Abstract
Purpose:
Dose-dense Methotrexate-Vinblastine-Adriamycin-Cisplatin (ddMVAC) and Gemcitabine-Cisplatin (GC) are accepted neoadjuvant regimens for muscle-invasive bladder cancer (BC). The aim of this study was to validate the score from a Coexpression extrapolation (COXEN) algorithm-generated gene expression model (GEM) as a biomarker in patients undergoing radical cystectomy.
Experimental Design:
Eligibility included cT2-T4a N0 M0, urothelial BC, ≥ 5 mm of viable tumor, cisplatin eligible, with plan for cystectomy; 237 patients were randomized between ddMVAC, given every 14 days for 4 cycles, and GC, given every 21 days for 4 cycles. The primary objective assessed pre-specified dichotomous treatment specific COXEN score as predictive of pT0 rate or ≤ pT1 (downstaging) at surgery.
Results:
Among 167 evaluable patients, the odds ratio for pT0 with the GC GEM score in GC-treated patients was 2.63 (p=0.10; 95% CI [0.82,8.36]); for the ddMVAC COXEN GEM score with ddMVAC treatment, the OR was 1.12 (p=0.82, 95% CI [0.42, 2.95]). The GC GEM score was applied to pooled arms (GC and ddMVAC) for downstaging with an OR of 2.33 (p=0.02; 95% CI [1.11, 4.89]). In an intention to treat analysis of eligible patients (n=227), pT0 rates for ddMVAC and GC were 28% and 30% (p = 0.75); downstaging was 47% and 40% (p = 0.27), respectively.
Conclusion:
Treatment-specific COXEN scores were not significantly predictive for response to individual chemotherapy treatment. The COXEN GEM GC score was significantly associated with downstaging in the pooled arms. Additional biomarker development is planned.
Introduction
Bladder cancer is the fifth most common cancer with 80,470 new cases and 17,670 deaths expected in the United States in 2019.1 The use of neoadjuvant, cisplatin-based combination chemotherapy is optimal care in eligible patients with muscle-invasive disease planning for radical cystectomy.2,3 Despite randomized data supporting this approach and increased utilization over time, the application of pre-operative neoadjuvant chemotherapy remains underutilized.4 No predictive biomarkers are currently used clinically to select individualized patient chemotherapy or determine the appropriateness of chemotherapy. Currently, there are two acceptable regimens utilized in this setting: gemcitabine with cisplatin (GC) or dose-dense Methotrexate-Vinblastine-Adriamycin-cisplatin (ddMVAC) chemotherapy.2,5-7
SWOG trial 8710 was pivotal in establishing the role of cisplatin-based combination therapy in bladder cancer.3 The study enrolled 317 patients with cT2-T4aN0M0, who were randomized between MVAC (traditional 28-day cycles) followed by cystectomy versus cystectomy alone. The median survival improved from 46 to 77 months with the addition of MVAC chemotherapy. The rate of pT0 was 15% without chemotherapy and 38% with the addition of chemotherapy in those who underwent cystectomy (31% of all randomized). Notably, regardless of treatment arm, those with pT0 had an 8-year overall survival estimate of approximately 75%, while those with any residual disease at cystectomy had an 8-year survival estimate of 30%. In a retrospective analysis of S8710, the overall survival was evaluated by the pathologic response at surgery.8 The median overall survival was 13.6 years in those with pT0, 10.6 years with pT1/pTIS/pTa, and 3.7 years in those with >pT1.
Neoadjuvant ddMVAC has been utilized in two recent single arm studies. In a study of 54 bladder cancer patients with cT2-T4a N0 or N1 disease, three cycles of ddMVAC were given preoperatively.9 Of the 40 evaluable patients, 38% had pT0 at the time of surgery and 52% had residual, non-muscle invasive disease at the time of cystectomy. In a second study of neoadjuvant ddMVAC in 39 patients with cT2-T4 and N0 or N1 disease, 49% achieved non-muscle invasive disease.10
Coexpression extrapolation (COXEN) is a predictive biomarker approach developed by Theodorescu and colleagues.11,12 Conceptually different than standard approaches, COXEN is the first demonstration that development of predictive biomarkers is possible in the absence of clinical response data from patients. Technically, COXEN is based on an in vitro assessment performed using the NCI-60, a bank of 60 well-characterized cell lines from a wide range of cancer types, which include drug sensitivity and gene expression data among other characterizations.13 Once the in vitro gene expression model (GEM) signature associated with response is established, it is correlated with a histologically relevant gene expression data set obtained from treatment naive human tumors to identify concordant genes. GEMs developed for multiagent regimens are a compilation of GEMs for each individual drug. Through this process, a correlation coefficient is derived for each individual gene in the GEM signature with those showing concordance in the human sample. Expression of this GEM signature can then be directly assessed to any transcriptionally profiled individual patient sample. A “COXEN score” is then generated from the GEM signature correlation coefficients and this is used to predict response (see Supplemental figure 1 for more details).
In S1314, we enrolled patients with muscle-invasive bladder cancer who were eligible for cisplatin-based multi-agent chemotherapy and in whom cystectomy was planned. Patients were randomized between neoadjuvant GC and ddMVAC chemotherapy with treatment assignment independent of the GEM signature derived COXEN score. The primary endpoint was pathologic response at the time of cystectomy. The purpose of the trial was to evaluate whether either the pre-specified GC or the ddMVAC COXEN score dichotomies were associated with favorable response to neoadjuvant chemotherapy defined as pT0 or ≤pT1 at radical cystectomy. This strategy could be evaluated in a subsequent prospective trial. We also assessed whether either score helped to predict which of the two regimens a patient would be more likely to respond. As a phase II study, this trial was not designed to use COXEN GEM scores to prospectively predict response to GC or ddMVAC chemotherapy.
Methods
Patients
The study was reviewed and received approval by the NCI Central Institutional Review Board (CIRB), and patients provided written, informed consent; it was conducted according to the Declaration of Helsinki guidelines. Eligible subjects had histologically-proven urothelial carcinoma of the bladder, stage cT2-T4a N0 M0 disease and a Zubrod performance status of 0 or 1. Those with mixed histology, including a component of urothelial carcinoma, were eligible. Those with small cell carcinoma, pure adenocarcinoma, and pure squamous cell carcinoma were excluded. Pathologic confirmation of 5 mm of viable tumor on the transurethral resection of bladder tumor (TURBT) specimen was required to provide adequate tissue for COXEN testing. TURBT and pelvic exam under anesthesia were performed within 56 days prior to registration. Patients with previous systemic cytotoxic chemotherapy for urothelial carcinoma, peripheral neuropathy ≥ Grade 2, Class III or IV heart failure, and hearing impairment > Grade 2 were excluded. Adequate organ function for chemotherapy was required, including sufficient bone marrow reserve and a calculated creatinine clearance of ≥ 60 mL/min, using the modified Cockcroft-Gault formula. The study was listed on clinicaltrials.gov with identifier: NCT02177695.
The subset of eligible patients was considered evaluable for the primary COXEN GEM assessment if they received at least 3 of 4 cycles of chemotherapy (or progressed while receiving chemotherapy), had adequate pretreatment tissue for COXEN GEM assessment, and either underwent a cystectomy within 100 days after chemotherapy or progressed prior to the expected cystectomy. The latter group was managed as non-responders.
Study design and treatment
S1314 was a phase II study with 1:1 randomization between GC and ddMVAC chemotherapy conducted by SWOG and other member groups of the National Clinical Trials Network (Figure 1). Randomization was balanced on two stratification factors: performance status (0 vs 1) and clinical T-stage (cT2 vs cT3/4). GC was administered as Gemcitabine 1,000 mg/m2 on days 1 and 8 with Cisplatin 70 mg/m2 on day 1 every 21 days for 4 cycles, with optional growth factor support. Dose-dense MVAC was administered as methotrexate (30 mg/m2) on day 1, vinblastine (3 mg/m2) on day 1 or 2, doxorubicin (30 mg/m2) on day 1 or 2, and cisplatin (70 mg/m2) on day 1 or 2 every 14 days for 4 cycles, with mandatory growth factor support use. At minimum, a bilateral standard pelvic lymph node dissection, including the external and internal iliac and obturator nodes, was to be performed at the time of the radical cystectomy. Patients were removed from protocol treatment for unacceptable toxicity, inability to receive adequate chemotherapy (e.g. a continuous delay more than 3 weeks, cumulative delay of 4 weeks, more than one dose reduction required, or less than 3 cycles of chemotherapy administered). Radiographic assessments of the chest, abdomen and pelvis by CT or MRI imaging were performed at baseline, in the preoperative period, every 3 months for one year, then every 6 months for 2 years, then annually until 5 years from registration.
Figure 1. Consort diagram.
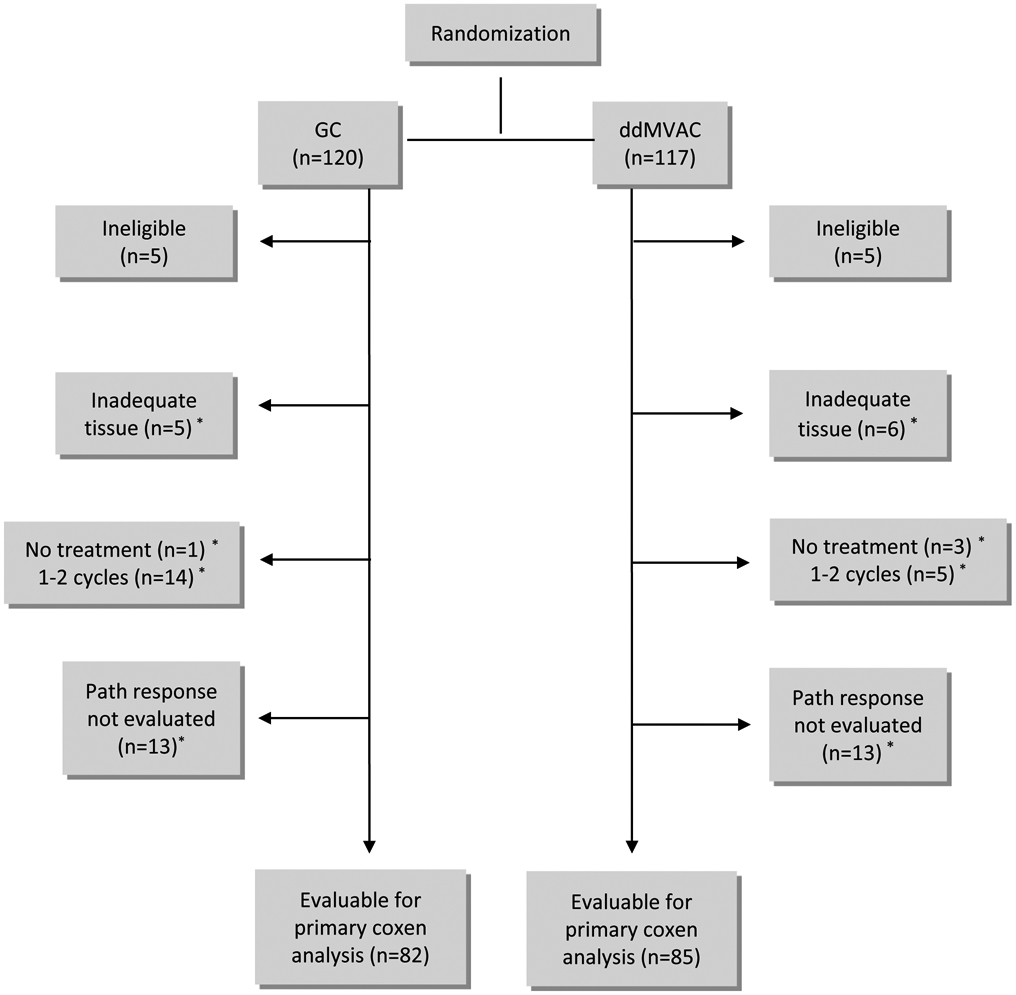
*These patients are included in secondary analysis of intent-to-treat comparison of path response rates between arms
The gene expression analysis for the COXEN determination was obtained via an Affymetrix U133A GeneChip via ALMAC laboratory (Durham, North Carolina). RNA was isolated from 10-micron slides and the samples were analyzed in 2 batches, with 7 patients having their samples run in both batches, as a quality control measure.
COXEN development
For the development of the COXEN score, the process started with gene expression modeling using the NCI60 panel for each agent (Methotrexate, Vinblastine, Adriamycin, Cisplatin and Gemcitabine). These data were then evaluated with human gene expression data to yield between 20 and 60 probes per agent. Model training and evaluation was then performed with additional data sets to develop the combined COXEN scores and the cut point for positivity for ddMVAC and GC regimens. The COXEN algorithms for each regimen and cut points for defining favorable versus unfavorable score were established prior to the start of the trial (see Supplemental figure 1 for additional details). The Laval cohort, comprised of bladder cancer patients treated with cystectomy and lymph node dissection with chemotherapy, was used to develop the COXEN GEM in both cases and consists of formalin-fixed paraffin embedded (FFPE) tissues of cystectomy specimens obtained from l'Hôpital de l'Hôtel-Dieu at Laval University, Québec, Canada as previously described (Supplemental Figure 1).14 Frozen robust multiarray analysis was used to normalize the microarray samples.15 The information utilized to calculate the individual drug scores is included as Supplemental Table 1.
Statistical analysis
The primary objective of S1314 was to evaluate whether either the pre-specified ddMVAC- or GC-specific COXEN GEM scores are associated with pathologic response at the time of cystectomy in patients treated with the respective neoadjuvant chemotherapy regimen. This was done by evaluating whether the treatment specific COXEN GEM score was associated with a complete response (pT0) rate or downstaging (≤ pT1) in the corresponding treatment group or in the pooled arms. Logistic regression was used to model pathologic response with the respective dichotomous COXEN GEM score (favorable/unfavorable), managed as a covariate with adjustment for the two stratification factors. All eligible, evaluable patients were included. The protocol also specified that if no interaction was found between the treatment specific GEM score and treatment arm, then data from both treatment arms could be combined to evaluate the predictive association of each GEM score with response to neoadjuvant chemotherapy more generally.
Another component of the primary objective was to assess in a preliminary fashion whether the COXEN GEM score is a regimen-specific predictive factor of pathologic response. This was also evaluated in a logistic regression model, fit separately for each COXEN score. In addition to stratification factors and dichotomous COXEN GEM score, an indicator for treatment arm and the interaction of treatment arm with COXEN GEM score was also included in the model, using the pooled sample. A significant interaction would suggest that the respective COXEN GEM score was able to differentiate whether a patient was more likely to respond to one chemotherapy regimen over another.
A secondary trial objective was to assess the difference in pT0 rate between the 21-day GC and 14-day ddMVAC arms in an intent-to-treat (ITT) analysis of all eligible, randomized patients, regardless of amount of treatment received, gene expression, or cystectomy status, using a logistic regression model adjusted for stratification factors. All ITT analyses report two-sided p values. We also assessed the safety and tolerability of 21-day GC and 14-day ddMVAC chemotherapy when given in the neoadjuvant setting for bladder cancer. All patients who received some protocol therapy were included in that analysis. Another secondary objective was to evaluate the value of gene expression profiling in predicting overall survival, but since survival data are not yet mature, that association will be evaluated in a subsequent report.
A sample size target of 184 eligible, evaluable patients was utilized to develop the statistical plan. The score from the COXEN GEM had previously yielded a sensitivity and specificity of 83% and 64%, respectively in a cohort of patients with muscle-invasive bladder cancer treated with neo-adjuvant MVAC chemotherapy.11 Applying a one-sided alpha of 0.05 and using 92 patients for each within-treatment arm analysis, there would be 99% statistical power to detect differences in pT0 rates where the absolute difference in the pT0 rate is 50% between the predicted responders and non-responders (based on favorable COXEN GEM score dichotomy) and 92% power to detect differences in pT0 rates of 30%. Although statistical power was known to be low, the ability of the regimen specific COXEN GEM score to direct which of the two neoadjuvant treatment regimens the patient should receive was investigated.
Results
A total of 237 patients was enrolled between 7/11/14 and 12/1/17. Ten patients were ineligible (6 without tissue, 2 without adequate disease assessment and 2 with kidney function out of window), leaving 227 patients. Of those, 11 had tissue that was inadequate for COXEN assessment, 23 received less than 3 cycles of chemotherapy, and among those who received 3-4 cycles, 26 did not have a cystectomy performed within 100 days, yielding 167 evaluable patients (Figure 1) for the primary COXEN GEM assessment. Non-evaluable patients were not included in the primary COXEN analysis, but were included in the ITT efficacy and safety evaluation. The median age was 64 years and the majority of evaluable patients were male, most common stage was cT2 and the majority of patents had a Zubrod performance status of 0 (Table 1). The proportion of favorable GC and ddMVAC COXEN GEM scores was similar in each treatment arm, although the proportion of favorable GC and ddMVAC GEM scores was higher in the ddMVAC treatment arm. The cross-classification of the favorable proportion of each COXEN GEM score and pathologic response are shown in Table 2 by treatment arm and in pooling both arms.
Table 1.
Patient Characteristics by Randomized Treatment Arm for the COXEN eligible and Intent-to-Treat groups
| COXEN-evaluable population | ITT analysis population | ||||
|---|---|---|---|---|---|
| GC (N=82) | ddMVAC (N=85) | GC (N=115) | ddMVAC (N=112) | ||
| Age (median, range) | 64.4 (34.5, 79.2) | 64.8 (33.1, 78.4) | 64.9 (34.5, 79.2) | 64.8 (33.1, 86.5) | |
| Sex | |||||
| Male | 64 (79%) | 75 (88%) | 92 (80%) | 99 (88%) | |
| Female | 18 (21%) | 10 (12%) | 23 (20%) | 13 (12%) | |
| Clinical Stage | |||||
| T2 | 74 (92%) | 73 (87%) | 102 (89%) | 98 (88%) | |
| T3 or T4a | 8 (8%) | 12 (13%) | 13 (11%) | 14 (12%) | |
| Zubrod Performance Status | |||||
| 0 | 61 (75%) | 67 (80%) | 88 (77%) | 86 (77%) | |
| 1 | 21 (25%) | 18 (20%) | 27 (23%) | 26 (23%) | |
| GC Score | |||||
| Favorable | 18 (21%) | 25 (30%) | 19 (22%) * | 29 (31%) * | |
| Not Favorable | 64 (79%) | 60 (70%) | 68 (78%) * | 64 (69%) * | |
| ddMVAC Score | |||||
| Favorable | 23 (28%) | 30 (36%) | 24 (28%) * | 31 (33%) * | |
| Not Favorable | 59 (72%) | 55 (64%) | 63 (72%) * | 62 (67%) * | |
n=47 patients included in the ITT analysis (n=28 on the GC arm, n=19 on the ddMVAC arm) were not evaluable for the primary COXEN analysis and therefore did not have COXEN scores generated.
Abbreviations:
GC - Gemcitabine, cisplatin
MVAC - Methotrexate, vinblastine, adriamycin, cisplatin
Table 2.
Cross-Classification of Treatment Arm, COXEN Score Dichotomy Status and Pathologic Response from Cystectomy
| Chemotherapy Response |
GC Arm (n=82) |
ddMVAC Arm (n=85) |
Pooled Treatment Arms (n=167) |
|||||
|---|---|---|---|---|---|---|---|---|
| Favorable GC Score |
Unfavorable GC Score |
Favorable MVAC score |
Unfavorable MVAC score |
Favorable GC Score |
Unfavorable GC Score |
Favorable MVAC Score |
Unfavorable MVAC Score |
|
| pT0 | 8 (44%) | 20 (31%) | 10 (26%) | 17 (38%) | 18 (42%) | 37 (30%) | 17 (32%) | 38 (33%) |
| ≤pT1, but not pT0 | 2 (12%) | 10 (16%) | 6 (15%) | 14 (31%) | 10 (23%) | 22 (18%) | 9 (17%) | 23 (20%) |
| Non-responders | 8 (44%) | 34 (57%) | 14(59%) | 24 (31%) | 15 (35%) | 65 (52%) | 27 (51%) | 53 (47%) |
| Total | 18 (100%) | 64 (100%) | 30 (100%) | 55 (100%) | 43 (100%) | 124 (100%) | 53 (100%) | 114 (100%) |
For the primary analysis, we determined the relationship of GC and ddMVAC COXEN GEM scores to pT0. The odds ratio for pT0 with respect to the GC biomarker in patients treated with GC was 2.63 (p=0.10; 95% CI [0.82,8.36]). This translates into an observed difference in pT0 response rates (Table 2) between favorable and unfavorable GC GEM is 44% versus 31%. For the ddMVAC COXEN GEM score with the endpoint of pT0 in those treated with ddMVAC, the odds ratio was 1.12 (p=0.82, 95% CI [0.42, 2.95]) (Table 3). The GC COXEN GEM score was applied to both arms for the outcome of downstaging (≤ pT1) with an odds ratio of 2.33 (p=0.02; 95% CI [1.11, 4.89]); when the ddMVAC COXEN GEM score was applied to both arms for the outcome of downstaging, the odds ratio was 0.90 (p=0.76; 95% CI [0.46, 1.75]) (Table 3). In this pooled analysis for the GC COXEN score, the sensitivity for pT0 and downstaging was 32% with a specificity of 81% (Table 3).
Table 3.
Results of Logistic Regression Modeling of COXEN Score and Pathologic Response at Cystectomy
| COXEN Score |
Outcome | Arm | N | Odds Ratio (95% CI)** |
P- value** |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| GC* | pT0 | GC | 82 | 2.63 (0.82, 8.36) |
0.10 | 29% (13%, 49%) |
81% (69%, 91%) |
44% (22%, 69%) |
69% (56%, 80%) |
| GC* | ≤pT1 | GC | 82 | 1.75 (0.60, 5.34) |
0.30 | 25% (13%, 41%) |
81% (66%, 91%) |
47% 34%, 60% |
53% 40%, 66% |
| ddMVAC* | pT0 | ddMVAC | 85 | 1.12 (0.42, 2.95) |
0.82 | 37% (19%, 58%) |
63% (46%, 78%) |
33% (17%, 53%) |
44% (17%, 53%) |
| ddMVAC* | ≤pT1 | ddMVAC | 85 | 0.92 (0.37, 2.27) |
0.86 | 34% (21%, 49%) |
63% (46%, 78%) |
53% (34%, 72%) |
69% (55%, 81%) |
| GC* | pT0 | Pooled | 167 | 1.84 (0.88, 3.83) |
0.10 | 33% (21%, 47%) |
78% (69%, 85%) |
42% (27%, 58%) |
70% (61%, 78%) |
| GC* | ≤pT1 | Pooled | 167 | 2.33 (1.11, 4.89) |
0.02 | 32% (23%, 43%) |
81% (71%, 89%) |
65% (49%, 79%) |
52% (43%, 61%) |
| ddMVAC* | pT0 | Pooled | 167 | 0.99 (0.49, 2.02) |
0.99 | 31% (19%, 45%) |
68% (58%, 76%) |
32% (20%, 46%) |
67% (58%, 76%) |
| ddMVAC* | ≤pT1 | Pooled | 167 | 0.90 (0.46, 1.75) |
0.76 | 30% (21%, 41%) |
66% (55%, 76%) |
49% (35%, 63%) |
46% (37%, 56%) |
favorable based on prespecified COXEN algorithm and cut point
adjusted for two stratification factors – clinical stage at baseline (T2 vs T3, T4a), PS (0 vs 1) in logistic regression model
pT0=complete pathologic response, ≤pT1 = downstaging
Pooled = GC + ddMVAC arms, PPV=positive predictive value, NPV = negative predictive value
Seven subjects had their samples run in two batches with GEM scores calculated and dichotomized for both GC and ddMVAC (14 determinations). One patient had a mismatch for both GC and MVAC GEM score classification, and one patient each had a mismatch in the MVAC GEM or GC GEM among the seven patients resulting in 4 of the 14 determinations in 3 patients not matching in the duplicate runs.
The predictive ability of the GC and ddMVAC COXEN GEM scores to assign specific chemotherapy treatments was assessed. No significant interaction between treatment arm and either COXEN GEM score was detected. Interaction p-values for the four models (complete response and downstaging outcomes for each of the two COXEN GEM scores) ranged from 0.43-0.88. Both the estimated Spearman and Pearson correlation coefficient between the GC and ddMVAC GEM scores were 0.39 (p=0.001), indicating moderate correlation.
Treatment and safety
The percentage of eligible subjects with adequate tissue receiving 3-4 cycles of chemotherapy was 86% for GC and 92% for ddMVAC (Figure 1). The proportion of pathologic complete response was comparable in both arms with 30% in the GC and 28% in the ddMVAC arms (p = 0.75). Similarly, the downstaging (partial or complete response combined) rate was similar in both arms with 40% in the GC and 47% in the ddMVAC groups (p = 0.27) (supplemental Table 2).
A summary of the adverse events is included in Table 4, with a complete listing in Supplemental Table 3. Febrile neutropenia was noted in 2 patients in the GC arm and 1 in the ddMVAC arm. In the intention-to-treat population (n=227), 100% in the ddMVAC and 60% in the GC arm received growth factor support. There were some clinically relevant differences in toxicities between the arms of the study with a higher incidence of mucositis, constipation, and diarrhea with ddMVAC, and higher incidence of thrombotic events, peripheral edema and neutropenia in the GC arm. There was one case of grade 5 toxicity categorized as related or likely related to therapy in the study, a patient treated with ddMVAC who died from cardiac arrest. There was one additional patient with grade 5 toxicity in the GC and two in the ddMVAC arm, categorized as unrelated or unlikely to be related to chemotherapy.
Table 4 -. Summary of Selected Adverse Events Number of Patients with a Given Type and Grade of Adverse Event.
Adverse Events Unlikely or Not Related to Treatment Excluded
| GC+CYST (n=114) Grade |
DDMVAC+CYST (n=109) Grade |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADVERSE EVENTS | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 |
| Alopecia | 95 | 16 | 3 | 0 | 0 | 0 | 62 | 27 | 20 | 0 | 0 | 0 |
| Anemia | 38 | 34 | 32 | 10 | 0 | 0 | 46 | 36 | 19 | 8 | 0 | 0 |
| Anorexia | 87 | 19 | 8 | 0 | 0 | 0 | 74 | 23 | 12 | 0 | 0 | 0 |
| Constipation | 86 | 21 | 7 | 0 | 0 | 0 | 74 | 29 | 6 | 0 | 0 | 0 |
| Cardiac arrest | 114 | 0 | 0 | 0 | 0 | 0 | 108 | 0 | 0 | 0 | 0 | 1 |
| Creatinine increased | 87 | 14 | 13 | 0 | 0 | 0 | 77 | 17 | 10 | 5 | 0 | 0 |
| Diarrhea | 102 | 11 | 1 | 0 | 0 | 0 | 85 | 19 | 3 | 2 | 0 | 0 |
| Edema limbs | 103 | 11 | 0 | 0 | 0 | 0 | 104 | 5 | 0 | 0 | 0 | 0 |
| Fatigue | 35 | 57 | 19 | 3 | 0 | 0 | 31 | 47 | 26 | 5 | 0 | 0 |
| Febrile neutropenia | 112 | 0 | 0 | 2 | 0 | 0 | 108 | 0 | 0 | 1 | 0 | 0 |
| Mucositis oral | 102 | 10 | 2 | 0 | 0 | 0 | 73 | 24 | 7 | 5 | 0 | 0 |
| Neutrophil count decreased | 72 | 6 | 16 | 17 | 3 | 0 | 95 | 3 | 2 | 5 | 4 | 0 |
| Peripheral sensory neuropathy | 103 | 8 | 3 | 0 | 0 | 0 | 96 | 10 | 3 | 0 | 0 | 0 |
| Platelet count decreased | 66 | 34 | 3 | 7 | 4 | 0 | 73 | 22 | 10 | 2 | 2 | 0 |
| Sinus tachycardia | 106 | 8 | 0 | 0 | 0 | 0 | 107 | 1 | 1 | 0 | 0 | 0 |
| Thromboembolic event | 104 | 1 | 5 | 3 | 1 | 0 | 106 | 0 | 2 | 1 | 0 | 0 |
| Tinnitus | 95 | 15 | 3 | 1 | 0 | 0 | 87 | 20 | 2 | 0 | 0 | 0 |
| Vomiting | 95 | 15 | 4 | 0 | 0 | 0 | 86 | 14 | 6 | 3 | 0 | 0 |
| White blood cell decreased | 74 | 17 | 18 | 5 | 0 | 0 | 97 | 5 | 2 | 4 | 1 | 0 |
| MAX. GRADE ANY ADVERSE EVENT* | 4 | 16 | 44 | 41 | 9 | 0 | 1 | 20 | 47 | 30 | 10 | 1 |
including AE’s not highlighted in this particular table
Discussion
The GC and ddMVAC COXEN scores were not significantly associated with response in their respective treatment arms. There was no evidence of an interaction between COXEN score and a specific treatment regimen in predicting response. Several factors may explain the lack of association with the primary objective. The statistical power analysis was based on 92 evaluable patients in each arm; however, despite increasing the overall accrual goal during the conduct of the trial, the number of evaluable patients was slightly below the number used in the power calculations, with 82 with GC and 85 with ddMVAC. This was largely driven by delayed/abandoned cystectomy and inadequate chemotherapy (i.e. < 3 cycles) and this may have impacted the power to detect an association in the primary analysis with respect to each COXEN marker.
It should be stressed that S1314 is a phase II, randomized study and was not powered to be a definitive study in this setting. Prospective phase II studies of this nature are a key aspect of the clinical evaluation of predictive biomarkers and necessary to inform the next step of development. As described, the GC marker has a signal of association with pathologic response, while the ddMVAC marker does not. It may also be possible that the observed modest statistical association with the GC COXEN score is a false positive. We observed an absolute difference in pT0 rate between favorable GC GEM and unfavorable of only 13% (44% vs. 31%, Table 2) while the study was adequately powered for differences in the range of 30%. Samples from 7 individuals were assessed in each of the two cohorts for both the MVAC and GC biomarkers; notably, 4 of 14 determinations were discrepant between the cohorts, which is of concern. Based on this finding, a larger number of replicates should be tested in future clinical application of this approach to better characterize the fidelity of these determinations across assessments.
Interestingly, the score from the GC GEM was associated with response when applied to the pooled arms with respect to downstaging, including p≤T1 (odds ratio of 2.33; p=0.02; 95% CT [1.11, 4.89]). This translates into a 65% downstaging for those with a favorable GC GEM and 48% downstaging for those categorized as unfavorable. The potential differences in performance of the GC versus ddMVAC COXEN GEM scores is not clear. It is possible that differences in the performance of these biomarkers may reflect an intrinsic limitation of COXEN principle, as GEMs developed for multiagent regimens are a compilation of GEMs for each drug individually. By this virtue, a significant amount of statistical noise is likely generated for regimens with the more drugs that are included (e.g. ddMVAC). There is planned additional testing of this hypothesis post hoc by evaluating GEM models for each individual drug used in each arm as well as in both arms and will report this separately.
S1314 provides a platform to develop and validate additional biomarkers in the bladder cancer neoadjuvant setting. Using the prospectively obtained clinical data and outcomes, assessments of circulating tumor cells, miRNA, DNA and SNP-based markers in these samples are ongoing. In addition, further refinement of the COXEN algorithm as well as the utility of GEM combinations in multiagent drug combination therapies will be evaluated as a future aim. While the overall survival (OS) data are not yet mature, we will evaluate the association of these GEM scores with OS in the future as these results mature.
While not primarily designed or powered to directly compare the clinical activity of GC and ddMVAC, this study does provide comparison data in a prospective randomized study. Overall, there was no clinically meaningful difference in the pathologic response between the arms and the observed pathologic response rates were similar to the rates observed in S8710 and other recent neoadjuvant chemotherapy trials.3,9,10 With respect to the adverse events, there were some regimen-specific differences observed between the treatment arms, which are not unexpected for these regimens.
In summary, this study did not find that COXEN score were predictive of pT0 rate or ≤ pT1. However, the GC COXEN score did show a statistically significant association with downstaging when applied to a pooled group including both treatment arms. This positive association of the COXEN derived GEM score and pathologic response in this setting validates a conceptual advance in biomarker development, namely that predictive biomarkers may be developed based on established cancer cell line drug response but in the absence of patient response data. This represents a novel translation of an in vitro-developed biomarker into a clinical application. Future plans include additional investigations of COXEN GEM uses and the evaluation of other biomarkers using this clinical data set.
Supplementary Material
Translational relevance.
The coexpression extrapolation (COXEN) algorithm represents a novel approach to generate predictive gene expression models (GEMs) for cancer therapy response, based on in vitro results. This methodology allows for the direct and rapid testing of new biomarkers in the clinical setting. S1314 is a randomized trial conducted by the National Clinical Trial Network of neoadjuvant GC and ddMVAC chemotherapy for bladder cancer and was prospectively designed to test COXEN generated GEMs in this setting. While neither chemotherapy specific COXEN GEM scores predicted for response in a statistically significant manner for a complete pathologic response, there was a signal of prediction when the GC score was tested across both treatment arms for pathologic downstaging. S1314 adds important randomized data comparing GC and ddMVAC neoadjuvant chemotherapy and the data generated from this study will be used as a platform to further refine the COXEN approach and assess other prespecified biomarkers.
Acknowledgements:
Support from NIH/NCI grant CA180888, CA180819, CA180820, CA180821, CA189830, CA180830, CA180834, CA189829, CA189971, CA180798, CA233230, CA180818, CA189822, CA189854, CA189972, CA180858, CA189872, CA189858, CA189958, CA189848, CA180835, CA189808, CA180826, CA180855, CA46368, CA13612 and CA46282.
Footnotes
These data have been presented in part at the American Society of Clinical Oncology (ASCO) Annual meeting, Chicago IL, June 3, 2019.
REFERENCES:
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7–34, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Flaig TW, Spiess PE, Agarwal N, et al. : NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J Natl Compr Canc Netw 16:1041–1053, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859–66, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Zaid HB, Patel SG, Stimson CJ, et al. : Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 83:75–80, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Sternberg CN, de Mulder PH, Schornagel JH, et al. : Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 19:2638–46, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, de Mulder P, Schornagel JH, et al. : Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42:50–4, 2006 [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–77, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Sonpavde G, Goldman BH, Speights VO, et al. : Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 115:4104–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. : Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32:1895–901, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choueiri TK, Jacobus S, Bellmunt J, et al. : Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 32:1889–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SC, Baras AS, Lee JK, et al. : The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Res 70:1753–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JK, Havaleshko DM, Cho H, et al. : A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A 104:13086–91, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoemaker RH: The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6:813–23, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Baras AS, Dancik G, et al. : A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol 12:137–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCall MN, Bolstad BM, Irizarry RA: Frozen robust multiarray analysis (fRMA). Biostatistics 11:242–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


