Abstract
BACKGROUND
The mechanism of left atrial (LA) remodeling is poorly understood. The aim of this longitudinal study was to investigate whether changes in NT-proBNP levels relate to alterations of LA structure and function over time in a multiethnic population.
METHODS
From the prospective cohort study, the Multi-Ethnic Study of Atherosclerosis, our analysis included 1,838 participants who underwent cardiac magnetic resonance imaging at the baseline and 10-year examinations, had NT-proBNP levels available at both time points, and did not develop heart failure, myocardial infarction, and/or atrial fibrillation. Multivariable linear regression was used to analyze the association between NT-proBNP level (log-transformed) at the 2 time points and change in LA volumes, LA emptying fractions (total, active, and passive), and LA longitudinal strain. Log NT-proBNP was categorized into Low-Low (N = 681), Low-High (N = 238), High-Low (N = 237), and High-High (N = 682) based on the median value at both time points.
RESULTS
With the Low-Low group as the reference group, the High-High group experienced a greater increase in LA maximum and minimum indexed volumes: 3.1 ml/m2 (95% confidence interval 1.98, 4.20) and 2.7 ml/m2 (1.89, 3.51), respectively. The High-High group also experienced a greater decrease in LA total, passive, active emptying fraction, and longitudinal strain: −3.3% (−4.46, −2.11), −0.9% (−1.80, −0.02), −4.2% (−5.55, −2.76), and −2.3% (−3.80, −0.72), respectively. The Low-High group had similar associations, but the effect sizes were not as high.
CONCLUSIONS
Adverse LA remodeling over 10 years of follow-up strongly correlates with prolonged elevated levels of intracardiac stress, as assessed by NT-proBNP levels.
Keywords: blood pressure, cardiac magnetic resonance imaging, hypertension, left atrium, multimodality tissue tracking, NT-proBNP
Graphical Abstract
Graphical Abstract.
Left atrium (LA) modulates left ventricular (LV) filling as it acts as a reservoir, a conduit to LV during early diastole, as well as a contractile booster pump in late diastole.1,2 Recent studies suggest that changes in LA structure and function have been associated with cardiovascular disease (CVD) and clinical outcomes including atrial fibrillation (AF), stroke, and all-cause mortality.2–5 LA structure and function have been identified as potentially useful prognostic markers in heart failure (HF) with both reduced (HFrEF) and preserved ejection fraction (HFpEF).4,6,7 LA also functions as a neurohumoral organ contributing to cardiovascular homeostasis by storing ANP and small amounts of BNP in the granules of atrial myocytes. The natriuretic peptide response is closely associated with myocardial wall stress and is known to be one of the criteria for the diagnosis of HF.8 LA volume may reflect chronicity and severity of elevated left-sided filling pressure, and has been associated with natriuretic peptides and outcomes in different HF states.9 Recently, the concept of atrial cardiomyopathy as a progressive fibrotic atrial disease has been evolved in risk for AF with stroke.10 Given the relatively thin atrial wall and its complex motion, accurate quantification of LA size and function by cardiac magnetic resonance (CMR) may be challenging. Feature tracking CMR (FT-CMR) was specifically developed to measure myocardial deformation in order to overcome those shortcomings, therefore providing a complete assessment of LA phasic volumes and function with excellent accuracy and reproducibility.2,11
Indeed, in the Multi-Ethnic Study on Atherosclerosis (MESA) NT-proBNP is the single most important predictor of both AF and incident HF among all phenotypic variables measured in the baseline MESA examination.12,13 Due to physiologic and pathologic alterations, levels of NT-proBNP change over time.14 However, it is still unclear whether changes in NT-proBNP levels are associated with LA remodeling and what role they play, in the pathogenesis of adverse LA remodeling. Hence, the aim of this study is to establish both cross-sectional and longitudinal relationships between NT-proBNP and LA structure and function in a multiethnic population free of clinical CVD at baseline.
METHODS
Study participants
Between July 2000 and August 2002, 6,814 men and women aged 45–84 years who were recruited from 6 US field centers (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and St Paul, MN) and were enrolled in a prospective observational cohort study, MESA. MESA included participants who identified their race/ethnicity as White, African American, Chinese American, or Hispanic and with subclinical CVD at baseline. Details of the MESA study design have been previously described.15 Ten-year follow-up examinations for the participants were done in 2010. All study protocols were reviewed and approved by institutional review boards of each field center and all participants signed written consent.15
At baseline, 5,004 participants had CMR, of these 4,223 had quantifiable LA imaging data available. Similarly, at 10 years, out of 3,045 participants who underwent a CMR, only 2,879 had quantifiable LA imaging data. After excluding participants without NT-proBNP there were 1,985 participants who had baseline and follow-up NT-proBNP and LA data from CMR. Of those, 147 participants developed HF, AF, and/or myocardial infarction during follow-up and were excluded from this analysis. A total of 1,838 participants were included for analysis. Only 1,633 participants have LA passive and active function available.
Multimodality tissue tracking image analysis
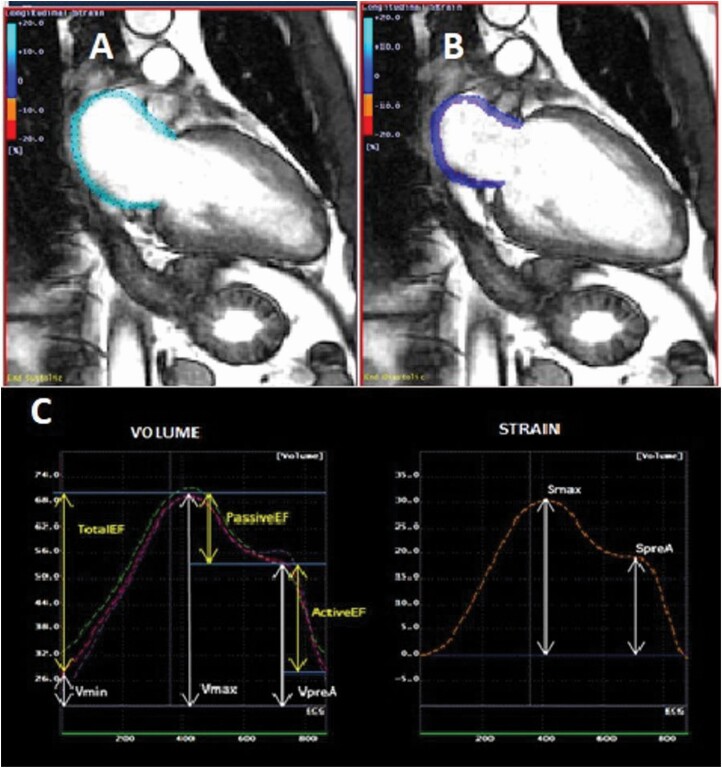
Details of the CMR protocol have been described previously.2 LA parameters were obtained using semi-automated MTT software version 5.0 (Toshiba, Tokyo, Japan) by a trained radiologist from baseline 4- and 2-chamber cine CMR images. LA endocardial and epicardial borders were drawn manually by using marked points by a single experienced operator at a reference frame, the ventricular end-systolic frame just before the mitral valve opening (Figure 1). The software then searches for pixel pattern that most closely matches the epicardial and endocardial contours in the subsequent frames using the marked points. For quality control, the user retraced the borders through all the subsequent frames if necessary. LA volume was calculated by using the biplane area-length modified Simpson method from apical 4- and 2-chamber views by generating a volume/time curve. Longitudinal strain curves for each atrial wall segment were generated.
Figure 1.
The figure illustrates phasic LA volumes, function (left), and longitudinal strain (right) corresponding to 1 cardiac cycle. (a and b) Feature tracking CMR imaging of LA in a 2- and 4-chamber view. (c) Vmax—maximum LA volume, Vmin—minimum LA volume, and VpreA—LA volume before atrial contraction, respectively. On the longitudinal strain curve (right), Smax—maximum LA longitudinal strain and SpreA—LA longitudinal strain before atrial contraction. Abbreviations: CMR, cardiac magnetic resonance; LA, left atrium.
Using the volume/time curve, measurements for LA volumes were extracted. All measured LA volumes were subsequently indexed according to body surface area. The parameters of LA function and volume derived from the curves:
-
•
Maximum LA volume (LAVmax): LA volume at end-systole before mitral valve opening.
-
•
Minimum LA volume (LAVmin): LA volume at end diastole after mitral valve closure.
-
•
Pre-atrial contraction (LAVpreA): LA volume before atrial contraction.
-
•
LA passive emptying fraction (LAPEF): .
-
•
LA active emptying fraction (LAAEF): .
-
•
LA total emptying fraction (LATEF): .
LA strain was calculated from the average of global values in the longitudinal direction at each time frame, determined by the software’s automatic division of the LA wall into equal segments. From the longitudinal strain curve, peak longitudinal LA strain (LASmax) is calculated. Excellent inter- and intraobserver reliability have been demonstrated.4
NT-proBNP
NT-proBNP levels were measured from frozen serum samples drawn at baseline and 10-year follow-up and stored at a temperature range of −70 °C to −80 °C. All measurements were performed on the Elecsys electrochemiluminescence immunoassay based on the double-antibody sandwich method (Roche Diagnostic, Indianapolis, IN, USA). A 250 µl serum sample previously unthawed or only thawed once was used for analysis. The measuring range was 5–35,000 pg/ml (defined by the lower detection limit and the maximum of the standard curve). The intraassay and interassay coefficients of variation for baseline examination: at 175 pg/ml, 2.7% and 3.2%; at 355 pg/ml, 2.4% and 2.9%; at 1,068 pg/ml, 1.9% and 2.6%; and at 4,962 pg/ml, 1.8% and 2.3%. The interassay CVs on 3 controls run with 10-year follow-up sample: 4.03%, 3.05%, and 3.18%.12,16
Statistical analysis
Continuous variables were expressed as mean ± SD or as median (interquartile range) based on the distribution of the variable, and categorical variables were expressed as percentages. NT-proBNP was log-transformed to approximate a normal distribution. The change in log NT-proBNP was divided into 4 categories based on the median values and median value was chosen for this analysis as an approximation to the point at which most slopes changed. Based on log NT-proBNP value relative to median at baseline and 10 years it was categorized into Low-Low, Low-High, High-Low, and High-High. Differences in baseline clinical characteristics, risk factors, LA, and LV MRI parameters by log NT-proBNP categories were tested using analysis of variance (continuous variables) or χ 2 tests (categorical variables) and Kruskal–Wallis test for skewed distribution.
For cross-sectional association, with LA parameters as the dependent variable and log NT-proBNP as the independent variable, we used linear regression at 10 years. Next, to assess for nonlinear association, the log NT-proBNP value at which the dependent variables had a substantial change in slope, i.e., the inflection point was determined using linear splines. Linear splines were performed by fitting the data to the regression equation and knots for the linear splines were chosen at log NT-proBNP value of 4.2 pg/ml. The log NT-proBNP value at which linear spline offered the best fit was chosen as the inflection point for the dependent variables. The line segments were constrained to be continuous between the 2 categories.
For longitudinal analysis, a delta variable was created for LA outcome variables. We performed several analyses with categorizing NT-proBNP values based on specific thresholds at baseline and 10-year follow-up such as different cutoffs which produced qualitatively similar trends. For both cross-sectional and longitudinal models, multivariable linear models were adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, lipid lowering medications, smoking status, LV mass, LV ejection fraction, and LV mass–volume ratio (measure of LV diastolic dysfunction). The variables included in the multivariable regression models were chosen a priori. For testing interactions of log NT-proBNP with gender and race/ethnicity on LA function, volume, and strain, the likelihood ratio test was used. All analyses were performed using STATA v15.0 (StataCorp), and significance was declared for P ≤ 0.05.
RESULTS
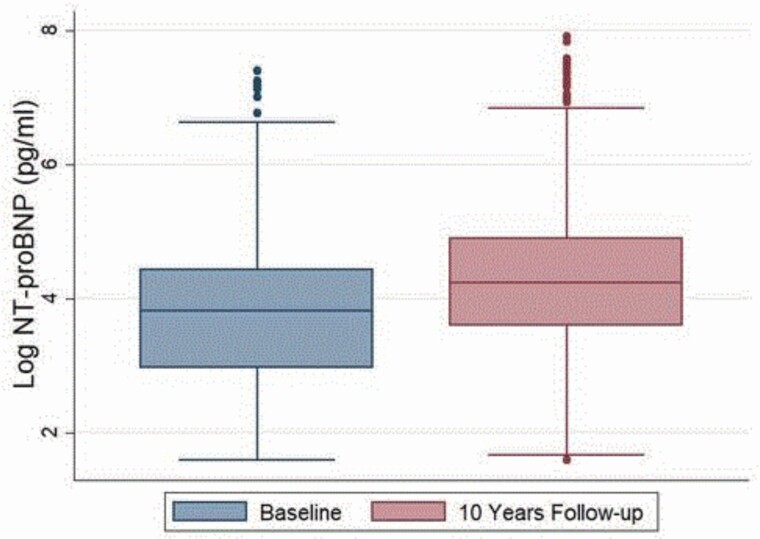
At baseline, the mean (SD) age was 59 (9.2) years and 52% were men. Of 1,838 participants, 43.9% were Caucasian, 21.6% were African American, 21.6% were Hispanic, and 13.3% were Chinese. The median log NT-proBNP at baseline was 3.81 pg/ml and at 10 years was 4.21 pg/ml (Figure 2). Over 10 years, NT-proBNP levels increased from a median (IQR) of 45.2 (19–85) to 69.5 (36.1–135.4). Based on clinical cutoffs of NT-proBNP for acute HF,17 only 7 (0.4%) participants and 14 (0.8%) participants had abnormal NT-proBNP levels at baseline and 10-year follow-up, respectively. Out of this, only 4 (0.2%) participants had persistent high NT-proBNP level over 10 years.
Figure 2.
Boxplot showing distribution of log NT-proBNP shown at baseline (blue) and at 10 years (red). Wilcoxon signed rank test showed statistically significant difference in the values of log NT-proBNP at baseline and at 10-year follow-up.
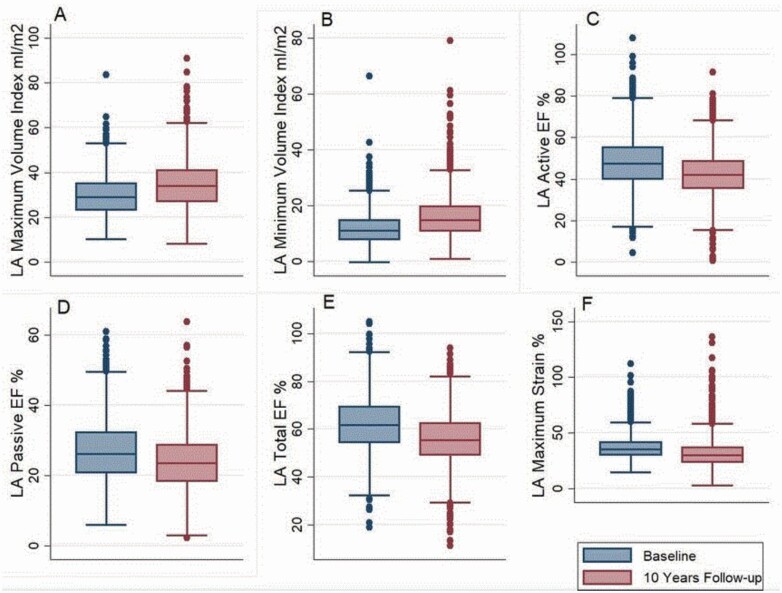
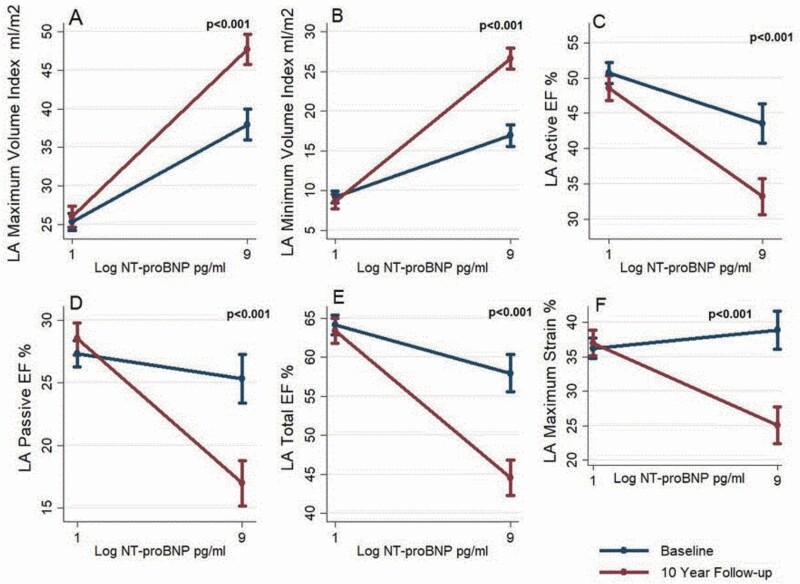
The mean LA volume indices where the mean maximum volume (LAVIMAX) showed an increase of 5.2 ml/m2, mean minimum volume (LAVIMIN) showed an increase of 4.1 ml/m2. On the contrary, the LA function showed a decline with the mean active emptying fraction (LAAEF) decreasing from 48.2% to 42.4%, passive emptying fraction (LAPEF) decreasing from 26.6% to 23.8%, and total emptying fraction (LATEF) from 62.1% to 55.7%. The maximum longitudinal strain had a similar trend of decline from a mean of 37.1% to 32.1% (Figure 3). Table 1 shows the characteristics of our study participants by log NT-proBNP categories. The relationship of LA volume, function, and strain with log NT-proBNP at baseline and at 10 years can be seen in Figure 4.
Figure 3.
Bar graph showing mean LA structural and functional parameters at baseline (blue) and 10-year follow-up (red). (a) LA Maximum Volume Index; (b) LA Minimum Volume Index; (c) LA Active EF; (d) LA Passive EF; (e) LA Total EF; (f) LA Maximum Strain. There is a statistically significant difference in the mean values of LA parameters at baseline and at 10-year follow-up using paired t-tests. Abbreviations: EF, emptying fraction; LA, left atrium.
Table 1.
Baseline characteristics of log NT-proBNP categories
| Low-Low | Low-High | High-Low | High-High | P value | |
|---|---|---|---|---|---|
| Participants, n (%) | 681 (37.1) | 238 (13.0) | 239 (13.0) | 680 (37.0) | |
| Age in years, mean (SD) | 55.0 (7.6) | 59.8 (8.8) | 56.7 (7.8) | 63.5 (9.2) | <0.001 |
| Males, n (%) | 446 (65.5) | 134 (56.3) | 88 (36.8) | 211 (31.0) | <0.001 |
| Race | |||||
| Caucasian American | 233 (34.2) | 91 (38.2) | 124 (51.9) | 360 (52.9) | |
| Black American | 166 (24.4) | 70 (29.4) | 42 (17.6) | 120 (17.7) | <0.001 |
| Hispanic American | 153 (22.5) | 53 (22.3) | 45 (18.8) | 136 (20.0) | |
| Chinese American | 129 (18.9) | 24 (10.1) | 28 (11.7) | 64 (9.4) | |
| BMI, kg/m2, mean (SD) | 27.65 (4.5) | 28.61 (5.2) | 27.75 (5.0) | 27.48 (5.3) | 0.02 |
| SBP, mm Hg, mean (SD) | 117.55 (15.4) | 124.66 (16.6) | 120.87 (22.6) | 126.98 (22.8) | <0.001 |
| DBP, mm Hg, mean (SD) | 72.80 (9.3) | 73.58 (9.1) | 71.27 (11.7) | 69.84 (10.55) | <0.001 |
| HDL cholesterol, mg/dl, mean (SD) | 47.68 (12.8) | 49.00 (14.2) | 53.45 (16.2) | 55.07 (15.6) | <0.001 |
| LDL cholesterol, mg/dl, mean (SD) | 119.76 (29.6) | 120.14 (35.3) | 110.97 (26.8) | 117.0 (31.9) | 0.001 |
| Total cholesterol, mg/dl, mean (SD) | 194.41 (34.6) | 195.84 (39.2) | 189.90 (35.6) | 196.47 (34.5) | 0.09 |
| Lipid lowering medication, n (%) | 87 (12.8) | 45 (18.9) | 35 (14.6) | 91 (13.4) | 0.12 |
| Diabetes mellitus, n (%) | 58 (8.5) | 42 (17.6) | 13 (5.5) | 59 (8.7) | <0.001 |
| Hypertension, n (%) | 169 (24.8) | 93 (39.1) | 86 (36.0) | 305 (44.9) | <0.001 |
| Antihypertensive medication use, n (%) | 141 (20.7) | 82 (34.5) | 75 (31.4) | 245 (36.0) | <0.001 |
| Current smoker, n (%) | 82 (12.0) | 29 (12.2) | 30 (12.6) | 62 (9.1) | 0.25 |
| NT-proBNP, pg/ml, median (IQR) | 15.34 (6.8–27.9) | 28.6 (18.3–37.8) | 66.9 (53.0–85.9) | 99.16 (67.0–149.7) | <0.001 |
| LA maximum volume index, ml/m2, mean (SD) | 28.07 (7.9) | 28.39 (8.7) | 30.23 (8.6) | 31.25 (9.4) | <0.001 |
| LA minimum volume index, ml/m2, mean (SD) | 10.88 (5.0) | 11.37 (5.6) | 11.83 (5.7) | 12.80 (6.5) | <0.001 |
| LA total emptying fraction, %, mean (SD) | 62.93 (11.4) | 61.87 (12.0) | 62.75 (12.1) | 61.06 (11.1) | 0.02 |
| LA passive emptying fraction, %, mean (SD) | 26.67 (9.4) | 26.71 (9.5) | 27.69 (9.4) | 26.16 (8.3) | 0.21 |
| LA active emptying fraction, %, mean (SD) | 49.38 (12.4) | 47.93 (14.0) | 48.24 (14.4) | 47.21 (16.7) | 0.04 |
| LA maximum strain, %, mean (SD) | 36.73 (10.2) | 36.70 (11.6) | 38.74 (12.1) | 37.13 (11.4) | 0.10 |
| LV ejection fraction, %, mean (SD) | 62.09 (5.6) | 62.42 (5.3) | 62.93 (5.6) | 63.25 (5.6) | 0.001 |
| LV mass, g, mean (SD) | 125.36 (27.7) | 125.44 (25.9) | 116.63 (28.7) | 115.32 (29.5) | <0.001 |
| LV end-diastolic volume index, ml/m2, mean (SD) | 71.19 (11.8) | 69.08 (12.6) | 71.18 (10.9) | 69.49 (12.2) | <0.001 |
| LV end-systolic volume index, ml/m2, mean (SD) | 26.93 (5.8) | 25.98 (6.2) | 26.34 (5.5) | 25.60 (6.8) | 0.001 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LA, left atrium; LDL, low-density lipoprotein; LV, left ventricle; SBP, systolic blood pressure.
Figure 4.
The figure shows the longitudinal changes of LA volume, function, and strain with log NT-proBNP at baseline and 10 years. x axis: log NT-proBNP; y axis: LA parameters. (a) LA Maximum Volume Index; (b) LA Minimum Volume Index; (c) LA Active EF; (d) LA Passive EF; (e) LA Total EF; (f) LA Maximum Strain. The values represent the adjusted mean and standard errors. Each line represents the starting point at lowest value of log NT-proBNP and ending with highest value of log NT-proBNP for baseline and 10-year follow-up. Lines are shown for average change in LA CMR parameters with log NT-proBNP at baseline (blue) and at 10-year follow-up (red). Marginal means obtained after adjusting for demographics and traditional cardiovascular risk factors. Abbreviations: CMR, cardiac magnetic resonance; EF, emptying fraction; LA, left atrium.
Table 2 shows the cross-sectional relationship between LA parameters with log NT-proBNP at 10-year follow-up by performing a linear spline regression. The inflection point for LA volume, function, and strain was at log NT-proBNP value of 4.2 pg/ml. LA volumes showed a positive association, and LA function and strain showed an inverse association with log NT-proBNP within log NT-proBNP >4.2 pg/ml, which plateaus for log NT-proBNP ≤4.2 pg/ml. The coefficients decrease when adjusted for demographics, cardiovascular risk factors. Similar trend was seen with additional adjustment for LV ejection fraction, mass, and mass–volume ratio.
Table 2.
Cross-sectional association between log NT-proBNP and LA parameters at 10 years
| Univariate | Multivariable | R 2 | |||
|---|---|---|---|---|---|
| Log NT-proBNP | Log NT-proBNP | Log NT-proBNP | Log NT-proBNP | ||
| ≤4.2 pg/ml | >4.2 pg/ml | ≤4.2 pg/ml | >4.2 pg/ml | ||
| LA maximum volume index, ml/m2 | 1.23* | 3.61* | 0.56 | 3.36* | 0.33 |
| (0.18, 2.28) | (2.86, 4.36) | (−0.42, 1.53) | (2.64, 4.09) | ||
| LA minimum volume index, ml/m2 | −0.04 | 3.76* | 0.06 | 3.30* | 0.30 |
| (−0.76, 0.68) | (3.24, 4.27) | (−0.64, 0.77) | (2.78, 3.82) | ||
| LA total emptying fraction, % | 1.10* | −4.75* | −0.12 | −3.94* | 0.30 |
| (0.05, 2.15) | (−5.51, −4.00) | (−1.12, 0.87) | (−4.68, −3.20) | ||
| LA passive emptying fraction, % | 0.04 | −2.50* | −0.21 | −1.08* | 0.36 |
| (−0.76, 0.84) | (−3.07, −1.92) | (−0.92, 0.50) | (−1.61, −0.55) | ||
| LA active emptying fraction, % | 1.30* | −4.07* | −0.07 | −4.22* | 0.20 |
| (0.18,2.43) | (−4.89, −3.26) | (−1.19, 1.06) | (−5.06, −3.39) | ||
| LA maximum strain, % | 1.90* | −3.72* | −0.06 | −3.02* | 0.27 |
| (0.53, 3.27) | (−4.70, −2.74) | (−1.36, 1.24) | (−3.99, −2.06) | ||
Coefficients and 95% confidence intervals (in brackets) for multivariable linear spline regression models to assess the cross-sectional associations at 10-year follow-up. The inflection point was chosen at log NT-proBNP value of 4.2 pg/ml. Multivariable model is adjusted for age, gender and race/ethnicity, systolic blood pressure, diastolic blood pressure, hypertension, antihypertensive medications, HDL cholesterol, LDL cholesterol, total cholesterol, lipid lowering medications, BMI, diabetes mellitus, smoking status, LV ejection fraction, LV mass, and mass–volume ratio. Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LA, left atrium; LDL, low-density lipoprotein; LV, left ventricle.
*If P value <0.05.
Table 3 shows the coefficients and 95% confidence intervals of change in LA parameters with log NT-proBNP from baseline to 10 years with LA parameters as dependent variables and log NT-proBNP as independent variables. There were 681 (37.1%) participants in the Low-Low, 238 (13.0%) in the Low-High, 237 (12.9%) in the High-Low, and 682 (37.1%) in the High-High groups. The Low-Low category was used as the reference. The High-High group experienced a greater increase in LAVIMAX and LAVIMIN: 3.1 ml/m2 (95% confidence interval 1.98, 4.20) and 2.7 ml/m2 (1.89, 3.51), respectively. The High-High group also experienced a greater decrease in LATEF, LAPEF, and LAAEF fractions: −3.3% (−4.46, −2.11), −0.9% (−1.80, −0.02), and −4.2% (−5.55, −2.76), respectively. Similarly, the High-High group experienced a decrease in LA longitudinal strain −2.3% (−3.80, −0.72). Similar significant associations were observed for the Low-High group compared with the Low-Low group, but little difference was observed for the High-Low group compared with the Low-Low group.
Table 3.
Longitudinal association of log NT-proBNP with LA parameters over 10 years
| Univariate | Multivariable | R 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Low-Low | Low-High | High-Low | High-High | Low-High | High-Low | High-High | ||
| Δ LA maximum volume index, ml/m2 | REF | 3.37 | −0.09 | 3.32 | 1.73 | 0.34 | 3.10 | 0.34 |
| (1.94, 4.79)* | (−1.52, 1.34) | (2.28, 4.35)* | (0.38, 3.09)* | (−1.01, 1.69) | (1.98, 4.20)* | |||
| Δ LA minimum volume index, ml/m2 | REF | 2.23 | 0.05 | 2.79 | 1.24 | 0.40 | 2.70 | 0.27 |
| (1.23, 3.23)* | (−0.95, 1.06) | (2.07, 3.52)* | (0.25, 2.23)* | (−0.59, 1.39) | (1.89, 3.51)* | |||
| Δ LA total emptying fraction, % | REF | −2.38 | −0.47 | −3.07 | −1.81 | −0.97 | −3.29 | 0.47 |
| (−3.87, 0.89)* | (−1.96, 1.02) | (−4.14, −1.99)* | (−3.25, −0.37)* | (−2.40, 0.47) | (−4.46, −2.11)* | |||
| Δ LA passive emptying fraction, % | REF | −2.30 | −0.68 | −2.30 | −1.20 | −0.55 | −0.91 | 0.60 |
| (−3.48, −1.11)* | (−1.90, 0.54) | (−3.16, −1.42)* | (−2.28, −0.12)* | (−1.65, 0.55) | (−1.80, −0.02)* | |||
| Δ LA active emptying fraction, % | REF | −2.13 | −0.36 | −2.55 | −2.15 | −1.11 | −4.15 | 0.52 |
| (−3.81, −0.45)* | (−2.08, 1.37) | (−3.78, −1.31)* | (−3.84, −0.46)* | (−2.83, 0.62) | (−5.55, −2.76)* | |||
| Δ LA maximum strain, % | REF | −1.80 | −2.91 | −1.69 | −1.78 | −1.72 | −2.26 | 0.43 |
| (−4.13, 0.54) | (−5.25, −0.57)* | (−3.37, −0.01)* | (−3.68, 0.12) | (−2.61, 0.17) | (−3.80, −0.72)* | |||
Coefficients and 95% confidence intervals (in brackets) for multivariable regression models to assess the change in associations with log NT-proBNP with changes in LA parameters over 10 years. Multivariable model adjusted for age, sex, race, systolic blood pressure, diastolic blood pressure, hypertension, antihypertensive medications, HDL cholesterol, LDL cholesterol, total cholesterol, lipid lowering medications, BMI, diabetes mellitus, smoking status, LV ejection fraction, LV mass, and mass–volume ratio. Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LA, left atrium; LDL, low-density lipoprotein; LV, left ventricle.
*If P value <0.05.
There was no statistically significant interaction between NT-proBNP group and gender for any of the CMR outcomes studied (all P > 0.05). There was no interaction between NT-proBNP group and race on LA volumes (LAVIMAX P value = 0.12, LAVIMIN P value = 0.08) but there was an NT-proBNP-race/ethnic interaction for LA function (LATEF P value = 0.001, LAPEF P value = 0.03, LAAEF P value = 0.05) and strain (P value = ≤0.001). White and Hispanics demonstrated similar trends and patterns as whole cohort in the associations between change in LA function and strain and log NT-proBNP, but these associations were not statistically significant for individuals of Chinese and Black Americans. The association of LA parameters and NT-proBNP was not statistically significant among participants with interim HF which could be due to small sample size.
DISCUSSION
Our study demonstrates that in a multiethnic population increasing levels of NT-proBNP over 10 years as well as high levels at both baseline and 10-year follow-up examination, were associated with greater increase in LA volume, and greater decrease in LA function and LA strain when compared with those with stable low levels over the 10-year follow-up period. Moreover, a drop in log NT-proBNP over 10 years was not associated with differences in LA parameters during that time. Additionally, Low-High and High-High groups showed statistically significant relationships with LA parameters inferring that the elevated levels of log NT-proBNP at the most recent examination was strongly correlated with increase in LA volume, as well as decrease in LA global function and strain. Importantly also LA parameters correlated with elevated log NT-proBNP independently of LV mass and volume.
In most previous studies, 2-dimensional echocardiography has been used for volume measurements of LA. Given the location of LA and its thin asymmetric wall, the assessment of the function is challenging and CMR has been shown to have excellent reproducibility and accuracy in the measurement of cardiac volumes, making it a gold standard modality.18 One study comparing LA volume measured using CMR and echocardiography showed that echocardiography consistently underestimated volumes compared with CMR.19,20 To our knowledge, there are no other studies assessing the association between NT-proBNP and phasic LA function using CMR. Ability to identify high cardiovascular risk is essential for prevention strategies and asymptomatic subjects may benefit from early risk stratification. However, traditional risk assessment tools have not been rigorously evaluated in asymptomatic older adult population. Additionally, the direct contribution of intracardiac stress in the initiation, progression, and acceleration of age-related remodeling is undisputed although the natural history at the population level remains largely unknown due to the absence of longitudinal studies. Hence, the results of our study add to the knowledge of the mechanisms behind NT-proBNP as a marker of adverse cardiac remodeling, in particular LA, which would aid in risk stratification of asymptomatic adults particularly those with high-risk burden.
LA remodeling is associated with incident hypertension and has emerged as a strong predictor of CVDs.21–23 Assessment of LA function is valuable in the evaluation of LV diastolic performance and LA volume index is now included as parameter to diagnose HF.4,24,25 Early phases of LA remodeling are adaptive and reversible while over time it becomes adverse, maladaptive, and permanent.20,26 Understanding the mechanism of LA remodeling and markers associated with it is crucial to the development of novel strategies for screening and management of hypertensive heart disease and CVDs. Studies have suggested that inflammation and oxidative stress may contribute to the process of adverse LA remodeling.20,21,27 In this regard, NT-proBNP has been associated with atrial dilatation, fibrosis, and dysfunction in AF and hypertensive heart disease, cross-sectionally.28–30 In MESA, studies have shown the association of elevated NT-proBNP with incident CVDs.12,31 In recent years, prognostic implications of serial measurements of NT-proBNP in different clinical settings such as HF and myocardial infarction.32,33 Furthermore, reduction in NT-proBNP level over time have been associated with better outcomes in both HFrEF and HFpEF.34,35 While most previous studies focused on ischemic LV function, our study, we demonstrated the associations between changes in NT-proBNP and changes in LA remodeling independent of LV structural and functional parameters including LV mass, volumes, and ejection fraction after excluding congestive heart failure (CHF), myocardial infarction, and AF. In lieu, a recent study showed that LA reservoir and conduit function were associated with higher BNP levels and are early markers of age-related remodeling, independent of LV morphology and function using echocardiography.36 Additionally, previous studies have suggested that LA myocardium could be more easily susceptible to remodeling compared with LV myocardium and thus could be an early marker of age-related remodeling independent of LV remodeling.36,37 Our findings, hence, suggest that disrupting the pathological process leading to continuous rise in NT-proBNP may help prevent adverse LA remodeling in older adults.
There are several hypotheses to explain the link between NT-proBNP and its change with LA parameters. The first and more intuitive possibility is that individuals with elevated NT-proBNP levels are more likely to have both clinical and subclinical CVDs. Second, we propose that the longitudinal increase in NT-proBNP, an index of enhanced myocardial stress, could lead to progressive subclinical LA dysfunction that encompasses an adverse response to neuroendocrine hormonal overstimulation, and hypoxia mechanisms leading to myocardial dysfunction and progressive cardiomyocyte death.31,38,39 The fact that increasing NT-proBNP levels remained significantly associated with LA remodeling despite adjustment for CVD risk factors such as diabetes, BMI, hypertension, and smoking indicate that LA remodeling accompanied by high NT-proBNP begin early in the course of progressive cardiac dysfunction. This suggests that early and aggressive control of risk factors associated with increase NT-proBNP levels documented in this and other studies could significantly alter progressive LA remodeling and dysfunction in older adults.
Limitations
First, the threshold adopted (median log NT-proBNP) is specific for this population. Second, the NT-proBNP included analysis was only measured at 2 time points—at baseline and 10-year follow-up. The measure of NT-proBNP at these 2 time points can be confounded by endocrine disorders or renal function which could not be accounted for in this study. Third, based on 2 cross-sectional examinations performed 10 years apart, our study was not designed to account for physiologic intraindividual variability in NT-proBNP levels. Fourth, we used linear methods to measure LA volumes which may have underestimated the true volumes even though this method has been widely validated.40 LA CMR-FT faces several challenges as opposed to ventricular deformation tracking that may limit the performance of the LA tracking. The LA myocardial wall is thinner than the ventricular myocardial wall and LA anatomy is more variable than ventricular anatomy. LA physiology is quite complex (reservoir function, conduit function, booster pump function). The presence of pulmonary veins and LA appendage can compromise tracking quality. Fifth, with this study design, we may have introduced survival bias as systematically healthier participants were more likely to return for a follow-up CMR examination.
This longitudinal analysis performed in a multiethnic, middle, to older age cohort demonstrates that elevated NT-proBNP at baseline and at the 10-year follow-up examination is strongly associated with adverse anatomical remodeling and contractile dysfunction of the LA over 10 years of follow-up. Future studies should expand on our findings to better understand the pathophysiology of intracardiac stress induced LA remodeling.
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
FUNDING
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Additional support was provided by grant R01 HL 127659 from NHLBI.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TS. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 2011; 12:421–430. [DOI] [PubMed] [Google Scholar]
- 2. Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K, Spragg D, Ashikaga H, Rickard J, Marine JE, Calkins H, Nazarian S. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging 2015; 8:e002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, De Lemos JA. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging 2017; 10:e0050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JAC. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging 2014; 7:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong AC, Liu K, Lewis CE, Sidney S, Colangelo LA, Kishi S, Ambale-Venkatesh B, Arynchyn A, Jacobs DR Jr, Correia LC, Gidding SS, Lima JA. Left atrial dimension and traditional cardiovascular risk factors predict 20-year clinical cardiovascular events in young healthy adults: the CARDIA study. Eur Heart J Cardiovasc Imaging 2014; 15:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, Klein AL, Marsan NA, Prior DL, Yu CM, Poppe KK, Doughty RN, Whalley GA; MeRGE Heart Failure Collaborators . Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009; 11:929–936. [DOI] [PubMed] [Google Scholar]
- 7. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K, Shah SJ. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 2016; 9:e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J 2011; 278:1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015; 8:295–303. [DOI] [PubMed] [Google Scholar]
- 10. Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol 2015; 65:2239–2251. [DOI] [PubMed] [Google Scholar]
- 11. Maceira AM, Cosín-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010; 12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida ALC, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure. Circ Heart Fail 2012; 5:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart 2013; 99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 14. Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA, Jacobs DR Jr. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism 2014; 63:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002; 156:871–881. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L35215769. Accessed 5 September 2019. [DOI] [PubMed] [Google Scholar]
- 16. Ying W, Zhao D, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Sharma K, Shah SJ, Heckbert SR, Lima JA, deFilippi CR, Budoff MJ, Post WS, Michos ED. Sex hormones and change in N-terminal pro-B-type natriuretic peptide levels: the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab 2018; 103:4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Januzzi JL Jr, Chen-Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, Peacock WF, Rivers EJ, Walters EL, Gaggin HK; ICON-RELOADED Investigators . N-terminal pro-B-type natriuretic peptide in the emergency department: the ICON-RELOADED Study. J Am Coll Cardiol 2018; 71:1191–1200. [DOI] [PubMed] [Google Scholar]
- 18. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90:29–34. [DOI] [PubMed] [Google Scholar]
- 19. Whitlock M, Garg A, Gelow J, Jacobson T, Broberg C. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol 2010; 106:1345–1350. [DOI] [PubMed] [Google Scholar]
- 20. Habibi M, Samiei S, Venkatesh BA, Opdahl A, Helle-Valle TM, Zareian M, Almeida ALC, Choi EY, Wu C, Alonso A, Heckbert SR, Bluemke DA, Lima JAC. Cardiac magnetic resonance-measured left atrial volume and function and incident atrial fibrillation. Circ Cardiovasc Imaging 2016; 9:e004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol 2017; 16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heckbert SR, Wiggins KL, Blackshear C, Yang Y, Ding J, Liu J, McKnight B, Alonso A, Austin TR, Benjamin EJ, Curtis LH, Sotoodehnia N, Correa A. Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study. Obesity 2017; 25:1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aljizeeri A, Gin K, Barnes ME, Lee PK, Nair P, Jue J, Tsang TS. Atrial remodeling in newly diagnosed drug-naive hypertensive subjects. Echocardiography 2013; 30:627–633. [DOI] [PubMed] [Google Scholar]
- 24. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355:251–259. [DOI] [PubMed] [Google Scholar]
- 25. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Édes I, Handoko ML, Heymans S, Pezzali N. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 26. Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017; 10:65–77. [DOI] [PubMed] [Google Scholar]
- 27. Markman TM, Habibi M, Venkatesh BA, Zareian M, Wu C, Heckbert SR, Bluemke DA, Lima JAC. Association of left atrial structure and function and incident cardiovascular disease in patients with diabetes mellitus: results from multi-ethnic study of atherosclerosis (MESA). Eur Heart J Cardiovasc Imaging 2017; 18:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao H, Xue L, Wu Y, Ma H, Chen L, Wang X, Zhu Q, Dai N, Chen Y. Natriuretic peptides and right atrial fibrosis in patients with paroxysmal versus persistent atrial fibrillation. Peptides 2010; 31:1531–1539. [DOI] [PubMed] [Google Scholar]
- 29. Tsioufis C, Stougiannos P, Taxiarchou E, Skiadas I, Chatzis D, Thomopoulos C, Lalos S, Stefanadis C, Kallikazaros I. The interplay between haemodynamic load, brain natriuretic peptide and left atrial size in the early stages of essential hypertension. J Hypertens 2006; 24:965–972. [DOI] [PubMed] [Google Scholar]
- 30. Uraizee I, Cheng S, Hung CL, Verma A, Thomas JD, Zile MR, Aurigemma GP, Solomon SD. Relation of N-terminal pro-B-type natriuretic peptide with diastolic function in hypertensive heart disease. Am J Hypertens 2013; 26:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu CY, Heckbert SR, Lai S, Ambale-Venkatesh B, Ostovaneh MR, McClelland RL, Lima JAC, Bluemke DA. Association of elevated NT-proBNP with myocardial fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2017; 70:3102–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN; Val-HeFT Investigators . Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol 2008; 52:997–1003. [DOI] [PubMed] [Google Scholar]
- 33. Kontos MC, Lanfear DE, Gosch K, Daugherty SL, Heidenriech P, Spertus JA. Prognostic value of serial N-terminal pro-brain natriuretic peptide testing in patients with acute myocardial infarction. Am J Cardiol 2017; 120:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, Solomon SD. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol 2016; 68:2425–2436. [DOI] [PubMed] [Google Scholar]
- 35. Jhund PS, Anand IS, Komajda M, Claggett BL, McKelvie RS, Zile MR, Carson PE, McMurray JJ. Changes in N-terminal pro-B-type natriuretic peptide levels and outcomes in heart failure with preserved ejection fraction: an analysis of the I-Preserve study. Eur J Heart Fail 2015; 17:809–817. [DOI] [PubMed] [Google Scholar]
- 36. Yoshida Y, Nakanishi K, Daimon M, Ishiwata J, Sawada N, Hirokawa M, Kaneko H, Nakao T, Mizuno Y, Morita H, Di Tullio MR, Homma S, Komuro I. Alteration of cardiac performance and serum B-type natriuretic peptide level in healthy aging. J Am Coll Cardiol 2019; 74:1789–1800. [DOI] [PubMed] [Google Scholar]
- 37. Brecht A, Oertelt-Prigione S, Seeland U, Rücke M, Hättasch R, Wagelöhner T, Regitz-Zagrosek V, Baumann G, Knebel F, Stangl V. Left atrial function in preclinical diastolic dysfunction: two-dimensional speckle-tracking echocardiography-derived results from the BEFRI Trial. J Am Soc Echocardiogr 2016; 29:750–758. [DOI] [PubMed] [Google Scholar]
- 38. Mitchell A, Misialek JR, Folsom AR, Duprez D, Alonso A, Jerosch-Herold M, Sanchez OA, Watson KE, Sallam T, Konety SH. Usefulness of N-terminal pro-brain natriuretic peptide and myocardial perfusion in asymptomatic adults (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2015; 115:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shemisa K, Bhatt A, Cheeran D, Neeland IJ. Novel biomarkers of subclinical cardiac dysfunction in the general population. Curr Heart Fail Rep 2017; 14:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, Bluemke DA, Lima JA, Venkatesh BA. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson 2015; 17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]