Abstract
Gallbladder cancer is an aggressive cancer that continues to be an important health care issue in certain regions of the world such as Southeast Asia and Latin America. Most patients are diagnosed at an advanced, unresectable stage and systemic therapy is their only option. Gallbladder cancer patients have traditionally been included in clinical trials for biliary tract cancer. Thus, systemic chemotherapy options for this cancer are similar to those for cholangiocarcinoma, including gemcitabine and cisplatin in the first line and FOLFOX in the second-line setting. Ongoing phase III clinical trials may change the systemic therapy paradigm for this cancer. Molecular profiling has indicated important genetic differences between gallbladder cancer and cholangio-carcinoma, which affects choice of targeted therapy. Her2/neu amplification, PIK3CA mutations and DNA repair genetic aberrations are relatively frequent and represent actionable targets for this cancer.
Keywords: Cancer of gallbladder, genes ErbB2, targeted therapy
Introduction
Gallbladder cancer is classified as a rare tumor but exacts a high mortality in Latin America and Asia and represents the most common biliary cancer worldwide (1). Surgery is the only curative treatment, with 5-year overall survival rates up to 63.2%; however, most gallbladder cancer patients are diagnosed with advanced-stage disease and have an extremely poor prognosis, with reported 5-year overall survival rates <5% (2). Gemcitabine plus cisplatin is considered as the standard first-line systemic chemotherapy regimen for advanced biliary tract cancers based on the results of the ABC-02 trial. In this trial, 36% of the 410 patients were diagnoses with gallbladder cancer (3). Promising anti-tumor activity has been noted with novel targeted therapies in biliary tract cancers, including fibroblast growth factor receptor (FGFR), MEK, ERBB2, isocitrate dehydrogenase-1 (IDH1) and the Poly ADP-ribose polymerase 1 (PARP1) inhibitors (4-7), among which ERBB2 is most applicable for gallbladder cancer (6,7).
Systemic chemotherapy for gallbladder cancer
Biliary tract cancers, including gallbladder cancer have a poor prognosis, with an estimated 5-year overall survival of less than 20%. For patients with advanced-stage or unrespectable biliary tract cancers, the first-line systemic chemotherapy is a combination of gemcitabine and cisplatin. However, this first-line standard of care has limited effectiveness, with median overall survival <1 year (3). In this phase III trial, 400 patients were enrolled and treated with gemcitabine plus cisplatin versus single agent gemcitabine. This trial reported a median progression-free survival of 8 months and an overall survival of 11 months. About 149 patients enrolled had gallbladder cancer and in a subset analysis, the doublet chemotherapy prolonged their overall survival as compared with gemcitabine alone (HR 0.61, range, 0.41–0.89). This study thus established gemcitabine and cisplatin as the standard of care for advanced unresectable gallbladder cancer. Other combination regimens have been investigated in gallbladder cancer, including gemcitabine plus oxaliplatin (GEMOX), gemcitabine + capecitabine and gemcitabine + S1 (8). These combinatorial regimens may have similar efficacy as gemcitabine plus cisplatin in the first-line setting.
Recently, a phase II trial of gemcitabine, cisplatin, and nab-paclitaxel on days 1 and 8 of 21-day cycles for biliary tract cancers patients, including gallbladder cancer was reported (9). The primary trial endpoint was progression-free survival and secondary endpoints included overall survival, overall response rate, and safety. Median follow-up was 12.2 months, and median progression free survival was 11.8 months (95% CI: 6.0–15.6). Partial response and disease control rates were 43% and 84%. Median overall survival was 19.2 months (95% CI: 13.2 to not estimable). Treatment efficacy was not impacted significantly by tumor type. Neutropenia was the most common toxicity (32%). This regimen is currently being investigated in the phase III setting for advanced biliary tract cancers (ClinicalTrials.gov Identifier: NCT03768414). In the European Union, 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) regimen is being investigated versus gemcitabine and cisplatin for advanced biliary tract cancers, including gallbladder cancer. The results of these studies may refine the current first line therapy options for gallbladder cancer (10).
Until recently, there was no established second-line therapy for biliary tract cancers as the standard of care. In a systemic review on second-line therapy biliary tract cancers treatment, based on 20 studies, the weighted overall response rate was estimated at 5.1% and the median progression free survival was 4 months (11). In another study, median overall survival for patients with biliary tract cancers from start of second line therapy was 11 months (95% CI: 8.8–13.1) and in particular 9.4 months (95% CI: 7.2–12.3) for the 24.8% patients with gallbladder cancer (12). Recently, in the ABC-06 trial, of 162 pts (81 in each arm) that were randomized to FOLFOX versus supportive care; 21% had gallbladder cancer (13). After 150 overall survival events, the adjusted HR was 0.69 (P=0.031) in favor of FOLFOX. Median overall survival was 6.2 months for the FOLFOX arm vs. 5.3 months for supportive care only arm. FOLFOX was thus reported as recommended standard in the second line setting for biliary tract cancer (BTC) and results in a modestly improved survival as compared with supportive care alone. Further details may help further guide the applicability of such recommendation once the complete report is published.
There remains no clinical evidence to suggest differential outcomes for gallbladder cancer vs. other biliary tract cancers with systemic chemotherapy. While inclusion of gallbladder cancer along with other biliary tract cancers in chemotherapy-based clinical trials is a reasonable approach at this time, it is anticipated in the future that the bundling exercise will be abandoned.
Next generation sequencing of gallbladder cancer: Western vs. Asian patients
As already introduced, significant differences exist in mutational spectrum of gallbladder cancer versus cholangiocarcinoma. For instance, Isocitrate Dehydrogenase 1 or 2 (IDH1/2), BAP1 mutations and FGFR fusions are more likely to occur in intrahepatic cholangiocarcinoma while KRAS, p53 and SMAD4 mutations are more common in extrahepatic cholangiocarcinoma. Gallbladder cancer on the other hand has the high frequency of ERBB2 amplifications (7,14).
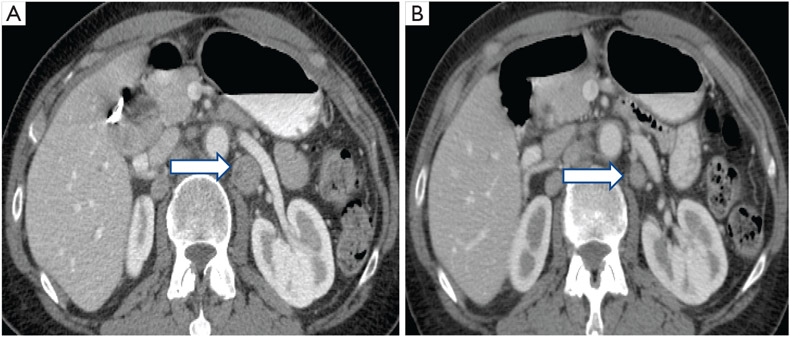
HER2/neu gene is a key driver of oncogenesis and its overexpression as a result of gene amplification is a critical target for therapy in breast cancer and gastric cancer group recently studied HER2/neu expression in 187 cases of gallbladder cancer; this is the largest reported series to date using the commonly accepted American Society of Clinical Oncology criteria (6,15). Thirteen per cent of patients were noted to have HER2/neu overexpression (3+ by immunohistochemistry) and radiological partial responses were noted with HER2/neu directed therapies. Figure 1 illustrates the benefits of HER 2/neu targeted therapy, noted in a case of gallbladder cancer that was involving retroperitoneal node in a 73-year-old female. After treatment with trastuzumab and pertuzumab, follow-up scans demonstrated improvement in adenopathy that was sustained over 5 months. Other targeted therapy options including EGFR-directed therapies are discussed elsewhere (16,17). Genetic variation in biliary tract cancers can also result from etiology and regions of the world.
Figure 1.
A 73-year-old female with metastatic retroperitoneal lymphadenopathy from gallbladder carcinoma with HER 2/neu amplification. Axial contrast-enhanced CT images demonstrate: (A) a 1.9-cm lymph node (arrow) posterior to the left renal vein. After 2 months of trastuzumab + pertuzumab, lymphadenopathy is decreased: (B) the lymph node (arrow) posterior to the left renal vein now measures 1.2 cm.
Trastuzumab and pertuzumab combination therapy was investigated in 11 patients with HER2-positive biliary cancer (HER2-amplified/overexpressed, n=8; HER2-mutated, n=3). At a median follow-up of 4.2 months (range, 2.0–12.0 months), 4 patients had partial responses and 3 had stable disease for >4 months, further suggesting the value of Her2/neu targeting in gallbladder cancer (18).
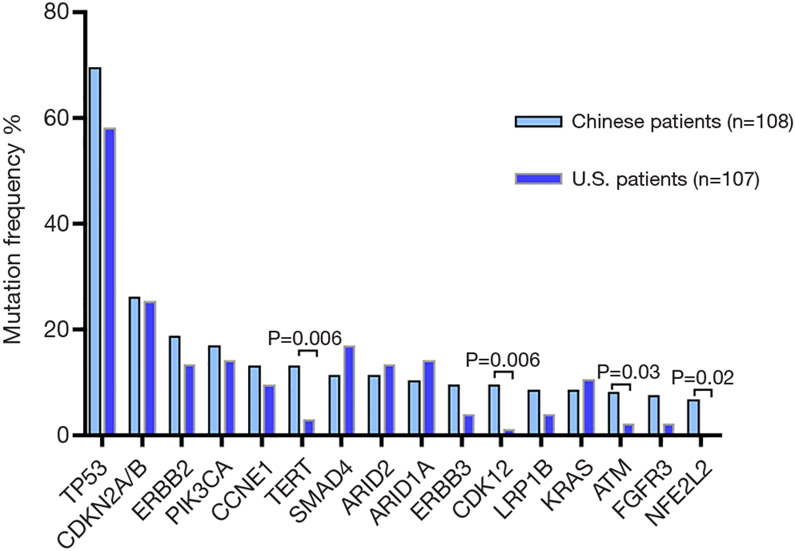
Next generation sequencing was investigated in cohorts of 108 Chinese and 107 US gallbladder cancer patients. The most frequent alterations were in TP53 (69%), CDKN2A/B (26%), ERBB2 (19%), PIK3CA (17%) and CCNE1 (13%) in the Chinese cohort; and TP53 (58%), CDKN2A/B (25%), SMAD4 (17%), ARID1A (14%) and PIK3CA (14%) ERBB2 (13%) in the US patients (Figure 2) (19). Out of the top 9 dysregulated genetic pathways in cancer, Chinese patients harbored more frequent mutations in ERBB family (31% vs. 19%, P=0.04). High frequency PI3K/mTOR pathway variation was observed in both Chinese (37%) and US cohort (33%) (P=0.5) Additionally, both Chinese and US gallbladder cancer patients exhibited a relatively high tumor mutational burden (TMB) (>10 muts/Mb) in 17.6% and 17.0%, respectively. This heterogeneity will have a significant impact on treatment decisions.
Figure 2.
Next generation sequencing in cohorts of 108 Chinese and 107 US gallbladder cancer patients.
Therefore, in the case of targeted therapeutics, it is important to account for biliary tract cancers type. The ‘one size fit all’ approach must be discouraged.
Targeted therapy and immunotherapy
While clinical trials for targeted therapeutics in gallbladder cancer have lagged behind the more commonly occurring gastrointestinal cancers, clinical trials of EGFR, MEK, VEGFR and PI3-Kinase inhibitors have been completed. Phase II/III randomized trials have failed to reveal the superiority of any targeted agent with chemotherapy for biliary tract cancers. These trials are depicted in Table 1. Two areas of particular interest in gallbladder cancer include Her2/neu and DNA repair gene alterations (6,20,21). The frequency of these genetic aberrations is 10–15% in the gallbladder cancer population, making these as potential targets for specific inhibitors. Case series and reports have already indicated the benefit of targeted agents in this population.
Table 1.
Randomized trials of targeted therapies in biliary tract cancers
| Study title | Study type | Targeted pathway | Progression-free survival | Overall survival |
|---|---|---|---|---|
| GEMOX ± erlotinib (20) | Randomized phase II | EGFR | Study: 5.2 months; control: 4.8 months (P=0.08) |
Study: 9.5 months; control: 9.5 months (P=0.6) |
| GEMOX ± Cetuximab (21) | Randomized phase II | EGFR | Study:6.1 months; control: 5.5 months (P=NS) |
Study: 11 months; control: 12.4 months (P=NS) |
| GEMOX ± Panitumumab (22) | Randomized phase II | EGFR | Study: 5.3 months; control: 4.4 months (P=0.27) |
Study: 9.9 months; control: 10.2 months (P=0.42) |
| Gemcitabine, cisplatin ± cediranib (23) | Randomized phase II | VEGFR | Study:8 months; control: 7.4 months (P=0.72) |
Study: 14 months; control: 12 months (P=0.62) |
| Gemcitabine, ± sorafenib (24) | Randomized phase II | Multi-targeted TKI | Study:3 months; control: 4.9 months (P=0.859) |
Study:8.4 months; control: 11.2 months (P=0.775) |
NS, not significant.
Immunotherapy with checkpoint inhibitors is now being explored in biliary tract cancers and preliminary data suggests modest efficacy with single agent check point inhibitors. The largest study in the regard thus far has been the KEYNOTE-158 study with Pembrolizumab (22). This study enrolled 104 patients with BTC, of whom 58% were PD-L1+. The response rate was reported at 6% with disease stability in 16% and the response duration was 6 to 16+ months. Immunotherapeutic combinations may have more promise for gallbladder cancer than single-agent checkpoint inhibitors. Nivolumab has been combined with gemcitabine and cisplatin in a phase I study for biliary tract cancers, with a 37% response rate and 15 months overall survival (23). Similar promising results have been reported with ongoing trials of pembrolizumab + GM-CSF and levantinib + pembrolizumab (24). Future randomized, controlled trials are required in this setting.
Conclusions
Systemic therapy for gallbladder cancer has evolved significantly over the past decade and there is now an accepted first- and second-lines chemotherapy regimen. Inclusion of gallbladder cancer along with other biliary tract cancers for systemic chemotherapy trials is reasonable at this time. However, in case of targeted and immunotherapies, genetic heterogeneity between gallbladder cancer and other biliary tract cancers must be considered. Next generation sequencing studies have indicated that Her2/neu, DNA repair and PI3-kinase genetic alterations may be potential areas for investigation in gallbladder cancer. Ongoing first line systemic chemotherapy, targeted therapeutics and immunotherapy trials may result in a paradigm shift for this disease.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349–64. [DOI] [PubMed] [Google Scholar]
- 2.D'Hondt M, Lapointe R, Benamira Z, et al. Carcinoma of the gallbladder: Patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol 2013;39:548–53. [DOI] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 4.Geynisman DM, Catenacci DV Toward personalized treatment of advanced biliary tract cancers. Discov Med 2012;14:41–57. [PubMed] [Google Scholar]
- 5.Yamashita S, Koay EJ, Passot G, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: A comprehensive analysis of 362 consecutive patients. Cancer 2017;123:1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res 2018;24:4154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javle M, Rashid A, Churi C, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol 2014;45:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelip JM, Edeline J, Blanc JF, et al. Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig Liver Dis 2019;51:318–20. [DOI] [PubMed] [Google Scholar]
- 11.Lamarca A, Hubner RA, David Ryder W et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328–38. [DOI] [PubMed] [Google Scholar]
- 12.Goff LW, Lowery MA, Jordan E, et al. Second-line chemotherapy (CTx) outcomes in advanced biliary cancers (ABC): A retrospective multicenter analysis. J Clin Oncol 2016;34:437. [Google Scholar]
- 13.Lamarca A, Palmer DH, Wasan HS, et al. A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC + mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol 2019;37:4003. [Google Scholar]
- 14.Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016;122:3838–47. [DOI] [PubMed] [Google Scholar]
- 15.Roa I, de Toro G, Schalper K, et al. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients With Advanced Gallbladder Cancer. Gastrointest Cancer Res 2014;7:42–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol 2016;7:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang P, Javle M, Pang F, et al. Somatic genetic aberrations in gallbladder cancer: comparison between Chinese and US patients. HepatoBiliary Surg Nutr 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javle MM, Hainsworth JD, Swanton C, et al. Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J Clin Oncol 2017;35:402.27893326 [Google Scholar]
- 19.Javle MM, Catenacci D, Jain A, et al. Precision medicine for gallbladder cancer using somatic copy number amplifications (SCNA) and DNA repair pathway gene alterations. J Clin Oncol 2017;35:4076. [Google Scholar]
- 20.Lamarca A, Barriuso J, McNamara MG, et al. Biliary Tract Cancer: State of the Art and potential role of DNA Damage Repair. Cancer Treat Rev 2018;70:168–77. [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Kwong LN, Javle M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr Treat Options Oncol 2016;17:58. [DOI] [PubMed] [Google Scholar]
- 22.Ueno M, Chung HC, Nagrial A, et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase 2 KEYNOTE-158 study. Ann Oncol 2018;29: viii205–70. [Google Scholar]
- 23.Ikeda M, Ueno M, Morizane C, et al. A multicenter, open-label, phase I study of nivolumab alone or in combination with gemcitabine plus cisplatin in patients with unresectable or recurrent biliary tract cancer. J Clin Oncol 2019;37:306. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Shi W, Zhao S, et al. Lenvatinib plus checkpoint inhibitors in patients (pts) with advanced intrahepatic cholangiocarcinoma (ICC): Preliminary data and correlation with next-generation sequencing. J Clin Oncol 2018;36:500. [Google Scholar]