Wide availability of the 3 vaccines approved by the U.S. Food and Drug Administration for emergency use against SARS-CoV-2 has led to reports of adverse reactions not seen during clinical trials: We now report a series of patients who developed CMR-proven myocarditis shortly after vaccination.
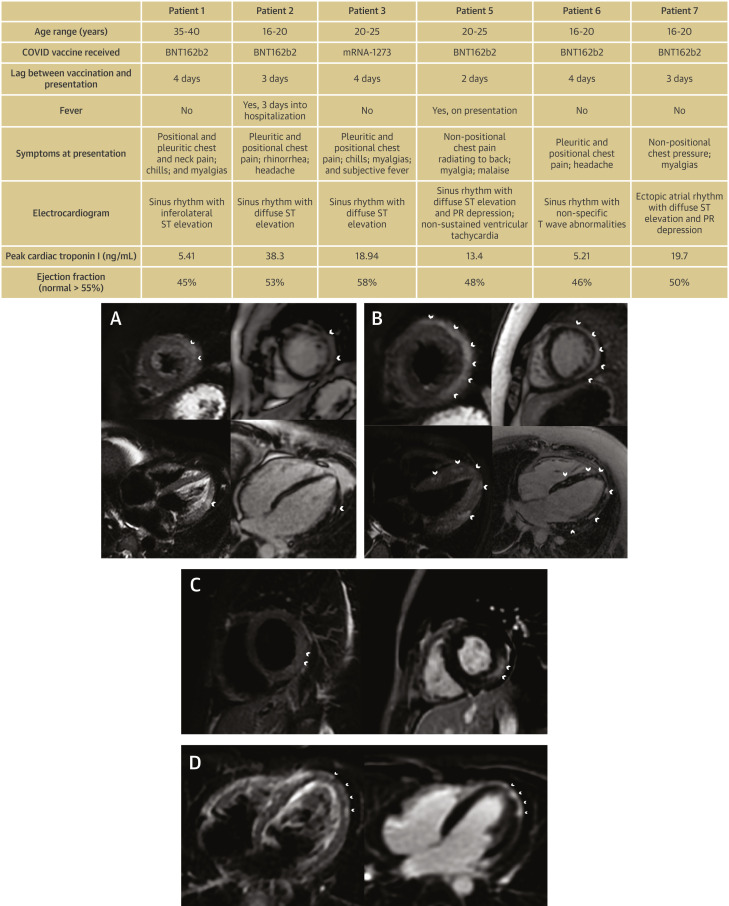
Six previously healthy men (17-37 years of age) with no infectious prodrome developed severe chest pain and elevated troponin I within 2 days-4 days of their second vaccination (Figure 1 ). Five patients had ST-segment elevation on presentation, with 4 demonstrating no coronary artery obstruction. All patients had negative nasopharyngeal SARS-CoV-2 PCR testing. CMR revealed patchy midmyocardial increased T2 signal with corresponding late gadolinium enhancement consistent with the acute inflammation of myocarditis (Figure 1). Five patients had abnormal left ventricular systolic function. None of the patients developed any other complications, and all were discharged home.
Figure 1.
Clinical Characteristics and Cardiac Magnetic Resonance Imaging of Patients Following SARS-CoV-2 Vaccination
(Top) Clinical characteristics of patients with myocarditis following SARS-CoV-2 vaccination. (Bottom) Cardiac magnetic resonance of myocarditis following vaccination. In each panel, T2-weighted sequences are on the left and late gadolinium (LGE) sequences are on the right. (A) Patient 1: short-axis and 4-chamber views demonstrating areas of increased T2 signal and LGE in the midwall of the lateral segments (arrowheads) in a patient who received their second SARS-CoV-2 vaccination 5 days earlier. (B) Patient 2: short-axis and 4-chamber views demonstrating increased T2 signal and LGE in the midwall and subepicardial layer throughout the left ventricle (arrowheads) in a patient who received their second SARS-CoV-2 vaccination 7 days earlier. (C) Patient 3: short-axis views demonstrating increased T2 signal and LGE in the mid wall and subepicardial layer of the mid-posterolateral segment (arrowheads) in a patient who received their second SARS-CoV-2 vaccination 6 days earlier. (D) Patient 6: 4-chamber view demonstrating areas of increased T2 signal and LGE in the subepicardial apical and apical lateral segments (arrowheads).
Large clinical trials of both BNT162b2 and mRNA-1273 in more than 70,000 individuals in the United States showed good safety profiles for both of the mRNA-based vaccines and no reports of myocarditis (1,2). However, myocarditis has been described after other vaccinations, such as seasonal influenza (3) and smallpox (4) and regulatory agencies are evaluating the risk of COVID-19 vaccine-associated myocarditis based on post-Emergency Use Authorization reports. CMR findings in patients with suspected COVID-19 vaccine-associated myocarditis have not been well described in published reports, and our report tries to document some of these changes. Although the clinical presentation, CMR findings, and temporal association strongly suggest the possibility of vaccine-associated myocarditis in our 6 patients, we cannot conclude definitively that COVID-19 vaccine was causative or that other etiologies for myocarditis can be definitively excluded in our patients. Nevertheless, clinicians should be suspicious of myocarditis in recently vaccinated patients with symptoms consistent with this diagnosis.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng M.P., Kozoriz M.G., Ahmadi A.A., Kelsall J., Paquette K., Onrot J.M. Post-vaccination myositis and myocarditis in a previously healthy male. Allergy Asthma Clin Immunol. 2016;12:6. doi: 10.1186/s13223-016-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keinath K., Church T., Kurth B., Hulten E. Myocarditis secondary to smallpox vaccination. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223523. [DOI] [PMC free article] [PubMed] [Google Scholar]