To the Editor:
We read with interest the recent letter published by Bril et al. recently published in Journal of Hepatology.1 The authors describe a possible case of COVID-19 vaccine-associated autoimmune hepatitis (AIH) in a 35-year-old woman 3 months post-partum. The patient presented with pruritis and jaundice 13 days after receiving a BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine, which may be the first report of COVID-19 vaccine-associated liver injury. As vaccination programs are being rolled out globally,2 many clinically significant side effects are starting to be identified, such as vaccine-induced immune thrombotic thrombocytopenia.3
Herein, we report the case of a 36-year-old Iraqi-born male physician who developed likely vaccine-induced AIH following COVID-19 vaccination. He has a past medical history of hypertension treated with olmesartan and laser eye surgery 2 weeks prior that required topical fluoroquinolone eye drops, 1 g of acetaminophen TDS, and 400 mg of ibuprofen TDS for 1 week total. He had no previous history of liver disease. Of note, he had his first dose of ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca) 26 days prior to presentation with a subsequent mild febrile reaction requiring 1 g of acetaminophen TDS, and 400 mg of ibuprofen TDS for 3 days. He was referred to our emergency department after a finding of markedly abnormal liver function tests on routine blood tests and was asymptomatic at the time.
His physical examination was unremarkable. Blood tests were significant for the following: bilirubin 17 μmol/L, alanine aminotransferase (ALT) 1,774 U/L, aspartate aminotransferase (AST) 633 U/L, gamma glutamyltransferase 136 U/L, alkaline phosphatase 118 U/L, albumin 45 g/L, and international normalized ratio 1.1. Serology was negative for hepatitis A, B, C and E, Epstein-Barr virus, cytomegalovirus, herpes simplex virus, and HIV. Antinuclear antibody was positive at a titre of 1:160 in a speckled pattern. Immunoglobulins were normal with an IgG of 12.8 g/L (ref 7.0–16.5 g/L). Anti-liver-kidney microsomal, anti-smooth muscle, anti-mitochondrial antibodies, and anti-soluble liver antigen were normal. His caeruloplasmin, transferrin saturation, alpha-1-antitrypsin level and creatine kinase levels were also normal. Abdominal ultrasound revealed a normal-sized liver. No thrombus was identified in the portal, splenic, hepatic, or superior mesenteric veins. An MRI cholangiogram revealed mild peri-portal oedema, no biliary dilatation, and smooth bile duct contour with no evidence of stricturing.
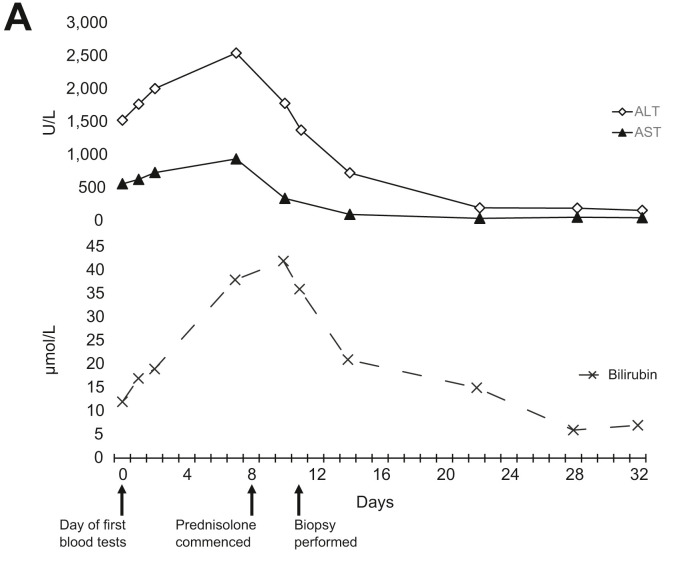
During ongoing close follow-up, he developed pruritus and had a corresponding worsening of his liver function tests (with a bilirubin of 38 μmol/L, ALT of 2550 U/L, and AST of 943 U/L). He was commenced on 60 mg of prednisolone daily, admitted to hospital, and had a liver biopsy 3 days after commencing the corticosteroids. His biopsy was significant for interface hepatitis with a mixed, predominantly lymphocytic, inflammatory cell infiltrate without significant fibrosis. Copper and iron stains were negative. These findings are consistent with an acute hepatitis of autoimmune aetiology. The post-biopsy pre-treatment Revised Original Score for Autoimmune Hepatitis4 is 15 (supporting a probable diagnosis of AIH). His prednisolone has been weaned over the following month and is now down to 20 mg/day, with an ALT of 163 U/L and bilirubin of 7 μmol/L (Fig. 1 ) after 24 days of corticosteroid therapy.
Fig. 1.
Trends of plasma biochemistry over time.
In contrast to the communique published by Bril et al.,1 our patient received the Oxford-AstraZeneca vaccine and did not have any apparent confounding factors such as pregnancy. This case supports the notion of COVID-19 vaccine-triggered autoimmune phenomena irrespective of the vaccine’s mechanism of action, though this is the first report of an adenovirus-based vaccine precipitating AIH. Similar to the previously described case by Bril et al.,1 causation cannot be definitively proven and it is possible that other factors, including drugs or toxins, may have contributed to the presentation. In this case, however, the patient is a practicing physician with excellent health literacy and we feel it is unlikely that other potential aetiologies were missed on history.
The case of this 36-year-old previously well man developing apparent AIH precipitated by a COVID 19-vaccine is another salient reminder to be vigilant of the rapidly changing landscape of potentially rare complications associated with novel vaccine agents and mass immunisation programs worldwide.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
DCC provided patient care as well as co-wrote the original manuscript and edited the final submission; DS co-wrote the original manuscript and edited the final submission; EF provided patient care, assisted with conceptualisation, and edited the final submission; WK provided patient care, assisted with conceptualisation, and edited the final submission; SKR provided patient care, assisted with conceptualisation, and edited the final submission.
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.06.014.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. April 15 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. April 9 2021 doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez F., Berg P.A., Bianchi F.B., Bianchi L., Burroughs A.K., Cancado E.L., et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.