Abstract
Background
the aim of this review was to analyze the implementation and impact of remote home monitoring models (virtual wards) for confirmed or suspected COVID-19 patients, identifying their main components, processes of implementation, target patient populations, impact on outcomes, costs and lessons learnt.
Methods
we carried out a rapid systematic review on models led by primary and secondary care across seven countries (US, Australia, Canada, The Netherlands, Ireland, China, UK). The main outcomes included in the review were: impact of remote home monitoring on virtual length of stay, escalation, emergency department attendance/reattendance, admission/readmission and mortality. The search was updated on February 2021. We used the PRISMA statement and the review was registered on PROSPERO (CRD: 42020202888).
Findings
the review included 27 articles. The aim of the models was to maintain patients safe in the appropriate setting. Most models were led by secondary care and confirmation of COVID-19 was not required (in most cases). Monitoring was carried via online platforms, paper-based systems with telephone calls or (less frequently) through wearable sensors. Models based on phone calls were considered more inclusive. Patient/career training was identified as a determining factor of success. We could not reach substantive conclusions regarding patient safety and the identification of early deterioration due to lack of standardized reporting and missing data. Economic analysis was not reported for most of the models and did not go beyond reporting resources used and the amount spent per patient monitored.
Interpretation
future research should focus on staff and patient experiences of care and inequalities in patients’ access to care. Attention needs to be paid to the cost-effectiveness of the models and their sustainability, evaluation of their impact on patient outcomes by using comparators, and the use of risk-stratification tools.
Keywords: Remote home monitoring, Virtual wards, COVID-19, SARS-CoV-2, Silent hypoxia, Rapid systematic review
Research in context.
Evidence before this study
Remote home monitoring models for other conditions have been studied, but their adaptation to monitor COVID-19 patients and the analysis of their implementation constitute gaps in research.
Added value of this study
The review covers a wide range of remote home monitoring models (pre-hospital as well as step-down wards) implemented in primary and secondary care sectors in eight countries and focuses on their implementation and impact on outcomes (including costs).
Implications of all the available evidence
The review provides a rapid overview of an emerging evidence base that can be used to inform changes in policy and practice regarding the home monitoring of patients during COVID-19. Future research should focus on the cost-effectiveness of the models and their sustainability, their impact on patient outcomes, and the use of risk-stratification tools.
Alt-text: Unlabelled box
1. Introduction
COVID-19 has rapidly spread across the world, leading to high rates of mortality and unprecedented pressure on healthcare systems. Delays in the presentation of patients with COVID-19 has led to patients arriving as emergencies with very low oxygen saturation, often without accompanying breathlessness (‘silent hypoxia’) [1]. These delayed presentations of severe COVID-19 lead to extended hospital admissions for patients, often requiring invasive treatment and potential admission to intensive care units (ICU) or death [2]. Remote home monitoring models (sometimes referred to as ‘virtual wards’) have been established to: (1) avoid unnecessary hospital admissions (appropriate care at the appropriate place), and (2) escalate cases of deterioration at an earlier stage to avoid invasive ventilation and ICU admission [3]. Some of these models have integrated the use of pulse oximetry to monitor oxygen levels and identify and treat cases of ‘silent hypoxia’ [2].
Remote home monitoring models have been implemented in the US, Australia, Canada, the Netherlands, Ireland, China and UK, with some variation in the frequency of patient monitoring, modality (a combination of telephone or video calls and use of applications or online portals), patient admission criteria, staffing used for patient monitoring and level of clinical oversight, and use of pulse oximetry [[4], [5], [6], [7], [8]].
There is a paucity of published literature on the models of care developed to implement remote home monitoring across different healthcare contexts during the COVID-19 pandemic, the experiences of staff implementing these models and patients receiving care, the use of data for monitoring progress, resources required, as well as the impact of these models on clinical, process and economic outcomes. The aim of this review was to address these gaps by identifying the nature and scale of remote home monitoring models implemented during COVID-19, their main components, processes of implementation, target patient populations and lessons learned. We sought to analyze and interpret evaluations of these models and their outcomes.
2. Methods
2.1. Design
We followed the review method proposed by Tricco et al. [9]. The rapid review method follows a systematic review approach but proposes adaptations to some of the steps to reduce the amount of time required to carry out the review. We used a large multidisciplinary team to review abstracts and full texts, and extract data; in lieu of dual screening and selection, a percentage of excluded articles was reviewed by a second reviewer, and software was used for data extraction and synthesis [9].
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [10] to guide the reporting of the methods and findings. The review protocol was registered with PROSPERO (CRD: 42020202888, registered 6 August 2020).
2.2. Research questions
The review sought to answer the following questions:
-
(1)
What are the aims and designs of remote home monitoring models?
-
(2)
What are the main stages involved in delivering remote home monitoring for COVID-19?
-
(3)
Which patient populations are considered appropriate for remote monitoring?
-
(4)
How is patient deterioration determined and flagged?
-
(5)
What are the expected outcomes of implementing remote home monitoring?
-
(6)
What is their impact on outcomes and costs?
-
(7)
What are the benefits and limitations of implementing these models?
2.3. Search strategy
We used a phased search approach [9] guided by the PICO framework (see Appendix 1). We carried out a series of search phases where we gradually added search terms based on the keywords used in the literature we identified. Appendix 1 includes the strategies used for each search phase, including the final search strategy. We searched for literature indexed in the following databases: MEDLINE, CINAHL PLUS, EMBASE, TRIP, medRxiv and Web of Science. Initial searches were carried out by CV on 9 July 2020 and updated on 21 August 2020, 21 September 2020 and 5 February 2021. Results were combined into Mendeley and duplicates were removed. The reference lists of included articles were manually screened to identify additional relevant publications.
2.4. Study selection, inclusion and exclusion criteria
One researcher (CVP) screened the articles in the title phase, and additional researchers (KS) cross-checked exclusions in the abstract and full-text phases (KS, MS). Disagreements were discussed until consensus was reached. The inclusion criteria used for study selection was: (1) focus on the monitoring of confirmed or suspected patients with COVID-19), (2) focus on pre-hospital monitoring, monitoring after Emergency Department (ED) presentation and step-down wards for early discharge, (3) focus on monitoring at home (excluding monitoring done while the patient is in healthcare facilities), and (4) published in English. Due to the rapidly expanding evidence-base on COVID-19, we included a wide range of publications (i.e. feature articles, descriptions of services, preprints) and did not limit the selection to evaluations of remote home monitoring. We excluded articles that did not focus on remote monitoring, were not aimed at monitoring patients with COVID-19 or were not published in English.
2.5. Data extraction and management
Data extraction was carried out using a form developed in REDCap (Research Electronic Data Capture) that included: the design and general characteristics of the model (triage process, patient reporting tools, patient monitoring tools), patient populations (patient number, mean age, comorbidities), main reported process and clinical outcomes (virtual length of stay, escalation, ED attendance, admission, mortality) and its potential economic impact. In the review protocol, we had indicated that we would also be extracting data on resting pulse oximeter readings, but we could not include this information in the data extraction form due to missing data in the articles. The form was developed after the initial screening of full-text articles. It was then piloted independently by two researchers using a random sample of five articles (CV and KS). Disagreements were discussed until consensus was reached. The data extraction form was finalized based on the findings from the pilot. Data extraction was cross-checked by three researchers (TG, CSJ and ST).
2.6. Data synthesis
Data were exported from REDCap and the main article characteristics were synthesized. The information entered in free text boxes was exported from REDCap and analyzed using framework analysis [11]. The initial categories for the framework were informed by our research questions but we were also sensitive to topics emerging from the data.
2.7. Quality assessment
Due to the descriptive nature of the articles and limited data in relation to study design, we did not assess the quality of the studies.
2.8. Role of the funding source
The funders had no role in study design, data collection and analysis and decision to publish the manuscript. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HS&DR, NIHR, NHS or the Department of Health and Social Care. All members of the research team had access to the data. The research team developed and decided to submit the manuscript for publication.
3. Results
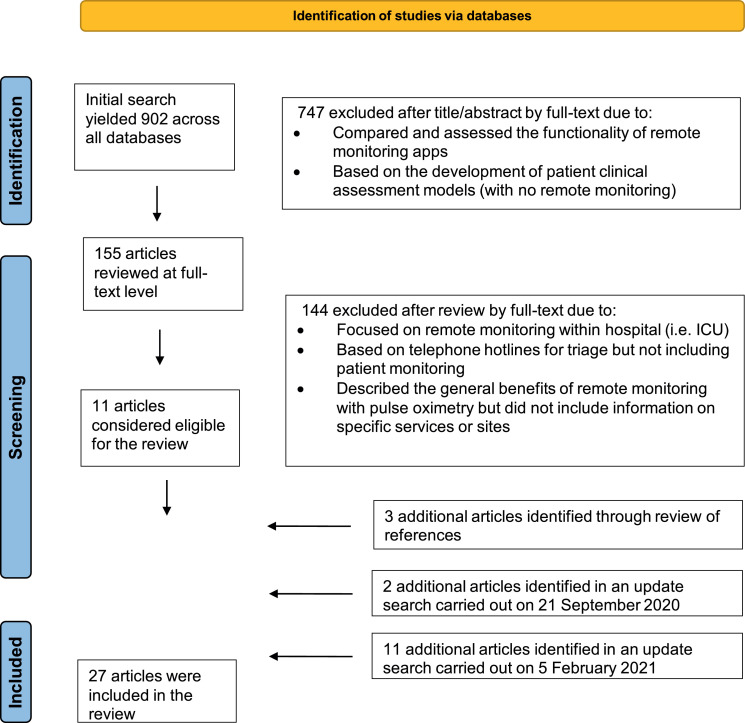
The initial search yielded 902 articles (Fig. 1). These were screened based on the title and abstract and type of article, resulting in 155 articles for full-text review. Full-text review of these articles led to 11 articles that met the inclusion criteria (reasons for exclusion can be found in Fig. 1). Three additional articles were identified by reviewing the bibliography, two articles were identified in an updated search carried out on 21 September 2020, and eleven articles were added in an updated search carried out on 5 February 2021, ultimately leading to 27 articles included in the review. We excluded articles that focused on monitoring that took place within hospital settings (i.e. ICU) or for other non-COVID-19 related conditions.
Fig. 1.
Study selection process.
3.1. Characteristics of the included remote home monitoring models
Eleven of the articles described remote home monitoring models implemented in the US, eight in the UK, two in Canada, two in the Netherlands and one each in China, Ireland, Brazil and Australia. Twelve of the articles described the service, six were identified as evaluations, seven as observational studies, one as a feasibility study and one (containing the example of two models) was a news feature (with a limited description of the services). Seventeen of the examples were published in peer-reviewed journals, nine were published in the form of preprints and one was a published conference abstract. The main characteristics of the included remote home monitoring examples are summarized in Appendix 2.
3.2. Aims and main designs of remote home monitoring models
The primary aim of the remote home monitoring models was to enable the early identification of deterioration for patients self-managing COVID-19 symptoms at home (including those who had not been admitted to hospital as well as those who had been discharged). The programme theory guiding these models was that if patients were able to take the required regular observations whilst remaining at home and communicate these to the healthcare professionals responsible for their care, then cases of deterioration could be identified early and acted upon. These actions could include changing their treatment protocol, referring them to primary care or to the emergency department for assessment and potential admission to hospital. A secondary aim of the models was their use to reduce the rate of hospital infection and demand for beds in the acute care sector, where admission to hospital could be prevented for patients considered suitable to be managed at home and those who had been admitted to hospital could be discharged earlier but continue under the remote care of a medical team (a team that varied in composition depending on the model).
Most of the remote home monitoring models included in the review (23 examples) were led by teams in secondary care. Three examples were primary care led and two were led by both secondary and primary care. Thirteen of the models functioned as pre-admission wards, in the sense that they sought to prevent the admission of patients to hospital or to identify cases of deterioration early (so those who should be referred could be admitted to hospital with lower rates of acuity). Five of the models functioned as “step-down” wards, that is, they were designed for patients who had been admitted to hospital (including ICU) where the medical team had identified that they could be discharged and safely monitored at home until their symptoms improved. Ten models functioned as pre-admission and step-down wards, organized according to two separate pathways.
3.3. Patient populations considered appropriate for remote monitoring
Most of the models established broad criteria for patient eligibility, defining the patient group as adult (over 18 yrs.) patients with COVID-19 symptoms (suspected and confirmed cases). Six of the models limited referrals to COVID-19 cases confirmed through testing [6,[12], [13], [14], [15]]. The model described by Hutchings et al. [6] excluded patients over 65 years with significant comorbidities. Shah et al.[16] excluded pregnant women and only included patients with SpO2 above 92% at initial assessment. We did not find any examples targeting socially and economically disadvantaged groups (although some models included support from social workers and mental health professionals) [4,17]. It is important to highlight that the size of the patient cohorts varied considerably (see Table 1 for patient numbers) and ranged from 12 patients to 6853. The models with the highest numbers of patients were implemented in the US. The comorbidities mentioned with greater frequency were hypertension, asthma and obesity.
Table 1.
Main characteristics of monitored patients.
| Type of model | Number patients | Mean age | Most common comorbidities | |
|---|---|---|---|---|
| Agarwal [17] | Pre-admission | 97 | Inconsistencies in reporting in the article (48.6, 43.6 and 43.8) | Asthma, hypertension, dyslipidemia and anxiety |
| Annis [8] | Pre-admission | 2255 | median 38* | NS |
| Grutters [26] | Step-down | 33 | 57 | NS |
| Margolius [4] | Pre-admission | 4213 | 42 | NS |
| Lam [20] | Pre-admission | 50 | median 44 | hypertension, malignant disease |
| Medina [13] | Pre-admission and step-down | 878 | NS | NS |
| O'Keefe [12] | Pre-admission | 496 | 47.6 | hypertension, obesity, asthma diabetes |
| Shah [16] | Pre-admission | 77 | median 44 | obesity and hypertension |
| Xu [21] | Pre-admission | 48 | median 37.5 | NS |
| Hutchings [6] | Pre-admission | 162 | median 38 | NS |
| Kricke [7] | Pre-admission | 6835 | 47** | NS |
| Ford [14] | Pre-admission and step-down | 154 | NS | NS |
| Maghrabi [18] | Step-down | 300 | 57 | Hypertension |
| Thornton[5] | Pre-admission and step-down | Example 1: 1042 Example 2: 244 | NS | NS |
| Morgan[28] | Pre-admission and step-down | 2348 | 40-49 | NS |
| O'Carroll [15] | Step-down | 18 | median 48 | NS |
| Bell [19] | Pre-admission | 192 | median 43 | NS |
| Gaeta [27] | Pre-admission | 488 | NS | NS |
| Gordon [37] | Step-down | 225 | Median 54 | NS |
| Kodama [25] | Step-down | 50 | NS | NS |
| Nunan [22] | Pre-admission and step-down | 273 | median 50.3 | NS |
| Pereira [38] | Pre-admission | 12 (COVID-19+ve) | 37.2 | Obesity, hypertension |
| Silven [39] | Pre-admission and step-down | 55 | NS | NS |
| Francis [40] | Pre-admission and step-down | 900 | 54.9 | Diabetes, Asthma |
| Vindrola-Padros [24] | Pre-admission and step-down | 2084 | NS | NS |
| Wilcock [23] | Pre-admission | 41 | 45.9 | NS |
| Clarke [41] | Pre-admission and step-down | 908 | 54 | NS |
*For the subset of 1496 patients who completed the programme; ** for a subset of 6,006 who completed a survey
NS=not specified
3.4. Stages of remote home monitoring
The articles described five main stages in remote home monitoring for COVID-19: (1) referral and triage to determine eligibility, (2) onboarding of patient to remote home monitoring service (provision of information to patient and/or career on monitoring process, mechanisms for escalation and self-care), (3) monitoring (including recording of observations, communication of the information, assessment of the information by the medical team), (4) escalation (if required), and (5) discharge from the pathway.
Patient information recorded at triage included: Patient demographics (age, sex, race/ethnicity, insurance type in the models in the US); Clinical variables (clinical signs and symptoms, medical history and medications); Health data for risk assessment and vital signs data (body temperature, heart rate, respiratory rate and oxygen saturation).
Three studies included some degree of detail in relation to the categorization of patients in relation to risk [14,17,18,19]. O'Keefe and colleagues [12] described and evaluated a risk assessment model based on age, medical history and symptom severity. This model was able to identify the need for hospitalization in initially non-severe COVID-19 patients.
In ten of the examples included in the review, monitoring was based on patient record of observations using a paper-based system and then communicating the information to a member of the medical team by telephone (see Appendix 2). Twelve of the examples relied on the use of an online mechanism, either through an app or online form. Three examples offered patients a telephone or an app option [20]. Another example relied on the use of wearable sensors to continuously monitor temperature readings and transfer these to the medical team [6]. Twenty of the models relied on the use of pulse oximetry from the beginning of implementation, four models did not use pulse oximetry, one model added pulse oximetry three weeks after implementation and two articles indicated that the use of pulse oximetry was being considered in the near future.
Escalation was actioned depending on pre-established thresholds. Not all articles have reported thresholds for escalation and most only refer to the worsening of symptoms. Shah et al. [16] indicated that patients on their remote home monitoring pathway were flagged as deteriorating if reporting SpO2 below 92% after a double reading. Xu et al. [21] used a SpO2 reading of below 93% or BP less than 90/60 mmHg. Some of the examples included in the review established safety-netting options in cases when patients could not be reached via phone such as calling the police so they could visit the patient at home [6].
Most patients were followed-up until their symptoms improved or the patient opted out of the pathway. Medina et al. [13] reported following up patients on the step-down pathway for 7 days post-discharge from hospital and those on the pre-admission pathway for 14 days. Shah et al. [16] followed-up patients on their pre-admission pathway for 7 days. Hutchings et al. [6] referred patients to their GP for follow-up after discharging them from the remote home monitoring pathway.
3.5. Expected outcomes of implementing remote home monitoring
The outcomes recorded in each remote home monitoring model are listed in Appendix 2. They can be grouped in three main categories: (1) process outcomes related to the remote home monitoring pathway, (2) process outcomes related to secondary care and (3) patient outcomes (including clinical and experience). Process outcomes related to the remote home monitoring pathway included: time from swab to assessment, time to escalation and ambulance attendance/emergency activation (i.e. calling 999 or 911). Process outcomes related to secondary care included length of stay. Outcomes considered at the patient level included: emergency department attendance/reattendance, hospital admission, ICU admission, readmission, mortality, ventilation or non-invasive ventilation needs, and patient satisfaction.
3.6. Impact on outcomes
It was difficult to carry out an analysis of the impact of remote home monitoring across all examples because not all articles reported data on the same outcomes (Table 2). Mortality rates were low, admission or readmission rates ranged from 0 to 29%, and ED attendance or reattendance ranged from 4 to 36%. Six of the models reported data on patient feedback, with high satisfaction rates [5,8,18,22,23,24,25,26].
Table 2.
Impact of remote home monitoring on selected outcomes.
| virtual LoS | Escalation | ED attendance/ reattendance | Admission/ readmission | Mortality | |
|---|---|---|---|---|---|
| Agarwal [17] | 8 days (median) | 5.10% | 4.2% | 0 | NS |
| Annis [8] | NS | NS | 4.0% | 0.6% | NS |
| Grutters* [26] | 13 days (mean) | 18 patients reassessed in hospital | NS | 9%** | 0 |
| Margolius* [4] | NS | NS | 7% | 1% | NS |
| Lam [20] | 12.5 days (only for 52% of patients) | 12% | NS | 8% | 0 |
| Medina [13] | NS | 10% | NS | 2%, 3%** | 9 patients but denominator is unclear |
| O'Keefe [12] | 13.1 days | NS | NS | 7.1% | NS |
| Shah [16] | NS | 25% | 36% | 29% | 2.6% |
| Xu [21] | NS | NS | NS | NS | 0 |
| Hutchings [6] | 8 days (only for 62 of the patients in the sample) | 5 patients | 2.5% | 1.9% | 0 |
| Kricke [7] | NS | NS | 7.7% | NS | NS |
| Ford [14] | NS | 14.3% referred to physician review; 3.9% physician to patient call; 2.6% to ED and admitted | 2.6% | 2.6% | NS |
| Maghrabi [18] | 3.5 days (median) | NS | 13% | 9%** | 0.66% |
| Thornton (for example 2) [5] | NS | NS | 11.9% | 7.4% | 0 |
| Morgan [28] | 12.7 days (mean) | 16.9% escalated to nurse review | 7.9% | 3.4% | NS |
| O'Carroll [15] | 12 days (median) | NS | NS | 4 patients | NS |
| Bell [19] | NS | NS | 16.7% | 3.6% | 0 |
| Gaeta [27] | NS | NS | 18.4% | 8.8% | 1.2% |
| Gordon [37] | unclear | NS | 4.9% | 1.3% | NS |
| Kodama [25] | NS | 26% | 6%**** | 2%**** | NS |
| Nunan [22] | NS | NS | 11.4% | 7.0% | 0.4% |
| Pereira [38] | NS | NS | NS | NS | NS |
| Silven [39] | NS | NS | NS | 9% | 0 |
| Francis [40] | NS | NS | NS | 8.1% | 2.0% |
| Vindrola-Padros [24] | NS | 10.4% | 8.3% | 6.4% | 1.1% |
| Wilcock [23] | 10.3 days (mean) | NS | NS | 7.3% | 1.9% *** |
| Clarke [41] | NS | NS | 5.7% | 4.4% | 3.1% |
*included data for patients on remote home monitoring pathway, **refers to readmission in cases of step-down, wards, ***of 52 initially recruited, **** these refer to very low patient numbers. LoS=length of stay, ED= emergency department, NS= not specified or not able to calculate based on data reported in the manuscript.
Remote home monitoring process outcomes were only included in six of the articles, with time from swab to assessment ranging from 2 to 3.7 days [12,17,20] and virtual length of stay from 3.5 days to 13 days (see Table 2). Only one article presented findings on reduction in length of stay, calculated at 5 days fewer per patient [26].
3.7. The economic impact
Very few of the selected studies for this rapid review provided a descriptive form of economic analysis, though some of them mentioned the potential for cost savings based on the utilization of virtual monitoring programs for other treatments in similar settings [14,22,26]. The study by Nunan et al. [22] found that setting up remote oximetry monitoring at the Royal Berkshire Hospital resulted in cost avoidance (in terms of bed days, saturation probes and staffing wages) that amounted to £107,600 per month. The amount spent per patient on remote monitoring varied by country and type of costs included in the analysis. The study from Gaeta et al. [27] reported a total cost of $621.8K (equivalent of £485.0K using purchasing power parity) for 621 COVID-19 patients that were monitored using outpatient telehealth follow-up in the Brooklyn Methodist Hospital. These costs included also costs of inpatient follow-up and averaged at £781.0 per monitored patient, whereas the mean cost per monitored patient reported in England varied from £400 to £553 for step-down and pre-hospital models respectively [24]. Some of the selected studies highlighted the fact that, during the pandemic, the intervention used existing resources and staff that were made available due to the emergency situation [7,12,14,28]. However, they also highlighted that, with the return to normal workloads in the health care system, a question of allocation of resources and sufficient staffing still remains.
4. Discussion
In this article, we have sought to make a contribution to the rapidly growing evidence-base on the use of remote home monitoring models for patients with confirmed or suspected COVID-19. The review has pointed to factors that need to be taken into consideration in relation to the design of these models. Most of the articles included in the review were led by secondary care but some authors argued that coordination between primary and secondary care could facilitate the implementation of remote home monitoring pathways [5,7,13]. Primary care led models might be more adaptable to evolving patient and system needs and easier to replicate in contexts with limited secondary care access and capacity [17]. Three articles reported on models that integrated mental health and social care support during and after patient monitoring, highlighting a wide range of patient needs [6,13,17].
Even though several of the examples used apps and other types of online platforms, discussions in relation to the use of health technology were limited. The use of apps for monitoring allowed the follow-up of a higher number of patients (compared to paper-only models) but some of the studies indicated that models based on telephone calls were more inclusive (i.e. including patients without internet access or technological literacy) [19]. Patient experience was captured in some of the articles we reviewed [8,26] but the analysis was limited. An analysis of patient experience and engagement is important as the literature on the use of remote patient monitoring for other conditions has demonstrated that higher levels of patient engagement with remote patient monitoring technology are associated with better patient outcomes [29].
Similarly, to other reviews on remote patient monitoring in other conditions, another limitation was the lack of attention placed on the implementation of the models and the failure to identify the programme theories guiding their design, factors that acted as barriers and facilitators and the extent to which the pathways were implemented according to their original plans [30]. This could be due to the limited evidence on COVID-19 and the management of patients with this disease at the time of designing and implementing these models as well as the general limited use of programme theories in the design of healthcare interventions that has already been documented in the literature [31].
Emerging international evidence has indicated that lower thresholds for oxygen saturation are associated with worse patient outcomes [2,32]. In the case of our review, even though some authors argued that pulse oximetry identified the need for hospitalization when using a cut-off of 92% [16], we could not reach conclusions in relation to patient safety and the degree to which remote home monitoring models can conclusively identify cases of deterioration at an earlier stage in the disease trajectory. The main reasons were lack of standardized reporting across articles in relation to these outcome measures and how these were measured, as well as the limitation that none of the articles used comparators.
Issues with using pulse oximetry were also highlighted, including: patient physiological measures needed to be recorded several times a day to correctly identify cases of deterioration, some remote home monitoring examples used standardized home pulse oximeters to avoid variability between different brands, pulse oximetry readings were made less accurate by nail polish, severe anemia, hyperbilirubinemia, hemoglobinopathies, or poor peripheral perfusion from severe vasoconstriction or poor cardiac output [16,33]. Some authors also argued that patient training was a key determining factor of the success of health information technology as it ensured readings and other observations were carried out accurately [6]. Remote home monitoring needed to be seen as an approach to maintain patients safe in the right setting, rather than as an admission avoidance model.
Remote home monitoring for COVID-19 patients was expected to have a positive economic impact, mainly due to cost savings in staff time and personal protective equipment (PPE) utilization, avoidance of infection of frontline medical staff and reduced hospitalizations [14,21]. However, the economic evidence in relation to these was limited. Very few of the selected studies included a simple descriptive form of economic analysis which included the cost per patient and the cost avoidances of using remote monitoring for patients with COVID-19. The selected studies have, however, raised the issue of resource allocation and funding, especially when it comes to the continuity of such programs after the first emergency situation. Most of the staff who worked on remote monitoring interventions for COVID-19 came from other services and the resources used were already existing. Yet, with the return to normal workloads, providing sufficient staff and enough resources may become a problem. Previous studies have indicated that remote monitoring in itself has contributed to increased efficiency in the use of resources (such as reduction in length of stay, increasing bed availability without compromising patient care safety, etc.) [15,21]. A cost-effectiveness analysis combining the results from the cost analyses with the quality-adjusted life years (QALYs) gained for remote home monitoring models for COVID-19 patients could help to inform decision making on adopting these models on a wider scale. This economic analysis would need to include costs and benefits beyond the actual remote home monitoring models considering a wider (e.g., NHS and social services or a societal) perspective, a valid control group, as well as a longer follow-up period.
This review has a series of limitations. The last search was carried out on 5 February 2021, so any articles published after this date were not included. We only included articles published in English, therefore potentially missing relevant information published in other languages. Furthermore, although we employed multiple broad search terms, it is possible that we missed articles that did not use these terms. Due to the variability in study designs, the descriptive nature of the articles and the combination of peer-reviewed and grey literature, we did not assess these for quality using standardized tools for assessment. We feel it is important to note that we found several cases of missing data and inconsistencies in the reporting of evaluations that would lead to low quality ratings. It would be important to carry out a full quality assessment of the evidence base on the remote monitoring of COVID-19 patients in the near future.
Despite the low quality of the data reported in the articles included in the review, the findings have shed light on important trends in the implementation of these models around the world, including demonstrating the fact that these models can be set-up rapidly to respond to the pressures of a pandemic. Furthermore, the review has pointed to areas that need to be explored further in the future. These could include an analysis of patient experience, beyond measures of satisfaction and the exploration of potential inequalities in patients’ access to remote home monitoring models or patients’ difficulties interacting with technology. Technological barriers have been reported in other studies of remote home monitoring and should not be overlooked when exploring the experiences of patients with COVID-19 [34,35].
Additional attention needs to be paid to the processes used to implement these models and how these might vary based on the healthcare sector, patient population, size, wave of the pandemic and approaches used for triage, monitoring and escalation. These models can also be expanded to monitor patients remotely for other conditions, in connection with COVID-19 as well as beyond. As mentioned earlier, primary care might need to play a more central role in the coordination of remote patient monitoring models, providing more holistic care for patients and reducing the demand on hospital services [36]. The evaluation of remote home monitoring, considering its impact on patient outcomes through the use of comparators is also required. We also need to consider the sustainability of these models during multiple epidemiological peaks, compare different approaches to remote home monitoring and assess their cost-effectiveness.
Funding
The study was funded by the National Institute for Health Research-NIHR (Health Services and Delivery Research, 16/138/17 – Rapid Service Evaluation Research Team; or The Birmingham, RAND and Cambridge Evaluation (BRACE) Centre Team (HSDR16/138/31).
Contributors
NJF, CSJ, TG, KS, MS, SMT and CVP contributed to the conceptualisation of the study. MS, KS, CSJ, TG, SMT, NC and CVP participated in the data curation, analysis and validation. NJF, MIK, AS and KK reviewed and provided feedback on the manuscript. All authors approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
All of the relevant data are included in the manuscript and supplementary files.
Declaration of Competing Interest
Mr Georghiou reports grants from National Institute for Health Research, NIHR, during the conduct of the study. All the other authors declare no conflict of interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100965.
Appendix. Supplementary materials
References
- 1.Levitan R.M. Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia. Acad Emerg Med. 2020;27(8):785–786. doi: 10.1111/acem.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal D. Oxygen and mortality in COVID-19 pneumonia: a comparative analysis of supplemental oxygen policies and health outcomes across 26 countries. Front Public Health. 2021 doi: 10.3389/fpubh.2021.580585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NHS. Pulse oximetry to detect early deterioration of patients with COVID-19 in primary and community care settings, 2020.
- 4.Margolius D., Hennekes M., Yao J., Einstadter D., Gunzler D., Chehade N., Sehgal A.R., Tarabichi Y., Perzynski A.T. On the front (phone) lines: results of a COVID-19 hotline. J Am Board Family Med. 2021;34(Supplement):S95–102. doi: 10.3122/jabfm.2021.S1.200237. Feb 1. [DOI] [PubMed] [Google Scholar]
- 5.Thornton J. The “virtual wards” supporting patients with covid-19 in the community. BMJ British Med J. 2020:369. doi: 10.1136/bmj.m2119. [DOI] [PubMed] [Google Scholar]
- 6.Hutchings O.R., Dearing C., Jagers D., Shaw M.J., Raffan F., Jones A., Taggart R., Sinclair T., Anderson T., Ritchie A.G. Virtual health care for community management of patients with COVID-19 in Australia: observational cohort study. J Med Internet Res. 2021;23(3):e21064. doi: 10.2196/21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kricke G. Rapid implementation of an outpatient Covid-19 monitoring program. NEJM. 2020 doi: 10.1056/CAT.20.0214. [DOI] [Google Scholar]
- 8.Annis T., Pleasants S., Hultman G. Rapid implementation of a COVID-19 remote patient monitoring program. J Am Med Inform Assoc. 2020;27(8):1326–1330. doi: 10.1093/jamia/ocaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tricco A., et al. . Rapid reviews to strengthen health policy and systems: a practical guide, 2017. [DOI] [PMC free article] [PubMed]
- 10.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gale N.K., Heath G., Cameron E. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methods. 2013;13(1):117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Keefe J.B., Tong E.J., Taylor Jr T.H., O'Keefe G.A., Tong D.C. Use of a telemedicine risk assessment tool to predict the risk of hospitalization of 496 outpatients with COVID-19: retrospective analysis. JMIR Public Health Surveill. 2021;7(4):e25075. doi: 10.2196/25075. Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina M., Babiuch C., Card M. Home monitoring for COVID-19. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc028. [DOI] [PubMed] [Google Scholar]
- 14.Ford D., Harvey J.B., McElligott J. Leveraging health system telehealth and informatics infrastructure to create a continuum of services for COVID-19 screening, testing, and treatment. J Am Med Inform Assoc. 2020 doi: 10.1093/jamia/ocaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll O., MacCann R., Reilly A. Remote monitoring of oxygen saturation in individuals with COVID-19 pneumonia. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.01492-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah S., Majmudar K., Stein A. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med. 2020;27(8):681–692. doi: 10.1111/acem.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal P., Mukerji G., Laur C., Chandra S., Pimlott N., Heisey R., Stovel R., Goulbourne E., Bhatia R.S., Bhattacharyya O., Martin D. Adoption, feasibility and safety of a family medicine–led remote monitoring program for patients with COVID-19: a descriptive study. CMAJ Open. 2021;9(2):E324. doi: 10.9778/cmajo.20200174. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maghrabi F., Bazaz R., Wilson E., et al S57 The development and implementation of a virtual discharge ward for patients with COVID-19 pneumonia: data on the first 300 patientsThorax 2021;76:A35-A36.
- 19.Bell L.C., Norris-Grey C., Luintel A., Bidwell G., Lanham D., Marks M., Baruah T., O'Shea L., Heightman M., Logan S. Implementation and evaluation of a COVID-19 rapid follow-up service for patients discharged from the emergency department. Clinical Medicine. 2021;21(1):e57. doi: 10.7861/clinmed.2020-0816. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam P.W., Sehgal P., Andany N. A virtual care program for outpatients diagnosed with COVID-19: a feasibility study. CMAJ Open. 2020;8(2):E407–EE13. doi: 10.9778/cmajo.20200069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H., Huang S., Qiu C. Monitoring and management of home-quarantined patients with COVID-19 using a WeChat-based telemedicine system: retrospective cohort study. J Med Internet Res. 2020;22(7):e19514. doi: 10.2196/19514. [published Online First: 2.7.2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunan J., Clarke D., Malakouti A., Tannetta D., Calthrop A., Xu X.H., Chan N.B., Khalil R., Li W., Walden A. Triage into the community for COVID-19 (TICC-19) patients pathway-service evaluation of the virtual monitoring of patients with COVID pneumonia. Acute Med. 2020;19(4):183–191. Jan 1. [PubMed] [Google Scholar]
- 23.Wilcock J., Grafton-Clarke C., Coulson T. What is the value of community oximetry monitoring in people with SARS-CoV-2? A prospective, open-label clinical study. medRxiv. 2021 Jan 1.
- 24.Vindrola-Padros C., Sidhu M.S., Georghiou T., Sherlaw-Johnson C., Singh K.E., Tomini S.M., Ellins J., Morris S., Fulop N.J. The implementation of remote home monitoring models during the COVID-19 pandemic in England. EClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100799. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama R., Arora S., Anand S., Choudhary A., Weingarten J., Francesco N., Chiricolo G., Silber S., Mehta P.H. Telemedicine and e-Health; 2020. Reengineering the Discharge Transition Process of COVID-19 Patients Using Telemedicine, Remote Patient Monitoring, and Around-the-Clock Remote Patient Monitoring from the Emergency Department and Inpatient Units. Dec 14. [DOI] [PubMed] [Google Scholar]
- 26.Grutters L.A., Majoor K.I., Mattern E.S.K. Home telemonitoring makes early hospital discharge of COVID-19 patients possible. J Am Med Inform Assoc. 2020:ocaa168. doi: 10.1093/jamia/ocaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaeta T., Chiricolo G., Mendoza C., Vaccari N., Melville L., Melniker L., Bove J. 124 impact of a novel telehealth follow-up protocol for at-risk emergency department patients discharged with presumptive or confirmed COVID-19. Ann Emerg Med. 2020;76(4) OctS49.28. [Google Scholar]
- 28.Morgan AU, Balachandran M, Do D, Lam D, Parambath A, Chaiyachati KH, Bonalumi NM, Day SC, Lee KC, Asch DA. Remote Monitoring of Patients with Covid-19: Design, implementation, and outcomes of the first 3,000 patients in COVID Watch. NEJM Catalyst Innovations in Care Delivery. 2020;1 [Google Scholar]
- 29.Su D., Michaud T.L., Estabrooks P. Diabetes management through remote patient monitoring: the importance of patient activation and engagement with the technology. Telemed Health. 2018;25(10):952–959. doi: 10.1089/tmj.2018.0205. [DOI] [PubMed] [Google Scholar]
- 30.Kew K.M., Cates C.J. Home telemonitoring and remote feedback between clinic visits for asthma. Cochrane Database of Syst Rev. 2016;(8) doi: 10.1002/14651858.CD011714.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidoff F., Dixon-Woods M., Leviton L. Demystifying theory and its use in improvement. BMJ Qual Saf. 2015;24(3):228. doi: 10.1136/bmjqs-2014-003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumdar S.R., Eurich D.T., Gamble J.M. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis. 2011;52(3):325–331. doi: 10.1093/cid/ciq076. [DOI] [PubMed] [Google Scholar]
- 33.Luks A.M., Swenson E.R. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17(9):1040–1046. doi: 10.1513/AnnalsATS.202005-418FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGillicuddy J.W., Weiland A.K., Frenzel R.M. Patient attitudes toward mobile phone-based health monitoring: questionnaire study among kidney transplant recipients. J Med Internet Res. 2013;15(1):e6. doi: 10.2196/jmir.2284. [published Online First: 08.01.2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seto E., Leonard K.J., Masino C. Attitudes of heart failure patients and health care providers towards mobile phone-based remote monitoring. J Med Internet Res. 2010;12(4):e55. doi: 10.2196/jmir.1627. [published Online First: 29.11.2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S. Strengthening the UK primary care response to COVID-19. BMJ. 2020;370:m3691. doi: 10.1136/bmj.m3691. [DOI] [PubMed] [Google Scholar]
- 37.Gordon W.J., Henderson D., DeSharone A., Fisher H.N., Judge J., Levine D.M., MacLean L., Sousa D., Su M.Y., Boxer R. Remote patient monitoring program for hospital discharged COVID-19 patients. Appl Clin Inf. 2020;11(05):792–801. doi: 10.1055/s-0040-1721039. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motta L.P., Silva P.P., Borguezan B.M., Amaral J.L., Milagres L.G., Bóia M.N., Ferraz M.R., Mogami R., Nunes R.A., Melo P.L. An emergency system for monitoring pulse oximetry, peak expiratory flow, and body temperature of patients with COVID-19 at home: development and preliminary application. PLoS ONE. 2021;16(3) doi: 10.1371/journal.pone.0247635. Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silven A.V., Petrus A.H., Villalobos-Quesada M., Dirikgil E., Oerlemans C.R., Landstra C.P., Boosman H., van Os H.J., Blanker M.H., Treskes R.W., Bonten T.N. Telemonitoring for patients with COVID-19: recommendations for design and implementation. J Med Internet Res. 2020;22(9):e20953. doi: 10.2196/20953. Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis N.A., Stuart B., Knight M., Vancheeswaran R., Oliver C., Willcox M., Barlow A., Moore M. Predictors of clinical deterioration in patients with suspected COVID-19 managed in a ‘virtual hospital 'setting: a cohort study. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-045356. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke J., Flott K., Crespo R.F., Ashrafian H., Fontana G., Benger J., Darzi A., Elkin S. Assessing the safety of home oximetry for COVID-19: a multi-site retrospective observational study. medRxiv. 2020 Jan 1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.