on worm immunity directly. Many sterile C. elegans mutants have been shown to survive longer upon infection than fertile worms. However, this is true for some sterility-inducing mutations and not others.[67] While in most species, the preponderance of data is from observation of females there is significant evidence that reproduction has immunological costs (and vice versa) in males as well.[68] Lastly, there are some notable exceptions where mating and reproduction leads to improved immunity.[5,69–71] These observations underscore the strong links between immunity and fertility, and emphasize that even in simple organisms it is shaped by many aspects of physiology and behavior besides resource tradeoff (see Box 1 for additional considerations and exceptions behind LHT).
MOLECULAR MECHANISMS UNDERLYING THE IMMUNITY-FERTILITY RELATIONSHIP ARE POORLY UNDERSTOOD
Despite the wealth of information from model organisms and humans on the mutual impacts of reproductive status and immune capability, our knowledge about the molecular underpinnings of this relationship is scant. If, as the overwhelming evidence suggests, the immunity-fertility relationship is reciprocal and fluid, then it follows logically that there must be signaling pathways that discern the animal’s physiological status and transmit this knowledge to core regulatory molecules that control these dynamics. The obvious candidates for these roles are likely to be signaling cascades and regulatory proteins that function in one, or both, of these processes. Indeed, studies in Drosophila and C. elegans have provided evidences for this premise that we describe below, although this research is still in its infancy.
CONSERVED SIGNALING PATHWAYS HAVE BEEN IMPLICATED IN THE IMMUNITY-FERTILITY AXIS
In Drosophila, two signaling pathways have been implicated in directly linking the immune and reproductive systems. These are the juvenile hormone (JH) and 20-hydroxyecdysone (20E) pathway, and the insulin/IGF1 (IIS) signaling pathway.[5] JH and 20E are major endocrine regulators in insects. JH facilitates larval growth and prevents metamorphosis, whereas 20E induces molting. Their balance mediates proper progression through development and metamorphosis.[72,73] Post-development, these hormones regulate multiple aspects of reproductive maturation, including oocyte maturation.[74, 75] But, during mating JH and 20E are mutually antagonistic. In many insect species, mating elevates JH levels and depresses 20E levels.[5] The two hormones also have opposing impacts on immunity: JH acts as an immune repressor in adults, whereas, 20E acts as an immune activator.[76,77] These strikingly opposite profiles have led to the suggestion that they mediate the immunity-fertility balance with JH promoting procreation and 20E enhancing immunity (Figure 3).[5] This is supported by evidence from other insects as well; in some species JH controls resource allocation between reproduction and immunity, whereas, in others it drives the tradeoff between reproduction and flight capacity.[77–79]
Reproduction and immunity in flies are also governed intimately by the insulin/IGF1 signaling (IIS) pathway that links nutritional status to growth and proliferation. IIS drives oogenesis in the female; egg production is diminished under reduced IIS signaling.[80] In contrast, IIS represses immune resistance so that low IIS activity increases immunity and immune-response pathways diminish IIS.[81] Interestingly, reduced IIS also leads to diminished JH levels in many instances, while low JH causes reduced IIS (Figure 2).[5,82] JH and 20E are restricted to insects, but the IIS pathway is a conserved from yeast to humans and performs similar anti-immunity functions in worms. Besides essential roles in development and maturation of the germline, IIS plays several roles in C. elegans adults during reproduction from meiosis progression to sperm guidance.[83,84] As in flies, IIS also represses innate immunity in worms (Figure 3). IIS inhibition results in elevated expression of a spectrum of anti-microbial genes as well as increased survival in the presence of numerous pathogens.[85]
The IIS and JH/20E pathways are the prominent candidates for mediating the immunity-fertility dialogue because of the strikingly opposing impacts they have on the two processes. However, other signaling cascades have very plausible roles in this relationship as well. For instance, in Drosophila, the immune-responsive Jun-N Kinase (JNK) cascade represses IIS signaling.[86] In fact, in both worms and flies immune-regulatory pathways often intersect with, or have roles in, reproduction. The TGFβ pathway plays an important protective role during pathogenesis in worms while mediating reproductive aging.[85,87] Similarly, the conserved p38 MAPK cascade, a cornerstone of worm immune responses, also controls apoptosis in the germline during infection.[85,88,89] In Drosophila, a canonical p38-mediated MAPK cascade contributes to defense against microbial infection too, and a protective role for TGFβ signaling upon infection by Micrococcus luteus has been identified.[90,91] Both pathways also influence fertility and reproductive fitness in flies.[92,93] Hence, it is conceivable that the mutual interactions of these pathways shape the transcriptional programs that determine reproductive health and immune status. Though the details vary, these functions are conserved between species.
CONSERVED TRANSCRIPTION FACTORS ARE INVOLVED IN THE IMMUNITY-FERTILITY DIALOGUE
Signal transduction cascades are conduits that transmit physiological and environmental information to downstream transcription factors that bring about requisite gene expression changes. So, it is expected that the signaling pathways involved in the immunity-fertility crosstalk impinge on transcription regulators. Indeed, reduced IIS signaling inhibits the activity of several proteins essential for triggering anti-microbial gene expression, including the conserved FOXO family members DAF-16 in worms and dFOXO in flies.[94] But, are there dedicated transcription factor(s) that integrate these signals to directly control the immunity-fertility crosstalk? Recent studies have revealed the existence of such “master regulator” proteins. We serendipitously discovered such a role for TCER-1, the C. elegans homolog of the human transcription elongation and splicing factor, TCERG1.[95,96] We first identified TCER-1 as a factor that conferred enhanced lifespan on C. elegans adults lacking a germline.[97] In investigating its functions in normal, fertile animals, we discovered that TCER-1 was essential for fertility and reproductive health. tcer-1 mutants laid fewer, and less healthy, eggs and showed signs of premature reproductive senescence.[98] Interestingly, we also found that tcer-1 mutants showed exceptional resistance against infection by P. aeruginosa and other Gram-positive and Gram-negative pathogens. Conversely, TCER-1 overexpression decreased resistance upon infection.[59] Given that pro-longevity genes often enhance stress resistance and immune resistance, this was an unexpected discovery as it suggested that TCER-1 is a novel pro-longevity factor that widely represses immunity. We further found that TCER-1 inhibits immunity only during the fertile stages of life and not after reproductive cessation, suggesting that it may repress immunity to divert cellular resources towards fertility. To test this, we asked if elevating TCER-1 levels can alleviate the decline in progeny production experienced by worms upon infection. Indeed, while normal animals exhibited a ~65–70% reduction in egg laying within 12 h of being exposed to P. aeruginosa, worms that overexpressed TCER-1 showed considerable protection against this fertility loss.[59] Hence, TCER-1 appears to provide a molecular link that determines the animal’s physiological status on the continuum of peak fertility to peak immune fitness (Figure 2). These discoveries are interesting not only because they reveal TCER-1 to be an important arbiter of the immunity-fertility crosstalk, arguably one of the first such factors to be identified, but also because they open avenues to discover the molecular basis of resource allocation, a question that has previously been inaccessible. If, for instance, TCER-1 (and other factors like it) directs resource allocation to tilt the balance between fertility and immunity, then these resource(s) can now be identified.
LIPID METABOLISM IS MODULATED BY REGULATORS OF IMMUNITY-FERTILITY AXIS
In both invertebrates and vertebrates, the primary cellular resource capable of meeting the high energetic demands of reproduction (i.e., production of eggs and the deposition of large quantities of fat, proteins and organelles in them) is stored fat. Fat also fulfills the high-energy required to mount immune response during infections across phyla.[99,100] Lipids also serve as key signaling molecules for orchestrating immune/stress tolerance gene expression and progression of many, if not all, steps of reproduction.[99] There are several strong lines of evidence in both flies and worms that suggest that cellular lipids may form a vital link between fertility and immunity. TCER-1 promotes longevity by mediating widespread changes in lipid anabolic and catabolic pathways and maintaining lipid homeostasis in germline-less worms, so it is highly likely that the protein also impacts lipid metabolism to support fertility and inhibit immunity.[98] Interestingly, another conserved pro-longevity factor, SKN-1, worm homolog of the human protein NRF2, has recently been identified as modulating lipid deposition into eggs upon infection.[101] The Curran lab found that exposure to a pathogenic strain of P. aeruginosa, but not an avirulent one, causes a rapid depletion of somatic lipids and a concomitant transfer of fats to the eggs reliant upon SKN-1 activity. SKN-1 gain-of-function mutants exhibit reduced somatic fat and increased lipid deposition in eggs even without pathogen exposure. These mutants are highly susceptible to pathogen-mediated death and restoring their somatic fat levels also restores their resistance against pathogen.[101]
Evidence for lipid allocation being an important link between immunity and fertility also comes from the studyof CEH-60 and UNC-62, C. elegans orthologs of the TALE class of homeodomain transcription factors, PBX and MEIS, respectively. In a recent study, Robert Dowen demonstrated that CEH-60 and UNC-62 act together in a complex to directly activate the expression of vitellogenin (VIT) proteins that transport fat into eggs, and to repress stress-responsive genes including those conferring immunity (Figure 3).[102] Consequently, ceh-60 mutants have reduced fat deposition in their eggs but hyperactivation of innate immunity genes and increased survival in the presence of P. aeruginosa.[102] Notably, the signaling cascades discussed above are also conserved regulators of lipid metabolism, and studies in other organisms also link fat to immune response. In Drosophila, JH is critical for incorporation of VITs and associated lipids into maturing oocytes, whereas pathogen exposure (or genetic activation of the immune response) leads to decreased triglyceride levels in the fly fat body through suppression of IIS.[75,103] The regulation of lipid metabolism by IIS is, in turn, conserved between flies, worms and mammals. Whether lipids are the only resource whose allocation directs the immunity-fertility balance, and the kind of qualitative and quantitative changes they may be subjected to during the process, remains unknown.
Another outstanding question about these recent discoveries pertain to their conservation in mammalian systems and their potential relevance to the immunity-fertility axis in humans. While it is a given that vertebrates and invertebrates clearly differ, it is noteworthy that many of the genes and molecules discussed here are conserved. TCERG1 is highly enriched in vertebrate oocytes, including mice, monkeys and humans.[104,105] Similar to our observations that TCER-1 declines with age in the worm germline, TCERG1 levels reportedly diminish significantly with age in oocytes of mice and women.[59,105,106] Additionally, mutants for the Arabidopsis homologue of TCER-1, AtPRP40C, show a late-flowering phenotype and increased resistance against P. syringae pv. maculicola infection, raising the enticing possibility of functional conservation.[107] Like SKN-1, its mammalian homolog, NRF2, plays key roles in the innate immune response.[108] NRF2 impacts oogenesis and spermatogenesis as well as regulation of lipid metabolism in adipocytes and the liver.[109–111] Similarly, FOXO-mediated expression of anti-microbial proteins is conserved from worms to mammals, as is the function in lipid homeostasis.[94] The interactions between PBX, MEIS and other TALE members are also conserved across species, and while they have mostly been studied for their roles in mammalian neuronal development, there is increasing evidence for their roles in lipid homeostasis.[112] Notably, a human CEH-60 ortholog, PBX1, has been shown to control maintenance of immune tolerance in T cells, and genetic variations in the Pbx1 gene have been associated with lupus susceptibility.[113]
CONCLUSIONS AND OUTLOOK
A wealth of evidence exists for the profound links between reproductive status and immune health in species ranging from worms to humans. But, deciphering the molecular mechanisms underlying this dynamic association has been challenging. Even in simple invertebrates, where tradeoff and resource allocation appear to be the overt drivers of this relationship, it has been difficult to identify the limiting resources or the molecular pathways controlling their distribution. However, recent discoveries in model organisms have begun revealing the existence of signaling pathways and transcriptional “master regulators” that integrate physiological and environmental signals to orchestrate the balance between reproductive fitness and immune health. The mutual relationships of these transcription factors and signaling molecules remain unknown, as do the identities of other players controlling this complex relationship. These are likely to be major foci of current and future investigations in the field (Box 2). As many of these genes and molecules are evolutionarily conserved, investigating their biology holds the promise of expanding our understanding of the immunity-fertility axis in humans and leveraging it in the service of human health.
BOX 2: Concluding Questions.
What is the molecular currency involved in the immunity-fertility tradeoff? Could lipids or other cellular building blocks play this role?
What are the “master regulator” proteins that link reproduction and immune status and what are their mechanisms of action?
How conserved are the mechanisms that regulate immunity-fertility tradeoffs between invertebrates and mammals, especially primates?
What are the long-term consequences of the immunity-fertility crosstalk on maternal post-reproductive health?
Are the global changes in modern human reproductive profiles influencing the evolution of our immune system?
ACKNOWLEDGMENTS
The authors acknowledge the support provided by grants from the National Institutes of Health to A.G. (R01AG051659) and J.L.Y. (R01GM104007) and a Children’s Hospital of Pittsburgh Research Advisory Committee (RAC) fellowship to N.N.
Funding information
National Institute on Aging, Grant/Award Number: R01AG051659; National Institute of General Medical Sciences, Grant/Award Number: R01GM104007
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated.
REFERENCES
- 1.Bonney EA (2016). Immune regulation in pregnancy: A matter of perspective? Obstet Gynecol. Clin. North Am, 43(4), 679–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis AP, Read JS, & Jamieson DJ (2014). Pregnancy and infection. N. Engl. J. Med, 371(11), 1075. [DOI] [PubMed] [Google Scholar]
- 3.Arck PC, & Hecher K (2013). Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med, 19(5), 548–556. [DOI] [PubMed] [Google Scholar]
- 4.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, … Yoshinaga K (2015). Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol, 16(4), 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwenke RA, Lazzaro BP, & Wolfner MF (2016). Reproduction-immunity trade-offs in insects. Annu. Rev. Entomol, 61, 239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen A, Kushnir VA, Barad DH, & Gleicher N (2014). Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol, 10(1), 37–50. [DOI] [PubMed] [Google Scholar]
- 7.Brazdova A, Senechal H, Peltre G, & Poncet P (2016). Immune aspects of female infertility. Int. J. Fertil. Steril, 10(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsum E, & Lof M (2007). Energy metabolism during human pregnancy. Annu. Rev. Nutr, 27, 277–292. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Schlitzer A, Placek K, Joosten LAB, & Schultze JL (2019). Innate and adaptive immune memory: An evolutionary continuum in the host’s response to pathogens. Cell Host Microbe, 25(1), 13–26. [DOI] [PubMed] [Google Scholar]
- 10.Muehlenbein MP, Hirschtick JL, Bonner JZ, & Swartz AM (2010). Toward quantifying the usage costs of human immunity: Altered metabolic rates and hormone levels during acute immune activation in men. Am. J. Hum. Biol, 22(4), 546–556. [DOI] [PubMed] [Google Scholar]
- 11.Rauw WM (2012). Immune response from a resource allocation perspective. Front. Genet, 3, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDade TW, Georgiev AV, & Kuzawa CW (2016). Trade-offs between acquired and innate immune defenses in humans. Evol. Med. Public Health, 2016(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wigby S, Suarez SS, Lazzaro BP, Pizzari T, & Wolfner MF (2019). Sperm success and immunity. Curr. Top. Dev. Biol, 135, 287–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stearns SC (1992). The evolution of life histories, Oxford, New York: Oxford University Press. [Google Scholar]
- 15.Flatt T, & Heyland A (2011). Mechanisms of life history evolution: The genetics and physiology of life history traits and trade-offs. Oxford; New York: Oxford University Press. [Google Scholar]
- 16.Lee KA (2006). Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol, 46(6), 1000–1015. [DOI] [PubMed] [Google Scholar]
- 17.Hales KG, Korey CA, Larracuente AM, & Roberts DM (2015). Genetics on the fly: A primer on the Drosophila model system. Genetics, 201(3), 815–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corsi AK, Wightman B, & Chalfie M (2015). A transparent window into biology: A primer on caenorhabditis elegans. Genetics, 200(2), 387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy K, Ezard THG, Jones OR et al. (2019). Animal life history is shaped by the pace of life and the distribution of age-specific mortality and reproduction. Nat Ecol Evol, 3, 1217–1224. 10.1038/s41559-019-0938-7. [DOI] [PubMed] [Google Scholar]
- 20.Olmos-Ortiz A, Flores-Espinosa P, Mancilla-Herrera I, Vega-Sanchez R, Diaz L, & Zaga-Clavellina V (2019). Innate immune cells and toll-like receptor-dependent responses at the maternal-fetal interface. Int. J. Mol. Sci, 20(15), 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlebacher A (2013). Immunology of the maternal-fetal interface. Annu. Rev. Immunol, 31, 387–411. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang Y, Mouillet JF, Coyne CB, & Sadovsky Y (2014). Review: Placenta-specific microRNAs in exosomes – good things come in nano-packages. Placenta, 35 Suppl, S69–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shynlova O, Lee YH, Srikhajon K, & Lye SJ (2013). Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reprod. Sci, 20(2), 154–167. [DOI] [PubMed] [Google Scholar]
- 24.Ernerudh J, Berg G, & Mjosberg J (2011). Regulatory T helper cells in pregnancy and their roles in systemic versus local immune tolerance. Am. J. Reprod. Immunol, 66(66 Suppl), 31–43. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Nakashima A, Shima T, & Ito M (2010). Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol, 63(6), 601–610. [DOI] [PubMed] [Google Scholar]
- 26.Bonney EA, & Onyekwuluje J (2003). The H-Y response in midgestation and long after delivery in mice primed before pregnancy. Immunol. Invest, 32(1–2), 71–81. [DOI] [PubMed] [Google Scholar]
- 27.Dutta S, Sengupta P, & Haque N (2020). Reproductive immunomodulatory functions of B cells in pregnancy. Int. Rev. Immunol, 39(2), 53–66. [DOI] [PubMed] [Google Scholar]
- 28.Ostensen M, Sicher P, Forger F, & Villiger PM (2005). Activation markers of peripheral blood mononuclear cells in late pregnancy and after delivery: A pilot study. Ann. Rheum. Dis, 64(2), 318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groer ME, Jevitt C, & Ji M (2015). Immune changes and dysphoric moods across the postpartum. Am. J. Reprod. Immunol, 73(3), 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, & Amino N (1997). Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. Am. J. Reprod. Immunol, 37(5), 368–377. [DOI] [PubMed] [Google Scholar]
- 31.Tay CC (1991). Mechanisms controlling lactational infertility. J. Hum. Lact, 7(1), 15–18. [DOI] [PubMed] [Google Scholar]
- 32.Dieterich CM, Felice JP, O’Sullivan E, & Rasmussen KM (2013). Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin. North Am, 60(1), 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, & Skountzou I (2017). H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog, 13(11), e1006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan L, Nguyen T, & McComb S (2013). From mice to women: The conundrum of immunity to infection during pregnancy. J. Reprod. Immunol, 97(1), 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, & Way SS (2011). Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe, 10(1), 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson DP, & Klein SL (2012). Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav, 62(3), 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patas K, Engler JB, Friese MA, & Gold SM (2013). Pregnancy and multiple sclerosis: Feto-maternal immune cross talk and its implications for disease activity. J Reprod. Immunol, 97(1), 140–146. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell AD (2016). Helminth infection during pregnancy: Insights from evolutionary ecology. Int. J. Womens Health, 8, 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mpairwe H, Tweyongyere R, & Elliott A (2014). Pregnancy and helminth infections. Parasite Immunol, 36(8), 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szekeres-Bartho J (2018). The role of progesterone in feto-maternal immunological cross talk. Med. Princ. Pract, 27(4), 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, & Lin QD (2010). Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol, 84(2), 164–170. [DOI] [PubMed] [Google Scholar]
- 42.Cornelius DC (2018). Preeclampsia: From inflammation to immunoregulation. Clin. Med. Insights Blood Disord, 11, 1179545X17752325,1179545X1775232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knutson AO, & Watters JJ (2020). All roads lead to inflammation: Is maternal immune activation a common culprit behind environmental factors impacting offspring neural control of breathing? Respir. Physiol. Neurobiol, 274, 103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, & Divanovic S (2016). Inflammation and preterm birth. J. Leukoc Biol, 99(1), 67–78. [DOI] [PubMed] [Google Scholar]
- 45.Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, & Ashkanani L (2001). Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod, 16(10), 2219–2226. [DOI] [PubMed] [Google Scholar]
- 46.Bonney EA (2013). Demystifying animal models of adverse pregnancy outcomes: Touching bench and bedside. Am. J. Reprod. Immunol, 69(6), n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robertson SA, Care AS, & Moldenhauer LM (2018). Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest, 128(10), 4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, & Armant DR (2013). Physiological and molecular determinants of embryo implantation. Mol. Aspects Med, 34(5), 939–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biteau N, Asencio C, Izotte J, Rousseau B, Fevre M, Pillay D, & Baltz T (2016). Trypanosoma brucei gambiense Infections in mice lead to tropism to the reproductive organs, and horizontal and vertical transmission. PLoS Negl. Trop Dis, 10(1), e0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho T, Trindade S, Pimenta S, Santos AB, Rijo-Ferreira F, & Figueiredo LM (2018). Trypanosoma brucei triggers a marked immune response in male reproductive organs. PLoS Negl. Trop Dis, 12(8), e0006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, … Diamond MS (2016). Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell, 165(5), 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, … Fikrig E (2017). Zika virus causes testicular atrophy. Sci. Adv, 3(2), e1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caine EA, Jagger BW, & Diamond MS (2018). Animal models of Zika virus infection during pregnancy. Viruses, 10(11), 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsetsarkin KA, Maximova OA, Liu G, Kenney H, Teterina N, Bloom ME, … Pletnev AG (2018). Routes of Zika virus dissemination in the testis and epididymis of immunodeficient mice. Nat. Commun, 9(1), 5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Short SM, Wolfner MF, & Lazzaro BP (2012). Female Drosophila melanogaster suffer reduced defense against infection due to seminal fluid components. J. Insect Physiol, 58(9), 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maures TJ, Booth LN, Benayoun BA, Izrayelit Y, Schroeder FC, & Brunet A (2014). Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science, 343(6170), 541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi C, & Murphy CT (2014). Mating induces shrinking and death in Caenorhabditis mothers. Science, 343(6170), 536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vezilier J, Nicot A, Gandon S, & Rivero A (2012). Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. Biol. Sci, 279(1744), 4033–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amrit FRG, Naim N, Ratnappan R, Loose J, Mason C, Steenberge L, … Ghazi A (2019). The longevity-promoting factor, TCER-1, widely represses stress resistance and innate immunity. Nat. Commun, 10(1), 3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mylonakis E, Ausubel FM, Perfect JR, Heitman J, & Calderwood SB (2002). Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA, 99(24), 15675–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang S-B, Choi J-G, Wei S, Park B-J, Chelliah R, & Oh D-H (2018). In vivo screening platform for shiga toxin-producing Escherichia coli (STEC) using Caenorhabditis elegans as a model. PLoS One, 13(2), e0193277–e0193277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang RJ, Breger J, Idnurm A, Gerik KJ, Lodge JK, Heitman J, … Mylonakis E (2005). Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a Caenorhabditis elegans progeny-based approach. Infect. Immun, 73(12), 8219–8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howick VM, & Lazzaro BP (2014). Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol. Biol, 14(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oku KP, Price TAR, & Wedell N (2019). Does mating negatively affect female immune defences in insects? Animal Biol, 69(1), 117–136. [Google Scholar]
- 65.Short SM, & Lazzaro BP (2010). Female and male genetic contributions to post-mating immune defence in female Drosophila melanogaster. Proc. Biol. Sci, 277(1700), 3649–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman T, Liddle LF, Kalb JM, Wolfner MF, & Partridge L (1995). Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature, 373(6511), 241–244. [DOI] [PubMed] [Google Scholar]
- 67.Miyata S, Begun J, Troemel ER, & Ausubel FM (2008). DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics, 178(2), 903–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKean KA, & Nunney L (2001). Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A, 98(14), 7904–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vigneron A, Jehan C, Rigaud T, & Moret Y (2019). Immune defenses of a beneficial pest: The mealworm beetle, Tenebrio molitor. Front. Physiol, 10, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galvez D, & Chapuisat M (2014). Immune priming and pathogen resistance in ant queens. Ecol. Evol, 4(10), 1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barribeau SM, & Schmid-Hempel P (2017). Sexual healing: Mating induces a protective immune response in bumblebees. J. Evol. Biol, 30(1), 202–209. [DOI] [PubMed] [Google Scholar]
- 72.Hiruma K, & Kaneko Y (2013). Hormonal regulation of insect metamorphosis with special reference to juvenile hormone biosynthesis. Curr. Top. Dev. Biol, 103, 73–100. [DOI] [PubMed] [Google Scholar]
- 73.Yamanaka N, Rewitz KF, & O’Connor MB (2013). Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol, 58, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flatt T, Tu MP, & Tatar M (2005). Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays, 27(10), 999–1010. [DOI] [PubMed] [Google Scholar]
- 75.Santos CG, Humann FC, & Hartfelder K (2019). Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci, 31, 43–48. [DOI] [PubMed] [Google Scholar]
- 76.Flatt T, Heyland A, Rus F, Porpiglia E, Sherlock C, Yamamoto R, … Silverman N (2008). Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J. Exp. Biol, 211(Pt 16), 2712–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwenke RA, & Lazzaro BP (2017). Juvenile hormone suppresses resistance to infection in mated female Drosophila melanogaster. Curr. Biol, 27(4), 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zera AJ, & Harshman LG (2001). The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Evol. Syst, 32(1), 95–126. [Google Scholar]
- 79.Zera AJ, & Zhao Z (2004). Effect of a juvenile hormone analogue on lipid metabolism in a wing-polymorphic cricket: Implications for the endocrine-biochemical bases of life-history trade-offs. Physiol. Biochem. Zool, 77(2), 255–266. [DOI] [PubMed] [Google Scholar]
- 80.Templeman NM, & Murphy CT (2018). Regulation of reproduction and longevity by nutrient-sensing pathways. J. Cell Biol, 217(1), 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Libert S, Chao Y, Zwiener J, & Pletcher SD (2008). Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol. Immunol, 45(3), 810–817. [DOI] [PubMed] [Google Scholar]
- 82.Tu MP, Yin CM, & Tatar M (2005). Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen. Comp. Endocrinol, 142(3), 347–356. [DOI] [PubMed] [Google Scholar]
- 83.Lopez AL 3rd, Chen J, Joo HJ, Drake M, Shidate M, Kseib C, & Arur S (2013). DAF-2 and ERK couple nutrient availability to meiotic progression during Caenorhabditis elegans oogenesis. Dev. Cell, 27(2), 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edmonds JW, Prasain JK, Dorand D, Yang Y, Hoang HD, Vibbert J, … Miller MA (2010). Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell, 19(6),858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim DH, & Ewbank JJ (2018). Signaling in the innate immune response. Worm. Book, 2018, 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang MC, Bohmann D, & Jasper H (2005). JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell, 121(1), 115–125. [DOI] [PubMed] [Google Scholar]
- 87.Luo S, Kleemann GA, Ashraf JM, Shaw WM, & Murphy CT (2010). TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell, 143(2), 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salinas LS, Maldonado E, & Navarro RE (2006). Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ, 13(12), 2129–2139. [DOI] [PubMed] [Google Scholar]
- 89.Aballay A, Drenkard E, Hilbun LR, & Ausubel FM (2003). Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr. Biol, 13(1), 47–52. [DOI] [PubMed] [Google Scholar]
- 90.Chen J, Xie C, Tian L, Hong L, Wu X, & Han J (2010). Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc. Natl. Acad. Sci. U. S. A, 107(48), 20774–20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clark RI, Woodcock KJ, Geissmann F, Trouillet C, & Dionne MS (2011). Multiple TGF-beta superfamily signals modulate the adult Drosophila immune response. Curr. Biol, 21(19), 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzanne M, Irie K, Glise B, Agnes F, Mori E, Matsumoto K, & Noselli S (1999). The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev, 13(11), 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monsivais D, Matzuk MM, & Pangas SA (2017). The TGF-beta family in the reproductive tract. Cold Spring Harb. Perspect. Biol, 9(10), a022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, … Hoch M (2010). FOXO-dependent regulation of innate immune homeostasis. Nature, 463(7279), 369–373. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez-Hernandez N, Boireau S, Schmidt U, Munoz-Cobo JP, Hernandez-Munain C, Bertrand E, & Sune C (2016). The in vivo dynamics of TCERG1, a factor that couples transcriptional elongation with splicing. RNA, 22(4), 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coiras M, Montes M, Montanuy I, Lopez-Huertas MR, Mateos E, Le Sommer C, … Sune C (2013). Transcription elongation regulator 1 (TCERG1) regulates competent RNA polymerase II-mediated elongation of HIV-1 transcription and facilitates efficient viral replication. Retrovirology, 10, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghazi A, Henis-Korenblit S, & Kenyon C (2009). A transcription elongation factor that links signals from the reproductive system to lifespan extension in Caenorhabditis elegans. PLoS Genet, 5(9), e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amrit FR, Steenkiste EM, Ratnappan R, Chen SW, McClendon TB, Kostka D, … Ghazi A (2016). DAF-16 and TCER-1 facilitate adaptation to germline loss by restoring lipid homeostasis and repressing reproductive physiology in C. elegans. PLoS Genet, 12(2), e1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hubler MJ, & Kennedy AJ (2016). Role of lipids in the metabolism and activation of immune cells. J. Nutr. Biochem, 34, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dolezal T, Krejcova G, Bajgar A, Nedbalova P, & Strasser P (2019). Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol, 109, 31–42. [DOI] [PubMed] [Google Scholar]
- 101.Nhan JD, Turner CD, Anderson SM, Yen CA, Dalton HM, Cheesman HK, … Curran SP (2019). Redirection of SKN-1 abates the negative metabolic outcomes of a perceived pathogen infection. Proc. Natl. Acad. Sci. U. S. A, 116(44), 22322–22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dowen RH (2019). CEH-60/PBX and UNC-62/MEIS coordinate a metabolic switch that supports reproduction in C. elegans. Dev. Cell, 49(2), 235–250.e7. [DOI] [PubMed] [Google Scholar]
- 103.DiAngelo JR, Bland ML, Bambina S, Cherry S, & Birnbaum MJ (2009). The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. U. S. A, 106(49), 20853–20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, … Cibelli JB (2006). The transcriptome of human oocytes. Proc. Natl. Acad. Sci. U. S. A, 103(38), 14027–14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng P, Patel B, McMenamin M, Moran E, Paprocki AM, Kihara M, … Latham KE (2005). Effects of follicle size and oocyte maturation conditions on maternal messenger RNA regulation and gene expression in rhesus monkey oocytes and embryos1. Biol. Reprod, 72(4), 890–897. [DOI] [PubMed] [Google Scholar]
- 106.Steuerwald NM, Bermudez MG, Wells D, Munne S, & Cohen J (2007). Maternal age-related differential global expression profiles observed in human oocytes. Reproductive BioMedicine Online, 14(6), 700–708. [DOI] [PubMed] [Google Scholar]
- 107.Hernando CE, Garcia Hourquet M, de Leone MJ, Careno D, Iserte J, Mora Garcia S, & Yanovsky MJ (2019). A role for pre-mRNA-PROCESSING PROTEIN 40C in the control of growth, development, and stress tolerance in Arabidopsis thaliana. Front. Plant Sci, 10, 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Battino M, Giampieri F, Pistollato F, Sureda A, de Oliveira MR, Pittalà V, … Nabavi SM (2018). Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol. Adv, 36(2), 358–370. [DOI] [PubMed] [Google Scholar]
- 109.Ma R, Liang W, Sun Q, Qiu X, Lin Y, Ge X, … Chen L (2018). Sirt1/Nrf2 pathway is involved in oocyte aging by regulating cyclin B1. Aging, 10(10), 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortes MM, Hoang YD, … Luderer U (2010). Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic. Biol. Med, 49(9), 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seo H-A, & Lee I-K (2013). The role of Nrf2: Adipocyte differentiation, obesity, and insulin resistance. Oxid. Med. Cell. Longev, 2013,2013,1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oriente F, Perruolo G, Cimmino I, Cabaro S, Liotti A, Longo M, … Beguinot F (2018). Prep1, A homeodomain transcription factor involved in glucose and lipid metabolism. Front. Endocrinol, 9, 346–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sengupta M, & Morel L (2011). The role of Pbx1 in T cells. Protein Cell, 2(12), 946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated.