Abstract
Sports nutrition supplements have previously been reported to contain undeclared doping substances. The use of such supplements can lead to general health risks and may give rise to unintentional doping violations in elite sports. To assess the prevalence of doping substances in a range of high-risk sports nutrition supplements available from Dutch web shops. A total of 66 sports nutrition supplements - identified as potentially high-risk products claiming to modulate hormone regulation, stimulate muscle mass gain, increase fat loss, and/or boost energy - were selected from 21 different brands and purchased from 17 web shops. All products were analyzed for doping substances by the UK life sciences testing company LGC, formerly known as the Laboratory of the Government Chemist, using an extended version of their ISO17025 accredited nutritional supplement screen. A total of 25 out of the 66 products (38%) contained undeclared doping substances, which included high levels of the stimulants oxilofrine, β-methylphenethylamine (BMPEA) and N,β-dimethylphenethylamine (NBDMPEA), the stimulant 4-methylhexan-2-amine (methylhexaneamine, 1,3-dimethylamylamine, DMAA), the anabolic steroids boldione (1,4-androstadiene-3,17-dione) and 5-androstene-3β,17α-diol (17α-AED), the beta-2 agonist higenamine and the beta-blocker bisoprolol. Based upon the recommended dose and the potential variability of analyte concentration, the ingestion of some products identified within this study could pose a significant risk of unintentional doping violations. In addition to inadvertent doping risks, the prescribed use of 3 products (4.5%) could likely impose general health risks.
Key points.
In this study, 38% of 66 high-risk sports supplements tested (which claimed to intensify workouts, promote muscle growth and fat loss) were found to contain doping agents.
4.5% of the products tested were found to contain doping agents in concentrations which can have acute negative health effects, and may result in a positive doping test.
The problem regarding the presence of undeclared doping agents in sports supplements has not diminished in recent decades.
Key words: Contamination, spiking, dietary supplements, prohibited substances, elite sport, health risks, doping violation
Introduction
An increasing part of the world population uses nutritional supplements (Bailey et al., 2011; Skeie et al., 2009; Imai et al., 2006). These supplements are mainly consumed for health reasons (Bailey et al., 2013; Dickinson et al., 2014; Wardenaar et al., 2016). Unfortunately, some nutritional supplements can also cause health problems (Palmer et al., 2003; Teschke et al., 2012; Zhou et al., 2013; Cohen, 2009; 2016). This is especially true since the United States effectuated the Dietary Supplement Health and Education Act in 1994 (NIH, 1994). It set the worldwide governmental standard on how to deal with dietary supplements. From this moment on, the supplement industry was no longer required to prove a supplement is safe and effective before they can sell it. Product quality and regulation is left up to the industry itself (Angell and Kassirer, 1998; Ayotte et al., 2001; Gurley et al., 2000; Catlin et al., 2000). As a result, some nutritional supplements can contain actual dosages divergent from the dosages communicated on the label (Angell and Kassirer, 1998; Ayotte et al., 2001; Gurley et al., 2000; Parasrampuria et al., 1998; Chen et al., 2009; Green et al., 2001). Some products can even contain ingredients not listed on the label at all (Ayotte et al., 2001; Catlin et al., 2000; Chen et al., 2009; Kamber et al., 2001; Green et al., 2001).
This lack of regulation can be particularly hazardous for elite-level athletes. They consume supplements far more than others, primarily for performance enhancement reasons (Green et al., 2001). In a recent study, 64% of elite athletes use supplements (Baltazar-Martins et al., 2019). 42% of these users in elite did not consult any professional. Among fitness enthusiasts and recreative weightlifters the number of supplement users may be even higher. Studies have reported percentages of gym visitors using supplements between 36 and 85% (El Khoury and Antoine-Jonville, 2012; Morrison et al., 2004).
In addition, supplements targeted at performance enhancement can be labeled as ‘high-risk’ as some of them contain harmful, undeclared ergogenic substances. Studies report contaminations with anabolic agents (Catlin et al., 2000; Kamber et al., 2001; Green et al., 2001; Geyer et al., 2002; 2003; De Cock et a.l, 2001; Schilt et al., 2002; Delbeke et al., 2002; Van der Merwe and Grobbelaar, 2004; Baume et al., 2006; Parr et al., 2007; Judkins et a.l, 2007; Martello et al., 2007), stimulants (Kamber et al., 2001; Schilt et al., 2002; Van der Merwe and Grobbelaar, 2004; Parr et al., 2007; Judkins et al., 2007; Martello et al., 2007; Jung et al., 2006; Vidal and Quandte, 2006) and beta-2 agonists (Parr et al., 2008). With the continued growth of the nutritional supplement market, these problems continue to evolve. Recently, dozens of ‘US legal for sale’ supplements were identified which declared designer steroids on their label (Rahnema et al., 2015). A number of sports nutrition supplements are even found to be ‘spiked’ with high amounts of newly developed designer stimulants (Rahnema et al., 2015; Cholbinski et al., 2014; Uralets et al., 2014; Wójtowicz et al., 2015; Cohen et al., 2014; 2016). These deliberate additions already have led to some severe health related sports incidents (Smith et al., 2014; Archer et al., 2015; Johnston et al., 2016).
Next to these health risks, the use of contaminate supplements may also cause elite athletes to infringe doping regulations. According to the world anti-doping rules, athletes are strictly liable for doping substances found in their urine during doping control procedures (WADA, 2015). At the same time, doping laboratories can sometimes detect concentrations as low as a few picograms per milliliter (Thevis et al., 2017). Consequently, it is estimated that up to 9% of all the positive doping tests nowadays are caused by elite athletes using poorly labeled sports nutrition supplements (Outram and Stewart, 2015).
Although the risks are quite clear, recent prevalence rates concerning the doping contamination of sports supplements that are supposed to enhance performance are lacking. As the only two comprehensive studies in this field are at least a decade old (Geyer et al., 2004; Judkins et al., 2007), we executed this study, assessing the current prevalence of doping contamination in sports nutrition supplements.
Methods
Selection of sports nutrition supplements
We searched for web shops targeting the Dutch market in the fourth quarter of 2014. We used several search prompts combining the following terms (translated from Dutch): “nutritional”, “supplements”, “online”, “buy”, “web shop”. Web shops were excluded if they did not provide an overview of the brands sold or if they did not sell sports nutrition supplements. We merged the overviews of the brands sold by the web shops into one database. Brands were excluded if they were listed by less than 50% of the web shops, if they did not sell sports nutrition supplements or if they executed specific screening (as part of a quality assurance programme) for doping substances. From brands that sold over 30 different dietary supplements, we only included the sports nutrition supplements which were part of the brands premium product line, or were part of the product lines with the strongest focus on improving ergogenic performance. From the brands included we made an inventory of the sport nutrition supplements they sold. Products were included if they emphasized one or more of the following performance enhancing claims: modulate hormone regulation, stimulate muscle mass gain, increase fat loss, or boost energy; such functional categories being identified as potentially posing a greater risk to the athlete.
Supplements were excluded if they listed one or more doping substances on their label or if their label contained a specific warning for drug tested athletes. Out of these preselected sports nutrition supplements we made an equally distributed purchase selection.
Purchase orders were placed in December 2014. If orders were cancelled, we tried other web shops. If products turned out to be unavailable altogether, we replaced them with other products. Free try out samples were included if they were already part of our preselection. In the end we sent out 66 products from 21 brands and 17 web shops for analysis with Gas Chromatography Mass Spectrometry (GCMS) and Liquid Chromatography Mass Spectrometry (LCMS) techniques (Figure 1).
Figure 1.
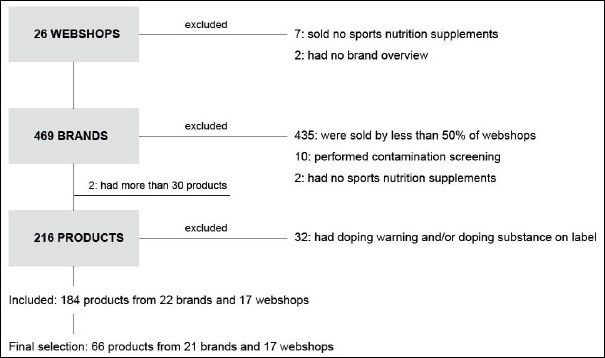
Outline of study protocol.
Sample preparation
Laboratory analysis was performed by LGC, formerly known as the Laboratory of the Government Chemist, a laboratory globally recognized in the field of anti-doping analysis, with ISO/IEC 17025 accreditation underlining their technical competence. LGC manage and administer the sports nutrition supplements safeguards systems Informed-Sport and Informed-Choice. On receipt of the samples, LGC reviewed the integrity of all products. Products that contained multiple components with different compositions were split into each constituent and analyzed separately. Powdered supplements were mixed before a homogenous 1 g portion per sample was taken for analysis. Tablets were crushed into a fine powder, and mixed before a 1 g sample was analyzed. Capsules and gel caps emptied, and the contents were blended and mixed with the chopped shells before a 1 g sample was taken for analysis.
High level screening analysis
LGC’s drug screening methods are designed to detect trace levels of compounds, typically in the low ng/g region. Therefore, due to the potential for doping substances to be present at elevated levels (based on the selection criteria employed within this study), an initial pre-screening analysis was conducted, with samples being tested in a strongly diluted form. This high level screening consisted of four different screening tests, targeting the detection of compounds in approximately the mg·g-1 region. The Basic Screen and Acidic/Neutral Screen covered the detection of compound classes such as stimulants, diuretics, narcotics and beta-blockers, an Atmospheric-Pressure Chemical Ionization (APCI) Screen covered the detection of glucocorticoids and compounds requiring ionization by APCI and a Steroid Screen covered the detection of anabolic steroids. All samples were included in the Basic, Acidic/Neutral and APCI Screen. For reasons of effectiveness, only the 24 products advertised to modulate hormone regulation or stimulate muscle mass gain were included for the Steroid Screen. In total, 27 individual samples were screened, as some products consisted of multiple components.
Basic Screen
Following sample preparation, 1 g of each sample was weighed into a 20 mL glass scintillation vial to which 10 mL methanol was added. The samples were then placed on a rotary mixer and solvent extracted for one hour. After mixing, the samples were centrifuged to remove particulate from the solvent. From this, a 100μL aliquot of supernatant was taken and diluted with 9900 μL of methanol, resulting in an effective working solution equivalent to 1g sample diluted in 1L methanol. From this working solution, 10μL aliquots were taken and placed into separate plastic HPLC vials. Internal standard (morphine-d3) was added to each vial as an injection marker. Next, the combined sample and internal standard mix was gently evaporated to dryness under oxygen-free nitrogen. Prior to analysis all samples were reconstituted with screen applicable solvents. The samples were analyzed by High Resolution - Liquid Chromatography Mass Spectrometry (HR-LCMS) using a Thermo LTQ Orbitrap. Full scan data was captured which was then referenced against compound databases containing in excess of 2000 compounds.
Acidic/neutral Screen
As with the basic screen, 1g of sample was effectively diluted with 1L of methanol and a 10μL aliquot taken for analysis. Hydrochlorothiazide-d2 (instead of morphine-d3) was used as an injection marker. Following evaporation and reconstitution samples were then analyzed by HR-LCMS using a Thermo Q-Exactive Orbitrap. Similar to the Basic Screen, the high resolution full scan mass spectrometry data was captured and referenced against compound databases containing in excess of 2000 compounds.
APCI Screen
Using the sample prepared for the acidic/neutral screen as detailed above, analysis was performed using Liquid Chromatography Mass Spectrometry - Multiple Reaction Monitoring (LCMS-MRM). Hydrocortisone-d2 was used as an injection marker. The instrument used was a Sciex 5500 Q-Trap triple quadrupole mass spectrometer, coupled to a Waters Acquity uHPLC system. Data was collected in MRM mode with up to three diagnostic transitions collected for each analyte.
Steroid Screen
1g of sample was weighed into a 20ml glass scintillation vial, to which 10mL methanol was added (as detailed above). Following extraction and centrifugation a 4 mL aliquot was taken. These samples were evaporated to a low volume using a Genevac rotary vacuum evaporator. To the concentrated samples, 4mL of an acetate buffer was added. This buffer contained the internal standard methyltestosterone. Subsequently, the samples were extracted using Solid-Phase Extraction to obtain a purified neutral fraction. After evaporation to dryness, samples were derivatized to form trimethylsilyl (TMS) ethers. Samples were analysed using an Agilent 6890 GC coupled to an Agilent 5973 Single Quadrupole MS system. Data was collected in both Selected Ion Monitoring (SIM) and Full-scan mode simultaneously.
Estimations of high level findings
Using the process of standard addition, the elevated findings revealed during the high level screening analysis were re-analyzed to estimate concentration levels. Target analytes were added to a series of volumetric flasks so that their final concentration levels would be equivalent to 0, 5, 10, 20, 40 and 80ngmL-1. Internal standard was added to each flask to give a concentration equivalent to 20ngmL-1. The 6 calibration samples were prepared in duplicate. In line with the original procedure, the samples of the high level screening products were diluted to bring their concentrations within instrumental range. 100μL of sample was added to all replicates, after which they were made up to volume with methanol. The same level of dilution used for the high level samples was also used for the System and Matrix blanks, with only one replicate of the final dilution for each prepared. Both System and Matrix blanks were spiked with internal standard only. Following completion of all replicates, 500μL of each was taken and evaporated to dryness prior to reconstitution into mobile phase. Samples were analysed using a Sciex 4000 Q trap MS coupled to a Waters Acquity uHPLC, using a method designed to separate amphetamine and methamphetamine from their β-methyl isomers. In addition to the samples and blanks, a mixed reference standard was also analyzed. Data was collected in MRM mode with three transitions monitored for each analyte. In order to provide an estimate of concentration within the samples, calibration curves were produced from which the linear equation Y = MX + C was obtained. In order for the curve to be considered acceptable, criteria of ± 15% were applied to each point. A minimum of 4 points were required, excluding the 0 replicate. Of the four points a maximum of 2 replicates from separate points could be excluded. With X being the subject and Y=0, the concentration within the dilution was obtained. By taking into account the overall dilution, the concentration per gram was calculated. It should be noted that this method of standard addition assumes 100% extraction of target analytes. Therefore, the calculated concentration should be considered as an approximate minimum value.
Low level screening analysis
After the high level screening analysis (employed to pre-screen samples and limit the risk of laboratory contamination) LGC executed low level screening analysis using their ISO/IEC 17025 accredited nutritional supplement screen, (the screen being extended with additional non-accredited compounds). The screening process is designed and validated to detect compounds in the low ng (nanogram) region and consists of three different tests: Steroid Test, Basic Test, and Acidic/Neutral Test. However, it should be noted that whilst the non-accredited compounds do not fall under the current scope of the ISO accreditation, analysis was still subject to the same quality control procedures. Furthermore, these tests do not give a precise estimation of the amount of analyte found. The tests only answer whether a doping substance is detected in the concerning product – the test being considered a qualitative analysis only. Such findings can typically be considered to be in the ng·g-1 region.
After sample preparation, a 1 g portion of each sample was obtained. Internal standards specific to each test were added to each sample to verify extraction efficiency. The internal markers consisted of a number of deuterated and isotopic analogues of the target analytes. Internal markers were added at levels equivalent to 10 or 100 ngg-1, depending on the specified method capability or reporting level of each compound. In addition to the samples, positive and negative controls were prepared to monitor batch performance. The negative controls were used to rule out potential process contamination and were only spiked with internal standards. The positive controls were spiked with both internal standards and all of the compounds being screened. All samples were then diluted and extracted with methanol by placing on a rotary mixer for 1 hour. After mixing, the samples were centrifuged to remove excess particulate from the solvent. Two separate portions of the supernatant were then removed.
Basic and Acidic/Neutral tests
One portion of the supernatant was treated with an acetate buffer and extracted using mixed mode Solid-Phase Extraction. This process was conducted to isolate separate basic and acidic/neutral fractions. Both fractions were then gently evaporated to dryness prior to reconstitution with mobile phase.
Steroid test
The second portion of supernatant was treated with a high pH phosphate buffer and extracted with Solid-Phase Extraction. The samples were then evaporated to dryness prior to reconstitution with NaOH. The samples were then liquid extracted with pentane prior to conducting a second Solid-Phase Extraction. After evaporation to dryness the samples were derivatized to form TBDMS ethers.
Product contamination figures
Products were reported as negative, positive or inconclusive. A positive result means high level or low level screening analysis revealed one or more doping substances in the product. A negative result means screening analysis did not reveal any doping substances or the substance detected was below its specified reporting limit. If no doping substances were revealed, but screening analysis did not fully succeed (one or more compounds were not recovered by the analytical process), the result for this product was marked inconclusive.
Estimations related to positive doping controls
In order to evaluate the potential risks of a contaminated product leading to a positive doping test, we had to compare the potential ingestion of doping substances from contaminated products with the minimum ingestion needed to obtain a positive doping test. We estimated the maximum ingestion by multiplying the estimated contamination level by the recommended maximum daily dose for each product.
For the high level findings, contamination levels were estimated using the process of standard addition. For the low level determinations, only a qualitative analysis was conducted; however the majority of findings can typically be considered in the low ngg-1 range (typically sub 100ng/g). Noting that a quantitative estimation cannot be assigned to these low level findings and to prevent false-negative risk estimations, we therefore decided to use a maximum sample calculation of 1000 ngg-1 for all low level screenings. By doing this we also further emphasize the potential risk posed by these products.
Next, we established the minimum ingestion needed for a positive doping test. For endogenous anabolic steroids - when administrated exogenously - it is expected to be in the low mg region (Strahm et al, 2015; Watson, et al, 2009). For the other doping substances the minimum ingestion for a positive doping control is estimated indirectly by combining the reporting levels set by WADA (2018a; 2018b) and data from limited number of available excretion studies and clinical reports (Wójtowicz et al, 2015; Van Eenoo et al, 2006; Koehler et al, 2007, Perrenoud et al, 2009; Feng, et al, 2012; NHI, 2019). It results in ballpark figures of 10 μg for higenamine, 50 μg for most stimulants and bisoprolol, 5 mg for cathine and 10 mg for the stimulants ephedrine and methylephedrine.
Potential health risks
Most medicines related to the detected contaminants have a maximum daily dose of several milligrams (Table 1). The only exception are medicines related to higenamine, which can have maximum daily doses around 100μg. However, according to Feng et al (Perrenoud et al, 2009) the highest safe dose of higenamine is established at 24 μg per kg of body weight. Therefore, we feel it is safe to assume that supplement contaminations below 1 mg - for the doping substances found in this study - are unlikely to cause moderate to severe health issues. For supplements contaminations above 1 mg, we compared the contaminant maximum daily intake to the maximum daily intake prescribed for comparable medicines. For the synthetic amphetamine-like ß-methylphenethylamine and N,ß-dimethylphenethylamine we used Attention Deficit Hyperactivity Disorder medicines Dexedrine and Ritalin as comparison (Catalent Pharma Solutions, 2017; Novartis Pharmaceuticals Corporation, 2017). Their maximum daily dose is set at 60 mg. For oxilofrine we used Carnigen as comparison. For this outdated medicine (1950-2010) the maximum daily dose was 40 mg of oxilofrine a day (Cohen et al, 2017).
Table 1.
Maximum dose of medicines containing doping substances
| WADA Doping Class | Substance | Maximum dose |
|---|---|---|
| Anabolic agents | testosterone | 1000 mg* |
| nandrolone | 50 mg* | |
| Stimulants | dexamphetamine | 40 mg** |
| methylphenidate | 60 mg** | |
| ephedrine | 50 mg** | |
| Beta-2 agonists | salbutamol | 40 mg* |
| salmeterol | 100 μg** | |
| formoterol | 72 μg** | |
| Beta-blocker | bisoprolol | 10 mg** |
* Maximum single dose
** Maximum daily dose (WADA, 2015)
Results
Screening results
Of the 66 sports nutrition supplements, 25 products (38%) tested positive for the presence of doping substances, 38 products (58%) tested negative, and the results of 3 products (4.5%) were inconclusive (Figure 2).
Figure 2.
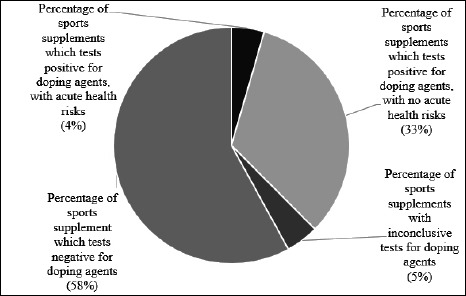
Overview of the main screening results.
The positive products contained 5 different anabolic steroids (21 findings), 9 different stimulants (25 findings), 1 beta-2 agonist (4 findings) and 1 beta-blocker (1 finding) (Figure 3).
Figure 3.
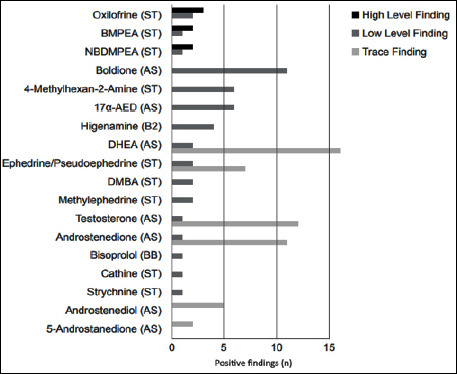
A vertical bar chart showing the detected doping agents, organized by number of hits.
Three products (4.5%) contained high levels of synthetically produced stimulants. Two products contained oxilofrine as well as β-methylphenethylamine (BMPEA) and N,β-dimethylphenethylamine (NBDMPEA). One product contained only high levels of oxilofrine (Table 2).
Table 2.
High level findings.
| Supplement | Compound | Equation | X | Weight (g) | Estimated concentration (mg·g-1) |
|---|---|---|---|---|---|
| F1 | BMPEA | Y=0.00254X – 0.07 | 27.6 | 1.08 | 26 |
| NBDMPEA | Y=0.0133X – 0.0141 | 1.06 | 1.08 | 1 | |
| oxilofrine | Y=0.00177X – 0.0209 | 11.8 | 1.08 | 11 | |
| F3 | BMPEA | Y=0.00704X – 0.407 | 57.8 | 1.19 | 49 |
| NBDMPEA | Y=0.0262X – 0.735 | 28.1 | 1.19 | 24 | |
| oxilofrine | Y=0.0173X – 0.82 | 47.4 | 1.19 | 40 | |
| G3 | oxilofrine | Y=0.00172X – 0.102 | 59.3 | 1.07 | 55 |
During low level screening, the anabolic steroid boldione was detected the most (11 findings) followed by the anabolic steroid 5-androstene-3β,17α-diol (17α-AED) and the stimulant 4-methylhexan-2-amine (both 6 findings), The beta-2 agonist higenamine was detected 4 times.
The beta-blocker detected was bisoprolol (Figure 3).
Doping control risk assessment
Based on the results of this survey, the use of some sport nutrition supplements pose a significant risk of doping violations (Table 3).
Table 3.
Maximum doping substance intake and doping control risk
| Supplement | Weight maximum daily dose | Compound (class) | Contamination calculation | Positive doping control threshold | Estimated potential risk |
|---|---|---|---|---|---|
| B5 | 1.5 g | higenamine | 1.5 μg | 10 μg | NO 2017 |
| F1 | 15 g | BMPEA † NBDMPEA † Oxilofrine † |
0.39 g 15 mg 0.17 g |
50 μg 50 μg 50 μg |
YES YES YES |
| F3 | 20 g | BMPEA † NBDMPEA † Oxilofrine † |
0.98 g 0.48 g 0.80 g |
50 μg 50 μg 50 μg |
YES YES YES |
| G2 | 3.0 g | Endogenous AAS (1) higenamine oxilofrine testosterone |
3.0 μg 3.0 μg 3.0 μg 3.0 μg |
1 mg 10 μg 50 μg 1 mg |
NO NO 2017 NO NO |
| G3 | 3.0 g | cathine ephedrine methylephedrine oxilofrine † strychnine testosterone |
3.0 μg 3.0 μg 3.0 μg 0.17 g 3.0 μg 3.0 μg |
5 mg 10 mg 10 mg 50 μg 50 μg 1 mg |
NO NO NO YES NO NO |
| H23 | 4.8 g | Endogenous AAS (6) ephedrine higenamine |
29 μg 4.8 μg 4.8 μg |
1 mg 10 mg 50 μg |
NO NO NO 2017 |
| O2 | 4.8 g | higenamine | 4.8 μg | 10 μg | NO 2017 |
| Q1 | 89 g | DMBA oxilofrine |
89 μg 89 μg |
50 μg 50 μg |
YES YES |
†= high level finding, 2017 = before September 2017 this product was estimated to potentially cause a positive doping test, 3=this product contained 3 components which were tested separately
Only for the three products with high level screening findings does this risk seem obvious. Maximum daily intake of the product with the highest risk is estimated to cause urine levels up to 20,000 times higher than the amount needed to provide a positive doping test. Of the products with only low screening findings only one product containing stimulants has the theoretical potential to do the same, but only if contamination levels are assumed to be at the high end of the ng·g-1 range. The risk is predominantly caused by the product’s high maximum recommended daily dose of 89 g. At the time of the study, the 4 products containing higenamine would also cause a clear potential risk for doping tested athletes. However, in September 2017 WADA introduced a reporting level for higenamine, which indicates that the laboratories are expected not to report higenamine urine levels below 10 ng·mL-1 (WADA, 2018a). Since then, the four detected higenamine findings are unlikely to cause doping violations anymore.
Health risk assessment
Only the three high level screening findings give maximum ingestion levels well above the previous established threshold level of 1 mg (Table 4).
Table 4.
Maximum intake compared to prescribed maximum dosages
| Product | Compound | Maximum intake | Prescibed Maximum dosage | Factor |
|---|---|---|---|---|
| F1 | BMPEA | 0.39 g | 60 mg | 6.5 |
| NBDMPEA | 15 mg | 60 mg | 0.3 | |
| oxilofrine | 0.17 g | 40 mg | 4.3 | |
| F3 | BMPEA | 0.98 g | 60 mg | 16 |
| NBDMPEA | 0.48 g | 60 mg | 8.0 | |
| oxilofrine | 0.80 g | 40 mg | 20 | |
| G3 | oxilofrine | 0.17 g | 40 mg | 4.3 |
Discussion
This study demonstrates that 38% of the selected high-risk sports nutrition supplements sold online contain undeclared doping substances. Based upon the recommended dose, the ingestion of some of these products could result in doping violations. The prescribed use of 3 products (4.5%) could impose general health risks.
In the present study we assessed the current prevalence of doping contamination in a range of high-risk sports nutrition supplements with specific functional claims. We selected 216 sports nutrition supplements claiming to modulate hormone regulation, stimulate muscle mass gain, increase fat loss, and/or boost energy. As we were interested in the unreported presence of doping substances, 32 products (15% preselection) were excluded for declaring a doping substance on the label and/or giving a specific warning for doping controlled athletes (Figure 1). Nevertheless, these products may pose significant risks for athletes concerning unintentional doping violations and general health.
We purchased and analyzed 66 products, 25 (38%) of which contained undeclared doping substances. As in previous prevalence studies 15-24% of high-risk sport supplements contained doping agents (Geyer et al., 2003; Judkins et al. 2007) (Table 5), the sports supplements industry doesn’t appear to be solving its contamination and spiking problem. Table 5 may at first sight give the impression that the situation is deteriorating, but Chi-Squared testing indicates this is not the case (data not shown).
Table 5.
Comparison of prevalence rate studies.
The difference can probably be best explained by the more extensive testing screen used in this study. Also the stronger focus on selecting products emphasizing performance enhancing claims, which are potentially higher risk products, is felt to be of influence.
The positive products contained 5 different anabolic steroids (21 findings), 9 different stimulants (25 findings), 1 beta-2 agonist (4 findings) and 1 beta-blocker (1 finding) (Figure 3). Three products were reported to contain high levels of contamination (Table 2). One contained oxilofrine (at least 55 mg·g-1), two others were shown to contain oxilofrine (40 and 11 mg·g-1) as well as β-methylphenethylamine (BMPEA, 49 and 26 mg·g-1) and N,β-dimethylphenethylamine (NBDMPEA, 24 and 1 mg·g-1). When taking the prescribed maximum daily dose of these products, the maximum intake of contaminants would exceed the maximum prescribed dose of referenced medication 6 out of 7 times. For each product as a whole, the three supplements exceeded the maximum prescribed doses of referenced medication by 4 to 44 times (Table 4). Ingestion of such excessive amounts could potentially lead to adverse health effects, including cardiac arrest (Novartis Pharmaceuticals Corporation, 2017) and coma (Catalent Pharma Solutions, 2017; Novartis Pharmaceuticals Corporation, 2017). Oxilofrine is a synthetically produced hydroxyl derivate of ephedrine. It was produced and sold as a medicine under the name Carnigen to treat low blood pressure from 1950 to 2010. It has been reported in nutritional supplements before (Cohen et al., 2017). The synthetically produced β-methylphenethylamine (BMPEA), an isomer of amphetamine, was also detected in nutritional supplements before (Cholbinski et al., 2014). As far as we know, and at the time of this study, the detection of the synthetically produced N,β-dimethylphenethylamine (NBDMPEA) in supplements is a first. It is isomeric with synthetic methamphetamine.
During low level screening, the anabolic steroid boldione was detected the most (11 findings) followed by the anabolic steroid 5-androstene-3β,17α-diol (17α-AED) and the stimulant 4-methylhexan-2-amine (both 6 findings). The beta-2 agonist higenamine was detected 4 times. During this screening, the beta-blocker bisoprolol was also detected once. Next to the high level findings, the stimulant oxilofrine was detected two more times and the stimulants BMPEA and NBDMPEA were both detected one more time.
The use of some sport nutrition supplements could lead to doping violations. Based on the data from this study and the available reference data, it is not possible to state exactly how many. Only for the three products with high level screening findings this risk seems obvious. WADA states that most stimulant urine levels should not be reported below 50 ngg-1. According to data from excretion studies (Van Eenoo et al., 2006; Koehler et al., 2007; Perrenoud et al., 2009), we expect that at least a single dose of 50 μg should be ingested to obtain this minimum urine level. However, when the maximum recommended daily dose is taken, the ingestion of the 7 high level contaminants (found within 3 products - Table 2) is expected to range between 15 mg - 0.98 g. This is 300-20,000 times higher than potentially needed to cause a positive doping test. Of the products with only low screening findings only one product containing stimulants has the theoretical potential to do the same, but only if contamination levels are assumed to be at the high end of the ng·g-1 region. The risk is then predominantly caused by the product’s high maximum recommended daily dose of 89 g. The assumptions made seem to infer that testing sport nutrition supplements at levels of 10-100 ng·g-1 are a good measure for elite athletes to mitigate the risk of accidentally infringing doping regulations.
Four products were reported to contain higenamine (norcoclaurine). Higenamine is a natural constituent of several botanicals (Chung et al., 2006; Patil et al., 1991). The four products containing higenamine all contained complex botanical ingredients, making it possible that the presence was due to natural presence instead of product contamination. At the time of the study, ingestion of these products would be a clear potential risk for doping tested athletes. Laboratories had a limit of detection as low as 2 pg·mL-1 and there was no reporting limit (Thevis et al., 2017). Based on data of excretion studies, we calculate that the ingestion of a single dose of 2 ng higenamine already had the potential to cause this level. However, WADA acknowledged this risk and introduced a reporting level of 10 ng·mL-1 for higenamine in September 2017 (Perrenoud et al., 2009). Since then, the four detected higenamine findings are unlikely to cause doping violations anymore. Hence, the introduction of the reporting levels for higenamine seems an adequate response from WADA to protect the good-willing athletes and to prevent honest mistakes.
The complex nature of botanical based products has potentially played part in most of the findings within this study. The most common compounds observed are steroids such as boldione and 5-androstene-3β,17α-diol (17α-AED). It is not fully understood as to why these observations are occurring. They may be the result of suboptimal production processes in the supplement chain. Another explanation may be the possible microbial conversion of plant sterols into low levels of anabolic steroids or natural presence (Patil et al., 1991; Saraphanchotiwitthaya and Sripalakit, 2016). The same explanations may apply to the findings of the natural occurring stimulants ephedrine (and/or pseudoephedrine), methylephedrine, norpseudoephedrine and strychnine (Behpour et al., 2012; Dingerdissen and McLaughlin, 1973; Medana et al., 2013). Since we were already aware of this risk before this study, we used minimum reporting levels for a number of doping substances related to microbial conversion or natural presence (Figure 3). Reporting levels were set at 10ng·g-1 for related anabolic steroids and 100ng·g-1 for pseudoephedrine/ephedrine. Findings below these levels were not considered positives. However, without the use of these reporting levels, another 8 products would have been regarded positive, bringing the total positive products up to 50%. The number of findings (doping substances detected) would have risen from 51 to 104.
Product ‘spiking’ (the deliberate action of a manufacturer to add undeclared substances to a product) could be considered an obvious explanation for the seven high level findings. For the low level findings of synthetic stimulants 4-methylhexan-2-amine (6 findings), 1,3-dimethylbutylamine (DMBA, 2 findings) and oxilofrine (2 findings) and beta-blocker bisoprolol (1 finding) cross-contamination seems more plausible. The complex nature of botanical based products was also felt to have played part in the three products which produced inconclusive results.
The present study shows that the current self-control strategy for the production of nutritional supplements is insufficient. A small amount of sports nutrition supplements are likely ‘spiked’ with newly developed designer compounds and may continue to cause health related sports incidents. Better regulation and controls are needed to prevent potential health issues amongst athletes and the general consumer alike. The possible natural presence of - and microbial conversion of plant sterols into – low levels of doping substances in botanical ingredients should be studied more extensively. Next to higenamine, it may also explain several other low level findings.
The problems outlined in this paper may be more serious than our estimates and calculations suggest. We have not taken into account the habit of some sports supplement users to use higher doses than the manufacturer specifies. In a study of users of pre-workout formulas, 14% of them admitted to exceeding the recommended maximum amount by a factor of two or more (Jagim et al., 2019).
Conclusion
In conclusion, many sports nutrition supplements sold online still contain undeclared doping substances. The prescribed use of such products significantly increases the risk of unintentional doping violations and may even impose general health risks. Food regulation authorities, doping controlled athletes, and WADA are advised to take appropriate actions.
Acknowledgements
ED, LS and OdH designed the study, selected the products and purchased them. LGC performed the analytical tests of the samples and analyzed the initial screening data, ED performed the subsequent calculations. ED, LvL and WK wrote the manuscript. The Dutch Ministry of Health, Welfare and Sport funded the project. The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare. The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author who was an organizer of the study.
Biographies

Erik DUIVEN
Employment
Director of Education at Doping Authority Netherlands.
Degree
MSc
Research interests
Education and knowledge dissemination.
E-mail: e.duiven@dopingautoriteit.nl

Luc J.C. VAN LOON
Employment
Professor of Physiology of Exercise and Nutrition at the Department of Human Biology at Maastricht University Medical Centre
Degree
PhD
Research interests
Skeletal muscle metabolism
E-mail: l.vanloon@maastrichtuniversity.nl

Laila SPRUIJT
Employment
Senior Educator Elite Sports at Doping Authority Netherlands.
Degree
MSc
Research interests
Education and information
E-mail: L.Spruijt@dopingautoriteit.nl

Willem KOERT
Employment
Science Officer at Doping Authority Netherlands.
Degree
MSc
Research interests
Doping agents, dietary supplements, personality disorders
E-mail: W.Koert@dopingautoriteit.nl

Olivier M. DE HON
Employment
Chief Operating Officer at Doping Authority Netherlands.
Degree
PhD
Research interests
Effectiveness of anti-doping policies
E-mail: O.Dehon@dopingautoriteit.nl
References
- Angell M., Kassirer J.P. (1998) Alternative medicine - the risk of untested and unregulated remedies. The New England Journal of Medicine 339, 839-841. https://doi.org/10.1056/NEJM199809173391210 10.1056/NEJM199809173391210 [DOI] [PubMed] [Google Scholar]
- Archer J. R., Dargan P. I., Lostia A. M., van der Walt J., Henderson K., Drake N., Sharma S., Wood D. M., Walker C. J., Kicman A. T. (2015) Running an unknown risk: a marathon death associated with the use of 1,3-dimethylamylamine (DMAA) Drug Testing and Analysis 7, 433-438. https://doi.org/10.1002/dta.1764 10.1002/dta.1764 [DOI] [PubMed] [Google Scholar]
- Ayotte C., Lévesque J. F., Clé roux M., Lajeunesse A., Goudreault D., Fakirian A. (2001) Sport nutritional supplements: quality and doping controls. Canadian journal of Applied physiology = Revue Canadienne de Physiologie Appliquee 26 Suppl, 120-129. https://doi.org/10.1139/h2001-047 10.1139/h2001-047 [DOI] [PubMed] [Google Scholar]
- Bailey R. L., Gahche J. J., Lentino C. V., Dwyer J. T., Engel J. S., Thomas P. R., Betz J. M., Sempos C. T., Picciano M. F. (2011) Dietary supplement use in the United States, 2003-2006. The Journal of Nutrition 141, 261-266. https://doi.org/10.3945/jn.110.133025 10.3945/jn.110.133025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. L., Gahche J. J., Miller P. E., Thomas P. R., Dwyer J. T. (2013) Why US adults use dietary supplements. JAMA Internal Medicine, 173, 355-361. https://doi.org/10.1001/jamainternmed.2013.2299 10.1001/jamainternmed.2013.2299 [DOI] [PubMed] [Google Scholar]
- Baltazar-Martins G., Brito de Souza D., Aguilar-Navarro M., Muñoz-Guerra J., Plata M., Del Coso J. (2019) Prevalence and patterns of dietary supplement use in elite Spanish athletes. Journal of the International Society of Sports Nutrition 16, 30. https://doi.org/10.1186/s12970-019-0296-5 10.1186/s12970-019-0296-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baume N., Mahler N., Kamber M., Mangin P., Saugy M. (2006) Research of stimulants and anabolic steroids in dietary supplements. Scandinavian Journal of Medicine & Science in Sports 16, 41-48. https://doi.org/10.1111/j.1600-0838.2005.00442.x 10.1111/j.1600-0838.2005.00442.x [DOI] [PubMed] [Google Scholar]
- Behpour M., Ghoreishi S. M., Khayatkashani M., Motaghedifard M. (2012) A new method for the simultaneous analysis of strychnine and brucine in Strychnos nux-vomica unprocessed and processed seeds using a carbon-paste electrode modified with multi-walled carbon nanotubes. Phytochemical Analysis: PCA 23, 95-102. https://doi.org/10.1002/pca.1327 10.1002/pca.1327 [DOI] [PubMed] [Google Scholar]
- Catalent Pharma Solutions (2017) Dexedrine. Available from URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017078s049lbl.pdf#page=9. [Accessed 01 Feb 2017].
- Catlin D. H., Leder B. Z., Ahrens B., Starcevic B., Hatton C. K., Green G. A., Finkelstein J. S. (2000) Trace contamination of over-the-counter androstenedione and positive urine test results for a nandrolone metabolite. JAMA 284, 2618-2621. https://doi.org/10.1001/jama.284.20.2618 10.1001/jama.284.20.2618 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhao L., Lu F., Yu Y., Chai Y., Wu Y. (2009) Determination of synthetic drugs used to adulterate botanical dietary supplements using QTRAP LC-MS/MS. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 26, 595-603. https://doi.org/10.1080/02652030802641880 10.1080/02652030802641880 [DOI] [PubMed] [Google Scholar]
- Chołbiński P., Wicka M., Kowalczyk K., Jarek A., Kaliszewski P., Pokrywka A., Bulska E., Kwiatkowska D. (2014) Detection of β-methylphenethylamine, a novel doping substance, by means of UPLC/MS/MS. Analytical and Bioanalytical Chemistry 406, 3681-3688. https://doi.org/10.1007/s00216-014-7728-5 10.1007/s00216-014-7728-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.S, Yun-Choi H.S., Hahn Y. (2006) High Performance Liquid Chromatographic Analysis of Higenamine Enantiomers in Aconite Roots. Natural Product Sciences 6, 20-24. [Google Scholar]
- Cohen P.A. (2009) Imported fenproporex-based diet pills from Brazil: a report of two cases. Journal of General Internal Medicine 24, 430-433. https://doi.org/10.1007/s11606-008-0878-4 10.1007/s11606-008-0878-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P.A., Travis J.C., Venhuis B.J. (2014) A methamphetamine analog (N,α-diethyl-phenylethylamine) identified in a mainstream dietary supplement. Drug Testing and Analysis 6, 805-807. https://doi.org/10.1002/dta.1578 10.1002/dta.1578 [DOI] [PubMed] [Google Scholar]
- Cohen P. A., Bloszies C., Yee C., Gerona R. (2016) An amphetamine isomer whose efficacy and safety in humans has never been studied, β-methylphenylethylamine (BMPEA), is found in multiple dietary supplements. Drug Testing and Analysis 8, 328-333. https://doi.org/10.1002/dta.1793 10.1002/dta.1793 [DOI] [PubMed] [Google Scholar]
- Cohen P. A. (2016) Emergency department visits and hospitalisations for adverse events related to dietary supplements are common. Evidence-Based Medicine 21, 79. https://doi.org/10.1136/ebmed-2015-110362 10.1136/ebmed-2015-110362 [DOI] [PubMed] [Google Scholar]
- Cohen P. A., Avula B., Venhuis B., Travis J. C., Wang Y. H., Khan I. A. (2017) Pharmaceutical doses of the banned stimulant oxilofrine found in dietary supplements sold in the USA. Drug Testing and Analysis 9, 135-142. https://doi.org/10.1002/dta.1976 10.1002/dta.1976 [DOI] [PubMed] [Google Scholar]
- De Cock K. J., Delbeke F. T., Van Eenoo P., Desmet N., Roels K., De Backer P. (2001) Detection and determination of anabolic steroids in nutritional supplements. Journal of Pharmaceutical and Biomedical Analysis 25, 843-852. https://doi.org/10.1016/S0731-7085(01)00396-X 10.1016/S0731-7085(01)00396-X [DOI] [PubMed] [Google Scholar]
- Delbeke F. T., Van Eenoo P., Van Thuyne W., Desmet N. (2002) Prohormones and sport. Journal of Steroid Biochemistry and Molecular Biology 83, 245-251. https://doi.org/10.1016/S0960-0760(02)00274-1 10.1016/S0960-0760(02)00274-1 [DOI] [PubMed] [Google Scholar]
- Dickinson A., Blatman J., El-Dash N., Franco J. C. (2014) Consumer usage and reasons for using dietary supplements: report of a series of surveys. Journal of the American College of Nutrition 33, 176-182. https://doi.org/10.1080/07315724.2013.875423 10.1080/07315724.2013.875423 [DOI] [PubMed] [Google Scholar]
- Dingerdissen J. J., McLaughlin J. L. (1973) Cactus alkaloids. XXI. Beta-phenethylamines from Dolichothele sphaerica. Journal of Pharmaceutical Sciences 62, 1663-1666. https://doi.org/10.1002/jps.2600621017 10.1002/jps.2600621017 [DOI] [PubMed] [Google Scholar]
- El Khoury D., Antoine-Jonville S. (2012) Intake of Nutritional Supplements among People Exercising in Gyms in Beirut City. Journal of Nutrition and Metabolism 703490. https://doi.org/10.1155/2012/703490 10.1155/2012/703490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Jiang J., Hu P., Zhang J. Y., Liu T., Zhao Q., Li B. L. (2012) A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy Chinese subjects. Acta Pharmacologica Sinica, 33, 1353-1358. https://doi.org/10.1038/aps.2012.114 10.1038/aps.2012.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer H, Mareck-Engelke U., Reinhart U., Thevis M., Schänzer W. (2002) Positive Dopingfälle mit Norandrosteron durch verunreinigte Nahrungsergänzungsmittel. Deutsche Zeitschrift für Sportmedizin 51, 378-382. [Google Scholar]
- Geyer H, Bredehöft M., Mareck U., Parr M., Schänzer W. (2003) High doses of the anabolic steroid metandienone found in dietary supplements. European Journal of Sport Science 3, 1-5. https://doi.org/10.1080/17461390300073102 10.1080/17461390300073102 [DOI] [Google Scholar]
- Geyer H., Parr M.K., Mareck U., Reinhart U., Schrader Y., Schänzer W. (2004) Analysis of non-hormonal nutritional supplements for anabolic-androgenic steroids - results of an international study. International Journal of Sports Medicine 25, 124-129. https://doi.org/10.1055/s-2004-819955 10.1055/s-2004-819955 [DOI] [PubMed] [Google Scholar]
- Green G. A., Catlin D. H., Starcevic B. (2001) Analysis of over-the-counter dietary supplements. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine 11, 254-259. https://doi.org/10.1097/00042752-200110000-00008 10.1097/00042752-200110000-00008 [DOI] [PubMed] [Google Scholar]
- Gurley B. J., Gardner S. F., Hubbard M. A. (2000) Content versus label claims in ephedra-containing dietary supplements. Journal of the American Society of Health-System Pharmacists 57, 963-969. https://doi.org/10.1093/ajhp/57.10.963 10.1093/ajhp/57.10.963 [DOI] [PubMed] [Google Scholar]
- Hong H., Chen H. B., Yang D. H., Shang M. Y., Wang X., Cai S. Q., Mikage M. (2011) Comparison of contents of five ephedrine alkaloids in three official origins of Ephedra Herb in China by high-performance liquid chromatography. Journal of Natural Medicines 65, 623-628. https://doi.org/10.1007/s11418-011-0528-8 10.1007/s11418-011-0528-8 [DOI] [PubMed] [Google Scholar]
- Imai T., Nakamura M., Ando F., Shimokata H. (2006) Dietary supplement use by community-living population in Japan: data from the National Institute for Longevity Sciences Longitudinal Study of Aging (NILS-LSA) Journal of Epidemiology 16, 249-260. https://doi.org/10.2188/jea.16.249 10.2188/jea.16.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagim A. R., Camic C. L., Harty P. S. (2019) Common Habits, Adverse Events, and Opinions Regarding Pre-Workout Supplement Use Among Regular Consumers. Nutrients, 11, 855. https://doi.org/10.3390/nu11040855 10.3390/nu11040855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D. I., Chang A., Viray M., Chatham-Stephens K., He H., Taylor E., Wong L. L., Schier J., Martin C., Fabricant D., Salter M., Lewis L., Park S. Y. (2016) Hepatotoxicity associated with the dietary supplement OxyELITE Pro™ - Hawaii, 2013. Drug Testing and Analysis 8, 319-327. https://doi.org/10.1002/dta.1894 10.1002/dta.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judkins C., Hall D., Hoffman K. (2007) Investigation into supplement contamination levels in the US market. Forham: HFL ltd.. Available from URL: https://trustedsupplement.typepad.com/Informed-Choice-Sports-Supplement-Research.pdf. [Accessed on 18 March 2021]. [Google Scholar]
- Jung J., Hermanns-Clausen M., Weinmann W. (2006) Anorectic sibutramine detected in a Chinese herbal drug for weight loss. Forensic Science International 161, 221-222. https://doi.org/10.1016/j.forsciint.2006.02.052 10.1016/j.forsciint.2006.02.052 [DOI] [PubMed] [Google Scholar]
- Kamber M., Baume N., Saugy M., Rivier L. (2001) Nutritional supplements as a source for positive doping cases? International Journal of Sport Nutrition and Exercise Metabolism 11, 258-263. https://doi.org/10.1123/ijsnem.11.2.258 10.1123/ijsnem.11.2.258 [DOI] [PubMed] [Google Scholar]
- Knapik J. J., Steelman R. A., Hoedebecke S. S., Austin K. G., Farina E. K., Lieberman H. R. (2016) Prevalence of Dietary Supplement Use by Athletes: Systematic Review and Meta-Analysis. Sports Medicine 46, 103-123. https://doi.org/10.1007/s40279-015-0387-7 10.1007/s40279-015-0387-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler K., Geyer H., Guddat S., Orlovius A., Parr M. K., Thevis M., Mester J., Schänzer W. (2007) Sibutramine Found in Chinese Herbal Slimming Tea and Capsules. In: Recent Advances In: Doping Analysis. Eds: Schänzer W., Geyer H., Gotzmann A., Mareck U. Köln: Sport und Buch Strauß. 367-370. [Google Scholar]
- Martello S., Felli M., Chiarotti M. (2007) Survey of nutritional supplements for selected illegal anabolic steroids and ephedrine using LC-MS/MS and GC-MS methods, respectively. Food additives and Contaminants 24, 258-265. https://doi.org/10.1080/02652030601013729 10.1080/02652030601013729 [DOI] [PubMed] [Google Scholar]
- Medana C., Calza P., Giancotti V., Dal Bello F., Aragno M., Baiocchi C. (2013) Study of the photocatalytic transformation of synephrine: a biogenic amine relevant in anti-doping analysis. Analytical and Bioanalytical Chemistry, 405, 1105-1113. https://doi.org/10.1007/s00216-012-6593-3 10.1007/s00216-012-6593-3 [DOI] [PubMed] [Google Scholar]
- Morrison L. J., Gizis F., Shorter B. (2004) Prevalent use of dietary supplements among people who exercise at a commercial gym. International Journal of Sport Nutrition and Exercise Metabolism 14, 481-492. [DOI] [PubMed] [Google Scholar]
- NIH., (1994) Dietary supplement health and education act of 1994, public law 103-417, 103rd congress. 1994. Available at: https://ods.od.nih.gov/About/DSHEA_Wording.aspx Accessed Jan 2, 2019.
- NIH (National Institutes of Health). (2017) Dietary Supplement Health and Education Act of 1994. Available from URL: https://ods.od.nih.gov/About/DSHEA_Wording.aspx. [Accessed on 1 February 2017].
- NIH (National Healthcare Institute) Netherlands (2019) Bisoprolol. Available from URL: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/b/bisoprolol. [Accessed on 31 January 2019].
- Novartis Pharmaceuticals Corporation (2017) Ritalin. Available from URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/010187s087lbl.pdf#page=11. [Accessed on 1 February 2017].
- Outram S., Stewart B. (2015) Doping through supplement use: a review of the available empirical data. International Journal of Sport Nutrition and Exercise Metabolism 25, 54-59. https://doi.org/10.1123/ijsnem.2013-0174 10.1123/ijsnem.2013-0174 [DOI] [PubMed] [Google Scholar]
- Palmer M. E., Haller C., McKinney P. E., Klein-Schwartz W., Tschirgi A., Smolinske S. C., Woolf A., Sprague B. M., Ko R., Everson G., Nelson L. S., Dodd-Butera T., Bartlett W. D., Landzberg B. R. (2003) Adverse events associated with dietary supplements: an observational study. Lancet 361(9352), 101-106. https://doi.org/10.1016/S0140-6736(03)12227-1 10.1016/S0140-6736(03)12227-1 [DOI] [PubMed] [Google Scholar]
- Parasrampuria J., Schwartz K., Petesch R. (1998) Quality control of dehydroepiandrosterone dietary supplement products. JAMA 280, 1565. https://doi.org/10.1001/jama.280.18.1565 10.1001/jama.280.18.1565 [DOI] [PubMed] [Google Scholar]
- Parr M. K., Geyer H., Hoffmann B., Köhler K., Mareck U., Schänzer W. (2007) High amounts of 17-methylated anabolic-androgenic steroids in effervescent tablets on the dietary supplement market. Biomedical Chromatography 21, 164-168. https://doi.org/10.1002/bmc.728 10.1002/bmc.728 [DOI] [PubMed] [Google Scholar]
- Parr M. K., Koehler K., Geyer H., Guddat S., Schänzer W. (2008) Clenbuterol marketed as dietary supplement. Biomedical Chromatography 22, 298-300. https://doi.org/10.1002/bmc.928 10.1002/bmc.928 [DOI] [PubMed] [Google Scholar]
- Patil S., Srivastava A., Shukla A., Phase N. (1991) Spectrophotometric estimation of 4-androstene-3, 17-dione and 1,4-androstadiene-3,17-dione during C-1(2)-dehydrogenation by Mycobacterium fortuitum NRRL B-8153. World Journal of Microbiology & Biotechnology 7, 626-627. https://doi.org/10.1007/BF00452847 10.1007/BF00452847 [DOI] [PubMed] [Google Scholar]
- Perrenoud L., Saugy M., Saudan C. (2009) Detection in urine of 4-methyl-2-hexaneamine, a doping agent. Journal of Chromatography B., Analytical Technologies in the Biomedical and Life Sciences 877, 3767-3770. https://doi.org/10.1016/j.jchromb.2009.09.013 10.1016/j.jchromb.2009.09.013 [DOI] [PubMed] [Google Scholar]
- Rahnema C. D., Crosnoe L. E., Kim E. D. (2015) Designer steroids - over-the-counter supplements and their androgenic component: review of an increasing problem. Andrology 3, 150-155. https://doi.org/10.1111/andr.307 10.1111/andr.307 [DOI] [PubMed] [Google Scholar]
- Saraphanchotiwitthaya A., Sripalakit P. (2016) Production of 4-androstene-3,17-dione and 1,4-androstadiene-3,17-dione from rice germ and wheat germ extracts by Mycobacterium sp. Biotechnology Letters 38, 1595-1602. https://doi.org/10.1007/s10529-016-2140-1 10.1007/s10529-016-2140-1 [DOI] [PubMed] [Google Scholar]
- Schilt R., Vlis E., van der Vaes W. (2002) Onderzoek naar het voorkomen van dopinggeduide stoffen in voedingsmiddelen in de aanloop naar de Olympische winterspelen in Salt Lake City. [Study to head off doping substances in foodstuff in the run up to the Olympic Winter Games in Salt Lake City]. Zeist, the Netherlands: TNO Nutrition and Food Research and the National Institute of Public Health and the Environment (RIVM), NOC*NSF and the Ministry of Health, Welfare and Sports. [Google Scholar]
- Skeie G., Braaten T., Hjartåker A., Lentjes M., Amiano P., Jakszyn P., Pala V., Palanca A., Niekerk E. M., Verhagen H., Avloniti K., Psaltopoulou T., Niravong M., Touvier M., Nimptsch K., Haubrock J., Walker L., Spencer E. A., Roswall N., Olsen A., Wallström P., Nilsson S., Casagrande C., Deharveng G., Hellström V., Boutron-Ruault M. C., Tjønneland A., Joensen A. M., Clavel-Chapelon F., Trichopoulou A., Martinez C., Rodríguez L., Frasca G., Sacerdote C., Peeters P. H. M., Linseisen J., Schienkiewitz A., Welch A. A., Manjer J., Ferrari P., Riboli E., Bingham S., Engeset D., Lund E., Slimani N. (2009) Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. European Journal of Clinical Nutrition 63 Suppl 4, 226-238. https://doi.org/10.1038/ejcn.2009.83 10.1038/ejcn.2009.83 [DOI] [PubMed] [Google Scholar]
- Smith T. B., Staub B. A., Natarajan G. M., Lasorda D. M., Poornima I. G. (2014) Acute myocardial infarction associated with dietary supplements containing 1,3-dimethylamylamine and Citrus aurantium. Texas Heart Institute Journal 41, 70-72. https://doi.org/10.14503/THIJ-12-2870 10.14503/THIJ-12-2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahm E., Mullen J. E., Gårevik N., Ericsson M., Schulze J. J., Rane A., Ekström L. (2015) Dose-dependent testosterone sensitivity of the steroidal passport and GC-C-IRMS analysis in relation to the UGT2B17 deletion polymorphism. Drug Testing and Analysis, 7, 1063-1070. https://doi.org/10.1002/dta.1841 10.1002/dta.1841 [DOI] [PubMed] [Google Scholar]
- Teschke R., Wolff A., Frenzel C., Schulze J., Eickhoff A. (2012) Herbal hepatotoxicity: a tabular compilation of reported cases. LiverIinternational: official journal of the International Association for the Study of the Liver 32, 1543-1556. https://doi.org/10.1111/j.1478-3231.2012.02864.x 10.1111/j.1478-3231.2012.02864.x [DOI] [PubMed] [Google Scholar]
- Thevis M., Kuuranne T., Geyer H., Schänzer W. (2017) Annual banned-substance review: analytical approaches in human sports drug testing. Drug Testing and Analysis 9, 6-29. https://doi.org/10.1002/dta.2139 10.1002/dta.2139 [DOI] [PubMed] [Google Scholar]
- Uralets V., App M., Rana S., Morgan S., Ross W. (2014) Designer phenethylamines routinely found in human urine: 2-ethylamino-1-phenylbutane and 2-amino-1-phenylbutane. Journal of Analytical Toxicology 38, 106-109. https://doi.org/10.1093/jat/bkt121 10.1093/jat/bkt121 [DOI] [PubMed] [Google Scholar]
- Van Eenoo P., Deventer K., Roels K., Delbeke F. T. (2006) Quantitative LC-MS determination of strychnine in urine after ingestion of a Strychnos nux-vomica preparation and its consequences in doping control. Forensic Science International 164, 159-163. https://doi.org/10.1016/j.forsciint.2005.12.029 10.1016/j.forsciint.2005.12.029 [DOI] [PubMed] [Google Scholar]
- Van der Merwe P.J., Grobbelaar E. (2004) Inadvertent doping through nutritional supplements is a reality. The South African Journal of Sports Medicine 16, 3-7. https://doi.org/10.17159/2078-516X/2004/v16i2a180 10.17159/2078-516X/2004/v16i2a180 [DOI] [PubMed] [Google Scholar]
- Vidal C., Quandte S. (2006) Identification of a sibutramine-metabolite in patient urine after intake of a “pure herbal” Chinese slimming product. Therapeutic Drug Monitoring 28, 690-692. https://doi.org/10.1097/01.ftd.0000245392.33305.b0 10.1097/01.ftd.0000245392.33305.b0 [DOI] [PubMed] [Google Scholar]
- WADA. (2015) World Anti-Doping Code Available from URL: https://www.wada-ama.org/sites/default/files/resources/files/wada-2015-world-anti-doping-code.pdf. [Accessed on 1 February 2017].
- WADA. (2018a) WADA Technical Document - TD2018MRPL. Available from URL: https://www.wada-ama.org/sites/default/files/resources/files/td2018mrpl_v1_finaleng.pdf. [Accessed on 11 February 2018].
- WADA. (2018b) WADA Technical Document - TD2018DL. Available from URL: https://www.wada-ma.org/sites/default/files/resources/files/td2018dl_v1_en.pdf. [Accessed 11 February 2018].
- Wardenaar F., van den Dool R., Ceelen I., Witkamp R., Mensink M. (2016) Self-Reported Use and Reasons among the General Population for Using Sports Nutrition Products and Dietary Supplements. Sports, 4, 33. https://doi.org/10.3390/sports4020033 10.3390/sports4020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P., Judkins C., Houghton E., Russell C., Maughan R. J. (2009) Urinary nandrolone metabolite detection after ingestion of a nandrolone precursor. Medicine and Science in Sports and Exercise 41, 766-772. https://doi.org/10.1249/MSS.0b013e31818edaeb 10.1249/MSS.0b013e31818edaeb [DOI] [PubMed] [Google Scholar]
- Wójtowicz M., Jarek A., Chajewska K., Turek-Lepa E., Kwiatkowska D. (2015) Determination of designer doping agent--2-ethylamino-1-phenylbutane--in dietary supplements and excretion study following single oral supplement dose. Journal of Pharmaceutical and Biomedical Analysis 115, 523-533. https://doi.org/10.1016/j.jpba.2015.07.025 10.1016/j.jpba.2015.07.025 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Yang L., Liao Z., He X., Zhou Y., Guo H. (2013) Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21,789 patients. European Journal of Gastroenterology & Hepatology 25, 825-829. https://doi.org/10.1097/MEG.0b013e32835f6889 10.1097/MEG.0b013e32835f6889 [DOI] [PubMed] [Google Scholar]


