Abstract
Several copy number variants (CNVs) have been identified to confer high risk for a range of neuropsychiatric conditions. Because of advances in genetic testing within clinical settings, patients are increasingly receiving diagnoses of copy number variant genomic disorders. However, clinical guidelines surrounding assessment and management are limited. This review synthesises recent research and makes preliminary recommendations regarding the clinical evaluation of patients with neuropsychiatric risk CNVs. We recommend multi-system assessment beyond the initial referral reason, recognition of the potential need for co-ordinated multidisciplinary care, and that interventions take account of relevant multimorbidity. The frequently complex needs of patients with CNVs across the life-course pose challenges for many health care systems and may be best provided for by the establishment of specialist clinics.
Current Opinion in Genetics and Development 2021, 68:26–34
This review comes from a themed issue on Molecular and genetic basis of disease
Edited by Jennifer Gladys Mulle, Patrick F Sullivan and Jens Hjerling-Leffler
For a complete overview see the Issue and the Editorial
Available online 15th January 2021
https://doi.org/10.1016/j.gde.2020.12.012
0959-437X/Crown Copyright © 2021 Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Several copy number variants (structural deletions, duplications or translocations, >1000 bp) [1] have been robustly associated with neurodevelopmental and neuropsychiatric outcomes [2]. One of the first reports of an association between CNVs and psychiatric risk, concerned the high prevalence of psychotic disorders (30%) in adults with 22q11.2 deletion syndrome (22q11.2DS) ascertained through medical genetic clinics [3]. Advances in genomic techniques have revealed a range of pathogenic CNVs that confer psychiatric risk, often via genome-wide association studies (GWAS) that compare the frequency of CNVs in neuropsychiatric patient cohorts and controls. Through this approach risk CNVs have been identified for intellectual disability [4], ADHD [5], autism [6], schizophrenia [7,8], depression [9•] and bipolar disorder [10].
Medical Genetics services exist within many healthcare systems, offering both patient testing and counselling; CNVs are increasingly being detected in clinical settings through the use of technologies such as chromosomal microarray and exome/whole genome sequencing. Patient referrals can be received from a range of medical specialities and are triggered when a clinician suspects a genetic aetiology. In the case of neuropsychiatric risk CNVs, referral reasons often include, intellectual disability, developmental delay and childhood psychiatric and behavioural problems, but referrals are often from beyond psychiatry and cut across many specialities, including cardiology, neurology, paediatrics, endocrinology and speech and language services [11, 12, 13]. The process of receiving a genetic diagnosis can take several years, with some families describing long and distressing ‘diagnostic odysseys’ before they learn what is causing their child’s health problems [14]. A clear solution to this would be to implement genetic testing more widely (Ledbetter et al. this issue). Neuropsychiatric CNVs have been implicated in the aetiology of congenital heart abnormalities [15,16], facial dysmorphology [17, 18, 19], endocrine abnormalities [20,21], epilepsy [22,23], and anthropometric variability [24]. This highlights the pleiotropic effects of CNVs and the heterogeneity in individual outcomes.
Although neuropsychiatric CNVs as a group are relatively common, they are individually rare and almost all can be considered to be ‘orphan diseases’ (i.e. diseases affecting less than 200 000 people in the United States, equivalent to a lifetime prevalence <0.06%) [25]. Although there is increasing academic and clinical interest in CNVs generally, and the scope of research is expanding, helped in part by big data population cohort approaches to identifying outcomes [26••], some have been studied more intensively than others. In particular, the literature on 22q11.2DS is relatively large compared to many other CNVs, as it was already known to clinicians as syndromes such as DiGeorge syndrome [27] or Velo-cardio-facial syndrome [28] decades before current genomic testing technologies were available. Most of the focus in the literature to date has been on characterising clinical, behavioural and cognitive outcomes of neuropsychiatric CNVs, whereas research on therapeutic choices for these ‘orphan’ diseases is currently limited [25].
Clinical evaluation
Multimorbidity in CNV carriers frequently requires multidisciplinary care
Neuropsychiatric CNVs have pleiotropic manifestations across physical, psychiatric and cognitive domains [29••,30,31], and therefore carriers often have a history of disparate care from different medical specialties without a unifying diagnosis. Importantly, substantial evidence is now emerging that phenotypic changes across several CNVs follow a prescribed time-course, allowing interventional opportunity for clinical management [29••,32,33]. Whilst patients with neuropsychiatric CNVs can initially present to many different specialities, once a CNV has been confirmed, there is a need to consider appropriate multi-disciplinary care including access to neuropsychiatric expertise as well as the need for holistic care from relevant practitioners such as physical and occupational therapists, genetic counsellors, dieticians and social workers.
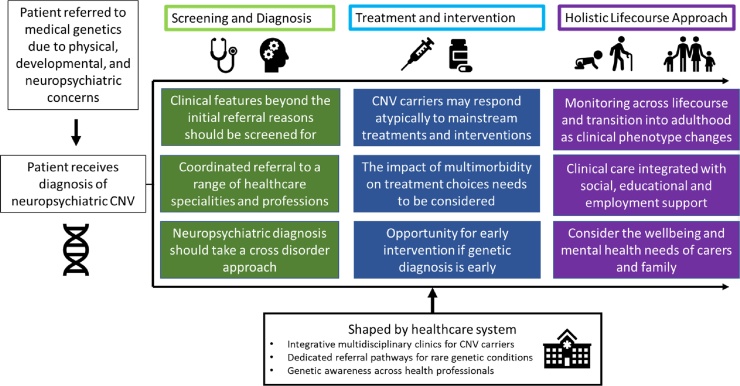
We make the following broad recommendations for clinical evaluation following a diagnosis of a neuropsychiatric CNV (see Figure 1). (1) Screening broadly for medical signs and symptoms beyond the initial referral reason, as neuropsychiatric CNVs have complex pleiotropic effects (Table 1 briefly summarises and links to further literature); (2) Treatment plans should take account of the fact that CNV carriers can have atypical reactions to common treatments and that multimorbidity may affect treatment effectiveness (hence the importance of recommendation 1); (3) Neuropsychiatric CNVs have differential effects across the life-course and hence clinical management should be age-specific.
Figure 1.
Ideal clinical examination.
Table 1.
Phenotypes of neuropsychiatric CNVs. These CNVs were selected on the basis of being robustly associated with neuropsychiatric conditions, and for being frequently diagnosed in medical genetic settings [29••,55]. It should be highlighted that the phenotypes listed below are not exhaustive, but highlight important features and link to useful references for clinicians and researchers
| Locus | Copy number change | Neuropsychiatric | Physical health | References |
|---|---|---|---|---|
| 1q21.1 | Deletion | ADHD | CHD | [69, 70, 71, 72] |
| ASD | Microcephaly | |||
| Epilepsy | Strabismus | |||
| ID | Facial dysmorphia | |||
| Mood Disorders | ||||
| SCZ | ||||
| 1q21.1 | Duplication | ADHD | CHD (inc ToF) | [69, 70, 71, 72, 73] |
| ASD | Macrocephaly | |||
| GAD | Facial dysmorphism | |||
| ID | ||||
| Mood Disorders | ||||
| SCZ | ||||
| 2p16.3 (NRXN1) | Deletion | ASD | CHD | [74, 75, 76] |
| ADHD | Craniofacial abnormalities | |||
| BPD | Seizures | |||
| Dysexecutive syndrome | ||||
| ID | ||||
| SCZ | ||||
| 3q29 | Deletion | ASD | GI abnormalities | [77, 78, 79, 80, 81] |
| BPD | Microcephaly | |||
| DD | Cleft palate | |||
| SCZ | Chest wall deformity | |||
| Cardiac malformations | ||||
| 15q11.2 | Deletion | DD | CHD | [82, 83, 84, 85] |
| Epilepsy | Ataxia/balance issues | |||
| NDDs | Facial dysmorphism | |||
| SCZ | ||||
| SLD | ||||
| 15q13.3 | Deletion | ASD | Mild facial dysmorphism | [8,86] |
| Epilepsy | Skeletal defects | |||
| OCD | ||||
| SCZ | ||||
| 16p11.2 | Deletion | ADHD | High BMI | [30,87] |
| ASD | Hypotonia | |||
| ID | Macrocephaly | |||
| 16p11.2 | Duplication | ADHD (Dup > Del) | Low BMI | [30,87,88] |
| ID | Microcephaly | |||
| SCZ | ||||
| 22q11.2 | Deletion | Anxiety Disorders | CHD | [32,47,51,58,61,89, 90, 91] |
| Early-onset Parkinson’s Disease | Craniofacial abnormalities | |||
| Psychotic disorder | Hypoparathyroidism | |||
| Immunodeficiency | ||||
| 22q11.2 | Duplication | ASD | CHD | [26••,92, 93, 94] |
| Protective against schizophrenia | Craniofacial abnormalities | |||
| Gastric reflux |
ASD: Autism Spectrum Disorder, ADHD: Attention Deficit Hyperactivity Disorder, BPD: Bipolar Disorder, ID: Intellectual Disability, DD: Developmental Delay, CHD: Congenital Heart Disease, GAD: Generalised Anxiety Disorder, GI: Gastrointestinal, ID: Intellectual Disability, NDDs: Neurodevelopmental Disorders, SCZ: Schizophrenia, SLD: Specific Learning Disorder.
Clinical evaluation: children
Children with neuropsychiatric CNVs display substantial clinical heterogeneity, which underscores the importance of accessing appropriate multi-specialist care, from speech and language therapists assisting language skills in one carrier with palatal abnormalities and delayed development, to an older child having growth difficulties during puberty. Collateral information from family members can give important insight into prenatal abnormalities or complications, developmental milestones and give an understanding of the patients’ social interactions. Genetic counselling and cascade genetic testing in family members may be appropriate to determine if the variant is de novo or inherited and whether other family members are affected.
A thorough history should be obtained from patients and their family, systematically covering medical and psychiatric morbidity. Children with CNVs have a range of neuropsychiatric deficits which cross traditional diagnostic boundaries and comorbidity is common [29••]. In managing these, clinicians should carefully consider the following: disparate disorders such as ID, ADHD, schizophrenia and autism have considerable overlap at phenomenological and genetic levels [34, 35, 36]. Moreover, comorbidity can result in diagnostic overshadowing, whereby all clinical symptoms are attributed to a primary diagnosis rather than to additional comorbidity. This occurs particularly in cases of ID [37], and can impede diagnosis of other treatable conditions. CNV carriers are also likely to experience clinically impairing symptoms which do not fit traditional categorical diagnoses, and clinicians should consider dimensional and functional domains of impairment such as attention, social functioning, affect and executive functioning [38] rather than focussing solely on categorical diagnostic criteria. One illustrative example is that many carriers do not meet criteria for autism diagnosis yet have clear functional impairments in domains such as social functioning [39•]. Also diagnosing psychiatric conditions within the context of multimorbid physical health problems can provide challenges, for instance cleft palate problems can make it difficult to assess the communication domain of autism [40]. Multi-informant interviews with parent and child are important, particularly for psychotic phenomena when the child may have experiences that the parent is not aware of [41]. It is important that the mental health needs of the child’s parents are not neglected, as compared to the general UK population parents of CNV carriers have are more likely to experience emotional distress [42•]; parents of child CNV carriers may need help to access respite care, social support, disability benefits, and counselling.
As well as psychiatric functioning, cognitive function should be gauged in children, formally through an educational assessment and neuropsychology if available. If access to these is limited locally, at the very least, a thorough clinical and educational history should be taken, augmented by bedside assessment of cognitive function as part of the mental state examination. It is advised that clinicians should consider the cognitive phenotype of the child in relation to that of family members who do not have the CNV. Cognitive function in CNV carriers may be modified by the effects of parental IQ [43], and a carrier with average cognitive function in the context of a highly functioning family may experience social and psychological impacts that need to be addressed.
It is also important for clinicians to consider whether treatments for typically developing children are suitable or require modification for CNV carriers for a variety of reasons including developmental delay, sensory impairments, multimorbidity of physical health problems, and sensitivity to adverse effects [44••]. For example, CNV carriers who experience cognitive and social difficulties, may find it difficult to access and benefit from therapies such as cognitive behavioural therapy and adaptations to therapies such as shorter sessions, frequent breaks and repetition of content should be considered [45]. There is also evidence that CNV carriers with autism benefit less from social skills training than children with autism but without a CNV [46•].
A comprehensive physical examination is vital to cover a range of potential physical comorbidities [47]. After an initial workup, coordinated care allows careful management of the evolving clinical needs of a carrier through their development. For 22q11.2 deletion, consensus guidelines exist which guide multidisciplinary clinics in conducting a thorough post-diagnosis screening [32], recommendations include immunologic evaluation, calcium levels investigation, parathyroid hormone level checks, ophthalmology, audiology, scoliosis examination, palate evaluation, renal ultrasound, and echocardiogram. For other neuropsychiatric CNVs there is emerging evidence of the complexity of their phenotypes (see Table 1), broadly we recommend that a physical examination that covers the major bodily systems including; heart and circulatory function [15,16], neurological examination [22,23], immunologic evaluation, endocrinology check [20,21], musculoskeletal examination, palate evaluation [19], audiology and ophthalmology. Physical assessment should also consider motor function, integrating physical and occupational therapists if necessary, as this is often impaired in carriers [31]. Follow-up screenings across development are necessary as the clinical phenotype changes with age and new medical features may emerge.
Clinical evaluation: adults
The clinical evaluation of adults with neuropsychiatric CNVs has specific considerations. Although it is likely that carriers with severe congenital and dysmorphic phenotypes will be identified by paediatric services, carriers with more subtle phenotypes may not be referred to genetic testing until adulthood. Adults may present with a personal or familial history of learning difficulties, neurodevelopmental problems, and congenital abnormalities [25,48••,49]. Although in many instances the CNV will have occurred de novo, the presence of a family history of these conditions may well impact on the health and wellbeing of the index case. The utility of genetic testing in adults has been demonstrated in genomic screening of a US healthcare system, where only a minority of adult CNV carriers (41 of 708) had previously received a genetic diagnosis [48••]. CNV carriers described relief at a genetic explanation for a ‘lifelong history of learning and behavioural struggles’.
The majority of clinical studies in CNV carriers have focused on children referred to genetics clinics, but a wide range of physical comorbidities in adults are emerging from population studies of CNV carriers such as those in the UK biobank [26••,50], and it is, therefore, important that adults are screened broadly for physical symptoms using a multidisciplinary approach. Medical phenotypes associated with CNVs in the UK biobank can be searched online (https://kirov.psycm.cf.ac.uk/).
Adult CNV carriers are at risk for major psychiatric disorders including schizophrenia and depression [8,9•], and childhood diagnoses can persist into adulthood [51]. Similar to our recommendations for children, management and treatment should be planned in the context of any cognitive or physical comorbidities, and pharmacotherapy should be based on a consideration of any medical comorbidities and sensitivity to adverse effects [44••]. For 22q11.2DS a literature is building on psychopharmacotherapy effectiveness and side effects [44••], particularly so for the administration of antipsychotics [52, 53, 54], but further research is needed for other CNVs.
The educational, relationship and employment needs of adult CNV carriers should be considered. Compared to the general population, CNV carriers as a group have lower educational achievement, struggle with employment, and have reduced fecundity [55,56•,57]. However, it should be highlighted that, although on average adult CNV carriers are more likely to experience such difficulties, there is considerable variability and many adults achieve highly in terms of academic and employment success [55]. Thus, it is important to consider carefully the needs of individual patients. Some CNV carriers are highly vulnerable and may require complex social and psychological support. For instance, individuals with 22q11.2DS are at increased risk of financial, emotional and sexual abuse [58]. There is also a need to consider specialist reproductive counselling, which should build on precedents set in 22q11.2DS, where risks of unsafe sex, and prenatal transmission need to be communicated appropriately [58,59], and should be consolidated and monitored across the reproductive age window [32].
For those carriers identified in childhood, effective transition to adult specialty care from paediatric services is crucial. The current reality in many healthcare systems is that support received from paediatric services often stops as soon as the child reaches the service’s age cut-off with little regard to whether the patient and family still need support, and whether care has been effectively transferred to relevant adult services. Early adulthood offers a clinical juncture for psychiatric reassessment and monitoring of known carriers, given this is this the time when late-onset psychopathology in particular psychosis may manifest [60]. Increasing recognition of CNVs and better longitudinal studies are also beginning to give insights into the long-term health of carriers. As the life-expectancy of CNV carriers increases with better care, attention should be paid to the possibility of neurodegenerative impact within these neurodevelopmental syndromes. For 22q11.2DS it has emerged that carriers have increased risk of early-onset Parkinson’s Disease [61].
Potential for early intervention
Genomic diagnoses are increasingly being made early in development, and provide potential for genomically informed early intervention to ameliorate later risk. This is already occurring for physical health; it has been recommended that all individuals diagnosed with 22q11.2DS have an echocardiogram regardless of presenting phenotype [32] to identify cardiac abnormalities early. There is also clear potential to develop early intervention approaches for psychiatric outcomes in CNV carriers. For instance, several studies have identified early behavioural predictors of psychotic outcomes in 22q11.2DS, [41,62, 63, 64, 65]. Among 16p11.2 deletion carriers, behavioural changes in satiety precede obesity [66], and early motor development may predict autism in 16p11.2 deletion carriers [67]. A barrier to translating this knowledge clinically is that these predictions have been at the level of the group, and clinical risk algorithms that can be applied to individuals are needed. Despite this, clinical practitioners should be alert for identifying early prodromal changes and be willing to refer to appropriate specialties. Clinicians should also ensure both patient and family are informed to the potential psychiatric sequalae as part of genetic counselling support following genetic diagnosis [68].
Conclusion
We have laid out recommendations for the clinical evaluation and management of individuals with neuropsychiatric risk CNVs based on current knowledge. We are aware that in most instances these are fairly generic, and constitute little more than good clinical practice, including the need to keep abreast of a rapidly developing field, and to consider the possibility of physical, psychiatric, psychological and social manifestations. To make further advances we need systematically to collect and collate more data on the pleiotropic outcomes of CNVs, life course changes, and the specific impact of different interventions. As other articles in this issue demonstrate, progress is being made, and the clinical knowledge base will improve as findings accrue from longitudinal studies of carriers, population studies and clinical case reports [25].
A current challenge to clinical management is the frequent need for multidisciplinary care across several specialties. Healthcare systems are not necessarily designed to support individuals who require coordinated management across multiple medical specialities. Solutions to this will vary from one healthcare system to another; for example, in the UK, General Practitioners are well placed to coordinate referral to other specialities, but many will require education in understanding the specific risks posed by different CNVs. A promising potential model would be to assign the family a health professional who coordinates care across specialities, navigating the family through the complex health problems of their child. Third sector organisations are currently trialling this model, including the UK ‘Same but Different’ organisation’s ‘Rare Navigator’ service (https://www.samebutdifferentcic.org.uk/rare-navigator). From a psychiatric perspective, there is clearly a need to embed an understanding genetics and genomics in residency training programmes [25], and a need for more cross disciplinary interactions with other specialities in particular medical genetics.
It can be argued that, given the complex admixture of developmental, psychiatric and physical outcomes of CNVs, carriers and their families should have access to specialist clinics in which practitioners have the knowledge and experience to coordinate management and to provide treatment, counselling and support. In this regard, some healthcare systems have introduced multidisciplinary clinics focused on individual CNVs such as 22q11.2 Deletion Syndrome (https://www.22qsociety.org/downloads/22q_Clinics_Around_The_World.pdf), others have created rare disease (for example the Centre for Rare Diseases at the University Hospital Birmingham, UK, https://www.uhb.nhs.uk/centre-for-rare-diseases.htm) and behavioural genetic services (for example the Autism Assessment and Behavioural Genetics Service at the Maudsley hospital, UK, https://www.slam.nhs.uk/our-services/service-finder-details?CODE=SU0295) that provide support for a range of genomic conditions. Further, serious consideration is needed by health systems and policymakers so such services can be offered more widely to those with CNVs and other genomic conditions.
In conclusion, individuals with neuropsychiatric CNVs require multi-system assessment and may need co-ordinated, individualised, multidisciplinary care depending on the nature and extent of multimorbidity. Assessment and care should also include consideration of a range of biological, psychological and social factors. Management should be developmentally appropriate, evolve across the life course, and clinicians should be alert for opportunities to instigate early intervention to ameliorate later risk. As CNVs and other genomic conditions are increasingly recognised, health care systems will need to adapt so they can provide appropriate individualised care to their patients.
Conflict of interest statement
MJO reports a grant from Takeda Pharmaceuticals outside of the submitted work. SJRAC and CJW declare no competing interests.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was funded by a Wellcome Trust Strategic Award (MJO, 503147), Health & Care Research Wales (MJO, Welsh Government, 507556), Medical Research Council Centre grant (MJO, G0801418), Medical Research Council Programme grant (MJO, G0800509), Medical Research Council and Medical Research Foundation grants (IMAGINE-ID study MJO; MR/L011166/1 and MR/N022572/1), and a Wellcome Trust ISSF Fellowship (SJRAC).
Appendix A. List of useful resources
We have curated a list of resources for clinicians and researchers that provides detailed information on the clinical phenotypes of a range of copy number variants.
Max Appeal! Consensus document for 22q11.2 Deletion Syndrome
The Consensus Document is a comprehensive but practical and accessible information resource which has had contributions from major centres across the UK, stakeholder organisations, families and over 50 experts (either as authors or advisers) working in the major clinical fields associated with 22q11 deletion. The Committee hopes that the guidance and information supplied will be of significant material benefit to all patients and families and those who provide care and support to them. In particular, given the heterogeneous clinical impact of 22q11DS, it is hoped that the document will be of broad professional interest, relevance and utility.
Simons Searchlight resource for autism associated genetic variants
Simons Searchlight is a partnership of leading scientists, researchers, and families. Simons Searchlight has compiled resources and information on a range of copy number variants and genes related to autism.
https://www.simonssearchlight.org/research/what-we-study/
Unique — The Rare Chromosome Disorder Support Group
Unique is a voluntary organization dedicated to promoting awareness of rare chromosomal abnormalities. The Unique charity has developed free Information Guides to specific chromosome and gene disorders, as well as guides translated into various languages. Guides written by Unique are reviewed on a voluntary basis by clinical geneticists and other professionals working in this field.
Medical phenotypes of CNV carriers in the UK biobank
Data from UK biobank on the medical phenotypes have been made searchable for researchers [26••,50] (https://kirov.psycm.cf.ac.uk/). It should be noted that data are provided ‘as is’, and without warranty, for scientific and educational use only.
Measuring the Impact on NeuroDevelopment of CNV (MIND·CNV)
This tool was created to help clinicians estimate the cognitive effect of undocumented deletions based on the results of Huguet et al. [95]. The tool estimates the loss of intellectual quotient (IQ) points related to a deletion as well as the estimated probability for that deletion to be de novo. (http://www.minds-genes.org/Site_EN/CNVsPredictionTools.html).
References
- 1.Lee C., Scherer S.W. The clinical context of copy number variation in the human genome. Expert Rev Mol Med. 2010;12:e8. doi: 10.1017/S1462399410001390. [DOI] [PubMed] [Google Scholar]
- 2.Kirov G., Rees E., Walters J. What a psychiatrist needs to know about copy number variants. Adv Psychiatr Treat. 2015;21:157–163. [Google Scholar]
- 3.Murphy K.C., Jones L.A., Owen M.J. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56 doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 4.Cooper G.M. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams N.M. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry. 2012;169:195–204. doi: 10.1176/appi.ajp.2011.11060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto D. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone J.L. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall C.R. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–32135. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Kendall K.M. Association of rare copy number variants with risk of depression. JAMA Psychiatry. 2019;76:818–825. doi: 10.1001/jamapsychiatry.2019.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this case-control study of 407 074 individuals in the UK Biobank study, neurodevelopmental Risk CNVs were associated with increased risk of depression in those without neurodevelopmental disorders.
- 10.Malhotra D., Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva M. European guidelines for constitutional cytogenomic analysis. Eur J Hum Genet. 2019;27:1–16. doi: 10.1038/s41431-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretelle F. Prenatal and postnatal diagnosis of 22q11. 2 deletion syndrome. Eur J Med Genet. 2010;53:367–370. doi: 10.1016/j.ejmg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Pletcher B.A. Indications for genetic referral: a guide for healthcare providers. Genet Med. 2007;9:385–389. doi: 10.1097/GIM.0b013e318064e70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thevenon J. Diagnostic odyssey in severe neurodevelopmental disorders: toward clinical whole‐exome sequencing as a first‐line diagnostic test. Clin Genet. 2016;89:700–707. doi: 10.1111/cge.12732. [DOI] [PubMed] [Google Scholar]
- 15.Brosig C.L. Preschool neurodevelopmental outcomes in children with congenital heart disease. J Pediatr. 2017;183:80–86. doi: 10.1016/j.jpeds.2016.12.044. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y. Association of rare recurrent copy number variants with congenital heart defects based on next generation sequencing data from family trios. Front Genet. 2019;10:819. doi: 10.3389/fgene.2019.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capkova Z. Differences in the importance of microcephaly, dysmorphism, and epilepsy in the detection of pathogenic CNVs in ID and ASD patients. PeerJ. 2019;7 doi: 10.7717/peerj.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallgrímsson B. Automated syndrome diagnosis by three-dimensional facial imaging. Genet Med. 2020:1–12. doi: 10.1038/s41436-020-0845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simioni M. Investigation of genetic factors underlying typical orofacial clefts: mutational screening and copy number variation. J Hum Genet. 2015;60:17–25. doi: 10.1038/jhg.2014.96. [DOI] [PubMed] [Google Scholar]
- 20.Cheung E.N.M. Prevalence of hypocalcaemia and its associated features in 22q11· 2 deletion syndrome. Clin Endocrinol. 2014;81:190–196. doi: 10.1111/cen.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters R.G. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463 doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessi M. Rare copy number variations and predictors in children with intellectual disability and epilepsy. Front Neurol. 2018;9:947. doi: 10.3389/fneur.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppola A. Diagnostic implications of genetic copy number variation in epilepsy plus. Epilepsia. 2019;60:689–706. doi: 10.1111/epi.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macé A. CNV-association meta-analysis in 191,161 European adults reveals new loci associated with anthropometric traits. Nat Commun. 2017;8:744. doi: 10.1038/s41467-017-00556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan P.F., Owen M.J. Increasing the clinical psychiatric knowledge base about pathogenic copy number variation. Am J Psychiatry. 2020;177:204–209. doi: 10.1176/appi.ajp.2019.19040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Crawford K. Medical consequences of pathogenic CNVs in adults: analysis of the UK Biobank. J Med Genet. 2019;56:131–138. doi: 10.1136/jmedgenet-2018-105477. https://kirov.psycm.cf.ac.uk/ [DOI] [PubMed] [Google Scholar]; This case-control study examined the medical phenotypes of 54 pathogenic neuropsychiatric CNVs in adults (each group ranging from 5 to 2843 persons) in comparison to 381 452 controls. Medical phenotypes associated with CNVs in the UK biobank can be searched online (https://kirov.psycm.cf.ac.uk/).
- 27.DiGeorge A. A new concept of the cellular base of immunology. J Pediatr. 1965;67:907–908. [Google Scholar]
- 28.Shprintzen R. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- 29••.Chawner S.J. Genotype–phenotype associations in children with copy number variants associated with high neuropsychiatric risk in the UK (IMAGINE-ID): a case-control cohort study. Lancet Psychiatry. 2019;6:493–505. doi: 10.1016/S2215-0366(19)30123-3. [DOI] [PubMed] [Google Scholar]; This study of 258 children identified via UK medical genetic clinics found that pathogenic neuropsychiatric risk CNVs have pleiotropic effects across a range of psychiatric, neurodevelopmental and cognitive domains.
- 30.Niarchou M. Psychiatric disorders in children with 16p11. 2 deletion and duplication. Transl Psychiatry. 2019;9:8. doi: 10.1038/s41398-018-0339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham A.C. Coordination difficulties, IQ and psychopathology in children with high-risk copy number variants. Psychol Med. 2019:1–10. doi: 10.1017/S0033291719003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassett A.S. Practical guidelines for managing patients with 22q11. 2 deletion syndrome. J Pediatr. 2011;159:332–339. doi: 10.1016/j.jpeds.2011.02.039. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison S. Cognitive deficits in childhood, adolescence and adulthood in 22q11.2 deletion syndrome and association with psychopathology. Transl Psychiatry. 2020;10:53. doi: 10.1038/s41398-020-0736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen M.J. Intellectual disability and major psychiatric disorders: a continuum of neurodevelopmental causality. Br J Psychiatry. 2012;200:268–269. doi: 10.1192/bjp.bp.111.105551. [DOI] [PubMed] [Google Scholar]
- 35.Simonoff E. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 36.Doherty J.L., Owen M.J. Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med. 2014;6:29. doi: 10.1186/gm546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thapar A. Intellectual disability and attention-deficit/hyperactivity disorder: what does the clinical and genetic overlap mean for practice and research? J Am Acad Child Adolesc Psychiatry. 2017;56:105–106. doi: 10.1016/j.jaac.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Baker K., Vorstman J.A. Is there a core neuropsychiatric phenotype in 22q11.2 deletion syndrome? Curr Opin Neurol. 2012;25:131–137. doi: 10.1097/WCO.0b013e328352dd58. [DOI] [PubMed] [Google Scholar]
- 39•.Chawner S. A genetics-first approach to dissecting the heterogeneity of autism: phenotypic comparison of autism risk copy number variants. Am J Psychiatry. 2021;178:77–86. doi: 10.1176/appi.ajp.2020.20010015. [DOI] [PMC free article] [PubMed] [Google Scholar]; This international study of 547 individuals with a CNV at either 16p11.2 or 22q11.2 loci found that many CNV carriers who do not meet criteria for an autism diagnosis nonetheless have clinical domain-based impairments in domains.
- 40.Eliez S. Autism in children with 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry. 2007;46:433–434. doi: 10.1097/CHI.0b013e31802f5490. [DOI] [PubMed] [Google Scholar]
- 41.Chawner S.J. The emergence of psychotic experiences in the early adolescence of 22q11. 2 deletion syndrome. J Psychiatr Res. 2019;109:10–17. doi: 10.1016/j.jpsychires.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Baker K. Childhood intellectual disability and parents’ mental health: integrating social, psychological and genetic influences. Br J Psychiatry. 2020:1–8. doi: 10.1192/bjp.2020.38. [DOI] [PubMed] [Google Scholar]; A UK study of 888 parents of child with a neurodevelopmental risk genetic variant highlighted the mental health needs parents are not neglected, as compared to the general UK population, which found that parents of CNV carriers were more likely to experience emotional distress.
- 43.Moreno-De-Luca A. The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11. 2 deletions. JAMA Psychiatry. 2015;72:119–126. doi: 10.1001/jamapsychiatry.2014.2147. [DOI] [PubMed] [Google Scholar]
- 44••.Mosheva M. Effectiveness and side effects of psychopharmacotherapy in individuals with 22q11. 2 deletion syndrome with comorbid psychiatric disorders: a systematic review. Eur Child Adolesc Psychiatry. 2019:1–14. doi: 10.1007/s00787-019-01326-4. [DOI] [PubMed] [Google Scholar]; This systematic review retrieved psychopharmocotherapy data from 182 patients with 22q11.2DS. Recommendations emphasised the importance of the potential interaction of pharmacotherapy on the common medical comorbidities seen in carriers.
- 45.Fjermestad K.W., Vatne T.M., Gjone H. Cognitive behavioral therapy for adolescents with 22q11. 2 deletion syndrome. Adv Mental Health Intell Disabil. 2015;9:30–39. [Google Scholar]
- 46•.Tammimies K. Association between copy number variation and response to social skills training in autism spectrum disorder. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-46396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This randomised clinical trial provides preliminary evidence that those with ASD and large pathogenic CNVs (>500 kb) may not experience the same benefit from social skills group training as individuals with ASD without a pathogenic CNV.
- 47.McDonald-McGinn D.M. 22q11. 2 deletion syndrome. Nat Rev Dis Primers. 2015;1:1–19. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Martin C.L. Identification of neuropsychiatric copy number variants in a health care system population. JAMA Psychiatry. 2020;77:1276–1285. doi: 10.1001/jamapsychiatry.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study screened for pathogenic neuropsychiatric CNVs within an entire healthcare system in the USA and identified pathogenic CNVs in 708 of 90 595 participants. Only 5.8% of those identified had a previously known genomic diagnosis despite the majority of carriers having clinically associated symptoms; anxiety and depression were enriched in the CNV group. Disclosure of genetic results often led to positive reactions from patients.
- 49.Foley C. Identifying schizophrenia patients who carry pathogenic genetic copy number variants using standard clinical assessment: retrospective cohort study. Br J Psychiatry. 2020;216:275–279. doi: 10.1192/bjp.2019.262. [DOI] [PubMed] [Google Scholar]
- 50.Owen D. Effects of pathogenic CNVs on physical traits in participants of the UK Biobank. BMC Genomics. 2018;19:867. doi: 10.1186/s12864-018-5292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider M. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry. 2014;171:627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhoeven W.M., Egger J.I. Atypical antipsychotics and relapsing psychoses in 22q11.2 deletion syndrome: a long-term evaluation of 28 patients. Pharmacopsychiatry. 2015;48:104–110. doi: 10.1055/s-0034-1398612. [DOI] [PubMed] [Google Scholar]
- 53.de Boer J. Adverse effects of antipsychotic medication in patients with 22q11. 2 deletion syndrome: a systematic review. Am J Med Genet A. 2019;179:2292–2306. doi: 10.1002/ajmg.a.61324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butcher N.J. Response to clozapine in a clinically identifiable subtype of schizophrenia. Br J Psychiatry. 2015;206:484–491. doi: 10.1192/bjp.bp.114.151837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendall K.M. Cognitive performance among carriers of pathogenic copy number variants: analysis of 152,000 UK biobank subjects. Biol Psychiatry. 2017;82:103–110. doi: 10.1016/j.biopsych.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 56•.Mosheva M. Education and employment trajectories from childhood to adulthood in individuals with 22q11. 2 deletion syndrome. Eur Child Adolesc Psychiatry. 2019;28:31–42. doi: 10.1007/s00787-018-1184-2. [DOI] [PubMed] [Google Scholar]; This study analysed the educational and occupational profiles of 22q11.2 deletion carriers and demonstrated that carriers demonstrate vast heterogeneity in attainment outcomes. Psychotic symptoms were not associated with employment, whereas cognitive and adaptive functions were a better predictor of school and occupational profiles.
- 57.Stefansson H. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–366. doi: 10.1038/nature12818. [DOI] [PubMed] [Google Scholar]
- 58.Fung W.L.A. Practical guidelines for managing adults with 22q11. 2 deletion syndrome. Genet Med. 2015;17:599–609. doi: 10.1038/gim.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inglis A., Morris E., Austin J. Prenatal genetic counselling for psychiatric disorders. Prenat Diagn. 2017;37:6–13. doi: 10.1002/pd.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Os J., Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 61.Mok K.Y. Deletions at 22q11. 2 in idiopathic Parkinson’s disease: a combined analysis of genome-wide association data. Lancet Neurol. 2016;15:585–596. doi: 10.1016/S1474-4422(16)00071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vorstman J.A. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72:377–385. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang S.X., Gur R.E. Longitudinal perspectives on the psychosis spectrum in 22q11. 2 deletion syndrome. Am J Med Genet A. 2017;176:2192–2202. doi: 10.1002/ajmg.a.38500. [DOI] [PubMed] [Google Scholar]
- 64.Schneider M. Ultra high risk status and transition to psychosis in 22q11. 2 deletion syndrome. World Psychiatry. 2016;15:259–265. doi: 10.1002/wps.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antshel K.M. Predicting cognition and psychosis in young adults with 22q11. 2 deletion syndrome. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maillard A. 16p11. 2 locus modulates response to satiety before the onset of obesity. Int J Obes. 2016;40:870–876. doi: 10.1038/ijo.2015.247. [DOI] [PubMed] [Google Scholar]
- 67.Bernier R. Developmental trajectories for young children with 16p11. 2 copy number variation. Am J Med Genet B Neuropsychiatr Genet. 2017;174:367–380. doi: 10.1002/ajmg.b.32525. [DOI] [PubMed] [Google Scholar]
- 68.van den Bree M.B. The internet is parents’ main source of information about psychiatric manifestations of 22q11.2 deletion syndrome (22q11.2DS) Eur J Med Genet. 2013;56:439–441. doi: 10.1016/j.ejmg.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunetti-Pierri N. Recurrent reciprocal 1q21. 1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40:1466. doi: 10.1038/ng.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costain G., Silversides C.K., Bassett A.S. The importance of copy number variation in congenital heart disease. NPJ Genomic Med. 2016;1:1–11. doi: 10.1038/npjgenmed.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernier R. Clinical phenotype of the recurrent 1q21. 1 copy-number variant. Genet Med. 2016;18:341–349. doi: 10.1038/gim.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linden S.C. The psychiatric phenotypes of 1q21 distal deletion and duplication. Transl Psychiatry. 2021 doi: 10.1038/s41398-021-01226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dolcetti A. 1q21. 1 microduplication expression in adults. Genet Med. 2013;15:282–289. doi: 10.1038/gim.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al Shehhi M. NRXN1 deletion syndrome; phenotypic and penetrance data from 34 families. Eur J Med Genet. 2019;62:204–209. doi: 10.1016/j.ejmg.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 75.Castronovo P. Phenotypic spectrum of NRXN1 mono‐and bi‐allelic deficiency: a systematic review. Clin Genet. 2020;97:125–137. doi: 10.1111/cge.13537. [DOI] [PubMed] [Google Scholar]
- 76.Viñas-Jornet M. A common cognitive, psychiatric, and dysmorphic phenotype in carriers of NRXN1 deletion. Mol Genet Genomic Med. 2014;2:512–521. doi: 10.1002/mgg3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mulle J.G. GeneReviews®[Internet] University of Washington; Seattle: 2017. 3q29 recurrent deletion. [Google Scholar]
- 78.Cox D.M., Butler M.G. A clinical case report and literature review of the 3q29 microdeletion syndrome. Clin Dysmorphol. 2015;24:89. doi: 10.1097/MCD.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glassford M.R. Novel features of 3q29 deletion syndrome: results from the 3q29 registry. Am J Med Genet A. 2016;170:999–1006. doi: 10.1002/ajmg.a.37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollak R.M. Neuropsychiatric phenotypes and a distinct constellation of ASD features in 3q29 deletion syndrome: results from the 3q29 registry. Mol Autism. 2019;10:30. doi: 10.1186/s13229-019-0281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pollak R.M. New phenotypes associated with 3q29 duplication syndrome: results from the 3q29 registry. Am J Med Genet A. 2020;182:1152–1166. doi: 10.1002/ajmg.a.61540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cox D.M., Butler M.G. The 15q11. 2 BP1–BP2 microdeletion syndrome: a review. Int J Mol Sci. 2015;16:4068–4082. doi: 10.3390/ijms16024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Der Meer D. Association of copy number variation of the 15q11. 2 BP1-BP2 region with cortical and subcortical morphology and cognition. JAMA Psychiatry. 2020;77:420–430. doi: 10.1001/jamapsychiatry.2019.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silva A.I. Reciprocal white matter changes associated with copy number variation at 15q11. 2 BP1-BP2: a diffusion tensor imaging study. Biol Psychiatry. 2019;85:563–572. doi: 10.1016/j.biopsych.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jønch A.E. Estimating the effect size of the 15Q11. 2 BP1–BP2 deletion and its contribution to neurodevelopmental symptoms: recommendations for practice. J Med Genet. 2019;56:701–710. doi: 10.1136/jmedgenet-2018-105879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharp A.J. A recurrent 15q13. 3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet. 2008;40:322–328. doi: 10.1038/ng.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinman K.J. 16p11. 2 deletion and duplication: characterizing neurologic phenotypes in a large clinically ascertained cohort. Am J Med Genet A. 2016;170:2943–2955. doi: 10.1002/ajmg.a.37820. [DOI] [PubMed] [Google Scholar]
- 88.D’Angelo D. Defining the effect of the 16p11. 2 duplication on cognition, behavior, and medical comorbidities. JAMA Psychiatry. 2016;73:20–30. doi: 10.1001/jamapsychiatry.2015.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campbell I.M. What is new with 22q? An update from the 22q and You Center at the Children’s Hospital of Philadelphia. Am J Med Genet A. 2018;176:2058–2069. doi: 10.1002/ajmg.a.40637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson O. Palatal evaluation and treatment in 22q11. 2 deletion syndrome. Am J Med Genet A. 2019;179:1184–1195. doi: 10.1002/ajmg.a.61152. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan K.E. Chromosome 22q11. 2 deletion syndrome and DiGeorge syndrome. Immunol Rev. 2019;287:186–201. doi: 10.1111/imr.12701. [DOI] [PubMed] [Google Scholar]
- 92.Rees E. Evidence that duplications of 22q11.2 protect against schizophrenia. Mol Psychiatry. 2014;19:37–40. doi: 10.1038/mp.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wenger T.L. 22q11. 2 duplication syndrome: elevated rate of autism spectrum disorder and need for medical screening. Mol Autism. 2016;7:1. doi: 10.1186/s13229-016-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu A. Genotypic and phenotypic variability of 22q11. 2 microduplications: an institutional experience. Am J Med Genet A. 2019;179:2178–2189. doi: 10.1002/ajmg.a.61345. [DOI] [PubMed] [Google Scholar]
- 95.Huguet G. Measuring and estimating the effect sizes of copy number variants on general intelligence in community-based samples. JAMA Psychiatry. 2018;75:447–457. doi: 10.1001/jamapsychiatry.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]