Abstract
High-grade serous ovarian carcinoma (HGSC) is the most lethal gynecologic malignancy. While immune checkpoint inhibitors against PD-L1 and CTLA-4 have shown significant effects in multiple tumor types, the response rate to single-agent immune checkpoint inhibitors is low in HGSC. Alternative biomarkers and targets must be identified to guide patient selection and new therapeutic strategies in HGSC. Here, we aim to investigate the clinical significance of novel immune modulators, including B7-H4, IDO1, Tim3, IL6, and IL-8, in patients with HGSC. A total of 48 patients with HGSCs, comprising 24 cases that were sensitive and 24 that were resistant to standard paclitaxel and carboplatin chemotherapy, were selected for our initial analysis. A NanoString assay including 33 immune-related genes was used to compare the expression of different immune regulatory molecules in the sensitive and resistant groups. Differentially expressed proteins were verified using multiplex immunohistochemical staining on tissue arrays of 202 patients with HGSCs who underwent primary surgery at MDACC. We analyzed the expression levels of immune checkpoints and compared expression profiles with clinicopathologic features including response, progression-free survival, and overall survival. HGSC tumors resistant to therapy expressed higher levels of B7-H4 (69.3%), IDO1 (71.8%), Tim3 (89.1%) and inflammatory factors IL-6 and IL-8 and expressed higher Tim3 in stromal components. High expression of B7-H4 and IDO1 was associated with significantly lower overall survival and progression-free survival. B7-H4 and IDO1 were co-expressed in 49.1% of studied cases. A panel of immunomodulatory proteins including B7-H4, IDO1, Tim3, IL-6, and IL-8 are expressed at high levels in HGSCs. These modulators represent novel targets to enhance immunotherapy in patients with HGSCs.
1. INTRODUCTION
Epithelial ovarian cancer is the most lethal gynecologic malignant tumor worldwide, accounting for 70%-80% of gynecologic cancer–associated deaths owing to resistance to therapy [1]. Despite intensive efforts over the past 40 years, drug resistance remains a major problem in advanced high-grade serous ovarian carcinoma (HGSC); the overall five-year cure rate for patients with advanced stage disease has only had modest improvements [2, 3]. Recently, immune checkpoint targeting drugs including inhibitors of PD-1 (also known as B7-H1), PD-L1 and CTLA-4, have been successful in multiple solid tumors, including melanoma and non–small cell lung cancer [1]. However, the majority of HGSC patients showed no on minimal effect in clinical trial [4], the PD-1 protein is only focally expressed in high-grade ovarian serous carcinoma [5], tumor immunity was considered to be harnessed in HGSCs [6]. Similarly, while CTLA-4–targeted drugs have been used for melanoma and urothelial and lung cancers, their therapeutic benefit has been limited in ovarian cancer, although it was reported combination of nivolumab plus ipilimumab group has a longer progression-free survival compared with the nivolumab only group (3.9 vs 2.0 months) [7]. The poor response rate is likely due to its unique immunosuppressive tumor microenvironment [8, 9]. Thus, it is important to identify alternative immune checkpoint inhibitors and other new markers for immune therapy.
In this study, we comprehensively examined the different components of the tumor immune microenvironment in drug-sensitive and drug-resistant cases using the NanoString technique and multiplex immunohistochemical staining. We showed that B7-H4 (another member of the B7 family) and IDO1 are expressed in HGSC patients with drug resistance and are associated with survival in patients with HGSC.
2. MATERIAL AND METHODS
2.1. Patient samples and NanoString analysis
In order to study the inflammatory molecules involved in drug resistance, among 660 HGSC cases archived by the Department of Pathology at The University of Texas MD Anderson Cancer Center (1993-2015), 48 patients who had upfront surgery followed by standard treatment with combined paclitaxel and carboplatin, comprising 24 drug-resistant and 24 drug-sensitive tumor samples were selected and sub-grouped for Nanostring (whole section of tissue were used) according to the criteria described below: resistant cases including those were none or partial response to treatment and tumor recurred within 6 months; For sensitive cases, complete clinical response and recurrence > 6 months. These clinicopathologic characteristics are summarized in Table S1. The overall survival the time from diagnosis to death and the progression-free survival time from the time from remission to death are defined as according the criteria described in RECIST criteria, WHO, 2000 [10, 11].
Whole sections for each case were cut and total RNA was extracted using the High Pure FFPE RNA isolation kit (Roche). A customized nCounter XT expression assay (NanoString Technologies), including 33 immune-related genes (Table S2) and four housekeeping genes (RPL13A, PPIA, GUSB, and TBP) as internal controls, was performed according to the manufacturer’s protocol. The data were analyzed using nSolver software version 3.0 (NanoString Technologies).
2.2. Patient cohort, tissue microarray, and multiplex immunohistochemical staining
To further verify the expression and distribution of inflammatory molecules disclosed by Nanostring, multiple IHC was performed on a series of tissue microarray (TMA) including 202 formalin-fixed, paraffin-embedded (FFPE) samples obtained from patients with HGSC who underwent primary surgery without neoadjuvant chemotherapy between 2009 and 2015 at MD Anderson Cancer Center. Tissue samples were debulking tumor followed by standard combined treatment with paclitaxel and carboplatin. Tissues were prepared in a TMA format as previously described [12]. There were 12 cases overlapped in experiments of Nanostring and multiple IHC. The clinicopathologic characteristics of these patients are summarized in Table S3. Overall survival was defined from the date of diagnosis until the event endpoint or the date of the end time of this study (April 30, 2019), while progression-free survival was defined from the date of surgery to tumor recurrence. The use of all tissues in this study was approved by the Institutional Review Board of MD Anderson Cancer Center.
To investigate the expression and distribution of B7-H4, IDO1, Tim3, VISTA, Lag3, and CD8, multiplex immunohistochemistry was performed on the tissue microarray sections (4 μm) staining with an Opal seven-color automation immunohistochemistry kit (PerkinElmer) and then scanning with the Vectra Polaris automated quantitative pathology imaging system (PerkinElmer) in Flow Cytometry & Cellular Imaging Facility at MD Anderson. Normal fallopian tube and ovarian cystadenoma was used as a negative control and the controls for antibodies and software training. Details of antibodies and the panel are listed in Tables S4 and S5. For B7-H4 and IDO1, the novel proteins may expressed by both cancer cells and stromal cells, samples were scored using the sum of both the positive percentage score (0: 0%; 1: > 0% to < 25%; 2: 25% to < 50%; 3: 50% to <75%; 4: >75%) and the density score (0: negative; 1: weak; 2: medium; 3: strong) by Vectra Polaris automated quantitative pathology imaging system and assessment from 3 independent pathologist (JL, NN, and YZ) as previously described [13]. Expression levels of these proteins were thus categorized as negative (0-1), weak (2-4) or strong (5-7). For Tim3, VISTA, Lag3, and CD8 which are markers widely used to distinguish variable subpopulations of lymphocytes, only the positive percentage scores were considered and analyzed by software of Vectra Polaris. Consequently, their expression levels were categorized as negative, (0), weak (1-2), or strong (3-4). The histologic findings and scores were reviewed by and consensus was reached among three pathologists (JL, NN, and YZ).
2.3. Statistical analysis
Data are presented as the mean ± standard deviation. Means were compared between three or more groups using one-way analysis of variance. Comparisons between two groups were performed using Student t-tests for means of continuous data and chi-square analysis for categorical data. Kaplan–Meier and Cox regression analysis were used to compare survival between groups. Statistical analyses were conducted using SPSS 19.0 statistical software (IBM). P < 0.05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Suppressive immune regulators and inflammatory cytokines were expressed at high levels in tumor tissues in cases resistant to therapy
First, we used NanoString analysis to examine 33 genes (Table S6) grouped into four clusters (Figure 1): T lymphocyte–related genes (FOXP3, CD4, CD8, PTPRC which codes for CD45RO), non–T lymphocyte–related cell markers (ICOS, CD160, ITGAE which code for CD103, CD14, MS4A1 which codes for CD20), immune regulators, and pro-inflammation molecules. Compared with the drug-sensitive group, the drug-resistant group had higher expression levels of B7-H4, IDO1, IL-6, and IL-8 (Table S6 and Figure 1). However, the widely studied novel immunotherapy targets PD-L1, and PD-L2, were minimally expressed in the drug-resistant group. Also expressed at low levels in the resistant group were CD8 (marker for cytotoxic T lymphocytes), CD20 (marker for B lymphocytes), CD103 (marker for regulatory T cells and alloantigen-induced regulatory CD8 cells), and CD14 (marker for macrophages). These results suggested that negative immune regulators (B7-H4 and IDO1) and pro-inflammatory molecules (IL-6 and IL-8) are produced at high levels in tumor tissues in drug-resistant cases while cell-mediated and humoral immunity are attenuated.
Figure 1.
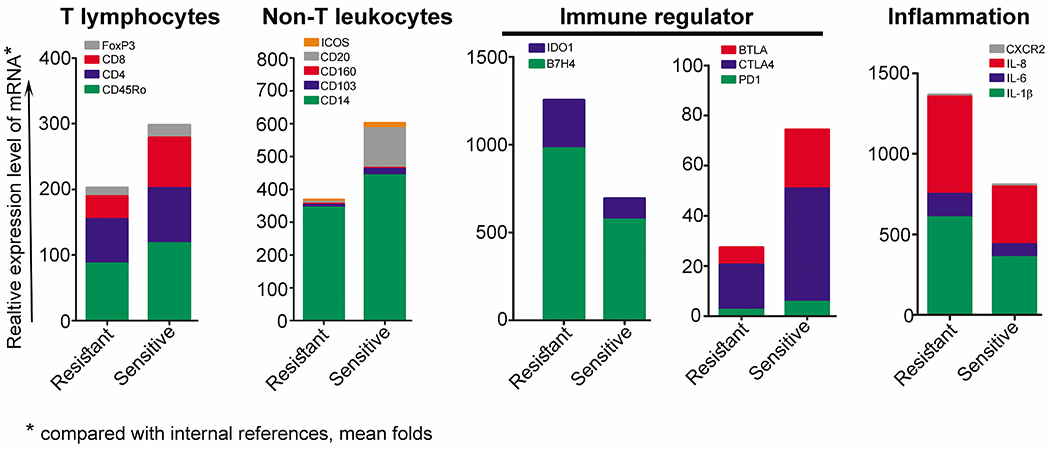
Expression levels of 33 genes in drug-resistant and drug-sensitive HGSC cases, analyzed by NanoString. Relative expression levels of B7-H4, IDO1, BRCA1/2, IL-6, and IL-8 were higher in the resistant group compared with the sensitive group.
3.2. Expression levels of B7-H4 and IDO1 in tumor cells were associated with immune-inhibitory effects mediated by Tim3 and VISTA
Histologic evaluation of HGSC was performed by independent pathologists (JL and YZ) via H&E staining and immunofluorescent of cytokeratin (CK, marker of epithelium. Figure S1). Among the 202 surgery-treated cases (Table S3, Table 1), there were 140 (69.3%), 145 (71.8%) and 180 (89.1%) cases show positive expression of B7-H4, IDO1 and Tim3 respectively, and this positive expression was significantly associated with advanced TNM stage (Table 1), but not with age. B7-H4 and IDO1 were both expressed in 68.6% of IDO1-positive cases and in 49.1% of all studied cases (Figure 2A and B), suggesting the close positive association of B7-H4 and IDO1 (Table S7, P=0.012) as inhibitors of immune response indicated as increased positivity of Tim3 and VISTA as discussed below.
Table 1.
Correlation of stage with immune checkpoints
| Stage |
|||||
|---|---|---|---|---|---|
| Protein | Group (score) | N | I/II(%) | III/IV(%) | P value |
| B7H4 | |||||
| Negative (0-1) | 62 | 29 (46.8) | 33 (53.2) | 0.027* | |
| Weak positive (2-4) | 49 | 20 (40.8) | 29 (59.1) | ||
| strong positive (5-7) | 91 | 19 (20.9) | 72 (79.1) | ||
| IDO1 | |||||
| Negative (0-1) | 57 | 27 (47.4) | 30 (52.6) | 0.042* | |
| Weak positive (2-4) | 122 | 35 (28.7) | 87 (71.3) | ||
| strong positive (5-7) | 23 | 6 (26.1) | 17 (73.9) | ||
| Tim3 | |||||
| Negative (0) | 22 | 10 (45.4) | 12 (54.5) | 0.035* | |
| Weak positive (1-2) | 152 | 52 (34.2) | 100 (65.8) | ||
| strong positive (3-4) | 28 | 6 (21.4) | 22 (78.6) | ||
| VISTA | |||||
| Negative (0) | 14 | 8 (44.4) | 6 (55.5) | 0.152 | |
| Weak positive (1-2) | 156 | 49 (31.4) | 107 (68.6) | ||
| strong positive (3-4) | 32 | 11 (34.4) | 21 (65.6) | ||
| CD8 | |||||
| Negative (0) | 44 | 14 (31.8) | 30 (68.2) | 0.074 | |
| Weak positive (1-2) | 134 | 43 (32.1) | 91 (67.9) | ||
| strong positive (3-4) | 24 | 11 (45.8) | 13 (54.2) | ||
Chi-square analysis.
significant difference.
Figure 2.
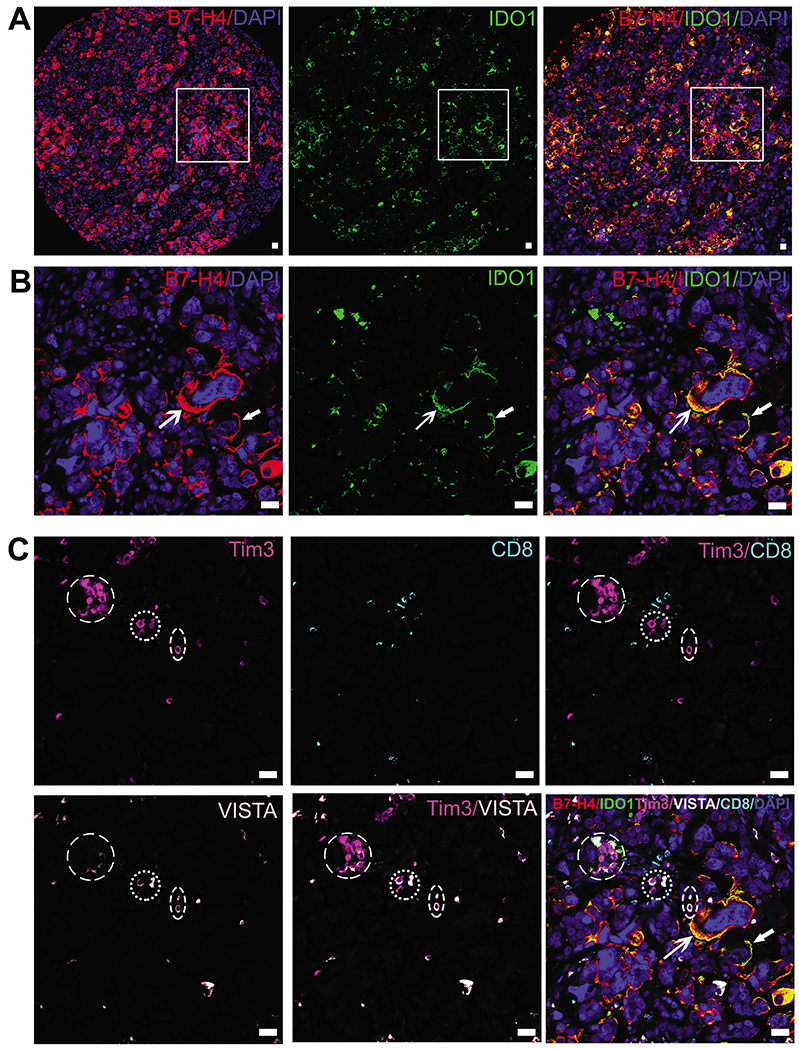
Representative images of co-expression of B7-H4 and IDO1 and the enrichment of Tim3-positive and VISTA-positive cells in the stroma. A and B: Co-expression of B7-H4 and IDO1 in membrane and cytoplasm of tumor cells, indicated by white arrows. Panels in B are magnifications of lined areas in A. C: Circled are clustered immune-suppressing stromal cells that were positive for Tim3 and VISTA and negative for CD8. These stromal cells were distributed close to the tumor cells positive for B7-H4 and IDO1. Bars, 50 μm.
B7-H4 and IDO1 were expressed both in the cell membrane and cytoplasm of cancer cells, but IDO1 was expressed predominantly in cell membrane (Figure 2A and B, indicated with arrow heads), although a few stromal cells were positive for B7-H4 and IDO. Expression of B7-H4 was positively associated with expression of Tim3 (P=0.004) but not with CD8 (P=0.056) (Table S7). Representative images depicting scoring are shown in Figure S2.
Positive signals for Tim3 and VISTA were found in stromal cells. There were co-expression of VISTA and CD8, but not Tim3 and CD8 (circled in Figure 2C). Expression of Tim3, an immune-suppressing regulator in cancer [14] which was predominantly localized to myeloid cells such as intratumoral dendritic cells [15], was positively associated with B7-H4, VISTA, and CD8 Table S7). Tumor cells positive for B7-H4 and IDO1 were distributed near stromal cells, which were enriched for Tim3 and VISTA (Figure 2C). However, there were no significant differences in expression of Tim3, VISTA, and CD8 in paired groups listed in Table S7. These results indicated that expression of B7-H4 and IDO1 in tumor cells is closely correlated with immune-inhibitory effects mediated by Tim3 and VISTA.
3.3. Expression levels of B7-H4 and IDO1 were correlated with a poor prognosis
As shown in Figure 3, Table S8, Table S9, the expression of B7-H4 and IDO1 was associated with poor overall survival (Figure 3A) but not with poorer progression-free survival in HGSC patients (Figure 3B). Overall survival durations were 44.6 ± 5.6 months for B7-H4 strong-positive and 55.5 ± 7.0 months for B7-H4 weak-positive cases, significantly shorter than that of the B7-H4–negative group (87.0 ± 9.5 months, P = 0.031. Table S8 and Figure 3A). Overall survival durations for the IDO1-positive group (strong and weak) were 48.4 ± 5.8 months and 59.9 ± 10.2 months, respectively, significantly shorter than that of the IDO1-negative group 94.6 ± 9.9 months, P = 0.024, Table S8 and Figure 3C). Progression-free survival durations for the IDO1-positive group (strong and weak) were 24.5 ± 6.3 months and 33.0 ± 21.5 months, respectively, significantly shorter than that of the IDO1-negative group (62.3 ± 13.3 months, P = 0.046, Table S9 and Figure 3D). However, strongly-positive and weakly-positive groups did not significantly differ in overall survival or progression-free survival. Neither were the expression levels of Tim3, VISTA, or CD8 significantly associated with either survival measure.
Figure 3.
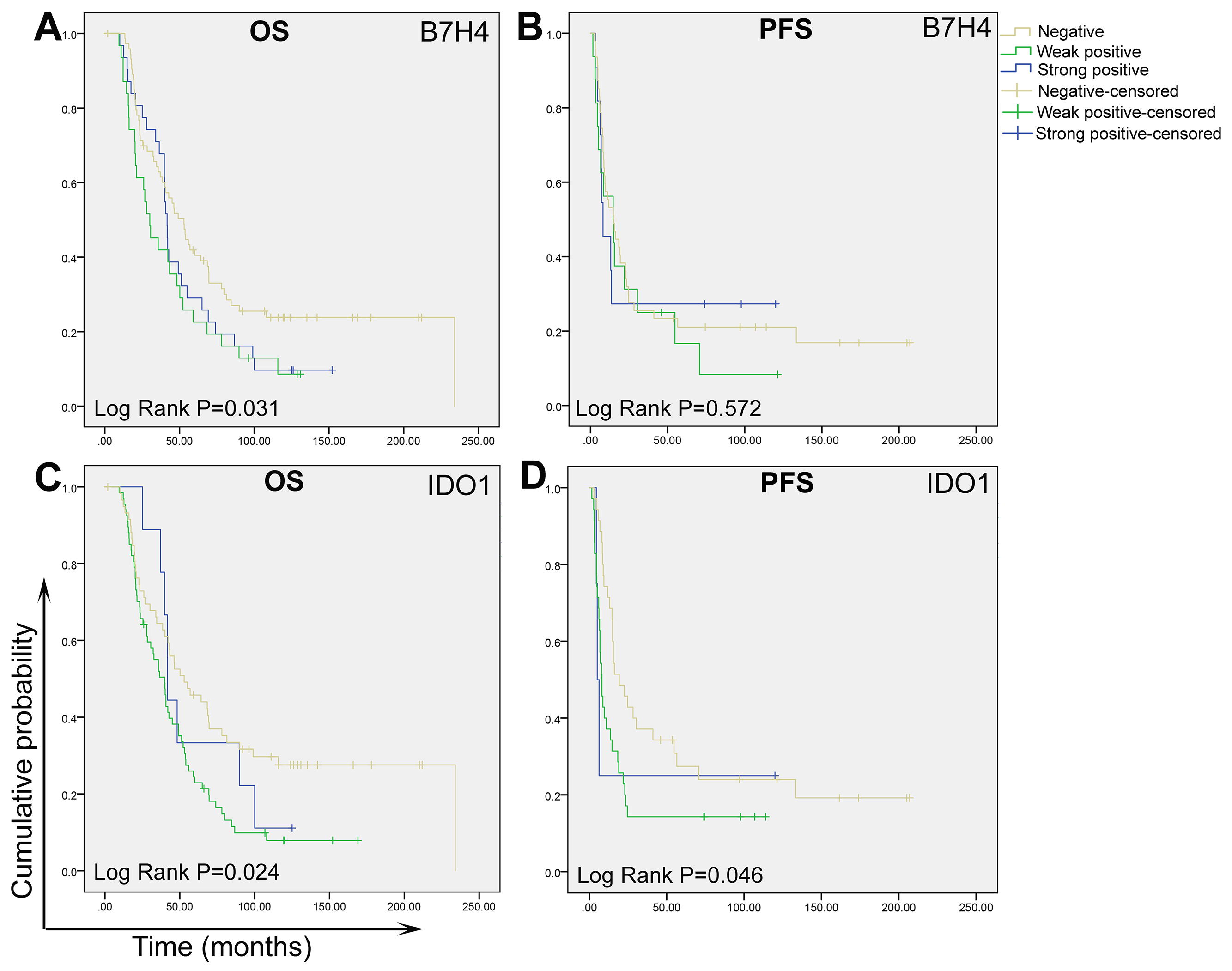
Association of overall survival (OS) and progression-free survival (PFS) with expression of B7-H4 and IDO1 in high-grade ovarian serous carcinoma.
4. DISCUSSION
In our analysis of immune modulators in HGSC, tissues treated with standard chemotherapy, we showed that immune inhibitors including B7-H4 and IDO1 and inflammation-related molecules including IL-6 and IL-8, but not CTLA-4 or PD-1, were elevated in resistant HGSCs. Furthermore, in HGSCs from patients who underwent surgery, high expression of B7-H4 and IDO1 together with Tim3 expression in stromal components was associated with advanced TNM stages. Expression of B7-H4 and IDO1 was associated with worse overall survival of HGSCs. Our findings suggest that these three newly discovered immune checkpoint inhibitors play major roles in the immune suppression of the tumor microenvironment in HGSCs.
B7-H4 is a member of the B7 family, including PD-1 (B7-H1), B7-H2, and B7-H3. In esophageal squamous cell cancer, B7-H4 was co-expressed in cancer stem cells, positively associated with cell cycle regulators such as cyclin D1 and p27, negatively associated with CD8-positive T cells, and associated with a poor prognosis [16]. High serum B7-H4 levels were also correlated with advanced TNM stage in patients with hepatocellular carcinoma and were thought to contribute to the tumorigenesis of the disease [17]. It has been suggested that B7-H4 be included in panels of markers involved in cancer stem cell maintenance [18]. In pancreatic cancer, B7-H4 was a negative prognostic marker, indicating more resistance to chemotherapy with gemcitabine [19]. In breast cancer, B7-H4 was highly expressed in both breast cancer and stroma cells, independently of the intrinsic subtypes of breast cancer [20]. B7-H4 expression on the tumor border also associated with increased distant spread and metastases in cervical cancer [21]. In murine models of urothelial carcinoma, blocking B7-H4 with antibodies reduced tumor size and increase infiltration of CD8+ T cells within stroma [22].
Several studies have been previously performed on B7-H3 or B7-H4 family in ovarian cancer. It has been reported that B7-H3 was expressed in both cancer cells and stroma cells, but B7-H4 was found restricted in cancer cells [23]. Yigit et al found B7-H4 is positive in macrophages which could play a key role in establish a suppressive microenvironment favoring cancer cells to escape immune elimination [24]. Pagnotti et al reported that B7-H4 is inversely related with lymphocyte infiltration in clear cell carcinoma but not in other type of epithelial ovarian cancer [25]. Simon et al found B7-H4 over-expression was related with advanced stage of malignant ovarian cancer [26], while Ye et al reported that no correlation was found between B7-H4 expression and clinicopathologic features of ovarian cancer, including stages, grade, tumor metastasis and histologic type in ovarian cancer, although B7-H4 expression was found significantly associated with a worse progression free survival [27]. Increased expression level of B7-H4 was positively related with expression of proliferative cell nuclear antigen, both of which were related with poor level of differentiation of ovarian cancer and lymph node metastasis [28].
We have previously showed that the percentage of B7-H4 positive cancer cells, but not the percentage pf B7-H3 positive cancer cells, was significantly higher in HGSC than in low-grade serous ovarian carcinoma [29]. In the current study, we combined percentage and density to evaluate the positivity of B7-H4, which was distributed mainly in the cytoplasm and membrane of cancer cells. B7-H4 was diffusely expressed in 68.5% of patients with HGSCs. Higher expression of B7-H4 was associated with drug resistance, poor overall survival and advanced pTNM stage of HGSC. Although patients with resistant tumors showed other changes, including increased densities of tumor-infiltrating lymphocytes (marked as positive for CD3, CD8, TIA-1, PD-1, or CD20), there was no change in the major immunosuppressive subpopulations, including regulatory T cells and IDO-positive cells. In addition, these changes had minimal effect on clinical outcomes.
IDO1 is another immune checkpoint inhibitor that has been studied in ovarian cancers, although their mechanisms of immune suppression remain poorly understood. IDO1 was extensively overexpressed in tumor cells of HGSCs, and this overexpression was associated with advanced pTNM stage and impaired survival [30]. The effect of IDO occurs at least in part via creating an immune-tolerant environment by inhibiting the recruitment of tumor-infiltrating leukocytes and enhancing the production of immunosuppressive cytokines, including TGF-β and IL-10, in ascites in mouse models [31]. In addition, IDO1 expression was positively correlated with B7-H4 expression of in tumor cells. However, contrary to expectation, IDO1 was found to promote rather than suppress anti-tumor immune escape by inducing the expression of IDO1 in tumor cells in an in vitro experiment [32]. IDO1 may function at least in part through modulating the expression of Tim 3. The overexpression of Tim3 enables tumor-infiltrating regulatory T cells to be more immunosuppressive than their counterparts in peripheral blood [33], and Tim 3 may serve as a biomarker for suppressed immune response in HGSCs [33].
We also found that IL-6 and IL-8 levels were elevated significantly in drug-resistant cases compared with the drug-sensitive group. The tumor-promoting effects of IL-6 and IL-8 are well studied in cancers of the breast, colon, pancreas, and lung, but not in ovarian cancer [34, 35]. In triple-negative breast cancers, inhibition of IL-6 and IL-8 expression dramatically inhibited colony formation and cell survival [36]. Stronger IL-6 and IL-8 expression predicted worse survival times [36]. In HER2-positive breast cancer, IL-6 contributes to the development of drug-resistant cancer cells and expanding population of cancer stem cells via an IL-6 feedback loop [37]. IL-6 was therefore considered to represent a new therapeutic target for cancer. Further study is needed to understand the roles and mechanisms of IL-6 and IL-8 in the development and drug resistance of ovarian cancer.
In summary, elevated expression of B7-H4 and IDO1 in tumor cells was associated with intrinsic drug resistance and was a negative prognostic indicator for ovarian HGSC. B7-H4 was highly co-expressed with IDO1, indicating a close synergistic function in creating an immunosuppressive microenvironment in the initiation and progression of ovarian cancer. Therefore, therapy targeting B7-H4 and IDO1 in combination may be a novel strategy to disrupt the immune barrier and improve the clinical outcomes of patients with HGSC. Further work is needed to understand the underlying mechanisms by which B7-H4 and IDO1 contribute to this disease.
Supplementary Material
Acknowledgments
Funding/Support:
This work is supported by The University of Texas MD Anderson Cancer Center SPORE grant (P50CA217685), MD Anderson Moonshot in Ovarian Cancer, the American Cancer Society, and the Frank McGraw Memorial Chair in Cancer Research. Multiplex IHC was performed in the Flow Cytometry & Cellular Imaging Facility – north campus, which is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672. This paper was edited by Sarah Bronson, ELS, at MD Anderson Cancer Center.
Footnotes
Conflict of interest: none.
REFERENCES
- [1].Fan CA, Reader J, Roque DM. Review of Immune Therapies Targeting Ovarian Cancer. Curr Treat Options Oncol 2018; 19, 74. [DOI] [PubMed] [Google Scholar]
- [2].Ghisoni E, Imbimbo M, Zimmermann S, Valabrega G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int J Mol Sci 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int J Mol Sci 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mills AM, Peres LC, Meiss A, Ring KL, Modesitt SC, Abbott SE, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy ML, Cote ML, Funkhouser E, Moorman PG, Peters ES, Schwartz AG, Terry PD, Wallace K, Schildkraut JM. Targetable Immune Regulatory Molecule Expression in High-Grade Serous Ovarian Carcinomas in African American Women: A Study of PD-L1 and IDO in 112 Cases From the African American Cancer Epidemiology Study (AACES). Int J Gynecol Pathol 2019; 38, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cho Y, Milane L, Amiji MM. Genetic and epigenetic strategies for advancing ovarian cancer immunotherapy. Expert Opin Biol Ther 2019; 19, 547–560. [DOI] [PubMed] [Google Scholar]
- [6].Martin de la Fuente L, Westbom-Fremer S, Arildsen NS, Hartman L, Malander S, Kannisto P, Masback A, Hedenfalk I. PD-1/PD-L1 expression and tumor-infiltrating lymphocytes are prognostically favorable in advanced high-grade serous ovarian carcinoma. Virchows Arch 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zamarin D, Burger RA, Sill MW, Powell DJ Jr., Lankes HA, Feldman MD, Zivanovic O, Gunderson C, Ko E, Mathews C, Sharma S, Hagemann AR, Khleif S, Aghajanian C. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol 2020, JCO1902059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10, 942–949. [DOI] [PubMed] [Google Scholar]
- [9].Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, Rahimi K, Shaw PA, Clarke BA, Nelson BH. Neoadjuvant Chemotherapy of Ovarian Cancer Results in Three Patterns of Tumor-Infiltrating Lymphocyte Response with Distinct Implications for Immunotherapy. Clin Cancer Res 2017; 23, 925–934. [DOI] [PubMed] [Google Scholar]
- [10].Subbiah V, Chuang HH, Gambhire D, Kairemo K. Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives. Diagnostics (Basel) 2017; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kurta ML, Edwards RP, Moysich KB, McDonough K, Bertolet M, Weissfeld JL, Catov JM, Modugno F, Bunker CH, Ness RB, Diergaarde B. Prognosis and conditional progression-free survival among patients with ovarian cancer. J Clin Oncol 2014; 32, 4102–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shen W, Niu N, Lawson B, Qi L, Zhang J, Li T, Zhang H, Liu J. GATA6: a new predictor for prognosis in ovarian cancer. Hum Pathol 2019; 86, 163–169. [DOI] [PubMed] [Google Scholar]
- [13].Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med 2012; 10, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bu M, Shen Y, Seeger WL, An S, Qi R, Sanderson JA, Cai Y. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol 2016; 37, 3949–3956. [DOI] [PubMed] [Google Scholar]
- [15].de Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, et al. TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 2018; 33, 60–74 e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Piao L, Yang Z, Jin J, Ni W, Qi W, Xuan Y. B7H4 is associated with stemness and cancer progression in esophageal squamous cell carcinoma. Hum Pathol 2018; 80, 152–162. [DOI] [PubMed] [Google Scholar]
- [17].Yuan L, Dong L, Yu G, Fan W, Zhang L, Wang P, Hu X, Zhao M. Aberrant expression of B7H4 may contribute to the development of hepatocellular carcinoma. Mol Med Rep 2016; 14, 5015–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Panaccione A, Guo Y, Yarbrough WG, Ivanov SV. Expression Profiling of Clinical Specimens Supports the Existence of Neural Progenitor-Like Stem Cells in Basal Breast Cancers. Clin Breast Cancer 2017; 17, 298–306 e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsiaousidou A, Tsaroucha AK, Lambropoulou M, Pitiakoudis M, Polychronidis A, Chatzitheoklitos E, Romanidis K, Simopoulos C. Increased B7H4 tissue expression correlates with high CA19.9 serum levels and a worse prognosis of pancreatic adenocarcinoma. Clin Exp Med 2016; 16, 351–356. [DOI] [PubMed] [Google Scholar]
- [20].Altan M, Kidwell KM, Pelekanou V, Carvajal-Hausdorf DE, Schalper KA, Toki MI, Thomas DG, Sabel MS, Hayes DF, Rimm DL. Association of B7-H4, PD-L1, and tumor infiltrating lymphocytes with outcomes in breast cancer. NPJ Breast Cancer 2018; 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Galazka K, Oplawski M, Windorbska W, Skret-Magierlo J, Koper K, Basta P, Mach P, Dutch-Wicherek M, Mazur A, Wicherek L. The immunohistochemical analysis of antigens such as RCAS1 and B7H4 in the cervical cancer nest and within the fibroblasts and macrophages infiltrating the cancer microenvironment. Am J Reprod Immunol 2012; 68, 85–93. [DOI] [PubMed] [Google Scholar]
- [22].Podojil JR, Glaser AP, Baker D, Courtois ET, Fantini D, Yu Y, et al. Antibody targeting of B7-H4 enhances the immune response in urothelial carcinoma. Oncoimmunology 2020; 9, 1744897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].MacGregor HL, Sayad A, Elia A, Wang BX, Katz SR, Shaw PA, et al. High expression of B7-H3 on stromal cells defines tumor and stromal compartments in epithelial ovarian cancer and is associated with limited immune activation. J Immunother Cancer 2019; 7, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol 2010; 117, 366–372. [DOI] [PubMed] [Google Scholar]
- [25].Pagnotti GM, Atkinson RM, Romeiser J, Akalin A, Korman MB, Shroyer KR. B7-H4 is Inversely Correlated With T-Cell Infiltration in Clear Cell but Not Serous or Endometrioid Ovarian Cancer. Appl Immunohistochem Mol Morphol 2019; 27, 515–522. [DOI] [PubMed] [Google Scholar]
- [26].Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW, Sarno MJ, Wolfert RL Diamandis EP. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol Oncol 2007; 106, 334–341. [DOI] [PubMed] [Google Scholar]
- [27].Ye Y, Wang JJ, Li SL, Wang SY, Jing FH. Does B7-H4 expression correlate with clinicopathologic characteristics and survival in ovarian cancer?: A systematic review and PRISMA-compliant meta-analysis. Medicine (Baltimore) 2018; 97, e11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zheng C, Yang R. RCD24, B7-H4 and PCNA expression and clinical significance in ovarian cancer. J BUON 2019; 24, 715–719. [PubMed] [Google Scholar]
- [29].Liang L, Jiang Y, Chen JS, Niu N, Piao J, Ning J, Zu Y, Zhang J, Liu J. B7-H4 expression in ovarian serous carcinoma: a study of 306 cases. Hum Pathol 2016; 57, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Indoleamine Ino K. 2,3-dioxygenase and immune tolerance in ovarian cancer. Curr Opin Obstet Gynecol 2011; 23, 13–18. [DOI] [PubMed] [Google Scholar]
- [31].Tanizaki Y, Kobayashi A, Toujima S, Shiro M, Mizoguchi M, Mabuchi Y, Yagi S, Minami S, Takikawa O, Ino K. Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of ovarian cancer by inducing an immunosuppressive environment. Cancer Sci 2014; 105, 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Opitz CA, Litzenburger UM, Opitz U, Sahm F, Ochs K, Lutz C, Wick W, Platten M. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells. PLoS One 2011; 6, e19823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fucikova J, Rakova J, Hensler M, Kasikova L, Belicova L, Hladikova K, Truxova I, Skapa P, Laco J, Pecen L, Praznovec I, Halaska MJ, Brtnicky T, Kodet R, Fialova A, Pineau J, Gey A, Tartour E, Ryska A, Galluzzi L, Spisek R. TIM-3 Dictates Functional Orientation of the Immune Infiltrate in Ovarian Cancer. Clin Cancer Res 2019. [DOI] [PubMed] [Google Scholar]
- [34].Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu HS, Hung SC. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer 2015; 136, 547–559. [DOI] [PubMed] [Google Scholar]
- [35].Holmer R, Goumas FA, Waetzig GH, Rose-John S, Kalthoff H. Interleukin-6: a villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat Dis Int 2014; 13, 371–380. [DOI] [PubMed] [Google Scholar]
- [36].Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res 2013; 73, 3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell 2012; 47, 570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


