Abstract
Acute itch is elicited by histamine, as well as non-histaminergic itch mediators including chloroquine, BAM8–22 and Ser-Leu-Ile-Gly-Arg-Leu (SLIGRL). When injected intradermally, histamine binds to histamine H1 and H4 receptors that activate transient receptor potential vanilloid 1 (TRPV1) to depolarize pruriceptors. Chloroquine, BAM8–22, and SLIGRL, respectively, bind to Mas-related G-protein-coupled receptors MrgprA3, MrgprC11, and MrgprC11/PAR2 that in turn activate transient receptor potential ankyrin 1 (TRPA1). In this study we tested if histamine, chloroquine, BAM8–22 and SLIGRL elicit thermal hyperalgesia and mechanical allodynia in adult male mice. We measured the latency of hindpaw withdrawal from a noxious heat stimulus, and the threshold for hindpaw withdrawal from a von Frey mechanical stimulus. Intraplantar injection of histamine resulted in significant thermal hyperalgesia (p < 0.001) and mechanical allodynia (p < 0.001) ipsilaterally that persisted for 1 h. Pretreatment with the TRPV1 antagonist AMG-517 (10 or 20 μg), but not the TRPA1 antagonist HC-030031 (50 or 100 μg), significantly attenuated the magnitude and time course of thermal hyperalgesia and mechanical allodynia elicited by histamine (p < 0.001 for both), indicating that these effects are mediated by TRPV1. In contrast, pretreatment with the TRPA1 antagonist significantly reduced thermal hyperalgesia and mechanical allodynia elicited by chloroquine (p < 0.001 for both), BAM-822 (p < 0.01, p < 0.001, respectively) and SLGRL (p < 0.05, p < 0.001, respectively), indicating that effects elicited by these non-histaminergic itch mediators require TRPA1. TRPV1 and TRPA1 channel inhibitors thus may have potential use in reducing hyperalgesia and allodynia associated with histaminergic and non-histaminergic itch, respectively.
Keywords: antinociception, hyperalgesia, allodynia, thermal withdrawal, mechanical withdrawal, pruritus
INTRODUCTION
Itch is defined as an unpleasant skin sensation associated with the desire to scratch, thereby removing exogenous stimuli such as parasites and plant particles. Several receptors and transducers implicated in itch sensation have been identified, including histamine receptors (Simone et al., 1991), protease-activated-receptor subtypes PAR-2 and −4 (Akiyama et al., 2015), members of the Mas-related G-protein coupled receptor family (MrgprA3, MrgprC11) (Liu et al., 2009, 2011), and the transient receptor potential vanilloid 1 (TRPV1) and ankyrin 1 (TRPA1) channels (Hung and Tan, 2018; Kittaka and Tominaga, 2017; Koivisto et al., 2018; Moore et al., 2018; Xie and Li, 2019). Histamine acting via histamine H1 and H4 receptors requires the opening of TRPV1 ion channels in order to depolarize pruriceptive nerve endings (Shim et al., 2007; Imamachi et al., 2009). MrgprA3 and C11 are histamine-independent receptors activated, respectively, by chloroquine (Liu et al., 2009) and BAM8–22 (Liu et al., 2011) and require co-expression of TRPA1 for itch (Wilson et al., 2011). The tethered peptide Ser-Leu-Ile-Gly-Arg-Leu (SLIGRL) elicits scratching in mice also via PAR2 and MrgprC11 (Liu et al., 2011). In humans, intradermal injections of histamine or BAM8–22 elicited itch at the injection site, surrounded by a localized area of alloknesis (itch elicited by innocuous low-threshold mechanical stimulation) and hyperknesis (itch elicited by high-threshold mechanical stimulation) (LasMotte et al., 2009; Sikand et al., 2011a, 2011b; Simone et al., 1991). Interestingly, a region of hyperalgesia (increased pain elicited by high-threshold mechanical stimulation that normally elicited only faint pricking pain) overlapped the region of alloknesis and hyperknesis. In a mouse model, low-threshold mechanical stimulation of skin surrounding a site of intradermal histamine injection elicited hindlimb scratches indicative of alloknesis (Akiyama et al., 2012a). Moreover, previous studies have shown that intraplantar injection of SLIGRL in mice elicited thermal hyperalgesia and mechanical allodynia (Vergnolle et al., 2001; Dai et al., 2004; Liu et al., 2011). These studies suggest that histamine and non-histaminergic pruritogens elicit painful as well as itchy dysesthesias. We have presently addressed the question of whether histamine and the non-histaminergic pruritogens chloroquine, BAM8–22 and SLIGRL elicit thermal hyperalgesia and/or mechanical allodynia in mice. We have additionally addressed the potential roles of TRPV1 and TRPA1 in mediating hyperalgesia and allodynia evoked by histamine as well as these non-histaminergic pruritogens. Portions of this study have been previously reported (Nozadze et al., 2018; Tsagareli et al., 2019).
EXPERIMENTAL PROCEDURES
Animal care and use
The experiments were performed on wild-type male mice <50 g in body weight, bred at the vivarium of Beritashvili Experimental Biomedicine Center (BMC). Males were used to be consistent with the authors’ prior studies and to avoid estrous cycle effects. The animals were kept under standard housing conditions (22 ± 2 °C, 65% humidity, lights from 6:00 a.m. to 8:00 p.m.), and fed by standard dry diet; water was freely available. The experimental protocol was approved by the local bioethics committee of the Beritashvili Experimental BMC. Guidelines of the International Association for the Study of Pain (IASP) regarding investigations of experimental pain in conscious animals were followed throughout (The Biomedical Researcher’s Handbook, 1987; Zimmermann, 1983). Every attempt was made to follow ARRIVE guidelines (https://arriveguidelines.org/).
Chemical injections
Histamine (0.25, 0.5 and 1 M/10 μL), chloroquine (0.25,0.5, and 1 mM/10 μL), BAM8–22 (2.5 nM/10 μL; 5 nM/10 μL; 12 nM/10 μL), the peptide SLIGRL, and TRPA1 antagonist HC-030031 were purchased from Sigma-Aldrich Chemicals, Co. (St. Louis, MO, USA). SLIGRL was dissolved in saline to obtain final doses of 7.5, 15 and 40 mM/10 μL. HC-030031 was dissolved in 30 μL 1% DMSO and saline to obtain final doses of 50 and 100 μg/30 μL, consistent with previous studies (Liu and Ji, 2012; Wang et al., 2015; Coavoy-Sánchez et al., 2016; Tsagareli et al., 2019).
Each chemical was injected intraplantar through a 30 G needle connected by PE 50 tubing to a Hamilton microsyringe. We used the intraplantar route to assess thermal hyperalgesia and mechanical allodynia on the plantar hindpaw, consistent with previous studies. The same volume of vehicle (isotonic saline) was microinjected in the same manner separately as a control. Mice were divided into groups with n = 6/group. Each group received two intraplantar injections in a volume of 10 μL, either saline or one of the three concentrations of a given agent, requiring 24 mice per pruritogen at three concentrations. Successive injections were separated by at least 7 days. Following the injection, the mouse was tested in either the thermal withdrawal (Hargreaves) test, or the mechanical paw withdrawal (von Frey) test using a counterbalanced design. Immediately following the injections we observed that many mice exhibited biting and licking directed to the injected hindpaw.
In a second set of experiments, the effect of intraplantar pretreatment with two different doses of the TRPV1 antagonist AMG-517 (10, 20 μg) (Garami et al., 2017) or two doses of the TRPA1 antagonist HC-030031 (50, 100 μg) on thermal or mechanical withdrawals elicited by intraplantar injection of two doses of each pruritogen was tested. Again, mice were divided into groups of 6. Groups of mice received one of two doses of either AMG-517 or HC-030031 in a volume of 30 μL injected intraplantar, followed 20 min later by one of two doses of histamine (0.25 or 0.5 M/10 μL) also injected intraplantar. In separate groups, mice similarly received one of two doses of HC-030031, followed 20 min later by one of two doses of either chloroquine (0.5 or 1 mM), BAM8–22 (5, 12 nM) or SLIGRL (15, 40 mM). This procedure was done twice for each mouse, once for either the Hargreaves or the von Frey test, and again at least 7 days later for the other test.
Behavioral tests
Behavioral tests were conducted starting immediately after intraplantar injection of the pruritogen. In prior studies, each pruritogen tested elicits itch-related scratching behavior that lasts approximately 20–30 min (see Discussion). The thermal and mechanical paw withdrawal tests were conducted during this period out to 120 min post-injection.
Thermal paw withdrawal (Hargreaves) test
Mice first were habituated to stand on a glass surface heated to 30 °C within a Plexiglass enclosure, over three separate daily sessions. For formal testing, baseline latencies for paw withdrawals evoked by radiant thermal stimulation were taken for each hind paw a minimum of three times/paw, with at least 5 min elapsing between tests of a given paw. A light beam (Plantar Test 390, IITC, Woodland Hills, CA, USA) was focused onto the plantar surface of one hind paw through the glass plate from below, and the latency from onset of the light to brisk withdrawal of the stimulated paw was measured. When tested with the investigator’s finger the stimulus elicited pain at latencies consistent with the paw withdrawal. Reductions in latency were considered to reflect thermal hyperalgesia as defined by the International Association for the Study of Pain (IASP) (https://www.iasp-pain.org/terminology?navItemNumber=576) and consistent with the literature. The other hind paw was similarly tested 30–60 s later. The mouse was then held gently and one hind paw received an intraplantar injection of one of the pruritogens or vehicle. The investigator was blinded as to the chemical injected. The mice then were placed back onto the glass plate and withdrawal latencies of both paws were measured at 3, 15, 30, 45, 60, and 120 min post-injection.
Mechanical paw withdrawal threshold (von Frey) test
Mice were first habituated to stand on a wire mesh surface. For formal testing, baseline withdrawals were assessed using an Electronic von Frey Aesthesiometer (2390, IITC, CA, USA). The plastic filament was pressed against the ventral paw from below. This device samples and holds force (g) at the moment that the hind paw withdrew from the filament. Each paw was tested for baseline mechanical withdrawals at least three times, with at least 5 min between successive measurements of a given paw. The mouse then received a unilateral intraplantar injection (see above) and was placed back onto the mesh surface. Mechanical paw withdrawals were measured at the same post-injection times as above for thermal paw withdrawals. Since mechanical stimuli eliciting withdrawals may not have been noxious (Barrot, 2012), reductions in threshold were considered to reflect mechanical allodynia as defined by IASP. The same groups of mice were used for thermal and mechanical withdrawal tests, with a minimum of 7 days in between successive tests to avoid possible carryover effects.
Statistics
All datasets from behavioral tests were confirmed to be normally distributed using the Kolmogorov–Smirnov test. Data were subjected to repeated measures analysis of variance (ANOVA). Comparisons between chemical and vehicle treatment of the same group of mice were made by paired t-test. Kruskal–Wallis ANOVA and subsequent Tukey test was used to assess differences between treatments. The data are expressed as mean ± s.e.m. Statistical significance was acknowledged if p < 0.05. The statistical software utilized was InStat 3.05 (GraphPad Software, Inc, San Diego, CA, USA).
RESULTS
Histamine
Intraplantar injection of histamine resulted in a significant reduction in thermal withdrawal latency and mechanical withdrawal threshold of the ipsilateral paw (Fig. 1A, C). For thermal withdrawals, there was a significant difference between the saline and all histamine groups combined (F(11,132) = 11.906, p < 0.0001, ANOVA), with post hoc tests showing significant differences at time points 5, 15, 30, 45 and 60 min (p < 0.01 for each) but not 120 min post-injection (p > 0.05) (Fig. 1A). The differences between groups receiving saline and each dose of histamine were statistically significant (p < 0.001 for each), although the differences between groups receiving each histamine dose were not indicating lack of dose-dependency. There was no statistically significant mirror image thermal hyperalgesic effect (p > 0.05) (Fig. 1B).
Fig. 1.
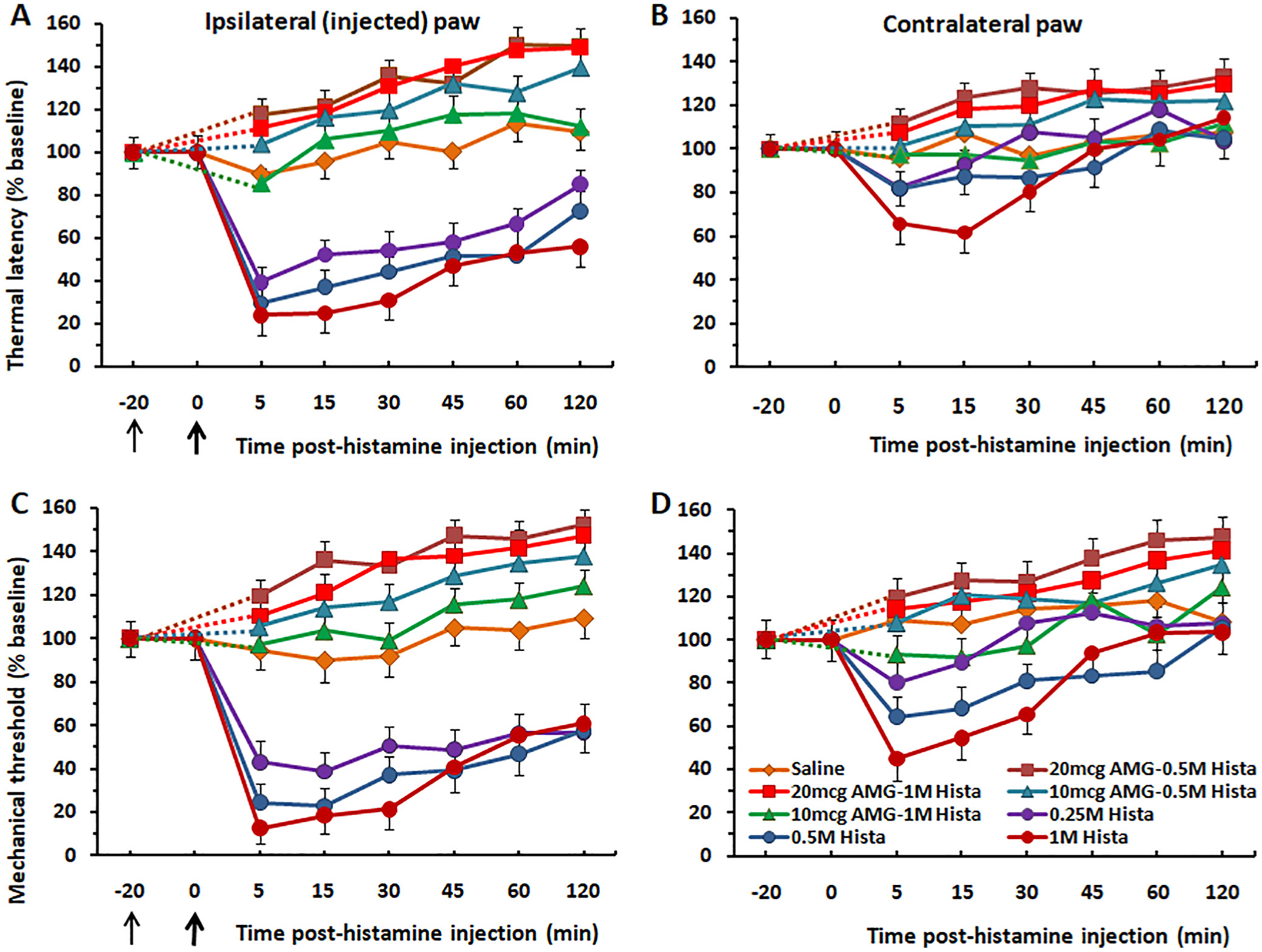
Thermal hyperalagesia and mechanical allodynia induced by intraplantar injection of histamine is prevented by pretreatment with the TRPV1 antagonist AMG-517. (A) Graph plots the mean thermal paw withdrawal latency following intraplantar injection of histamine (at bold arrow) at three different doses in the ipsilateral paw. Each histamine dose significantly reduced latencies compared to controls (p < 0.001) with no significant difference among histamine doses. Intraplantar injection of AMG-517 (thin arrow at −20 min) at both doses (50, 100 μg) significantly attenuated thermal hyperalgesia elicited by two different concentrations of histamine tested (0.5, and 1 M). (B) Format as in (A) for contralateral paw. There was no significant mirror image effect. (C) Graph plots mean mechanical withdrawal thresholds vs. time post-histamine injection in the ipsilateral paw. Histamine significantly reduced thresholds compared to vehicle controls (p < 0.001) with no significant difference among histamine doses. Both doses of AMG-517 prevented mechanical allodynia elicited by intraplantar histamine. (D) There was a significant mirror image allodynia effect at 5 min post-injection (p < 0.05). The allodynia effect was prevented by AMG-517. Thin black arrow: time of injection of AMG-517. Thick arrow: time of histamine injection. Dashed lines connect data for AMG-517 treatment groups with baseline at −20 min. Error bars: SEM.
Histamine also significantly reduced mechanical withdrawal thresholds compared to vehicle controls (F(11,132) = 13.376, p < 0.0001, ANOVA) with no significant difference among histamine doses (Fig. 1C). The differences between groups receiving saline and each dose of histamine were statistically significant at each time point post injection (p < 0.01 for each). There was also a significant mirror image mechanical allodynia effect only at 5 min post-injection (p < 0.05) (Fig. 1D). This mirror image effect may have been due to a spreading hyperalgesia/allodynia.
AMG-517 pretreatment completely prevented histamine-evoked thermal hyperalgesia and mechanical allodynia (Fig. 1A, C). Since there were no significant differences among the histamine or AMG-517 doses, data were pooled. There were significant differences between the AMG-517 pretreatment and histamine-only groups for thermal (F(7,568) = 51.426, p < 0.0001, ANOVA), and mechanical withdrawal of the ipsilateral hindpaw (F(7,568) = 65.635, p < 0.0001, ANOVA). For the thermal paw withdrawal test (ipsilateral paw), there were significant differences between the AMG-517 pretreatment and histamine-only groups at 5, 15, and 30 min post-injection (p < 0.0001 for each) (Fig. 1A). Similarly, for the von Frey test (ipsilateral paw), there were significant differences between the AMG-517 pretreatment and histamine-only groups at 5, 15, and 30 min post-injection (p < 0.001 for each) (Fig. 1C). AMG-517 also prevented the mirror image allodynia effect. For the contralateral (non-injected) paw, there were significant differences in thermal withdrawal latency between the AMG-517 pre-treatment and histamine groups at 5, 15 and 30 min post-injection (p < 0.05 for each) (Fig. 1B). Similar results were obtained for the von Frey test at 5, 15 and 30 min post-injection (p < 0.05 for each (Fig. 1D).
In contrast, intraplantar pretreatment with the TRPA1 antagonist HC-030013 at two doses (50 and 100 μg/30 μL) did not significantly affect thermal hyperalgesia or mechanical allodynia produced by either dose of histamine injected in the same hindpaw (Fig. 2). For the thermal withdrawal test (ipsilateral paw), there was no significant difference between AMG-517 pretreatment and histamine-only groups, at 5, 15 or 30 min post-injection of histamine (p > 0.05 for each) (Fig. 1A). Similarly, there were no significant differences between AMG-517-pretreatment and histamine-only groups on the contralateral paw at any time point (p > 0.05 for all) (Fig. 1B). The same was true for von Frey withdrawal thresholds, which were not different between AMG-517-pretreatment and histamine-only groups at any time point for either the ipsilateral (injected) or contralateral hindpaw (p > 0.05 for all) (Fig. 1C, D).
Fig. 2.
Thermal hyperalgesia and mechanical allodynia induced by intraplantar injection of histamine is not reduced by the TRPA1 antagonist HC-030031. Format as in Fig. 1. (A) Thermal withdrawal, ipsilateral paw. The significant thermal hyperalgesia induced by histamine was unaffected by pretreatment with HC-030031. (B) Thermal withdrawal, contralateral paw. The trend toward a weak mirror image hyperalgesic effect was not statistically significant. (C) Mechanical withdrawal, ipsilateral paw. The significant mechanical allodynia induced by histamine was unaffected by pretreatment with HC-030031. (D) Mechanical withdrawal, contrateral paw. The weak mirror image effect was not statistically significant. Thin black arrow: time of injection of HC-030031. Thick arrow: time of histamine injection. Dashed lines connect data for HC-030031 treatment groups with baseline at −20 min. Error bars: SEM.
Chloroquine
Intraplantar injection of chloroquine induced significant reductions in thermal withdrawal latency (F(11,132) = 11.53, p < 0.0001, ANOVA) and mechanical withdrawal threshold (F(11,132) = 12.44, p < 0.0001, ANOVA) of the ipsilateral paw (Fig. 3A, C). Each concentration of chloroquine significantly reduced thermal latencies and mechanical thresholds compared to the saline control group (p < 0.001) but not compared to each other, indicating a lack of dose-dependency. For the thermal paw withdrawal test (ipsilateral paw), there was a significant difference between the saline and chloroquine groups at 5, 15, 30, 45 and 60 (p < 0.05 for each) but not 120 min post-injection (Fig. 3A). The same was true for mechanical withdrawal thresholds (Fig. 3C). There were no significant mirror image effects in the contralateral paw for thermal or mechanical stimuli (Fig. 3B, D).
Fig 3.
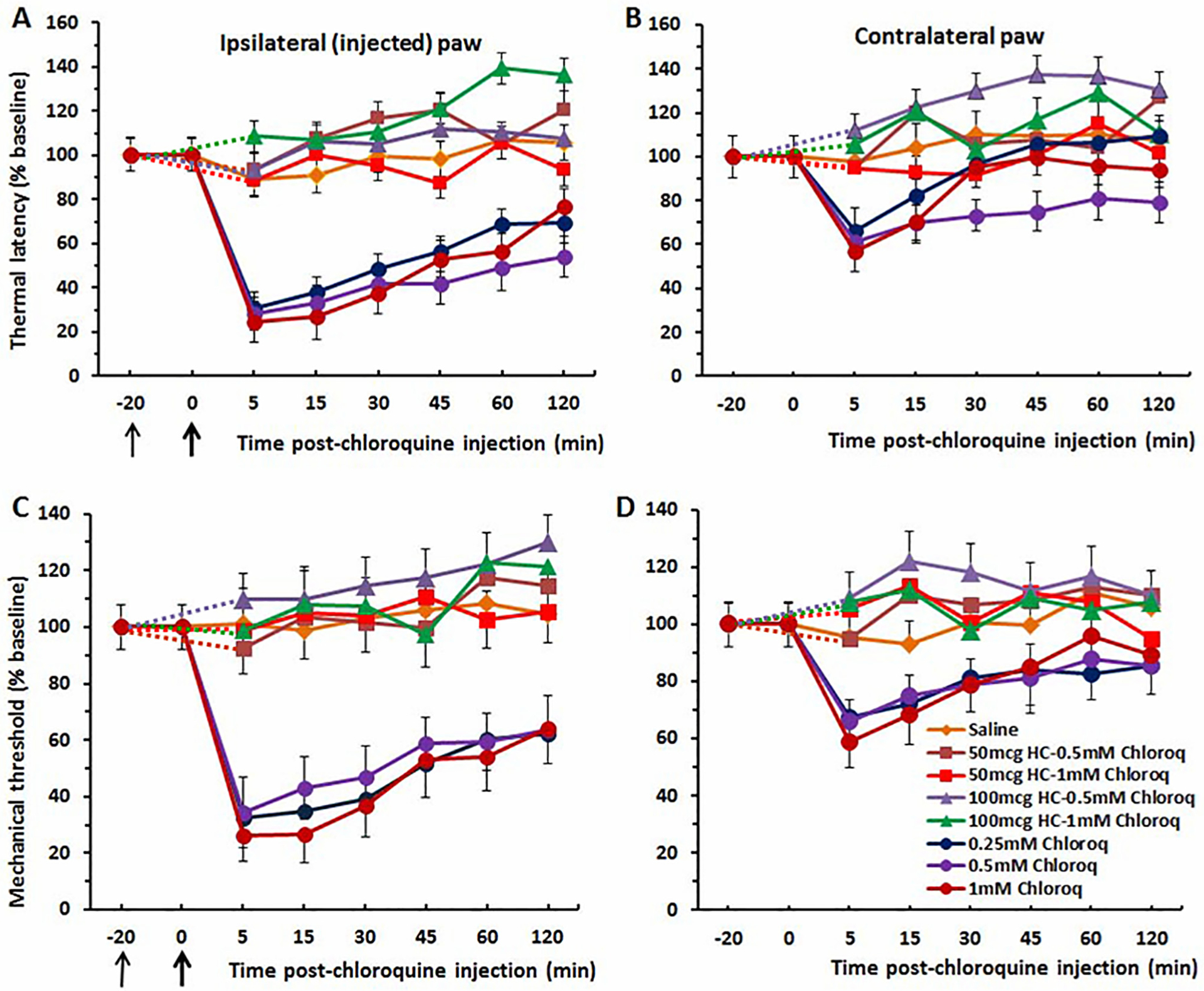
Thermal hyperalgesia and mechanical allodynia induced by intraplantar injection of chloroquine is reduced by the TRPA1 antagonist HC-030031. (A) Thermal withdrawal, ipsilateral paw. Chloroquine significantly reduced latencies compared to controls with no significant difference among chloroquine doses. Intraplantar injection of HC-030031 (thin arrow at −20 min) significantly attenuated thermal hyperalgesia elicited by chloroquine. (B) Thermal withdrawal, contralateral paw. The trend toward a small mirror image hyperalgesic effect was not significant. (C) Mechanical withdrawal, ipsilateral paw. Chloroquine significantly reduced thresholds compared to vehicle controls with no significant difference among chloroquine doses. HC-030031 prevented mechanical allodynia elicited by intraplantar chloroquine. (D) Mechanical withdrawal, contralateral paw. The trend toward a mirror image allodynia effect was not statistically significant. Thin black arrow: time of injection of HC-030031. Thick arrow: time of chloroquine injection. Dashed lines connect data for HC-030031 treatment groups with baseline at −20 min. Error bars: SEM.
Intraplantar pretreatment with the TRPA1 channel antagonist HC-030031 at two concentrations (50 and 100 μg/30 μL) attenuated thermal hyperalgesia produced by chloroquine injected in the same hindpaw. For the thermal paw withdrawal test (ipsilateral paw), there were significant differences between HC-030031 pretreatment groups and chloroquine-only groups at 5, 15, and 30 min post-injection (p < 0.001 for each) (Fig. 3A). The weaker mirror image thermal hyperalgesic effects were also prevented by pretreatment with HC-030031 (Fig. 3B). For the mechanical paw withdrawal (von Frey) test, significant differences were similarly observed at 5, 15 and 30 min post-injection (p < 0.001 for each) (Fig. 3C). The weaker mirror image mechanical allodynia effects were also prevented by pretreatment with HC-030031 (Fig. 3D).
BAM8–22
BAM8–22 significantly reduced thermal withdrawal latencies (F(11,132) = 9.562, p < 0.0001, ANOVA) (Fig. 4A) and mechanical withdrawal thresholds (F(11,132) = 10.174, p < 0.0001, ANOVA) (Fig. 4C), compared to the vehicle groups. For the thermal paw withdrawal test (ipsilateral paw), there were significant differences between the saline and BAM8–22 injected groups at 5 and 15 min post-injection (p < 0.001 for both), and at 30 min (p < 0.05) with no significant difference between the two BAM8–22 doses. For the von Frey test (ipsilateral paw), there were significant differences between saline and BAM8–22 groups at 5, 15, 30 and 45 min post-injection (p < 0.05 for each) (Fig. 4C) with no significant difference between the two BAM8–22 doses. In the contralateral paw there was a significant mirror image thermal hyperalgesic effect at 5 min post-injection (p < 0.05) but no significant mechanical allodynia (Fig. 4B, D).
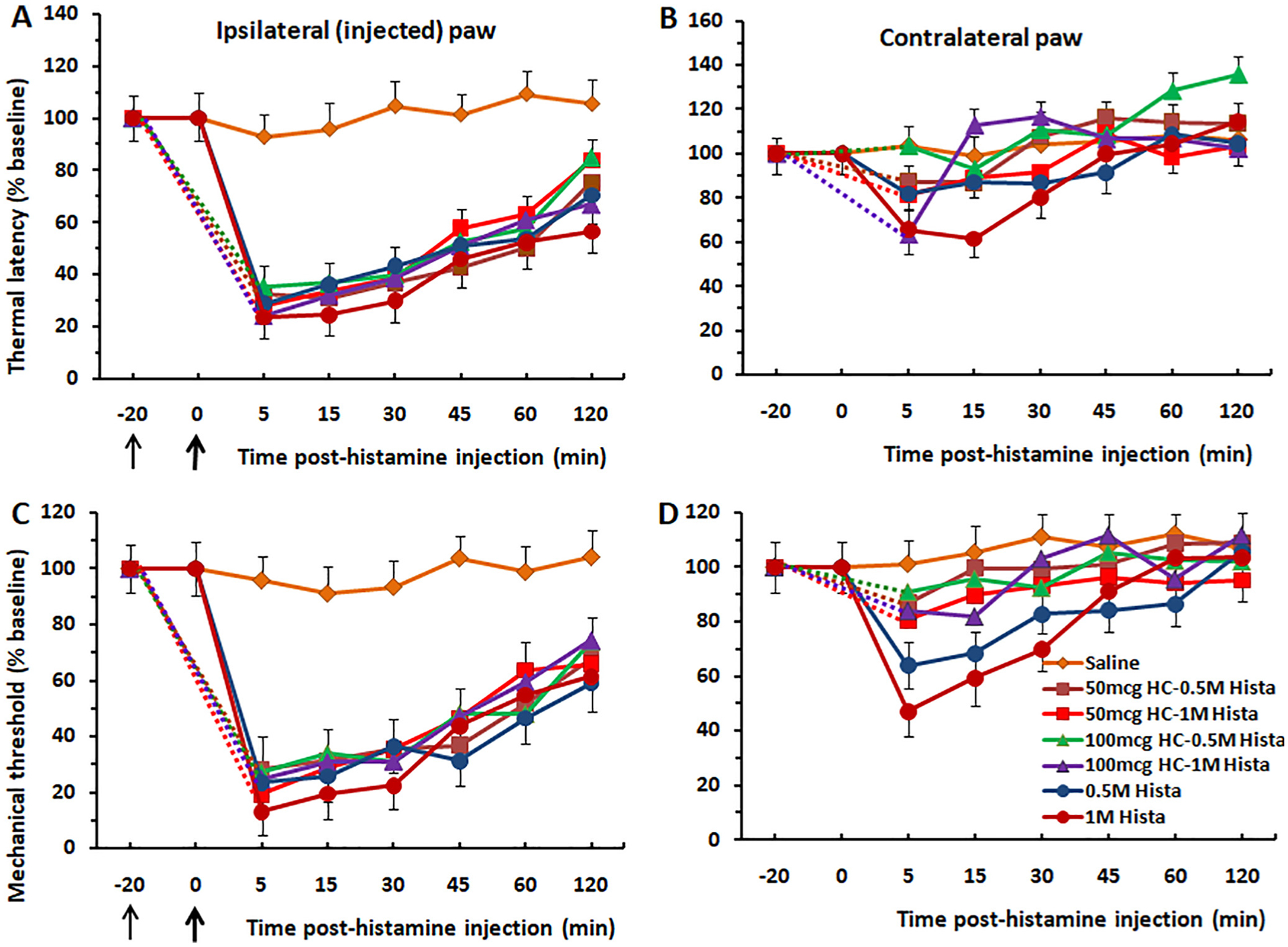
Fig. 4.

BAM8–22 induces thermal hyperalgesia and mechanical allodynia in a manner that is reduced by HC-030031. (A) Thermal withdrawal, ipsilateral paw. BAM8–22 significantly reduced latencies compared to saline controls, but with no significant difference between the two BAM8–22 doses. Pretreatment with HC-030031 significantly attenuated thermal hyperalgesia elicited by BAM8–22. (B) Thermal withdrawal, contralateral hindpaw. There was a significant mirror image hyperalgesic effect at 5 min post-injection of BAM8–22 that was significantly reduced by pretreatment with HC-030031. (C) Mechanical withdrawal, ipsilateral paw. BAM8–22 significantly reduced mechanical paw withdrawal force compared to vehicle controls. HC-030031 pretreatment significantly reduced mechanical allodynia elicited by intraplantar BAM8–22. (D) Mechanical withdrawal, contralateral paw. The trend toward a mirror image mechanical allodynia effect was not statistically significant. Thin black arrow: time of injection of HC-030031. Thick arrow: time of BAM8–22 injection. Dashed lines connect data for HC-030031 treatment groups with baseline at −20 min. Error bars: SEM..
The TRPA1 channel antagonist HC-030031 attenuated thermal hyperalgesia and mechanical allodynia produced by BAM8–22 injected in the same hindpaw. For the thermal paw withdrawal test, there were significant differences between the HC-030031 pre-injected and BAM8–22-only groups at 5, 15 and 30 min post-injection (p < 0.01 for each) (Fig. 4A). For the mechanical paw withdrawal (von Frey) test, similar significant differences were observed at 5, 15 and 30 min post-injection (p < 0.001 for each) (Fig. 4C). The weaker mirror image thermal hyperalgesia and mechanical allodynia induced by BAM8–22 were significantly reduced by pretreatment with HC-030031 (Fig. 4B, D).
SLIGRL
SLIGRL significantly reduced thermal withdrawal latencies (F(11,132) = 9.443, p < 0.0001, ANOVA) and mechanical withdrawal thresholds (F(11,132) = 12.505, p < 0.0001, ANOVA) (Fig. 5A, C). Differences between saline and SLIGRL-injected groups were significant at 5, 15 and 30 min post-injection for thermal withdrawal latency (p < 0.05 for each), and at 5 and 15 min post-injection for mechanical withdrawal thresholds (p < 0.05 for both), with no significant difference between the two BAM8–22 doses for either test. There was a significant mirror image mechanical allodynia at 5 min (p < 0.05) post-injection (Fig. 5D), but no significant thermal hyperalgesia (p > 0.05) in the contralateral paw (Fig. 5B).
Fig. 5.

SLIGRL induced thermal hyperalgesia and mechanical allodynia that was reduced by HC-030031. (A) Thermal withdrawal latency, ipsilateral paw. SLIGRL significantly reduced latencies compared to controls with no significant difference between SLIGRL doses. The higher dose of HC-030031 significantly reduced thermal hyperalgesia, but with no significant difference at the lower dose (50 μg HC-030031–15 mM SLIGRL). (B) Thermal withdrawal, contralateral hindpaw. There was no significant thermal hyperalgesic effect. (C) Mechanical withdrawal, ipsilateral paw. SLIGRL significantly reduced mechanical paw withdrawal force compared to vehicle controls with no significant difference among doses. HC-030031 significantly reduced mechanical allodynia elicited by intraplantar SLIGRL. (D) Mechanical withdrawal, contralateral paw. There was a significant mirror image mechanical allodynia effect at 5 min post-injection of SLIGRL that was significantly reduced following HC-030031. Thin black arrow: time of injection of HC-030031. Thick arrow: time of SLIGRL injection. Dashed lines connect data for HC-030031 treatment groups with baseline at −20 min. Error bars: SEM.
Intraplantar pre-treatment with the TRPA1 channel antagonist HC-030031 attenuated thermal hyperalgesia and mechanical allodynia produced by SLIGRL injected in the same hindpaw. For the thermal paw withdrawal test (ipsilateral paw), there were significant differences between HC-030031 pre-treatment and SLIGRL-only groups at 5, 15 and 30 min post-injection (p < 0.05 for each) (Fig. 5A). For the mechanical paw withdrawal test, significant differences were similarly observed at 5, 15 and 30 min post-injection (p < 0.001 for each) (Fig. 5C). The weaker mirror image thermal hyperalgesia and mechanical allodynia were also significantly reduced by pretreatment with HC-030031 (Fig. 5B, D).
DISCUSSION
The present findings showed a significant thermal hyperalgesia and mechanical allodynia induced by histamine as well as the non-histaminergic pruritogens chloroquine, BAM8–22 and SLIGRL. Histamine-evoked thermal hyperalgesia and mechanical allodynia were prevented by pretreatment with a TRPV1 but not TRPA1 antagonist, indicating that histaminergic hyperalgesia and allodynia require TRPV1 but not TRPA1. In contrast, thermal hyperalgesia and mechanical allodynia induced by chloroquine, BAM8–22 and SLIGRL were significantly attenuated or prevented by the TRPA1 antagonist, implying a critical role for TRPA1. These results confirm previous reports that SLIGRL elicits thermal hyperalgesia and mechanical allodynia (Vergnolle et al., 2001), in a PAR-2 (Liu et al., 2011) and TRPA1-dependent manner (Dai et al., 2004).
Each of the pruritogens tested presently elicits itch-related scratching behavior in animals (Kuraishi et al., 1995; Shimada et al., 2006; Liu et al., 2009; Wilson et al., 2011). Recent studies using the “cheek” model show that injection of these pruritogens into cheek skin elicits hindlimb scratch bouts that are reduced by μ-opioid anatagonists but not morphine, while algogens elicit forelimb wipes that are reduced by morphine but not μ-antagonists (Shimada and LaMotte, 2008; Akiyama et al., 2010). BAM8–22 elicits itch sensation in humans (Sikand et al., 2011a, 2011b), and chloroquine is well known to induce itch in patients being treated for malaria (Ajayi et al., 2004). Chloroquine acts via MrgprA3 (Liu et al., 2009), BAM8–22 acts via MrgprC11 and SLIGRL acts via MrgprC11 and PAR2 to elicit scratching behavior (Liu et al., 2011; Wilson et al., 2011). In the present study we tested if thermal hyperalgesia or mechanical allodynia is elicited by similar doses of each of these pruritogens. We used intraplantar injections, because this allowed thermal and mechanical testing of the hindpaw consistent with many previous studies. We believe that intraplantar injection of the pruritogens tested presently elicits itch, based on previous studies reporting that intraplantar injection of the itch mediator, serotonin (5-hydroxytryptamine, 5-HT) elicited biting behavior in rats (Klein et al., 2011b) and mice that was reduced by a μ-opiate receptor antagonist (Hagiwara et al., 1999), consistent with biting being an itch-related behavior.
The thermal hyperalgesia and mechanical allodynia elicited by histamine, chloroquine, BAM8–22 and SLIGRL are indicative of primary hyperalgesia that occurs rapidly via peripheral sensitization of pruriceptors by local inflammatory mediators (LaMotte et al., 1991; Simone, 1992; Treede et al., 1992; Woolf, 1995; Petho and Reeh, 2012; Viana, 2018). Pruriceptors for non-histaminergic itch are thought to be polymodal C-fiber nociceptors, while those for histaminergic itch are mechano-insensitive C-fibers (Schmelz et al., 1997; Johanek et al., 2008; Namer et al., 2008). Sensitization of pruriceptive C-fibers might contribute to the alloknesis, hyperknesis, hyperalgesia and allodynia observed following intradermal injections of histamine or BAM8–22 (Simone et al., 1991; LaMotte et al., 2009; Sikand et al., 2009, 2011b). Subsets of small-diameter dorsal root ganglion (DRG) or trigeminal ganglion (TG) neurons are activated by histamine, chloroquine, SLIGRL and BAM8–22 (Liu et al., 2009; Klein et al., 2011a; Wilson et al., 2011; Akiyama et al., 2012b; Han et al., 2013; Roberson et al., 2013; Pradhananga and Shim, 2015; Ganchingco et al., 2019) with most also activated by algogens such as capsaicin. SLIGRL directly sensitized the responses of DRG cells to chloroquine and BAM8–22, consistent with sensitization of chloroquine- and BAM8–22-evoked scratching behavior by SLIGRL (Akiyama et al., 2012b). In addition to peripheral sensitization, central sensitization of pain signaling pathways might also underlie the presently-observed thermal hyperalgesia and mechanical allodynia (Latremoliere and Woolf, 2009). It was recently reported that histamine and chloroquine excite ON cells and inhibit OFF cells in the rostral ventromedial medulla (RVM) similar to effects of pain-evoking stimuli (Follansbee et al., 2018). It is thus possible that the thermal hyperalgesia and mechanical allodynia elicited by pruritogens partly involves activation of ON cells that exert pronociceptive effects on spinal nociceptive transmission (Fields et al., 1991).
Intradermal insertion of single or multiple denatured cowhage spicules loaded with histamine or BAM8–22 acutely induced a dominant sensation of itch accompanied by subdominant nociceptive sensations of stinging, pricking and burning. Moreover, surrounding areas of skin exhibited dysesthesias including alloknesis, hyperknesis and mechanical hyperalgesia (LaMotte et al., 2009; Sikand et al., 2009, 2011a, 2011b). These findings indicate that itch and pain coexist simultaneously, and that histamine and BAM8–22 can acutely elicit hyperalgesia and allodynia in addition to itchy dysesthesias.
In a model of contact dermatitis involving local cutaneous application of squaric acid dibutyl ether (SADBE), mice exhibited thermal hyperalgesia and mechanical allodynia (Zhang et al., 2019). In humans, heating the SADBE-treated area to above 41 °C elicited itch indicative thermal alloknesis (Pall et al., 2015). These studies suggest that chronic itch or contact hypersensitivity conditions might produce peripheral or central sensitization. In support of peripheral sensitization, experimental models of chronic dry skin itch and contact hypersensitivity resulted in sensitization of DRG cells to non-histaminergic pruritogens but not to histamine (Akiyama et al., 2010; Fu et al., 2014).
TRPA1 and TRPV1 play roles in chronic as well as acute itch and pain (Kittaka and Tominaga, 2017; Hung and Tan, 2018; Koivisto et al., 2018; Moore et al., 2018; Xie and Li, 2019). We presently observed that hyperalgesia and allodynia elicited by acute injection of histamine was significantly reduced or prevented by pretreatment with the TRPV1 but not TRPA1 antagonist, consistent with a role for TRPV1 in acute histamine-mediated itch (Shim et al., 2007; Imamachi et al., 2009). The prevention by the TRPA1 antagonist of hyperalgesia and allodynia elicited by non-histaminergic pruritogens is consistent with a role for TRPA1 in acute non-histaminergic itch (Wilson et al., 2011). In models of chronic itch, spontaneous scratching was significantly lower in TRPA1-deficient mice compared to wild-types (Wilson et al., 2013; Follansbee et al., 2019. In a model of chronic inflammatory pain employing complete Freund’s adjuvant (CFA), mechanical allodynia, but not thermal hyperalgesia was significantly reduced by TRPA1 antagonists (Petrus et al., 2007; Eid et al., 2008; da Costa et al., 2010; Lennertz et al., 2012). Moreover, the TRPA1 antagonist HC-030031 significantly attenuated the mechanically-evoked responses of mechano-cold-sensitive C-fibers in CFA treated animals (Lennertz et al., 2012). TRPA1-expressing primary C-fiber afferents exhibited enhanced mechanical responses in CFA-treated animals (Dunham et al., 2008). These results imply that TRPA1-dependent peripheral sensitization of mechano-sensitive C-fiber afferents contributes to mechanical allodynia under conditions of inflammatory pain. Future studies are required to determine if chronic itch conditions are associated with TRPV1-or TRPA1-mediated thermal hyperalgesia and/or mechanical allodynia, if and how this differs from inflammatory pain models, and what the underlying mechanisms are.
In conclusion, the present study aimed to investigate if acute administration of pruritogens was accompanied by painful dysesthesias in an animal model. We found that histamine and the non-histaminergic pruritogens chloroquine, BAM8–22, and SLIGRL induced thermal hyperalgesia and mechanical allodynia that appear to coexist with itch. Histamine acts via TRPV1 but not TRPA1, while the non-histaminergic pruritogens act via TRPA1, underscoring the importance of these ion channels in mediating enhanced pain that accompanies acute itch elicited by these agents. Future animal studies are needed to determine if chronic itch is also associated with these painful dysesthesias. One implication of these findings is that TRP channel antagonists might prove to be useful in the clinical treatment of increased pain and allodynia that may be symptoms in patients suffering from chronic itch.
SOURCE OF FUNDING
This work was supported by Rustaveli National Science Foundation of Georgia (#217076) to M.G.T.
Abbreviations:
- ANOVA
analysis of variance
- PAR
protease-activated-receptor
- SLIGRL
Ser-Leu-Ile-Gly-Arg-Leu
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
Footnotes
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Ajayi AA, Kolawole BA, Udoh SJ (2004) Endogenous opioids, muopiate receptors and chloroquine-induced pruritus: a double-blind comparison of naltrexone and promethazine in patients with malaria fever who have an established history of generalized chloroquine induced itching. Int J Dermatol 43(12):972–977. 10.1111/j.1365-4632.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E (2010) Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 151(2):378–383. 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E (2012a) Mouse model of touch-evoked itch (alloknesis). J Invest Dermatol 132(7):1886–1891. 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E (2012b) Cross-sensitization of histamine-independent itch in mouse primary sensory neurons. Neuroscience 226:305–312. 10.1016/j.neuroscience.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Lerner EA, Carstens E (2015) Protease-activated receptors and itch. Handb Exp Pharmacol 226:219–235. 10.1007/978-3-662-44605-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M (2012) Tests and models of nociception and pain in rodents. Neuroscience 1(211):39–50. 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Coavoy-Sánchez SA, Rodrigues L, Teixeira SA, Soares AG, Torregrossa R, Wood ME, Whiteman M, Costa SKP, Muscará MN (2016) Hydrogen sulfide donors alleviate itch secondary to the activation of type-2 protease activated receptors (PAR-2) in mice. Pharmacol Res 113(Pt A):686–694. 10.1016/j.phrs.2016.09.030. [DOI] [PubMed] [Google Scholar]
- da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB (2010) The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 148(3):431–437. 10.1016/j.pain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K (2004) Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci 24:4293–4299. 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham JP, Kelly S, Donaldson LF (2008) Inflammation reduces mechanical thresholds in a population of transient receptor potential channel A1-expressing nociceptors in the rat. Eur J Neurosci 27(12):3151–3160. 10.1111/j.1460-9568.2008.06256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO (2008) HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain 4:48. 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P (1991) Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 14:219–245. 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Follansbee T, Akiyama T, Fujii M, Davoodi A, Nagamine M, Iodi Carstens M, Carstens E (2018) Effects of pruritogens and algogens on rostral ventromedial medullary ON and OFF cells. J Neurophysiol 120(5):2156–2163. 10.1152/jn.00208.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follansbee T, Zhou Y, Wu X, Delahanty J, Nguyen A, Domocos D, Iodi Carstens M, Hwang ST, Carstens E (2019) Signs of chronic itch in the mouse imiquimod model of psoriasiform dermatitis: sex differences and roles of TRPV1 and TRPA1. Itch 4(3). 10.1097/itx.0000000000000025 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Biomedical Investigator’s Handbook for Researchers Using Animal Models. Washington, D.C.: Found Biom Res, 1987. [Google Scholar]
- Fu K, Qu L, Shimada SG, Nie H, LaMotte RH (2014) Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett 579:190–194. 10.1016/j.neulet.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchingco JRC, Fukuyama T, Yoder JA, Bäumer W (2019) Calcium imaging of primary canine sensory neurons: Small-diameter neurons responsive to pruritogens and algogens. Brain Behav 9 (12). 10.1002/brb3.1428 e01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A, Ibrahim M, Gilbraith K, Khanna R, Pakai E, Miko A, Pinter E, Romanovsky AA, Porreca F, Patwardhan AM (2017) Transient receptor potential vanilloid 1 antagonists prevent anesthesia-induced hypothermia and decrease postincisional opioid dose requirements in rodents. Anesthesiology 127(5):813–823. 10.1097/ALN.0000000000001812. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Nojima H, Kuaishi Y (1999) Serotonin-induced biting of the hind paw is itch-related response in mice. Pain Res 14:53–59. 10.11154/pain.14.53. [DOI] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X (2013) A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16(2):174–182. 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CY, Tan CH (2018) TRP channels in nociception and pathological pain. Adv Exp Med Biol 1099:13–27. 10.1007/978-981-13-1756-9_2. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK (2009) TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A 106(27):11330–11335. 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M (2008) A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28(30):7659–7669. 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittaka H, Tominaga M (2017) The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol Int 66 (1):22–30. 10.1016/j.alit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E (2011a) Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol 106 (3):1078–1088. 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Sawyer CM, Zanotto KL, Ivanov MA, Cheung S, Carstens MI, Furrer S, Simons CT, Slack JP, Carstens E (2011b) A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats. J Neurophysiol 105(4):1701–1710. 10.1152/jn.00922.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto A, Jalava N, Bratty R, Pertovaara A (2018) TRPA1 antagonists for pain relief. Pharmaceuticals (Basel) 11(4). 10.3390/ph11040117.pii: E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M (1995) Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol 275(3):229–233. 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF (1991) Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66(1):190–211. 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Green BG, Zelterman D (2009) Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 101(3):1430–1443. 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10 (9):895–926. 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennertz RC, Kossyreva EA, Smith AK, Stucky CL (2012) TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS ONE 7(8). 10.1371/journal.pone.0043597 e43597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ji RR (2012) Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull 28(2):145–154. 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139 (7):1353–1365. 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X (2011) The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 4(181). 10.1126/scisignal. 2001925 ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB (2018) Regulation of pain and itch by TRP channels. Neurosci Bull 34(1):120–142. 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M (2008) Separate peripheral pathways for pruritus in man. J Neurophysiol 100(4):2062–2069. 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozadze I, Tsiklauri N, Gurtskaia G, Tsagareli M (2018) The role of transient receptor potential (TRPA1) channels in pruritus. Georgian Med News 25(280–281):134–137. [PubMed] [Google Scholar]
- Pall PS, Hurwitz OE, King BA, LaMotte RH (2015) Psychophysical measurements of itch and nociceptive sensations in an experimental model of allergic contact dermatitis. J Pain 16 (8):741–749. 10.1016/j.jpain.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petho G, Reeh PW (2012) Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 92(4):1699–1775. 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain 3:40. 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhananga S, Shim WS (2015) Caffeic acid exhibits anti-pruritic effects by inhibition of multiple itch transmission pathways in mice. Eur J Pharmacol 762:313–321. 10.1016/j.ejphar.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ (2013) Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 16(7):910–918. 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE (1997) Specific C-receptors for itch in human skin. J Neurosci 17 (20):8003–8008. 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U (2007) TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 27(9):2331–2337. 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH (2008) Behavioral differentiation between itch and pain in mouse. Pain 139(3):681–687. 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG (2006) Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530(3):281–283. 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH (2011a) BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31(20):7563–7567. 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH (2011b) Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain 152(11):2485–2494. 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH (2009) Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144(1–2):66–75. 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA (1992) Neural mechanisms of hyperalgesia. Curr Opin Neurobiol 2(4):479–483. 10.1016/0959-4388(92)90183-l. [DOI] [PubMed] [Google Scholar]
- Simone DA, Alreja M, LaMotte RH (1991) Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res 8 (3):271–279. 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN (1992) Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol 38(4):397–421. 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Tsagareli MG, Nozadze I, Tsiklauri N, Gurtskaia G (2019) TRPA1 channel is involved in SLIGRL-evoked thermal and mechanical hyperalgesia in mice. Med Sci 7(4). 10.3390/medsci7040062.pii: E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL (2001) Proteinase activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med 7 (7):821–826. 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Viana F (2018) Nociceptors: thermal allodynia and thermal pain. Handb Clin Neurol 156:103–119. 10.1016/B978-0-444-63912-7.00006-0. [DOI] [PubMed] [Google Scholar]
- Wang XL, Tian B, Huang Y, Peng XY, Chen LH, Li JC, Liu T (2015) Hydrogen sulfide-induced itch requires activation of Cav3.2 T-type calcium channel in mice. Sci Rep 25(5):16768. 10.1038/srep16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor mediated itch. Nat Neurosci 14(5):595–602. 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM (2013) The ion channel TRPA1 is required for chronic itch. J Neurosci 33(22):9283–9294. 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (1995) An overview of the mechanisms of hyperalgesia. Pulm Pharmacol 8(4–5):161–167. 10.1006/pulp.1995.1021. [DOI] [PubMed] [Google Scholar]
- Xie B, Li XY (2019) Inflammatory mediators causing cutaneous chronic itch in some diseases via transient receptor potential channel subfamily V member 1 and subfamily A member 1. J Dermatol 46(3):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Malewicz NM, Xu X, Pan J, Kumowski N, Zhu T, Shimada SG, Nie H, LaMotte RH (2019) Differences in itch and pain behaviors accompanying the irritant and allergic contact dermatitis produced by a contact allergen in mice. Pain Rep 4 (5). 10.1111/1346-8138.14749e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110. 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


