Abstract
We describe the spatiotemporal course of cortical high-gamma activity, hippocampal ripple activity and interictal epileptiform discharges during an associative memory task in 15 epilepsy patients undergoing invasive EEG. Successful encoding trials manifested significantly greater high-gamma activity in hippocampus and frontal regions. Successful cued recall trials manifested sustained high-gamma activity in hippocampus compared to failed responses. Hippocampal ripple rates were greater during successful encoding and retrieval trials. Interictal epileptiform discharges during encoding were associated with 15% decreased odds of remembering in hippocampus (95% confidence interval 6–23%). Hippocampal interictal epileptiform discharges during retrieval predicted 25% decreased odds of remembering (15–33%). Odds of remembering were reduced by 25–52% if interictal epileptiform discharges occurred during the 500–2000 ms window of encoding or by 41% during retrieval. During encoding and retrieval, hippocampal interictal epileptiform discharges were followed by a transient decrease in ripple rate. We hypothesize that interictal epileptiform discharges impair associative memory in a regionally and temporally specific manner by decreasing physiological hippocampal ripples necessary for effective encoding and recall. Because dynamic memory impairment arises from pathological interictal epileptiform discharge events competing with physiological ripples, interictal epileptiform discharges represent a promising therapeutic target for memory remediation in patients with epilepsy.
Keywords: intracranial EEG, gamma oscillations, interictal spikes, epileptiform discharges, epilepsy
Henin et al. report that hippocampal interictal epileptiform discharges acutely impair declarative memory in a regionally and temporally specific manner, likely by disrupting physiological hippocampal ripples needed for successful encoding and recall.
Introduction
Memory dysfunction affects 40–50% of patients with epilepsy. In a community-based survey, patients ranked memory problems as the most important cognitive comorbidity of epilepsy, adversely affecting daily functioning, work participation, and school performance.1 These subjective complaints range from difficulties with remembering names and phone numbers (semantic memory) to what happened during last year’s family vacation (episodic memory).2 While multiple factors are involved,3 interictal epileptiform discharges (IEDs) are pathological bursts of neuronal activity between seizures which can dynamically impair cognition. In 1939, Schwab demonstrated that ‘subclinical EEG discharges’ increased reaction time or resulted in failure to respond to the stimulus.4 Subsequently, Aarts introduced the term ‘transitory cognitive impairment’ (TCI) to describe this functional disruption. Because IEDs are transiently associated with memory impairment, they may provide a target for therapeutic intervention.5
Intracranial EEG studies with epilepsy surgery patients have advanced our understanding of human cognition, including the deleterious impact of IEDs. Intracranial EEG offers high spatiotemporal precision and superior signal-to-noise ratio6 for characterizing the neurophysiology of memory, including recordings from deep structures (e.g. hippocampus and entorhinal cortex) inaccessible by other methods. Increased gamma activity in dominant mesial temporal and frontal regions predicts successful encoding of word lists7,8 or word pairs.9 Recently, hippocampal sharp-wave ripple events have been correlated with successful retrieval of word pairs10,11 and visual episodic memories.12 However, these intracranial EEG studies describing physiological processes including gamma activity and ripples typically exclude trials with IEDs, discarding them as ‘noise’.
Accumulating evidence demonstrates that IEDs can impair encoding, maintenance, consolidation, and retrieval of verbal learning.5,13–17 Left temporal and parietal neocortical IEDs are associated with impaired memory for word list items and word pairs.14,17 IEDs outside the seizure onset zone (SOZ) in higher order visual processing regions have been associated with impaired encoding and retrieval performance for words.17 Despite this, our understanding of the relationship of IEDs and human memory is largely correlational,14,16,18 without a clear mechanism of disruption. Studies assessing word-list learning or verbal paired associate tasks found the greatest effects of IEDs occurring in higher-order visual neocortical areas (i.e. fusiform gyrus, inferior temporal gyrus) or parietal lobe.14,17 It is unclear whether IEDs disrupt sensory processing of words or memory function per se and if they do, the mechanism of disruption is unknown.18 IEDs may be more frequent during drowsy or distracted states, and thus only indirectly associated with poor memory performance.18,19
In this study, we aimed to establish the relationship between high gamma activity (HGA) and hippocampal ripples and pathological IEDs. Previously, physiological and pathological events have been investigated independently. We hypothesized that characterizing the spatiotemporal course of physiological activity during a memory task would reveal when and where in the brain IEDs exert the greatest impact and provide a potential mechanism for how IEDs disrupt cognitive processing. We selected a face-profession association task, which potentially represents a more clinically relevant probe of episodic memory than word-list recall or word-pair association tasks.7–9,16,17,20 Our face-profession task depends on bilateral hippocampal function,21–23 thus permitting an opportunity to test how IEDs, often abundant in the mesial temporal lobe, interrupt mnemonic processes. We examined the spatiotemporal time course of HGA, a robust index of local cortical activity24 across widespread brain areas during encoding and retrieval, distinguishing between successfully remembered versus forgotten pairs. As hippocampal activation was critical for encoding and retrieval, we next compared the hippocampal ripple rate of successful versus failed trials. To ensure that detected HGA and ripple rate were physiological events and not pathological high frequency oscillations, which are increased in epileptogenic cortex25,26 and often coupled with IEDs,25 we excluded electrodes inside the SOZ and trials with IEDs. Next, we evaluated the impact of IEDs by brain region and with respect to the SOZ. Because hippocampal IEDs had a consistently adverse effect on memory, we examined their impact by time course, predicting a greater impact on memory if IEDs occurred during the trial when hippocampal activity was critical. Finally, to investigate how IEDs may disrupt memory function, we examined the temporal relationship between IEDs and hippocampal ripples.
Materials and methods
Face-profession association task
We used 120 colour images of distinct human faces with neutral expression from the Chicago Face Database (Fig. 1A).27 The image set comprised 59 male, 61 female, and an equal proportion of White, Black, Hispanic, and Asian faces, paired with 120 emotionally neutral, single-word professions, 4–10 letters long, selected from the US Bureau for Labor Statistics database. Since epilepsy patients typically have impaired performance compared to healthy subjects,14–16,28 we calibrated the task difficulty per subject to achieve a balanced distribution between successful and failed encoding trials. Trial sets ranged from 1 to 10 pairs per set. Task stimuli were presented by computer using custom software (MATLAB, Psychophysics Toolbox). Each face-profession pair was shown for 5 s, with a 1-s interstimulus interval that was marked by a plus sign. To ensure attention and sensory processing of test stimuli, patients were instructed to read the profession aloud and make a mental association. To prevent rehearsal after the encoding block, a brief distraction task was presented (‘Count backwards from 15’). The task laptop sent a pulse to a trigger input channel in the EEG amplifier to synchronize task stimuli with the electrophysiological recordings. The time stamps associated with the pulses were used to annotate the intracranial EEG recordings. For the last 10 patients, we recorded audio responses, enabling analysis of HGA and hippocampal ripples aligned temporally with the onset of the patient’s vocalized response.
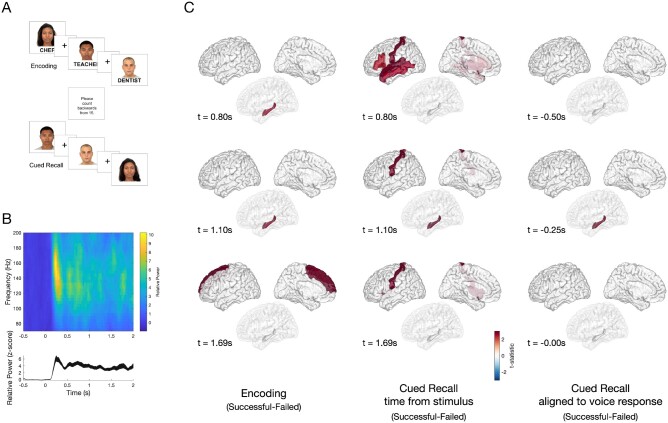
Figure 1.
Task design and differences in HGA between successful and failed associative memory. (A) A computerized program presented task stimuli and recorded subject spoken responses. During encoding, each face-profession pair was shown for 5 s, with a 1-s interstimulus interval (ISI), which was marked by a plus symbol. To ensure attention and sensory processing of test stimuli, subjects were instructed to read the profession aloud and make a mental association. To prevent rehearsal, a brief distraction task followed the encoding block, during which subjects were asked to count backwards from 15. During cued recall, subjects were shown only the faces from the prior set and asked to say aloud the associated profession. The cued recall period lasted for as long as the subject needed to provide a response. Voice response was recorded for the last 10 subjects and scored for accuracy. (B) Example spectrogram (top) of the raw data recorded in the occipital cortex, and high gamma activity (bottom, HGA 60–170 Hz) normalized to the −500 prestimulus baseline, with a peak at 250 ms after stimulus presentation. (C) Group-level differences in HGA by time for correctly versus incorrectly recalled face-profession pairs thresholded at P < 0.05 (cluster-corrected) during encoding (left), cued recall (middle) and vocal-aligned cued recall (right). Left: During encoding, increased HGA in hippocampus beginning approximately +0.80s after stimulus presentation, with increased HGA in superior frontal region beginning approximately +1.69 s distinguished between successful and failed trials. Middle: During cued recall, increased HGA at +0.80 s after face stimulus presentation in inferior frontal gyrus, postcentral, superior temporal and middle temporal gyrus, and later at +1.10 s in hippocampus distinguish between successful and failed trials P < 0.05, cluster-corrected). Right: To disambiguate the contribution of vocalization to cued recall, the difference between successful and failed trials was determined, timed in response to the vocalization in 10 patients. A difference in hippocampal HGA was seen beginning at −250 ms prior to vocalization (all significant clusters identified at a significance threshold P < 0.05 using a cluster-based permutation test).
We tested for memory function using a cued recall paradigm. This paradigm allows greater experimental control compared to free recall designs, including the ability to precisely measure the spatiotemporal relationship between physiological and pathological events. A cued recall paradigm may be more sensitive to clinical memory dysfunction compared to recognition memory tasks.29 During recall, subjects were shown only faces from the prior set and asked to speak aloud the associated profession. The cued recall segment lasted for as long as the subject needed to provide a response. Sets during which subjects freely stated the associated profession paired with the face stimulus were scored as correct trials. Trials during which the subject responded ‘pass’ or gave the incorrect answer were scored as incorrect trials.
Antiseizure medications were reduced to record seizures during the patient’s hospital stay. Cognitive testing was performed ≥6 h after the last seizure. If a patient had a seizure or non-epileptic seizure during testing, all concurrent and subsequent trials were excluded from analysis. All participants provided informed consent with procedures approved by the institutional review board at NYU Langone.
Intracranial EEG data acquisition and electrode localization
Brain activity was recorded from implanted stainless steel or platinum-iridium depth electrodes or subdural electrode grids embedded in silastic sheets. Decisions for implantation, placement of electrodes, and the duration of monitoring were made by the clinical team and without reference to this study. Subdural grids and strips covered extensive portions of lateral and medial frontal, parietal, occipital, and temporal cortices of both hemispheres (Fig. 2). Recordings were made using: (i) Natus NicoletOne C64 clinical amplifier, bandpass filtered from 0.16 Hz to 250 Hz, with a 512-Hz sampling rate; or (ii) a Natus Quantum clinical amplifier, with a 2000-Hz sampling rate, which was later down-sampled to 512 Hz following anti-aliasing filtering. Electrode localization was performed using automated processes and expert review. The location of each electrode relative to the cortical surface was determined from post-implantation CT scan or MRI brain co-registered to the pre-implant T1-weighted MRI brain scan.30 Co-registered, skull-stripped T1 images were non-linearly registered to an MNI-152 template and electrode locations were then extracted in Montreal Neurological Institute (MNI) space. Each electrode was assigned to a brain region of interest based on the Desikan-Killiany anatomical atlas.31 Hippocampal depth electrode location was confirmed by expert review (A.L., S.H.).
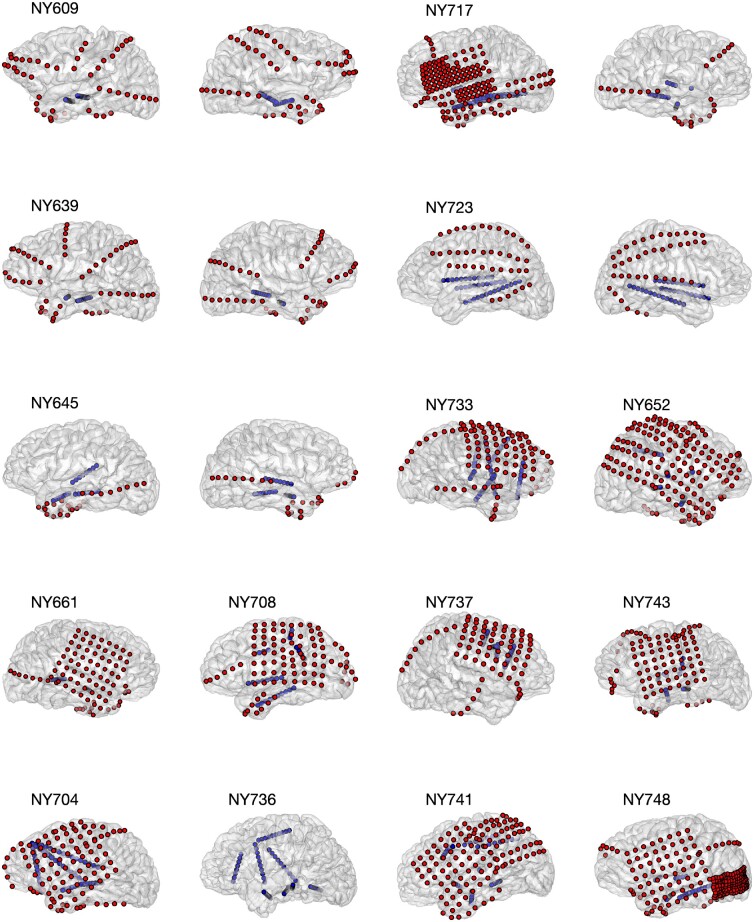
Figure 2.
Electrode coverage for 15 subjects. We recorded from a total of 1646 electrodes in 15 subjects. Five patients had bilateral depths and strips (Patients NY609, NY639, NY645, NY723, NY736). Six patients had left hemisphere subdural grids, strips, and depths (Patients NY704, NY 708, NY717, NY741, NY743, NY748). Four patients had right hemisphere subdural grids, strips, and depths (Patients NY652, NY661, NY733, NY737). Grid and strip electrodes are shown in red; depth electrodes are shown in blue. Patient NY737 did not have hippocampal depth electrodes and therefore was excluded from ripple analysis.
Intracranial EEG analysis
Standard artefact rejection techniques, such as notch filtering for line noise and harmonics (60, 120, 180 Hz), detrending, and baseline correction were applied. In addition, EEG artefacts were removed by omitting trials where the raw signal exceeded 5 standard deviations (SD) above the mean.32 Finally, visual inspection of individual trials excluded trials with excessive non-physiological noise. Intracranial EEG analysis focused on identifying differences in the spectral-temporal features between successful and failed encoding trials. All analyses were performed using a bipolar montage, conducted across regions and trials. After identifying electrodes in the hippocampus, entorhinal cortex, and regions of the neocortex, we performed time-frequency analyses in the −500 ms to 2000 ms from time of the cue presentation. For 10 subjects with recordings of spoken responses, we manually marked the onset of the vocalization and derived vocal-aligned responses for each trial in −2000 ms to 2000 ms around the vocal onset.
Intracranial EEG recordings were segmented into encoding and cued recall periods, comparing trials that were later successfully recalled versus forgotten. For recall, we performed two analyses: one aligned to cue presentation and another aligned against vocalization. HGA activity of the raw EEG traces was estimated using multi-taper spectral analysis (Chronux Toolbox, 250-ms windows, 10-ms steps; 60–170 Hz), normalized per frequency bin to the −0.5 to −0.05 s pre-stimulus baseline, and then averaged across frequency bands. For vocalization-aligned responses, baseline normalization was performed across the entire −2 to +2 s interval from vocalization onset to adjust for the large variability in response time relative to cue onset. For each patient, HGA across all electrodes was averaged within a region of interest based on the Desikan-Killiany anatomical atlas.31 Finally, to assess for differences between conditions (successful versus failed encoding) within a given region of interest, a non-parametric cluster-based permutation test was used to determine significance of any power changes between conditions and control for multiple comparisons.33 Briefly, temporal clusters are identified in the test set (e.g. adjacent time points which exceed the critical t-value using a dependent samples t-test, two-sided). Next, a null distribution was formed from 1000 random permutations of the condition labels, and clusters were identified in this set using the same criteria. Clusters in the test set were deemed significant if the summed t-statistic in the observed cluster exceeded that of the maximum cluster in the null distribution 95% of the time (P < 0.05, cluster-corrected).
Seizure onset zone
We sought to identify electrodes within the SOZ, which has impaired function compared to non-SOZ regions.17 The SOZ was determined by the clinical team, by correlating clinical seizures with intracranial EEG seizures. SOZ electrodes were excluded from all analyses correlating hippocampal ripples and IEDs with memory performance, and when examining the temporal relationship between IEDs and ripples. We performed a secondary analysis of ripple rate in SOZ to assess its functional reserve.
Interictal epileptiform discharge detection
Given the significant inter-rater variability in IED annotation among experts,34 we used an automated IED detection algorithm35 combined with validation by two experts (A.L., S.H.). This algorithm identifies brief outliers in the signal envelope (Hilbert envelope of the bandpass filtered signal between 10 Hz and 60 Hz) by adaptively modelling the distribution of the background activity (log-normal distribution of the signal envelope in 5-s windows, 4-s overlap), and determining if the signal voltage exceeds 3.3× the mode + median of the envelope of the modelled background activity.35 All spikes in the 0-ms to +2000-ms window were identified for each electrode for both encoding and recall trials, and compared between correct and incorrect trials.
Interictal epileptiform discharge analysis and modelling
To assess the impact of IEDs on memory, we fit a mixed-effects model to the IED counts per region of interest. A generalized linear mixed-effects model (GLMM) with a logit link function permitted modelling of a binary outcome of each trial (remembered versus forgotten),17 as:
| (1) |
where, πij is the probability of successful recall for subject i in trial j, IEDij is the number of spikes in trial j for subject i, and bi ∼ N(0, σ2) is the random-effects intercept for each subject i, accounting for subject-specific variation in recall performance. Effect sizes (odds ratio, OR) and confidence intervals (CI) were calculated from logistic regression estimates of the fixed-effects [e.g. OR = exp(β1)]. Significance of the model coefficients were determined using an F-test36 and false discovery rate (FDR) corrected across all regions of interest. IEDs in more than one region were considered independently.17
Ripple detection
All electrode pairs (bipolar montage) with at least one contact located within the hippocampus (including anterior and posterior regions) were selected for analysis for each patient. To reduce the likelihood of detecting pathological ripples, electrodes within the SOZ and trials with IEDs were excluded from initial ripple analysis. Ripple detection was performed by filtering the raw intracranial EEG between 80 Hz and 120 Hz, then extracting the power envelope using the Hilbert transform. Events where the envelope of the filtered response exceeded 2 SDs and measured 20–200 ms in duration were marked as ripple events.10 These filter settings reduced the potential of detecting pathological high frequency oscillations (HFOs), which have a centroid peak between 150 Hz and 200 Hz.37 HFOs are potential biomarkers of epileptogenicity and have been negatively correlated with HGA and memory performance.37 Ripple rate was calculated in 125-ms bins, as the number of ripple events detected per second. Ripple duration and spectral qualities were inspected. To examine differences in ripple rate between successful and failed encoding, the average ripple rate was computed across all electrodes for each patient, and a non-parametric cluster-based permutation test was used to identify the effect of condition (successful versus failed) across patients.
A secondary analysis examined the impact of IEDs on ripples during encoding, cued retrieval, and voice-aligned retrieval, by comparing the hippocampal ripple rates before and after the IED detection. First, all IEDs across all hippocampal electrodes were pooled and binned in 500-ms windows within encoding, recall, and voice aligned trials, from 500 ms before to 2000 ms after cue onset. Ripple rate was calculated in the 500-ms window before and after each IED was detected, excluding ripples within 50 ms before and after the IED to avoid detection of pathological HFOs. Ripple rates before and after IEDs were compared using a paired Wilcoxon’s signed-rank test, then adjusted for multiple comparisons using FDR corrections.38 This measure tested the null hypothesis that the ripple rate before and after the IED were the same.
Data availability
The data that support these findings are available upon reasonable request from the corresponding authors.
Results
Data collection
We recorded from 15 patients with epilepsy undergoing intracranial EEG monitoring for surgical evaluation from New York University Langone Hospital (NYULH). All study activities were approved by the NYU Langone Institutional Review Board and all patients provided informed consent to participate in the study. We included seven males and eight females, with an average age of 30.5 (range 15–55, SD 12.4). Fourteen were right-handed and one was ambidextrous; average IQ was 98.8 (range 82–132, SD 15.6). Table 1 summarizes additional demographic and clinical characteristics. Most patients scored below normative values on a standardized verbal memory task, the Rey Auditory Verbal Learning Test (RAVLT, z = −1.5, SD = 2.4; Supplementary Table 1), and visual memory task, the Rey Ostereith Copy Figure Test (ROCFT, z = −2.5, SD = 2.4; Supplementary Table 1). Patients’ implantations included a combination of subdural grids, strips and depth (e.g. hippocampal) electrodes (Fig. 2). For one patient (Patient NY737), electrode coverage did not include the hippocampus. We analysed 1646 electrodes in this study. SOZs were diverse (Table 1). Eight patients underwent surgical resection, six were recommended for neurostimulation (responsive, vagus nerve, or deep brain stimulation), and one patient did not receive a surgical intervention due to a failure to record clinical seizures.
Table 1.
Demographic and clinical characteristics of subjects
| Subject | Age | Sex | Handness | Average set size | IQ | Coverage | SOZ | Outcome |
|---|---|---|---|---|---|---|---|---|
| NY609 | 45 | F | R | 2.2 | 95 | Bilateral | R mesial temporal | R anterior temporal lobe resection |
| NY639 | 18 | M | R | 8.8 | 91 | Bilateral | L hemisphere | RNS |
| NY645 | 38 | M | R | 4.3 | 82 | Bilateral | R mesial temporal | RNS |
| NY652 | 20 | F | R | 4.1 | 74 | R hemisphere | R temporal, parietal, occipital | R temporal, parietal, occipital corticectomy |
| NY661 | 28 | M | R | 9.2 | 132 | R hemisphere | R temporal | R anterior temporal lobe resection |
| NY704 | 55 | F | R | 3.5 | 108 | L hemisphere | L basal and lateral temporal neocortex | Tailored L anterior temporal lobe resection |
| NY708 | 41 | F | R | 1.4 | 116 | L hemisphere |
Unclear Non-epileptic seizures |
No surgical intervention |
| NY717 | 49 | F | R | 3.3 | 115 | Bilateral | R temporal pole and hippocampus | RNS |
| NY723 | 26 | M | R | 2.4 | NA | Bilateral | B mesial temporal lobes | RNS |
| NY733 | 15 | M | R | 4 | 107 | R hemisphere | R orbitofrontal, cingulate, insula | Tailored R frontal corticectomy |
| NY736 | 29 | M | R | 4 | 96 | LH depths | L mesial temporal cortex | L anterior temporal lobe resection |
| NY737 | 20 | F | R | 4 | 91 | L hemisphere | R middle frontal, cingulate, pars triangularis/opercularis, precentral | R frontal corticectomy |
| NY741 | 24 | M | B | 2.1 | 82 | L hemisphere | L frontal, parietal, temporal, occipital | RNS, VNS, or DBS |
| NY743 | 30 | F | R | 2 | 98 | L hemisphere | L posterior insula, periopercular | RNS |
| NY748 | 19 | F | R | 3.4 | 96 | L hemisphere | L temporal neocortical | Tailored L post inferior temporal corticectomy |
Fifteen subjects were recruited from a single epilepsy centre. Subjects had an average age of 30.5 years (range 15–55, SD 12.4), 53% female, right-handed, with a mean IQ of 98.8 (range 82–132, SD 15.6). Patients were implanted with a combination of strategies: bilateral strips and depths; right and left hemisphere grids, strips, and depths. SOZs determined by intracranial EEG monitoring and clinical outcomes are reported, which could include surgery or a therapeutic device: deep brain stimulation (DBS); responsive neurostimulation (RNS), or vagal nerve stimulation (VNS). F = female; L = left; M = male; R = right.
All patients performed the face-profession visual association task (Fig. 1A). The face-profession task robustly activates both hippocampi in healthy subjects.23 Since epilepsy patients typically have impaired performance compared to healthy subjects,14–16,28 we calibrated the task difficulty for each subject to achieve a balanced distribution between successful and failed encoding trials. Subject performance varied from 1 to 10 trials per set, between 24 to 119 trials total per subject. Low total trial numbers were due to poor performance, lethargy, ensuing seizure, and/or time limitations due to clinical factors. Because trial set size varied by subject, direct comparisons of performance across subjects was not possible.
Differences in high gamma activity in hippocampus and frontal regions distinguish remembering
Intracranial EEG recordings were segmented into encoding and cued recall periods, comparing trials which were later successfully recalled versus forgotten. As expected, HGA (60–170 Hz) demonstrated an early increase in several cortical areas, including primary visual cortex (Fig. 1B) and visual association cortices, such as fusiform gyrus and inferior temporal gyrus, immediately after stimulus presentation (Fig. 1C). Later, beginning approximately +0.80 s after stimulus presentation, HGA in hippocampus distinguished successful and failed encoding trials (P < 0.05, cluster-corrected, additionally; Supplementary Fig. 1). Increased HGA in the superior frontal region beginning at +1.69 s also characterized successful encoding (Fig. 1C;P < 0.05, cluster-corrected). During cued recall, increased HGA at +0.80 s after stimulus presentation in inferior frontal, postcentral, superior and middle temporal gyri, and later at +1.10 s in hippocampus and postcentral gyrus distinguished successful and failed recall trials (Fig. 1C;P < 0.05, cluster-corrected). To disambiguate the cognitive from the motor vocalization response components and to account for variable response times, we examined the difference between correct and incorrect responses timed to the vocalization in 10 patients with voice recordings. We found a difference in hippocampal HGA occurring before vocalization, starting approximately −250 ms before vocalization (Fig. 3C;P < 0.05, cluster-corrected).
Figure 3.
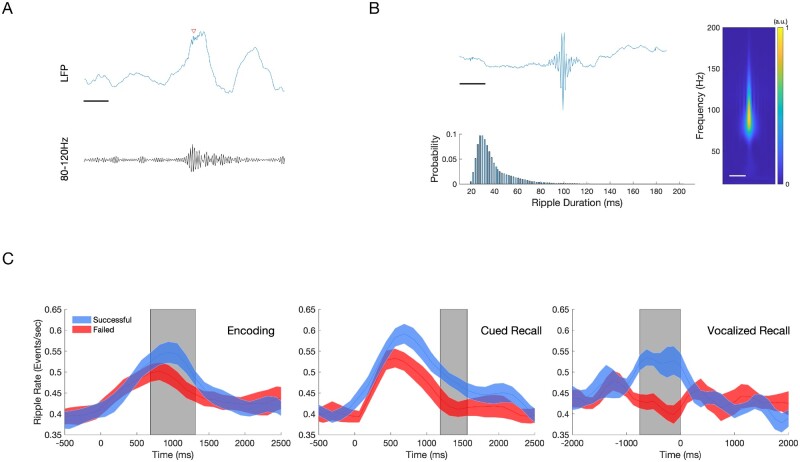
Hippocampal ripples during encoding and recall predict successful associative memory. Ripple events were detected using a bipolar montage from the electrodes located in or closest to the hippocampus, using a previously published method (80–120 Hz, 20–200 ms duration).10 To reduce the detections of pathological high frequency oscillations (HFOs), detections were restricted to regions outside of SOZ, and with trials which did not contain an IED. (A) Sample of a raw EEG tracing (blue) with detected ripple event (red arrow), with bandpass filtered (80–120 Hz) tracing (black). Scale bar = 125 ms (B) Characteristics of all detected hippocampal ripples. An average of 1955 ripple events were detected per patient across all conditions. Top: Grand averaged ripple response (left) and spectrogram (right, 10–200 Hz) demonstrates a peak frequency between 80 Hz and 100 Hz. Scale bars = 125 ms. Bottom: Histogram showing detected ripple duration, which follows skewed log distribution. Mean ripple duration 39.8 ms, SD 18.3 ms. (C) Average ripple rate between Successful and Failed Associative Memory Trials (mean ± standard error of the mean). Top: Successful encoding is characterized by a higher hippocampal ripple rate (blue) compared to failed encoding (red) between 750 ms and1375 ms after stimulus presentation (grey box, n = 14, P < 0.05, cluster-corrected). Middle: Successful cued recall is characterized by a higher ripple rate compared to failed cued recall between +1250 ms and +1625 ms after stimulus presentation (grey box, n = 14, P < 0.05, cluster-corrected). Bottom: Successful cued recall is characterized by a higher ripple rate from −750 ms to 0 ms aligned voice response (grey box, n = 9, P < 0.05, cluster-corrected).
Hippocampal ripples increased in successful versus failed remembering
Hippocampal ripples were measured from all depth electrodes within the hippocampus for each patient. Ripple detection was performed using a published method that has demonstrated the relationship between ripples in hippocampus and middle temporal gyrus and successful retrieval (80–120 Hz, 20–200 ms duration).10 We detected an average of 1955 (±799) hippocampal ripple events across all test blocks per patient, after excluding electrodes in the SOZ. An example raw trace of a detected ripple event is shown in Fig. 3A. We observed that ripples are brief events possessing oscillatory features characterized by a spectral centroid occurring between 80 Hz and 120 Hz (Fig. 3B). Ripples had a median duration of 39 ms [±18 ms, interquartile range (IQR)] and showed a skewed log distribution (Fig. 3B, bottom).
To examine whether the incidence of detected hippocampal ripple events during encoding and retrieval stages correlated with memory performance, we computed the ripple rate (Hz) of detected events in 125-ms bins from −500 ms to 2000 ms relative to the picture onset. While baseline ripple rates in the 500 ms preceding stimulus presentation were similar preceding all trials, successful encoding trials were characterized by a greater ripple rate approximately +750 to +1375 ms after stimulus presentation (Fig. 3C, top; P < 0.05, cluster-corrected). Likewise, successful recall trials were characterized by a greater increase in ripple rate occurring approximately +1250 until +1625 ms after stimulus presentation (Fig. 3C, middle; P < 0.05, cluster-corrected). For the 10 patients with voice recording, successful trials had significantly increased ripple events in the 750 ms window preceding the vocal response (Fig. 3C, bottom; P < 0.05, cluster-corrected).
In contrast, hippocampal ripples in the SOZ during encoding and cued recall did not differ between successful and failed trials (Supplementary Fig. 2). However, even in the SOZ, ripple rate differentiated successful and failed trials when trials were aligned to the vocal response (Supplementary Fig. 2).
Interictal epileptiform discharges in hippocampus decrease the odds of remembering
IEDs occurring during either the encoding or retrieval stages of the associative memory task were identified and pooled by anatomical region. We detected 13 484 IEDs across all test blocks and electrodes for all patients (mean and SD, 898 ± 342 events). IED rate varied from zero to two events per trial across subjects (Supplementary Fig. 4). We blindly reviewed ∼12% of detected events, classifying them as a true or a false IED detection (A.L., S.H.). This analysis revealed a positive predictive rate of 92% and false positive rate of 8% among the detected IEDs. Figure 4A shows the raw tracing of an example IED and its spectrogram.
Figure 4.
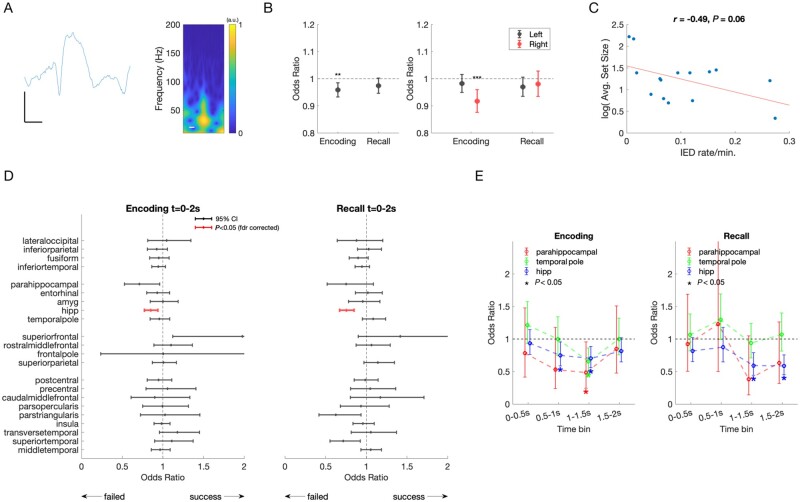
IEDs and effect on memory performance. (A) Example raw tracing and spectrogram of a detected IED. Scale bars = 25 µV, 125 ms. (B) Left: IEDs recorded in any brain region during encoding predicted a 4% decreased odds of remembering (OR = 0.96, CI = 0.93–0.99, P = 0.002, F-test). IEDs in any brain region during cued recall trended towards decreased odds of remembering (P = 0.0760, F-test). Right: IEDs occurring in the right hemisphere predicted a 9% decreased odds of remembering (P = 0.0002, F-test). There was no difference in odds of remembering for IEDs in the left hemisphere during encoding, or either hemisphere during recall. Error bars represent 95% confidence intervals. (C) Relationship between block size, IQ, and IED rate. Subjects varied in performance, ranging between 1 and 10 stimuli presented per block, larger sets indicating superior task performance across patients. There was a trend towards a negative correlation between IED rate and log set size [r(13) = −0.49, P = 0.06]. (D) Odds of successful memory for IEDs occurring during encoding and recall, by brain region. Mean odds of successful memory per IED occurring during encoding (left) and cued recall (right). Error bars represent 95% CI. Odds < 1 indicate a decreased odds of successful remembering if an IED occurred during the trial. After correction for multiple comparisons, a significant decrease in remembering occurs for IEDs in hippocampus [red, encoding: OR = 0.85, CI = 0.77–0.94), F(1,6221) = 10.5, P = 0.001; recall: OR = 0.75, CI = 0.67–0.85, F(1,5956) = 21.8, P < 0.001]. (E) Odds of successful memory for IEDs occurring in selected brain regions, by 500 ms time bin. Mean odds of successful memory per IED occurring during encoding (left), demonstrate that odds of remembering are further decreased by 25–52% if IEDs occurred in hippocampus, parahippocampal gyrus, and temporal pole occur between 500–2000 ms. During recall (right), mean odds of successful memory per IED in hippocampus decreased by 41% when IEDs occurred between 1000 ms and 2000 ms after stimulus presentation. Error bars represent 95% CI. Significant changes indicated by an asterisk (P < 0.05, F-test).
SOZ was determined clinically by correlating seizure behaviour to ictal intracranial EEG changes. IEDs occurring in any brain region outside the SOZ during encoding decreased the odds of remembering [Fig. 4B, left; OR = 0.96, CI = 0.93–0.99, F(1,136290) = 9.2247, P = 0.002]. IEDs occurring in any brain region during the cued recall stage was associated with a trend towards poorer performance [Fig. 4B;F(1,131202) = 3.1477, P = 0.08]. Further, an IED occurring in the right, but not left hemisphere during encoding was associated with significantly decreased odds of remembering [Fig 4B, right; OR = 0.92, CI = 0.88–0.96, F(1,61266) = 13.8, P < 0.001]. There was a non-significant negative correlation between the frequency of IEDs and trial set size [r(13) = −0.49, P = 0.06; Fig. 4C]. Verbal IQ did not predict trial set size.
When evaluating the odds of remembering related to IEDs occurring in distinct brain regions during the encoding period, the largest impact on performance was when IEDs occurred in the hippocampus. An IED in the hippocampus was associated with decreased odds of remembering by 15% (95% CI: 6–23%; Fig. 4D). During cued recall, an IED in hippocampus and outside of SOZ reduced odds of remembering by 25% (15–33%; Fig. 4D).
Given the time course of HGA and hippocampal ripple activity predicting successful versus failed trials, we predicted that IEDs during critical time windows would have an even greater effect. When the impact of IEDs during encoding was evaluated in a more time-resolved manner, we found that an IED in hippocampus occurring during the 500-ms to 1500-ms window after stimulus presentation was associated with decreased odds of remembering between 25% and 30% (Fig. 4E). IEDs in the temporal pole during the 1500-ms to 2000-ms window decreased odds of remembering by 34%. IEDs in parahippocampal cortex during the 1000–1500 ms after stimulus were associated with a decreased odds of 52%. Similarly, during cued recall, IEDs in hippocampus during the 1000–2000-ms window decreased odds of recall by 41% (Fig. 4E).
Hippocampal interictal epileptiform discharges slow response time
In general, response times for all correct trial responses were delivered more quickly compared to incorrect responses or responses where subjects ‘passed’ (mean = 1.74 s ± 1.6 SD for correct trials versus mean = 5.87 s ± 4.6 for incorrect/pass trials, Z = −14.2, P < 0.001, Wilcoxon’s rank sum test) (Supplementary Fig. 6). For all response types (correct, incorrect, pass), trials with an IED were slower compared to trials without an IED when trials were pooled across patients [correct: medianIED− = 1.25 s (1.10 IQR); medianIED+ = 1.61 s (1.49 IQR), Z = −3.89, P < 0.001; incorrect: medianIED− = 2.05 s (2.62 IQR); medianIED+ = 4.15 s (3.93 IQR), Z = −5.19, P < 0.001, pass: medianIED− = 3.01 s (4.03 IQR); medianIED+ = 6.13 s (4.35 IQR), Z = −3.74, P < 0.001; Wilcoxon's rank sum test].
Hippocampal interictal epileptiform discharges decrease ripple rate
Because both IEDs and ripple rates were inversely related with memory performance, we examined their temporal relationship on a finer temporal scale. Hippocampal ripples generally decreased after the IED for during encoding and cued recall (Fig. 5A and B). However, this decrease in ripple rate reached significance during the 0.5–1.0 s and 1.0–1.5 s time bins during encoding. Additionally, ripple rate demonstrated the greatest decrease after IEDs occurring in the time bin aligned to vocal onset during cued recall (Fig. 5C). We further analysed ripple rate decreases following IEDs along the longitudinal axis of the hippocampus, and found that IED induced reductions in ripple rates were primarily driven by activity in anterior hippocampus (Supplementary Fig. 7).
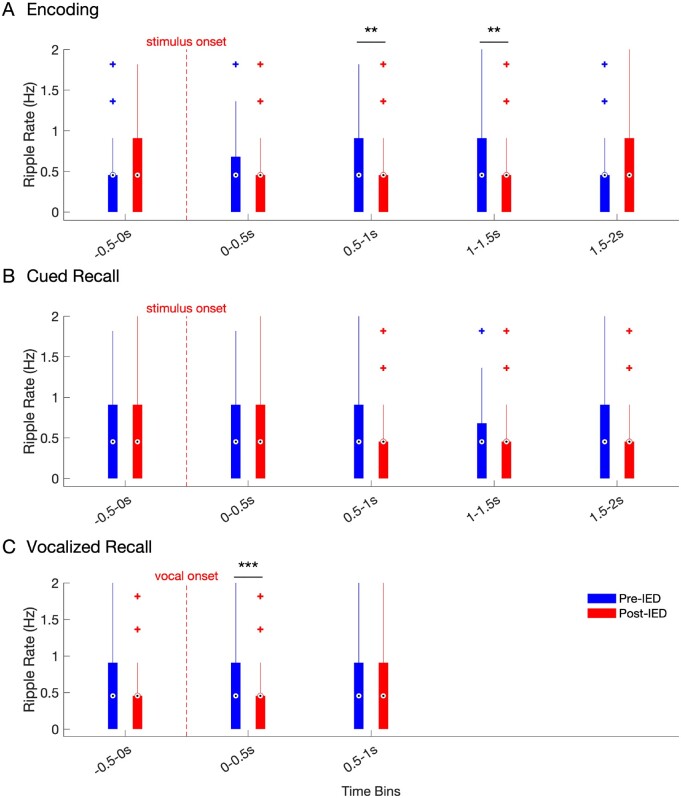
Figure 5.
Hippocampal ripple rate in the pre and post-IED window during encoding and recall. Ripple rates across all hippocampal electrodes in 500 ms pre- (blue) versus 500 ms post-IED (red), binned by time of detected IEDs (500-ms bin windows) for IEDs detected during (A) encoding, (B) cued recall, and (C) voice-aligned recall. Box and whisker plots represent the median (circles) and IQR (bars), along with extreme values (whiskers and outliers). A reduction in ripple rate after an IED event was found during the 0.5–1 s (Z = 2.8838, nIEDs = 209, P = 0.004) and 1–1.5 s (Z = 3.0873, nIEDs=266, P = 0.002) windows during encoding. In addition, ripple rates were significantly reduced in the time window during (0–0.5 s) vocalization during cued recall (Z = 3.9, nIEDs = 177, P < 0.001).
Discussion
We examined the contribution of physiological (HGA and hippocampal ripples) and pathological (IED) events in successful and failed associative memory in epilepsy patients. An increase in frontal and hippocampal HGA and hippocampal ripple rate during encoding predicted successful memory. During the encoding and cued recall stages, physiological differences between successful and error trials peaked between 500 ms and 1500 ms after stimulus presentation, or within 750 ms of the spoken response. Conversely, pathological IEDs occurring in hippocampus during encoding and retrieval reduced the likelihood of remembering. A hippocampal IED during encoding decrease the odds of remembering by 15% (CI 6–23%). A hippocampal IED during cued recall decreased the odds of remembering by 25% (CI 15–33%). IEDs occurring during the 500–2000-ms window of encoding or retrieval had an even greater negative behavioural impact. Hippocampal IEDs observed during encoding and recall were generally followed by a decrease in ripple rate, although this difference only reached significance between 500 ms and 1500 ms during encoding and 0 to +500 ms around vocal onset. Overall, our findings suggest that IEDs impair associative memory in a regionally and temporally specific manner, likely by competing with physiological memory processes (i.e. hippocampal ripples) needed to encode and recall.
Hippocampal ripples predict associative memory performance
Our findings advance understanding of the contribution of physiological ripples to memory performance. Hippocampal ripple events detected from local field potential (LFP) recordings in humans undergoing invasive monitoring for epilepsy surgery have been shown to correlate with successful retrieval of word pairs10,11 and visual episodic memories.12 Recently, LFP ripples in the middle temporal gyrus have been shown to organize sequences of single unit firing activity in humans in a phase-locked manner during successful memory retrieval.11 The sequence of single unit firing carries item-specific information, with the temporal order of unit firing during encoding replayed during retrieval in remembered trials.11 Similar to prior studies,10–12 we also observed an increase in ripple activity during the 750 ms prior to voiced retrieval predicting successful memory. Together, these studies represent a translational link to ripple activity observed in rodents during spatial navigation, which are replayed during wakefulness39 and sleep.40,41
Whereas previous human studies demonstrated ripples’ contribution to retrieval processes, we extend these findings to demonstrate the role of hippocampal ripple events during successful encoding and retrieval. To capture physiological, and not pathological, high frequency oscillations, we excluded SOZ electrodes. We also excluded trials with IEDs, which often have superimposed pathological HFO events overriding the peak of the waveform.42,43 Concordant with previous results, we found that ripples possessed an oscillatory waveform, a centroid peak between 80 Hz and 100 Hz, a mean duration of ∼30 ms, and followed a skewed log distribution. These features resemble ripples in rodents42 suggesting that the events are not filtered high gamma events. The detected ripple rate at our threshold was approximately one event per second, comparable to previous human studies.10,12 Most importantly, physiological ripple events had a strong temporal correlation with successful encoding and recall.
Our finding that ripple rate from hippocampus within the SOZ distinguished between successful and failed trials only during the voice-aligned recall condition, but not during encoding or cued recall, suggests that the SOZ preserves some physiological function, albeit less than tissue outside the SOZ.37
Hippocampal interictal epileptiform discharges decrease memory performance and prolong reaction time
Our work supports and extends prior work on the role of IEDs in memory dysfunction by detailing IED spatiotemporal dynamics, especially regarding the role of the hippocampus. Early studies using intracranial depth recordings in humans demonstrated that hippocampal IEDs during a Sternberg (letter) working memory experiment impaired performance if they occurred during the maintenance or recognition stage.16 One study including 80 surgical patients across multiple surgical centres demonstrated that IEDs during the encoding and recall epochs impaired verbal memory performance, with stronger effects seen for left hemisphere IEDs occurring in inferior temporal, medial temporal, and parietal regions.14 However, hippocampal IEDs predicted reduced memory only if they occurred during the recall phase, but not encoding phase.14 Another study in epilepsy surgical patients showed that IEDs occurring in the left fusiform gyrus, and middle and inferior temporal gyrus, and outside of the SOZ, during encoding of a word list decreased odds of recall by 8–15% per IED event.17 The authors hypothesized that IEDs disrupted visual encoding or recognition of word forms. In prior work, it is surprising that hippocampus and nearby structures have been inconsistently implicated in IED-related memory disruption given their critical role in temporarily binding new information for later retrieval.44–46 It is unclear from previous studies why IEDs would disrupt one stage of learning but not the other within the same task.
We show consistent and large effects of IEDs in hippocampus and adjacent neocortical regions during both encoding and recall, amplified during physiologically important windows of the trial. Our large effect sizes may be due to the appropriate selection of a face-profession association task, which differs from working memory paradigms and verbal free recall in several ways: (i) the task is associative in nature and demands linking semantic and visual information (a key function of the hippocampus)44,46; and (ii) it is a cued recall paradigm, allowing for precise temporal alignment between HGA, ripple events, and IEDs to stimulus presentation and vocal response. Here, we examined how the HGA and hippocampal ripple time courses could predict when and where IEDs might have maximal impact. We found that IEDs in mesial temporal structures occurring within 500–1500 ms—around the same time when HGA and ripple activity distinguished successful from failed memory—had a larger deleterious effect, decreasing odds of recall by 25–52% per IED. This is a truly large effect, implying that IEDs occurring within critical time windows will interfere with the learning process with a high probability.
Not only did IEDs increase forgetting, but slowed reaction times. Slower responses could reflect disruption in associative computation, but alternatively slower processing of the visual stimuli or execution of the motor command, depending on the IED location. Previously, focal and generalized IEDs have prolonged reaction times in a driving simulation task,47,48 and increased crash rate when virtual obstacle is presented.48 IEDs prolonged reaction time in a variable manner, with generalized discharges affecting reaction time greater than focal discharges48; and longer and higher voltage IEDs exerting a greater effect.49
Hippocampal interictal epileptiform discharges outside the seizure onset zone impair memory performance
Similar to previous reports,17 we found that IEDs within the SOZ generally did not impair memory performance. Only IEDs outside of the SOZ diminished performance. This suggests that physiological processes, such as ripples, were already compromised in the epileptogenic cortex. We found a positive, consistent relationship with hippocampal ripple activity and memory performance when SOZ tissue was excluded from analysis. However, when hippocampal tissue within the SOZ was analysed separately, we only observed the increase in ripple rate predicting performance with voice aligned recall, but not during encoding or cued recall (Supplementary Fig. 2). Our findings suggest some preserved function in the SOZ compared to surrounding brain tissue outside of the SOZ Supplementary Fig. 2.37 The diminished reserve in SOZ is likely due to greater neuronal loss and damage.50
Hippocampal interictal epileptiform discharges decrease ripple rate
By relating the temporal evolution of IEDs to hippocampal ripples, we demonstrate a potential mechanism for IED-induced disruption of mnemonic processing, at least in the anterior hippocampus. Previous studies focused on either physiological HGA or ripple activity10,12 or pathological IEDs.14,16,17 We have shown that IEDs occurring within the critical 1000–2000 ms period after cue presentation corresponds to a decrease in ripple activity required for successful recall. While IEDs have previously been demonstrated to interfere with sleep-dependent memory consolidation,13 our work suggests that IEDs also interfere with the physiological processes supporting memory encoding and recall. IEDs likely trigger a prolonged cortical downstate,13 as supported by a recent study demonstrating IED modulation of inhibitory interneurons in the medial temporal lobe.51 The state-dependent decrease in ripple activity after IEDs imply that IEDs are not merely an epiphenomenon of a drowsy or distracted state,18 but rather trigger decreases in physiological ripples necessary for memory.
Limitations
The limitations of our study include a moderate sample size of patients, which limit analysis of how clinical and demographic factors—such as gender, epilepsy duration and severity, and education—affect memory performance. In addition, given that intracranial recordings are composed of LFPs, we could not directly measure ripple content. We relied on the features of the filtered ripple event such as duration and frequency, their correlation with memory performance, differing time course from HGA to deduce that the signals were indeed physiological ripples. Strict exclusion of pathological cortex and trials and prior studies combining LFP with single unit recordings11 provide additional support. Future studies may utilize a combination of LFP with single unit recordings to reveal ripple content. Finally, studies in human subjects are limited by the inability to induce timed IEDs and prove their causative role in disrupting physiological models to impact memory.
Conclusions
Earlier animal studies found that most hippocampal IEDs act as pathological ripples, recruiting a much larger population of the neuronal pool and in a narrower time window than the physiologically protracted events during ripples.52 The competition between physiological ripples and pathological IED rate in a kindling model led to deterioration of performance on a cheeseboard maze task.13 Together, these findings and our work suggest that epileptic IEDs hijack physiological processes essential for effective encoding and recall of episodic memories.
Assuming that transient memory impairment arises from pathological IED events competing with physiological ripples, hippocampal IEDs represent a promising therapeutic target for memory remediation in patients with epilepsy and Alzheimer’s disease.53 However, before IEDs can be considered a therapeutic target, assessment of performance in a real-time manner using a wider variety of clinically meaningful memory tasks is needed. Furthermore, closed-loop termination of IEDs during cognitive testing in rodents and humans54 are needed to prove that blocking IEDs can indeed restore memory.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Lucia Melloni and Lila Davachi for early advice on task design.
Funding
S.H., A.S., H.B., and A.L. receive support from the National Institute of Health (NIH, K23-NS 104252, RO1 MH 107396), and the Zimin Foundation. A.F. receives support from the NIH (R01 NS 109367, R01 NS125641, R21 MH122829), and National Science Foundation (NSF 1912286). D.F. receives support from the NIH (R01 NS06209207, NIH R01 NS109367, CDC U48DP006396-01SIP 19-003), Neuropace, Inc, Epilepsy Foundation, and Epitel. O.D. receives support from the NIH (R01 MH107396, U01 NS099705, R01 MH111417, U01 NS090415, R01 MH116978, R01 HL151490), Tuberous Sclerosis Alliance, Epilepsy Foundation of American, and the National Science Foundation. G.B. receives support from the NIH (U01 NS090583, R01 MH107396, R01 MH122391). W.D. receives support from the NIH (R01 NS062092, R01 MH116978).
Competing interests
OD has equity and/or compensation from the following companies: Privateer Holdings, Tilray, Receptor Life Sciences, Qstate Biosciences, Tevard, Empatica, Engage, Egg Rock/Papa & Barkley, Rettco, SilverSpike, and California Cannabis Enterprises (CCE). He has received consulting fees from GW Pharma, Cavion and Zogenix.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- HGA
high gamma activity
- IED
interictal epileptiform discharge
- SOZ
seizure onset zone
References
- 1. Fisher RS, Vickrey BG, Gibson P, et al. The impact of epilepsy from the patient's perspective I. Descriptions and subjective perceptions. Epilepsy Res. 2000;41:39-51. [DOI] [PubMed] [Google Scholar]
- 2. Lemesle B, Planton M, Pages B, et al. Accelerated long-term forgetting and autobiographical memory disorders in temporal lobe epilepsy: one entity or two? Rev Neurol (Paris). 2017;173:498-505. [DOI] [PubMed] [Google Scholar]
- 3. Mazarati A. Epilepsy and forgetfulness: one impairment, multiple mechanisms. Epilepsy Curr. 2008;8:25-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwab RS. A method of measuring consciousness in attacks of petit mall epilepsy. Arch Neurol Psychiatry. 1939;41:215-217. [Google Scholar]
- 5. Aarts JH, Binnie CD, Smit AM, et al. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107:293-308. [DOI] [PubMed] [Google Scholar]
- 6. Lachaux JP, Axmacher N, Mormann F, et al. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98:279-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sederberg PB, Kahana MJ, Howard MW, et al. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809-10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sederberg PB, Schulze-Bonhage A, Madsen JR, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2006;17:1190-1196. [DOI] [PubMed] [Google Scholar]
- 9. Greenberg JA, Burke JF, Haque R, . et al. Decreases in theta and increases in high frequency activity underlie associative memory encoding. Neuroimage. 2015;114:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaz AP, Inati SK, Brunel N, et al. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science. 2019;363:975-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaz AP, Wittig JH Jr., Inati SK, et al. Replay of cortical spiking sequences during human memory retrieval. Science. 2020;367:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norman Y, Yeagle EM, Khuvis S, et al. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science. 2019;365:eaax1030. [DOI] [PubMed] [Google Scholar]
- 13. Gelinas JN, Khodagholy D, Thesen T, et al. Interictal epileptiform discharges induce hippocampal-cortical coupling in temporal lobe epilepsy. Nat Med. 2016;22:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horak PC, Meisenhelter S, Song Y, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia. 2017;58:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krauss GL, Summerfield M, Brandt J, et al. Mesial temporal spikes interfere with working memory. Neurology. 1997;49:975-980. [DOI] [PubMed] [Google Scholar]
- 16. Stemmer N, Strekalova E, Djogo N, et al. Generation of amyloid-beta is reduced by the interaction of calreticulin with amyloid precursor protein, presenilin and nicastrin. PloS One. 2013;8:e61299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ung H, Cazares C, Nanivadekar A, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. 2017;140:2157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleen JK, Kirsch HE.. The nociferous influence of interictal discharges on memory. Brain. 2017;140:2072-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung LW. Hippocampal interictal spikes induced by kindling: relations to behavior and EEG. Behav Brain Res. 1988;31:75-84. [DOI] [PubMed] [Google Scholar]
- 20. Jang AI, Wittig JH Jr., Inati SK, et al. Human cortical neurons in the anterior temporal lobe reinstate spiking activity during verbal memory retrieval. Curr Biol. 2017;27:1700-5 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staresina BP, Davachi L.. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suthana NA, Donix M, Wozny DR, et al. High-resolution 7T fMRI of human hippocampal subfields during associative learning. J Cogn Neurosci. 2015;27:1194-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Theysohn N, Qin S, Maderwald S, et al. Memory-related hippocampal activity can be measured robustly using FMRI at 7 tesla. J Neuroimaging. 2013;23:445-451. [DOI] [PubMed] [Google Scholar]
- 24. Mukamel R, Gelbard H, Arieli A, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951-954. [DOI] [PubMed] [Google Scholar]
- 25. Weiss SA, Alvarado-Rojas C, Bragin A, et al. Ictal onset patterns of local field potentials, high frequency oscillations, and unit activity in human mesial temporal lobe epilepsy. Epilepsia. 2016;57:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiss SA, Banks GP, McKhann GM Jr., et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain. 2013;136:3796-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma DS, Correll J, Wittenbrink B.. The Chicago face database: a free stimulus set of faces and norming data. Behav Res Methods. 2015;47:1122-1135. [DOI] [PubMed] [Google Scholar]
- 28. Fell J, Klaver P, Elger CE, et al. The interaction of rhinal cortex and hippocampus in human declarative memory formation. Rev Neurosci. 2002;13:299-312. [DOI] [PubMed] [Google Scholar]
- 29. Cassel A, Morris R, Koutroumanidis M, et al. Forgetting in temporal lobe epilepsy: when does it become accelerated? Cortex. 2016;78:70-84. [DOI] [PubMed] [Google Scholar]
- 30. Yang AI, Wang X, Doyle WK, et al. Localization of dense intracranial electrode arrays using magnetic resonance imaging. Neuroimage. 2012;63:157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968-980. [DOI] [PubMed] [Google Scholar]
- 32. Fell J, Klaver P, Lehnertz K, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259-1264. [DOI] [PubMed] [Google Scholar]
- 33. Maris E, Oostenveld R.. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177-190. [DOI] [PubMed] [Google Scholar]
- 34. Jing J, Herlopian A, Karakis I, et al. Interrater reliability of experts in identifying interictal epileptiform discharges in electroencephalograms. JAMA Neurol. 2020;77:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janca R, Jezdik P, Cmejla R, et al. Detection of interictal epileptiform discharges using signal envelope distribution modelling: application to epileptic and non-epileptic intracranial recordings. Brain Topogr. 2015;28:172-183. [DOI] [PubMed] [Google Scholar]
- 36. Korn EL, Graubard BI.. Simultaneous testing of regression coefficients with complex survey data: use of bonferroni t statistics. Am Stat. 1990;44:270-276. [Google Scholar]
- 37. Liu S, Parvizi J.. Cognitive refractory state caused by spontaneous epileptic high-frequency oscillations in the human brain. Sci Transl Med. 2019;11:eaax7830. [DOI] [PubMed] [Google Scholar]
- 38. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289-300. [Google Scholar]
- 39. Jadhav SP, Kemere C, German PW, et al. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buzsaki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson MA, McNaughton BL.. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676-679. [DOI] [PubMed] [Google Scholar]
- 42. Bragin A, Engel J, Wilson CL, et al. High-frequency oscillations in human brain. Hippocampus. 1999;9:137-142. [DOI] [PubMed] [Google Scholar]
- 43. Waldman ZJ, Camarillo-Rodriguez L, Chervenova I, et al. Ripple oscillations in the left temporal neocortex are associated with impaired verbal episodic memory encoding. Epilepsy Behav. 2018;88:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eichenbaum H. What H.M. taught us. J Cogn Neurosci. 2013;25:14-21. [DOI] [PubMed] [Google Scholar]
- 45. Leranth C, Szeidemann Z, Hsu M, et al. AMPA receptors in the rat and primate hippocampus: a possible absence of GluR2/3 subunits in most interneurons. Neuroscience. 1996;70:631-652. [DOI] [PubMed] [Google Scholar]
- 46. Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195-231. [DOI] [PubMed] [Google Scholar]
- 47. Krestel HE, Nirkko A, von Allmen A, et al. Spike-triggered reaction-time EEG as a possible assessment tool for driving ability. Epilepsia. 2011;52:e126-9-e129. [DOI] [PubMed] [Google Scholar]
- 48. Nirkko AC, Bernasconi C, von Allmen A, et al. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia. 2016;57:832-840. [DOI] [PubMed] [Google Scholar]
- 49. Cohen E, Antwi P, Banz BC, et al. Realistic driving simulation during generalized epileptiform discharges to identify electroencephalographic features related to motor vehicle safety: feasibility and pilot study. Epilepsia. 2020;61:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sutula TP, Hagen J, Pitkanen A.. Do epileptic seizures damage the brain? Curr Opin Neurol. 2003;16:189-195. [DOI] [PubMed] [Google Scholar]
- 51. Reed CM, Mosher CP, Chandravadia N, et al. Extent of single-neuron activity modulation by hippocampal interictal discharges predicts declarative memory disruption in humans. J Neurosci. 2020;40:682-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riekkinen P, Buzsaki G, Riekkinen P Jr, et al. The cholinergic system and EEG slow waves. Electroencephalography and Clinical Neurophysiology. 1991;78:89-96. [DOI] [PubMed] [Google Scholar]
- 53. Lam AD, Deck G, Goldman A, et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat Med. 2017;23:678-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat Neurosci. 2015;18:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support these findings are available upon reasonable request from the corresponding authors.